Abstract
Obesity affects both medical and surgical outcomes in renal transplant recipients (RTRs). Dietary diversity, an important component of a healthy diet, might be a useful nutritional strategy for monitoring patients with obesity. In this cross-sectional study, the data of 85 eligible RTRs were analyzed. Demographic data, routine laboratory data, and 3-day dietary data were collected. Participants were grouped into nonobesity and obesity groups based on body mass index (BMI) (for Asian adults, the cutoff point is 27 kg/m2). Dietary diversity score (DDS) was computed by estimating scores for the six food groups emphasized in the Food Guide. The mean age and BMI of participants were 49.7 ± 12.6 years and 24.0 ± 3.8 kg/m2, respectively. In the study population, 20.0% (n = 17) were obese. DDS was significantly lower in obese participants than in those who were not obese (1.53 ± 0.87 vs. 2.13 ± 0.98; p = 0.029). In addition, DDS was correlated with nutrition adequacy of the diet. Multivariate analysis showed that the odds of obesity decreased with each unit increase in DDS (odds ratio, 0.278; 95% confidence interval, 0.101–0.766; p = 0.013). We conclude that patients with higher dietary diversity have a lower prevalence of obesity.
Keywords: renal transplant recipients, obesity, dietary diversity, nutrient adequacy
1. Introduction
The prevalence of chronic kidney disease (CKD) in Taiwan is extremely high. Kidney transplantation is the best treatment for patients with end-stage of CKD [1]. However, obesity, which is abnormal or excessive body fat accumulation, is common in renal transplant recipients (RTRs). Up to 50% of RTRs experience weight gain after transplantation [2]. Findings derived from a national transplant database in United States revealed that the proportion of obese RTRs increased by 116% from 1987 to 2001 [3]. Obesity-related medical comorbidities and concerns regarding post-transplant outcomes were described in detail previously [4]. An analysis of more than 190,000 RTRs from the Scientific Registry of Transplant Recipients database revealed that obesity is associated with delayed graft function, graft failure, proteinuria, and acute rejection [5]. In addition, obesity is one of the risk factors for cardiovascular disease (CVD), hypertension, hyperlipidemia, insulin resistance and metabolic syndromes, which can lead to graft loss and the CVD-related mortality in RTRs [6,7,8,9]. Consequently, strategies designed to prevent post-transplant weight gain and other consequences associated with obesity in RTRs might improve the survival of patients and grafts.
Factors contributing to obesity during the post-transplant period have been well-researched [10]. Among these factors, inappropriate dietary intake, which is a major lifestyle-related risk factor for many chronic diseases, plays an important role in overweight and obesity among RTRs [10]. In general, RTRs have many dietary restrictions for a long time before transplantation. After successful kidney transplantation, improved renal function and appetite result in better nutrition status and advanced weight gain. In addition, an unrestricted and liberalized diet even leads to overnutrition problems in this population. Indices of dietary intake and dietary quality may be more informative for monitoring the overall diet and clinical outcomes in RTRs.
Few studies have investigated the well-established recommendation of healthy eating for RTRs, especially in Taiwan. In 2009, the Dietitians Association of Australia published guidelines for the nutritional management of adult RTRs, with particular focus on nutrient recommendations [11]. In our previous study [12], we observed that RTRs had inadequate intake of most nutrients, and that compliance with dietary recommendations was poor. The National Kidney Foundation (NKF) published a health guide for transplantation and recommended a healthful, balanced diet that includes foods from food guides, such as a variety of fresh fruits and vegetables, lean meats, reduced-fat dairy products, and whole grains, as well as a diet low in salt and high in fiber, for RTRs [13]. Studies investigating the connection between eating a balanced diet and clinical outcomes in RTRs are warranted.
Dietary diversity score (DDS), one of the indicators used for assessing dietary quality [14], is used to evaluate the diversity within food groups based on a healthful, balanced diet recommended in national food guides [15]. Studies have examined the association between DDS and obesity in the general population [14]; however, this index has rarely been employed in studies on RTRs. In this study, we hypothesize that DDS can be used as an independent determinant of obesity in RTRs.
2. Materials and Methods
2.1. Study Design and Settings
From September 2016 to June 2018, stable RTRs who were regularly followed up at the Department of Urology of Chang Gung Memorial Hospital were enrolled into this cross-sectional study. Participants were recruited through advertisements. Data regarding RTR demographics, anthropometric assessments, laboratory examinations, and dietary intake were collected by well-trained staff according to standardized methods and procedures. Moreover, participants were required to maintain their regular medications.
