Abstract
Background
Data on the epidemiology of herpes zoster (HZ), particularly in the unvaccinated immunocompetent population, are needed to assess disease burden and the potential impact of vaccination.
Methods
The study at a large health care organization comprised: (1) incidence estimated from immunocompetent adults aged ≥50 years unvaccinated with zoster vaccine live who had incident HZ in 2011–2015; (2) proportion of HZ-related nonpain complications assessed by double abstraction of electronic health records (EHRs) of 600 incident patients 2011–2015; (3) HZ-related hospitalizations among HZ patients diagnosed in 2015; (4) HZ-related death determined from automated data and EHRs; and (5) recurrent HZ identified from a cohort initially diagnosed with HZ in 2007–2008 and followed through 2016.
Results
HZ incidence rate was 9.92/1000 person-years (95% confidence interval [CI], 9.82–10.01). Proportions of cutaneous, neurologic, and other complications were 6.40% (95% CI,1.73%–11.07%), 0.77% (95% CI, .00%–2.36%), and 1.01% (95% CI, .00%–2.93%), respectively. Only 0.86% of patients had an HZ-related hospitalization. The case-fatality rate was 0.04%. Recurrence rate was 10.96/1000 person-years (95% CI, 10.18–11.79) with 10-year recurrence risk of 10.26% (95% CI, 9.36%–11.23%).
Conclusions
These recent HZ epidemiology data among an immunocompetent, unvaccinated population measure real-world disease burden.
Keywords: herpes zoster, epidemiology, older adults, incidence, complications, mortality, hospitalization, recurrence
(See the Editorial Commentary by Harpaz, on pages 708–11.)
Before the licensure and broad uptake of a live varicella vaccine, most of the US population experienced primary infection with varicella zoster virus (VZV) [1]. As the primary infection resolves, the virus establishes latency in dorsal root ganglia and can reactivate to cause herpes zoster (HZ). Zoster vaccine live (ZVL) has been recommended since 2006 for immunocompetent adults aged ≥60 years [1]. Prior to the availability of ZVL, HZ affected nearly 1 million people in the United States annually [2]. A new recombinant zoster vaccine (RZV) containing VZV glycoprotein-E and the AS01B adjuvant showed high efficacy and acceptable safety in phase 3 clinical trials and was licensed in 2017 [3, 4]. The Advisory Committee on Immunization Practices recommended to preferentially administer RZV to individuals aged ≥50 years, regardless of history of HZ or prior vaccination with ZVL [5].
Population-based data on the epidemiology of HZ in adults, especially those who are immunocompetent and who have not received zoster vaccination, are needed in order to better understand the present burden of disease, as well as to critically evaluate the potential impact of an RZV vaccination program [6]. We have previously reported on the incidence of postherpetic neuralgia (PHN) and herpes zoster ophthalmicus (HZO) in an integrated health care organization [7–9]. Therefore, the objectives of this study were to estimate the incidence of HZ, the proportion of HZ-related complications, the proportion of HZ patients that require hospitalization, HZ case-fatality rate, and HZ recurrence rate, among an immunocompetent and ZVL-unvaccinated population aged ≥50 years.
METHODS
Setting
This study (GSK study identifier, HO-17-18378) was conducted using electronic health records (EHRs) at Kaiser Permanente Southern California (KPSC), an integrated health care organization that provides prepaid comprehensive health care to more than 4.6 million members. EHRs included information on sociodemographics, health care utilization (outpatient, emergency department, and inpatient encounters), diagnoses, laboratory tests, pharmacy utilization, vaccination records, membership history, and death. The demographic composition at KPSC is representative of the Southern California population [10, 11]. Compared to the racial/ethnic distribution in the United States, KPSC membership is composed of twice as many Asian and 3 times as many Hispanic individuals. The study was approved by the institutional review board of KPSC with a waiver for the requirement of informed consent.
Incidence of HZ
The incidence of HZ was calculated among individuals who had never been vaccinated with ZVL. Eligible individuals were KPSC members aged ≥50 years at any time between 1 January 2011 and 31 December 2015 and had at least 1 year of membership before entering the cohort. Members with any history of ZVL vaccination or an HZ diagnostic code prior to cohort entry were excluded from this analysis. Individuals with HIV, leukemia, or lymphoma diagnoses, or individuals who had immunosuppressing agents dispensed within the year prior to entering the cohort were excluded. The list of immunosuppressing agents was provided in our previous publication [12]. Therefore, only immunocompetent individuals at the beginning of follow-up were included. Incident HZ patients were identified by international classification of diseases (ICD)-9 codes 053.xx (or ICD-10 codes B02.xx beginning from October 2015) from hospital, outpatient, and emergency department settings during the follow-up period. A sensitivity analysis was performed, with incident HZ defined as HZ diagnostic codes plus antiviral medications (acyclovir, valacyclovir, or famciclovir) dispensed within ±7 days of the HZ diagnosis date.
Each individual was followed until (1) incident HZ, (2) receipt of ZVL, (3) end of membership with KPSC (including death), or (4) 31 December 2015, whichever occurred first. The number of incident HZ patients was described by age category (50–54, 55–59, 60–64, 65–69, 70–74, 75–79, and ≥80 years old). Person-time accumulated from each individual during the interval was summed as the denominator and counted for each age category. The 95% confidence interval (CI) was calculated by assuming the occurrence of HZ followed a Poisson distribution.
