Abstract
To understand the role of intestinal mucosal microbiota on mental stress-related diarrhoea, we collected the intestinal mucosa of mice treated with Folium senna extract gavage combined with restraint and tail pinch stress for 7 days; and intestinal mucosal microbiota characteristics were analyzed by 16S rRNA Pacbio SMRT gene full-length sequencing. The results showed that the diversity (i.e., alpha diversity including the Chao1, Simpson, ACE, and Shannon indices and beta diversity including the NMDS of weighted UniFrac distances) and composition of the microbial community in the intestinal mucosa of mice with diarrhoea and repeated stress changed significantly (P < 0.05). In the co-occurrence network, Staphylococcus sciuri and Escherichia fergusonii was identified as putative keystone species. Moreover, the characteristics of the intestinal microbial species was analyzed by LEfSe, Metastats, and group difference, and ten altered gut microbiota species can be used as characteristic microbes in the mice with diarrhoea and repeated stress: the abundances of Stigmatella aurantiaca, Candidatus arthromitus sp. SFB-mouse, Erythrobacter gaetbuli, Desulfitobacterium hafniense, Ochrobactrum pituitosum, and Candidatus arthromitus sp. SFB-mouse-NL in the model group were significantly lower than those in the control group (P < 0.05); whereas Microbacterium dextranolyticum, Klebsiella pneumoniae, Escherichia sp. BBDP27, and Streptococcus danieliae were enriched in the control group (P < 0.05). Collectively, mental stress-related diarrhoea increased the intestinal microbiota diversity. The species associated with mental stress-related diarrhoea including Microbacterium dextranolyticum, Klebsiella pneumoniae, Escherichia sp. BBDP27, and Streptococcus danieliae were significantly enriched; while the species which are beneficial to mental stress-related diarrhoea are Stigmatella aurantiaca, Candidatus arthromitus sp. SFB-mouse, Erythrobacter gaetbuli, Desulfitobacterium hafniense, Ochrobactrum pituitosum, and Candidatus arthromitus sp. SFB-mouse-NL for its significantly depleted.
Keywords: Functional gastrointestinal disorders, Diarrhoea, Psychological distress, Intestinal microbiota, Intestinal mucosa, Folium senna extract
Introduction
Gastrointestinal symptoms, especially diarrhoea, can be triggered by the psychological distress (Berens et al. 2019). The psychological distress have a detrimental impact, manifesting in functional gastrointestinal disorders (FGID) including irritable bowel syndrome (IBS) (Hearn et al. 2020) and celiac disease (CD) (Coburn et al. 2019). The prevalence of FGID is high, with a population-based study suggesting 62% for at least one gastrointestinal symptom according to the Rome IV criteria (Agrawal et al. 2020). 50–60% of the people who suffering from psychiatric conditions have been recorded to have progression of the gastrointestinal disorders (Agrawal et al. 2020). Clinical evidence shows that the psychological distress could predict gastrointestinal disorders and chronic diarrhoea in humans, in which 18% reported suffering psychological distress (Clevers et al. 2019). Diarrhoea caused by psychological distress is currently considered as one of the worst public health problems due to the number of cases, difficulty of treatment, healthcare costs, and quality-of-life issues for affected individuals.
Our understanding of the etiology and the pathogenesis of diarrhoea related to the psychological distress remained limited. It is now evident that microbial factors play key roles in its pathophysiology. Researches have demonstrated that microbiota such as Escherichia coli O and Clostridium difficile- contribute to the host nervous system (Jia et al. 2018; Holtmann et al. 2017), which is highlighted associations between diarrhoea and mental stress. The microbiota-derived metabolites can serve as neurochemicals to induce the expression of norepinephrine (NE), 5-hydroxytryptamine (5-HT), and dopamine (DA) and increased the expression of Aquaporin-3 (AQP-3) to alter the intestinal permeability (Holtmann et al. 2017). The increased intestinal permeability is dominated by diarrhoea associated with intestinal bacterial overgrowth (Pimentel et al. 2020). Furthermore, the intestinal mucosal barrier primarily composed of biological and immunologic barriers is the key role in the interaction with the brain-gut axis by regulation of endocrine and neurologic functions (Plaza-Diaz et al. 2019). Moreover the microbiota colonized in the intestinal mucosa (i.e., intestinal mucosal biological barrier) is more sensitive and characteristic to the intestinal nerve response (Sokol et al. 2020). But there are no studies have comprehensively examined the microbiotal composition and characteristics of the diarrhoea related to psychological stress.
Further research on the associations of the intestinal mucosa microbiome with diarrhoea related to psychological stress is warranted. Referring to our previous researches (Yuan et al. 2020; Liu et al.2020), in this study the animal model of diarrhoea with mental stress was established by the Folium senna extract gavage combined with restraint and tail pinch stress for 7 days in KM mice. Our previous study have shown that Folium sennae has a good immediate and effective cathartic effect (Long et al. 2018), so it can be used to construct the model of animal diarrhoea to support research on the disturbance of microbiota triggered by food intake (Lu et al. 2020). And the repeated tail pinch and restraint are often used to imitate environmental stressors to investigate the development of chronic unpredictable mild stress, since it could cause forms of mental stress such as pain and restlessness (Huang et al. 2020; Guerrero-Bautista et al. 2019). In addition, in our study a new improved technique of 16S rRNA gene sequencing, PacBio Single molecule real-time (SMRT) sequencing technology, was used to accurately obtain the information of rRNA gene full-length sequence (Johnson et al. 2019; Hsieh et al. 2019). Pacbio SMRT sequencing technology can greatly improve ability to analyze the diversity of microbiota at the species level to screen potential biomarkers to connect diarrhoea and mental stress.
In our previous research (Zhang et al. 2020), we found that Folium sennae can alter the intestinal bacterial characteristic and intervene the tryptophan metabolism of intestinal microflora, such as Streptococcus, Sutterella and Dorea. So considering the role of tryptophan secondary metabolites on gastrointestinal nerve responding to mental stress, we investigated the microbiotal characteristics of the intestinal fecal of mice treated with Folium senna gavage combined with environmental stressors including restraint and tail pinch stress in this study. But in our study on the intestinal microbiota diversity of Folium senna gavage combined with restraint and tail pinch stress, we found the differences of composition and abundance of species were not significant in intestinal fecal of mice with diarrhoea and repeated stress (Yuan et al. 2020). Recent studies have found that the microbiota colonized in the intestinal mucosa is more sensitive and characteristic to the intestinal nerve response (Sokol et al. 2020). Thus, we hypothesized that there was a greater sensitivity to changes in intestinal microflora diversity and community structure in the intestinal mucosa of stress-related diarrhoea mice. The aim of the current study was to systematically characterize overall differences in intestinal mucosa microbial communities of mice with diarrhoea and repeated stress using the Pacbio SMRT gene full-length sequencing. The current study will first clarify the mucosa microbial characteristics of diarrhoea related to repeated mental stress and the potential biomarkers of diarrhoea with repeated mental stress.
