Abstract
The epithelial-mesenchymal transition (EMT) serves vital roles in the angiogenesis, cell invasion and metastasis of various malignant tumors, including bladder cancer. Traditional Chinese medicinal herbs have been demonstrated to exhibit anticancer properties. The present study aimed to screen the sensitivity of bladder cancer to natural compounds by using six classic anti-inflammatory and detoxifying herbs, including the ethanol extract of Paris polyphylla (PPE), Scutellaria barbata, Pulsatillae decoction, Dahuang Huanglian Xiexin decoction, Bazhengsan and Hedyotis diffusa combined with S. barbata, were used to treat bladder cancer cells in vitro. Bladder cancer was more sensitive to PPE compared with the other tested herbs, and PPE significantly suppressed bladder cancer cell migration and invasion. Thus, the present study focused on PPE. Bladder cancer cells were treated with monomer components of PPE, including polyphyllin (PP) I, PPII, PPVI and PPVII. The results demonstrated that PPII treatment significantly inhibited cancer cell migration and invasion, increased the expression level of E-cadherin and decreased the levels of N-cadherin, snail family transcriptional repressor 2, twist family bHLH transcription factor 1, matrix metallopeptidase (MMP) 2 and MMP9 compared with those in the control group (untreated cells). These results suggested that PPII treatment may suppress bladder cancer cell migration and invasion by regulating the expression of EMT-associated genes and MMPs. Therefore, PPE and PPII may have antimetastatic effects and PPII may serve as a potential therapeutic option for inhibiting bladder cancer metastasis.
Keywords: urinary carcinoma, Paris polyphylla, epithelial-mesenchymal transition, invasion, migration
Introduction
Human bladder cancer is a heterogeneous disease that is one of the most common types of cancer worldwide, with ~550,000 new diagnosed cases annually (1). In the Western world, bladder cancer is the 4th most common malignancy in men; the incidence in women is approximately one-third of that in men (2). Bladder cancer is classified into non-muscle-invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC): ~75% of patients with bladder cancer exhibit NMIBC, and 15–20% of NMIBCs progress to MIBCs; the remaining 25% of patients exhibit MIBC at first diagnosis (3,4). However, MIBC is highly aggressive, and patients with invasive bladder cancer exhibit a poor prognosis with a 5-year survival rate of <50% (5). The treatment options for bladder cancer include radiation alone, chemotherapy alone, combination treatment with radiation and chemotherapy or surgery, and the selected treatment is dependent on the age of the patient, the aggressiveness of the disease and drug effectiveness (6). Despite the various treatment options, metastasis is fatal for patients; therefore, uncovering the underlying mechanism of metastasis and screening for antimetastatic drugs is urgently needed.
Chronic inflammation increases the risk of cancer and contributes to tumor development through the induction of oncogenic mutations, enhanced angiogenesis and early tumor promotion (7). Tumor-associated inflammation is associated with tumor progression; evidence has indicated that inflammation impacts every step of tumorigenesis, from initiation through promotion to invasion and metastasis (8). Traditional Chinese medicine provides the material basis and molecular structure framework for the screening of antitumor drugs. Heat-clearing and detoxifying herbs have been reported to serve important roles in anti-inflammation, tumor cells death and suppression of tumor proliferation as well as metastasis (9). Paris polyphylla (chonglou) is a heat-clearing and detoxifying herb mainly used in traditional Chinese medicine to treat headaches, fevers and wounds, as well as for neutralizing snake poison (10). Extracts from Paris polyphylla have also been demonstrated to exert anticancer effects in breast (11), lung (12) and ovarian (13) cancer. In the Chinese Pharmacopoeia, Polyphyllin (PP) I, PPII, PPVI and PPVII are the key components for identifying Paris polyphylla (14). Therefore, these four extracts have gained increasing attention and have been chosen to be studied in cancer treatment.
The epithelial-mesenchymal transition (EMT) serves vital roles in angiogenesis, cell invasion and metastasis (15). The key changes during EMT include a decrease in the levels of the epithelial marker E-cadherin (CDH1) and increases in those of the mesenchymal marker N-cadherin (CDH2), as well as snail family transcriptional repressor 2 (SNAI2) and twist family bHLH transcription factor 1 (TWIST1), which are two transcriptional inhibitors of CDH1 (16). PPs, especially PPI, have been demonstrated to suppress tumor cell invasion and migration in vitro. PPI, which is an active component of Paris polyphylla, suppresses metastasis in hepatocellular carcinoma by downregulating the formation of vasculogenic mimicry via the TWIST1/VE-cadherin pathway (17). PPI has also been demonstrated to inhibit gastric cancer cell invasion by suppressing the EMT-associated CIP2A/PP2A/Akt signaling pathway (18). However, to the best of our knowledge the role and the underlying mechanism of PPs, especially PPII, in the inhibition of bladder cancer metastasis have not been studied.
The aim of the present study was to screen sensitive heat-clearing and detoxifying herbs and their effective components in bladder cancer. In addition, the present study aimed to identify the inhibitory effect and mechanism of PPII, a vital component of ethanol extracts of Paris polyphylla (PPE), on the invasion and migration of human bladder cancer cells in vitro.
Materials and methods
Cell culture
Human bladder cancer cell lines T24 and 5637 (kindly provided by Professor Shengtian Zhao, Shandong Provincial Hospital, Jinan, China) were cultured in RPMI-1640 medium (cat. no. CM10041; Macgene Technology Co., Ltd.) supplemented with 10% (v/v) fetal bovine serum (FBS, cat. no. 04-001-1ACS; Biological Industries). Cells were incubated in a humidified atmosphere with 5% CO2 at 37°C.
