Abstract
Background
Clinical trials of interventions for preventing malaria in pregnancy often use measures of malaria at delivery as their primary outcome. Although the objective of these interventions is to improve birth outcomes, data on associations between different measures of malaria at delivery and adverse birth outcomes are limited.
Methods
Data came from 637 Ugandan women enrolled in a randomized controlled trial of intermittent preventive treatment of malaria in pregnancy. Malaria at delivery was detected using peripheral and placental blood microscopy, placental blood loop-mediated isothermal amplification (LAMP), and placental histopathology. Multivariate analyses were used to estimate associations between measures of malaria at delivery and risks of low birth weight (LBW), small for gestational age (SGA), and preterm birth (PTB).
Results
Detection of malaria parasites by microscopy or LAMP was not associated with adverse birth outcomes. Presence of malaria pigment detected by histopathology in ≥30% of high-powered fields was strongly associated with LBW (adjusted risk ratio [aRR] = 3.42, P = .02) and SGA (aRR = 4.24, P < .001) but not PTB (aRR = 0.88, P = .87).
Conclusions
A semiquantitative classification system based on histopathologically detected malaria pigment provided the best surrogate measure of adverse birth outcomes in a high-transmission setting and should be considered for use in malaria in pregnancy intervention studies.
Keywords: low birth weight, malaria in pregnancy, placental malaria, preterm birth, small for gestational age
In a high-transmission setting, semiquantitative classification of malaria pigment by histopathology was the best surrogate measure of adverse birth outcomes. Detection of malaria parasites by microscopy or loop-mediated isothermal amplification of DNA was not associated with any adverse birth outcomes.
Malaria in pregnancy (MiP) remains a major public health problem. In sub-Saharan Africa, where the burden of malaria is higher than any other region and access to preventive measures is limited [1], an estimated 11 million women were at risk for MiP in 2018 [2]. Most women in sub-Saharan Africa are semi-immune to malaria and remain asymptomatic when infected with Plasmodium falciparum during pregnancy. However, parasites can sequester in the placenta and compromise its function, contributing to adverse birth outcomes that include low birth weight (LBW), small for gestational age (SGA), and preterm birth (PTB) [3]. To protect women against the adverse effects of MiP, the World Health Organization recommends intermittent preventive treatment of MiP (IPTp) with sulfadoxine pyrimethamine (SP) [4]. However, the effectiveness of IPTp with SP has been threatened by the spread of high level parasite resistance [5].
Although prevention of adverse birth outcomes (primarily LBW) has been a focus of policy recommendations [6], most clinical trials evaluating interventions to prevent MiP have used measures of malaria at delivery as their primary outcome. These include detection of malaria parasites in peripheral or placental blood by microscopy, rapid diagnostic tests, and/or deoxyribonucleic acid (DNA) amplification, as well as the use of placental histopathology to detect malaria parasites, hemozoin pigment, and inflammatory changes associated with placental malaria [7]. Placental histopathology can detect malaria pigment (hemozoin) persisting from placental infections occurring earlier in gestation [8]. Although such malaria-specific outcomes are intended to serve as surrogates of adverse birth outcomes, there is no consensus on what outcome(s) should be used and limited data on the relationships between different measures of malaria at delivery and specific adverse birth outcomes.
Given widespread resistance to SP in many parts of sub-Saharan Africa, 3 recent randomized controlled trials (RCTs) from East Africa focused on the artemisinin-based combination therapy dihydroartemisinin-piperaquine (DP) as an alternative to SP for IPTp [9–11]. All 3 studies showed that DP was more effective than SP at reducing the risk of malaria at delivery, but none were able to detect any significant differences in adverse birth outcomes. For the present study, we utilized data from the most recent of these RCTs conducted in Uganda to assess associations between various measures of malaria at delivery using placental blood microscopy, placental blood loop-mediated isothermal amplification (LAMP), and placental histopathology with different adverse birth outcomes including LBW, SGA, and PTB.
