Abstract
p21-activated kinase 6 (PAK6), a member of the serine/threonine kinase family, has been reported to be involved in numerous types of cancers. The present study aimed to investigate the role of PAK6 in cervical cancer. In the present study, PAK6 expression was evaluated in tissue microarrays and cell lines by using immunohistochemistry and western blotting. The mRNA level of PAK6 was evaluated by reverse transcription quantitative PCR. The Wnt/β-catenin signaling-related protein expression was detected by western blotting following short hairpin (sh)RNA-mediated PAK6 knockdown or PAK6 overexpression. Cell proliferation was determined using Cell Countink Kit-8. Migration, invasion and colony formation were further assessed following PAK6 knockdown or overexpression. Co-immunoprecipitation (Co-IP) and fluorescence colocalization microscopy were used to detect the interaction between PAK6 and GSK3β. The results from tissue microarray revealed that the expression levels of PAK6 in cervical cancer tissues were upregulated. The downregulation of PAK6 expression levels using shRNA not only decreased cell growth and proliferation, but it also inhibited the migration and invasion of HeLa cells. Conversely, the overexpression of PAK6 promoted the proliferation, migration and invasion of HeLa cells. In addition, the expression levels of proteins involved in the Wnt/β-catenin signaling pathway were modified in the PAK6 knockdown group, including downregulation of GSK3β phosphorylation and Cyclin D1 protein, and upregulation of β-catenin phosphorylation and E-cadherin. In contrast, following the overexpression of PAK6, the Wnt/β-catenin signaling pathway was activated. Further investigation using fluorescence microscopy and Co-IP assays indicated that PAK6 may interact with GSK3β. In conclusion, the findings of the present study suggested that PAK6 may serve a role in promoting cervical cancer through activating the Wnt/β-catenin signaling pathway.
Keywords: cervical cancer, p21-activated kinase 6, Wnt/β-catenin signaling pathway, cell transformation
Introduction
Cervical cancer is the leading cause of cancer-associated mortality among women worldwide and the estimated age-standardized incidence of cervical cancer was 13.1 per 100,000 women (1). Several studies have suggested that both genetic and epigenetic changes may serve important roles in carcinogenesis (2,3); however, the development of targeted molecular therapy for cervical cancer is unsatisfactory. It is therefore crucial to improve the therapeutic strategies by determining the underlying mechanisms involved in cervical cancer.
The p21-activated kinase (PAK) family is a group of serine/threonine kinases, which contain a Cdc42/Rac-interactive binding domain and a Ste20-related kinase domain (4). Based on the sequence homology and regulatory properties, PAKs have been classified into two groups: Group I (PAK1-3) and Group II (PAK4-6) (5). Previous studies have revealed that the PAK family was involved in numerous cellular functions, such as cell motility, cell survival and gene regulation (6,7). The overexpression or amplification of PAKs has been detected in several types of human tumor, including breast cancer, neurofibromatosis, colon cancer and lung cancer (8,9), and it has been reported to be involved in several cancer signaling pathways, including the NF-κB, Ras, AKT, Raf and p53 pathways (10–13). Thus, the different biological roles of PAKs have positioned them as attractive cancer therapeutic targets.
PAK6, which was originally cloned as an androgen receptor-interacting protein, is a unique member of the PAK family (14,15). Although PAK6 expression levels were reported to be overexpressed in primary and metastatic prostate cancer (13), and hypothesized to be a useful biomarker of adenocarcinoma (16), the current knowledge of the role of PAK6 in the progression of other types of cancer remains insufficient, with very little known about its underlying mechanism. Thus, the present study aimed to determine the role of PAK6 in the oncogenesis of cervical cancer, one of the most common types of cancer to affect women, and to identify possible molecular mechanisms.
Materials and methods
Cell culture
The human cervical cancer cell lines, HeLa and C33A, and the 293T cell line were obtained from the China Center for Type Culture Collection. All cells were cultured in DMEM (HyClone; Cytiva), supplemented with 10% heat-inactivated FBS (Invitrogen; Thermo Fisher Scientific, Inc.), 100 IU/ml penicillin and 100 µg/ml streptomycin, and placed at 37°C in a humidified incubator containing 5% CO2.
Immunohistochemistry (IHC)
A total of 50 specimens with complete clinical information on the clinical metastatic status of cervical cancer were obtained from the CR501a tissue microarray (Alenabio). This microarray included 40 cases of squamous cell carcinoma, 2 cases of adenocarcinoma, 4 cases of adenosquamous carcinoma and 4 paracancerous tissues. Specimens were blocked using goat serum (1:10; cat. no. AR1009, Boster Biological Technology) for 20 min at room temperature, followed by incubation with PAK6 antibody (1:50; cat. no. 13539–1-AP; ProteinTech Group, Inc.) at 4°C overnight. Specimens were incubated with secondary antibody (goat anti-rabbit; 1:100; cat. no. S0001; Affinity Biosciences) at 37°C for 2 h. Signal was detected using DAB detection kit (cat. no. AR1022; Boster Biological Technology). The histological classifications and clinical staging were performed in accordance with the International Federation of Gynecology and Obstetrics (FIGO) classification system (17). PAK6 expression levels were subsequently determined using IHC. All tissue sections were examined qualitatively in five randomly selected fields by two separate researchers using an Olympus CX31 light microscope (magnification, ×40; Olympus Corporation). The scores were determined using an immunoreactivity score (18) (IRS; IRS=intensity score × extent score). The intensity score for PAK6 was determined as follows: i) 0, negative; ii) 1, weak; iii) 2, moderate; and iv) 3, strong. The extent scores were determined as follows: i) 0, <10%; ii) 1, 10–30%; iii) 2, 30–65%; and iv) 3, >65%, according to the percentage of positively stained cells. The IHC results for PAK6 were assigned to one of four categories according to the IRS: i) IRS of ≤1 was defined as (−); ii) 1<IRS ≤3 as (+); iii) 3<IRS ≤6 as (++); and iv) IRS>6 as (+++). The tissue microarray testing was approved by the Medical Ethics Committee of Huazhong University of Science and Technology (Wuhan, China) and all patients provided written, informed consent.