Reagents
PPI (cat. no. A0386), PPII (cat. no. A0387), PPVI (cat. no. A0389) and PPVII (cat. no. A0390) of ≥98% purity were purchased from Chengdu Must Bio-Technology Co., Ltd. and dissolved in DMSO (cat. no. D8370; Sigma-Aldrich; Merck KGaA). Antibodies against CDH1 (cat. no. 20874-1-AP) and ACTB (cat. no. 66009-1-Ig) were purchased from Proteintech Group, Inc. Antibodies against CDH2 (cat. no. ab98952), TWIST1 (cat. no. ab50581) and SNAI2 (cat. no. ab27568) were obtained from Abcam, and antibodies against matrix metallopeptidase (MMP) 2 (cat. no. 87809) and MMP9 (cat. no. 13667) were obtained from Cell Signaling Technology, Inc. The secondary antibodies HRP-labeled Goat Anti-Rabbit IgG (H+L) (cat. no. A0208) and HRP-labeled Goat Anti-Mouse IgG (H+L) (cat. no. A0216) were purchased from Beyotime Institute of Biotechnology.
Compound extraction
PPE were prepared using EtOH/H2O (60/40, v/v) for 2 h twice under reflux conditions to generate a crude extract, followed by filtration with 150 µm filter cloth. The filtrates were combined, concentrated and separated from the liquid reaction mixture by water precipitation. Following 12-h incubation at 4°C, the extracted solution was filtered, precipitated and dried. Finally, dry powders were obtained and dissolved in DMSO to be used for bladder cell treatment. A total of five compounds including Scutellaria barbata, Pulsatillae decoction, Dahuang Huanglian Xiexin decoction, Bazhengsan and Hedyotis diffusa combined with S. barbata were immersed in 10X pure water for 1 h. Subsequently, the compounds were boiled twice over a light flame, and the filtrates were merged and condensed into extracts using a rotary evaporator. The condensed extracts were freeze-dried, and each powder was weighed and dissolved in water prior to experimental use.
Cell viability assay
The sensitivity to the six extracts and cisplatin of bladder cancer cells was detected using the Cell Counting Kit-8 (CCK-8; cat. no. CK04; Dojindo Molecular Technologies, Inc.) according to the manufacturer's instructions. Briefly, T24 (6.0×103 cells/well) and 5637 (8.0×103 cells/well) cells were cultured in quintuplicate in 96-well plates at 37°C overnight and treated with the drugs at different concentrations: [Cisplatin, T24 (0, 0.5, 1, 2, 4, 8 µg/ml) and 5637 (0, 1, 3, 9, 27, 81 µg/ml)]; [PPE, T24 (0, 1, 2, 4, 8, 16 µg/ml) and 5637 (0, 1, 3, 9, 27, 81 µg/ml)]; [Scutellaria barbata, T24 (0, 0.1, 0.2, 0.4, 0.8, 1.6 mg/ml) and 5637 (0, 0.25, 0.5, 1, 2, 4 mg/ml)]; Hedyotis diffusa combined with S. barbata, T24 and 5637 (0, 0.25, 0.5, 1, 2, 4 mg/ml); [Pulsatillae decoction, T24 (0, 0.25, 0.5, 1, 2, 4 mg/ml) and 5637 (0, 0.05, 0.1, 0.2, 0.4, 0.8 mg/ml)]; [Dahuang Huanglian Xiexin decoction; T24 (0, 0.25, 0.5, 1, 2, 4 mg/ml) and 5637 (0, 0.05, 0.1, 0.2, 0.4, 0.8 mg/ml)]; and Bazhengsan, T24 and 5637 (0, 0.25, 0.5, 1,2, 4 mg/ml) for 48 h. Subsequently, the medium was removed, and the cells were incubated with 90 µl new culture medium supplemented with 10 µl CCK-8 reagent for 2–4 h. Absorption at 450 nm was detected using a spectrophotometer (Type, 1510; Thermo Fisher Scientific Inc.).
Wound healing assay
The bladder cancer cells T24 (6.5×105 cells/well) and 5637 (8.0×105 cells/well) were seeded in 6-well plates. At 24 h, when the cells had reached 90% confluence, linear scratch wounds were created on the cell monolayers in triplicate using a sterile 200-µl pipette tip. The cells were maintained in RPMI-1640 medium containing 1% FBS. Images were captured at 0, 24 and 48 h using an inverted fluorescence microscope (Vert. A1; Carl Zeiss AG) to measure mean distance between edges of wound area. Representative images of wound treated with PPE were observed at ×50 magnification; images of wound for T24 cells treated with PPII were observed at ×50 and 5637 cells at ×100 magnification.
Transwell assay
A 24-well plate was used to determine the effect of drugs on bladder cancer invasion. Bladder cancer cells were harvested and resuspended in serum-free medium supplemented with drugs at different concentrations (PPE for T24 cells at 0, 0.6 and 1.2 µg/ml; and for 5637 at 0, 0.95 and 1.9 µg/ml; PPII for cells at 0, 0.1 and 0.2 µg/ml). An 8-µm pore size filter was pre-coated with Matrigel (cat. no. 354234; BD Biosciences) at 37°C for 30 min, and the lower chamber was filled with medium containing 10% FBS. Suspended bladder cancer cells (2.5×104 cells/well) were seeded in the upper chamber and incubated for 48 h at 37°C. Subsequently, the filter was fixed with 4% paraformaldehyde fix solution (cat. no. P0099; Beyotime Institute of Biotechnology) for 15 min and stained with 0.1% crystal violet for 30 min at room temperature. Finally, the cells and Matrigel on the upper chamber were scraped with a cotton swab, and the invasive cells on the lower surface were counted under an inverted fluorescence microscope (Vert. A1; Carl Zeiss AG). Three fields for each sample were captured at ×100 magnification.
