Abstract
The aim of the present study was to explore the value of shear wave elastography (SWE) in the differential diagnosis of cervical disease and to evaluate the infiltration of cervical cancer. A total of 40 inpatients with cervical cancer, 40 inpatients with cervical benign lesion and 40 healthy volunteers encountered between October 2014 and January 2017 were enrolled. All patients and volunteers underwent conventional ultrasound (US) and SWE examinations. The malignancy and the size (including long, tranverse and anteroposterior diameter) of the lesion were assessed on US. The elastic score, strain ratio, shear wave speed (SWS) and the size of lesions were determined on SWE. Infiltration of the uterus and vaginal vault were also evaluated on US and SWE. The SWS values of cervical cancers, cervical benign lesions and normal cervixes groups were compared. The results suggested that the optimal cut-off elasticity score for predicting cervical cancers was 3 points. The strain ratio between the cervical cancers and the cervical benign lesions exhibited a significant difference (P<0.01). The mean value of SWS for cervical cancers was significantly higher than that of cervical benign lesions and normal cervix (P<0.05). Regarding the lesion size and volume, SWE and pathological measurements were larger than those determined by US (P<0.05 for each). The lesion volume on SWE and pathological measurements exhibited no significant difference (P>0.05). Compared to the pathological diagnosis of focal infiltration of uterus and vaginal vault, the diagnostic accuracy of SWE was higher than that of US. In conclusion, SWE may be used to differentiate between cervical benign lesions and cervical cancers. The elastic score, strain ratio and SWS of cervical cancers were higher than those of cervical benign lesions. Furthermore, SWE is able to evaluate the infiltration of cervical cancer.
Keywords: ultrasound, shear wave elastography, diagnosis, infiltration, cervical cancer
Introduction
Cervical cancer is one of the most common malignant tumor types of the female reproductive system in China; it poses a global health burden and poses a serious threat to the health and lives of females. Cervical cancer is prone to invasion of adjacent tissues and migration (1–4).
Early diagnosis and staging of cervical cancer are the basis of treatment. The clinical stage of cervical cancer is dependent on the tumor size, vaginal or parametrial infiltration, rectal or bladder invasion and distant metastasis. In general, surgery is the major method for early-stage cervical cancer, while radiotherapy is the major method for intermediate- and late-stage cervical cancer. Therefore, pre-operative evaluation of cervical cancer is important.
The imaging methods for cervical cancer include ultrasound (US), CT and MRI. MRI has become the preferred non-invasive method for evaluating the infiltration of cervical cancer (5). However, compared with MRI examination that has a high cost and long duration, transvaginal US for cervical cancer has advantages including low cost and convenience (6–8). However, conventional US cannot provide any information on tissue stiffness.
Shear wave elastography (SWE) is a novel US technology that is able to quantitatively evaluate the stiffness of tissue. The stiffness changes with the variation in the pathology and is an important characteristic based on which cervical malignant and benign lesions may be distinguished. The evaluation methods of SWE include elasticity score, strain ratio and shear wave speed (SWS). Among these, SWS and the elastic strain ratio are quantitative parameters, while the elastic score is a semi-quantitative measure. SWE has been widely used in the diagnosis of breast and thyroid lesions (9,10). With its maturity progressing, its application in cervical lesions has increased (11–16). Since SWE is a supplement technology to US, it may be performed during B-mode US examination. The stiffness and infiltration of cervical lesions may be observed at the same time. SWE cannot only diagnose cervical cancer but also provide information on the presence of vaginal or parametrial infiltration. Thus, it may provide valuable information for clinical.
The present study aimed to qualitatively and quantitatively evaluate the diagnostic value of SWE for cervical cancer, and its ability to evaluate the local infiltration of cervical cancer.
Materials and methods
Study population
All of the patients were retrospectively enrolled in the present study from Linyi People's Hospital (Linyi, China) between October 2014 and January 2017. The patients with suspected cervical lesions underwent US and SWE examinations in a routine manner. The inclusion criteria for cervical lesions were as follows: i) Confirmed by histopathology; and ii) cervical primary disease. The exclusion criteria were as follows: i) Poor SWE quality; and ii) no histopathological results. Finally, 40 inpatients with cervical cancer and 40 inpatients with benign cervical lesions were enrolled. Another 40 healthy volunteers who came to the hospital for routine heath check-ups, without cervical diseases of the same age were selected.
