Abstract
Background: The present study aims to evaluate the diagnostic roles of various immunohistochemical (IHC) markers in thymic tumors, including thymic carcinoma (TC) and thymoma (TM). Methods: Eligible studies were obtained by searching the PubMed databases and screening the searched articles. Thirty-eight articles were used in the present meta-analysis and included 636 TCs and 1861 TMs. Besides, for IHC markers with statistical significance, a diagnostic test accuracy review was performed. Results: The comparison of various IHC expressions between TC and TM was performed for 32 IHC markers. Among these IHC markers, there were significant differences between TC and TM for beta-5t, B-cell lymphoma 2 (Bcl-2), calretinin, CD1a, CD5, carcinoembryonic antigen (CEA), cytokeratin19 (CK19), CD117, glucose transporter 1 (Glut-1), insulin-like growth factor 1 receptor (IGF-1R), mesothelin, MOC31, mucin1 (MUC1), p21, and terminal deoxynucleotidyl transferase (TdT). Markers with higher expressions in TCs were Bcl-2, calretinin, CD5, CEA, CD117, Glut-1, IGF-1R, mesothelin, MOC31, MUC1, and p21. Among these markers, there were no significant differences between TC and TM type B3 in immunohistochemistries for Bcl-2 and CK19. On the other hand, β-catenin and CD205 showed a considerable difference in IHC expressions between TC and TM type B3, but not between TC and overall TM. In diagnostic test accuracy review, MUC1 and beta-5t were the most useful markers for TC and TM, respectively. Conclusions: Taken together, our results showed that the expression rates for various IHC markers significantly differed between TC and TM. The IHC panel can be useful for differentiation from limited biopsied specimens in daily practice.
Keywords: thymus, thymic carcinoma, thymoma, immunohistochemistry, meta-analysis, diagnostic test accuracy review
1. Introduction
Thymic epithelial tumors (TETs), which originate from thymic epithelial cells, include thymoma (TM), thymic carcinoma (TC), and thymic neuroendocrine tumors [1,2]. These TETs have different biological functions, histological findings, and genomic profiles [2,3]. TMs can be classified into type A, AB, B1, B2, B3, and C (TC) based on the World Health Organization (WHO) classification [1]. According to the WHO classification, TETs, regardless of subtype or histology, are classified as malignant tumors [1,4]. The incidence of TC is approximately 22% [5], with squamous cell carcinoma being the most common subtype of TC, accounting for approximately 70% of all TCs [1,6]; accurate differentiation between thymic squamous cell carcinoma and TM type B3 is required [1]. Further, focal squamous differentiation and keratinization can be found in other types of TC, such as lymphoepithelioma-like carcinoma and basaloid carcinoma [1]. In the diagnosis of anterior mediastinal tumors, the direct invasion and metastasis of pulmonary squamous cell carcinoma are required to differentiate between these tumors and TC. The pathologic diagnosis of an anterior mediastinal tumor is essential when determining treatment modality and prognosis [2]. TC patients generally show a higher stage and worse prognosis compared to TM patients [3,7], but the differential diagnosis is challenging when the given specimen is a small biopsied tissue. Previous studies reported the diagnostic implications of various immunohistochemical (IHC) markers, including CD117, a marker closely related to the invasion and metastasis of tumor cells, which is highly expressed in TC [2]. In addition, Nakagawa et al. reported that the combination of CD117 and CD5 was useful for differentiating between thymic and lung squamous cell carcinoma [8]. Insulin-like growth factor-1 receptor (IGF-1R) was proposed as a potential therapeutic target for TET and is more frequently expressed in TC than in TM [9,10,11].
Although various IHC expressions were reported, single specific markers for each TET are not available. In our study, we evaluated the IHC expression patterns of TCs and TMs, alongside the performance of a diagnostic test accuracy review for various IHC markers.
2. Materials and Methods
2.1. Published Study Search and Selection Criteria
Relevant articles were obtained by searching the PubMed database through 31 January 2020. For searching, the keywords used were as follows: “thymic carcinoma or thymoma” and “immunohistochemistry”. The titles and abstracts of all searched articles were screened for inclusion and exclusion. Included articles had the information for the immunohistochemistry of the TC and TM. However, case reports, non-original articles, or those written in English were excluded from the present study. The PRISMA checklist shown in the supplementary Table S1.
2.2. Data Extraction
Data associated with various IHC expressions of TC and TM were extracted from each of the eligible studies [2,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44]. Two independent authors extracted all of the data. Extracted data were the author’s information, study location, number of patients analyzed, and tumor subtypes of TM. In addition, the expression rates by IHC markers were investigated in TC and TM.
2.3. Statistical Analyses
A meta-analysis was performed using the Comprehensive Meta-Analysis software 2.0 package (Biostat, Englewood, NJ, USA). The expression rates of various IHC markers were investigated by dividing them into TC and TM markers. Comparisons of IHC expressions between TC and TM type B3 were also performed. Heterogeneity between the studies was checked using Q and I2 statistics and expressed as p-values. Additionally, sensitivity analysis was conducted to assess the heterogeneity of eligible studies and the impact of each study on the combined effects. Due to the use of various evaluation criteria and tumor types in the eligible studies, a random-effect model rather than a fixed-effect model was determined to be more suitable for this meta-analysis. The Begg’s funnel plot and Egger’s test were performed to assess publication bias, with fail-safe N and trim-fill tests additionally used to confirm the degree of publication bias if found. The results were considered statistically significant at p < 0.05. The diagnostic test accuracy review of various IHC markers was performed using R software ver. 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria). We calculated the pooled sensitivity, specificity and diagnostic odds ratio (OR) according to individual data collected from each eligible study. By plotting the sensitivity and 1-specificity of each study, the summary receiver operating characteristic curve (SROC) was able to be constructed with curve fitting performed via linear regression. Due to all of the data being heterogeneous, accuracy data were pooled by fitting the SROC and measuring the area under the curve (AUC).