Western blotting
The total proteins were obtained from bladder cancer cells treated with PPII for 48 h at different concentrations (0, 0.1, 0.2 and 0.4 µg/ml). Cell lysates were acquired using RIPA cell lysis buffer and centrifuged at 16,363 × g at 4°C for 15 min. A bicinchoninic acid assay kit (cat. no. P0012S; Beyotime Institute of Biotechnology) was used to determine the protein concentration, and 10% SDS-PAGE was applied to separate equal amounts of proteins (40 µg protein loaded per lane). After the proteins were transferred to PVDF membranes, the membranes were blocked with 5% nonfat milk for 1 h at room temperature and incubated with primary antibodies at a 1:1,000 dilution at 4°C overnight. Appropriate secondary antibodies conjugated with horseradish peroxidase at 1:2,000 dilution were used to incubate membrane-bound primary antibodies at room temperature for 1 h, followed by development of immunoblots using an enhanced chemiluminescence kit (cat. no. WBKLS0050; Merck KGaA). The densitometry analysis was performed using Image-Pro Plus version.6.0 (Media Cybernetics Inc.).
Statistical analysis
All experiments were conducted at least three times. Data are presented as the mean ± SEM. Statistical analysis was performed using GraphPad Prism 5.0 software (GraphPad Software, Inc.) by one-way ANOVA with Dunnett's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Bladder cancer cells are sensitive to PPE
To identify heat-clearing and detoxifying herbs that affect bladder cancer cells, 5637 and T24 cells were challenged with PPE (Fig. S1B), S. barbata (Fig. S1C), Hedyotis diffusa combined with S. barbata (Fig. S1D), Pulsatillae decoction (Fig. S1E), Dahuang Huanglian Xiexin decoction (Fig. S1F) and Bazhengsan (Fig. S1G) for 48 h at different concentrations. Cisplatin was used as a positive control (Fig. S1A). Bladder cancer cells T24 and 5637 were more sensitive to PPE compared with the other herbs. The IC50 of each compound in different cells was calculated and analyzed; the results demonstrated that PPE treatment exerted a stronger inhibitory effect on the viability of T24 and 5637 cells compared with that of other compounds, with IC50 values of 4.43±0.08 and 7.87±0.39 µg/ml, respectively (Table I), at 48 h. The inhibitory effect of PPE on bladder cancer cells was similar to that of the positive control cisplatin.
Table I.
IC50 values of six classic heat-clearing and detoxicating herbs and cisplatin
| IC50 | ||
|---|---|---|
| Compound | T24 cells | 5637 cells |
| Cisplatin, µg/ml | 1.30±0.17 | 2.62±0.30 |
| PPE, µg/ml | 4.43±0.08 | 7.87±0.39 |
| Scutellaria barbata, mg/ml | 0.75±0.06 | 0.67±0.04 |
| Pulsatillae decoction, mg/ml | 0.45±0.02 | 0.16±0.01 |
| Dahuang Huanglian Xiexin decoction, mg/ml | 0.82±0.02 | 0.14±0.03 |
| Bazhengsan, mg/ml | 1.84±0.09 | 0.85±0.05 |
| Hedyotis diffusa combined with S. barbata, mg/ml | 0.67±0.04 | 0.45±0.03 |
IC50, half-maximal inhibitory concentration; PPE, ethanol extract of Paris polyphylla.
PPE suppresses the migration and invasion of bladder cancer cells
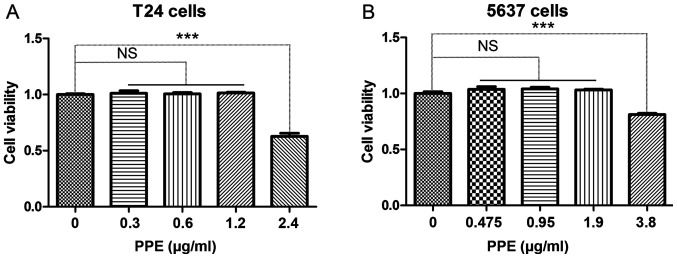
In order to determine the appropriate drug concentration, CCK-8 was used to evaluate the viability of bladder cancer cells following PPE treatment. The results demonstrated that PPE inhibited the viability of T24 (Fig. 1A) and 5637 (Fig. 1B) cells at >1.2 and 1.9 µg/ml, respectively (P<0.001). Therefore, different concentrations of PPE were used in subsequent experiments to treat T24 (0, 0.6 and 1.2 µg/ml) and 5637 (0, 0.95 and 1.9 µg/ml) cells.
Figure 1.
Effects of PPE on the viability of bladder cancer cells. (A) T24 cells; (B) 5637 cells. Experiments were conducted in triplicate. NS, not significant; ***P<0.001. PPE, ethanol extracts of Paris polyphylla.