2.2. Patient Recruitment
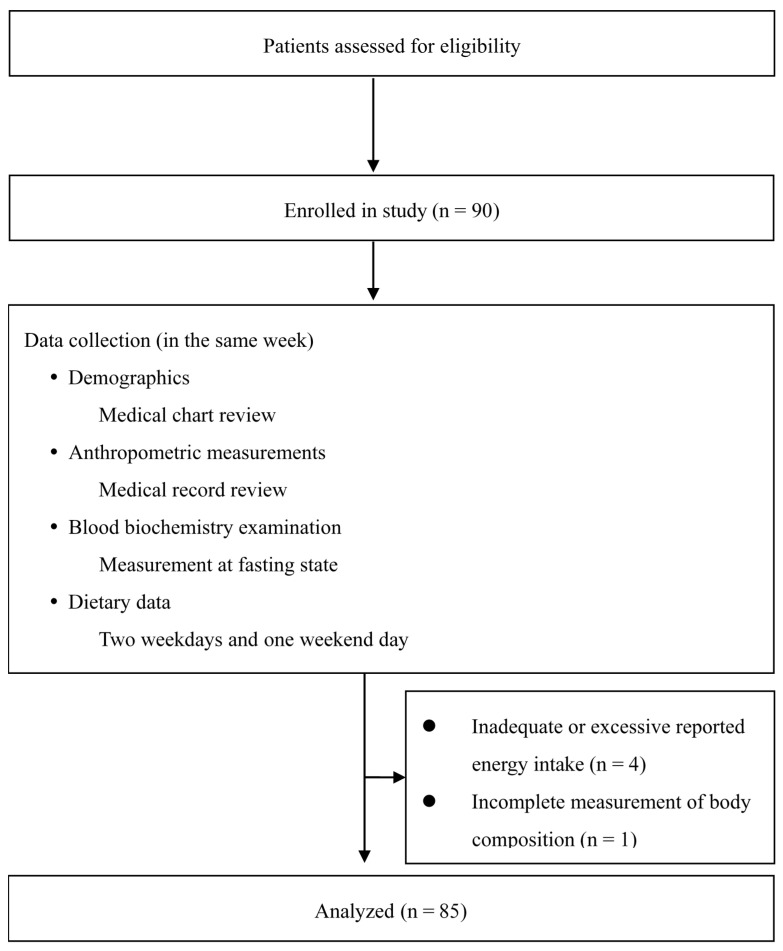
Patients aged >18 years with stable graft kidney function who received maintenance immunosuppressive therapy (a calcineurin inhibitor-, antimetabolite-, and steroid-based regimen) were included. We checked urine albumin to creatinine ratio (ACR) for deterioration of graft kidney function. Prior to this study, the patients were informed of the purpose of the research. We excluded patients who had weight change >3 kg, acute rejection, glomerular filtration rate variation >25%, and change in immunosuppressive regimen 3 months prior to recruitment, as described previously [16]. Moreover, patients with malignant tumors, acute infection, history of dementia or cognitive impairment, inadequate or excessive reported energy intake, or missing assessment data were excluded. Finally, data of 85 RTRs were assessed. A flowchart of enrollment and study procedures is provided in Figure 1.
Figure 1.
Flowchart of patient enrollment and study procedures.
2.3. Demographic Data and Anthropometric Assessment
The demographic data collected included age, sex, height, and weight and were obtained from patients’ medical charts. We calculated body mass index (BMI) as weight (in kilograms) adjusted for the square of height (in meters). Patients were grouped into nonobesity and obesity groups according to the BMI cutoff point for obesity (BMI ≥ 27 kg/m2) suggested by the Taiwan Ministry of Health and Welfare (MOHW) [17]. Information on donor source, transplant vintage, and immunosuppressants was also collected through chart review.
2.4. Biochemical Parameters
Fasting blood samples were collected by registered nurses and were analyzed at the clinical laboratory using standardized methods. The following parameters were analyzed: albumin, blood urea nitrogen, creatinine, hemoglobin A1c, total cholesterol, triglycerides, high density lipoprotein-cholesterol, low density lipoprotein-cholesterol, high-sensitivity C-reactive protein, and insulin. The homeostatic model assessment of insulin resistance was used to assess insulin resistance, as described previously [18].
2.5. Dietary Data
All patients completed 3-day dietary records (2 weekdays and 1 weekend day) before they visited the well-trained dietitian at the latest follow-up. Moreover, 24-h dietary recall was completed through face-to-face interviews to confirm dietary records. Energy and nutrient intake was estimated using validated and reliable nutrient analysis software (COFIT Pro, Version 1.0.0; Cofit HealthCare Inc., Taipei, Taiwan) [19], which is based on the Taiwan MOHW Food and Drug Administration database [20]. The consumption of the six major food groups emphasized in Taiwan MOHW Food Guide (2017) was investigated: (1) whole grains and grain crops; (2) soybeans, fish, eggs, and meat; (3) vegetables; (4) fruits; (5) dairy products; and (6) oil and nuts.
2.6. Physical Activity
We used the short version of the International Physical Activity Questionnaire [21] to evaluate patients’ habitual physical activity levels. During the dietetic interview, patients self-described how much time (days per week and minutes per day) they spent performing physical activity of different intensity levels (vigorous, moderate, walking, and sitting). A metabolic equivalent (MET) value was calculated and is reported in MET-min/day, which has been previously described in detail [22,23].
2.7. DDS Calculation
We applied sex-specific serving sizes for each food group emphasized in the Taiwan food guide [24]. According to the guide, every Taiwanese adult should eat the following each day, based on the individual energy requirement: whole grains and grain crops (6–16 servings); soybeans, fish, eggs, and meat (3–8 servings); dairy products (1.5–2 servings); vegetables (3–5 servings); fruits (2–4 servings); and oil and nuts (4–8 servings). A score of 1 was given for participants who consumed at least one half-serving for each of the six food groups. The scores ranged from 0 to 6.
2.8. Nutrient Adequacy of the Diet: Nutrient Adequacy Ratio (NAR) and Mean Adequacy Ratio (MAR)
The NAR of 14 nutrients was selected on the basis of Dietary Reference Intakes (DRIs) in Taiwan MOHW: vitamins B1, B2, B6, B12, A, C, and E; niacin; folic acid; calcium; magnesium; phosphorus; iron; and zinc [25]. NAR was calculated for each nutrient as the ratio of a participant’s daily intake to age- and sex-specific standard recommended amounts. MAR was calculated as the mean of the NAR of 14 nutrients. The formulas of NAR and MAR are presented below [26]:
2.9. Ethical Considerations
All the standardized procedures used in this study were approved by the Chang Gung Medical Foundation Institutional Review Board (IRB201600954B0). All patients signed the informed consent form before the study was conducted.