Vector construction and transfection
Short hairpin RNAs (shRNA/sh) targeting PAK6 (shPAK6) and the negative control (NC; scramble vector) were obtained from Shanghai GeneChem Co., Ltd. (Table I). The plasmid was linearized by restriction endonuclease double digestion with AgeI and EcoR1, and the shPAK6 (GCAGGCTATTCCGAAGCAT) or shPAK6 NC (TTCTCCGAACGTGTCACGT) sequences were inserted to construct lentiviral vectors.
Table I.
Sequences of p21-activated kinase 6 shRNA with AgeI and EcoR1 sites.
| shRNA | Sequence (5′→3′) |
|---|---|
| Top strand | 5′–GAATTCGCAGGCTATTCCGAAGCATTTCAAGAGAATGCTTCGGAATAGCCTGCTTTTTT-3′ |
| Bottom strand | 3′-CGTCCGATAAGGCTTCGTAAAGTTCTCTTACGAAGCCTTATCGGACGAAAAAAGAGCTC−5′ |
shRNA, short hairpin RNA; AgeI + Sense + loop + Antisense + termination signal.
To overexpress PAK6, a recombinant lentiviral vector containing the full-length cDNA of PAK6 was used, and empty vector was used as negative control. Briefly, the primer sequences were designed and synthesized by Shanghai GeneChem Co., Ltd. (Table II). The PAK6 fragment, amplified by reverse transcription-quantitative PCR using mRNA extracted from HeLa cells, was subsequently cloned into the EcoR1 and XhoI sites of the linearized pSico-eGFP-Flag plasmid (FenghuiShengwu) to generate the recombinant lentiviral vector.
Table II.
Primers for amplifying the full length of p21-activated kinase 6 cDNA.
| Primer | Sequence (5′→3′) |
|---|---|
| Forward | 5′-GGCCTCGAGATGTTCCGCAAGAAAAAGA-3′ (XhoI) |
| Reverse | 5′-CTGGAATTCCGCAGGTGGAGGTCTGCTTT-3′ (EcoR1) |
The lentiviral vectors were co-transfected with the three packaging plasmids (pRSV-Rev, pMD2.G and pCMV–VSV-G, Hedgehog Bio Science and Technology Ltd.) into 293T cells (1×106) using Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) to produce the lentiviral particles. The ratio of lentiviral vectors was as follows: pRSV-Rev: pMD2.G: pCMV–VSV-G was 2:1:1. For a 60 mm dish, 3, 1.5, and 1.5 µg of those plasmids was used individually. Following incubation at 37°C for 72 h, the lentiviruses were collected by centrifugation (1,200 × g, 4°C, 5 min) and used to infect the HeLa cells (1×106), which were sub-cultured (with 3 µg/ml puromycin) for four generations to obtain the stably transfected cell line. The changes in the expression levels of PAK6, phosphorylated (p)-β-Catenin, β-Catenin, glycogen synthase kinase (GSK)3β, p-GSK3β, cyclin D1 and E-cadherin in the stably transfected cells were analyzed using western blotting.
In addition, pDsRed-N1 and pEGFP-N1 plasmids (Shanghai Beinuo Biotech, Co., Ltd.) were introduced to construct PAK6/pDsRed-N1 and GSK3β/pEGFP-N1 recombinants, based on the designed primers (Table III) following the steps described above. For a 6-well culture plate, 4 µg plasmid was co-transfected into 293T cells (2×105 cells/well) for 48 h using Lipofectamine® 2000 reagent. Transfected cells were used for fluorescence colocalization microscopy analysis.
Table III.
Primers for amplifying PAK6 and GSK3β.
| Gene | Primer sequence (5′→3′) |
|---|---|
| PAK6 | F: GGCCTCGAGATGTTCCGCAAGAAAAAGA (XhoI) |
| R: CTGGAATTCCGCAGGTGGAGGTCTGCTTT (EcoRI) | |
| GSK3β | F: AGACTCGAGATGTCAGGGCGGCCCAGAA (XhoI) |
| R: GACGAATTCCGGTGGAGTTGGAAGCTGATG (EcoRI) |
PAK6, p21-activated kinase 6; GSK3β, glycogen synthase kinase 3β; F, forward; R, reverse.
Reverse transcription-quantitative PCR (RT-qPCR)
The mRNA level of PAK6 was evaluated by RT-qPCR. Total RNA was extracted by TRIzol Reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and reversed transcribed into single-strand cDNA using Superscript II reverse transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.). The cDNA amplification was performed using SYBR Premix Ex Tap (Tli RNaseH Plus; Takara) on the BIO-RAD CFX96 system. The following thermocycling conditions were used: Initial denaturation at 95°C for 3 min, followed by 40 cycles of 95°C for 15 sec and 95°C for 1 min, finally dissociation at 95°C for 10 min. β-actin was used as an internal control. Fold-changes were calculated using the relative quantification (2−ΔΔCq) method (19). The primers (Shanghai GeneChem Co., Ltd.) used are as follows: PAK6, forward 5′-GACTCCATCCTGCTGACCCTC-3′; reverse 5′-CACCTCAGTGGCATACAAAGACC-3′; β-actin, forward 5′-ACCAGTTCGCCATGGATGAC-3′; reverse 5′-TGCCGGAGCCGTTGTC-3′.