US examination
The transvaginal US and SWE images were obtained with the Aixplorer US system (SuperSonic Imagine) and an SE12-3 endocavity transducer was selected (frequency, 3–12 MHz).
All patients had emptied their bladder prior to the examination and placed on the examination bed in the lithotomy position. A condom containing US gel was placed over the end-fire probe. The probe entered the vaginal vault slowly. The operator evaluated the structure of the cervix, uterus and ovary, focusing on the shape and echo of the cervix, and observed whether any lesions were present. The operator recorded the long, transverse and anteroposterior diameter, shape, echo, infiltration and involvement of the lesion. Color Doppler US was then performed.
The lesion was considered to be cervical cancer if it had the following features on US: Unclear boundary between the lesion and surrounding tissues, irregular shape, uterine effusion and abundant blood flow signals on color Doppler US. A circular lesion with regular shape, clear boundary and sparse blood flow signals was considered as a cervical benign lesion.
Evaluation of whether the uterus had been infiltrated on US were according to the following criteria: i) No uterus infiltration was indicated if the lesion did not exceed the cervix; ii) suspected uterus infiltration was present if the boundary between the lesion and the intrauterine orifice was not clear and iii) in cases with uterus infiltration, the lesion invaded upward across the cervical orifice (17).
Conversely, the following criteria was used to evaluate whether the vaginal vault had been infiltrated on US: i) No vaginal vault infiltration was present if the lesion did not exceed the cervix or if the lesion reached the vault, but the vaginal vault was clearly displayed; ii) in cases of suspected vaginal vault infiltration, the boundary between the lesion and the vaginal vault was not clear and iii) vaginal vault infiltration was indicated if the lesion reached or exceeded the vault, and the vaginal vault was not displayed clearly.
SWE examination
Subsequently, SWE was performed by one radiologist with 3 years' experience in SWE. The probe was moved gently and smoothly to the vaginal vault without compression, thus avoiding the pre-compression stiffening artifact on SWE. The lesion was positioned in the center of the screen and the mode was switched to SWE. The optimal settings with an elastic modulus ranging from 0 kPa (blue, soft) to 180 kPa (red, hard) were applied. The patients were requested to hold their breath and the probe was stabilized for 3–5 sec. The image was frozen after the color was completely filled in the sampling box of SWE. A round ‘Q-box’ region of interest was placed inside the SWE image for measurement of the tissue stiffness in units m/sec. In the present study, two Q-boxes were placed. One was placed in the lesion area and the diameter was adjusted according to the size of the lesion to include the maximum diameter of the lesion. The other was placed in the normal cervical tissue around the lesion and the diameter was fixed to 5 mm. The system automatically calculated the ratio of the average stiffness between the lesion and adjacent normal tissues (strain ratio) (Fig. 1). Measurements were performed in triplicate. The average values were calculated for the subsequent statistical analysis. The measurements of strain ratio were not performed for two patients with cervical cancer due to the large tumor size.
Figure 1.