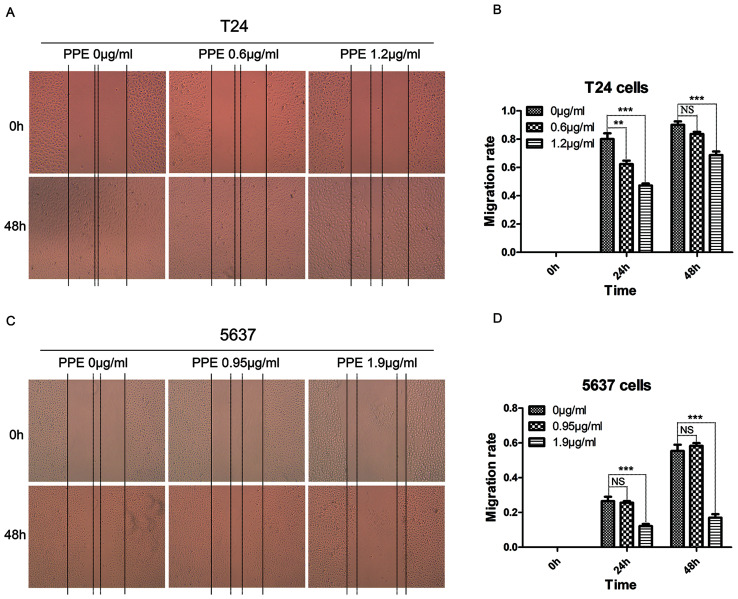
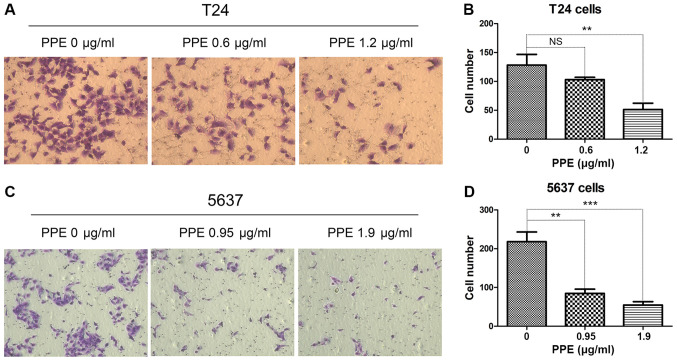
To investigate the effects of PPE on bladder cancer invasion and migration, wound healing and Transwell assays were performed in T24 and 5637 cells treated with PPE. The results revealed that PPE significantly suppressed the wound closure of T24 cells at 1.2 µg/ml (Fig. 2A and B) and 5637 cells (Fig. 2C and D) at 1.9 µg/ml (P<0.001). The effect of PPE on the invasiveness of bladder cancer cells was further examined, and the results demonstrated that PPE suppressed the invasive ability of T24 (Fig. 3A and B, P<0.01 at 1.2 µg/ml) and 5637 (Fig. 3C and D, P<0.01 at 0.95 µg/ml and P<0.001 at 1.9 µg/ml) cells compared with that of the control group. Therefore, PPE inhibited the invasive and migratory abilities of T24 and 5637 bladder cancer cells.
Figure 2.
Effects of PPE on the migration of bladder cancer cells. (A and B) T24 cells; (C and D) 5637 cells. Magnification, ×50. Experiments were conducted in triplicate. NS, not significant; **P<0.01; ***P<0.001. PPE, ethanol extracts of Paris polyphylla.
Figure 3.
Effects of PPE on the invasion of bladder cancer cells. (A and B) T24 cells; (C and D) 5637 cells. Representative images of T24 and 5637 invasion were captured at ×100 magnification. Experiments were conducted in triplicate. NS, not significant; **P<0.01; ***P<0.001. PPE, ethanol extracts of Paris polyphylla.
Bladder cancer cells are sensitive to PPII
To determine which component from PPE served a role in the anticancer activity of PPE in bladder cancer cells, the essential components of PPE, including PPI (Fig. S2B), PPII (Fig. S2C), PPVI (Fig. S2D) and PPVII (Fig. S2E) were used to treat T24 and 5637 cells at different concentrations. Cisplatin was also used as a positive control (Fig. S2A). According to the IC50 values, T24 and 5637 cells were more sensitive to PPII compared with PPI, PPVI and PPVII (Table II).
Table II.
IC50 values of four components from Paris polyphylla and cisplatin.
| IC50, µg/ml | ||
|---|---|---|
| Compound | T24 cells | 5637 cells |
| Cisplatin | 1.15±0.11 | 3.25±0.33 |
| Polyphyllin I | 0.42±0.04 | 2.48±0.13 |
| Polyphyllin II | 0.64±0.03 | 0.71±0.23 |
| Polyphyllin VI | 0.84±0.02 | 3.79±0.40 |
| Polyphyllin VII | 0.53±0.02 | 3.51±0.27 |
IC50, half-maximal inhibitory concentration.
PPII suppresses bladder cancer cell migration and invasion
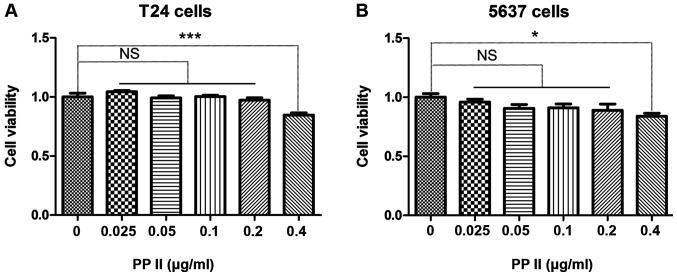
The bladder cancer cells T24 (Fig. 4A) and 5637 (Fig. 4B) were treated with 0, 0.025, 0.05, 0.1, 0.2 or 0.4 µg/ml PPII for 48 h. The viability of bladder cancer cells was inhibited when the concentration of PPII was 0.4 µg/ml (P<0.05). Therefore, the antimetastatic activity of PPII was further analyzed using 0, 0.1 and 0.2 µg/ml PPII.
Figure 4.
Effects of PPII on the viability of bladder cancer cells. (A) T24 cells; (B) 5637 cells. Experiments were conducted in triplicate. NS, not significant; *P<0.05; ***P<0.001. PPII, polyphyllin II.