3. Results
3.1. Selection and Characteristics of the Studies
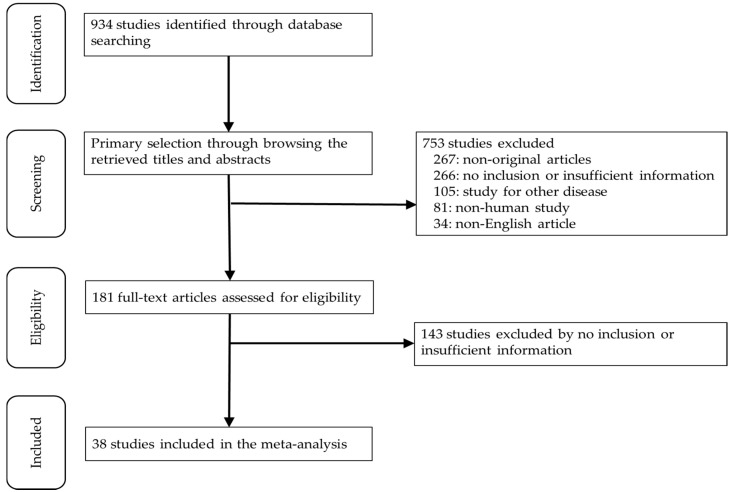
In this study, 934 relevant articles were searched for on the PubMed database and reviewed for a meta-analysis. Of these, 409 articles had no or lack of sufficient information for a meta-analysis. In addition, 267 were excluded due to non-original articles. Among the remaining articles, 220 reports were excluded for the following reasons: articles in other diseases (n = 105), non-human studies (n = 81), and a language other than English (n = 34) (Figure 1). Finally, 38 eligible articles were selected and included for the meta-analysis (Table 1). These studies included 2497 patients, including TC (n = 636) and TM (n = 1861).
Figure 1.
Flow chart of the searching strategy.
Table 1.
Main characteristics of the eligible studies.
| Study | Location | Number of Patients | ||||||
|---|---|---|---|---|---|---|---|---|
| Thymic Carcinoma | Thymoma | Type A | Type AB | Type B1 | Type B2 | Type B3 | ||
| Adam 2014 [9] | Germany | 24 | 45 | |||||
| Chen 1996 [10] | Taiwan | 26 | 15 | |||||
| Cui 2011 [11] | China | 4 | 39 | 2 | 5 | 7 | 14 | 11 |
| Dorfman 1997 [12] | USA | 24 | 41 | |||||
| Du 2016 [13] | China | 22 | 21 | 21 | ||||
| Girard 2009 [14] | USA | 7 | 38 | 8 | 22 | 8 | ||
| Girard 2010 [15] | USA | 7 | 56 | 5 | 12 | 8 | 21 | 10 |
| Hayashi 2013 [16] | Japan | 18 | 17 | 17 | ||||
| Henley 2002 [17] | USA | 6 | 36 | |||||
| Hino 1997 [18] | Japan | 19 | 17 | |||||
| Hirabayashi 1997 [19] | Japan | 4 | 36 | |||||
| Hiroshima 2002 [20] | Japan | 10 | 36 | 8 | 8 | 7 | 7 | 6 |
| Kaira 2011 [21] | Japan | 17 | 5 | 5 | ||||
| Khoury 2010 [22] | USA | 12 | 54 | 17 | ||||
| Kornstein 1997 [23] | USA | 24 | 85 | |||||
| Laury 2011 [24] | USA | 5 | 9 | |||||
| Lee 2019 [25] | Korea | 30 | 110 | 11 | 31 | 28 | 16 | 19 |
| Mimae 2011 [26] | Japan | 37 | 103 | 6 | ||||
| Mimae 2012 [27] | USA | 37 | 103 | 6 | ||||
| Nakagawa 2005 [8] | Japan | 20 | 50 | 10 | 10 | 10 | 10 | 10 |
| Nonaka 2007 [28] | USA | 16 | 58 | 9 | 19 | 7 | 16 | 7 |
| Omatsu 2012 [29] | Japan | 22 | 22 | 1 | 1 | 7 | 7 | 6 |
| Pan 2003 [30] | Taiwan | 22 | 35 | 9 | 10 | 4 | 7 | 5 |
| Petrini 2010 [31] | Italy | 13 | 105 | |||||
| Remon 2017 [32] | France | 12 | 84 | 4 | 25 | 8 | 27 | 20 |
| Rieker 2006 [33] | Germany | 4 | 30 | 8 | 6 | 5 | 6 | 5 |
| Song 2012 [34] | China | 15 | 87 | 3 | 29 | 5 | 22 | 28 |
| Stefanaki 1997 [35] | Greece | 2 | 29 | |||||
| Su 2015 [36] | China | 20 | 16 | 16 | ||||
| Suzuki 2018 [37] | Japan | 10 | 7 | |||||
| Tateyama 1999 [38] | Japan | 7 | 18 | |||||
| Thomas 2016 [39] | USA | 34 | 29 | |||||
| Thomas de Montpréville 2015 [40] | France | 16 | 75 | 5 | 17 | 11 | 25 | 17 |
| Tsuchida 2008 [41] | Japan | 17 | 20 | 5 | 4 | 6 | 5 | |
| Weissferdt 2011 [42] | USA | 31 | 60 | 30 | ||||
| Wu 2019 [2] | China | 22 | 128 | 11 | 35 | 19 | 40 | 23 |
| Yamada 2011 [43] | Japan | 13 | 41 | 3 | 17 | 7 | 10 | 4 |
| Zucali 2010 [44] | Italy | 8 | 101 | 15 | 28 | 24 | 8 | 24 |
3.2. Comparison of Immunohistochemical Expressions between Thymic Carcinoma and Thymoma
First, the significant differences in IHC expressions between TC and TM were investigated. There were significant differences in immunohistochemistry for beta-5t, B-cell lymphoma 2 (Bcl-2), calretinin, CD1a, CD5, carcinoembryonic antigen (CEA), cytokeratin19 (CK19), CD117, Glut-1, IGF-1R, mesothelin, MOC31, MUC1, p21, and TdT (Table 2). Among these markers, Bcl-2, calretinin, CD5, CEA, CD117, glucose transporter 1 (Glut-1), insulin-like growth factor 1 receptor (IGF-1R), mesothelin, MOC31, mucin1 (MUC1), and p21 showed significantly higher expressions in TC than in TM. On the other hand, TMs have shown higher expressions of beta-5t, CD1a, CK19, and terminal deoxynucleotidyl transferase (TdT) than TC. In comparison with TC, the significant differences in IHC expressions for beta-catenin and CD205 were found in TM type B3, but not in overall TM. Among markers with a significant difference, the estimated expression rates of CD205 were 0.650 (95% CI 0.461–0.801) and 0.958 (95% CI 0.757–0.994) in TC and TM type B3, respectively (Table 3). In addition, the estimated expression rates of Glut-1 and IGF-1R were significantly higher in TC than in TM type B3. However, the Glut-1 and IGF-1R expression rates of TM type B3 were 52.6% and 64.6%, respectively.