METHODS
Study Setting and Participants
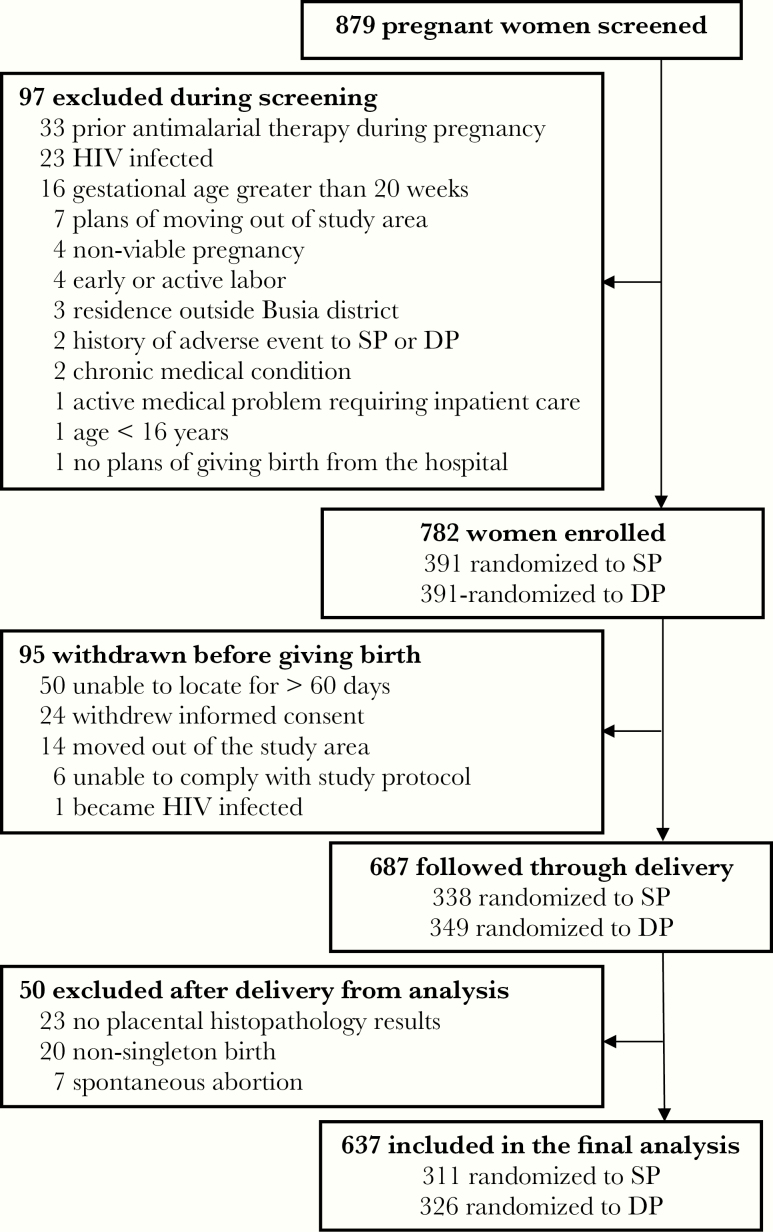
Data for the present study came from a previously published double-blind, RCT comparing monthly IPTp with SP versus monthly IPTp with DP in Busia District, an area in southeastern Uganda where malaria transmission is perennial and holoendemic [11]. In brief, between September 2016 and May 2017, 782 human immunodeficiency virus-uninfected pregnant women at least 16 years of age and between 12 and 20 gestation weeks were enrolled and allocated to treatment groups, receiving either SP or DP given every 4 weeks starting at 16 or 20 weeks gestational age. For the present study, all women with a singleton delivery at ≥28 weeks gestational age with placental histopathology results were included (Figure 1).
Figure 1.
Flowchart of study participants from the parent clinical trial and those included in this study. DP, dihydroartemisinin-piperaquine; HIV, human immunodeficiency virus; SP, sulfadoxine-pyrimethamine.
Study Procedures
Study participants were provided a long-lasting, insecticide-treated net at enrollment and received all medical care at a dedicated study clinic throughout their pregnancy. Routine visits were conducted every 4 weeks and women were encouraged to come to the clinic whenever they felt ill. Study participants were encouraged to deliver at Masafu General Hospital where the study clinic was located. Women delivering at home were visited by study staff at delivery or as soon as possible afterwards. At delivery, a standardized assessment was completed including evaluation for birth weight, gestational age (based on ultrasound dating at enrollment), and collection of biological specimens including maternal blood, placental blood, and placental tissue.
Laboratory Procedures
Maternal and placental thick blood smears were stained using 2% Giemsa for 30 minutes and examined for asexual parasites using a light microscope by experienced microscopists. Slides were read in duplicate, and any discrepant readings were resolved by a third reader. Parasite DNA were extracted from dried placental blood spots using chelex extraction and detected using a LAMP kit (Eiken Chemical, Tokyo, Japan) as previously described [12]. For placental histopathology, the basal plate was trimmed into a 3-mm slice and dehydrated through a series of ethanol washes, cleared in xylene, and embedded in paraffin wax blocks. A 3-μm thick section from each tissue block was obtained using a rotary microtome, and sections were mounted onto glass slides via a flotation water bath. Slides were baked in a hot air oven at 60°C for 30 minutes, deparaffinized in xylene, dehydrated through a series of ethanol washes, stained with hematoxylin and eosin, and mounted with organic media. The histopathological process underwent extensive quality assurance to eliminate formalin pigment and minimize the effect of other artifacts. Placental tissue was assessed for the presence of parasites, malaria pigment (hemozoin) in fibrin and macrophages, and intervillous inflammation using a standardized case record form (Appendix 1 and 2) based on previously published methods by Muehlenbachs et al [13].