Proportion of HZ-Related Complications
To determine the proportion of unvaccinated HZ patients with cutaneous, neurologic, or other complications, 600 patients with an HZ diagnosis who also had at least 2 additional HZ-related medical encounters (occurring 2011–2015) within 6 months following the initial HZ diagnosis date were sampled for further review. Patients were randomly sampled from 4 age strata (50–59, 60–69, 70–79, and ≥80 years old), each containing 150 patients. Medical charts within the 6-month period were independently reviewed by 2 research associates for ascertainment of complications.
Complications were included only if they were confirmed to be related to HZ or were possibly related to HZ. Confirmed complications were those that were clearly noted by the care provider as HZ-related or were located at the site of the initial HZ rash with no clear alternate cause specified. Possibly HZ-related complications were those in which the cause of the condition was unclear, the condition was noted as possibly HZ-related, or it was unclear whether the location matched the HZ site. The 2 abstractors compared their results and resolved any discrepancies, consulting the physician coinvestigator (B. A.) as needed.
Terms for conditions in the cutaneous complications category included disseminated cutaneous zoster, bacterial superinfection, cellulitis, zoster gangrenosum, zoster hemorrhagicus, septicemia, hypo/hyperpigmentation, granulomatous skin lesions, pseudolymphoma, manifestation of psoriasis (Köbner phenomenon), scar formation/atrophic scars/hypertrophic scars, persisting zoster (HZ rash that did not resolve within 10 days of incident HZ diagnosis date), and other skin complications (those occurring at least 10 days after the incident HZ diagnosis date and not including skin complications on the eye/eyelid).
Conditions in the neurologic complications category included meningoencephalitis/encephalitis, aseptic meningitis/meningitis, granulomatous arteritis, segmental pareses, cranial nerve palsies, peripheral nerve palsies, Ramsay-Hunt syndrome/HZ oticus, Guillain-Barré syndrome, transverse myelitis/ascending myelitis/myelitis, motor neuropathy, autonomic dysfunction, granulomatous cerebral angiitis, diaphragmatic paralysis, sensory loss, deafness, vestibular dysfunction, Bell’s-like palsy/motor nerve palsies, otitis externa, and other neurologic complications (nonpain or non-PHN related).
Conditions in the other complications category included bronchitis, pleuritis, esophagitis, gastritis, enterocolitis, peritonitis, cystitis, myositis, pericarditis, visceral VZV dissemination/disseminated HZ, pneumonia, hepatitis, abdominal hernias, bladder dysfunction, pneumonitis, pancreatitis, myocarditis, arthritis, and other.
Proportions were weighted by the percentage of incident HZ patients that had ≥2 visits for HZ within 6 months after initial HZ diagnosis to reflect the distribution of complications among all incident HZ patients. The overall proportion was calculated by adjusting the sampling ratios using the age-specific distribution of the HZ patients. The 95% CIs were estimated assuming the occurrence of HZ complications followed a binomial distribution. Partial sampling variance was also considered when calculating the CI for the overall proportion.
HZ-Related Hospitalizations
HZ-related hospitalizations within 6 months of incident HZ diagnosis were identified from the inpatient care setting for all HZ patients diagnosed in 2015. Medical records were independently reviewed by 2 research associates to determine if the hospitalization was attributable to HZ. The results of the 2 independent reviews were compared to resolve any discrepancies, with consultation from the physician coinvestigator (B. A.) as needed. Hospitalization attributable to HZ was defined as meeting at least 1 of the following criteria: (1) HZ or an HZ complication was listed as the reason for hospitalization; (2) HZ or an HZ complication was the primary discharge diagnosis; (3) HZ-related pain or treatment or procedure for HZ-related pain was the reason for hospitalization; (4) HZ or an HZ complication was not the reason for admission but was recognized, evaluated, or treated during hospitalization; (5) hospitalization was for nursing home admission evaluation for a patient having difficulties with self-care because of an HZ complication. The age-specific proportion of HZ-related hospitalizations among incident patients and hospitalization rate among the 2015 population were reported.
HZ Case Fatality and Mortality Rate
HZ-related death was determined from 2 sources among the incident HZ patients identified during 2011–2015. First, automated data were used to identify patients in which HZ was documented as the cause of death in the mortality record. Second, because HZ was not commonly documented as the underlying cause of death, the physician coinvestigator reviewed hospitalized HZ patients who either died during hospitalization or died within 6 months after discharge to determine if the cause of death was related to HZ complications. Standard definitions for underlying cause of death and contributing cause of death from death certificates were used [13]. The age-specific proportion of HZ-related death among incident patients (case-fatality rate) and mortality rate among the 2011–2015 population were reported.
HZ Recurrence
We estimated the incidence rate of recurrent HZ among the unvaccinated population who had an initial HZ episode between 1 January 2007 and 31 December 2008. To increase the positive predictive value of HZ patient identification, incident patients of initial HZ and recurrent HZ were identified using HZ diagnosis codes plus antiviral medications (acyclovir, valacyclovir, or famciclovir) dispensed within ±7 days of the HZ diagnosis date.
