Key Points
Question
Are the modified Atkins diet and low glycemic index therapy diet noninferior to the ketogenic diet with regard to seizure reduction at 24 weeks among children aged 1 to 15 years with drug-resistant epilepsy?
Findings
In this randomized clinical trial of 158 children with drug-resistant epilepsy, the median reduction in seizure burden was similar between the ketogenic diet, modified Atkins diet, and low glycemic index therapy diet, although the noninferiority of the modified Atkins diet and low glycemic index therapy diet was not proven. The adverse events were least with the low glycemic index therapy diet, and 1 adverse event may be avoided for every 4.3 children treated with the low glycemic index therapy diet compared with a ketogenic diet.
Meaning
The findings of this trial indicate that guidelines should support the use of the ketogenic, modified Atkins, and low glycemic index therapy diets for management of drug-resistant epilepsy; each dietary therapy should be discussed with caregivers in terms of the benefit in reducing seizure burden and the risk of adverse events.
Abstract
Importance
The ketogenic diet (KD) has been used successfully to treat children with drug-resistant epilepsy. Data assessing the efficacy of the modified Atkins diet (MAD) and low glycemic index therapy (LGIT) diet compared with the KD are scarce.
Objective
To determine whether the MAD and LGIT diet are noninferior to the KD among children with drug-resistant epilepsy.
Design, Setting, and Participants
One hundred seventy children aged between 1 and 15 years who had 4 or more seizures per month, had not responded to 2 or more antiseizure drugs, and had not been treated previously with the KD, MAD, or LGIT diet were enrolled between April 1, 2016, and August 20, 2017, at a tertiary care referral center in India.
Exposures
Children were randomly assigned to receive the KD, MAD, or LGIT diet as additions to ongoing therapy with antiseizure drugs.
Main Outcomes and Measures
Primary outcome was percentage change in seizure frequency after 24 weeks of dietary therapy in the MAD cohort compared with the KD cohort and in the LGIT diet cohort compared with the KD cohort. The trial was powered to assess noninferiority of the MAD and LGIT diet compared with the KD with a predefined, noninferiority margin of −15 percentage points. Intention-to-treat analysis was used.
Results
One hundred fifty-eight children completed the trial: KD (n = 52), MAD (n = 52), and LGIT diet (n = 54). Intention-to-treat analysis showed that, after 24 weeks of intervention, the median (interquartile range [IQR]) change in seizure frequency (KD: −66%; IQR, −85% to −38%; MAD: −45%; IQR, −91% to −7%; and LGIT diet: −54%; IQR, −92% to −19%) was similar among the 3 arms (P = .39). The median difference, per intention-to-treat analysis, in seizure reduction between the KD and MAD arms was −21 percentage points (95% CI, −29 to −3 percentage points) and between the KD and LGIT arms was −12 percentage points (95% CI, −21 to 7 percentage points), with both breaching the noninferiority margin of −15 percentage points. Treatment-related adverse events were similar between the KD (31 of 55 [56.4%]) and MAD (33 of 58 [56.9%]) arms but were significantly less in the LGIT diet arm (19 of 57 [33.3%]).
Conclusions and Relevance
Neither the MAD nor the LGIT diet met the noninferiority criteria. However, the results of this study for the LGIT diet showed a balance between seizure reduction and relatively fewer adverse events compared with the KD and MAD. These potential benefits suggest that the risk-benefit decision with regard to the 3 diet interventions needs to be individualized.
Trial Registration
ClinicalTrials.gov Identifier: NCT02708030
This randomized clincal trial assesses whether addition of either the modified Atkins diet or low glycemic index therapy diet to ongoing antiseizure drug therapy was noninferior to the ketogenic diet with regard to seizure reduction at 24 weeks among children aged 1 to 15 years with drug-resistant epilepsy.
Introduction
The International League Against Epilepsy defines drug-resistant epilepsy as ‘‘failure of adequate trials of 2 tolerated, appropriately chosen and used antiepileptic drug schedules (whether as monotherapies or in combination) to achieve sustained seizure freedom.”1(p28) Drug-resistant epilepsy responds poorly to pharmacologic management and frequently requires intervention via other modalities, including surgery, vagus nerve stimulation, and dietary therapy. However, standards of care for drug-resistant epilepsy management have not been well defined. In addition, the coexistent motor, language, and memory deficits may render some patients unsuitable for curative epilepsy surgery.