Measures of Malaria Assessed at Delivery
The presence of malaria parasites from maternal blood and placental blood was assessed by microscopy, whereas the presence of parasite DNA from placental blood was assessed by LAMP (all binary variables). Data from placental histopathology was assessed using the following methods: (1) binary classification defined as the presence of any parasites or malaria pigment; (2) bulmer classification system defined as uninfected (no evidence of parasites or pigment), active infection (parasites detected, no malaria pigment in fibrin), active-chronic (parasites detected and malaria pigment in fibrin), or past-chronic (parasites not detected, malaria pigment in fibrin) [14]; and (3) semiquantitative measures based on intervillous inflammation (none, intermediate, massive) and the proportion of high-power fields (HPFs) with malaria pigment seen in fibrin as previously described [13].
Adverse Birth Outcomes
The following adverse birth outcomes were classified as binary variables: LBW (<2500 grams), SGA (<10th percentile relative to an external growth reference), and PTB (<37 weeks gestational age).
Statistical Analysis
Statistical analyses were performed using STATA software version 14.2. Continuous variables were summarized as means and standard deviations. Categorical variables were summarized as frequencies and percentages. Proportions were compared using a χ 2 test or Fisher’s exact test. Associations between measures of malaria assessed at delivery and adverse birth outcomes were estimated using multivariate log-binomial regression models with standard errors and expressed as a risk ratio (RR). P < .05 were considered statistically significant.
RESULTS
Characteristics of Study Participants
Among 879 women screened, 97 (11.0%) did not meet criteria for enrollment. Of 782 women enrolled, 95 (12.1%) withdrew before delivery and 50 (6.4%) were excluded after delivery, yielding 637 women for analyses (Figure 1). At enrollment, mean maternal age was 24 years, 24.0% were primigravidae, 51.8% had microscopic parasitemia, and 82.3% had microscopic or submicroscopic parasitemia (Table 1). During pregnancy, women randomized to IPTp with SP had a significantly higher prevalence of parasitemia and incidence of malaria compared with women randomized to DP (Table 1). Likewise, women randomized to IPTp with SP had a significantly higher prevalence of binary measures of malaria at delivery compared with women randomized to IPTp with DP, including maternal blood microscopy (8.4% vs 0.3%, P < .001), placental blood microscopy (8.8% vs 0.3%, P < .001), placental blood LAMP (22.3% vs 2.2%, P < .001), and any evidence of parasites or malaria pigment by histopathology (61.7% vs 28.2%, P < .001). In contrast, there were no significant differences in the risk of individual adverse birth outcomes between the 2 IPTp arms, with an overall prevalence of 7.2%, 11.0%, and 5.8% for LBW, SGA, and PTB, respectively (Table 1). Of note, 5 women included in the analyses had stillbirths, all of which were LBW and SGA, and 3 of which were PTB.
Table 1.
Characteristics of Study Participants
| Study Period | Characteristic | All | Randomized to Monthly SP | Randomized to Monthly DP | |
|---|---|---|---|---|---|
| At Enrollment | Number of participants | 637 | 311 | 326 | |
| Age in years, mean (SD) | 24.0 (5.8) | 24.0 (6.0) | 23.9 (5.7) | ||
| Gestational age in weeks, mean (SD) | 15.6 (2.3) | 15.7 (2.4) | 15.4 (2.3) | ||
| Gravidity, n (%) | 1 | 153 (24.0%) | 81 (26.1%) | 72 (22.1%) | |
| 2 | 150 (23.6%) | 65 (20.9%) | 85 (26.1%) | ||
| ≥3 | 334 (52.4%) | 165 (53.1%) | 169 (51.8%) | ||
| Parasite prevalence, n (%) | Microscopic | 330 (51.8%) | 156 (50.2%) | 174 (53.4%) | |
| Microscopic or submicroscopic | 524 (82.3%) | 260 (83.6%) | 264 (81.0%) | ||
| During Pregnancy | Parasite prevalencea, n/N (%) | Microscopic | 1114/4348 (25.6%) | 787/2110 (37.3%) | 347/2238 (15.5%) |
| Microscopic or submicroscopic | 2338/4348 (53.8%) | 1496/2110 (70.9%) | 842/2238 (37.6%) | ||
| Incidence of malaria, episodes per person years | 0.34 | 0.60 | 0.09 | ||
| At Delivery | Binary measures of malaria in pregnancy, n/N (%) | Maternal blood microscopy | 27/635 (4.3%) | 26/310 (8.4%) | 1/325 (0.3%) |
| Placental blood microscopy | 28/633 (4.4%) | 27/307 (8.8%) | 1/326 (0.3%) | ||
| Placental blood LAMPb | 74/624 (11.9%) | 67/301 (22.3%) | 7/323 (2.2%) | ||
| Histopathology | 284/637 (44.6%) | 192/311 (61.7%) | 92/326 (28.2%) | ||
| Adverse birth outcomes, n (%) | Low birth weight | 46 (7.2%) | 23 (7.4%) | 23 (7.1%) | |
| Small for gestational age | 70 (11.0%) | 33 (10.6%) | 37 (11.4%) | ||
| Preterm birth | 37 (5.8%) | 20 (6.4%) | 17 (5.2%) |
Abbreviations: DP, dihydroartemisinin-piperaquine; LAMP, loop-mediated isothermal amplification; SD, standard deviation; SP, sulfadoxine-pyrimethamine.
aDuring routine visits.
bLAMP of DNA.