Recurrent HZ was defined as having HZ at ≥6 months after the most recent diagnosis of HZ. Subjects with initial HZ were followed until (1) recurrent HZ, (2) receipt of ZVL, (3) end of membership with KPSC (including death), or (4) 31 December 2016, whichever occurred first. We extended the follow-up period to 31 December 2016 to provide a better estimate of overall incidence and long-term risk of HZ recurrence. Only the first recurrence was included in the analysis.
Similar to the HZ incidence estimations, incidence of recurrent HZ was estimated by age category (50–54, 55–59, 60–64, 65–69, 70–74, 75–79, and ≥80 years old). We also used Kaplan-Meier methods to calculate the cumulative incidence of HZ recurrence and stratified the risk curve by decade of age.
RESULTS
The incidence rates of HZ are presented in Table 1. The incidence rate measured using diagnostic codes was 9.92 (95% CI, 9.82–10.01) per 1000 person-years. It generally increased with age, from 7.20/1000 person-years in the 50–54 years group to 13.99/1000 person-years in the ≥80 years group. There were 26 545 out of 1 360 248 (1.95%) cohort members who became immunosuppressed during the follow-up period. Among the 26 545 members, 909 went on and developed HZ. Two of the 909 cases were HZ-related death. Accounting for censoring when individuals became immunosuppressed during follow-up resulted in minimal change in the incidence estimate (9.82 per 1000 person-years; 95% CI, 9.72–9.91). Overall, 85% of the incident HZ diagnoses had antiviral medications. The incidence rate of HZ defined by diagnostic codes plus antiviral medications was 8.40 (95% CI, 8.31–8.49) per 1000 person-years, increasing from 6.21/1000 person-years in the 50–54 years group to 11.61/1000 person-years in the ≥80 years group.
Table 1.
Incidence of Herpes Zoster Among an Immunocompetent, Unvaccinated Population ≥50 Years Old From Kaiser Permanente Southern California, 2011–2015
| Age at HZ Diagnosis, y | Incident HZ Patients Identified by Diagnostic Codes | Incident HZ Patients Identified by Diagnostic Codes Plus Antiviral Medicationsa | ||||||
|---|---|---|---|---|---|---|---|---|
| Total Number of Person-Years | Incident Patients, n | Incidence /1000 Person-Years | 95% CI | Total Number of Person-Years | Incident Patients, n | Incidence/ 1000 Person-Years | 95% CI | |
| 50–54 | 1 071 337 | 7709 | 7.20 | 7.04–7.36 | 1 072 774 | 6660 | 6.21 | 6.06–6.36 |
| 55–59 | 1 001 850 | 8633 | 8.62 | 8.44–8.80 | 1 004 256 | 7421 | 7.39 | 7.22–7.56 |
| 60–64 | 729 944 | 7450 | 10.21 | 9.98–10.44 | 731 980 | 6251 | 8.54 | 8.33–8.75 |
| 65–69 | 479 795 | 5624 | 11.72 | 11.42–12.03 | 481 195 | 4786 | 9.95 | 9.67–10.23 |
| 70–74 | 317 937 | 4092 | 12.87 | 12.48–13.27 | 319 080 | 3466 | 10.86 | 10.51–11.23 |
| 75–79 | 224 031 | 3200 | 14.28 | 13.80–14.79 | 225 100 | 2659 | 11.81 | 11.37–12.27 |
| ≥80 | 299 156 | 4185 | 13.99 | 13.57–14.42 | 300 656 | 3491 | 11.61 | 11.23–12.00 |
| Total | 4 124 050 | 40 893 | 9.92 | 9.82–10.01 | 4 135 041 | 34 734 | 8.40 | 8.31–8.49 |
Abbreviations: CI, confidence interval; HZ, herpes zoster.
aAntiviral medications (acyclovir, valacyclovir, or famciclovir) dispensed within ±7 days of HZ diagnosis date.
The proportions of cutaneous, neurologic, and other complications among incident HZ patients are presented in Table 2. Cutaneous complications occurred in 6.40% (95% CI, 1.73%–11.07%), neurologic complications in 0.77% (95% CI, .00%–2.36%), and other complications in 1.01% (95% CI, .00%–2.93%), among all HZ patients. When including possibly HZ-related patients, the proportion became 7.02% (95% CI, 2.17%–11.87%), 1.36% (95% CI, .00%–3.54%), and 1.70% (95% CI, .00%–4.06%), respectively. Common cutaneous complications included scar formation/atrophic scars/hypertrophic scars (n = 17), persisting zoster (n = 16), hypo/hyperpigmentation or discoloration (n = 12), and cellulitis (n = 12, including 3 possibly HZ-related patients). Common neurologic complications included Bell’s-like palsy/motor nerve paralysis (n = 6, including 1 possibly HZ-related patient), sensory loss (n = 6, including 5 possibly HZ-related patients), and cranial nerve palsies (n = 5, including 3 possibly HZ-related patients). Other common complications included other miscellaneous complications (n = 16, including 7 possibly HZ-related patients) and visceral VZV dissemination (n = 1).