Western blotting
Total protein was extracted from cells using a lysis buffer (150 mM NaCl; 50 mM Tris-HCl, pH 7.4; 2 mM EDTA; 1% NP-40; 0.1% SDS), containing a protease inhibitor cocktail (cat. no. ab65621; Abcam). Total protein was quantified using BCA (Beyotime Institute of Biotechnology), and proteins (20 µg) were separated by 12% SDS-PAGE. The separated proteins were subsequently transferred onto a PVDF membrane and blocked in 5% skim milk at room temperature for 1 h. The membranes were then incubated at 4°C overnight with the following primary antibodies: PAK6 (1:1,000; cat. no. 13539-1-AP; ProteinTech Group Inc.), β-catenin (1:1,000; cat. no. 51067-2-AP; ProteinTech Group Inc.), cyclinD1 (1:1,000; cat. no. 60186-1-Ig; ProteinTech Group Inc.), E-cadherin (1:1,000; cat. no. 20874-1-AP; ProteinTech Group Inc.) and GAPDH (1:10,000; cat. no. 10494-1-AP; ProteinTech Group Inc.); GSK3β (1:1,000; cat. no. AF7814; Affinity Biosciences), p-GSK3β (1:1,000; cat. no. AF2016; Affinity Biosciences), p-β-catenin (1:2,000; cat. no. DF2989; Affinity Biosciences). Following the primary antibody incubation, the membranes were incubated with a horseradish peroxidase-conjugated secondary antibodies (anti-mouse and anti-rabbit; cat. nos. SA00001-1 and SA00001-2, respectively; 1:10,000; ProteinTech Group, Inc.) for 1 h at room temperature. Bands were detected using enhanced chemiluminescence substrate (Tanon Science and Technology Co., Ltd.). The expression levels were semi-quantified using ImageJ 1.51K software (National Institutes of Health).
Colony formation assay
A total of 200 stably transfected HeLa cells were incubated in 5 ml DMEM (HyClone; Cytiva) supplemented with 10% FBS (Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 mg/ml streptomycin in a 6-cm dish at 37°C for 1 week. Once visible to the naked eye, colonies were fixed with 4% paraformaldehyde for 30 min at room temperature and stained with 0.1% crystal violet at room temperature for 15 min. The colonies were then visualized using an inverted microscope and counted. Data were analyzed using Image J 1.51 software.
Cell proliferation assay
A Cell Counting Kit-8 (CCK-8) assay (Beijing Solarbio Science & Technology Co., Ltd.) was used to analyze the cell proliferation, according to the manufacturer's protocol. Briefly, 5×103 stably transfected HeLa cells/well were seeded into 96-well plates. Following 0, 24, 48, 72 and 96 h of incubation at 37°C, 10 µl CCK-8 solution was added/well at 37°C for 2 h. The absorbance of each well was measured at a wavelength of 450 nm to determine cell proliferation.
Cell migration and invasion assays
The stably transfected HeLa cells (1×105) were plated into a 24-well Transwell insert with DMEM containing 0.1% FBS (pore size, 8.0-µm), which was pre-coated with Matrigel (Corning Inc.) at 37°C for 5 h for the invasion assay. A volume of 500 µl DMEM supplemented with 10% FBS was plated in the lower chambers. Following incubation at 37°C for 48 h, the non-migratory/invasive cells remaining on the top surface of the membrane were removed by scraping, and the migratory/invasive cells in the lower chamber were fixed with 4% paraformaldehyde at room temperature for 30 min and stained with 0.1% crystal violet at room temperature. The stained cells were counted in five randomly selected visual fields using a light microscope (magnification, ×20).
Co-immunoprecipitation (Co-IP)
Cells were lysed in RIPA lysis buffer (Beyotime Institute of Biotechnology), containing protease inhibitors (Roche Diagnostics) for 30 min. Following centrifugation at 13,000 × g for 15 min 4°C, the 1/10 volume of supernatant was collected as input, and half of the remaining supernatant was incubated with 20 µl/ml protein A/G sepharose beads (Beyotime Institute of Biotechnology) at 4°C for 1 h to remove non-specific hybrid proteins. Following centrifugation (12,000 × g; 4°C; 5 min), the lysates were incubated with 2 µg anti-PAK6 rabbit polyclonal antibody (cat. no. 13539-1-AP; ProteinTech Group, Inc.) or negative control rabbit IgG (cat. no. A7016; Beyotime Institute of Biotechnology) at 4°C overnight and then rotated at 4°C with a mixture of protein A/G sepharose beads (20 µl/ml) for 4 h. The beads were then washed 3 times with RIPA buffer, and the bound proteins were boiled in 2X Laemmli buffer and further analyzed using western blotting.
Fluorescence colocalization microscopy analysis
The 293T cells transfected with PAK6/pDsRed-N1 and GSK3β/pEGFP-N1 recombinants were cultured at 37°C for 48 h, and then washed several times with PBS. Images of PAK6/pDsRed-N1 and GSK3β/pEGFP-N1 positive cells were captured using Olympus FV1000-IX81 microscope (FluoView 1000-IX81; Olympus Corporation).
Statistical analysis
Data analysis was performed using GraphPad Prism 6.0 software (GraphPad Software, Inc.) and SPSS version 13 software (SPSS, Inc.). The data are presented as the mean ± SD. A one-way ANOVA was used to determine the statistical differences between the groups presented in all figures. The data presented in Tables IV and V were analyzed using a Chi-squared test. Each experiment was performed three times. P<0.05 was considered to indicate a statistically significant difference.
Table IV.
Expression levels of PAK6 in cervical carcinoma and paracarcinoma tissues.
| PAK6 expression levels (n) | ||||||
|---|---|---|---|---|---|---|
| Histologic type | Cases (n) | (−) | (+/++) | (+++) | Positive rate (%) | P-value |
| Cervical cancer | 46 | 8 | 27 | 11 | 82.61 | <0.01 |
| Paracarcinoma tissue | 4 | 4 | 0 | 0 | 0.00 | |
PAK6, p21-activated kinase 6.
Table V.
Association between PAK6 expression levels and clinicopathological parameters in cervical cancer.
| PAK6 expression levels (n) | ||||||
|---|---|---|---|---|---|---|
| Variable | Cases (n) | (−) | (+/++) | (+++) | Positive rate (%) | P-value |
| Age | >0.05 | |||||
| <45 | 21 | 3 | 13 | 5 | 85.71 | |
| ≥45 | 25 | 5 | 14 | 6 | 80.00 | |
| Pathological type | >0.05 | |||||
| Adenocarcinoma | 2 | 1 | 1 | 0 | 50.00 | |
| Adenosquamous carcinoma | 4 | 1 | 2 | 1 | 75.00 | |
| Squamous cell carcinoma | 40 | 6 | 24 | 10 | 85.00 | |
| International federation of gynecology and obstetrics stage | >0.05 | |||||
| I | 35 | 7 | 26 | 2 | 80.00 | |
| II | 8 | 1 | 1 | 6 | 87.50 | |
| III + IV | 3 | 0 | 0 | 3 | 100.00 | |
| Grade | <0.05 | |||||
| 1 | 6 | 4 | 2 | 0 | 33.33 | |
| 2 | 12 | 2 | 8 | 2 | 83.33 | |
| 3 | 22 | 0 | 14 | 8 | 100.00 | |
PAK6, p21-activated kinase 6.