2.10. Statistical Analysis
SAS version 9.4 software (SAS Institute, Cary, NC, USA) was used to perform all statistical analyses, and the significance level was set at p < 0.05. Data are presented as mean ± standard deviation, percentage, correlation coefficient, or odds ratio (OR) with 95% confidence interval (CI), as appropriate. The Shapiro–Wilk test and Q–Q plot were used to evaluate normality. Patients were divided into obesity and nonobesity groups, and the results were compared using Student’s t test and the Wilcoxon rank-sum test, as appropriate. We conducted multivariate logistic regression analysis adjusted for age, sex, total energy intake, and physical activity to identify independent risk factors for obesity, with DDS as the binary outcome variable.
3. Results
Table 1 summarizes the demographic, anthropometric, laboratory, and dietary characteristics of the study population. The mean age of participants was 49.72 ± 12.60 years, and 20.0% (n = 17) were obese. Among the 85 RTRs, the post-transplantation duration was 8.83 ± 5.97 years, and 82.4% (n = 70) of transplants were identified as originating from deceased donors. All patients were receiving maintenance immunosuppression regimens: 54 (63.5%) were receiving tacrolimus (FK506), and 31 (36.5%) were receiving cyclosporine.
Table 1.
Demographic, anthropometric, clinical, and nutritional characteristics of the 85 renal transplant recipients 1,2.
| All (n = 85) | Nonobesity (n = 68) | Obesity (n = 17) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Demographics | |||||||||
| Male/female | 46/39 | 34/34 | 12/5 | ||||||
| Age, y | 49.72 ± 12.60 | 50.4 | ± | 12.84 | 47 | ± | 11.54 | ||
| Post-transplant, y | 8.83 | ± | 5.97 | 8.47 | ± | 6.27 | 10.31 | ± | 4.44 |
| Tacrolimus/cyclosporine used | 54/31 | 43/25 | 11/6 | ||||||
| Deceased/living donors | 70/15 | 57/11 | 13/4 | ||||||
| Anthropometry | |||||||||
| Height, cm | 161.39 | ± | 8.61 | 160.87 | ± | 8.73 | 163.47 | ± | 8.01 |
| Body weight, kg | 62.88 | ± | 13.26 | 58.84 | ± | 10.51 | 79.06 | ± | 10.59 * |
| BMI, kg/m2 | 24 | ± | 3.83 | 22.64 | ± | 2.86 | 29.46 | ± | 1.84 * |
| Laboratory | |||||||||
| Albumin, g/dL | 4.34 | ± | 0.3 | 4.36 | ± | 0.26 | 4.23 | ± | 0.43 |
| Blood urea nitrogen, mg/dL | 24.05 | ± | 11.59 | 24.38 | ± | 12.5 | 22.75 | ± | 6.97 |
| Creatinine, mg/dL | 1.43 | ± | 0.76 | 1.42 | ± | 0.8 | 1.45 | ± | 0.61 |
| Total cholesterol, mg/dL | 208.2 | ± | 45.34 | 210.18 | ± | 48.34 | 200.29 | ± | 30.46 |
| Triglycerides, mg/dL | 157.92 | ± | 122.19 | 152.44 | ± | 129.36 | 179.82 | ± | 87.6 * |
| HDL-C, mg/dL | 52.25 | ± | 17.79 | 53.62 | ± | 18.25 | 46.76 | ± | 15.04 |
| LDL-C, md/dL | 121.45 | ± | 37.59 | 121.74 | ± | 40.51 | 120.29 | ± | 23.31 |
| HbA1c, % | 6.06 | ± | 1.01 | 6.05 | ± | 1.09 | 6.11 | ± | 0.55 |
| Insulin, μU/mL | 8.56 | ± | 13.04 | 6.7 | ± | 3.32 | 16.03 | ± | 27.8 * |
| hs-CRP, mg/dL | 5.16 | ± | 12.2 | 5.13 | ± | 13.26 | 5.29 | ± | 6.68 |
| Dietary intake | |||||||||
| Energy, kcal/day | 1872.58 | ± | 377.8 | 1857.59 | ± | 354.7 | 1932.56 | ± | 466.63 |
| Carbohydrate, g/day | 207.22 | ± | 47.34 | 203.95 | ± | 41.43 | 220.31 | ± | 65.94 |
| Protein, g/day | 67.39 | ± | 14.06 | 66.91 | ± | 13.76 | 69.32 | ± | 15.51 |
| Fat, g/day | 84.89 | ± | 22.41 | 85.52 | ± | 22.67 | 82.37 | ± | 21.81 |
| Whole grains and grain crops, servings | 10.54 | ± | 2.69 | 10.43 | ± | 2.41 | 11.01 | ± | 3.67 |
| Soybeans, fish, eggs, and meat, servings | 5.81 | ± | 1.65 | 5.75 | ± | 1.65 | 6.04 | ± | 1.68 |
| Vegetables, servings | 2.5 | ± | 1.04 | 2.56 | ± | 1.09 | 2.27 | ± | 0.81 |
| Fruits, servings | 1.21 | ± | 1.02 | 1.19 | ± | 1.01 | 1.3 | ± | 1.08 |
| Dairy products, servings | 0.19 | ± | 0.34 | 0.22 | ± | 0.37 | 0.1 | ± | 0.19 |
| Oils and nuts, servings | 10 | ± | 3.18 | 10 | ± | 3.28 | 10.02 | ± | 2.85 |
| Others | |||||||||
| eGFR, mL/min/1.73 m2 | 54.71 | ± | 21.48 | 54.3 | ± | 21.8 | 56.35 | ± | 20.68 |
| HOMA-IR | 2.35 | ± | 4.96 | 1.71 | ± | 1.3 | 4.93 | ± | 10.72 $ |
| Physical activity, MET-min/day | 1034.66 | ± | 414.57 | 944.86 | ± | 347.04 | 1393.86 | ± | 476.02 * |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HOMA-IR, homoeostatic model assessment of insulin resistance; HDL-C, high density lipoprotein-cholesterol; hs-CRP, high-sensitivity C-reactive protein; LDL-C, low density lipoprotein-cholesterol; MET, metabolic equivalents. 1 Values are shown as the mean ± standard deviation or number, as appropriate. 2 Statistical analyses were conducted using Student’s t test or Wilcoxon rank-sum test, as appropriate. * p < 0.05; $ p = 0.0593.