Table 2.
Meta-analysis for the odds ratio of various immunohistochemical expressions between thymic carcinoma and thymoma.
| Marker | Number of Subsets |
Fixed Effect [95% CI] |
Heterogeneity Test [p-Value] |
Random Effect [95% CI] |
Egger’s Test [p-Value] |
|---|---|---|---|---|---|
| Androgen receptor | 3 | 0.362 [0.120, 1.091] | 0.063 | 0.740 [0.065, 8.450] | 0.480 |
| beta-5t | 2 | 0.002 [0.000, 0.030] | 0.564 | 0.002 [0.000, 0.030] | - |
| beta-catenin | 2 | 0.829 [0.254, 2.704] | 0.022 | 0.512 [0.027, 9.722] | - |
| Bcl-2 | 4 | 2.461 [1.043, 5.807] | 0.637 | 2.461 [1.043, 5.807] | 0.871 |
| Calretinin | 1 | 19.429 [2.218, 170.165] | 1.000 | 19.429 [2.218, 170.165] | - |
| CD15 | 2 | 4.139 [1.413, 12.127] | 0.022 | 2.263 [0.130, 39.382] | - |
| CD1a | 2 | 0.052 [0.012, 0.223] | 0.073 | 0.028 [0.001, 0.623] | - |
| CD205 | 2 | 0.221 [0.064, 0.759] | 0.019 | 0.137 [0.006, 3.046] | - |
| CD5 | 11 | 52.560 [26.424, 104.547] | 0.972 | 52.560 [26.424, 104.547] | 0.034 |
| CEA | 2 | 45.273 [5.567, 368.160] | 0.505 | 45.273 [5.567, 368.160] | - |
| CK19 | 2 | 0.061 [0.016, 0.224] | 0.364 | 0.061 [0.016, 0.224] | - |
| CK5/6 | 4 | 0.191 [0.080, 0.459] | 0.022 | 0.294 [0.054, 1.607] | 0.283 |
| c-Kit | 12 | 41.444 [23.767, 72.267] | 0.771 | 41.444 [23.767, 72.267] | 0.024 |
| Cyclin D1 | 2 | 0.407 [0.128, 1.298] | 0.006 | 1.140 [0.022, 58.476] | - |
| E-cadherin | 3 | 0.340 [0.170, 0.680] | 0.001 | 0.400 [0.064, 2.516] | 0.167 |
| EGFR | 6 | 0.311 [0.130, 0.741] | 0.014 | 0.314 [0.066, 1.493] | 0.964 |
| Estrogen receptor | 1 | 0.319 [0.012, 8.254] | 1.000 | 0.319 [0.012, 8.254] | - |
| Glut-1 | 4 | 11.607 [3.003, 44.862] | 0.100 | 15.187 [2.082, 110.780] | 0.019 |
| HBME | 2 | 2.776 [0.337, 22.853] | 0.088 | 2.763 [0.076, 100.781] | - |
| IGF-1R | 6 | 10.216 [5.611, 18.602] | 0.005 | 9.465 [2.869, 31.221] | 0.806 |
| Mesothelin | 3 | 39.842 [12.067, 131.542] | 0.876 | 39.842 [12.067, 131.542] | 0.386 |
| MOC31 | 2 | 18.019 [4.366, 75.113] | 0.874 | 18.019 [4.366, 75.113] | - |
| MUC1 | 3 | 44.866 [11.273, 178.576] | 0.786 | 44.866 [11.273, 178.576] | 0.249 |
| p21 | 2 | 10.270 [2.862, 36.849] | 0.716 | 10.270 [2.862, 36.849] | - |
| p53 | 7 | 2.554 [1.077, 6.055] | 0.029 | 3.199 [0.759, 13.481] | 0.487 |
| p63 | 3 | 0.239 [0.094, 0.610] | 0.013 | 0.264 [0.028, 2.482] | 0.924 |
| PAX8 | 3 | 0.371 [0.107, 1.288] | 0.065 | 0.539 [0.058, 4.989] | 0.505 |
| Progesterone receptor | 2 | 1.681 [0.170, 16.597] | 0.597 | 1.681 [0.170, 16.597] | - |
| Survivin | 2 | 1.251 [0.358, 4.378] | 0.103 | 0.733 [0.056, 9.558] | - |
| TdT | 2 | 0.015 [0.003, 0.085] | 0.206 | 0.014 [0.001, 0.126] | - |
| Thrombomodulin | 1 | 0.449 [0.023, 8.896] | 1.000 | 0.449 [0.023, 8.896] | - |
| WT-1 | 1 | 4.953 [0.193, 127.130] | 1.000 | 4.953 [0.193, 127.130] | - |
CI, confidence interval.
Table 3.