Comparison of Different Measure of Malaria Assessed at Delivery and Associations With Adverse Birth Outcomes
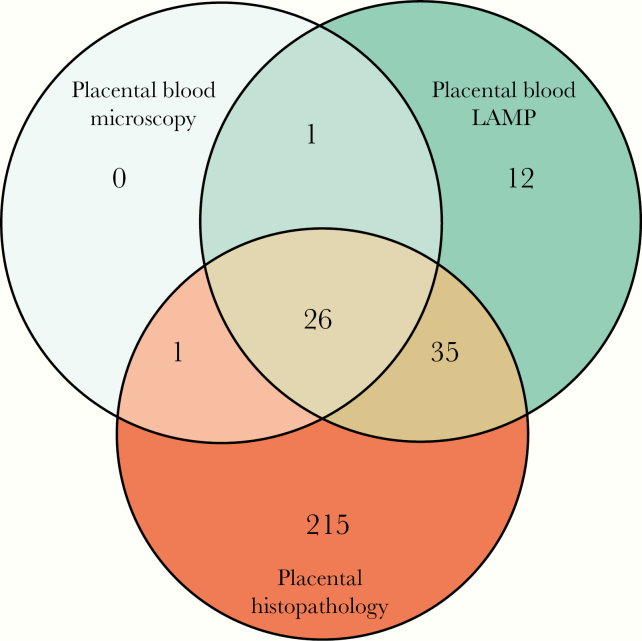
The prevalence of malaria assessed at delivery was similar and relatively low based on maternal (4.3%) and placental (4.4%) blood microscopy, but it increased considerably when using LAMP to detect parasite DNA in placental blood (11.9%), and it was highest when using histopathology to detect parasites or malaria pigment (44.6%) (Table 1). A total of 290 cases had evidence of malaria by placental blood microscopy, placental blood LAMP, or placental histopathology (Figure 2). No cases were detected using only placental blood microscopy, 12 (4.1%) cases were detected using only placental blood LAMP, and 215 (74.1%) cases were detected with placental histopathology alone.
Figure 2.
Venn diagram of binary measures of malaria at delivery using different methods. LAMP, loop-mediated isothermal amplification.
The detection of parasites in placental or maternal blood by microscopy was not associated with any adverse birth outcomes (Table 2). The detection of parasite DNA in placental blood using LAMP was not associated with LBW or SGA, although there was a nonsignificant trend towards an increased risk of PTB (adjusted RR [aRR] = 1.71; 95% confidence interval [CI], 0.76–3.82; P = .19). However, the detection of any parasites or malaria pigment by histopathology was associated with an increased risk of SGA (aRR = 2.11; 95% CI, 1.25–3.54; P = .005) and a nonsignificant trend towards an increased risk of LBW (aRR = 1.75; 95% CI, 0.94–3.26; P = .08), but it was not associated with PTB. Placental histopathology findings were further assessed using the Bulmer classification system. Of note, among 284 samples that were positive by histopathology, only malaria pigment was detected in 257 (90.4%). Compared with uninfected samples, those in which only parasites were detected (active infection) were associated with an increased risk of LBW and SGA, but not PTB, although only 3 samples met this criteria and CIs were wide. Those in which parasites and malaria pigment were detected (active-chronic infection) were not associated with any adverse birth outcomes. Those in which only malaria pigment was detected (past-chronic infection) were associated with an increased risk of SGA (aRR = 2.14; 95% CI, 1.27–3.60; P = .004) but not LBW or PTB (Table 2).
Table 2.