Table 2.
Proportion of Confirmed or Possible Herpes Zoster-Related Cutaneous, Neurologic, and Other Complications Among an Immunocompetent, Unvaccinated Population ≥50 Years Old From Kaiser Permanente Southern California, 2011–2015
| Age at Initial HZ Diagnosis, y | Incident HZ Patients, n | Patients With ≥2 HZ Visits in 6 mo After Incident HZ, n | Sampled HZ Patients, n | Cutaneous Complications | Neurologic Complications | Other Complications | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Confirmed, n | Confirmed or Possible, n | Weighted Proportion,a % (95% CI) | Confirmed, n | Confirmed or Possible, n | Weighted Proportion,a % (95% CI) | Confirmed, n | Confirmed or Possible, n | Weighted Proportion,a % (95% CI) | |||||||
| Confirmed | Confirmed or Possible | Confirmed | Confirmed or Possible | Confirmed | Confirmed or Possible | ||||||||||
| 50–54 | 7709 | 4949 | 67 | 4 | 4 | 3.83 (.19–7.47) | 3.83 (.19–7.48) | 0 | 1 | 0.00 (.00–∞) | 0.96 (.00–2.82) | 1 | 1 | 0.96 (.00–2.82) | 0.96 (.00–1.13) |
| 55–59 | 8633 | 5920 | 83 | 3 | 4 | 2.48 (.00–5.23) | 3.30 (.14–6.46) | 1 | 1 | 0.83 (.00–2.44) | 0.83 (.00–2.44) | 0 | 0 | 0.00 (.00–∞) | 0.00 (.00–∞) |
| 60–64 | 7450 | 5431 | 88 | 6 | 7 | 4.97 (1.13–8.81) | 5.80 (1.68–9.92) | 1 | 2 | 0.83 (.00–2.44) | 1.66 (1.00–3.93) | 1 | 4 | 0.83 (.00–2.44) | 3.31 (.14–3.66) |
| 65–69 | 5624 | 4393 | 62 | 7 | 8 | 8.82 (2.66–14.97) | 10.08 (3.56–16.60) | 1 | 1 | 1.26 (.00–3.71) | 1.26 (1.00–3.71) | 0 | 0 | 0.00 (.00–∞) | 0.00 (.00–∞) |
| 70–74 | 4092 | 3393 | 92 | 9 | 9 | 8.11 (3.08–13.15) | 8.11 (3.08–13.15) | 1 | 2 | 0.90 (.00–2.66) | 1.80 (1.00–4.27) | 3 | 3 | 2.70 (.00–5.71) | 2.70 (.00–3.16) |
| 75–79 | 3200 | 2738 | 58 | 9 | 9 | 13.28 (5.30–21.25) | 13.28 (5.30–21.25) | 0 | 1 | 0.00 (.00–∞) | 1.48 (1.00–4.34) | 2 | 2 | 2.95 (.00–6.97) | 2.95 (.00–3.49) |
| ≥80 | 4185 | 3640 | 150 | 20 | 22 | 11.60 (6.86–16.33) | 12.76 (7.83–17.68) | 3 | 4 | 1.74 (.00–3.69) | 2.32 (.08–4.56) | 3 | 7 | 1.74 (.00–3.69) | 4.06 (1.12–7.00) |
| Totalb | 40 893 | 30 464 | 600 | 58 | 63 | 6.40 (1.73–11.07) | 7.02 (2.17–11.87) | 7 | 12 | 0.77 (.00–2.36) | 1.36 (.00–3.54) | 10 | 17 | 1.01 (.00–2.93) | 1.70 (.00–4.06) |
Abbreviations: CI, confidence interval; HZ, herpes zoster.
aProportions were weighted to reflect the actual distribution of complications among incident HZ patients.
bThe overall proportion was calculated by adjusting the sampling ratios using the age-specific distribution of the HZ patients.
HZ-related hospitalizations and case-fatality rates are presented in Table 3. Among 8160 HZ incident patients diagnosed in 2015, only 70 (0.86%) were determined to have had an HZ-related hospitalization. The hospitalization rate among the population was 8.49/100 000 person-years (95% CI, 6.72–10.73) and sharply increased with age. The case-fatality rate of HZ was extremely low. Among 40 893 HZ patients diagnosed between 2011 and 2015, only 18 (0.04%) deaths were determined to be HZ-related. Ten out of the 18 patients (56%) were aged ≥80 years. The mortality rate among the population was 0.23 (95% CI, .14–0.37) per 100 000 person-years. HZ was determined as an underlying cause of death for 3 (0.007% or 0.04 per 100 000 person-years among all HZ cases) of the 18 deaths (VZV meningoencephalitis/encephalopathy). For the remaining 15 deaths, HZ was considered a contributing factor.
Table 3.