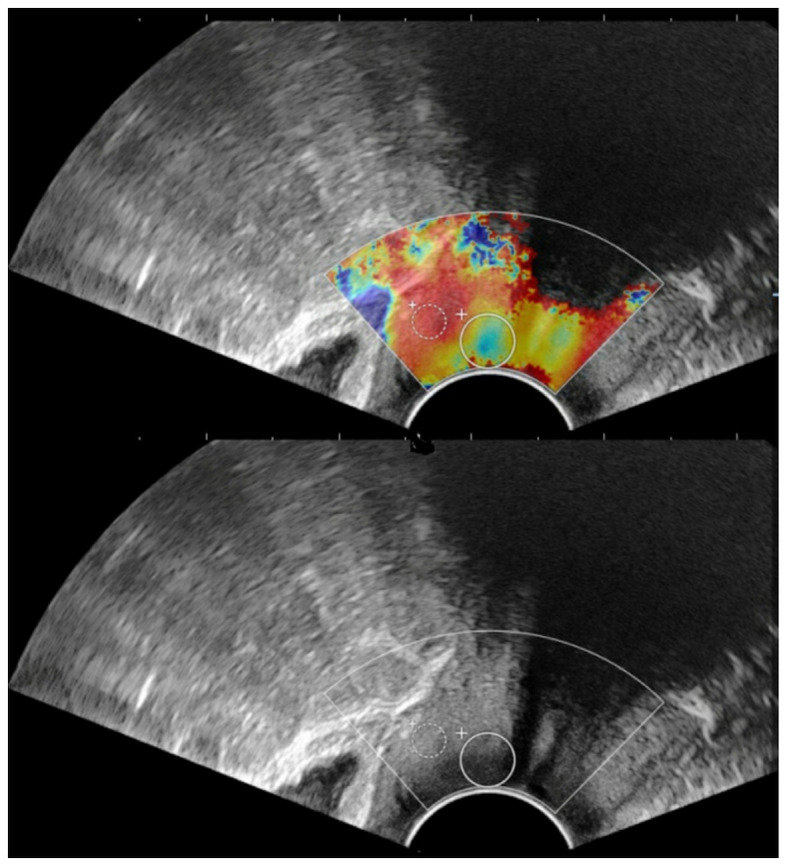
Measurement of strain ratio on shear wave elastography image. The big Q-box is the area of the lesion and the small Q-box is the area of normal tissues.
Elastic score
The lesions were divided into 5 grades (from soft to hard) according to the SWE images: Lesions or tissues that were mainly blue or blue were scored as 1 point; lesions that were mainly blue and green, with a small amount of red in the center, were scored as 2 points; lesions displaying with mixed signals of blue, green and red were scored as 3 points; lesions that were mainly red, with a small green area were scored as 4 points; those lesions that were almost fully red and may only have contained a small blue/green area in the surrounding area of the lesion were scored as 5 points.
Statistical analysis
The data were analyzed using SPSS (version 19.0; IBM, Corp). Normal distribution was evaluated using the Kolmogorov-Smirnov test. Continuous variables were expressed as the mean ± standard deviation if they had a normal distribution. Unpaired Student's t-test was used for comparison between the two groups. The SWS values of the three groups (cervical cancers, cervical benign lesions and normal cervixes) were evaluated using one-way analysis of variance (ANOVA), followed by the least significant difference (LSD) post-hoc test. Cancer volume was calculated as follows: Long × transverse × anteroposterior diameter of each cancer lesion, based on the measurement of US, SWE and pathology, respectively. Spearman's correlation coefficient was used to assess the differences in cancer volume. P<0.05 was considered to indicate a statistically significant difference.
Results
Basic patient characteristics
The basic data of the patients of the three groups are presented in Table I. The mean age of the cervical cancer group was 50.20 years ±8.32 (age range, 42–60 years), the cervical benign lesion group was 44.67 years ±7.38 (age range, 32–60 years) and the normal cervix group was 38.44±7.12 years (age range, 25–65 years). The average age of the patients with cervical cancer was higher than that of the patients with benign cervical lesions and normal cervix; however, there was no significant difference (all P>0.05), this result might due to the small sample size. For patients with cervical cancer, the major symptoms included irregular vaginal bleeding (30 patients), bleeding during sexual intercourse (36 patients) and increased leucorrhea with a peculiar smell (28 patients), and the duration was >6 months. Physical examination revealed cervical enlargement with cervical deformation in 24 patients and cervical mass in 34 patients. For patients with cervical benign lesions, the symptoms of cervical benign lesions included abnormal leucorrhea (26 patients) and vaginal contact bleeding (10 patients), while 4 patients were asymptomatic. Physical examination revealed cervical enlargement in 12 patients, cervical mass in 26 patients and no obvious abnormality in 2 patients.
Table I.