Associations Between Different Definitions of Malaria Assessed at Delivery and Adverse Birth Outcomes
| Definition of Malaria Assessed at Delivery | Low Birth Weight | Small for Gestational Age | Preterm Birth | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | aRRa (95% CI) | P Value | n (%) | aRRa (95% CI) | P Value | n (%) | aRRa (95% CI) | P Value | ||
| Reference | Reference | Reference | ||||||||
| Maternal blood microscopy | Negative (n = 608) | 44 (7.2%) | - | - | 67 (11.0%) | - | - | 35 (5.8%) | - | - |
| Positive (n = 27) | 2 (7.4%) | 0.86 (0.22–3.41) | .83 | 3 (11.1%) | 0.75 (0.25–2.24) | .61 | 2 (7.4%) | 1.03 (0.26–4.09) | .96 | |
| Placental blood microscopy | Negative (n = 605) | 43 (7.1%) | - | - | 67 (11.1%) | - | - | 35 (5.8%) | - | - |
| Positive (n = 28) | 2 (7.1%) | 0.80 (0.20–3.18) | .75 | 2 (7.1%) | 0.45 (0.12–1.75) | .25 | 2 (7.1%) | 0.93 (0.23–3.71) | .91 | |
| Placental blood LAMP | Negative (n = 550) | 38 (6.9%) | - | - | 57 (10.4%) | - | - | 27 (4.9%) | - | - |
| Positive (n = 74) | 5 (6.8%) | 0.89 (0.36–2.22) | .81 | 10 (13.5%) | 1.10 (0.59–2.07) | .76 | 7 (9.5%) | 1.71 (0.76–3.82) | .19 | |
| Histopathology (binary) | Negative (n = 353) | 18 (5.1%) | - | - | 23 (6.5%) | - | - | 17 (4.8%) | - | - |
| Positive (n = 284) | 28 (9.9%) | 1.75 (0.94–3.26) | .08 | 47 (16.6%) | 2.11 (1.25–3.54) | .005 | 28 (9.9%) | 1.09 (0.54–2.20) | .82 | |
| Bulmer classification | Uninfected (n = 353) | 18 (5.1%) | - | - | 23 (6.5%) | - | - | 17 (4.8%) | - | - |
| Active infection (n = 3) | 1 (33.3%) | 6.73 (1.28–35.6) | .03 | 1 (33.3%) | 5.43 (1.04–28.3) | .04 | 0 (0%) | NA | - | |
| Active chronic (n = 24) | 3 (12.5%) | 2.01 (0.59–6.88) | .27 | 2 (8.3%) | 0.88 (0.21–3.64) | .86 | 3 (12.5%) | 1.64 (0.47–5.76) | .44 | |
| Past chronic (n = 257) | 24 (9.3%) | 1.65 (0.87–3.12) | .13 | 44 (17.1%) | 2.14 (1.27–3.60) | .004 | 17 (6.6%) | 1.06 (0.51–2.18) | .88 |
Abbreviations: aRR, adjusted risk ratio; CI, confidence interval; NA, not applicable.
aAdjusted for gravidity.
Associations Between Additional Histopathological Findings and Adverse Birth Outcomes
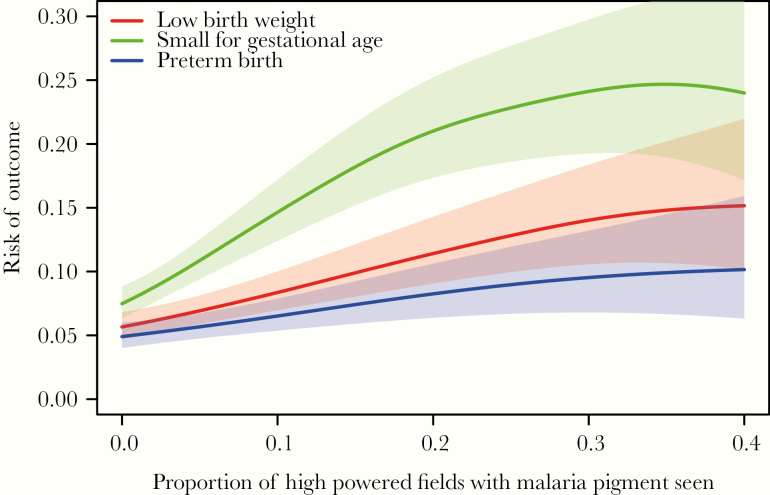
Given the diversity of data available from placental histology, we further explored other features as originally proposed by Muehlenbachs et al [13]. Only 4 of 637 (0.6%) samples had evidence of intervillous inflammation identified; therefore, this feature was not explored further. In contrast, a continuous measure of malaria pigment deposition, defined as the proportion of HPFs with malaria pigment seen, correlated well with LBW and SGA (Figure 3). Based on these visual relationships, we created a grading scale of malaria pigment deposition defined as none (no pigment seen), mild (<10% of HPFs), moderate (10% to <30% HPFs), and severe (≥30% HPFs). Increasing severity along our grading scale was associated with an increasing risk of both LBW and SGA but not PTB (Table 3). Compared with samples with no pigment seen, “severe” malaria pigment deposition was associated with a greater than 3-fold risk in LBW (aRR = 3.42; 95% CI, 1.26–9.29; P = .02) and over a 4-increase in risk of SGA (aRR vs 4.24; 95% CI, 1.99–9.02; P < .001) after controlling for the detection of parasites by any method and gravidity. Of note, the detection of parasites and gravidity were not significantly associated with any adverse birth outcomes after controlling for the severity of malaria pigment deposition (Table 3).
Figure 3.
Relationships between the proportion of high-powered fields with malaria pigment seen and adverse birth outcomes using lowess smoothing with standard errors represented by shaded areas. Data truncated for proportion of high-powered fields with malaria pigment seen >0.4 due to lack of precision.
Table 3.