Herpes Zoster-Related Hospitalizations (2015) and Mortality (2011–2015) Among an Immunocompetent, Unvaccinated Population ≥50 Years Old From Kaiser Permanente Southern California
| Age at HZ Diagnosis, y | HZ-Related Hospitalizations 1 January 2015–31 December 2015 | HZ-Related Mortality 1 January 2011–31 December 2015 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Incident Patients, n | Hospitalizations, n (%) | 95% CI | Rate per 100 000 Person-Years | 95% CI | Incident Patients, n | Deathsa, n (%) | 95% Cl | Rate per 100 000 Person-Years | 95% CI | |
| 50–54 | 1537 | 5 (0.33) | .04–.61 | 2.32 | .97–5.59 | 7709 | 2 (0.03) | .00–.06 b | 0.12 | .03–.49 |
| 55–59 | 1769 | 5 (0.28) | .04–.53 | 2.48 | 1.03–5.97 | 8633 | 0 (0.00) | … | 0.00 | … |
| 60–64 | 1421 | 14 (0.99) | .47–1.50 | 9.74 | 5.77–16.45 | 7450 | 3 (0.04) | .00–.09 | 0.23 | .07–.70 |
| 65–69 | 1200 | 11 (0.92) | .38–1.46 | 11.05 | 6.12–19.95 | 5624 | 1 (0.02) | .00–.05 | 0.10 | .01–.71 |
| 70–74 | 796 | 2 (0.25) | .00–.60 | 3.13 | .78–12.53 | 4092 | 2 (0.05) | .00–.12 | 0.26 | .06–1.02 |
| 75–79 | 603 | 10 (1.66) | .64–2.68 | 23.23 | 12.50–43.17 | 3200 | 0 (0.00) | … | 0.00 | … |
| ≥80 | 834 | 23 (2.76) | 1.65–3.87 | 39.57 | 26.30–59.55 | 4185 | 10 (0.24) | .09–.39 | 1.29 | .70–2.40 |
| Total | 8160 | 70 (0.86) | .66–1.06 | 8.49 | 6.72–10.73 | 40 893 | 18 (0.04) | .02–.06 | 0.23 | .14–.37 |
Abbreviations: CI, confidence interval; HZ, herpes zoster.
aHZ related deaths were identified in 1 of the following ways: (1) HZ was documented as a cause of death in the mortality record, or (2) among hospitalized HZ patients who either died during hospitalization or died within 6 months after discharge, cause of death was determined to be related to HZ complications via chart review.
bNegative lower bounds were set to zero.
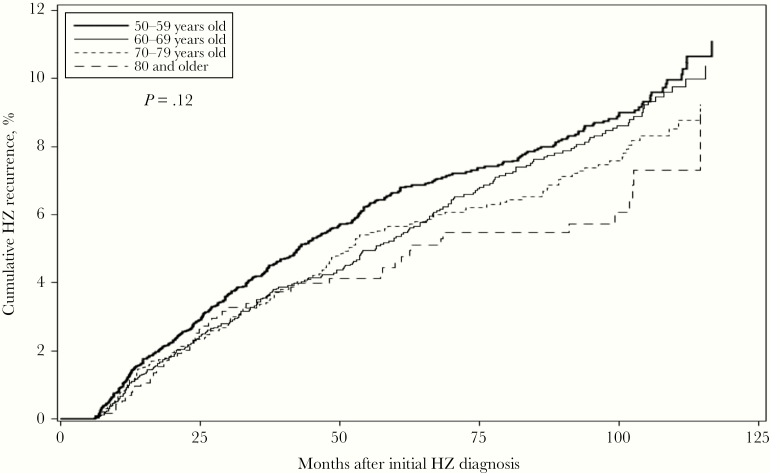
The incidence rate of recurrent HZ is presented in Table 4. Overall, the incidence rate of recurrence was 10.96 (95% CI, 10.18–11.79) per 1000 person-years. The incidence rate of recurrent HZ decreased from 12.74 (95% CI, 10.90–14.90) per 1000 person-years in the 50–54 years group to 8.92 (95% CI, 6.85–11.61) per 1000 person-years in the ≥80 years group. The cumulative incidence of HZ recurrence, stratified by age, is shown in Figure 1. The 10-year cumulative incidence of recurrence was 11.11% (95% CI, 9.60%–12.84%), 10.37% (95% CI, 8.87%–12.11%), 9.23% (95% CI, 7.62%–11.15%), and 8.96% (95% CI, 5.79%–13.74%) for individuals aged 50–59 years, 60–69 years, 70–79 years, and ≥80 years, respectively. Overall, the 2-, 4-, 6-, 8-, and 10-year cumulative incidence of recurrence was 2.50% (95% CI, 2.21%–2.82%), 4.77% (95% CI, 4.36%–5.23%), 6.59% (95% CI, 6.08%–7.13%), 8.03% (95% CI, 7.45%–8.66%), and 10.26% (95% CI, 9.36%–11.23%), respectively. The Kaplan-Meier curves were not statistically significantly different by age group using the log-rank test (P = .12).
Table 4.