The mean daily consumption of the six major food groups was as follows: whole grains and grain crops, 10.54 ± 2.69 servings; soybeans, fish, eggs, and meat, 5.81 ± 1.65 servings; dairy products, 0.19 ± 0.34 serving; vegetables, 2.50 ± 1.04 servings; fruits, 1.21 ± 1.02 serving; and oils and nuts, 10.0 ± 3.18 servings. As shown in Table 2, the mean DDS was 2.01 ± 0.98 in this study (range, 0–4). The maximum and minimum scores of diversity were found for oil and nuts (0.95 ± 0.21) and dairy products (0 ± 0), respectively.
Table 2.
Dietary diversity scores and diversity scores of food groups in obese and nonobese renal transplant recipients 1,2.
| All (n = 85) | Nonobesity (n = 68) | Obesity (n = 17) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Whole grains and grain crops | 0.24 ± 0.43 | 0.25 | ± | 0.44 | 0.18 | ± | 0.39 | ||
| Soybeans, fish, eggs, and meat | 0.54 | ± | 0.5 | 0.59 | ± | 0.5 | 0.35 | ± | 0.49 |
| Vegetables | 0.15 | ± | 0.36 | 0.19 | ± | 0.4 | 0 | ± | 0 |
| Fruits | 0.13 | ± | 0.34 | 0.13 | ± | 0.34 | 0.12 | ± | 0.33 |
| Dairy products | 0 | ± | 0 | 0 | ± | 0 | 0 | ± | 0 |
| Oils and nuts | 0.95 | ± | 0.21 | 0.97 | ± | 0.17 | 0.88 | ± | 0.33 |
| Total | 2.01 | ± | 0.98 | 2.13 | ± | 0.98 | 1.53 | ± | 0.87 * |
1 Values are shown as mean ± standard deviation. 2 Statistical analyses were conducted using Student’s t test or Wilcoxon rank sum test, as appropriate. * p < 0.05.
Notably, the scores of diversity were positively correlated with total energy and macronutrient intake (renergy = 0.59, p < 0.001; rcarbohydrate = 0.49, p < 0.001; rprotein = 0.66, p < 0.001; rfat = 0.37, p = 0.001). Table 3 provides the correlation coefficients between DDS and NARs. A positive and significant correlation was found between DDS and most of the NARs as well as MAR.
Table 3.
Correlation between dietary diversity score and nutrient adequacy for selected nutrients 1.
| Nutrient Adequacy Of The Diet | Coefficient of Dietary Diversity Score |
p Value |
|---|---|---|
| Vitamin A (RE) | 0.15 | 0.1826 |
| Vitamin B1 | 0.43 | <0.0001 |
| Vitamin B2 | 0.41 | 0.0001 |
| Vitamin B6 | 0.49 | <0.0001 |
| Vitamin B12 | 0.28 | 0.0096 |
| Vitamin C | 0.09 | 0.4109 |
| Vitamin E (α-TE) | 0.08 | 0.4809 |
| Niacin | 0.50 | <0.0001 |
| Folic acid | 0.38 | 0.0004 |
| Iron | 0.28 | 0.0118 |
| Zinc | 0.40 | 0.0002 |
| Calcium | 0.18 | 0.1121 |
| Phosphorus | 0.50 | <0.0001 |
| Magnesium | 0.46 | <0.0001 |
| Mean adequacy ratio | 0.50 | <0.0001 |
Abbreviations: RE, retinol equivalent; α-TE, α-tocopherol equivalent. 1 Statistical analyses were conducted using Spearman’s rank correlation adjusted for age and sex.
Comparison of Patients with and without Obesity
No statistically relevant differences were observed in age or total energy and macronutrient intake between the obesity and nonobesity groups (Table 1 and Table 2). However, obese patients had significantly lower DDS scores (1.5 ± 0.9 vs. 2.07 ± 1.0; p = 0.038). On the basis of suggestions in Taiwan’s food guide, none of our RTRs consumed sufficient dairy products daily. Moreover, compared with nonobese RTRs, obese RTRs consumed insufficient daily vegetables, had significantly higher BMI, and showed a worse lipid profile (high level of total triglycerides) and insulin resistance.