Results
PAK6 expression levels are upregulated in cervical cancer tissues and in C33A and HeLa cells
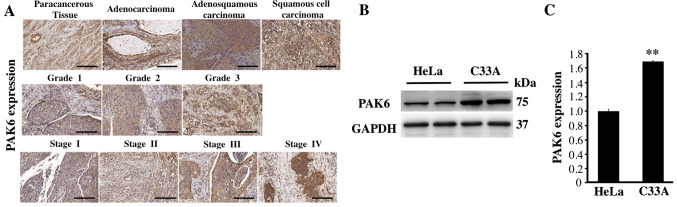
The expression levels of PAK6 were analyzed using IHC and a commercial tissue microarray to investigate the function of PAK6 in the development and progression of cervical carcinoma. PAK6 staining was observed in both the cytoplasm and nuclei of the positive cells in all of the different cervical tissue types, but primarily in the cytoplasm (Fig. 1A). The positive rate of PAK6 expression in 46 cases of cervical cancer was 82.61%, which was significantly increased compared with the expression levels observed in the 4 paracancerous tissues (P<0.01; Fig. 1A; Table IV). The positive rates of PAK6 in cervical adenocarcinoma, adenosquamous carcinoma and squamous cell carcinoma were 50.00, 75.00 and 85.00%, respectively, with no significant differences observed between the three groups (P>0.05; Table V). In addition, the positive rates of PAK6 expression in stages I, II and III + IV of cervical cancer (according to the clinical classification of FIGO staging) were 80.00, 87.50 and 100.00%, respectively. Finally, the positive rates of PAK6 expression in grade 1, 2 and 3 of cervical cancer were 33.33, 83.33 and 100.00%, respectively (Fig. 1A; Table V). The expression levels of PAK6 were also analyzed in both C33A and HeLa cells; significantly upregulated expression levels of PAK6 were identified in the C33A cells compared with the HeLa cells (P<0.01; Fig. 1B and C). These results suggested that PAK6 may serve an important role in cervical cancer.
Figure 1.
PAK6 expression in cervical cancer tissues and in C33A and HeLa cells. (A) Immunohistochemistry was used to analyze the expression levels of PAK6 in cervical cancer tissues. Scale bars, 200 µm. (B) Expression levels of PAK6 in C33A and HeLa cells were analyzed using western blotting. (C) Semi-quantification of the PAK6 protein expression levels presented in part (B) using ImageJ software. **P<0.01 vs. HeLa cells. PAK6, p21-activated kinase 6.
Inhibition of PAK6 attenuates the proliferation, migration and invasion of HeLa cells
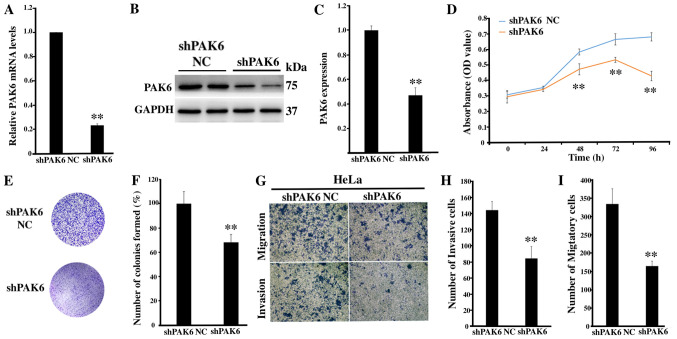
To determine the role of PAK6 in cervical carcinogenesis, lentiviral-mediated RNA interference targeting PAK6 was used to investigate any biological changes in the HeLa cell line. The transfection efficiency of shPAK6 in HeLa cells was subsequently determined; the mRNA and protein expression levels of PAK6 were significantly downregulated in the shPAK6-transfected cells compared with the shPAK6 NC group (both P<0.01; Fig. 2A-C). Notably, the proliferation rate (P<0.01; Fig. 2D) and the number of cell colonies formed (P<0.01; Fig. 2E and F) were significantly reduced in the shPAK6-transfected cells compared with the shPAK6 NC-transfected cells. In addition, the invasive and migratory abilities of HeLa cells following PAK6 knockdown were investigated. The cells transfected with shPAK6 were revealed to have a significantly reduced invasive and migratory capacity compared with the cells transfected with the shPAK6 NC (Fig. 2G-I). These results suggested that inhibiting PAK6 expression levels may affect a number of hallmarks of cancer, including the cell proliferative, migratory and invasive abilities of HeLa cells.
Figure 2.
Effect of the knockdown of PAK6 expression levels on the proliferation, migration and invasion of HeLa cells. (A) PAK6 mRNA expression levels were analyzed in stably shPAK6-transfected HeLa cells. (B) PAK6 protein expression levels were analyzed in stably shPAK6-transfected HeLa cells using western blotting. (C) Semi-quantification of PAK6 expression levels presented in part (B). (D) Cell Counting Kit-8 assays and (E) colony formation assays were used to analyze the proliferative rate of shPAK6-transfected HeLa cells. (F) Semi-quantification of the number of colonies formed from part (E). (G) Cell migration and invasion were determined in stably shPAK6-transfected HeLa cells, (magnification ×200). (H) Semi-quantification of the number of invasive cells from part (G). (I) Semi-quantification of the number of migratory cells from part (G). **P<0.01 vs. shPAK6 NC. PAK6, p21-activated kinase 6; sh, short hairpin RNA; NC, negative control.