Herpes Zoster Recurrence Among an Immunocompetent, Unvaccinated Population ≥50 Years Old From Kaiser Permanente Southern California, 2007–2016
| Incidence of HZ Recurrence | Cumulative Incidence | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at HZ Diagnosis, y | Incident Patientsa, n | Recurrent Patientsb, n | Number of Person-Years | Incidence/1000 Person-Years | 95% CI | 2-y Risk, % | 95% CI | 4-y Risk, % | 95% CI | 6-y Risk, % | 95% CI | 8-y Risk, % | 95% CI | 10-y Risk, % | 95% CI |
| 50–59 | 4286 | 2.81 | 2.33–3.38 | 5.52 | 4.81–6.32 | 7.22 | 6.39– 8.15 | 8.70 | 7.76–9.76 | 11.11 | 9.60–12.84 | ||||
| 50–54 | 2029 | 157 | 12 321 | 12.74 | 10.90–14.90 | ||||||||||
| 55–59 | 2257 | 142 | 12 730 | 11.15 | 9.46–13.15 | ||||||||||
| 60–69 | 3604 | 2.29 | 1.82–2.88 | 4.24 | 3.56–5.05 | 6.54 | 5.64–7.58 | 8.32 | 7.25–9.54 | 10.37 | 8.87–12.11 | ||||
| 60–64 | 2003 | 110 | 10 587 | 10.39 | 8.62–12.53 | ||||||||||
| 65–69 | 1601 | 106 | 9094 | 11.66 | 9.64–14.10 | ||||||||||
| 70–79 | 2518 | 2.33 | 1.78–3.06 | 4.56 | 3.73–5.57 | 6.07 | 5.07–7.25 | 7.38 | 6.23–8.73 | 9.23 | 7.62–11.15 | ||||
| 70–74 | 1447 | 78 | 8179 | 9.54 | 7.64–11.91 | ||||||||||
| 75–79 | 1071 | 65 | 5991 | 10.85 | 8.51–13.83 | ||||||||||
| ≥80 | 1308 | 55 | 6168 | 8.92 | 6.85–11.61 | 2.32 | 1.58–3.42 | 3.99 | 2.94–5.41 | 5.47 | 4.12–7.24 | 5.73 | 4.32–7.59 | 8.96 | 5.79–13.74 |
| Total | 11 716 | 713 | 65 070 | 10.96 | 10.18–11.79 | 2.50 | 2.21–2.82 | 4.77 | 4.36–5.23 | 6.59 | 6.08–7.13 | 8.03 | 7.45–8.66 | 10.26 | 9.36–11.23 |
Abbreviations: CI, confidence interval; HZ, herpes zoster.
aIncident HZ patients from 2007 to 2008 were identified from HZ diagnosis codes plus antiviral medications (acyclovir, valacyclovir, or famciclovir dispensed within ±7 days of HZ diagnosis date).
bRecurrent HZ was defined as having HZ diagnosis codes plus antiviral medications (acyclovir, valacyclovir, or famciclovir dispensed within ±7 days of HZ diagnosis date) ≥6 months after the most recent diagnosis of HZ.
Figure 1.
Cumulative incidence of herpes zoster recurrence among an immunocompetent, unvaccinated population ≥50 years old from Kaiser Permanente Southern California, 2007–2016. Abbreviation: HZ, herpes zoster.
DISCUSSION
In this study, we provide estimates from a large integrated health care network on the incidence of HZ, as well as data on HZ-associated complications, hospital admissions, mortality, and recurrence in unvaccinated, immunocompetent adults ≥50 years old. Data included in this study were obtained prior to RZV approval, and therefore provide timely updates to better assess the potential impact of the uptake of RZV on HZ epidemiology. Our results also provide estimations for parameters that are needed in cost-effectiveness and budget impact analyses to help inform policy decisions.
Estimations of age-specific incidence of HZ vary across studies, mainly due to population compositions (eg, distribution of sex and race, inclusion of immunocompromised patients, or differences in vaccination coverage). In contrast to active surveillance used in clinical trials [3, 4, 14], our study depended on real-world care seeking, which is influenced by many factors such as community, physician practice, patient comorbidities, timing of presentation, and HZ severity.
We found that approximately 8%–10% of patients with HZ experienced complications in addition to pain, similar to that in the ≥50-year-old population reported by Yawn et al in 2007 [2]. The proportion of HZ-related complications in our study needs to be interpreted with caution. Medical record review was targeted to identify patients with cutaneous, neurologic, or other complications, excluding PHN and HZO. Patients with pain only documented in the medical record were not included as having complications. In addition, we used criteria to define complications that would more accurately reflect utilization and therefore provide a better estimation of the costs associated with HZ. For example, we defined persisting zoster as an HZ rash that did not resolve within 10 days of the HZ diagnosis date, which is considerably shorter than the definition used in other studies [15]. However, costs incurred from care sought for an HZ rash persisting beyond 10 days could be an important factor to include in economic projections. Nevertheless, we acknowledge that health-seeking behavior and accessibility of care likely play a role in our estimations. With a prepaid and highly accessible system like KPSC, the proportion of complications may be higher than those estimated from other health care systems.