Dietary therapies have been reported to be effective and safe, and can be administered synergistically with other treatment options.2 The classic ketogenic diet (KD) is a high-fat, adequate protein, low-carbohydrate diet. The KD has been shown to be effective in randomized clinical trials, and benefits have been reported across various retrospective and prospective observational studies.3,4 However, some families and patients find adherence to the KD difficult, and it has an established adverse effect profile.5 Therefore, other diets, such as the modified Atkins diet (MAD) and low glycemic index therapy (LGIT) diet, have been investigated.6,7 Results from various studies have indicated that the MAD is as effective as the KD for drug-resistant epilepsy management, but the evidence is limited.6,8
In 2018, the International Ketogenic Consensus Guideline stated that the diet should be “individualized based on the family and child situation, rather than perceived efficacy,”2(p181) and that there was reasonable class III evidence to support its use. However, a Cochrane review, which included 7 randomized clinical trials assessing the efficacy of the KD in drug-resistant epilepsy, concluded that other more palatable diets “may have a similar effect on seizure control as classical KD but this assumption requires more investigation.”9(p2) This randomized trial was undertaken to assess whether addition of either the MAD or LGIT diet to ongoing antiseizure drug therapy was noninferior to the KD with regard to seizure reduction at 24 weeks among children aged 1 to 15 years with drug-resistant epilepsy.
Methods
Study Design
This noninferiority randomized clinical trial was conducted at All India Institute of Medical Sciences, New Delhi, India, between April 1, 2016, and August 20, 2017. The trial protocol was approved by the All India Institute of Medical Sciences Institutional Ethics Committee. Written informed consent was obtained from caregivers of participating children. Children between age 1 and 15 years with drug-resistant epilepsy presenting to the pediatric neurology outpatient clinic were considered for inclusion. Drug-resistant epilepsy was defined as seizure frequency of 4 or more seizures per month and treatment failure of 2 or more prescribed antiseizure drugs in maximum tolerated doses.8 For West syndrome, drug-resistant epilepsy was defined as more than 4 spasm clusters per month despite treatment with 2 or more antiseizure drugs and either adrenocorticotropic hormone or vigabatrin. Exclusion criteria included surgically remediable cause of drug-resistant epilepsy, inborn errors of metabolism, and known chronic systemic disorder. Children treated with the KD, MAD, or LGIT diet in the past were also excluded. A detailed study protocol is presented in Supplement 1. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for equivalence and noninferiority trials.
Enrolled children underwent a 4-week run-in period. During the run-in period, baseline investigations were undertaken, and syrups were reformulated to tablets that were then ground and either sprinkled over food or mixed with water to facilitate ingestion by young children. Parents were advised to maintain a daily seizure log. Demographic data and medical history were chronicled. Changes to the child’s diet were not advised during the run-in period. No changes in antiseizure drug dosages were made during the run-in period or during the 24-week intervention phase. Following the run-in period, children were randomly assigned to 1 of 3 interventions: KD, MAD, or LGIT diet. Computer-generated, random, permuted blocks stratified by age (1-5, >5-10, and >10-15 years) were used to generate a randomization list. Sealed and serially numbered opaque envelopes were used for allocation concealment. The dietitian and one of us (V.S.) directly involved with diet prescription could not be blinded to treatment. Participants, other study personnel, and those who analyzed the data were blinded.
Tailored diet prescriptions were developed for each patient based on food preferences and staple diet of the family. A gradual-initiation, nonfasting protocol was used for introducing the KD, which involved administering 75% of total daily caloric requirement on the day of the KD initiation and gradually increasing it to full calorie level over 2 to 4 weeks, as tolerated by the child.10 Classic KD was started at a 1:1 ratio (lipids:nonlipids). Lipid content was gradually increased to 2:1, 2.5:1, 3:1, and 4:1 every 48 hours while urinary ketosis and tolerance were monitored. The MAD group patients followed the Johns Hopkins protocol.11 Initiation of the LGIT diet involved restricting high glycemic index (>55) food items and limiting carbohydrates to approximately 10% of daily calories. All families were educated about the diet and provided with a detailed menu plan. The MAD and LGIT diets were started in the outpatient setting, while children were admitted for initiating the KD. For the MAD and LGIT diets, a list of food replacement options was also provided. Diets were supplemented with vitamins and minerals. Each patient’s caregiver maintained a daily log of meals, seizure frequency, urinary ketones, and dietary intolerance symptoms. Patients were followed up as outpatients at 4, 12, and 24 weeks after intervention initiation. Twice-weekly telephone calls were made to ensure dietary adherence, monitor for adverse events, and address any caregivers’ concerns.
Seizure frequency was assessed from the daily log (eAppendix 1 in Supplement 1). Mean and median number of seizures at a time point were calculated from seizure counts in the preceding 28 days and expressed as percentage change from baseline. Diet tolerance and adverse events were evaluated in interviews with caregivers. Evaluation details performed at each hospital visit are reported in eAppendix 2 and eAppendix 3 in Supplement 1. Serum was isolated within 2 hours of blood sample collection at baseline and at 24 weeks and stored at −80 °C. Levels of copper, selenium, and zinc in all serum samples were measured in a single batch.