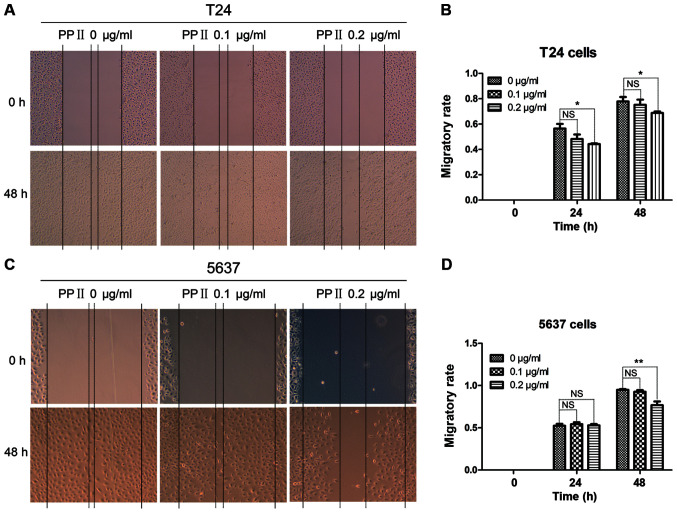
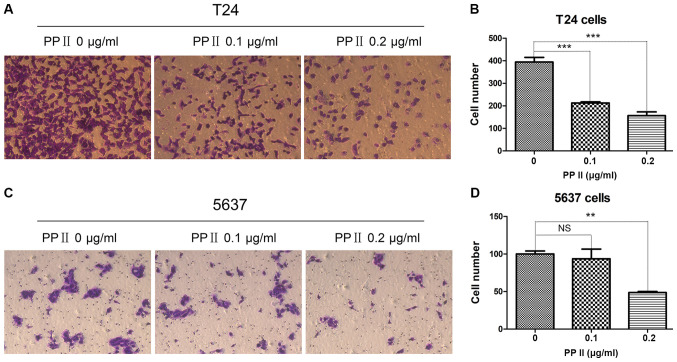
To further investigate the effects of PPII on bladder cancer cell migration and invasion, T24 and 5637 cells were treated with 0, 0.1 and 0.2 µg/ml PPII for 48 h. Cell migration was analyzed using a wound healing assay. PPII significantly inhibited the migratory rates of T24 cells at 0.2 µg/ml (Fig. 5A and B; P<0.05) and 5637 cells at 0.2 µg/ml treated for 48 h (Fig. 5C and D; P<0.01) compared with those in the control group. The invasive ability of bladder cancer cells was examined by a Transwell assay. The results indicated that PPII also significantly decreased the ability of T24 cells (Fig. 6A and B; P<0.001) and 5637 cells at 0.2 µg/ml (Fig. 6C and D; P<0.01) to traverse the membrane. These results suggested that PPII may inhibit the invasive and migratory abilities of bladder cancer cells.
Figure 5.
Effects of PPII on the migration of bladder cancer cells. (A and B) T24 cells, magnification, ×50; (C and D) 5637 cells, magnification, ×100. Experiments were conducted in triplicate. NS, not significant; *P<0.05; **P<0.01. PPII, polyphyllin II.
Figure 6.
Effects of PPII on the invasion of bladder cancer cells (magnification, ×100). (A and B) T24 cells; (C and D) 5637 cells. Experiments were conducted in triplicate. NS, not significant; **P<0.01; ***P<0.001. PPII, polyphyllin II.
PPII affects the expression of EMT-associated proteins and MMPs
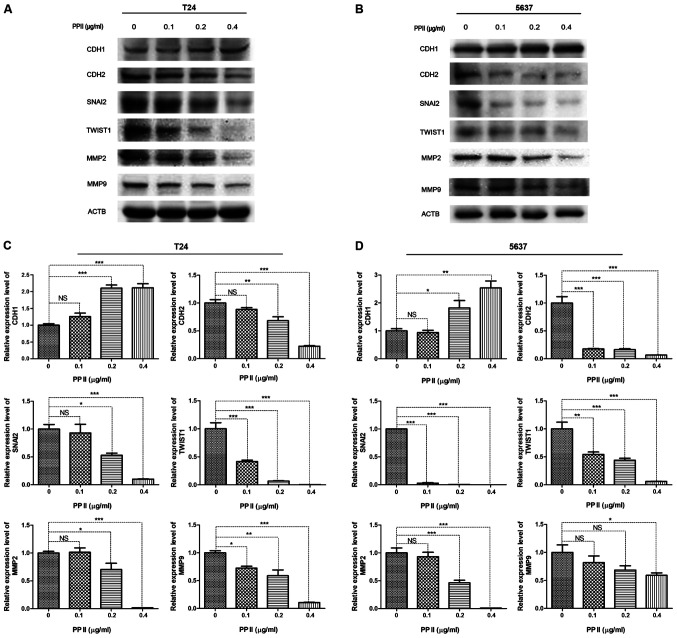
Since EMT-inducing transcription factors promote cancer metastasis, the present study examined whether PPII inhibited bladder cancer migration and invasion by regulating the expression of EMT-associated proteins. As markers of EMT, the expression levels of CDH1 and CDH2 were detected by western blot analysis. The results demonstrated that the expression of CDH1 in T24 (Fig. 7A) and 5637 (Fig. 7B) cells was significantly increased following PPII treatment, whereas that of CDH2 was decreased compared with the control group. In addition, the expression levels of the transcriptional repressors of CDH1, including SNAI2 and TWIST1, were decreased in PPII-treated cells compared with those in the untreated control group (Fig. 7C and D). The expression levels of gelatinases MMP2 and MMP9, which serve crucial roles in maintaining extracellular matrix homeostasis and tumor invasion, were also significantly inhibited by PPII compared with those in the control group (Fig. 7C and D). These results suggested that PPII-induced migration and invasion inhibition may occur through the suppression of EMT and MMPs expression.