Given that the study included individuals who were immunocompetent at baseline, hospitalization associated with HZ was low but increased substantially with age. Among more than 8000 incident HZ patients in 2015, less than 1% had an HZ-related hospitalization and mainly in the oldest old. Similarly, in the ZOE-50 and ZOE-70 combined analysis, Kovac et al reported 1.05% (5/477) of incident HZ patients in the placebo group being hospitalized for HZ [16]. The hospitalization rate for adults ≥80 years was particularly high (about 40/100 000 person-years), but lower than in a previous study: Jackson et al reported HZ-associated hospitalization rates (also confirmed with medical records) ranging from 10/100 000 persons in adults 60–69 years of age to 65/100 000 persons in adults ≥80 years of age in the United States [17]. Data on HZ-related mortality in immunocompetent populations are sparse in the literature. After detailed review of medical records, only 18 (0.04%) deaths were considered HZ-related in our study. Patients aged ≥80 years are at a much higher risk of HZ-related death, likely due to HZ aggravating comorbidities that lead to functional decline and loss of independence. Our finding of substantially higher rates of HZ-related hospitalization and death in adults ≥80 years of age suggests the importance of preventing HZ in this older population.
Data on the incidence of HZ recurrence are critical for economic analyses of vaccination programs [18]. However, limited data are available. Previous estimations for the risk of recurrence were based on studies with small sample sizes or short follow-up durations [19, 20]. Population composition, case definitions, and follow-up duration vary, making comparisons across studies difficult [21]. In our recurrence analysis, identification of the initial HZ episode and HZ recurrence required both a diagnostic code and a dispense record of antiviral medications. This increased the positive predictive value and excluded patients with carry-forward diagnostic codes only. Our estimates were comparable to those estimated in a previous study that used medical record review to identify recurrence [20]. Among 1131 patients aged ≥50 years with incident HZ (about 8% immunocompromised population), the incidence of recurrence was 9.2/1000 person-years in the 50–69 year olds and 9.0/1000 person-years in the ≥70 year olds with an average 6.9 years of follow-up [20]. Our estimation is 10.96/1000 person-years with an average 5.6 years of follow-up. While the results are similar, our study has more power with 6 times the number of person-years, and our study excluded immunocompromised persons. These advantages allow for clearer interpretation of the data than previous studies [22, 23]. Our data suggest a linear increase of recurrence over time instead of a delayed recurrence after a recent HZ episode. The 10-year cumulative incidence of recurrence estimated by our study suggests that each year approximately 1% of the immunocompetent patients with an initial HZ at ≥50 years will experience a recurrence. Our data do not support the hypothesis that an HZ episode prevents recurrence [24]. The role of endogenous boosting in preventing HZ needs further examination.
Our study had some potential limitations. First, it was not feasible to perform medical record review of all the incident and recurrent HZ patients given the large sample size of our study. Nevertheless, the incidence rate estimated in this study was similar to those reported in the placebo group in clinical trials that mainly included immunocompetent subjects. The recurrence rates are also similar to those reported in the same age group from a study with similar follow-up duration that used medical record review to confirm recurrence. Secondly, medical record review for complications was conducted for a sample of 600 incident HZ patients from the outpatient, emergency department, and inpatient settings. Rare complications may have been missed; however, by reviewing and confirming hospitalized patients and mortality patients, we were able to estimate the incidence of more severe HZ patients and HZ-related fatality. Finally, to reduce the probability of including follow-up visits of an initial episode when estimating HZ recurrence, we specified that recurrent HZ had to occur at least 6 months after the most recent visit with an HZ diagnostic code. Hence, we may have missed patients with recurrence occurring soon after the initial episode.
In conclusion, our study provides important estimations for base case values in an immunocompetent population that are needed for vaccine economic model analyses. Our data suggest that HZ-related mortality is infrequent in immunocompetent HZ patients. Mortality and hospitalization mainly occur in the oldest old. The incidence rate and recurrence rate had different trends with increased age. The linear increase of recurrence over time does not support the hypothesis of HZ preventing recurrence. Although utilization data may be affected by health-seeking behavior or accessibility to care, they directly reflect the real-world disease burden to health care systems.
Notes
Acknowledgments. The authors thank Business and Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Grégory Leroux coordinated manuscript development and editorial support.
Author contributions. H. F. T., K. B., B. A., Y. L., H. T., C. Z., B. C., B. J. P., D. V. O., and L. S. S. were involved in the design of the study. H. F. T., K. B., B. A., Y. L., H. T., Y. T., C. Z., B. C., and L. S. S. collected or generated the data. All authors analyzed and/or interpreted the data and participated to the development of this manuscript and in its critical review with important intellectual contributions. All authors had access to relevant aggregated study data and gave approval of the final manuscript before submission. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The work described was carried out in accordance with ICMJE recommendations for conduct, reporting, editing, and publications of scholarly work in medical journals. The corresponding author had final responsibility to submit for publication.
Financial support. This work was supported by GlaxoSmithKline Biologicals SA (GSK study identifier, HO-17-18378) and was involved in all stages of study conduct, including analysis of the data. GlaxoSmithKline Biologicals SA also provided funding for editorial support, manuscript coordination, and journal-specific fees associated with publication of this manuscript.