Overexpression of PAK6 promotes the proliferation, migration and invasion of HeLa cells
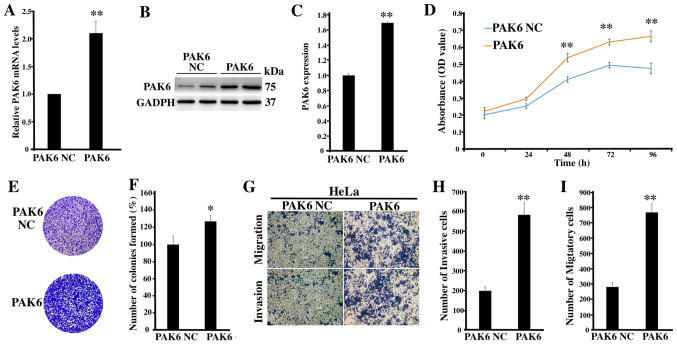
To further determine the role of PAK6 in cervical cancer, PAK6 was overexpressed to investigate the biological changes of HeLa cells, of which the transfection was identified as being successful (P<0.01; Fig. 3A-C). The proliferative rate (P<0.01; Fig. 3D) and the number of cell colonies formed (P<0.05; Fig. 3E and F) in the PAK6 overexpression group were significantly increased compared with the PAK6 NC group. Moreover, according to the results of the Transwell and Matrigel assays, the number of invasive and migratory cells in the PAK6 overexpression group were significantly increased compared with the PAK6 NC group (both P<0.01; Fig. 3G-I). Collectively, these findings suggested that PAK6 overexpression may promote the cell proliferative, migratory and invasive abilities of HeLa cells.
Figure 3.
Effects of the overexpression of PAK6 on the proliferation, migration and invasion of HeLa cells. (A) PAK6 mRNA expression levels in stable PAK6 overexpressing HeLa cells were analyzed. (B) PAK6 protein expression levels were analyzed in stable PAK6 overexpressing HeLa cells using western blotting. (C) Semi-quantification of PAK6 expression levels presented in part (B). (D) Cell Counting Kit-8 assays and (E) colony formation assays were used to analyze the proliferative rate of stable PAK6 overexpressing HeLa cells. (F) Semi-quantification of the number of colonies formed from part (E). (G) Cell migration and invasion were determined in stable overexpressing PAK6 HeLa cells, (magnification ×200). (H) Semi-quantification of the number of invasive cells from part (G). (I) Semi-quantification of the migratory cell number from part (G). *P<0.05, **P<0.01 vs. PAK6 NC. PAK6, p21-activated kinase 6; NC, negative control.
Effect of PAK6 knockdown or overexpression on the Wnt/β-catenin signaling pathway in HeLa cells
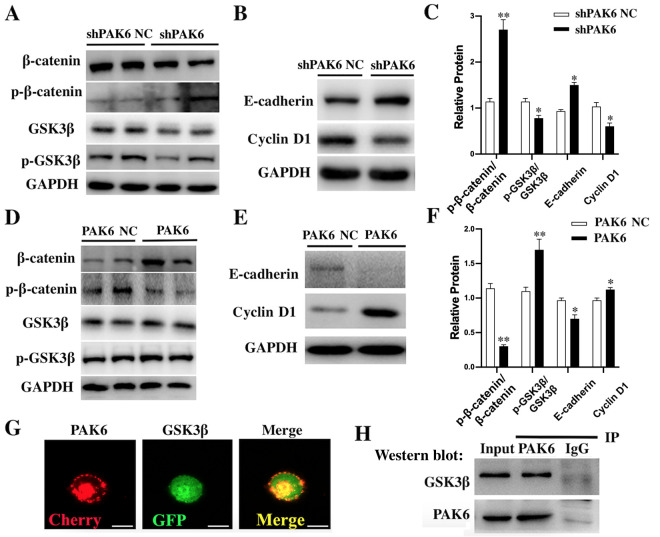
According to the western blotting results, the ratio p-GSK3β/GSK3β and the downstream Cyclin D1 proteins were significantly downregulated in shPAK6-transfected HeLa cells compared with the shPAK6 NC-transfected cells (all P<0.05; Fig. 4A-C). However, the ratio p-β-catenin/β-catenin (P<0.01) and E-cadherin (P<0.05) were significantly upregulated in PAK6 knockdown cells compared with the shPAK6 NC group (Fig. 4A-C). In contrast, the ratio p-GSK3β/GSK3β (P<0.01) and the expression of Cyclin D1 (P<0.05) in stable PAK6 overexpressing HeLa cells were all significantly upregulated (Fig. 4D-F), while the ratio p-β-catenin/β-catenin (P<0.01) and E-cadherin levels (P<0.05) were significantly downregulated, compared with the PAK6 NC group (Fig. 4D-F). Taken together, these findings suggested that PAK6 may have a promoting role in the progression of cervical cancer by activating the Wnt/β-catenin signaling pathway.
Figure 4.
Effect of PAK6 knockdown or overexpression on the Wnt/β-catenin signaling pathway in HeLa cells. Western blotting was used to analyze the expression levels of (A) β-catenin, p-β-catenin, GSK3β and p-GSK3β, and (B) E-cadherin and Cyclin D1 in stably shPAK6-transfected HeLa cells. (C) Semi-quantification of the expression levels of proteins in parts (A) and (B). *P<0.05, **P<0.01 vs. shPAK6 NC. Western blotting was used to analyze the expression levels of (D) β-catenin, p-β-catenin, GSK3β and p-GSK3β, and (E) E-cadherin and cyclin D1 in stable PAK6 overexpressing HeLa cells. (F) Semi-quantification of the expression levels of proteins in parts (D) and (E). *P<0.05, **P<0.01 vs. PAK6 NC. (G) Immunofluorescence was used to demonstrate the co-localization of PAK6 and GSK3β. Scale bars, 10 µm. (H) Co-IP was used to analyze the interaction between PAK6 and GSK3β. PAK6, p21-activated kinase 6; sh, short hairpin RNA; NC, negative control; p-, phosphorylated; GSK3β, glycogen synthase kinase 3β; IP, immunoprecipitation.