The primary outcome was percentage change in seizure frequency from baseline at 24 weeks of therapy. The primary outcome measure was assessed in a blinded manner; daily logs were coded with unique identification numbers and assessed by an individual (R.M.P.) blinded to intervention. Secondary outcomes, which were analyzed 24 weeks after intervention initiation, included proportion of patients with greater than 50% seizure reduction from baseline, proportion of patients showing improvement in social quotient on the Vineland Social Maturity Scale (scores of 85-110 considered normal; higher scores, higher levels of function),12 changes in T score on the Child Behavior Checklist (normal score, <60; borderline, 60-63; and clinically impaired, >63),13 and changes in serum levels of copper, selenium, and zinc. Urinary ketone levels, recorded by parents as numeric values of 0 to 4 on urinary dipstick, were used to assess the level of ketosis. The median urinary ketone level over the past 4 weeks was associated with mean and median percentage decline in seizure frequency. The rate of seizure decline was calculated for each week using the formula 100 × (y − x)/x, where y indicates the mean or median number of daily seizures in the preceding week and x indicates the mean or median baseline number of daily seizures during run-in. Adherence to the prescribed diet was assessed from daily logs, which included the number of meals given and portions left uneaten. The proportion of prescribed diet that was consumed was computed every 4 weeks. A minimum 80% value was required for inclusion in the final analysis.
Sample size calculations were based on the study by Kim et al,8 who reported that, after 6 months of the KD and MAD, the KD group had 33.8% and the MAD group had 44.6% of baseline seizure frequency. Guided by this 10.8–percentage point difference, the predetermined noninferiority margin was set at a 15–percentage point difference between the treatment arms. Testing for this margin with 80% power at an α level of .05, assuming an SD of 30% for outcomes after 24 weeks of treatment, requires 50 patients per group. Assuming a 10% dropout rate, the required sample size was 165.
Statistical Analysis
The primary outcome of percentage seizure reduction at 24 weeks was analyzed by computing the effect size (mean or median difference) and determining its 95% CI. The MAD and LGIT diet were considered noninferior to the KD when the lower limit of the 95% CI of effect size was greater than −15 percentage points. Results were checked for distribution normality, and mean or median values were used as appropriate. The intention-to-treat (ITT) population included all patients who fulfilled eligibility criteria and were assigned an intervention. With ITT analysis, for patients who could not be contacted at 24 weeks of intervention, the number of mean or median daily seizures over the last 4 weeks of last contact with the patient was used for primary end-point calculation. The per-protocol population was defined to exclude randomly assigned patients who did not receive the full allocated treatment up to 24 weeks. For secondary outcomes, the intervention groups were compared using the χ2 test or Fisher exact test for categorical variables and standard unpaired, 2-tailed t test or Wilcoxon-Mann-Whitney test for continuous variables. P values <.05 were considered statistically significant. Adverse events were summarized per treatment group and compared as proportions with a χ2 test. Statistical analysis was conducted using R, version 3.5.1 (R Foundation).
Results
Of 214 children with drug-resistant epilepsy who were screened, 170 were randomly assigned to receive the KD (n = 55), MAD (n = 58), or LGIT diet (n = 57) and were included in ITT analysis (Figure 1). Twelve patients were withdrawn, and the remaining 158 patients (KD, 52; MAD, 52; and LGIT diet, 54) were included in per-protocol analyses. Their baseline characteristics are shown in Table 1. The 3 groups were similar for median baseline daily seizures (KD, 9; MAD, 8.5; and LGIT diet, 9; P = .99), proportion of patients with structural epilepsy (KD, 33; MAD, 41; and LGIT diet, 41; P = .33), and proportion of patients requiring 4 or more antiseizure drugs (KD, 20; MAD, 31; and LGIT diet, 31; P = .16). Baseline clinical examination was normal in 9 patients (16.4%) receiving a KD compared with 2 children (3.4%) receiving an MAD and 2 children (3.5%) receiving an LGIT diet (P = .01). Details of drug-resistant epilepsy causes and antiseizure drug use are given in eAppendix 4 and eAppendix 5 in Supplement 1, respectively. Mean (SD) dietary adherence was significantly better with the LGIT diet (94.3% [2.6%]) compared with the KD (91.4% [2%]) or MAD (90.6% [2.4%]). All patients had greater than 80% adherence throughout the study (eAppendix 6 in Supplement 1).
Figure 1. Flow of Patients.
GLUT-1 indicates glucose transporter type 1; MTS, mesial temporal sclerosis; and OPD, outpatient department.
Table 1. Demographic and Clinical Characteristics at Baseline.