Meta-analysis for the odds ratio of various immunohistochemical expressions between thymic carcinoma and thymoma type B3.
| Marker | Type | Number of Subsets | Fixed Effect [95% CI] |
Heterogeneity Test [p-Value] |
Random Effect [95% CI] |
Egger’s Test [p-Value] |
|---|---|---|---|---|---|---|
| beta-5t | Thymic carcinoma | 2 | 0.031 [0.004, 0.188] | 0.877 | 0.031 [0.004, 0.188] | - |
| Thymoma type B3 | 2 | 0.948 [0.706, 0.993] | 0.511 | 0.948 [0.706, 0.993] | - | |
| beta-catenin | Thymic carcinoma | 1 | 0.750 [0.448, 0.917] | 1.000 | 0.750 [0.448, 0.917] | - |
| Thymoma type B3 | 1 | 0.118 [0.030, 0.368] | 1.000 | 0.118 [0.030, 0.368] | - | |
| CD1a | Thymic carcinoma | 2 | 0.127 [0.036, 0.360] | 0.155 | 0.096 [0.012, 0.489] | - |
| Thymoma type B3 | 2 | 0.847 [0.680, 0.935] | 0.682 | 0.847 [0.680, 0.935] | - | |
| CD205 | Thymic carcinoma | 2 | 0.650 [0.461, 0.801] | 0.371 | 0.650 [0.461, 0.801] | - |
| Thymoma type B3 | 2 | 0.958 [0.757, 0.994] | 0.679 | 0.958 [0.757, 0.994] | - | |
| CD5 | Thymic carcinoma | 5 | 0.722 [0.610, 0.812] | 0.678 | 0.722 [0.610, 0.812] | 0.318 |
| Thymoma type B3 | 5 | 0.100 [0.039, 0.233] | 0.358 | 0.096 [0.035, 0.236] | 0.110 | |
| CEA | Thymic carcinoma | 1 | 0.750 [0.522, 0.892] | 1.000 | 0.750 [0.522, 0.892] | - |
| Thymoma type B3 | 1 | 0.029 [0.002, 0.336] | 1.000 | 0.029 [0.002, 0.336] | - | |
| c-Kit | Thymic carcinoma | 11 | 0.688 [0.607, 0.759] | 0.142 | 0.692 [0.591, 0.778] | 0.532 |
| Thymoma type B3 | 11 | 0.099 [0.060, 0.160] | 0.944 | 0.099 [0.060, 0.160] | 0.005 | |
| Glut-1 | Thymic carcinoma | 4 | 0.952 [0.862, 0.985] | 0.827 | 0.952 [0.862, 0.985] | 0.017 |
| Thymoma type B3 | 4 | 0.495 [0.351, 0.640] | 0.105 | 0.526 [0.296, 0.745] | 0.621 | |
| IGF-1R | Thymic carcinoma | 5 | 0.820 [0.720, 0.890] | 0.580 | 0.820 [0.720, 0.890] | 0.147 |
| Thymoma type B3 | 5 | 0.632 [0.495, 0.751] | 0.179 | 0.646 [0.468, 0.791] | 0.573 | |
| Mesothelin | Thymic carcinoma | 1 | 0.417 [0.185, 0.692] | 1.000 | 0.417 [0.185, 0.692] | - |
| Thymoma type B3 | 1 | 0.028 [0.002, 0.322] | 1.000 | 0.028 [0.002, 0.322] | - | |
| MOC31 | Thymic carcinoma | 1 | 0.500 [0.244, 0.756] | 1.000 | 0.500 [0.244, 0.756] | - |
| Thymoma type B3 | 1 | 0.118 [0.030, 0.368] | 1.000 | 0.118 [0.030, 0.368] | - | |
| MUC1 | Thymic carcinoma | 3 | 0.849 [0.706, 0.930] | 0.140 | 0.897 [0.666, 0.975] | 0.034 |
| Thymoma type B3 | 3 | 0.270 [0.144, 0.449] | 0.051 | 0.198 [0.048, 0.549] | 0.462 | |
| p21 | Thymic carcinoma | 1 | 0.667 [0.376, 0.869] | 1.000 | 0.667 [0.376, 0.869] | - |
| Thymoma type B3 | 1 | 0.118 [0.030, 0.368] | 1.000 | 0.118 [0.030, 0.368] | - | |
| TdT | Thymic carcinoma | 2 | 0.070 [0.018, 0.242] | 0.611 | 0.070 [0.018, 0.242] | - |
| Thymoma type B3 | 2 | 0.865 [0.690, 0.949] | 0.336 | 0.865 [0.690, 0.949] | - |
CI, confidence interval.
3.3. Diagnostic Test Accuracy Review for Immunohistochemical Markers
The diagnostic test accuracy reviews were performed for candidates of IHC markers, which were showed the statistical differences between TC and TM type B3. Five positive markers, including CD5, CD117, Glut-1, and IGF-1R, MUC1, and four negative markers, including beta-5t, CD1a, CD205, and TdT, were included in the present analysis (Table 4). Among these markers, the most effective positive and negative markers may be MUC1 and beta-5t, 0.932 (95% CI 0.686–0.988), 0.847 (95% CI 0.505–0.968), 46.251 ( 95% CI 11.634–183.877), 0.921 and 1.000 ( 95% CI 0.927–1.000), 1.000 (95% CI 0.942–1.000), 571.396 (95% CI 33.356–9788.053), 0.985), in sensitivity, specificity, diagnostic OR, and AUC on SROC, respectively; Table 4. The orders of AUC on SROC were MUC1, Glut-1, CD117, IGF-1R, and CD5 in positive markers and were beta-5t, TdT, CD1a, and CD205 in negative markers.
Table 4.
Diagnostic test accuracy review of various immunohistochemical markers for differentiation between thymic carcinoma and thymoma type B3.