Basic characteristics of patients and healthy volunteers.
| Group | Cases (n) | Age (years)a | Symptoms | Pathological result |
|---|---|---|---|---|
| Cervical cancer | 40 | 50.20±8.32 (42–60) | Irregular vaginal bleeding (n=30), sexual intercourse bleeding (n=36), and increased leucorrhea with a peculiar smell (n=28) | Squamous cell carcinoma (n=28) Adenocarcinoma (n=6) Others (n=6) |
| Cervical benign lesion | 40 | 44.67±7.38 (32–60) | Leucorrhea abnormality (n=26), contact vaginal bleeding (n=10), and asymptomatic (n=4) | Myoma (n=16) Polypoid lesion (n=24) |
| Normal cervix | 40 | 38.44±7.12 (25–65) | None (n=40) | Normal |
Values are expressed as the mean ± standard deviation (range).
Characteristics on US imaging
The normal cervix is cylindrical, with strong banded echoes along the long axis of the cervix in the center and uniform internal echoes. The diameter of cervical benign lesions was 29.72±6.23 mm in the long, 30.12±6.59 mm in the transverse and 23.81±6.23 mm in the anteroposterior diameter. Among them, 16 patients had cervical myoma and 24 patients had cervical polyp. Regarding the diameter of cervical cancers, the long diameter was 35.45±9.96 mm, the transverse diameter was 34.08±3.58 mm and the anteroposterior diameter was 24.52±11.30 mm. All data are presented in Table IV.
Table IV.
Comparison among US, SWE and pathology for the measurement of cervical cancers.
| Measure (mm) | US | SWE | Pathology | ta | tb |
|---|---|---|---|---|---|
| Long diameter | 29.72±6.23 | 34.87±6.87 | 37.02±6.98 | 2.483 | 3.489 |
| Transverse diameter | 30.12±6.59 | 35.08±6.23 | 36.88±7.01 | 2.446 | 3.142 |
| Anteroposterior diameter | 23.81±6.23 | 28.11±6.98 | 29.60±6.58 | 2.055 | 2.858 |
| Calculation (ml) | US | SWE | Pathology | rc | rd |
| Volume | 21.31±0.26 | 34.24±0.30 | 39.45±0.32 | 0.992 | 0.890 |
P<0.05 compared with US and SWE.
P<0.01 compared with US and pathology.
r Correlation between SWE and surgical pathology.
r Correlation between US and surgical pathology. Volume was calculated as follows: Long × transverse × anteroposterior diameter. US, conventional ultrasound; SWE, shear wave elastography.
A total of 29 cervical benign lesions (12 myomas and 17 polyps) were correctly diagnosed by US. The diagnostic accuracy rate was 72.5% (29/40). Furthermore, 32 cervical cancers were correctly diagnosed by US and the diagnostic accuracy was 80.0%.
Characteristics on SWE imaging
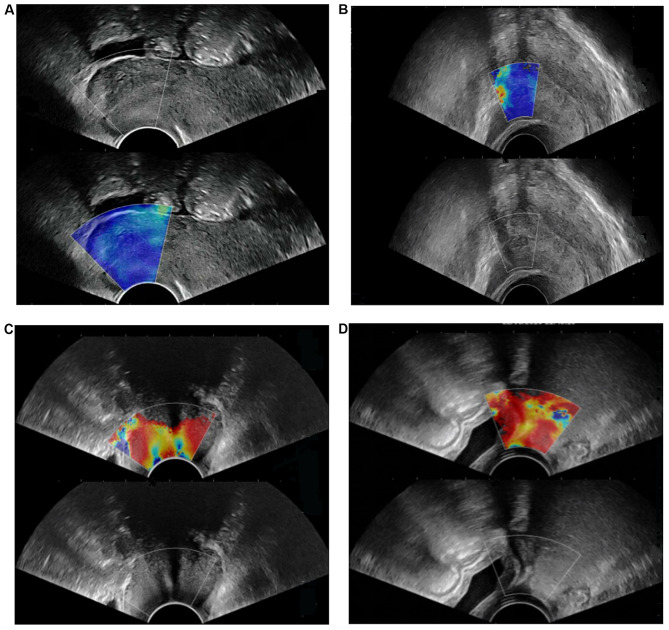
The elastic score of the 40 patients with a normal cervix was 1. A normal cervix displays as uniform blue on SWE images, occasionally with small red and green areas. The upper vaginal wall, cervical canal and cervical serosa are uniformly and continuously red (Fig. 2A). The results of the 40 benign cervical lesions were as follows: Elastic score 1 (26 patients), 2 (12 patients) and 3 (2 patients), 95% of patients had an elastic score of ≤2 (38 patients; Fig. 2B). The elastic score of the 40 cervical cancers was 5 points in 4 patients, 4 points in 32 patients, 3 points in 2 patients and 2 points in 2 patients, of which 38 patients had ≥3 points (Fig. 2C). The distribution of the elastic score of cervical benign lesions and cervical cancers are provided in Fig. 3.