| Variable | No. (%) | ||
|---|---|---|---|
| KD (n = 55) | MAD (n = 58) | LGIT diet (n = 57) | |
| Age, mo | |||
| Mean (SD) | 62.2 (38.1) | 62.3 (40.2) | 63.8 (37.0) |
| Median (IQR) | 52 (31-89) | 51 (31-79) | 54 (32.5-82) |
| Male sex | 37 (67.3) | 49 (84.5) | 42 (73.7) |
| Diagnosis | |||
| Structural epilepsy | 33 (60.0) | 41 (70.7) | 41 (71.9) |
| Genetic epilepsy | 16 (29.1) | 12 (20.7) | 14 (24.6) |
| Genetic epilepsy with structural abnormality | 6 (10.9) | 5 (8.6) | 2 (3.5) |
| Age at first seizure, mo | |||
| Mean (SD) | 6.74 (11.46) | 9.5 (21.03) | 9.19 (18.92) |
| Median (IQR) | 2 (0-8) | 2 (0-8) | 0 (0-8.5) |
| Type of seizure at enrollment | |||
| Spasms | 33 (60.0) | 41 (70.7) | 38 (66.7) |
| Myoclonic (other than spasms) | 26 (47.2) | 23 (39.7) | 21 (36.8) |
| Tonic | 31 (56.4) | 33 (56.9) | 39 (68.4) |
| GTCS | 3 (5.5) | 3 (5.2) | 1 (1.8) |
| Focal | 10 (18.2) | 11 (18.9) | 17 (29.8) |
| Absence | 13 (23.6) | 14 (24.1) | 13 (22.8) |
| Multifocal | 31 (56.3) | 35 (60.3) | 41 (71.9) |
| Antiepileptic drugs | |||
| 2-3 | 35 (63.6) | 27 (46.6) | 26 (45.6) |
| ≥4 | 20 (36.4) | 31 (53.4) | 31 (54.4) |
| Median (IQR) | 3 (3-4) | 4 (3-4) | 4 (3-4) |
| Developmental delay | |||
| GM | 41 (74.5) | 46 (79.3) | 43 (75.4) |
| FM | 44 (80.0) | 49 (89.1) | 49 (85.9) |
| Language | 50 (90.9) | 50 (86.2) | 52 (91.2) |
| Sociocognitive | 51 (92.7) | 52 (89.7) | 54 (94.7) |
| Clinical examination | |||
| Normal | 9 (16.4) | 2 (3.4) | 2 (3.5) |
| Pyramidal signs | 36 (65.5) | 43 (74.1) | 45 (78.9) |
| Extrapyramidal signs | 10 (18.2) | 13 (22.4) | 10 (17.5) |
| Cranial nerve palsy | 13 (23.6) | 17 (29.3) | 12 (21.1) |
| Baseline daily seizures | |||
| Mean (SD) | 20.2 (25.1) | 20 (29.3) | 20.1 (38.7) |
| Median (IQR) | 9 (6-19) | 8.5 (5-20) | 9 (4.5-19.5) |
Abbreviations: FM, fine motor; GM, gross motor; GTCS, generalized tonic clonic seizures; IQR, interquartile range; KD, ketogenic diet; LGIT, low glycemic index therapy; MAD, modified Atkins diet.
Outcomes
Twenty-four weeks after intervention, the median daily seizure frequency was 3.3 (interquartile range [IQR], 1.2-14) with the KD, 4 (IQR, 0.5-10) with the MAD, and 4 (IQR, 0.4-11) with the LGIT diet (Table 2). After 24 weeks of intervention, the median change in seizure frequency was similar among the 3 arms in both the ITT (KD: −66%; IQR, −85% to −38%; MAD: −45%; IQR, −91% to −7%; and LGIT diet: −54%; IQR, −92% to −19%; P = .39) and per-protocol populations (P = .57) (Table 2). The median difference in change in seizure frequency between the KD and MAD was −21 percentage points (95% CI, −29 to −3 percentage points) in ITT and −10 percentage points (95% CI, −26 to 5 percentage points) in per-protocol analysis. The median difference in change in seizure burden between the KD and LGIT diet was −12 percentage points (95% CI, −21 to 7 percentage points) in ITT analysis and −7 percentage points (95% CI, −17 to 10 percentage points) in per-protocol analysis (Table 3).
Table 2. Seizure Frequency.
| Variable | No. (%) | ||
|---|---|---|---|
| KD (n = 52) | MAD (n = 52) | LGIT diet (n = 54) | |
| Seizure frequency at 24 wk | |||
| Median (IQR) | 3.3 (1.2 to 14) | 4 (0.5-10) | 4 (0.4-11) |
| Mean (SD) | 9.4 (14) | 11 (19) | 8.7 (12) |
| Achieved specific cutoff points after 24 wk of intervention | |||
| Complete resolution | 6 (11.5) | 8 (15.4) | 9 (16.7) |
| >90% Reduction | 6 (11.5) | 6 (11.5) | 8 (14.8) |
| >50% Reduction | 23 (44.2) | 13 (25.0) | 15 (27.8) |
| ≤50% Reduction | 13 (25.0) | 14 (26.9) | 15 (27.8) |
| Increase in seizure frequency at 24 wk | 4 (7.7) | 11 (21.2) | 7 (12.9) |
| % Change in seizure frequency | |||
| Per-protocol analysis | |||
| Median (IQR) | −67 (−87 to −37) | −57 (−92 to −5.5) | −60 (−92 to −24) |
| Mean (SD) | −60 (33) | −48 (46) | −55 (40) |
| Intention-to-treat analysis | |||
| Median (IQR) | −66 (−85 to −38) | −45 (−91 to −7) | −54 (−92 to −19) |
| Mean (SD) | −60 (32) | −46 (45) | −52 (41) |
Abbreviations: IQR, interquartile range; KD, ketogenic diet; LGIT, low glycemic index therapy; MAD, modified Atkins diet.