We examined the association between DDS and obesity using crude and multivariate-adjusted ORs with 95% CIs. With each unit increase in DDS, the crude odds of obesity significantly decreased by 49.5% (OR, 0.505; 95% CI, 0.275–0.928). This association remained significant after adjusting for age, sex, total energy intake, and physical activity (adjusted OR, 0.278; 95% CI, 0.101–0.766; p = 0.013).
4. Discussion
The present cross-sectional study showed that higher dietary diversity was significantly associated with 72% lower odds of obesity in RTRs. In addition, the results indicate that DDS is a good indicator of nutrition adequacy of diets (Table 3). We also observed that more nonobese than obese RTRs appeared to consume the recommended daily servings of each of the food groups, which was indicated as higher assigned scores in each component of DDS (Table 2). These results are more likely to be a direct/specific effect of such dietary quality on weight management in RTRs.
Previous studies show the high prevalence of obesity in RTRs. Johnson et al. observed that up to 50% of RTRs gained more than 10% body weight during the first year after kidney transplantation [27]. Armstrong et al. found that 21% of RTR had obesity after 7 years of transplantation [28]. Obesity is an ineffective prognostic factor in RTRs and may be involved in the adverse cardiovascular outcomes [28], progression of proteinuria, graft failure and acute rejection [5]. Appropriate dietary advice may enable the development of targeted strategies for treating obese RTRs.
DDS is a good indicator for assessing dietary quality in the general population [29,30,31] and diet-related health conditions, such as risk factors for cardiovascular disease [32], metabolic syndrome [33], and cause-specific mortality [34]. Our results suggest that monitoring dietary diversity based on food guides may be a useful method for evaluating the risk of obesity in RTRs. In the present study, an inverse association was observed between DDS and obesity, which is consistent with previous findings [35,36,37]. Oldewage-Theron and Egal asserted that adult women in South Africa had significantly higher BMI with lower DDS [35]. Azadbakht and Esmaillzadeh showed that Iranian female youth with higher DDS had lower abdominal adiposity and obesity [36]. Recently, Abris et al. also found a significant negative correlation between DDS and the prevalence of obesity among Filipino women [37]. According to our review of the relevant literature, many studies have investigated the association between DDS and obesity; however, studies focusing on patients with chronic kidney disease, particularly RTRs, are scant. Better dietary quality, as indicated by higher DDS, could have a profound influence on obesity prevention.
Other studies have provided controversial findings: there was a positive [38,39] or no [40,41] significant association between dietary diversity and obesity. Salehi-Abargouei et al. asserted that the inconsistency in these results can be explained as follows [14]: (1) food group variability defined by researchers to determine DDS; (2) different methods used for assessing dietary intake (e.g., food frequency questionnaire or dietary recall); (3) weighted scores based on the nutritional value or not; and (4) differences in numbers and characteristics of the study population, such as geographical differences or socioeconomic status. Our findings may not be generalizable to the entire transplant population. Further well-designed large-scale longitudinal studies using the similar approach of DDS to assess dietary quality are required to verify whether our observations can be extrapolated to other populations.
Another notable result of this study is that our obese RTRs seem not to have followed the recommendations in the food guide, which is indicated as lower scores for each component of DDS. In addition, the total scores of DDS were significantly lower in obese RTRs than in nonobese RTRs. Some evidence indicates an association between food guide adherence and overweight/obesity. So et al. reported that Canadians adults who followed Canada’s food guide recommendations, especially regarding the minimum servings of vegetables and fruits, had a lower prevalence of overweight/obesity and BMI [42]. A cross-sectional study from Australia also showed that low compliance with dietary guidelines was related to an approximately three-fold higher risk of being obese [43]. Our results showed that eating a variety and adequate amount of food, such as vegetables (which are low-energy density foods), does not add substantial energy to the overall caloric intake in the diet and results in favorable weight status.
In this study, positive correlations were found between DDS and nutrient adequacy for most micronutrients, implying that higher dietary diversity decreases the risk of micronutrient inadequacy. In addition, statistically positive relationships were identified between DDS and MAR (r = 0.50), consistent with the findings of studies conducted in Mali (r = 0.39) [44], the Philippines (r = 0.44) [45], and China (r = 0.37) [46]. NAR and MAR are indicators used to evaluate an individual’s daily intake of nutrients relative to the recommended intake. In this study, we used Taiwan DRIs for 14 nutrients as the reference values to calculate NAR. Our results provide detailed nutrient intake and quality information, showing that dietary compliance reflected by DDS corresponds to adequate nutrient intake. However, we found no relationship between DDS and nutrient adequacy for vitamins A, E, and C and calcium. The reasons for this result are unclear, but this finding implies that researchers assessing dietary quality should capture different dimensions together, such as dietary diversity and nutrient adequacy.
The present study also showed that few obese and nonobese RTRs consumed dairy products (Table 1), and that their intake of dairy products was inadequate; thus, the diversity scores of dairy products were 0 (Table 2). To the best of our knowledge, few studies have evaluated dietary intake in RTRs and have focused on food sources. Rho et al. found that Korean RTRs consumed less food derived from dairy products and had inadequate calcium intake [47]. This phenomenon is not surprising, because RTRs are required to avoid phosphorus-rich dairy products for a long time before kidney transplantation. In addition, the trend of lower dairy product consumption has been commonly found in Taiwan [48]. On the basis of NKF health guidance for transplantation [49], adult RTRs need approximately two servings of dairy products per day to maintain calcium and phosphorus levels. Education on a balanced diet and dietary consultation should be provided to RTRs.