Figure 2.
Elastic score of cervical cancer, cervical benign lesion and normal cervix on SWE imaging. (A) Determination of the elastic score of normal cervix on SWE. Normal cervix displayed as uniform blue with a small green area on the SWE image. The elastic score was 1 point. (B) Determination of the elastic score of a cervical benign lesion. The myoma (confirmed by pathology) displayed as blue-green mixed but mostly blue, and occasionally with small red areas. The elastic score was 2 points. (C) Determination of the elastic score of cervical cancer. The cancer (confirmed by pathology) displays mainly in red with occasional blue areas. The elastic score was 4 points. (D) Determination of the elastic score of cervical cancer with vaginal vault infiltration. The cancer (confirmed by pathology) displayed as a diffuse red area with no blue or green areas. The elastic score was 5 points. SWE, shear wave elastography.
Figure 3.
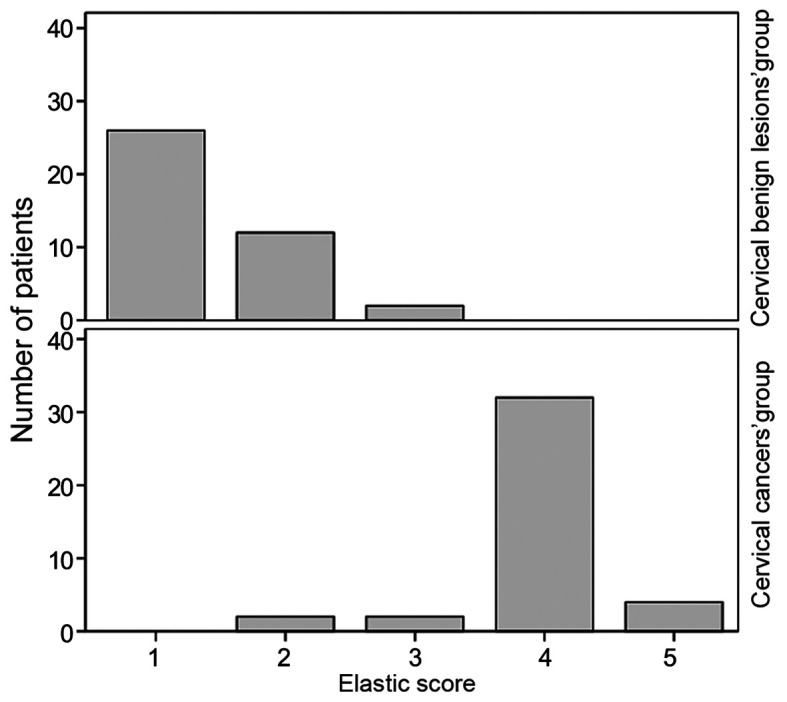
Distribution of elastic score of benign cervical lesions and cervical cancers.
The average elastic strain ratio of cervical benign lesions was 0.75±0.33 and the average elastic strain ratio of cervical cancer was 3.31±1.47. The distribution of the elastic strain ratio of all lesions is provided in Table II. The results indicated that the strain ratio of cervical cancer was distributed in the range of 1.5–7.5, while it was 0–2.0 for cervical benign lesions. The strain ratio between the two groups exhibited a significant difference (P<0.01).
Table II.
Distribution of elastic strain ratio of all lesions.
| Elastic strain ratio | Cervical benign lesions (n=38) | Cervical cancers (n=38) |
|---|---|---|
| ≤0.5 | 6 | 0 |
| 0.5< ratio ≤1.0 | 22 | 0 |
| 1.0< ratio ≤1.5 | 10 | 0 |
| 1.5< ratio ≤2.0 | 2 | 22 |
| 3.0< ratio ≤4.5 | 0 | 8 |
| 4.5< ratio ≤6.0 | 0 | 4 |
| ≥6.0 | 0 | 4 |
The SWS values of the three groups are provided in Table III. One-way ANOVA demonstrated significant differences in SWV among the three groups. Furthermore, multiple comparisons analyzed with the LSD post-hoc test demonstrated that the SWS value of cervical cancers was significantly higher than that of cervical benign lesions and normal cervixes (P<0.05 for both). However, there was no significant difference between the SWS values of cervical benign lesions and normal cervixes (P>0.05).