Table 3. Difference in Seizure Reduction With the 3 Treatment Strategiesa,b.
| Comparison between interventions | Per-protocol analysis | Intention-to-treat analysis | ||
|---|---|---|---|---|
| Median (95% CI) | Mean (95% CI) | Median (95% CI) | Mean (95% CI) | |
| KD-MAD | −10 (−26 to 5) | −12 (−28 to 3.2) | −21 (−29 to 3) | −14 (−28 to 0.61) |
| KD-LGIT | −7 (−17 to 10) | −5.7 (−20 to 8.4) | −12 (−21 to 7) | −8.1 (−22 to 5.5) |
Abbreviations: KD, ketogenic diet; LGIT, low glycemic index therapy; MAD, modified Atkins diet.
In both the per-protocol and intention-to-treat analyses, the lower limit of the 95% CI of the median difference and mean difference in seizure reduction between MAD and KD, as well as between LGIT diet and KD, breached the noninferiority margin of −15 percentage points.
Data are given as percentage points.
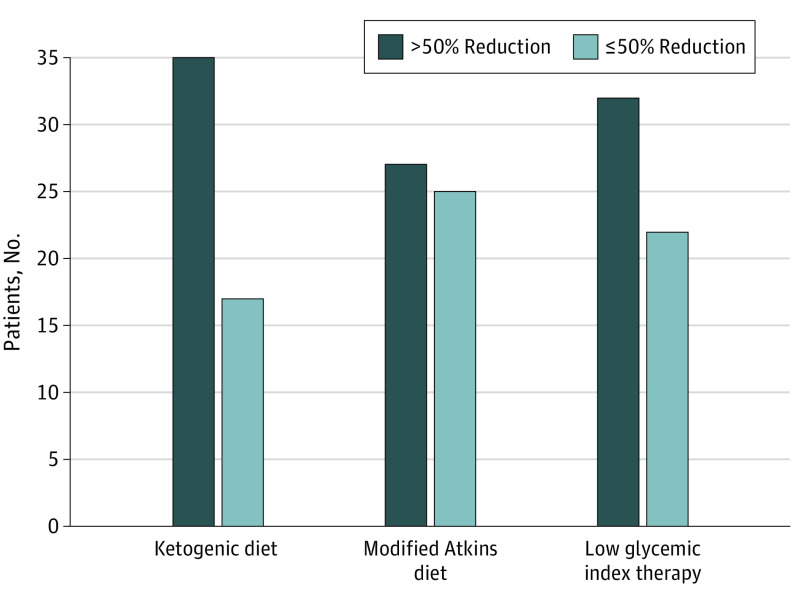
Proportions of patients with greater than 50% seizure reduction at 24 weeks were comparable across the 3 arms: KD, 35 of 52 (67.3%); MAD, 27 of 52 (51.9%); and LGIT diet, 32 of 54 (59.3%). The odds ratio (OR) between KD and MAD was 0.16 (95% CI, 0.86-4.22); between KD and LGIT diet, 1.42 (95% CI, 0.64-3.13); and between MAD and LGIT diet, 0.74 (95% CI, 0.34-1.60) (Figure 2). The change in seizure frequency was not associated with urinary ketone levels (eAppendix 7 in Supplement 1). There was rapid seizure reduction over the initial 4 weeks of the study with the KD and MAD, while the decrease was gradual over 10 to 12 weeks with the LGIT diet (eAppendix 8 in Supplement 1). Post hoc subgroup analysis by age (1-5, >5-10, and >10-15 years) was performed for percentage reduction in seizures and proportion of children with greater than 50% seizure reduction. All subgroups were comparable for reduction in seizure burden between the 3 interventions (eAppendix 9 in Supplement 1). At 24 weeks after the intervention, the mean social quotient improved in 83 children (54.2%) (eAppendix 10A in Supplement 1). The improvement was most notable with the KD (34 of 52 [65.4%]) followed by the LGIT diet (29 of 54 [53.7%]) and MAD (20 of 52 [38.5%]) (P = .02). After 24 weeks, the change in mean total T score was nonsignificant and similar for the 3 interventions (eAppendix 10B in Supplement 1).
Figure 2. Numbers of Patients With Greater Than 50% Reduction vs 5% or Less Reduction in Daily Seizure Frequency 24 Weeks After Intervention.
Proportions of patients with greater than 50% reduction in seizure frequency with ketogenic diet, modified Atkins diet, and low glycemic index therapy diet were comparable between ketogenic diet and modified Atkins diet (odds ratio [OR], 1.42; 95% CI, 0.64-3.13), between KD and MAD (OR, 0.16; 95% CI, 0.86-4.22), and between MAD and LGIT diet (OR, 0.74; 95% CI, 0.34-1.60).