Associations Between a Histopathological Grading Scale and Adverse Birth Outcomes
| Risk Factors | Low Birth Weight | Small for Gestational Age | Preterm Birth | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | aRRa (95% CI) | P Value | n (%) | aRRa (95% CI) | P Value | n (%) | aRRa (95% CI) | P Value | ||
| Reference | Reference | Reference | ||||||||
| Severity based on proportion of HPFs with malaria pigment seen | None (n = 356) | 19 (5.3%) | - | - | 24 (6.7%) | - | - | 17 (4.8%) | - | - |
| Mild (n = 146) | 11 (7.5%) | 1.35 (0.64–2.82) | .43 | 20 (13.7%) | 1.87 (1.05–3.33) | .03 | 8 (5.5%) | 0.94 (0.40–2.20) | .88 | |
| Moderate (n = 107) | 10 (9.4%) | 1.59 (0.71–3.57) | .26 | 17 (15.9%) | 2.00 (1.05–3.80) | .04 | 10 (9.4%) | 1.34 (0.57–3.18) | .50 | |
| Severe (n = 28) | 6 (21.4%) | 3.42 (1.26–9.29) | .02 | 9 (32.1%) | 4.24 (1.99–9.02) | <.001 | 2 (7.1%) | 0.88 (0.19–4.12) | .87 | |
| Parasites detected | Absent (n = 610) | 42 (6.9%) | - | - | 67 (11.0%) | - | - | 34 (5.6%) | - | |
| Present (n = 27) | 4 (14.8%) | 1.17 (0.41–3.31) | .77 | 3 (11.1%) | 0.47 (0.15–1.41) | .18 | 3 (11.1%) | 1.46 (0.44–4.76) | .54 | |
| Gravidity | Nonprimigravida (n = 484) | 30 (6.2%) | - | - | 41 (8.5%) | - | - | 22 (4.6%) | - | - |
| Primigravida (n = 153) | 16 (10.5%) | 1.18 (0.60–2.32) | .63 | 29 (19.0%) | 1.54 (0.92–2.56) | .10 | 15 (9.8%) | 1.93 (0.92–4.04) | .08 |
Abbreviations: aRR, adjusted risk ratio; CI, confidence interval; HPFs, high-power fields.
aMultivariate analysis including all risk factors listed.
The Role of Intermittent Preventive Treatment of Malaria in Pregnancy Regimen and Gravidity on the Risk of Severe Pigment Deposition
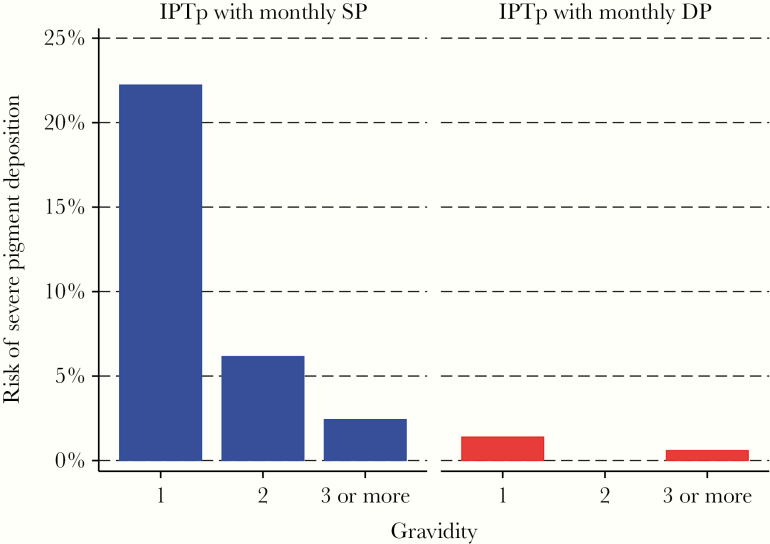
Although only 28 placental samples (4.4%) had evidence of severe malaria pigment deposition, a woman’s IPTp regimen and gravidity were strongly associated with this outcome (Figure 4). Women randomized to receive IPTp with SP had an 8.4% risk of severe malaria pigment deposition compared with only 0.6% among women randomized into the IPTp with DP treatment group. In addition, having microscopic parasitemia at enrollment was associated with severe malaria pigment deposition among women randomized to receive IPTp with SP (11.5% vs 5.2%, P = .04) but not women randomized to receive IPTp with DP (0.7% vs 0.6%, P = .92). With respect to gravidity, primigravidae had a 12.4% risk of severe malaria pigment deposition compared with 2.7% among secundigravidae (P = .001) and 1.5% among multigravida women (P < .001). Indeed, among primigravidae who received IPTp with SP, the risk of severe malaria pigment deposition was 22.2% compared with only 1.8% in the rest of the study population (Figure 4) (P < .001).
Figure 4.
Relationships between gravidity and the risk of severe pigment deposition stratified by intermittent preventive treatment of malaria in pregnancy (IPTp) arm. DP, dihydroartemisinin-piperaquine; SP, sulfadoxine-pyrimethamine.