Materials and methods
Experiment materials and reagents preparation
Five hundred grams of Folium sennae used in this study originated in Yunnan Province and provided by out-patient pharmacies of First Hospital of Hunan University of Chinese Medicine. Referring to our previous experiment, the Folium sennae filtrate was evaporated and concentrated into 500 mL (1 g/mL crude drug) decoction in a 75 °C rotary evaporator and preserved at 4 °C.
Total microbial genomic DNA samples were extracted using the OMEGA DNA isolation kit (Omega, D5625-01, USA) following the manufacturer’s instructions. PCR amplification of the nearly full-length bacterial 16S rRNA genes was performed using the forward primer 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and the reverse primer 1492R (5′-ACCTTGTTACGACTT-3′).
Animals and procedures
10 SPF KM mice (10-wk-old male, 20 ± 2 g on average) were purchased from Hunan Slaccas Jingda Laboratory (SJA) Animal Company (Hunan, China), with licence number SCXK (Xiang) 2016-0002. All the experimental animals were carried out in a shielded environment at the Animal Experiment Center of the Hunan University of Chinese Medicine with license number SYXK (Xiang) 2015-0003, ensuring ventilation, light avoidance, cleanliness and quietness, at a relative humidity of 47–53% and temperature of 23–25 °C, and were fed with unified standard feed purchased from Hunan SJA Laboratory Animal Company (Hunan, China).
The method of animal modeling in this study refers to our previous researches (Yuan et al. 2020; Liu et al. 2020). All the mice were randomly divided into two groups: five mice in the control group (gcm) and five mice in the model group (gmm). The mice were intragastrically administered once a day: at 9 am, the mice in the model group were received 0.35 mL of Folium senna extract while the mice in the control group were given the same dose of distilled water for 7 days continuously. At 3 pm, the mice in the model group were restrained in a constraint tube, and the distal 1/3 of the tail was pinched with a clip for 1 h each time for each day. The mice in the control group were without intervention.
Each mouse in two groups was sacrificed by cervical dislocation at day 8 for collecting intestinal mucosa samples. Intestine from the jejunum to rectum was stripped and flushed with PBS for removing contents. Then the intestinal tissue samples were cut longitudinally and the intestinal mucosa was scraped. The intestinal mucosa were collected immediately frozen at – 80 °C for DNA extraction.
Total DNA extraction from intestinal mucosa samples
Total microbial genomic DNA of intestinal mucosa samples were extracted following the manufacturer’s instructions and stored at – 20 °C. The quantity and quality of extracted DNAs were measured using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and agarose gel electrophoresis, respectively.
PCR amplification and sequencing analysis
The extracted DNA was amplified with two-step PCR, with sample-specific 16-bp barcodes were incorporated into the forward and reverse primers for multiplex sequencing in the second PCR step. The amplification system was prepared as follows: 5 μL of Q5 reaction buffer (5 ×), 5 μL of Q5 High-Fidelity GC Bufer (5 ×), 0.25 μL of Q5 High-Fidelity DNA Polymerase (5 U/μL), 2 μL (2.5 mM) of dNTPs, 1 μL (10 μmol/L) each of the forward and reverse primers, 2 μL of DNA template, and 8.75 μL of ddH2O. After thermal cycling a total of PCR amplicons were purified with Agencourt AMPure Beads (Beckman Coulter, Indianapolis, IN) and quantified using the PicoGreen dsDNA Assay Kit (Invitrogen, Carlsbad, CA, USA). Single Molecule Real Time (SMRT) sequencing technology was performed using the PacBio Sequel platform at Shanghai Personal Biotechnology Co., Ltd (Shanghai, China), and PacBio circular consensus sequencing (CCS) reads were derived. Raw sequences were initially processed through the PacBio SMRT Link portal (version 5.0.1.9585), and the raw sequences read with exact matches to the barcodes were assigned to respective samples and identified as valid sequences.
After chimera detection, the remaining high-quality sequences were clustered into OTUs at 97% sequence identity with UCLUST. OTUs containing less than 0.001% of total sequences across all samples were discarded.
Bioinformatics and statistical analysis
Sequence data analyses were mainly performed using QIIME and R packages (v3.2.0). Alpha diversity indices were computed using the OTU table in QIIME. Beta diversity analysis was performed to investigate the structural variation of microbial communities across samples using UniFrac distance metrics. Co-occurrence analysis was performed by calculating Spearman’s rank correlations between predominant taxa. Correlations with RHO > 0.6 and P < 0.01 were visualized as co-occurrence network using Cytoscape. Using the software of Mothur, the statistical algorithm of metastats (https://metastats.cbcb.umd.edu/) was called to test the sequence size (i.e., absolute abundance) difference of each taxon at the level of species. LEfSe (Linear discriminant analysis effect size) was performed to detect differentially abundant taxa across groups using the default parameters. Statistical analyses were performed with R 3.6.1 statistical software using the Student’s paired or unpaired t test. P < 0.05 was considered as statistically significance.
Results
Diversity analysis of intestinal mucosal microbiota
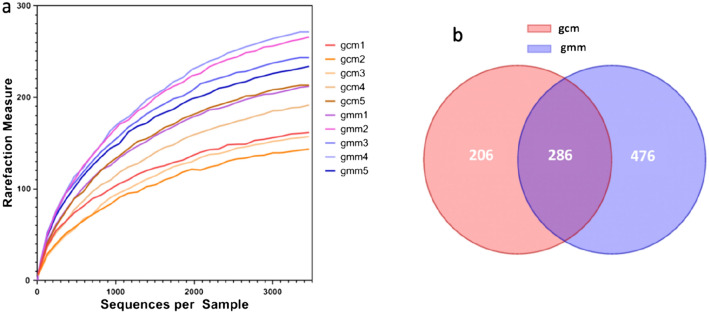
The number of OTUs can be compared in different samples under the same sequencing depth in the sparse curve, so as to measure the diversity of each sample to a certain extent. As shown in Fig. 1, the sparse curve flattens after 500 bps means the sample size was enough to estimate the community richness.
Fig. 1.