Adverse Events
Adverse events noted among 83 of 170 patients (48.8%) were comparable between the KD (31 of 55 [56.4%]) and MAD (33 of 58 [56.9%]) but were significantly less with the LGIT diet (19 of 57 [33.3%]) (eAppendix 11 in Supplement 1). The commonest clinical adverse event was vomiting, which was noted in 28 patients (50.9%) receiving the KD, 26 patients (44.8%) receiving the MAD, and 18 patients (31.6%) receiving the LGIT diet (eAppendix 12 in Supplement 1). Investigation-based adverse events were reported in 59 patients (34.7%) (KD, 21 [38.2%]; MAD, 24 [41.4%]; and LGIT diet, 14 [24.6%]; P = .14). Two patients receiving the LGIT diet and 1 patient receiving the KD were noted to have thrombocytopenia during evaluation at 24 weeks. Both patients were also receiving sodium valproate. Ten patients, all receiving concomitant zonisamide therapy, were found to have hypercalciuria at 24 weeks. In addition, 2 patients receiving the MAD developed scurvy, detected at 24 weeks in one child and at 12 weeks in the other, who was withdrawn from the study. Both of these children presented with extreme irritability and incessant crying. Owing to unavailability of resources for serum vitamin C assay, the diagnosis of scurvy was based on characteristic radiologic findings and clinical response to vitamin C therapy.
Three patients developed a prolonged QTc interval. For 2 of these children, the prolonged interval was detected at 24 weeks’ assessment, while for the third patient, the observation was made following hospital admission for respiratory tract infection, which necessitated diet discontinuation. The baseline QTc intervals for these 3 patients were 0.30, 0.31, and 0.36 seconds; following intervention, the intervals were 0.51, 0.54, and 0.52 seconds. Serum samples were additionally tested for zinc, selenium, and copper levels; preintervention and postintervention comparisons are provided in eAppendix 13 in Supplement 1. Children receiving the KD and MAD had significant decreases in their serum selenium levels, although the levels remained within the reference range. Preintervention/postintervention and intergroup anthropometric comparisons did not show any statistically significant differences (eAppendix 14 in Supplement 1).
Discussion
In this randomized clinical trial involving children with drug-resistant epilepsy, the MAD and LGIT diets were not noninferior to the KD with respect to seizure reduction at 24 weeks after diet initiation. In ITT analysis, median seizure reductions were 66% for the KD, 45% for the MAD, and 54% for the LGIT diet. The median difference in seizure frequency between the MAD and KD (−21 percentage points; 95% CI, −29 to 3 percentage points) and between the LGIT diet and KD (−12 percentage points; 95% CI, −21 to 7 percentage points) both breached the noninferiority limit of −15 percentage points. Hence, the trial failed to demonstrate that the LGIT diet or MAD is noninferior to the KD in children with drug-resistant epilepsy. However, the proportions of patients with greater than 50% seizure reduction at 24 weeks were similar across the 3 diets.
Our results should be interpreted in 5 main aspects that go beyond the noninferiority domain. First, this trial addressed the treatment strategies in terms of seizure reduction at a given time, expressed as percentage change with respect to baseline seizures. Most previous literature expresses the outcomes as proportions of patients with greater than 50% seizure reduction.3,7,14 Our approach gives a more absolute seizure reduction with each strategy. The proportion of children with greater than 50% seizure reduction after 24 weeks of the interventions was 67.3% with the KD, 51.9% with the MAD, and 59.3% with the LGIT diet. To put things into perspective, the median number of daily seizures at the beginning of the trial was nearly 9 per day per group. After 24 weeks of intervention, the per-day seizure burden had decreased to 3 with the KD and 4 with both the MAD and LGIT diet. For the subsequent analysis, a noninferiority margin of −15 percentage points implied a difference of nearly 1 to 2 seizures per day for a child having nearly 9 seizures per day at the start of the study. Both the MAD and LGIT diet were not noninferior to the KD as the lower limit of the 95% CI of effect size crossed the margin of −15 percentage points. The seizure reduction noted with the KD and LGIT diet is comparable with published reports; however, the present study demonstrated lesser reduction of seizures with the MAD.3,7,8,14 This difference can be partially accounted for by the fact that a diet was started late in the clinical course in our study, probably rendering the patients more drug resistant. The differences in seizure reduction with 3 interventions can be partly accounted for by the type of patients in each arm. Nine patients in the KD group had a normal neurologic examination compared with 2 each in the MAD and LGIT diet groups (P = .01). In addition, 20 patients in the KD group required 4 or more antiseizure drugs compared with 31 patients in both of the other groups. This difference may suggest that the patients in the MAD and LGIT diet groups had more refractory causes of seizures than those in the KD group.