Figure 7.
The expression of EMT-associated proteins and MMPs was examined by western blotting after (A) T24 and (B) 5637 cells were treated with different concentrations of PPII. ACTB was used as a loading control. The relative expression of proteins in (C) T24 and (D) 5637 cells treated with PPII at different concentrations was calculated. Experiments were conducted in triplicate. NS, not significant; *P<0.05; **P<0.01; ***P<0.001. PPII, polyphyllin II; ACTB, β-actin; CDH1, E-cadherin; CDH2, N-cadherin; SNAI2, snail family transcriptional repressor 2; TWIST1, twist family bHLH transcription factor 1; MMP, matrix metallopeptidase.
Discussion
Although neoadjuvant chemotherapy, surgery and combinational chemotherapy have been widely used in bladder cancer treatment, it is still one of the most common tumors with a poor prognosis and a low 5-year survival rate (1). Drug resistance is a major obstacle to bladder cancer chemotherapy, and >50% of NMIBC patients will recur in the future (19). Of note, bladder cancer has become the most expensive disease among all types of cancer due to the need for life-long recurrence monitoring (20), and bladder cancer metastasis is still a fatal disease. Thus, there is an urgent need to identify effective drugs to inhibit the progression of bladder cancer, especially metastasis, and to elucidate the detailed underlying molecular mechanisms. Increasing evidence has indicated that heat-clearing and detoxifying Chinese medicines exert anticancer effects without significant toxic effects (21–24). Chinese medicine provides a library with which to develop potential drugs for the prevention of tumor formation and metastasis.
Tumor metastasis is considered the primary lethal factor for patients with cancer (25). Therefore, the prevention of cancer metastasis is the key means to improving the survival rate of patients with cancer (26). In the present study, with the aim of identifying natural antimetastatic drugs against bladder cancer and investigating the associated molecular mechanisms, the effects of six heat-clearing and detoxifying compounds on the viability of bladder cancer cells were evaluated. The results demonstrated that bladder cancer cells were sensitive to PPE, which had an inhibitory effect on cell migration and invasion. To further investigate the antimetastatic mechanisms of PPE, the major components PPI, PPII, PPVI and PPVII were applied to treat bladder cancer cells; the results demonstrated that cancer cells were more sensitive to PPII compared with the other polyphyllins. When PPE, PPII and cisplatin were used to treat T24 and 5637 cells, respectively, the IC50 value of PPII was lower compared with that of PPE and cisplatin. In addition, exposure to PPII significantly inhibited bladder cancer cell invasion and migration by regulating the expression of EMT-associated proteins and MMPs.
EMT is a key step in cancer metastasis characterized by high expression of mesenchymal markers and low expression of epithelial markers (27,28). SNAI2 and TWIST1 are the major transcription factors regulating EMT that contribute to cancer metastasis via enhanced cell invasion (29). SNAI2 is an essential mediator of TWIST1-induced EMT (30); thus, the expression of these two EMT inducers was estimated in the present study and their levels obviously decreased after PPII treatment. In addition, CDH1 and CDH2, which are EMT markers, were dysregulated in bladder cancer cells, consistent with a previous study (31). Of note, the role and involvement of MMPs in cancer metastasis have been extensively investigated (32,33). MMP2 and MMP9 are widely studied MMPs in cancer progression and serve important roles in regulating tumor angiogenesis, invasion and metastasis mainly through degrading the extracellular matrix (34). In the present study, PPII treatment significantly increased the expression of CDH1 and decreased that of CDH2, SNAI2, TWIST1, MMP2 and MMP9 in bladder cancer cells compared with the untreated control. These results indicated that PPII may inhibit bladder cancer cell invasion and migration by regulating the expression of EMT-related proteins and MMPs.
Anticancer activities of extracts of Paris polyphylla have been previously reported. PPE suppressed the proliferation and promoted apoptosis of human osteosarcoma cells (35). Furthermore, an aqueous extract of Paris polyphylla was previously demonstrated to suppress ovarian cancer proliferation and migration in vitro via downregulation of peroxisome proliferator-activated receptor γ coactivator 1α (36). However, the effect of PPE on bladder cancer metastasis has not been investigated. In the present study, using six heat-clearing and detoxifying Chinese medicines, it was demonstrated that PPE significantly suppressed bladder cancer migration and invasion. To identify the effective component of bladder cancer, PPI, PPII, PPVI and PPVII were used to treat bladder cancer cells. The results demonstrated that bladder cancer cells exhibited the highest sensitivity to PPII; the antitumor effects of PPII have been previously demonstrated in other cancer cell, such as HepaRG (37). However, the role of PPII in inhibiting the metastasis of bladder cancer has not been studied. The present study broadened the antimetastatic scope of PPII and verified that PPII exerted an inhibitory effect on the migration and invasion of bladder cancer cells. However, the antimetastatic roles of the other polyphyllins should not be ignored; PPI has been extensively studied and proposed to serve antimetastatic functions, and its roles in ovarian (38) and lung (39) cancer have been identified. Bladder cancer cells were more sensitive to PPII compared with other polyphyllins in the present study, so PPII was selected for the experiments, but the antimetastatic functions of PPI, PPVI and PPVII in bladder cancer will be verified in future studies.