To investigate the mechanism underlying the effects of PAK6 on the biological characteristics of cervical cancer cells, the interaction between PAK6 and GSK3β was investigated using fluorescence microscopy and Co-IP. PAK6 and GSK3β were observed to be highly expressed in the cell membrane, cytoplasm and nucleus, indicated by the red and green fluorescence, respectively (Fig. 4G). In the merged slice, the markedly high levels of yellow fluorescence suggested that PAK6 and GSK3β were co-localized in the cells. The results of the Co-IP further confirmed their interaction; following PAK6 immunoprecipitation, a positive band was detected after probing for the anti-GSK3β antibody (Fig. 4H).
Discussion
Manser et al (20) first identified PAKs as molecules that interacted with small Rho-like G proteins in 1994. PAKs have been discovered to serve important roles in numerous cellular biological processes, including cell cycle regulation, cell polarity, cytoskeletal reorganization, gene transcription and translation (21). More importantly, PAKs were reported to be overexpressed in numerous types of human malignancy, such as colon and breast cancers (22–24). The present study discovered that the expression levels of PAK6 were upregulated in cervical cancer tissues, and that the downregulation of PAK6 expression levels by shRNA decreased cell growth and proliferation, and inhibited the migratory and invasive abilities of HeLa cells. Also, the expression levels of proteins related to the Wnt/β-catenin signaling pathway, including β-catenin, p-GSK3β and cyclin D1, were all downregulated following PAK6 knockdown. In contrast, following the overexpression of PAK6, the expression levels of β-catenin, p-GSK3β and cyclin D1 were all upregulated. Further analysis by fluorescence microscopy and Co-IP suggested that PAK6 may interact with GSK3β. Thus, these results indicated that PAK6 may serve a role in promoting cervical cancer by activating the Wnt/β-catenin signaling pathway.
Although the six PAKs in mammals are divided into two groups, each member in Group 1 (PAK1-3) shares >90% homology in its kinase domain, while Group 2 members (PAK4-6) share 50% homology in the kinase domain, suggesting that there may be some overlapping functions between PAKs (25). However, PAKs also present significant differences in their tissue distribution and subcellular localization, which may partly explain the organ-specific effects of these molecules (26). A previous study reported that transfected PAK6 was primarily localized in the cytoplasm and on the plasma membrane in HeLa cells; however, the presence of lower levels of nuclear PAK6 could not be ruled out, indicating that PAK6 may be mostly cytoplasmic, but a small fraction might be nuclear (27). Therefore, specific antibodies should be used to evaluate the cellular and tissue distribution of the endogenous PAK6 protein in future studies. In addition, IHC analysis demonstrated that the expression levels of PAK6 in cervical cancer tissues were not only significantly upregulated compared with the paracancerous tissues, but were also associated with the FIGO stage and degree of malignancy of the cancers, suggesting that PAK6 expression level may be increased at a higher grade and that PAK6 may be involved in the occurrence and development of cervical cancer and may be a potential therapeutic target.
Numerous studies have revealed that PAK4 and PAK5 serve crucial roles in cell processes involved in tumorigenesis and tumor development, including anchorage-independent growth, apoptosis and survival, migration and invasion. During mid-cell division, spindle positioning and anchoring have been discovered to require the involvement of PAK4 (28). In addition, PAK4 attenuated the stability of the p57Kip2 protein through the ubiquitin-proteasome pathway, resulting in the increased proliferation of breast cancer cells (29). PAK4 suppression downregulated the expression levels of cyclin A1, D1 and E1, and upregulated the expression levels of p27 and p21, leading to G1 arrest in pancreatic cancer cells (30). Moreover, PAK4 knockdown reduced the activation of several pro-survival pathways, including the NF-κB, ERK and JNK signaling pathways (31,32). Similar to PAK4, PAK5 knockdown delayed the G0/G1 phase of the human gastric cancer, liver cancer and glioma cell cycle, thereby inhibiting cell proliferation (33). Furthermore, PAK5 was reported to promote the migration and invasion of glioma and breast cancer cells via the PAK5/early growth response protein1/matrix metalloproteinase 2 signaling pathway (34). However, compared with PAK4 and PAK5, the role of PAK6 in the development of cancer remains unclear. To investigate the functional role of PAK6 in the occurrence and development of cervical cancer, the present study used knockdown and overexpression experiments. Following the knockdown of the PAK6 gene in HeLa cells, the number of cell colonies formed was significantly reduced compared with the control group, in addition to the proliferation rate. The migratory and invasive abilities were also significantly decreased. In contrast, following the overexpression of PAK6 in HeLa cells, the number of cell colonies formed were significantly increased compared with the PAK6 NC group and the proliferation rate was increased. In addition, the migratory and invasive abilities were significantly increased. These results indicated that PAK6 may have the ability to promote cell growth, proliferation, migration and invasion in the onset and development of cervical cancer.
Previous studies have revealed that the Wnt/β-catenin signaling pathway, which is essential for the maintenance of cervical cancer cells and epithelial-mesenchymal transition-associated stem cell-like features, was prominently involved in the development and invasion of cervical cancer (35,36). In the present study, the expression levels of PAK6 protein were closely related to Wnt-related proteins. PAK6 knockdown significantly downregulated the ratio p-GSK3β/GSK3β and the downstream Cyclin D1 proteins in HeLa cells, while the ratio p-β-catenin/β-catenin and E-cadherin were significantly upregulated, indicating that the Wnt/β-catenin signaling pathway may be inhibited. Moreover, following the overexpression of PAK6, the ratio p-GSK3β/GSK3β and the expression of Cyclin D1 were significantly upregulated in HeLa cells, while the ratio p-β-catenin/β-catenin and E-cadherin levels were downregulated, suggesting that the Wnt/β-catenin signaling pathway may be activated.