Table III.
Distribution and comparison of SWS values among three groups.
| Group | SWS value (m/sec)a | SWS mean value (m/sec)b | F-value | P-value |
|---|---|---|---|---|
| Normal cervix | 0.58–3.02 | 1.52±0.51 | 42.91 | 0.035 |
| Cervical benign lesion | 0.65–3.34 | 1.61±0.60 | ||
| Cervical cancer | 1.52–4.66 | 2.97±0.55 |
Values are expressed as
range
mean ± standard deviation. SWS, shear wave speed.
Evaluation of the size and volume of cervical cancer on SWE images
The long, transverse and anteroposterior diameter of cervical cancers measured by US, SWE and surgical pathology is displayed in Table IV. The results indicated that SWE measurements were closer to the surgical pathological measurements and there was no significant difference between them (P>0.05). The long, transverse and anteroposterior diameter of cervical cancers measured by SWE and surgical pathology were significantly larger than those determined by US (P<0.05 and P<0.01, respectively). The volume of cervical cancers calculated by SWE and US was positively correlated with the volume calculated by surgical pathology (r=0.992 and r=0.890, respectively).
On US, 10 patients were determined to have uterus infiltration, 22 patients were indicated to have no uterus infiltration and 8 patients had suspected uterus infiltration. On SWE, uterus infiltration was detected in 12 patients and no infiltration was observed in 24 patients, while 4 patients had suspected uterus infiltration. Surgical pathology indicated that uterus infiltration was present in 12 patients, no infiltration was observed in 24 patients and infiltration was suspected in 4 patients (Table V).
Table V.
Value of US and SWE for evaluating the uterus and vaginal vault infiltration compared with pathology.
| Uterus infiltration | Vaginal vault infiltration | |||||
|---|---|---|---|---|---|---|
| Observation | US | SWE | Pathology | US | SWE | Pathology |
| Infiltration | 10 | 12 | 12 | 6 | 8 | 8 |
| Suspected infiltration | 8 | 4 | 4 | 10 | 6 | 4 |
| No-infiltration | 22 | 24 | 24 | 24 | 26 | 28 |
| Conformity | 30 | 40 | / | 26 | 38 | / |
| Non-conformity | 10 | 0 | / | 14 | 2 | / |
| χ2 | 18.00 | 11.08 | ||||
| P-value | 0.005 | <0.001 | ||||
χ2 and P-value, conformity of US vs. SWE. US, conventional ultrasound; SWE, shear wave elastography.
On US, 6 patients were determined to have vaginal vault infiltration, 10 patients had suspected vaginal vault infiltration and 24 patients had no vaginal vault infiltration. SWE indicated that vaginal vault infiltration was present in 8 patients (Fig. 2D), no infiltration was observed in 26 patients and 6 patients had suspected infiltration. Surgical pathology suggested that vaginal vault infiltration was present in 8 patients, 4 patients had suspected infiltration and no infiltration was observed in 28 patients. There was a significant difference between SWE and US (P<0.05; Table V).
Discussion
In the present study, the elastic score was 1 point in 39 volunteers with a normal cervix, accounting for 97.5%, which was consistent with the study by Thomas et al (12). The SWE images of normal cervixes were uniform blue, indicating that the normal cervix was relatively soft and homogeneous. The cervical canal, cervical serosa layer and vaginal segment displayed in red color, the stiffness of which was lower than that of normal cervix. This may be linked to uterine secretions and the soft nature of the cervical canal.
In the present study, among the cervical benign lesions, 38 lesions had an elastic score of ≤2 points. Two lesions had an elastic score of 3 points, with blue color accounting for a large proportion of tissue on SWE imaging. The two lesions were pathologically confirmed as myoma with calcification. Therefore, their elastic scores were higher than those of others.