Potential conflicts of interest. H. F. T., K. B., B. A., Y. L., H. T., Y. T., C. Z., B. C., and L. S. S. are employees of Kaiser Permanente Southern California, which has been contracted by the GSK group of companies for the conduct of the present study. The following research contracts with pharmaceutical companies unrelated to the present work are reported: Dynavax (K. B., B. A., B. C., and L. S. S.), GSK (H. F. T., K. B., B. A., Y. L., H. T., Y. T., C. Z., B. C., and L. S. S.), Merck (Y. T.), Novavax (H. F. T., B. A., Y. L., L. S. S.), Seqirus (H. F. T., K. B., B. A., Y. L., H. T., and L. S. S.). H. F. T. served as a paid consultant for GSK. B. J. P. and D. V. O. are employees of GSK. B. J. P. holds shares in GSK.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: IDWeek 2019, 2–6 October 2019, Washington, DC (abstract ID, 674336).
References
- 1. Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices (ACIP) , Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57:1–30; quiz CE2–4. [PubMed] [Google Scholar]
- 2. Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc 2007; 82:1341–9. [DOI] [PubMed] [Google Scholar]
- 3. Cunningham AL, Lal H, Kovac M, et al. ; ZOE-70 Study Group Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med 2016; 375:1019–32. [DOI] [PubMed] [Google Scholar]
- 4. Lal H, Cunningham AL, Godeaux O, et al. ; ZOE-50 Study Group Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372:2087–96. [DOI] [PubMed] [Google Scholar]
- 5. Dooling KL, Guo A, Patel M, et al. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep 2018; 67:103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harpaz R, Leung JW. The epidemiology of herpes zoster in the United States during the era of varicella and herpes zoster vaccines: changing patterns among older adults. Clin Infect Dis 2019; 69:341–4. [DOI] [PubMed] [Google Scholar]
- 7. Zheng C, Luo Y, Mercado C, et al. Using natural language processing for identification of herpes zoster ophthalmicus cases to support population-based study. Clin Exp Ophthalmol 2019; 47:7–14. [DOI] [PubMed] [Google Scholar]
- 8. Bruxvoort KJ, Liang AS, Harpaz R, et al. Patient report of herpes zoster pain: incremental benefits of zoster vaccine live. Vaccine 2019; 37:3478–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tseng HF, Lewin B, Hales CM, et al. Zoster vaccine and the risk of postherpetic neuralgia in patients who developed herpes zoster despite having received the zoster vaccine. J Infect Dis 2015; 212:1222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Derose SF, Contreras R, Coleman KJ, Koebnick C, Jacobsen SJ. Race and ethnicity data quality and imputation using U.S. census data in an integrated health system: the Kaiser Permanente Southern California experience. Med Care Res Rev 2013; 70:330–45. [DOI] [PubMed] [Google Scholar]
- 11. Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J 2012; 16:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tseng HF, Smith N, Harpaz R, Bialek SR, Sy LS, Jacobsen SJ. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA 2011; 305:160–6. [DOI] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention. U.S. Standard Certificate of Death, 2003https://www.cdc.gov/nchs/data/dvs/DEATH11-03final-ACC.pdf. Accessed 28 February 2019.
- 14. Oxman MN, Levin MJ, Johnson GR, et al. ; Shingles Prevention Study Group A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–84. [DOI] [PubMed] [Google Scholar]
- 15. John AR, Canaday DH. Herpes zoster in the older adult. Infect Dis Clin North Am 2017; 31:811–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kovac M, Lal H, Cunningham AL, et al. ; ZOE-50/70 Study Group Complications of herpes zoster in immunocompetent older adults: incidence in vaccine and placebo groups in two large phase 3 trials. Vaccine 2018; 36:1537–41. [DOI] [PubMed] [Google Scholar]
- 17. Jackson LA, Reynolds MA, Harpaz R. Hospitalizations to treat herpes zoster in older adults: causes and validated rates. Clin Infect Dis 2008; 47:754–9. [DOI] [PubMed] [Google Scholar]
- 18. Prosser LA, Harpaz R, Rose AM, et al. A cost-effectiveness analysis of vaccination for prevention of herpes zoster and related complications: input for national recommendations. Ann Intern Med 2019; 170:380–8. [DOI] [PubMed] [Google Scholar]
- 19. Tseng HF, Chi M, Smith N, Marcy SM, Sy LS, Jacobsen SJ. Herpes zoster vaccine and the incidence of recurrent herpes zoster in an immunocompetent elderly population. J Infect Dis 2012; 206:190–6. [DOI] [PubMed] [Google Scholar]
- 20. Yawn BP, Wollan PC, Kurland MJ, St Sauver JL, Saddier P. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc 2011; 86:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open 2014; 4:e004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shiraki K, Toyama N, Daikoku T, Yajima M; Miyazaki Dermatologist Society Herpes zoster and recurrent herpes zoster. Open Forum Infect Dis 2017; 4:ofx007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakamura Y, Miyagawa F, Okazaki A, et al. Clinical and immunologic features of recurrent herpes zoster (HZ). J Am Acad Dermatol 2016; 75:950–956.e1. [DOI] [PubMed] [Google Scholar]
- 24. Weinberg A, Zhang JH, Oxman MN, et al. ; US Department of Veterans Affairs (VA) Cooperative Studies Program Shingles Prevention Study Investigators Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J Infect Dis 2009; 200:1068–77. [DOI] [PMC free article] [PubMed] [Google Scholar]