Second, the daily seizure log allowed for assessment of the rate of decline in seizures with each intervention. Administration of the KD was associated with an approximate 50% decline in seizure frequency in the first 4 weeks, and a further 10% reduction in seizure frequency was noted over the subsequent 20 weeks. Seizure reduction with the MAD was also rapid, with an approximate 40% decrease in the number of seizures by 4 weeks; seizure frequency plateaued between 40% and 50% over the next 20 weeks. With the LGIT diet, however, the seizure decline was gradual, with 50% reduction attained between 10 and 12 weeks. This understanding is necessary before a patient is considered to be a nonresponder and also to give a realistic perspective to caregivers. The rate of decline was considerably more rapid with the lipid-rich KD and MAD compared with the LGIT diet, which is primarily associated with restriction of carbohydrates to low glycemic index foods. While the KD and MAD are associated with ketosis, the exact role of ketone bodies in seizure control is unclear. Some animal studies have suggested that acetoacetate might reduce glutamate release at hippocampal synapses, while other studies have failed to show any association between ketone bodies and synaptic transmission.15,16,17 Studies have suggested an association between seizure control and serum levels of β-hydroxybutyrate.18 We measured urinary ketone levels and failed to show any association with change in seizure frequency, although serum ketone level estimation might have been more appropriate.
Third, this study assessed the effect of dietary strategies on drug-resistant epilepsy as a complete group and not etiologic or syndromic subcategories, which increases the generalizability of results and captures the complexity of clinical practice. Most enrolled children had spasms or generalized or multifocal seizures. The children with focal epilepsy were underrepresented because most children with lesional focal epilepsy were candidates for epilepsy surgery and, hence, were excluded from the study.
Fourth, this study demonstrated an improvement in social quotient with all 3 interventions; this improvement was statistically significantly better with the KD compared with the MAD, while the response was comparable between the KD and LGIT diet, as well as between the MAD and LGIT diet. In a randomized clinical trial assessing the cognitive and behavioral effect of the KD in children and adolescents with drug-resistant epilepsy, participants receiving the KD had lower levels of anxious and mood-disturbed behavior.19 To our knowledge, there are no comparable studies for MAD and LGIT diet interventions. Although the delineation of mechanisms explaining the improvement in social quotient is beyond the scope of this study, the improvement in social quotient can be partly attributed to the reduced seizure burden with each of the interventions. The change in the T score was not significant in our trial, and it is possible that the 24-week interval of observation was too brief to observe a change in this measure.
Fifth, all interventions were associated with adverse events. The KD and MAD were associated with poor dietary tolerance with vomiting, difficulties with palatability, diarrhea, and constipation.3,6,14 In addition, lipid profiles in these patients were altered owing to prolonged ingestion of high-lipid diets. Nephrocalcinosis and increased urinary calcium excretion in patients receiving the KD and MAD can be related to fat malabsorption and chronic acidosis, partly attributable to concurrent zonisamide therapy. In contrast, the LGIT diet was associated with minimal adverse events, none of which was life threatening. These reduced adverse events associated with the LGIT diet suggest a trade-off between the efficacy and harmful effects of the intervention. Although the risk-benefit trade-off was not considered at the beginning of the intervention, our results suggest that the LGIT diet led to lesser seizure reduction than the KD (average difference of approximately 1 seizure per day). However, absolute risk reduction of adverse events was approximately 23% among study participants treated with the LGIT diet compared with children receiving the KD. Hence, 1 adverse event can be avoided for every 4.3 children treated with the LGIT diet compared with the KD.
Strengths and Limitations
The strengths of our study are its embedment within the clinical practice setting with the inclusion of all children with drug-resistant epilepsy, irrespective of the underlying cause, allowing for generalizability of its results. To our knowledge, this is the first trial to analyze the 3 primary dietary options for drug-resistant epilepsy—KD, MAD, and LGIT diet—for seizure reduction, adverse events, and cognitive effects. The dropout rate of less than 10% in each of the 3 arms and dietary adherence greater than 80% further strengthen the results.
The trial also has limitations. The scientific weight of the study would have been better if the SD in each cohort was smaller. However, this was a close representation of a clinical setting with different drug-resistant forms of epilepsy responding to different degrees and at different rates. The study would also have been improved by blinding all involved individuals. However, it was impossible to blind dietitians, as the diet prescriptions required close parental interaction. The use of daily logs maintained by caregivers would have missed some seizures, including nocturnal seizures, and runs the risk of introducing subjective errors. In addition, a selection bias cannot be ruled out because this was a single-center study.
Conclusions
The data from this study show that all 3 dietary regimens—KD, MAD, and LGIT diet—significantly reduce the seizure burden in children with drug-resistant epilepsy. This information supports the use of all 3 dietary therapies. Still, the results are inconclusive with regard to noninferiority of the MAD and LGIT diet. The risk profiling illustrates that the LGIT diet is associated with the least number of and least severe adverse events, while the other 2 diets are more likely to be associated with serious and life-threatening events. It appears that each dietary intervention should be assessed in terms of the benefit in reducing seizure burden and the risk of adding adverse events before starting the KD, MAD, or LGIT diet in children.