Rarefaction curve diagrams (a) and Veen diagram (b) of OTUs. a The abscissa represents the sequences randomly selected per sample, and the ordinate represents the number of OTUs found at the corresponding depth. The fatter the curve is, the more suficient the sequencing result; gcm 1–5, control groups 1–5, gmm 1–5 model groups 1–5. b OUT numbers. Red, the number of OTUs in gcm group; purple, the number of OTUs in gcm group
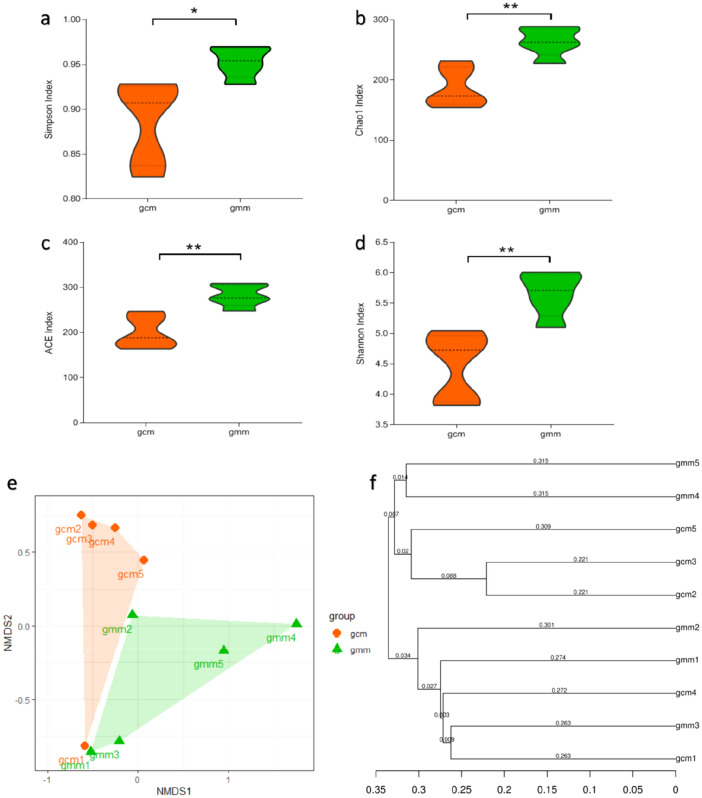
To investigate variances of diversity of intestinal mucosal microbiota between groups gmm and gcm, microbial alpha diversity including the Chao1, Simpson, ACE, and Shannon indices were used to estimate richness and diversity (Yin et al. 2020). We found that alpha diversity was significantly increased in group gmm compared with the control group (P < 0.05). These results indicate that intraindividual bacterial diversity in mice with diarrhoea and repeated stress distinctly differed from normal mice (Fig. 2a–e). Moreover, the NMDS of weighted UniFrac distances were used to measure beta diversity in groups (Han et al. 2016). The main purpose of beta diversity analysis is to investigate the similarity of community structure among different samples, and the results showed that the intestinal mucosal microbial diversity of the mice with diarrhoea and repeated stress (gmm) was significantly different from that of the control group (gcm) in weighted UniFrac distances (ANOSIM R = 0.2480, P = 0.041, Fig. 2f).
Fig. 2.
Diversity of the microbial community in the intestinal mucosa of the mice with diarrhoea and repeated stress (gcm) and the mice given distilled water (gmm). Alpha diversity was evaluated based on the Simpson (a), Chao1 (b), ACE (c), and Shannon (d) indices of the OTU levels. Principal coordinate analysis of beta diversity was based on the weighted UniFrac (e) and UPGMA clustering (f) analyses of weighted UniFrac distance matrix. Each point represents a sample. The closer the points are, the more similar the structures of the communities. gcm control group, gmm model group. *P < 0.05; **P < 0.01
Altered component analysis of the dominant microbiota
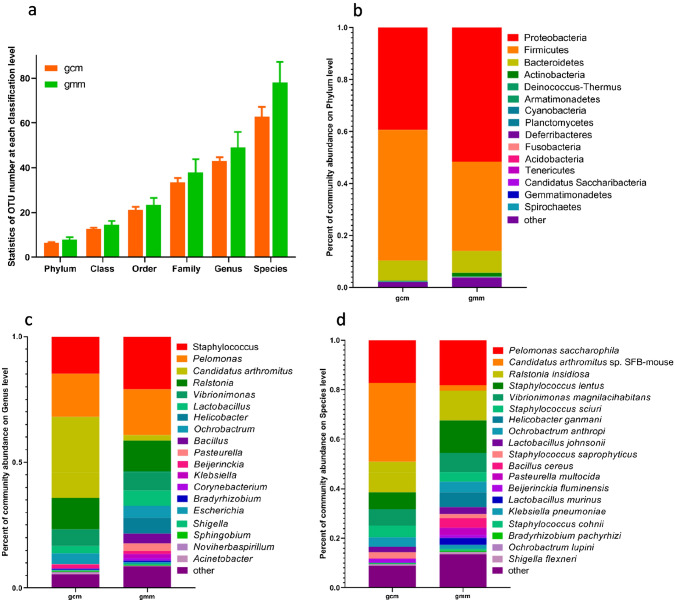
As shown in Fig. 3a, taxonomic analysis identified the OTU number of microbiota at each classification level. At all the six microbiota levels, the number of microbial taxa in mice with diarrhoea and repeated stress was higher than that in control group, with no statistically significant difference (P > 0.05). OTUs were identified into 15 prokaryotic phyla from the gene sequences (the sequences that could not be classified into any known groups were specified as other), and the relative abundance of 15 phyla varied between group gcm and gmm (Fig. 3b). The dominant (relative abundance > 5%) phyla in group gcm and gmm were Proteobacteria (39.32% vs. 51.61%), Firmicutes (50.37% vs. 34.34%), and Bacteroidetes (7.63% vs. 8.40%), and these dominant phyla accounted for over 94% of all sequences.
Fig. 3.