| Marker | Included Studies | Sensitivity (%) [95% CI] |
Specificity (%) [95% CI] |
Diagnostic OR [95% CI] |
AUC on SROC |
|
|---|---|---|---|---|---|---|
| Thymic carcinoma | CD5 | 5 | 0.731 [0.622, 0.817] | 0.967 [0.756, 0.996] | 23.936 [7.693, 74.478] | 0.725 |
| c-kit | 11 | 0.709 [0.613, 0.790] | 0.925 [0.873, 0.957] | 23.623 [11.900, 46.894] | 0.910 | |
| Glut-1 | 4 | 0.942 [0.856, 0.978] | 0.464 [0.225, 0.720] | 11.823 [2.879, 48.549] | 0.916 | |
| IGF-1R | 3 | 0.875 [0.760, 0.939] | 0.250 [0.136, 0.415] | 4.050 [1.087, 15.085] | 0.758 | |
| MUC1 | 3 | 0.932 [0.686, 0.988] | 0.847 [0.505, 0.968] | 46.251 [11.634, 183.877] | 0.921 | |
| Thymoma type B3 | beta-5t | 2 | 1.000 [0.927, 1.000] | 1.000 [0.942, 1.000] | 571.396 [33.356, 9788.053] | 0.985 |
| CD1a | 2 | 0.743 [0.628, 0.832] | 0.952 [0.504, 0.997] | 35.919 [1.606, 803.371] | 0.871 | |
| CD205 | 2 | 1.000 [0.931, 1.000] | 0.335 [0.165, 0.504] | 11.735 [1.368, 100.632] | 0.785 | |
| TdT | 2 | 0.879 [0.718, 0.954] | 0.933 [0.769, 0.983] | 93.458 [14.682, 594.912] | 0.958 |
CI, confidence interval; OR, odds ratio; AUC, area under the curve; SROC, summary receiver operating characteristic.
4. Discussion
Although the prognosis of each subtype of TETs is not clear, the prognosis of Type B3 TMs is clearly different from other subtypes of TMs and TC [22,45,46]; however, histological similarities are often found in thymic squamous cell carcinoma and TM type B3 [4,22,45]. Due to the potential importance of differentiation between TC and TM type B3, various IHC markers, such as CD117 and CD5, were introduced and studied [1,4,8,12,30,47,48]. However, the accuracy of using these markers as a diagnostic test was not clarified [22,45,46]. This study presented the first meta-analysis and diagnostic test accuracy review of the diagnostic roles of various IHC markers in TETs, including TC and TM.
TETs can be differentiated by histological characteristics [1]; however, similar histologic findings are present between the subtypes of TET. Because each subtype exhibits different clinical behaviors and outcomes, a precise diagnosis is essential [14,49]. The treatment of choice for TETs is surgical resection, where possible [50]. In inoperable cases, the preoperative diagnosis may be more important in regard to treatment decisions, such as chemotherapy [51]. However, other malignant and benign tumors can also occur in the thymus. The diagnostic goals for biopsy specimens could lie in defining malignant or benign tumors and differentiating between TETs and others. However, specimens obtained via needle biopsy have some limitations in regard to histological diagnosis. Immunohistochemistry is useful for the diagnosis of small biopsied specimens. In addition, information regarding various protein expression patterns is useful in regard to understanding tumorigenesis and developing targeted drugs.
Various tumors can occur in the anterior mediastinum, including TETs, germ cell tumors, and metastatic tumors. Differentiating between TET subtypes can be useful when deciding on treatments and predicting prognoses. Due to the similar histological findings of TC and TM type B3, diagnoses from small biopsied specimens are challenging in daily practice. However, single and specific IHC markers for each tumor are not yet defined. Specific markers have high expression in the target and low expression in the reference. According to our results, positive markers may be suitable for CD5, CD117, MUC1, and Glut-1 in differentiating TC from TM type B3. On the other hand, beta-5t, CD1a, and TdT are considered to be negative markers. In this study, we initially evaluated the statistical differences between TC and TM using odds ratios. In addition, we analyzed the expression rates of each IHC marker with statistical significance between TC and TM. These results should be considered before applying diagnostic markers in daily practice. Diagnostic implications, regardless of the statistical significance, are limited when IHC expression rates are high in both compared subgroups. Therefore, our results showed that the estimated expression rates were useful for the selection of an IHC panel.
Kim et al. suggested that an IHC panel using EZH2, CD117, and CD205 was useful for differentiation between TC and TM type B3 [46]. In the previous study, EZH2 showed higher sensitivity (88.9%) and specificity (100%) in differentiating between TC and TM type B3 [46]. However, because the raw data for EZH2 immunohistochemistry were not shown, the present study could not analyze these results. Based on our results, CD205 exhibited significantly lower expression in TC than in TM type B3. However, the CD205 expression rates of TC and TM type B3 were 65.0% and 95.8%, respectively. This difference in expression rate was not useful for differentiation between two tumor groups; further, information regarding negative markers was not shown [46]. Some markers, such as beta-5t, CD1a, and TdT, showed higher expressions in TM type B3 than in TC, which may present negative marker candidates for TC. Therefore, an IHC panel using positive and negative markers could be useful for the diagnosis of TC and TM, and an IHC panel consisting of positive and negative markers for the diagnosis of an anterior mediastinal mass is recommended. This diagnostic test accuracy review was performed for IHC markers with significant differences in expression between TC and TM type B3. Positive markers included CD5, CD117, Glut-1, IGF-1R, MUC1 for TC, beta-5t, CD1a, CD205, and TdT for TM type B3. Markers with high sensitivity, specificity, and diagnostic odds ratios were considered to be more effective. Among the markers highly expressed in TC, Glut-1 showed the highest expression rate, 95.2%. However, in TM type B3, the expression rate of Glut-1 was 52.6% (95% CI 29.6–74.5%). In the diagnostic test accuracy review, Glut-1 showed the highest diagnostic odds ratio (46.251, 95% CI 11.634–183.877). MUC1 expression in TM type B3 ranged from 0% to 77.8% [13,21,36], indicating a limited diagnostic role of MUC1, as well as Glut-1. Based on these criteria, the important markers were shown to be CD5 and CD117 (positive) and TdT and beta-5t (negative) for TCs.
The direct invasion and metastasis of primary lung cancers in the anterior mediastinum can be detected [1]. Based on the histological findings, it is difficult to differentiate between lung and thymic squamous cell carcinomas. Among the markers expressed in TC, CD5, CD117, and CD205 are uncommonly expressed in primary lung cancers [8], thereby presenting these markers as possible candidates in the IHC panel of thymic tumors when differentiating primary lung cancers. According to our results, CD205 may be comparable in differentiation from tumors of lung origin because it is expressed in both TC and TM. In addition, CD205 is useful for defining thymic origin. Taken together, an IHC panel containing CD205 as a positive and a negative marker would be more effective.