DISCUSSION
We investigated associations between measures of malaria at delivery, including peripheral and placental blood microscopy, placental blood LAMP, and placental histopathology, and specific adverse birth outcomes including LBW, SGA, and PTB. The proportion of women with evidence of malaria at delivery was higher when placental histopathology was used to detect parasites or malaria pigment (44.6%) compared with methods used to detect parasite DNA (LAMP, 11.9%), or parasites by microscopy using maternal (4.3%) or placental (4.4%) blood. Parasite detection in placental or maternal blood by microscopy was not associated with any adverse birth outcomes. Likewise, parasite DNA detection by LAMP was not associated with any adverse birth outcomes. These findings are likely due to a lack of sensitivity of these methods for detecting placental infection with malaria parasites earlier in pregnancy, which may play an important role in the development of adverse birth outcomes.
The presence of parasites or malaria pigment assessed by placental histopathology was associated with an increased risk of SGA but not LBW or PTB. Detection of only parasites in placental tissue (active infection) was associated with an increased risk of LBW and SGA, but this occurred in <1% of samples. The detection of only malaria pigment in placental tissue (past-chronic infection) was much more common and associated with a significant increase in the risk of SGA but not LBW or PTB. Further assessment of pigment deposition in fibrin based on the Muehlenbachs et al [13] classification system showed that severe malaria pigment deposition in fibrin was strongly associated with an increased risk of LBW and SGA but not PTB. Thus, in this high-transmission setting, severe malaria pigment deposition in fibrin indicative of heavy placental infection with malaria parasites earlier in pregnancy was associated with measures of intrauterine growth retardation but not early labor. We were not surprised to find that women randomized to monthly IPTp with DP had a much lower risk of severe malaria pigment deposition in fibrin compared with women randomized to SP, reflecting the more potent antimalarial effect of DP.
Studies that have assessed for associations between the presence of parasites or malaria pigment at delivery and adverse birth outcomes have provided mixed results. A study from the mid-1990s in Zanzibar showed that babies born to women with parasites and malaria pigment in the placental tissue had lower birth weight compared with those born to women with uninfected placentas [15]. In a study from Uganda in 2014, the presence of placental parasites detected by microscopy, LAMP, or histopathology was associated with an increased risk of PTB, with trends for an increased risk of LBW and SGA; however, the detection of malaria pigment alone was not associated with any adverse birth outcomes [16]. In a study from the mid-1990s in Tanzania, massive intervillous inflammatory infiltration was associated with LBW, whereas the presence of parasites or pigment was associated with PTB [17]. A study in Ghana from 2000 to 2001 reported that the detection of parasites by microscopy or polymerase chain reaction was not associated with LBW or PTB, but the detection of histidine-rich protein 2 antigen in placental blood was associated with an increased risk of LBW [18]. In a study from Tanzania published in 2010, massive intervillous inflammatory infiltration and severe pigment deposition were both independently associated with an increased risk of LBW [13].
Our study also showed that among women randomized to IPTp with SP, there was a strong inverse relationship between gravidity and the risk of severe pigment deposition. This finding is consistent with the well described phenomenon that even when pregnancy-associated malaria is common, adverse consequences are dependent on the ability to control parasitemia, which is influenced by the development of gravidity-specific immunity [19]. In addition, we found that IPTp with DP was associated with a marked reduction in the risk of severe pigment deposition across all gravidities. This may help explain the findings from the parent clinical trial where compared to IPTp with SP, IPTp with DP was associated with a dramatic reduction in the burden of malaria, but only a modest decrease in the risk of adverse birth outcomes, which was not statistically significant [11]. In a high-transmission, intensity setting such as ours, although DP may be highly effective at preventing MiP, the benefit in terms of the prevention of adverse birth outcomes is only apparent in a relatively small proportion of women at risk for high-grade placental infection (ie, severe pigment deposition).
This study is not without limitations. First, women were enrolled after they had been pregnant 3 to 4 months. If placental infection occurred before enrollment, malaria pigment would likely have persisted up to 6 months even after parasite clearance [8]. Thus, we are unable to draw conclusions about the risk of adverse birth outcomes that may be attributable to malaria exposure before enrollment in the study. In addition, our study participants were from a small geographical area in eastern Uganda with high malaria transmission intensity. Thus, our findings may not be generalizable to lower transmission areas. One strength of this study was the presence of a histopathology laboratory that utilized optimized standard operating procedures, extensive training, and experience to ensure high-accuracy histopathology reads. The quality of histopathology likely contributed to our ability to detect associations with adverse birth outcomes. Compared with other diagnostic methods that have been used historically, high-quality placental histopathology requires a greater investment in infrastructure and training.