Composition profiles of microbiota colonized in intestinal mucosa in the mice with diarrhoea and repeated stress (gcm) and the mice given distilled water (gmm). a Mean OTUs at each classification level; Phylum-level bacteria (b), Genus-level bacteria (c), and Species-level bacteria (d) were significantly different between the 2 groups; data were showed as relative abundance (%) of top 20 most abundant in each group. The abscissa is arranged according to group. The ordinate shows the relative abundance. The same colour represents the same classification unit, and longer bars indicate higher relative abundances of units
When the OTUs were analysed at genus level, a total of 147 genera were detected in all samples and relative abundance of top 20 was shown in Fig. 3c. The relative abundance of the dominant genera in two groups were obviously different: the mice with diarrhoea and repeated stress (gmm) was dominated by Staphylococcus (21.02%), Pelomonas (18.20%), Ralstonia (12.15%), Vibrionimonas (7.67%), Helicobacter(6.39%), and Lactobacillus(6.04%) which accounted for 71.47% of all sequences; in the control group (gcm), the dominant genera were Candidatus arthromitus (32.17%), Pelomonas (17.22%), Ralstonia (12.46%), and Vibrionimonas (6.65%), accounting for over 68.50% of all sequences.
A total of 266 species were detected in all samples and relative abundance of top 20 was shown in Fig. 3d. Compared with group gcm, the dominant (relative abundance > 5%) species of reduction in group gmm were Candidatus arthromitus sp. SFB-mouse (31.96% vs. 2.15%) and Ralstonia insidiosa (12.24% vs. 12.02%); the dominant species increased in group gmm were Pelomonas saccharophila (12.22% vs. 18.20%), Staphylococcus lentus (6.81% vs. 13.15%), and Vibrionimonas magnilacihabitans (6.65% vs. 7.67%).
Co-occurrence network analysis of the species colonized in intestinal mucosa of mice
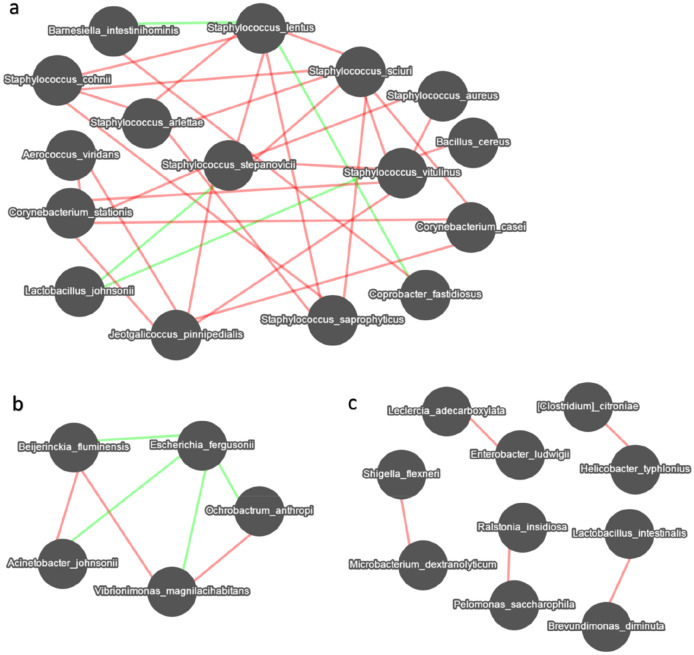
To explore the functional correlation between the species colonized in intestinal mucosa of mice, a correlation matrix was calculating using the Spearman’s correlation coefficients between microbial communities at the species level (Ossowicki et al. 2020; Sun et al. 2020). As shown in Fig. 4, a total of 31 significant species mainly clustered into 2 networks and 5 interrelationships determined based on an RHO > 0.6 and P < 0.01. Specifically, Staphylococcus sciuri, Staphylococcus stepanovicii, Staphylococcus vitulinus were significantly associated with seven species (Fig. 4a). In addition, Escherichia fergusonii was negatively correlated with four species including Beijerinckia fluminensis, Acinetobacter johnsonii, Vibrionimonas magnilacihabitans, and Ochrobactrum anthropi (Fig. 4b). There are five interrelationships shown as Fig. 4c, Lactobacillus intestinalis was positively correlated Brevundimonas diminuta; Leclercia adecarboxylata was positively correlated Enterobacter ludwigii. These correlation data suggested the mice with diarrhoea and repeated stress exhibited abnormal colonization of Staphylococcus and Escherichia, which may result in significant taxonomic perturbations in the intestinal mucosal microbiome.
Fig. 4.
Co-occurrence network analysis of dominant species (top50). The connection between the nodes indicates that there is a correlation between the two species; the red line indicates a positive correlation; the green line indicates a negative correlation. The more connections a node has, the more association it has with other species. Correlations with RHO > 0.6 and P < 0.01 were visualized as co-occurrence network
Key biomarkers for mice with diarrhoea and repeated stress
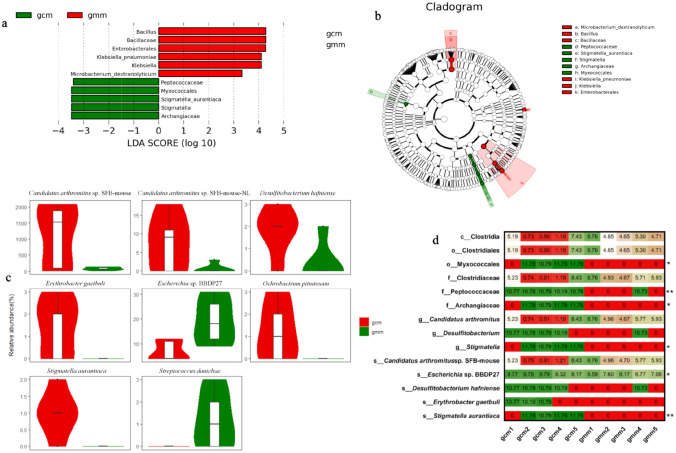
LEfSe is an analysis method based on linear discriminant analysis (LDA) effect size combined linear discriminant analysis with Kruskal Wallis and Wilcoxon rank sum test to screen key biomarkers (i.e. key community members) (Ozkul et al. 2020). LEfSe was used to determine whether specific bacterial taxa were differentially enriched in the mice with diarrhoea and repeated stress (gmm) compared with the control mice (gcm). Using a logarithmic LDA score cutoff of 2, we identified nine discriminatory microbial taxas as key discriminants (P < 0.05, Fig. 5a), and the different level taxon was shown in Fig. 5b. Three species including Stigmatella aurantiaca, Microbacterium dextranolyticum, and Klebsiella pneumoniae were identified as key biomarkers: Stigmatella aurantiaca was significantly overrepresented in the intestinal mucosa of group gcm, whereas Microbacterium dextranolyticum, and Klebsiella pneumoniae were enriched in group gmm.
Fig. 5.