This study has a limitation in that we performed the diagnostic test accuracy review for individual IHC markers, but it was difficult to conduct the diagnostic test accuracy review for a combination of markers due to limited eligible study information. Therefore, the recommended IHC panels could not clarify the diagnostic role of the differentiation of thymic masses.
5. Conclusions
In conclusion, our results showed that significant differences in IHC expression between TC and TM identified positive markers, including CD5, CD117, Glut-1, IGF-1R, and MUC1, and negative markers, including beta-5t, CD1a, CD205, and TdT against TC. An IHC panel including positive and negative markers, as well as CD205, could be useful to differentiate between thymic masses in daily practice.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4418/10/7/460/s1, Table S1. PRISMA Checklist.
Author Contributions
Conceptualization, J.-H.J.; methodology, J.-S.P.; software, J.-S.P.; data curation, N.-Y.K.; writing—original draft preparation, J.-H.J. and J.-S.P.; writing—review and editing, D.-W.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Travis W.D., Brambilla E., Burke A.P., Marx A., Nicholson A.G. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th ed. International Agency for Research on Cancer; Lyon, France: 2015. [DOI] [PubMed] [Google Scholar]
- 2.Wu Z., Xue S., Zheng B., Ye R., Xu G., Zhang S., Zeng T., Zheng W., Chen C. Expression and significance of c-kit and epithelial-mesenchymal transition (EMT) molecules in thymic epithelial tumors (TETs) J. Thorac. Dis. 2019;11:4602–4612. doi: 10.21037/jtd.2019.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly R.J., Petrini I., Rajan A., Wang Y., Giaccone G. Thymic malignancies: From clinical management to targeted therapies. J. Clin. Oncol. 2011;29:4820–4827. doi: 10.1200/JCO.2011.36.0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marx A., Strobel P., Badve S.S., Chalabreysse L., Chan J.K., Chen G., de Leval L., Detterbeck F., Girard N., Huang J., et al. ITMIG consensus statement on the use of the WHO histological classification of thymoma and thymic carcinoma: Refined definitions, histological criteria, and reporting. J. Thorac. Oncol. 2014;9:596–611. doi: 10.1097/JTO.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Y., Zhao H., Hu D., Fan L., Shi J., Fang W. Surgical treatment and prognosis of thymic squamous cell carcinoma: A retrospective analysis of 105 cases. Ann. Thorac. Surg. 2013;96:1019–1024. doi: 10.1016/j.athoracsur.2013.04.078. [DOI] [PubMed] [Google Scholar]
- 6.Okereke I.C., Kesler K.A., Freeman R.K., Rieger K.M., Birdas T.J., Ascioti A.J., Badve S., Nelson R.P., Loehrer P.J. Thymic carcinoma: Outcomes after surgical resection. Ann. Thorac. Surg. 2012;93:1668–1672. doi: 10.1016/j.athoracsur.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Rashid O.M., Cassano A.D., Takabe K. Thymic neoplasm: A rare disease with a complex clinical presentation. J. Thorac. Dis. 2013;5:173–183. doi: 10.3978/j.issn.2072-1439.2013.01.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakagawa K., Matsuno Y., Kunitoh H., Maeshima A., Asamura H., Tsuchiya R. Immunohistochemical KIT (CD117) expression in thymic epithelial tumors. Chest. 2005;128:140–144. doi: 10.1378/chest.128.1.140. [DOI] [PubMed] [Google Scholar]
- 9.Adam P., Hakroush S., Hofmann I., Reidenbach S., Marx A., Strobel P. Thymoma with loss of keratin expression (and giant cells): A potential diagnostic pitfall. Virchows Arch. 2014;465:313–320. doi: 10.1007/s00428-014-1606-6. [DOI] [PubMed] [Google Scholar]
- 10.Chen F.F., Yan J.J., Chang K.C., Lai W.W., Chen R.M., Jin Y.T. Immunohistochemical localization of Mcl-1 and bcl-2 proteins in thymic epithelial tumours. Histopathology. 1996;29:541–547. doi: 10.1046/j.1365-2559.1996.d01-540.x. [DOI] [PubMed] [Google Scholar]
- 11.Cui F., He J., Liu J., Chen Y. Protein expression status of p53 and epidermal growth factor receptor in thymoma. Oncol. Lett. 2011;2:459–463. doi: 10.3892/ol.2011.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorfman D.M., Shahsafaei A., Chan J.K. Thymic carcinomas, but not thymomas and carcinomas of other sites, show CD5 immunoreactivity. Am. J. Surg. Pathol. 1997;21:936–940. doi: 10.1097/00000478-199708000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Du M.J., Shen Q., Yin H., Rao Q., Zhou M.X. Diagnostic roles of MUC1 and GLUT1 in differentiating thymic carcinoma from type B3 thymoma. Pathol. Res. Pract. 2016;212:1048–1051. doi: 10.1016/j.prp.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Comprehensive genomic analysis reveals clinically relevant molecular distinctions between thymic carcinomas and thymomas. Clin. Cancer Res. 2009;15:6790–6799. doi: 10.1158/1078-0432.CCR-09-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girard N., Teruya-Feldstein J., Payabyab E.C., Riely G.J., Rusch V.W., Kris M.G., Zakowski M.F. Insulin-like growth factor-1 receptor expression in thymic malignancies. J. Thorac. Oncol. 2010;5:1439–1446. doi: 10.1097/JTO.0b013e3181e392a8. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi A., Fumon T., Miki Y., Sato H., Yoshino T., Takahashi K. The evaluation of immunohistochemical markers and thymic cortical microenvironmental cells in distinguishing thymic carcinoma from type b3 thymoma or lung squamous cell carcinoma. J. Clin. Exp. Hematop. 2013;53:9–19. doi: 10.3960/jslrt.53.9. [DOI] [PubMed] [Google Scholar]