Our study has several strengths and limitations. To the best of our knowledge, this is the first study to investigate associations between DDS and obesity in RTRs. The results should be interpreted cautiously because of the cross-sectional study design. Studies investigating dietary intake and trends of RTRs are scant, especially longitudinal studies. Further well-designed prospective studies and control trials are required to assess whether our observations can be extrapolated to other RTRs. In this study, we used 3-day dietary records, including workdays and off days, and a 24-h recall to determine dietary quality. These methods have been utilized to evaluate the dietary intake of RTRs and assess the nutrition-related problem [47]. In addition, we used individual-specific requirements for each food group, which may be more representative, to demonstrate the relationships of usual or habitual intake with adherence to dietary guidelines and the risk of obesity. However, different methods for assessing dietary intake and determining DDS may contribute to the inconsistent findings. Therefore, developing validated DDS as a dietary assessment tool is highly desirable. In the current study, we did not collect data on alcohol drinking habits, which are recognized as a major public health concern. Finally, our findings may still be limited by other unmeasured or residual confounding factors, such as the relatively small number of obese RTRs and the metabolic consequences of immunosuppressive agents.
5. Conclusions
We observed lower odds of obesity among RTRs consuming a balanced, diverse, and guideline-recommended diet. As DDS increased, the risk of micronutrient deficiency decreased. On the basis of evidence-based dietetic practice, the dietary recommendations for RTRs should emphasize adherence to dietary guidelines and increasing consumption of dairy products and vegetables for obesity prevention. Our study also introduced a novel strategy to alleviate obesity—the major complication after renal transplantation—which involves monitoring for the adequate intake of dietary food groups.
Acknowledgments
The authors thank all medical staff and patients who participated in the study from Chang Gung Memorial Hospital.
Author Contributions
Conceptualization, I.-H.L., T.V.D., H.-H.W., Y.-J.C., and T.-C.W.; Data curation, I.-H.L., T.V.D., S.-W.N., I.-H.T., H.-H.W., Y.-J.C., C.-Y.C., and T.-C.W.; Formal analysis, I.-H.L., T.V.D., C.-Y.C., and T.-C.W.; Investigation, I.-H.L., H.-H.W., and Y.-J.C.; Methodology, I.-H.L., T.V.D., and T.-C.W.; Project administration, I.-H.L., S.-W.N., I.-H.T., H.-H.W., Y.-J.C., and C.-Y.C.; Resources, I.-H.L., S.-W.N., I.-H.T., H.-H.W., and Y.-J.C.; Software, I.-H.L., T.V.D., C.-Y.C., and T.-C.W.; Supervision, T.-C.W.; Validation, T.-C.W.; Visualization, I.-H.L., T.V.D., and T.-C.W.; Writing—Original draft, I.-H.L., and T.-C.W.; Writing—Review and editing, I.-H.L., T.V.D., and T.-C.W. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by Chang Gung Memorial Hospital in Taiwan (CMRPG3F2001-2). The funder had no role in the decision to collect data, data analysis, or reporting of the results.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Abecassis M., Bartlett S.T., Collins A.J., Davis C.L., Delmonico F.L., Friedewald J.J., Hays R., Howard A., Jones E., Leichtman A.B., et al. Kidney transplantation as primary therapy for end-stage renal disease: A National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) conference. Clin. J. Am. Soc. Nephrol. 2008;3:471–480. doi: 10.2215/CJN.05021107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baum C.L. Weight gain and cardiovascular risk after organ transplantation. J. Parenter. Enter. Nutr. 2001;25:114–119. doi: 10.1177/0148607101025003114. [DOI] [PubMed] [Google Scholar]
- 3.Friedman A.N., Miskulin D.C., Rosenberg I.H., Levey A.S. Demographics and trends in overweight and obesity in patients at time of kidney transplantation. Am. J. Kidney Dis. 2003;41:480–487. doi: 10.1053/ajkd.2003.50059. [DOI] [PubMed] [Google Scholar]
- 4.Kaur G., Yerram P. Kidney Transplant Management. Springer; Berlin/Heidelberg, Germany: 2019. Post Kidney Transplant: Obesity; pp. 133–149. [Google Scholar]
- 5.Kwan J.M., Hajjiri Z., Metwally A., Finn P.W., Perkins D.L. Effect of the obesity epidemic on kidney transplantation: Obesity is independent of diabetes as a risk factor for adverse renal transplant outcomes. PLoS ONE. 2016;11:e0165712. doi: 10.1371/journal.pone.0165712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shirali A.C., Bia M.J. Management of cardiovascular disease in renal transplant recipients. Clin. J. Am. Soc. Nephrol. 2008;3:491–504. doi: 10.2215/CJN.05081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howard R.J., Patton P.R., Reed A.I., Hemming A.W., Van der Werf W.J., Pfaff W.W., Srinivas T.R., Scornik J.C. The changing causes of graft loss and death after kidney transplantation. Transplantation. 2002;73:1923–1928. doi: 10.1097/00007890-200206270-00013. [DOI] [PubMed] [Google Scholar]
- 8.Young J.B., Neumayer H.-H., Gordon R.D. Pretransplant cardiovascular evaluation and posttransplant cardiovascular risk. Kidney Int. 2010;78:S1–S7. doi: 10.1038/ki.2010.209. [DOI] [PubMed] [Google Scholar]
- 9.Porrini E., Delgado P., Torres A. Metabolic syndrome, insulin resistance, and chronic allograft dysfunction. Kidney Int. 2010;78:S42–S46. doi: 10.1038/ki.2010.422. [DOI] [PubMed] [Google Scholar]
- 10.Tantisattamo E. Post-transplant weight gain and obesity: An opportunity for renal dietary management. Adv. Obes. Weight Manag. Control. 2017;7:276–279. [Google Scholar]
- 11.Fry K., Patwardhan A., Ryan C., Trevillian P., Chadban S., Westgarth F., Chan M. Development of evidence-based guidelines for the nutritional management of adult kidney transplant recipients. J. Renal Nutr. 2009;19:101–104. doi: 10.1053/j.jrn.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Lin I.H., Wong T.C., Nien S.W., Chou Y.T., Chiang Y.J., Wang H.H., Yang S.H. Dietary Compliance Among Renal Transplant Recipients: A Single-Center Study in Taiwan. Transpl. Proc. 2019;51:1325–1330. doi: 10.1016/j.transproceed.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 13.National Kidney Foundation Foods to Avoid after Transplantation. [(accessed on 11 March 2020)];2020 Available online: https://www.kidney.org/atoz/content/foods-avoid-after-transplantation.