Key biomarkers for mice with diarrhoea and repeated stress. a Cladogram generated from the LEfSe analysis indicating the phylogenetic distribution from phylum to species of the microbiota. Diagram of taxonomic units with significant differences between groups. The ordinate represents the taxa with significant differences, and the abscissa represents the LDA scores visualized as bars. The bars are ordered by score to describe the differences in each sample. The longer the bar is, the more significant the difference. b Diagram of intergroup difference in taxonomic units based on the classification tree. Histogram of LDA scores to identify differentially abundant bacterial species (LDA score ≥ 2, P < 0.05). The cladogram shows all the hierarchical relationships among the taxonomic units from phylum to species. The node sizes correspond to the average relative abundances of the taxa. The colour indicates a significant difference in the taxa and high abundance in the sample; green colour represents gcm group, red colour represents gmm group. The letters identify taxa had obvious differences. c Abundance distribution between groups at the species level. The 8 absolute abundance difference at the level of species was test by the statistical algorithm of metastats (P < 0.05). d Relative abundance heat map. Red color means low relative abundance; green color means high relative abundance. *P < 0.05; **P < 0.01
Metastats is a classical statistical method of microecology (Wang et al. 2019), which tests the difference of sequence size (i.e., absolute abundance) between samples (groups) of different level taxon. As shown in Fig. 5c, six species including Stigmatella aurantiaca, Candidatus arthromitus sp. SFB-mouse, Erythrobacter gaetbuli, Desulfitobacterium hafniense, Ochrobactrum pituitosum, and Candidatus arthromitus sp. SFB-mouse-NL were significantly overrepresented in the group gcm, whereas Escherichia sp. BBDP27 and Streptococcus danieliae were enriched in group gmm.
Statistical analysis of differences of relative abundance between groups in different level taxon was shown in Fig. 5d: the depth of the heat map color represents the relative abundance, and significant differences in the proportions of two intestinal microbes were found at the species level. The abundance of Stigmatella aurantiaca was absent expression, and that the abundances of Escherichia sp. BBDP27 substantially increased from 0.20 to 0.56% in intestinal mucosa of the mice with diarrhoea and repeated stress.
Discussion
Combined with our previous studies on the diversity and structure of intestinal microflora in diarrhoea caused by various stressors, the results of the current study on the intestinal mucosal microflora in mental stress-related diarrhoea are of guiding significance for FGID clinical. After the treatment of Folium senna gavage combined with restraint and tail pinch stress, the diversity index and the NMDS of weighted UniFrac distances was significantly different, which showed that the diversity and composition of the microbial community in the intestinal mucosa of mice with diarrhoea and repeated stress changed significantly. Specifically, the diversity of microflora in the intestinal mucosa of mice with diarrhoea and repeated stress is more abundant, which also means that the bacteria are overgrown. It was found clinically that the risk of FGID (especially stress-related diarrhoea) was 4.5 times higher than that of those without small intestinal bacterial overgrowth (SIBO) (Shah et al. 2020). On the contrary, our previous studies have shown that dietary intake of Folium sennae and the antibiotic abuse can cause diarrhoea and reduce the diversity of intestinal microflora (Long et al. 2017, 2018; Zeng et al. 2019). Whether the SIBO can be a characteristic manifestation of diarrhoea is controversial (Pimentel et al. 2020; Sundin et al. 2020; Shah et al. 2020). The study shows that compared with Folium sennae induced diarrhoea the mental stress induced diarrhoea seems more likely to develop SIBO. Moreover, the difference of microbiota community structure in intestinal mucosa is more significant compared with our previous studies on fecal diversity sequencing of mice with diarrhoea and repeated stress (Yuan et al. 2020). In other words, the microbiota colonized in the intestinal mucosa is more sensitive and characteristic to response between environmental stressors and the physiological function of the gastrointestinal tract. Therefore, the current study supports the intestinal mucosal biological barrier effect on stress-related diarrhoea.
The altered microbial community structure in diarrhoea caused by repeated mental stress is one of the characteristics of the damage of intestinal mucosal biological barrier function. Bacteroides and Firmicutes are the dominant bacteria in human intestine, and the Firmicutes/Bacteroidetes ratio (F/B) is associated with glycolipid metabolism (Yang et al. 2019) regulate key metabolic pathways that are necessary for growth, reproduction, and immunity, metabolism of the host (Xie et al. 2020). In the current study, the F/B decreased from 6.60 to 3.88; and it is consistent with the clinical research results of chronic stress mental disease, which suggests that patients with chronic stress mental disease have a lower level of F/B (Ahmed et al. 2020). According to the results of co-occurrence network analysis, Staphylococcus and Escherichia fergusonii were found to have an intervention effect on the overall change of microbial community structure. Staphylococcus (Wahbi et al. 2020) and Escherichia (Karki et al. 2020) are major pathogens predominantly associated with digestive disorders and bacterial infections. The chronic inflammation of intestine or host such as visceral hypersensitivity is the basic pathogenesis of postinflammatory and neuropathic pain caused by mental stress-related diarrhoea (Theofanous et al. 2020). In terms of generic level, it was found that the abundance of Streptococcus has increased significantly in this research. In our previous study, ten genera were found to have statistical significance in the mice with Folium sennae intervention. The increased abundance of Paraprevotella, Streptococcus, Epulopiscium, Sutterella and Mycoplasma and the decreased abundance of Adlercreutzia, Lactobacillus, Dehalobacterium, Dorea and Oscillospira were found significantly. Compared with the current research, Streptococcus is a key genus to diarrhoea caused by Folium sennae for its increased expression in both two studies.