Further investigations using immunofluorescence and Co-IP identified that PAK6 may interact with GSK3β, which may be the trigger that activates the Wnt/β-catenin signaling pathway. GSK3β is considered to be an important molecule in the canonical Wnt signaling pathway, negatively regulating the phosphorylation and degradation of β-catenin (37). In the present study, following the knockdown of PAK6, the expression levels of p-GSK3β and β-catenin were discovered to be significantly downregulated in HeLa cells. In contrast, the overexpression of PAK6 resulted in the upregulated expression levels of β-catenin and p-GSK3β in HeLa cells, activating the Wnt signaling pathway in the cytoplasm. These results indicated that GSK3β regulated β-catenin positively, instead of negatively, in the Wnt signaling pathway in HeLa cells. A previous study reported that GSK3β served a positive regulatory role in Wnt signaling, by phosphorylating the LDL-receptor related protein 6, which is the co-receptor of the Wnt protein (38). In the current study, the results of Co-IP results revealed that PAK6 and GSK3β were expressed in the cell membrane, cytoplasm and nucleus, and were co-localized in the cell, providing a spatial basis for the interaction between the two proteins. It is therefore hypothesized that PAK6 may serve as a Wnt signal to interact with GSK3β on the cell or nuclear membrane in HeLa cells, thereby affecting the entry and exit of β-catenin into the nucleus, and positively regulating β-catenin in the Wnt signaling pathway. However, the mechanism underlying the interactions between PAK6 and GSK3β remain unclear and further study is required.
In conclusion, the findings of the present study indicated that PAK6 may promote the growth, proliferation, migration and invasion of HeLa cells through the Wnt/β-catenin signaling pathway. The functional and mechanistic investigations suggested that the interactions between PAK6 and GSK3β, which may positively regulate β-catenin, may be the trigger for the activation of the Wnt/β-catenin signaling pathway in the pathogenesis of cervical cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
QY, GLL and GW designed the experiments; QY, YCZ, YSC and AH performed the experiments; QY, GW, TX and YC analyzed the data; and QY, GLL and GW prepared and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Medical Ethics Committee of Huazhong University of Science and Technology (Wuhan, China) and all patients provided written, informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, Bray F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob Health. 2020;8:e191–e203. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitham HK, Hawes SE, Chu H, Oakes JM, Lifson AR, Kiviat NB, Sow PS, Gottlieb GS, Ba S, Sy MP, Kulasingam SL. A comparison of the natural history of hpv infection and cervical abnormalities among HIV-positive and HIV-negative women in Senegal, Africa. Cancer Epidemiol Biomarkers Prev. 2017;26:886–894. doi: 10.1158/1055-9965.EPI-16-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomih A, Smith JS, North KE, Hudgens MG, Brewster WR, Huang Z, Skaar D, Valea F, Bentley RC, Vidal AC, et al. DNA methylation of imprinted gene control regions in the regression of low-grade cervical lesions. Int J Cancer. 2018;143:552–560. doi: 10.1002/ijc.31350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu X, Busby J, John C, Wei J, Yuan X, Lu ML. Direct interaction between AR and PAK6 in androgen-stimulated PAK6 activation. PLoS One. 2013;8:e77367. doi: 10.1371/journal.pone.0077367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang K, Baldwin GS, Nikfarjam M, He H. p21-activated kinase signalling in pancreatic cancer: New insights into tumour biology and immune modulation. World J Gastroenterol. 2018;24:3709–3723. doi: 10.3748/wjg.v24.i33.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow HY, Dong B, Valencia CA, Zeng CT, Koch JN, Prudnikova TY, Chernoff J. Group I Paks are essential for epithelial-mesenchymal transition in an Apc-driven model of colorectal cancer. Nat Commun. 2018;9:3473. doi: 10.1038/s41467-018-05935-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu H, Liu S, Zhang G, Bin Wu, Zhu Y, Frederick DT, Hu Y, Zhong W, Randell S, Sadek N, et al. PAK signalling drives acquired drug resistance to MAPK inhibitors in BRAF-mutant melanomas. Nature. 2017;550:133–136. doi: 10.1038/nature24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han K, Zhou Y, Tseng KF, Hu H, Li K, Wang Y, Gan Z, Lin S, Sun Y, Min D. PAK5 overexpression is associated with lung metastasis in osteosarcoma. Oncol Lett. 2018;15:2202–2210. doi: 10.3892/ol.2017.7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siekmann IK, Dierck K, Prall S, Klokow M, Strauss J, Buhs S, Wrzeszcz A, Bockmayr M, Beck F, Trochimiuk M, et al. Combined inhibition of receptor tyrosine and p21-activated kinases as a therapeutic strategy in childhood ALL. Blood Adv. 2018;2:2554–2567. doi: 10.1182/bloodadvances.2018020693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabra H, Brunner M, Mandati V, Wehrle-Haller B, Lallemand D, Ribba AS, Chevalier G, Guardiola P, Block MR, Bouvard D. β1 integrin-dependent Rac/group I PAK signaling mediates YAP activation of Yes-associated protein 1 (YAP1) via NF2/merlin. J Biol Chem. 2017;292:19179–19197. doi: 10.1074/jbc.M117.808063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raghavan S, Venkatraman G, Rayala SK. Cloning and functional characterization of human Pak1 promoter by steroid hormones. Gene. 2018;646:120–128. doi: 10.1016/j.gene.2017.12.039. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Si W, Liu X, He L, Ren J, Yang Z, Yang J, Li W, Liu S, Pei F, et al. JMJD6 promotes melanoma carcinogenesis through regulation of the alternative splicing of PAK1, a key MAPK signaling component. Mol Cancer. 2017;16:175. doi: 10.1186/s12943-017-0744-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rane CK, Minden A. P21 activated kinase signaling in cancer. Semin Cancer Biol. 2019;54:40–49. doi: 10.1016/j.semcancer.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Kumar R, Sanawar R, Li X, Li F. Structure, biochemistry, and biology of PAK kinases. Gene. 2017;605:20–31. doi: 10.1016/j.gene.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang M, Siedow M, Saia G, Chakravarti A. Inhibition of p21-activated kinase 6 (PAK6) increases radiosensitivity of prostate cancer cells. Prostate. 2010;70:807–816. doi: 10.1002/pros.21114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee EJ, McClelland M, Wang YP, Long F, Choi SH, Lee JH. Distinct DNA methylation profiles between adenocarcinoma and squamous cell carcinoma of human uterine cervix. Oncol Res. 2010;18:401–408. doi: 10.3727/096504010X12644422320744. [DOI] [PubMed] [Google Scholar]