- 17.Henley J.D., Koukoulis G.K., Loehrer P.J. Epidermal growth factor receptor expression in invasive thymoma. J. Cancer Res. Clin. Oncol. 2002;128:167–170. doi: 10.1007/s00432-001-0319-9. [DOI] [PubMed] [Google Scholar]
- 18.Hino N., Kondo K., Miyoshi T., Uyama T., Monden Y. High frequency of p53 protein expression in thymic carcinoma but not in thymoma. Br. J. Cancer. 1997;76:1361–1366. doi: 10.1038/bjc.1997.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirabayashi H., Fujii Y., Sakaguchi M., Tanaka H., Yoon H.E., Komoto Y., Inoue M., Miyoshi S. p16INK4, pRB, p53 and cyclin D1 expression and hypermethylation of CDKN2 gene in thymoma and thymic carcinoma. Int. J. Cancer. 1997;73:639–644. doi: 10.1002/(SICI)1097-0215(19971127)73:5<639::AID-IJC5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 20.Hiroshima K., Iyoda A., Toyozaki T., Supriatna Y., Shibuya K., Shimamura F., Haga Y., Yoshida S., Fujisawa T., Ohwada H. Proliferative activity and apoptosis in thymic epithelial neoplasms. Mod. Pathol. 2002;15:1326–1332. doi: 10.1097/01.MP.0000038463.67854.84. [DOI] [PubMed] [Google Scholar]
- 21.Kaira K., Murakami H., Serizawa M., Koh Y., Abe M., Ohde Y., Takahashi T., Kondo H., Nakajima T., Yamamoto N. MUC1 expression in thymic epithelial tumors: MUC1 may be useful marker as differential diagnosis between type B3 thymoma and thymic carcinoma. Virchows Arch. 2011;458:615–620. doi: 10.1007/s00428-011-1041-x. [DOI] [PubMed] [Google Scholar]
- 22.Khoury T., Chandrasekhar R., Wilding G., Tan D., Cheney R.T. Tumour eosinophilia combined with an immunohistochemistry panel is useful in the differentiation of type B3 thymoma from thymic carcinoma. Int. J. Exp. Pathol. 2011;92:87–96. doi: 10.1111/j.1365-2613.2010.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kornstein M.J., Rosai J. CD5 labeling of thymic carcinomas and other nonlymphoid neoplasms. Am. J. Clin. Pathol. 1998;109:722–726. doi: 10.1093/ajcp/109.6.722. [DOI] [PubMed] [Google Scholar]
- 24.Laury A.R., Perets R., Piao H., Krane J.F., Barletta J.A., French C., Chirieac L.R., Lis R., Loda M., Hornick J.L., et al. A comprehensive analysis of PAX8 expression in human epithelial tumors. Am. J. Surg. Pathol. 2011;35:816–826. doi: 10.1097/PAS.0b013e318216c112. [DOI] [PubMed] [Google Scholar]
- 25.Lee G.J., Lee H., Woo I.S., Kim T., An H.J., Choi H.J., Lee Y.S., Lee K.Y., Lee J., Kang J.H. High expression level of SOX2 is significantly associated with shorter survival in patients with thymic epithelial tumors. Lung Cancer. 2019;132:9–16. doi: 10.1016/j.lungcan.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 26.Mimae T., Tsuta K., Takahashi F., Yoshida A., Kondo T., Murakami Y., Okada M., Takeuchi M., Asamura H., Tsuda H. Steroid receptor expression in thymomas and thymic carcinomas. Cancer. 2011;117:4396–4405. doi: 10.1002/cncr.26061. [DOI] [PubMed] [Google Scholar]
- 27.Mimae T., Tsuta K., Kondo T., Nitta H., Grogan T.M., Okada M., Asamura H., Tsuda H. Protein expression and gene copy number changes of receptor tyrosine kinase in thymomas and thymic carcinomas. Ann. Oncol. 2012;23:3129–3137. doi: 10.1093/annonc/mds147. [DOI] [PubMed] [Google Scholar]
- 28.Nonaka D., Henley J.D., Chiriboga L., Yee H. Diagnostic utility of thymic epithelial markers CD205 (DEC205) and Foxn1 in thymic epithelial neoplasms. Am. J. Surg. Pathol. 2007;31:1038–1044. doi: 10.1097/PAS.0b013e31802b4917. [DOI] [PubMed] [Google Scholar]
- 29.Omatsu M., Kunimura T., Mikogami T., Hamatani S., Shiokawa A., Masunaga A., Kitami A., Suzuki T., Kadokura M., Morohoshi T. Immunohistochemical analysis of thymic carcinoma focusing on the possibility of molecular targeted and hormonal therapies. Gen. Thorac. Cardiovasc. Surg. 2012;60:803–810. doi: 10.1007/s11748-012-0160-x. [DOI] [PubMed] [Google Scholar]
- 30.Pan C.C., Chen P.C., Chou T.Y., Chiang H. Expression of calretinin and other mesothelioma-related markers in thymic carcinoma and thymoma. Hum. Pathol. 2003;34:1155–1162. doi: 10.1053/j.humpath.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Petrini I., Zucali P.A., Lee H.S., Pineda M.A., Meltzer P.S., Walter-Rodriguez B., Roncalli M., Santoro A., Wang Y., Giaccone G. Expression and mutational status of c-kit in thymic epithelial tumors. J. Thorac. Oncol. 2010;5:1447–1453. doi: 10.1097/JTO.0b013e3181e96e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Remon J., Abedallaa N., Taranchon-Clermont E., Bluthgen V., Lindsay C.R., Besse B., Thomas de Montpreville V. CD52, CD22, CD26, EG5 and IGF-1R expression in thymic malignancies. Lung Cancer. 2017;108:168–172. doi: 10.1016/j.lungcan.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 33.Rieker R.J., Joos S., Mechtersheimer G., Blaeker H., Schnabel P.A., Morresi-Hauf A., Hecker E., Thomas M., Dienemann H., Schirmacher P., et al. COX-2 upregulation in thymomas and thymic carcinomas. Int. J. Cancer. 2006;119:2063–2070. doi: 10.1002/ijc.22078. [DOI] [PubMed] [Google Scholar]