Moreover, microbiota at the species level was analyzed to screen potential biomarkers to connect diarrhoea and mental stress. In the current study, three microecological analysis methods, including LEfSe, Metastats, and group difference analysis, were used to screen biomarkers of diarrhoea related to mental stress. In aggregate, the abundances of Stigmatella aurantiaca, Candidatus arthromitus sp. SFB-mouse, Erythrobacter gaetbuli, Desulfitobacterium hafniense, Ochrobactrum pituitosum, and Candidatus arthromitus sp. SFB-mouse-NL in the model group were significantly lower than those in the control group, whereas Microbacterium dextranolyticum, Klebsiella pneumoniae, Escherichia sp. BBDP27, and Streptococcus danieliae were enriched in the control group, and these species could be used as characteristic microbes in the model group. The characteristics of abundance variety of species of diarrhoea related to mental stress showed the increase of the pathogenic bacteria including Klebsiella pneumoniae, Escherichia sp. BBDP27, and Streptococcus danieliae. Klebsiella pneumoniae and Escherichia are important opportunistic pathogens commonly defined as highly drug-resistant superbugs capable of causing invasive disease (Chen et al. 2020; Palmer et al. 2020), and drug-resistant bacterial infection is very associated with chronic recurrent diarrhoea and inflammation. The species characteristics of abundances variety of diarrhoea related to mental stress showed the decrease of the probiotics including Stigmatella aurantiaca, Candidatus arthritis, and Desulfitobacterium hafniense. Bioactive secondary metabolite produced by Stigmatella aurantiaca are kinds of potential biopharmaceutical resources, especially angiotensin-converting microbial enzyme might cleave Aβ peptides and delay neurodegeneration (Jalkute and Sonawane 2015). The bioactive compound from Desulfitobacterium hafniense, has an effect on radical scavenging and antilipoperoxidant activity, as the same as Candidatus arthritis, which is related to nervous system damage for the reactive oxygen species generated by oxidative stress (Begines et al. 2019; Wakita et al. 2019). Furthermore, when investigating the microbiotal characteristics of the intestinal fecal of mice treated with Folium senna gavage combined with environmental stressors including restraint and tail pinch stress, we found species including Bacteroides vulgatus, Helicobacter ganmani, Staphylococcus lentus and Lactobacillus murinus, were significantly enriched, while species such as Candidatus arthromitus sp. SFB-mouse and Lactobacillus johnsonii, were significantly depleted (Yuan et al. 2020). Colonization of Candidatus arthromitus sp. SFB-mouse in both intestinal mucosa and feces decreased or even disappeared, which indicates its important role in stress-related diarrhoea.
Surprisedly, it was found that Stigmatella aurantiaca has the potential to mark mental stress-related diarrhoea in all the three differential analysis. Stigmatella aurantiaca is a gram-negative bacterium belonging to the genus Myxobacteria which are efficient chitin degraders (Sharma and Subramanian 2017). Stigmatella aurantiaca has been known as a rich source of secondary metabolites such as angiotensin-converting biological enzyme and myxobacterial extracts, with the potential neuroprotective effects contribute to host primary human neurons (Dehhaghi et al. 2019). In the current study, absent expression of Stigmatella aurantiaca in the intestinal mucosa of mice with diarrhoea and repeated stress accompanied by SIBO. Antibiotics can inhibit SIBO and have a therapeutic benefit in patients with stress-related diarrhoea (Chey et al. 2020). Stigmatella aurantiaca belongs to the bacteriolytic group of myxobacteria with function of degrading and utilizing living bacteria. The secondary metabolites of Stigmatella aurantiaca as a kind of methoxymethacrylate fungicides can be used as biological antibiotics with low cytotoxicity and high safety (Panter et al. 2019; Müller et al. 2019). Based on our previous experience in the cultivation of Debaryomyces hansenii for the treatment of antibiotic-related diarrhoea (He et al. 2017, 2019), Stigmatella aurantiaca is an important resource for potential treatment to mental stress-related diarrhoea. Therefore, it is worthy of further research.
Conclusion
In summary, the current study revealed shifts in the diversity and compositions of microbiota colonized in intestinal mucosa of mice with diarrhoea and repeated stress. Microbiota characteristics such as microbial diversity enrichment, microbial community reconstruction were found significantly associated with mental stress-related diarrhoea. The altered gut microbiota species with distinct characteristics were detected, such as Stigmatella aurantiaca, Candidatus arthromitus sp. SFB-mouse, Erythrobacter gaetbuli, Desulfitobacterium hafniense, Ochrobactrum pituitosum, Microbacterium dextranolyticum, Klebsiella pneumoniae, Escherichia sp. BBDP27, Streptococcus danieliae, and Candidatus arthromitus sp. SFB-mouse-NL. The altered species can be acted as potential biomarkers for the mental stress-related diarrhoea.
Further studies should assess the role of the characteristic microbiota in intestinal mucosal biological barrier and broaden the analysis to evaluate the effect of secondary metabolites from the characteristic microbiota on host response to get more complete picture of the pathogenesis of FGID, with the hope of developing the biological supplements for therapeutic targets in FGID therapy.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81874460).
Author contributions
ZT designed the study; HS and XP performed the experiments; CZ and TL analyzed the data; CZ wrote the paper; HS checked the paper. The decision to submit the manuscript for publication was made by all the authors.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Human and animal rights statement
The study was approved by the Animal Ethics and Welfare Committee of Hunan University of Chinese Medicine.
Contributor Information
Tianhao Liu, Email: lthlearner@126.com.
Zhoujin Tan, Email: tanzhjin@sohu.com.
References
- Agrawal L, Korkutata M, Vimal SK, Yadav MK, Bhattacharyya S, Shiga T. Therapeutic potential of serotonin 4 receptor for chronic depression and its associated comorbidity in the gut. Neuropharmacology. 2020;166(1):107969. doi: 10.1016/j.neuropharm.2020.107969. [DOI] [PubMed] [Google Scholar]