In conclusion, the results of the present study demonstrated that PPE inhibited bladder cancer migration and invasion and that the PPII component served an important role in the inhibitory effects. These results suggested that PPII may be a potential candidate for antimetastatic drug design. In addition, the results of the present study demonstrated that PPII, at least in part, targeted the expression of EMT-associated proteins and MMPs to suppress bladder cancer cell migration and invasion. Thus, these results lay the foundation for further studies of the antimetastatic mechanisms of PPE and PPII.
Supplementary Material
Acknowledgements
The authors would like to thank Professor Shengtian Zhao (Shandong Provincial Hospital, Jinan, China) for providing the T24 and 5637 cell lines.
Funding
The present study was supported by the National Nature Science Foundation (grant nos. 81573989, 81673807, 81674014 and 81873330), the SDU-KI Cooperative Research Project of Qilu Hospital of Shandong University (grant no. SDU-KI-2019-13) and the Shandong Province: Taishan Scholars Project (grant no. tsqn201909185).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
ZYL conceived and designed the study. WPN, LX, ZYL, YXG, WS and YHJ conducted the experiments. JWL, YZ, HQL, CCM and ZHS performed the statistical analysis. WPN, LX, ZHS, HQL and YXG drafted the manuscript. JWL, WS, YHJ, CCM, YZ and ZYL revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Richters A, Aben KKH, Kiemeney LALM. The global burden of urinary bladder cancer: An update. World J Urol. 2019 Nov 1; doi: 10.1007/s00345-019-02984-4. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: A global overview and recent trends. Eur Urol. 2017;71:96–108. doi: 10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Sharma S, Ksheersagar P, Sharma P. Diagnosis and treatment of bladder cancer. Am Fam Physician. 2009;80:717–723. [PubMed] [Google Scholar]
- 4.Allory Y, Beukers W, Sagrera A, Flández M, Marqués M, Márquez M, van der Keur KA, Dyrskjot L, Lurkin I, Vermeij M, et al. Telomerase reverse transcriptase promoter mutations in bladder cancer: High frequency across stages, detection in urine, and lack of association with outcome. Eur Urol. 2014;65:360–366. doi: 10.1016/j.eururo.2013.08.052. [DOI] [PubMed] [Google Scholar]
- 5.Kim WJ, Kim EJ, Kim SK, Kim YJ, Ha YS, Jeong P, Kim MJ, Yun SJ, Lee KM, Moon SK, et al. Predictive value of progression-related gene classifier in primary non-muscle invasive bladder cancer. Mol Cancer. 2010;9:3. doi: 10.1186/1476-4598-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milowsky MI, Rumble RB, Booth CM, Gilligan T, Eapen LJ, Hauke RJ, Boumansour P, Lee CT. Guideline on Muscle-Invasive and Metastatic Bladder Cancer (European Association of Urology Guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement. J Clin Oncol. 2016;34:1945–1952. doi: 10.1200/JCO.2015.65.9797. [DOI] [PubMed] [Google Scholar]
- 7.Quirk JT, Kupinski JM. Chronic infection, inflammation, and epithelial ovarian cancer. Med Hypotheses. 2001;57:426–428. doi: 10.1054/mehy.2001.1326. [DOI] [PubMed] [Google Scholar]
- 8.Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Liang Y, He C. Anticancer activities and mechanisms of heat-clearing and detoxicating traditional Chinese herbal medicine. Chin Med. 2017;12:20. doi: 10.1186/s13020-017-0140-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Man S, Gao W, Wei C, Liu C. Anticancer drugs from traditional toxic Chinese medicines. Phytother Res. 2012;26:1449–1465. doi: 10.1002/ptr.4609. [DOI] [PubMed] [Google Scholar]
- 11.Lee MS, Yuet-Wa JC, Kong SK, Yu B, Eng-Choon VO, Nai-Ching HW, Chung-Wai TM, Fung KP. Effects of polyphyllin D, a steroidal saponin in Paris polyphylla, in growth inhibition of human breast cancer cells and in xenograft. Cancer Biol Ther. 2005;4:1248–1254. doi: 10.4161/cbt.4.11.2136. [DOI] [PubMed] [Google Scholar]
- 12.He H, Zheng L, Sun YP, Zhang GW, Yue ZG. Steroidal saponins from Paris polyphylla suppress adhesion, migration and invasion of human lung cancer A549 cells via down-regulating MMP-2 and MMP-9. Asian Pac J Cancer Prev. 2014;15:10911–10916. doi: 10.7314/APJCP.2014.15.24.10911. [DOI] [PubMed] [Google Scholar]
- 13.Xiao X, Zou J, Bui-Nguyen TM, Bai P, Gao L, Liu J, Liu S, Xiao J, Chen X, Zhang X, et al. Paris saponin II of Rhizoma Paridis - a novel inducer of apoptosis in human ovarian cancer cells. Biosci Trends. 2012;6:201–211. doi: 10.5582/bst.2012.v6.4.201. [DOI] [PubMed] [Google Scholar]
- 14.Commission CP. 1st. Vol. 1. People's Medical Publishing House; Beijing: 2015. Pharmacopoeia of The People's Republic of China. [Google Scholar]