- 34.Song N., Chen G., Zhang P., Liu M., He W.X., Jiang G.N. Diagnostic and clinical significance of KIT(CD117) expression in thymic epithelial tumors in China. Asian Pac. J. Cancer Prev. 2012;13:2745–2748. doi: 10.7314/APJCP.2012.13.6.2745. [DOI] [PubMed] [Google Scholar]
- 35.Stefanaki K., Rontogianni D., Kouvidou C.H., Bolioti S., Delides G., Pantelidaki A., Sotsiou F., Kanavaros P. Expression of p53, mdm2, p21/waf1 and bcl-2 proteins in thymomas. Histopathology. 1997;30:549–555. doi: 10.1046/j.1365-2559.1997.5730805.x. [DOI] [PubMed] [Google Scholar]
- 36.Su X.Y., Wang W.Y., Li J.N., Liao D.Y., Wu W.L., Li G.D. Immunohistochemical differentiation between type B3 thymomas and thymic squamous cell carcinomas. Int. J. Clin. Exp. Pathol. 2015;8:5354–5362. [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki A., Hirokawa M., Takada N., Higuchi M., Tanaka A., Hayashi T., Kuma S., Miyauchi A. Utility of monoclonal PAX8 antibody for distinguishing intrathyroid thymic carcinoma from follicular cell-derived thyroid carcinoma. Endocr. J. 2018;65:1171–1175. doi: 10.1507/endocrj.EJ18-0282. [DOI] [PubMed] [Google Scholar]
- 38.Tateyama H., Eimoto T., Tada T., Hattori H., Murase T., Takino H. Immunoreactivity of a new CD5 antibody with normal epithelium and malignant tumors including thymic carcinoma. Am. J. Clin. Pathol. 1999;111:235–240. doi: 10.1093/ajcp/111.2.235. [DOI] [PubMed] [Google Scholar]
- 39.Thomas A., Chen Y., Berman A., Schrump D.S., Giaccone G., Pastan I., Venzon D.J., Liewehr D.J., Steinberg S.M., Miettinen M., et al. Expression of mesothelin in thymic carcinoma and its potential therapeutic significance. Lung Cancer. 2016;101:104–110. doi: 10.1016/j.lungcan.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas de Montpreville V., Quilhot P., Chalabreysse L., De Muret A., Hofman V., Lantuejoul S., Parrens M., Payan M.J., Rouquette I., Secq V., et al. Glut-1 intensity and pattern of expression in thymic epithelial tumors are predictive of WHO subtypes. Pathol. Res. Pract. 2015;211:996–1002. doi: 10.1016/j.prp.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 41.Tsuchida M., Umezu H., Hashimoto T., Shinohara H., Koike T., Hosaka Y., Eimoto T., Hayashi J.I. Absence of gene mutations in KIT-positive thymic epithelial tumors. Lung Cancer. 2008;62:321–325. doi: 10.1016/j.lungcan.2008.03.035. [DOI] [PubMed] [Google Scholar]
- 42.Weissferdt A., Moran C.A. Pax8 expression in thymic epithelial neoplasms: An immunohistochemical analysis. Am. J. Surg. Pathol. 2011;35:1305–1310. doi: 10.1097/PAS.0b013e3182260735. [DOI] [PubMed] [Google Scholar]
- 43.Yamada Y., Tomaru U., Ishizu A., Kiuchi T., Marukawa K., Matsuno Y., Kasahara M. Expression of proteasome subunit beta5t in thymic epithelial tumors. Am. J. Surg. Pathol. 2011;35:1296–1304. doi: 10.1097/PAS.0b013e3182237f5d. [DOI] [PubMed] [Google Scholar]
- 44.Zucali P.A., Petrini I., Lorenzi E., Merino M., Cao L., Di Tommaso L., Lee H.S., Incarbone M., Walter B.A., Simonelli M., et al. Insulin-like growth factor-1 receptor and phosphorylated AKT-serine 473 expression in 132 resected thymomas and thymic carcinomas. Cancer. 2010;116:4686–4695. doi: 10.1002/cncr.25367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kojika M., Ishii G., Yoshida J., Nishimura M., Hishida T., Ota S.J., Murata Y., Nagai K., Ochiai A. Immunohistochemical differential diagnosis between thymic carcinoma and type B3 thymoma: Diagnostic utility of hypoxic marker, GLUT-1, in thymic epithelial neoplasms. Mod. Pathol. 2009;22:1341–1350. doi: 10.1038/modpathol.2009.105. [DOI] [PubMed] [Google Scholar]
- 46.Kim B.S., Kim J.K., Kang C.H., Kim Y.T., Jung K.C., Won J.K. An immunohistochemical panel consisting of EZH2, C-KIT, and CD205 is useful for distinguishing thymic squamous cell carcinoma from type B3 thymoma. Pathol. Res. Pract. 2018;214:343–349. doi: 10.1016/j.prp.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 47.Berezowski K., Grimes M.M., Gal A., Kornstein M.J. CD5 immunoreactivity of epithelial cells in thymic carcinoma and CASTLE using paraffin-embedded tissue. Am. J. Clin. Pathol. 1996;106:483–486. doi: 10.1093/ajcp/106.4.483. [DOI] [PubMed] [Google Scholar]
- 48.Hishima T., Fukayama M., Fujisawa M., Hayashi Y., Arai K., Funata N., Koike M. CD5 expression in thymic carcinoma. Am. J. Pathol. 1994;145:268–275. [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas de Montpreville V., Ghigna M.R., Lacroix L., Besse B., Broet P., Dartevelle P., Fadel E., Dorfmuller P. Thymic carcinomas: Clinicopathologic study of 37 cases from a single institution. Virchows Arch. 2013;462:307–313. doi: 10.1007/s00428-013-1371-y. [DOI] [PubMed] [Google Scholar]
- 50.Detterbeck F.C., Asamura H., Crowley J., Falkson C., Giaccone G., Giroux D., Huang J., Kim J., Kondo K., Lucchi M., et al. The IASLC/ITMIG thymic malignancies staging project: Development of a stage classification for thymic malignancies. J. Thorac. Oncol. 2013;8:1467–1473. doi: 10.1097/JTO.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 51.Girard N., Ruffini E., Marx A., Faivre-Finn C., Peters S. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015;26(Suppl. 5):v40–v55. doi: 10.1093/annonc/mdv277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.