- 17.Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri. Int J Gynaecol Obstet. 2018;143(Suppl 2):S2–S36. doi: 10.1002/ijgo.12611. [DOI] [PubMed] [Google Scholar]
- 18.Lee DW, Ryu HS, Jin MS, Lee KH, Suh KJ, Youk J, Kim JY, Min A, Lee HB, Moon HG, et al. Immune recurrence score using 7 immunoregulatory protein expressions can predict recurrence in stage I–III breast cancer patients. Br J Cancer. 2019;121:230–236. doi: 10.1038/s41416-019-0511-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 21.Zhao ZS, Manser E. PAK family kinases: Physiological roles and regulation. Cell Logist. 2012;2:59–68. doi: 10.4161/cl.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu G, Li X, Guo B, Ke Q, Dong M, Li F. PAK5-mediated E47 phosphorylation promotes epithelial-mesenchymal transition and metastasis of colon cancer. Oncogene. 2016;35:1943–1954. doi: 10.1038/onc.2015.259. [DOI] [PubMed] [Google Scholar]
- 23.Ramos-Alvarez I, Jensen RT. P21-activated kinase 4 in pancreatic acinar cells is activated by numerous gastrointestinal hormones/neurotransmitters and growth factors by novel signaling, and its activation stimulates secretory/growth cascades. Am J Physiol Gastrointest Liver Physiol. 2018;315:G302–G317. doi: 10.1152/ajpgi.00005.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dou Q, Chen HN, Wang K, Yuan K, Lei Y, Li K, Lan J, Chen Y, Huang Z, Xie N, et al. Ivermectin induces cytostatic autophagy by blocking the PAK1/Akt axis in breast cancer. Cancer Res. 2016;76:4457–4469. doi: 10.1158/0008-5472.CAN-15-2887. [DOI] [PubMed] [Google Scholar]
- 25.Aburatani T, Inokuchi M, Takagi Y, Ishikawa T, Okuno K, Gokita K, Tomii C, Tanioka T, Murase H, Otsuki S, et al. High expression of P21-activated kinase 5 protein is associated with poor survival in gastric cancer. Oncol Lett. 2017;14:404–410. doi: 10.3892/ol.2017.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang F, Li X, Sharma M, Zarnegar M, Lim B, Sun Z. Androgen receptor specifically interacts with a novel p21-activated kinase, PAK6. J Biol Chem. 2001;276:15345–15353. doi: 10.1074/jbc.M010311200. [DOI] [PubMed] [Google Scholar]
- 27.Lee SR, Ramos SM, Ko A, Masiello D, Swanson KD, Lu ML, Balk SP. AR and ER interaction with a p21-activated kinase (PAK6) Mol Endocrinol. 2002;16:85–99. doi: 10.1210/mend.16.1.0753. [DOI] [PubMed] [Google Scholar]
- 28.Bompard G, Rabeharivelo G, Cau J, Abrieu A, Delsert C, Morin N. P21-activated kinase 4 (PAK4) is required for metaphase spindle positioning and anchoring. Oncogene. 2013;32:910–919. doi: 10.1038/onc.2012.98. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Wang D, Zhang H, Wang C, Dai W, Cheng Z, Wang G, Li F. P21-activated kinase 4 regulates the cyclin-dependent kinase inhibitor p57(kip2) in human breast cancer. Anat Rec (Hoboken) 2013;296:1561–1567. doi: 10.1002/ar.22754. [DOI] [PubMed] [Google Scholar]
- 30.Tyagi N, Bhardwaj A, Singh AP, McClellan S, Carter JE, Singh S. p-21 activated kinase 4 promotes proliferation and survival of pancreatic cancer cells through AKT- and ERK-dependent activation of NF-κB pathway. Oncotarget. 2014;5:8778–8789. doi: 10.18632/oncotarget.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He W, Zhao Z, Anees A, Li Y, Ashraf U, Chen Z, Song Y, Chen H, Cao S, Ye J. p21-activated kinase 4 signaling promotes japanese encephalitis virus-mediated inflammation in astrocytes. Front Cell Infect Microbiol. 2017;7:271. doi: 10.3389/fcimb.2017.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu X, Feng J, Zeng D, Ding Y, Yu C, Yang B. PAK4 confers cisplatin resistance in gastric cancer cells via PI3K/Akt- and MEK/ERK-dependent pathways. Biosci Rep. 2014;34:e00094. doi: 10.1042/BSR20130102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang ZP, Jiang BG, Gu XF, Zhao B, Ge RL, Zhang FB. P21-activated kinase 5 plays essential roles in the proliferation and tumorigenicity of human hepatocellular carcinoma. Acta Pharmacol Sin. 2014;35:82–88. doi: 10.1038/aps.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han ZX, Wang XX, Zhang SN, Wu JX, Qian HY, Wen YY, Tian H, Pei DS, Zheng JN. Downregulation of PAK5 inhibits glioma cell migration and invasion potentially through the PAK5-Egr1-MMP2 signaling pathway. Brain Tumor Pathol. 2014;31:234–241. doi: 10.1007/s10014-013-0161-1. [DOI] [PubMed] [Google Scholar]
- 35.Sun X, Liu Y. Activation of the Wnt/β-catenin signaling pathway may contribute to cervical cancer pathogenesis via upregulation of twist. Oncol Lett. 2017;14:4841–4844. doi: 10.3892/ol.2017.6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang YH, Chu TY, Ding DC. WNT/β-Catenin signaling pathway regulates non-tumorigenesis of human embryonic stem cells co-cultured with human umbilical cord mesenchymal stem cells. Sci Rep. 2017;7:41913. doi: 10.1038/srep41913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mao XW, Xiao JQ, Li ZY, Zheng YC, Zhang N. Effects of microRNA-135a on the epithelial-mesenchymal transition, migration and invasion of bladder cancer cells by targeting GSK3β through the Wnt/β-catenin signaling pathway. Exp Mol Med. 2018;50:e429. doi: 10.1038/emm.2017.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taelman VF, Dobrowolski R, Plouhinec JL, Fuentealba LC, Vorwald PP, Gumper I, Sabatini DD, De Robertis EM. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell. 2010;143:1136–1148. doi: 10.1016/j.cell.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.