- 14.Salehi-Abargouei A., Akbari F., Bellissimo N., Azadbakht L. Dietary diversity score and obesity: A systematic review and meta-analysis of observational studies. Eur. J. Clin. Nutr. 2016;70:1–9. doi: 10.1038/ejcn.2015.118. [DOI] [PubMed] [Google Scholar]
- 15.Kant A.K., Graubard B.I. A comparison of three dietary pattern indexes for predicting biomarkers of diet and disease. J. Am. Coll. Nutr. 2005;24:294–303. doi: 10.1080/07315724.2005.10719477. [DOI] [PubMed] [Google Scholar]
- 16.Heng A.-E., Montaurier C., Cano N., Caillot N., Blot A., Meunier N., Pereira B., Marceau G., Sapin V., Jouve C. Energy expenditure, spontaneous physical activity and with weight gain in kidney transplant recipients. Clin. Nutr. 2015;34:457–464. doi: 10.1016/j.clnu.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Taiwan Ministry of Health and Welfare Health Promotion Administration Promoting Your Health. [(accessed on 11 March 2020)];2018 Available online: https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=542&pid=9737.
- 18.Shoji T., Emoto M., Nishizawa Y. HOMA index to assess insulin resistance in renal failure patients. Nephron. 2001;89:348. doi: 10.1159/000046098. [DOI] [PubMed] [Google Scholar]
- 19.Wang J.-S., Hsieh R.-H., Tung Y.-T., Chen Y.-H., Yang C., Chen Y.C. Evaluation of a technological image-based dietary assessment tool for children during pubertal growth: A pilot study. Nutrients. 2019;11:2527. doi: 10.3390/nu11102527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taiwan Food and Drug Administration Taiwanese Food Composition and Nutrient Database. [(accessed on 7 December 2018)];2017 Available online: https://consumer.fda.gov.tw/Food/TFND.aspx?nodeID=178.
- 21.Liou Y.M., Jwo C.J., Yao K.G., Chiang L.-C., Huang L.-H. Selection of appropriate Chinese terms to represent intensity and types of physical activity terms for use in the Taiwan version of IPAQ. J. Nurs. Res. 2008;16:252–263. doi: 10.1097/01.JNR.0000387313.20386.0a. [DOI] [PubMed] [Google Scholar]
- 22.Craig C.L., Marshall A.L., Sjöström M., Bauman A.E., Booth M.L., Ainsworth B.E., Pratt M., Ekelund U., Yngve A., Sallis J.F. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 23.Lee P.H., Macfarlane D.J., Lam T.H., Stewart S.M. Validity of the international physical activity questionnaire short form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011;8:115. doi: 10.1186/1479-5868-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taiwan Ministry of Health and Welfare Health Promotion Administration Dietary Guidelines to Daily Food Guide. [(accessed on 11 March 2020)];2018 Available online: https://www.hpa.gov.tw/Pages/EBook.aspx?nodeid=1208.
- 25.Taiwan Ministry of Health and Welfare Health Promotion Administration Dietary Reference Intakes. [(accessed on 11 March 2020)];2013 Available online: https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=544&pid=725.