- Ahmed SA, Elhefnawy AM, Azouz HG, Roshdy YS, Ashry MH, Ibrahim AE, Meheissen MA. Study of the gut microbiome profile in children with autism spectrum disorder: a single tertiary hospital experience. J Mol Neurosci. 2020 doi: 10.1007/s12031-020-01500-3. [DOI] [PubMed] [Google Scholar]
- Begines P, Biedermann D, Valentová K, Petrásková L, Pelantová H, Maya I, Fernández-Bolaños JG, Křen V. Chemoenzymatic synthesis and radical scavenging of sulfated hydroxytyrosol, tyrosol, and acetylated derivatives. J Agric Food Chem. 2019;67(26):7281–7288. doi: 10.1021/acs.jafc.9b01065. [DOI] [PubMed] [Google Scholar]
- Berens S, Schaefert R, Baumeister D, Gauss A, Eich W, Tesarz J. Does symptom activity explain psychological differences in patients with irritable bowel syndrome and inflammatory bowel disease? Results from a multi-center cross-sectional study. J Psychosom Res. 2019;126(1):109836. doi: 10.1016/j.jpsychores.2019.109836. [DOI] [PubMed] [Google Scholar]
- Chen Y, Marimuthu K, Teo J, Venkatachalam I, Cherng BPZ, De Wang L, Prakki SRS, Xu W, Tan YH, Nguyen LC, Nguyen TH, Ng OT, Gan YH. Acquisition of plasmid with Carbapenem-resistance gene blaKPC2 in Hypervirulent Klebsiella pneumonia Singapore. EMERG Infect Dis. 2020;26(3):549–559. doi: 10.3201/eid2603.191230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chey WD, Shah ED, DuPont HL. Mechanism of action and therapeutic benefit of rifaximin in patients with irritable bowel syndrome: a narrative review. Therap Adv Gastroenterol. 2020;13(1):321880901. doi: 10.1177/1756284819897531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers E, Törnblom H, Simrén M, Tack J, Van OL. Relations between food intake, psychological distress, and gastrointestinal symptoms: a diary study. United European Gastroenterol J. 2019;7(7):965–973. doi: 10.1177/2050640619839859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn S, Rose M, Sady M, Parker M, Suslovic W, Weisbrod V, Kerzner B, Streisand R, Kahn I. Mental health disorders and psychosocial distress in pediatric celiac disease. J Pediatr Gastroenterol Nutr. 2019;1:e2605. doi: 10.1097/MPG.0000000000002605. [DOI] [PubMed] [Google Scholar]
- Dehhaghi M, Tan V, Heng B, Braidy N, Mohammadipanah F, Guillemin GJ. Neuroprotective effect of myxobacterial extracts on quinolinic acid-induced toxicity in primary human neurons. Neurotox Res. 2019;35(2):281–290. doi: 10.1007/s12640-018-9945-8. [DOI] [PubMed] [Google Scholar]
- Guerrero-Bautista R, Do Couto BR, Hidalgo JM, Cárceles-Moreno FJ, Molina G, Laorden ML, Núñez C, Milanés MV. Modulation of stress- and cocaine prime-induced reinstatement of conditioned place preference after memory extinction through dopamine D3 receptor. Biol Psychiatry. 2019;92(1):308–320. doi: 10.1016/j.pnpbp.2019.01.017. [DOI] [PubMed] [Google Scholar]
- Han CS, Martin MA, Dichosa AEK, Daughton AR, Frietze S, Kaplan H, Gurven MD, Alcock J. Salivary microbiomes of indigenous Tsimane mothers and infants are distinct despite frequent premastication. PeerJ. 2016;4(1):e2660. doi: 10.7717/peerj.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Long C, Liu Y, Guo Y, Xiao N, Tan Z (2017) Effects of Debaryomyces hansenii treatment on intestinal microorganisms in mice with antibiotics-induced diarrhea. 3 Biotech 7(5) [DOI] [PMC free article] [PubMed]
- He Y, Tang Y, Peng M, Xie G, Li W, Tan Z. Influence of Debaryomyces hansenii on bacterial lactase gene diversity in intestinal mucosa of mice with antibiotic-associated diarrhea. PLoS ONE. 2019;14(12):e225802. doi: 10.1371/journal.pone.0225802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearn M, Whorwell PJ, Vasant DH. Stigma and irritable bowel syndrome: a taboo subject? Lancet Gastroenterol Hepatol. 2020;1253(19):30348–30356. doi: 10.1016/S2468-1253(19)30348-6. [DOI] [PubMed] [Google Scholar]
- Holtmann G, Shah A, Morrison M. Pathophysiology of functional gastrointestinal disorders: a holistic overview. Dig Dis. 2017;1(1):5–13. doi: 10.1159/000485409. [DOI] [PubMed] [Google Scholar]
- Hsieh P, Vollger MR, Dang V, Porubsky D, Baker C, Cantsilieris S, Hoekzema K, Lewis AP, Munson KM, Sorensen M, Kronenberg ZN, Murali S, Murali BJ, Chiatante G, Maggiolini FAM, Blanché H, Underwood JG, Antonacci F, Deleuze JF, Eichler EE. Adaptive archaic introgression of copy number variants and the discovery of previously unknown human genes. Science. 2019;366(6463):eaax2083. doi: 10.1126/science.aax2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YL, Zeng NX, Chen J, Niu J, Luo WL, Liu P, Yan C, Wu LL. Dynamic changes of behaviors, dentate gyrus neurogenesis and hippocampal miR-124 expression in rats with depression induced by chronic unpredictable mild stress. Neural Regen Res. 2020;15(6):1150–1159. doi: 10.4103/1673-5374.270414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkute CB, Sonawane KD. Evaluation of a possible role of Stigmatella aurantiaca ACE in Aβ peptide degradation: a molecular modeling approach. J Mol Microbiol Biotechnol. 2015;25(1):26–36. doi: 10.1159/000370114. [DOI] [PubMed] [Google Scholar]
- Jia Z, Chen A, Bao F, He M, Gao S, Xu J, Zhang X, Niu P, Wang C. Effect of nisin on microbiome-brain-gut axis neurochemicals by Escherichia coli-induced diarrhea in mice. Microb Pathog. 2018;119(1):65–71. doi: 10.1016/j.micpath.2018.04.005. [DOI] [PubMed] [Google Scholar]
- Johnson JS, Spakowicz DJ, Hong BY, Petersen LM, Demkowicz P, Chen L, Leopold SR, Hanson BM, Agresta HO, Gerstein M, Sodergren E, Weinstock GM. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat Commun. 2019;10(1):5029. doi: 10.1038/s41467-019-13036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki P, Mohiuddin SG, Kavousi P, Orman MA. Investigating the effects of osmolytes and environmental pH on bacterial persisters. Antimicrob Agents Chemother. 2020;2(24):e02393–e2419. doi: 10.1128/AAC.02393-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long C, Liu Y, He L, Yu R, Li D, Tan Z, Hui H. Bacterial lactase genes diversity in intestinal mucosa of dysbacterial diarrhea mice treated with Qiweibaizhu powder. 3 Biotech. 2018;8(10):423. doi: 10.1007/s13205-018-1460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long CX, He L, Guo YF, Liu YW, Xiao NQ, Tan ZJ. Diversity of bacterial lactase genes in intestinal contents of mice with antibiotics-induced diarrhea. World J Gastroenterol. 2017;23(42):7584–7593. doi: 10.3748/wjg.v23.i42.7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YW, Wu Y, Hui HY, Tan ZJ (2020) Establishment of a mouse model of Ganqichengpi diarrhea and the efficacy of Tongxieyaofang prescription. Chin J Appl Environ Biol 26(4):e09026. 10.19675/j.cnki.1006-687x.2019.09026