Among cervical cancers, 38 lesions had an elastic score of ≥3 points, which was higher than that of cervical benign lesions. In this group, only 2 lesions had elastic score of 2 points, and the green area was larger than the blue area. The two lesions were pathologically confirmed to have necrosis. The stiffness of the necrosis and liquefaction area was lower than that of the surrounding tumor tissue. However, shear waves are not able to spread in liquids. It may be assumed that internal necrosis may decrease the stiffness of the lesion, which is consistent with a previous study (18). Therefore, the elastic score was vulnerable to the influence of necrotic areas in the lesion, which was also one of the limitations of the elastic score.
The average strain ratio of cervical benign lesions was 0.53±0.20 and 2.68±0.64 for cervical cancers. The strain ratio of cervical cancer was higher than that of benign lesions and the result was consistent with the study of Lu et al (19). However, due to the small sample size, it was not possible to determine the cut-off value of the strain ratio in the diagnosis of cervical benign lesions and cervical cancers. Future studies with a larger sample size are required to validate the present results.
The mean SWS of cervical cancers was significantly higher than that of cervical benign lesions and normal cervix, which was consistent with the study of Su et al (13). Normal cervical tissue includes a small amount of muscle fibers and collagen fibers. Malignant tumors are heterogeneous with dense cells and small stroma. The SWE features were associated with intratumoral heterogeneity in mechanical elasticity. Therefore, the stiffness of cervical cancer lesions is higher than that of cervical benign lesions and normal cervical tissue. The present study also suggested that the accuracy of SWE in evaluating the infiltration of vaginal vault and uterus was higher than that of US. In addition, the long, transverse and anteroposterior diameter of lesions measured on SWE was closer to that measured by surgical pathology. SWE was superior to US in evaluating the infiltration range of cervical cancer, which was consistent with the previous study (13).
Of note, the present study had certain limitations. First, the sample size was relatively small. Secondly, the cut-off value of the strain ratio was not determined due to the small sample size. Furthermore, intra- and inter-operator reproducibility was not performed in the present study, as US is operator dependent. The rationale for using one operator is based on real clinical practice. According to Wang et al (20), one operator is acceptable for SWE examination in real clinical practice.
In conclusion, SWE is not only able to differentiate between cervical benign lesions and cervical cancers by qualitative and quantitative analysis but also evaluate the infiltration range of cervical cancer.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- SWE
shear wave elastography
- US
ultrasound
- SWS
shear wave speed
Funding
The present study was funded by the National Natural Science Foundation of China (grant no. 81771843).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
BF drafted the initial manuscript and analyzed the results. HZ, ZS, JL and SW made substantial contributions to acquisition and interpretation of data. JL designed this study, and revised the manuscript critically for important intellectual content. HZ and SW made substantial contributions to acquisition and analysis of data. ZS revised the manuscript and improved the language expression. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Linyi People's Hospital (Linyi, China). Patients who participated in this research had complete clinical data and written informed consent was obtained from the patients or their guardians prior to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics. 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 4.Zheng R, Zeng H, Zhang S, Chen T, Chen W. National estimates of cancer prevalence in China, 2011. Cancer Lett. 2016;370:33–38. doi: 10.1016/j.canlet.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Fu C, Feng X, Bian D, Du W, Wang X, Zhao Y. Basic T1 perfusion magnetic resonance imaging evaluation of the therapeutic effect of neoadjuvant chemotherapy in locally advanced cervical cancer. Int J Gynecol Cancer. 2013;23:1270–1278. doi: 10.1097/IGC.0b013e31829db950. [DOI] [PubMed] [Google Scholar]