- 15.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 17.Xiao T, Zhong W, Zhao J, Qian B, Liu H, Chen S, Qiao K, Lei Y, Zong S, Wang H, et al. Polyphyllin I suppresses the formation of vasculogenic mimicry via Twist1/VE-cadherin pathway. Cell Death Dis. 2018;9:906. doi: 10.1038/s41419-018-0902-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Zhang Y, Huang P, Liu X, Xiang Y, Zhang T, Wu Y, Xu J, Sun Z, Zhen W, Zhang L, et al. Polyphyllin I inhibits growth and invasion of cisplatin-resistant gastric cancer cells by partially inhibiting CIP2A/PP2A/Akt signaling axis. J Pharmacol Sci. 2018;137:305–312. doi: 10.1016/j.jphs.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Hu H, Meng Q, Lei T, Zhang M. Nucleophosmin1 associated with drug resistance and recurrence of bladder cancer. Clin Exp Med. 2015;15:361–369. doi: 10.1007/s10238-014-0288-3. [DOI] [PubMed] [Google Scholar]
- 20.Fankhauser CD, Mostafid H. Prevention of bladder cancer incidence and recurrence: Nutrition and lifestyle. Curr Opin Urol. 2018;28:88–92. doi: 10.1097/MOU.0000000000000452. [DOI] [PubMed] [Google Scholar]
- 21.Hsiao WL, Liu L. The role of traditional Chinese herbal medicines in cancer therapy - from TCM theory to mechanistic insights. Planta Med. 2010;76:1118–1131. doi: 10.1055/s-0030-1250186. [DOI] [PubMed] [Google Scholar]
- 22.Chow SE, Chang YL, Chuang SF, Wang JS. Wogonin induced apoptosis in human nasopharyngeal carcinoma cells by targeting GSK-3β and ΔNp63. Cancer Chemother Pharmacol. 2011;68:835–845. doi: 10.1007/s00280-010-1552-1. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Lv J, Lei X, Li S, Zhang Y, Meng L, Xue R, Li Z. Baicalein reduces the invasion of glioma cells via reducing the activity of p38 signaling pathway. PLoS One. 2014;9:e90318. doi: 10.1371/journal.pone.0090318. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Weifeng T, Feng S, Xiangji L, Changqing S, Zhiquan Q, Huazhong Z, Peining Y, Yong Y, Mengchao W, Xiaoqing J, et al. Artemisinin inhibits in vitro and in vivo invasion and metastasis of human hepatocellular carcinoma cells. Phytomedicine. 2011;18:158–162. doi: 10.1016/j.phymed.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Valastyan S, Weinberg RA. Tumor metastasis: Molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fidler IJ, Kripke ML. The challenge of targeting metastasis. Cancer Metastasis Rev. 2015;34:635–641. doi: 10.1007/s10555-015-9586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 28.Radisky DC. Epithelial-mesenchymal transition. J Cell Sci. 2005;118:4325–4326. doi: 10.1242/jcs.02552. [DOI] [PubMed] [Google Scholar]
- 29.Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell. 2009;15:195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 30.Casas E, Kim J, Bendesky A, Ohno-Machado L, Wolfe CJ, Yang J. Snail2 is an essential mediator of Twist1-induced epithelial mesenchymal transition and metastasis. Cancer Res. 2011;71:245–254. doi: 10.1158/0008-5472.CAN-10-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F, Liu Y. TGF beta induced upregulation of malat1 promotes bladder cancer metastasis by associating with suz12. Clin Cancer Res. 2014;20:1531–1541. doi: 10.1158/1078-0432.CCR-13-1455. [DOI] [PubMed] [Google Scholar]
- 32.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 33.Halbersztadt A, Haloń A, Pajak J, Robaczyński J, Rabczynski J, St Gabryś M. The role of matrix metalloproteinases in tumor invasion and metastasis. Ginekol Pol. 2006;77:63–71. (In Polish) [PubMed] [Google Scholar]
- 34.Klein G, Vellenga E, Fraaije MW, Kamps WA, de Bont ES. The possible role of matrix metalloproteinase (MMP)-2 and MMP-9 in cancer, e.g., acute leukemia. Crit Rev Oncol Hematol. 2004;50:87–100. doi: 10.1016/j.critrevonc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Yao N, Ren K, Wang Y, Jin Q, Lu X, Lu Y, Jiang C, Zhang D, Lu J, Wang C, et al. Paris polyphylla suppresses proliferation and vasculogenic mimicry of human osteosarcoma cells and inhibits tumor growth in vivo. Am J Chin Med. 2017;45:575–598. doi: 10.1142/S0192415X17500343. [DOI] [PubMed] [Google Scholar]
- 36.Wang CW, Tai CJ, Choong CY, Lin YC, Lee BH, Shi YC, Tai CJ. Aqueous extract of Paris polyphylla (AEPP) inhibits ovarian cancer via suppression of peroxisome proliferator activated receptor gamma coactivator (PGC) 1alpha. Molecules. 2016;21:727. doi: 10.3390/molecules21060727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang W, Dong X, You L, Sai N, Leng X, Yang C, Yin X, Ni J. Apoptosis in HepaRG and HL-7702 cells inducted by polyphyllin II through caspases activation and cell-cycle arrest. J Cell Physiol. 2019;234:7078–7089. doi: 10.1002/jcp.27462. [DOI] [PubMed] [Google Scholar]
- 38.Gu L, Feng J, Xu H, Luo M, Su D. Polyphyllin I inhibits proliferation and metastasis of ovarian cancer cell line HO 8910PM in vitro. J Tradit Chin Med. 2013;33:325–333. doi: 10.1016/S0254-6272(13)60174-0. [DOI] [PubMed] [Google Scholar]
- 39.Lou W, Chen Y, Zhu KY, Deng H, Wu T, Wang J. Polyphyllin I overcomes EMT-associated resistance to erlotinib in lung cancer cells via IL-6/STAT3 pathway inhibition. Biol Pharm Bull. 2017;40:1306–1313. doi: 10.1248/bpb.b17-00271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.