CONCLUSIONS
In summary, among several different measures of malaria at delivery, only results based on placental histopathology were associated with adverse birth outcomes in a high-transmission setting. Furthermore, a semiquantitative classification system based on the severity of malaria pigment deposition was most informative in this study. Although prior studies provide somewhat conflicting results, there is a growing body of evidence that placental histopathology is the most sensitive method for the detection of malaria at delivery and that the rich data provided by histopathology can be more predictive adverse birth outcomes compared with simple binary measures that have been commonly used in the past. For clinical trials of interventions for the prevention of MiP, the use of malaria-specific outcomes as surrogate measures of adverse birth outcomes is a common practice and can greatly reduce sample size requirements. However, there is no consensus on which malaria-specific outcomes should be used in clinical trials. There is a need to develop standardized approaches of classifying malaria-specific outcomes in pregnancy and to evaluate these approaches in different epidemiological settings.
Supplementary Material
Notes
Acknowledgments. We acknowledge all laboratory and clinical staff of Infectious Diseases Research Collaboration for their tremendous work in data collection and all the women who consented to participate in the study.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention
Financial support. The Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grant Number P01 HD059454) and the National Institutes of Health/Fogarty International Center (Grant Number TW007375) funded this work.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Rogerson SJ, Desai M, Mayor A, Sicuri E, Taylor SM, Eijk van AM. Burden, pathology, and costs of malaria in pregnancy: new developments for an old problem. Lancet Infect Dis 2018; 18:107–18. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. World Malaria Report 2019. Geneva: World Health Organization; 2019. [Google Scholar]
- 3. Desai M, ter Kuile FO, Nosten F, et al. . Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 2007; 7:93–104. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization. A strategic framework for malaria prevention and control during pregnancy in the African region. Brazzaville: World Health Organization Regional Office for Africa; 2004: pp 1–5. [Google Scholar]
- 5. Gutman J, Kalilani L, Taylor S, et al. . The A581G mutation in the gene encoding Plasmodium falciparum dihydropteroate synthetase reduces the effectiveness of sulfadoxine-pyrimethamine preventive therapy in Malawian pregnant women. J Infect Dis 2015; 211:1997–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. WHO Malaria Policy Advisory Committee and Secretariet. Malaria Policy Advisory Committee to the WHO: conclusions and recommendations of eighth biannual meeting (September 2015). Malar J 2016; 15:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fried M, Muehlenbachs A, Duffy PE. Diagnosing malaria in pregnancy: an update. Expert Rev Anti Infect Ther 2012; 10:1177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Muehlenbachs A, Nabasumba C, McGready R, et al. . Artemether-lumefantrine to treat malaria in pregnancy is associated with reduced placental haemozoin deposition compared to quinine in a randomized controlled trial. Malar J 2012; 11:1– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Desai M, Gutman J, L’lanziva A, et al. . Intermittent screening and treatment or intermittent preventive treatment with dihydroartemisinin-piperaquine versus intermittent preventive treatment with sulfadoxine-pyrimethamine for the control of malaria during pregnancy in western Kenya: an open-label, three-group, randomised controlled superiority trial. Lancet 2015; 386:2507–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kakuru A, Jagannathan P, Muhindo MK, et al. . Dihydroartemisinin-piperaquine for the prevention of malaria in pregnancy. N Engl J Med 2016; 374:928–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kajubi R, Ochieng T, Kakuru A, et al. . Monthly sulfadoxine – pyrimethamine versus dihydroartemisinin – piperaquine for intermittent preventive treatment of malaria in pregnancy: a double-blind, randomised controlled superiority trial. Lancet 2019; 6736:1–12. [DOI] [PubMed] [Google Scholar]
- 12. Hopkins H, González IJ, Polley SD, et al. . Highly sensitive detection of malaria parasitemia in a malaria-endemic setting: performance of a new loop-mediated isothermal amplification kit in a remote clinic in Uganda. J Infect Dis 2013; 208:645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Muehlenbachs A, Fried M, McGready R, et al. . A novel histological grading scheme for placental malaria applied in areas of high and low malaria transmission. J Infect Dis 2010; 202:1608–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bulmer JN, Rasheed FN, Francis N, Morrison L, Greenwood BM. Placental malaria. I. Pathological classification. Histopathology 1993; 22:211–8. [DOI] [PubMed] [Google Scholar]
- 15. Leopardi O, Naughten W, Salvia L, et al. . Malaric placentas. A quantitative study and clinico-pathological correlations. Pathol Res Pract 1996; 192:892–8; discussion 899–900. [DOI] [PubMed] [Google Scholar]
- 16. Kapisi J, Kakuru A, Jagannathan P, et al. . Relationships between infection with Plasmodium falciparum during pregnancy, measures of placental malaria, and adverse birth outcomes. Malar J 2017; 16:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Menendez C, Ordi J, Ismail MR, et al. . The impact of placental malaria on gestational age and birth weight. J Infect Dis 2000; 181:1740–5. [DOI] [PubMed] [Google Scholar]
- 18. Mockenhaupt FP, Bedu-addo G, Gaertner Von C, et al. . Detection and clinical manifestation of placental malaria in southern Ghana. Malar J 2006; 10:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rogerson SJ, Hviid L, Duffy PE, Leke RF, Taylor DW. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect Dis 2007; 7:105–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.