- 6.Gaurilcikas A, Vaitkiene D, Cizauskas A, Inciura A, Svedas E, Maciuleviciene R, Di Legge A, Ferrandina G, Testa AC, Valentin L. Early-stage cervical cancer: Agreement between ultrasound and histopathological findings with regard to size and extent of local disease. Ultrasound Obstet Gyneeol. 2011;38:707–715. doi: 10.1002/uog.9037. [DOI] [PubMed] [Google Scholar]
- 7.Ghi T, Giunchi S, Kuleva M, Santini D, Savelli L, Formelli G, Casadio P, Costa S, Meriggiola MC, Pelusi G. Three-dimensional transvaginal sonography in local staging of cervical carcinoma: Description of a novel technique and preliminary results. Ultrasound Obstet Gynecol. 2007;30:778–782. doi: 10.1002/uog.5147. [DOI] [PubMed] [Google Scholar]
- 8.Togashi K, Nishimura K, Sagoh T, Minami S, Noma S, Fujisawa I, Nakano Y, Konishi J, Ozasa H, Konishi I, et al. Carcinoma of the cervix: Staging with MR imaging. Radiology. 1989;171:245–251. doi: 10.1148/radiology.171.1.2928532. [DOI] [PubMed] [Google Scholar]
- 9.Li DD, Xu HX, Guo LH, Bo XW, Xu JM, Zhang YF, Zhang K. Combination of two-dimensional shear wave elastography with ultrasound breast imaging reporting and data system in the diagnosis of breast lesions: A new method to increase the diagnostic performance. Eur Radiol. 2016;26:3290–3300. doi: 10.1007/s00330-015-4163-8. [DOI] [PubMed] [Google Scholar]
- 10.Park SY, Choi JS, Han BK, Ko EY, Ko ES. Shear wave elastography in the diagnosis of breast non-mass lesions: Factors associated with false negative and false positive results. Eur Radiol. 2017;27:3788–3798. doi: 10.1007/s00330-017-4763-6. [DOI] [PubMed] [Google Scholar]
- 11.Thomas A. Imaging of the cervix using sonoelastography. Ultrasound Obstet Gynecol. 2006;28:356–357. doi: 10.1002/uog.3813. [DOI] [PubMed] [Google Scholar]
- 12.Thomas A, Kummel S, Gemeinhardt O, Fischer T. Real-time sonoelastography of the cervix: Tissue elasticity of the normal and abnormal cervix. Acad Radiol. 2007;14:193–200. doi: 10.1016/j.acra.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Su Y, DU L, Wu Y, Zhang J, Zhang X, Jia X, Cai Y, Li Y, Zhao J, Liu Q. Evaluation of cervical cancer detection with acoustic radiation force impulse ultrasound imaging. Exp Ther Med. 2013;5:1715–1719. doi: 10.3892/etm.2013.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nitta E, Kanenishi K, Itabashi N, Tanaka H, Hata T. Real-time tissue elastography ofuterine sarcoma. Arch Gynecol Obstet. 2014;289:463–465. doi: 10.1007/s00404-013-2974-x. [DOI] [PubMed] [Google Scholar]
- 15.Xie M, Zhang X, Jia Z, Ren Y, Wang W. Elastography a sensitive tool for the evaluation of neoadjuvant chemotherapy in patients with high grade serous ovarian carcinoma. Oncol Lett. 2014;8:1652–1656. doi: 10.3892/ol.2014.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bakay OA, Golovko TS. Use of elastography for cervical cancer diagnostics. Exp Oncol. 2015;37:139–145. doi: 10.31768/2312-8852.2015.37(2):139-145. [DOI] [PubMed] [Google Scholar]
- 17.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Lee SH, Moon WK, Cho N, Chang JM, Moon HG, Han W, Noh DY, Lee JC, Kim HC, Lee KB, Park IA. Shear-wave elastographic features of breast cancers: Comparison with mechanical elasticity and histopathologic characteristics. Invest Radiol. 2014;49:147–155. doi: 10.1097/RLI.0000000000000006. [DOI] [PubMed] [Google Scholar]
- 19.Lu R, Xiao Y, Liu M, Shi D. Ultrasound elastography in the differential diagnosis of benign and malignant cervical lesions. J Ultrasound Med. 2014;33:667–671. doi: 10.7863/ultra.33.4.667. [DOI] [PubMed] [Google Scholar]
- 20.Wang Q, Guo LH, Li XL, Zhao CK, Li MX, Wang L, Liu XY, Xu HX. Differentiating the acute phase of gout from the intercritical phase with ultrasound and quantitative shear wave elastography. Eur Radiol. 2018;28:5316–5327. doi: 10.1007/s00330-018-5529-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.