- Lu J, Mao D, Li X, Ma Y, Luan Y, Cao Y, Luan Y. Changes of intestinal microflora diversity in diarrhea model of KM mice and effects of Psidium guajava L. as the treatment agent for diarrhea. J Infect Public Health. 2020;13(1):16–26. doi: 10.1016/j.jiph.2019.04.015. [DOI] [PubMed] [Google Scholar]
- Müller JI, Kusserow K, Hertrampf G, Pavic A, Nikodinovic-Runic J, Gulder TAM. Synthesis and initial biological evaluation of myxocoumarin B. Org Biomol Chem. 2019;17(7):1966–1969. doi: 10.1039/c8ob02273a. [DOI] [PubMed] [Google Scholar]
- Ossowicki Adam, Tracanna Vittorio, Petrus Marloes L. C., van Wezel Gilles, Raaijmakers Jos M., Medema Marnix H., Garbeva Paolina. Microbial and volatile profiling of soils suppressive to of wheat . Proc Royal Soc B Biol Sci. 2020;287:20192527. doi: 10.1098/rspb.2019.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkul C, Yalinay M, Karakan T. Structural changes in gut microbiome after Ramadan fasting: a pilot study. Benef Microbes. 2020;1(1):1–8. doi: 10.3920/BM2019.0039. [DOI] [PubMed] [Google Scholar]
- Palmer J, Mortzfeld BM, Piattelli E, Silby MW, McCormick B, Bucci V. Microcin H47: a class IIb microcin with potent activity against multi-drug resistant Enterobacteriaceae. ACS Infect Dis. 2020 doi: 10.1021/acsinfecdis.9b00302. [DOI] [PubMed] [Google Scholar]
- Panter F, Krug D, Müller R. Novel methoxymethacrylate natural products uncovered by statistics-based mining of the Myxococcus fulvus secondary metabolome. ACS Chem Biol. 2019;14(1):88–98. doi: 10.1021/acschembio.8b00948. [DOI] [PubMed] [Google Scholar]
- Pimentel M, Saad RJ, Long MD, Rao SSC. ACG clinical guideline: small intestinal bacterial overgrowth. Am J Gastroenterol. 2020;115(2):165–178. doi: 10.14309/ajg.0000000000000501. [DOI] [PubMed] [Google Scholar]
- Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Mechanisms of action of probiotics. Adv Nutr. 2019;10(1):S49–S66. doi: 10.1093/advances/nmy063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Talley NJ, Jones M, Kendall BJ, Koloski N, Walker MM, Morrison M, Holtmann GJ. Small intestinal bacterial overgrowth in irritable bowel syndrome: a systematic review and meta-analysis of case-control studies. Am J Gastroenterol. 2020;115(2):190–201. doi: 10.14309/ajg.0000000000000504. [DOI] [PubMed] [Google Scholar]
- Sharma Gaurav, Subramanian Srikrishna. Unravelling the Complete Genome of Archangium gephyra DSM 2261T and Evolutionary Insights into Myxobacterial Chitinases. Genome Biology and Evolution. 2017;9:1304–1311. doi: 10.1093/gbe/evx066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol H, Brot L, Stefanescu C, Auzolle C, Barnich N, Buisson A, Fumery M, Pariente B, Le Bourhis L, Treton X, Nancey S, Allez M, Seksik P. Prominence of ileal mucosa-associated microbiota to predict postoperative endoscopic recurrence in Crohn’s disease. Gut. 2020;69(3):462–472. doi: 10.1136/gutjnl-2019-318719. [DOI] [PubMed] [Google Scholar]
- Sun C, Zhanf B, Cen Z, Qin W, Wen X. Sludge retention time affects the microbial community structure: a large-scale sampling of aeration tanks throughout China. Environ Pollut. 2020;261(1):114140. doi: 10.1016/j.envpol.2020.114140. [DOI] [PubMed] [Google Scholar]
- Sundin J, Aziz I, Nordlander S, Polster A, Hu YOO, Hugerth LW, Pennhag AAL, Engstrand L, Törnblom H, Simrén M, Öhman L. Evidence of altered mucosa-associated and fecal microbiota composition in patients with Irritable Bowel Syndrome. Sci Rep. 2020;10(1):593. doi: 10.1038/s41598-020-57468-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofanous SA, Florens MV, Appeltans I, Denadai SA, Wood JN, Wouters MM, Boeckxstaens GE. Ephrin-B2 signaling in the spinal cord as a player in post-inflammatory and stress-induced visceral hypersensitivity. Neurogastroenterol Motil. 2020;1(31):e13782. doi: 10.1111/nmo.13782. [DOI] [PubMed] [Google Scholar]
- Wahbi W, Siam R, Kegere J, El-Mehalmey WA, Mamdouh W. Novel inulin electrospun composite nanofibers: prebiotic and antibacterial activities. ACS Omega. 2020;5(6):3006–3015. doi: 10.1021/acsomega.9b03957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakita Y, Saiki A, Kaneda H, Segawa S, Tsuchiya Y, Kameya H, Okamoto S. Analysis of free radical production capacity in mouse faeces and its possible application in evaluating the intestinal environment: a pilot study. Sci Rep. 2019;9(1):19533. doi: 10.1038/s41598-019-56004-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Rao Y, Guo X, Liu N, Liu S, Wen P, Li S, Li Y. Oral microbiome in patients with oesophageal squamous cell carcinoma. Sci Rep. 2019;9(1):19055. doi: 10.1038/s41598-019-55667-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F, Liu Z, Liu M, Chen L, Ding W, Zhang H. Amino acids regulate glycolipid metabolism and alter intestinal microbial composition. Curr Protein Pept Sci. 2020;2(18):e0216. doi: 10.2174/1389203721666200219100216. [DOI] [PubMed] [Google Scholar]
- Yang Meng, Bose Shambhunath, Lim Soo-Kyoung, Kim Hojun. Preventive Effects of Pyungwi-san against Dextran Sulfate Sodium- and clostridium difficile-induced inflammatory bowel disease in mice. International Journal of Molecular Sciences. 2019;20:6346. doi: 10.3390/ijms20246346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z, Zhang CY, Xinxin P, Sun L, Long CX, Tan ZJ. Intestinal microbiota characteristics of mice treated with Folium senna decoction gavage combined with restraint and tail pinch stress. 3 Biotech. 2020;10(4):180. doi: 10.1007/s13205-020-02172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng A, Peng M, Liu H, Guo Z, Xu J, Wang S, He L, Tan Z. Effects of Debaryomyces hansenii treatment on intestinal mucosa microecology in mice with antibiotic-associated diarrhea. PLoS ONE. 2019;14(11):e224730. doi: 10.1371/journal.pone.0224730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Shao H, Li D, Xiao N, Tan Z. Role of tryptophan-metabolizing microbiota in mice diarrhea caused by Folium sennae extracts. BMC Microbiol. 2020;20(1):185. doi: 10.1186/s12866-020-01864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]