- 26.Ruel M.T. Operationalizing dietary diversity: A review of measurement issues and research priorities. J. Nutr. 2003;133:3911S–3926S. doi: 10.1093/jn/133.11.3911S. [DOI] [PubMed] [Google Scholar]
- 27.Johnson C.P., Gallagher-Lepak S., Zhu Y.-R., Porth C., Kelber S., Roza A.M., Adams M.B. Factors influencing weight gain after renal transplantation. Transplantation. 1993;56:822–826. doi: 10.1097/00007890-199310000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Armstrong K.A., Campbell S.B., Hawley C.M., Nicol D.L., Johnson D.W., Isbel N.M. Obesity is associated with worsening cardiovascular risk factor profiles and proteinuria progression in renal transplant recipients. Am. J. Transplant. 2005;5:2710–2718. doi: 10.1111/j.1600-6143.2005.01073.x. [DOI] [PubMed] [Google Scholar]
- 29.Vandevijvere S., De Vriese S., Huybrechts I., Moreau M., Van Oyen H. Overall and within-food group diversity are associated with dietary quality in Belgium. Public Health Nutr. 2010;13:1965–1973. doi: 10.1017/S1368980010001606. [DOI] [PubMed] [Google Scholar]
- 30.Mirmiran P., Azadbakht L., Esmaillzadeh A., Azizi F. Dietary diversity score in adolescents-a good indicator of the nutritional adequacy of diets: Tehran lipid and glucose study. Asia Pac. J. Clin. Nutr. 2004;13:56–60. [PubMed] [Google Scholar]
- 31.Oldewage-Theron W.H., Kruger R. Food variety and dietary diversity as indicators of the dietary adequacy and health status of an elderly population in Sharpeville, South Africa. J. Nutr. Elder. 2008;27:101–133. doi: 10.1080/01639360802060140. [DOI] [PubMed] [Google Scholar]
- 32.Azadbakht L., Mirmiran P., Esmaillzadeh A., Azizi F. Dietary diversity score and cardiovascular risk factors in Tehranian adults. Public Health Nutr. 2006;9:728–736. doi: 10.1079/PHN2005887. [DOI] [PubMed] [Google Scholar]
- 33.Azadbakht L., Mirmiran P., Azizi F. Dietary diversity score is favorably associated with the metabolic syndrome in Tehranian adults. Int. J. Obes. 2005;29:1361–1367. doi: 10.1038/sj.ijo.0803029. [DOI] [PubMed] [Google Scholar]
- 34.Kant A.K., Schatzkin A., Ziegler R.G. Dietary diversity and subsequent cause-specific mortality in the NHANES I epidemiologic follow-up study. J. Am. Coll. Nutr. 1995;14:233–238. doi: 10.1080/07315724.1995.10718501. [DOI] [PubMed] [Google Scholar]
- 35.Oldewage-Theron W.H., Egal A.A. A cross-sectional baseline survey investigating the relationship between dietary diversity and cardiovascular risk factors in women from the Vaal Region, South Africa. J. Nurs. Educ. Pract. 2014;4:50. doi: 10.5430/jnep.v4n1p50. [DOI] [Google Scholar]
- 36.Azadbakht L., Esmaillzadeh A. Dietary diversity score is related to obesity and abdominal adiposity among Iranian female youth. Public Health Nutr. 2011;14:62–69. doi: 10.1017/S1368980010000522. [DOI] [PubMed] [Google Scholar]
- 37.Abris G.P., Provido S.M.P., Hong S., Yu S.H., Lee C.B., Lee J.E. Association between dietary diversity and obesity in the Filipino Women’s Diet and Health Study (FiLWHEL): A cross-sectional study. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0206490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jayawardena R., Byrne N.M., Soares M.J., Katulanda P., Yadav B., Hills A.P. High dietary diversity is associated with obesity in Sri Lankan adults: An evaluation of three dietary scores. BMC Public Health. 2013;13:314. doi: 10.1186/1471-2458-13-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ponce X., Ramirez E., Delisle H. A more diversified diet among Mexican men may also be more atherogenic. J. Nutr. 2006;136:2921–2927. doi: 10.1093/jn/136.11.2921. [DOI] [PubMed] [Google Scholar]
- 40.Ajani S. An assessment of dietary diversity in six Nigerian states. Afr. J. Biomed. Res. 2010;13:161–167. [Google Scholar]
- 41.Savy M., Martin-Prével Y., Sawadogo P., Kameli Y., Delpeuch F. Use of variety/diversity scores for diet quality measurement: Relation with nutritional status of women in a rural area in Burkina Faso. Eur. J. Clin. Nutr. 2005;59:703–716. doi: 10.1038/sj.ejcn.1602135. [DOI] [PubMed] [Google Scholar]
- 42.So H., McLaren L., Currie G. The relationship between health eating and overweight/obesity in Canada: Cross-sectional study using the CCHS. Obes. Sci. Pract. 2017;3:399–406. doi: 10.1002/osp4.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hendrie G.A., Golley R.K., Noakes M. Compliance with dietary guidelines varies by weight status: A cross-sectional study of Australian adults. Nutrients. 2018;10:197. doi: 10.3390/nu10020197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hatløy A., Torheim L.E., Oshaug A. Food variety—A good indicator of nutritional adequacy of the diet? A case study from an urban area in Mali, West Africa. Eur. J. Clin. Nutr. 1998;52:891–898. doi: 10.1038/sj.ejcn.1600662. [DOI] [PubMed] [Google Scholar]
- 45.Kennedy G.L., Pedro M.R., Seghieri C., Nantel G., Brouwer I. Dietary diversity score is a useful indicator of micronutrient intake in non-breast-feeding Filipino children. J. Nutr. 2007;137:472–477. doi: 10.1093/jn/137.2.472. [DOI] [PubMed] [Google Scholar]
- 46.Zhao W., Yu K., Tan S., Zheng Y., Zhao A., Wang P., Zhang Y. Dietary diversity scores: An indicator of micronutrient inadequacy instead of obesity for Chinese children. BMC Public Health. 2017;17:440. doi: 10.1186/s12889-017-4381-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rho M.R., Lim J.H., Park J.H., Han S.S., Kim Y.S., Lee Y.H., Kim W.G. Evaluation of nutrient intake in early post kidney transplant recipients. Clin. Nutr. Res. 2013;2:1–11. doi: 10.7762/cnr.2013.2.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee M.-S., Wahlqvist M.L., Peng C.-J. Dairy foods and health in Asians: Taiwanese considerations. Asia Pac. J. Clin. Nutr. 2015;24:S14. doi: 10.6133/apjcn.2015.24.s1.03. [DOI] [PubMed] [Google Scholar]
- 49.National Kidney Foundation Diet and Transplantation. [(accessed on 11 March 2020)];2020 Available online: https://www.kidney.org/atoz/content/nutritrans.