Trial Protocol
Data Sharing Statement
References
- 1.Kwan P, Brodie MJ. Definition of refractory epilepsy: defining the indefinable? Lancet Neurol. 2010;9(1):27-29. doi: 10.1016/S1474-4422(09)70304-7 [DOI] [PubMed] [Google Scholar]
- 2.Kossoff EH, Zupec-Kania BA, Auvin S, et al. ; Charlie Foundation; Matthew’s Friends; Practice Committee of the Child Neurology Society . Optimal clinical management of children receiving dietary therapies for epilepsy: updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open. 2018;3(2):175-192. doi: 10.1002/epi4.12225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neal EG, Chaffe H, Schwartz RH, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7(6):500-506. doi: 10.1016/S1474-4422(08)70092-9 [DOI] [PubMed] [Google Scholar]
- 4.Cross JH, Neal EG. The ketogenic diet—update on recent clinical trials. Epilepsia. 2008;49(suppl 8):6-10. doi: 10.1111/j.1528-1167.2008.01822.x [DOI] [PubMed] [Google Scholar]
- 5.Kang HC, Chung DE, Kim DW, Kim HD. Early- and late-onset complications of the ketogenic diet for intractable epilepsy. Epilepsia. 2004;45(9):1116-1123. doi: 10.1111/j.0013-9580.2004.10004.x [DOI] [PubMed] [Google Scholar]
- 6.Kossoff EH, Cervenka MC, Henry BJ, Haney CA, Turner Z. A decade of the modified Atkins diet (2003–2013): results, insights, and future directions. Epilepsy Behav. 2013;29(3):437-442. doi: 10.1016/j.yebeh.2013.09.032 [DOI] [PubMed] [Google Scholar]
- 7.Pfeifer HH, Lyczkowski DA, Thiele EA. Low glycemic index treatment: implementation and new insights into efficacy. Epilepsia. 2008;49(suppl 8):42-45. doi: 10.1111/j.1528-1167.2008.01832.x [DOI] [PubMed] [Google Scholar]
- 8.Kim JA, Yoon JR, Lee EJ, et al. Efficacy of the classic ketogenic and the modified Atkins diets in refractory childhood epilepsy. Epilepsia. 2016;57(1):51-58. doi: 10.1111/epi.13256 [DOI] [PubMed] [Google Scholar]
- 9.Martin K, Jackson CF, Levy RG, Cooper PN. Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database Syst Rev. 2016;2:CD001903. doi: 10.1002/14651858.CD001903.pub3 [DOI] [PubMed] [Google Scholar]
- 10.Bergqvist AG, Schall JI, Gallagher PR, Cnaan A, Stallings VA. Fasting versus gradual initiation of the ketogenic diet: a prospective, randomized clinical trial of efficacy. Epilepsia. 2005;46(11):1810-1819. doi: 10.1111/j.1528-1167.2005.00282.x [DOI] [PubMed] [Google Scholar]
- 11.Kossoff EH, McGrogan JR, Bluml RM, Pillas DJ, Rubenstein JE, Vining EP. A modified Atkins diet is effective for the treatment of intractable pediatric epilepsy. Epilepsia. 2006;47(2):421-424. doi: 10.1111/j.1528-1167.2006.00438.x [DOI] [PubMed] [Google Scholar]
- 12.Doll EA. The Measurement of Social Competence: A Manual for the Vineland Social Maturity Scale. Educational Test Bureau Educational Publishers; 1953. doi: 10.1037/11349-000 [DOI] [Google Scholar]
- 13.Achenbach TM, Rescorla LA. ASEBA Preschool Forms & Profiles. Research Center for Children, Youth and Families, University of Vermont; 2000. [Google Scholar]
- 14.Sharma S, Jain P, Gulati S, Sankhyan N, Agarwala A. Use of the modified Atkins diet in Lennox Gastaut syndrome. J Child Neurol. 2015;30(5):576-579. doi: 10.1177/0883073814527162 [DOI] [PubMed] [Google Scholar]
- 15.Thio LL, Wong M, Yamada KA. Ketone bodies do not directly alter excitatory or inhibitory hippocampal synaptic transmission. Neurology. 2000;54(2):325-331. doi: 10.1212/WNL.54.2.325 [DOI] [PubMed] [Google Scholar]
- 16.Greene AE, Todorova MT, Seyfried TN. Perspectives on the metabolic management of epilepsy through dietary reduction of glucose and elevation of ketone bodies. J Neurochem. 2003;86(3):529-537. doi: 10.1046/j.1471-4159.2003.01862.x [DOI] [PubMed] [Google Scholar]
- 17.Juge N, Gray JA, Omote H, et al. Metabolic control of vesicular glutamate transport and release. Neuron. 2010;68(1):99-112. doi: 10.1016/j.neuron.2010.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchhalter JR, D’Alfonso S, Connolly M, et al. The relationship between d-β-hydroxybutyrate blood concentrations and seizure control in children treated with the ketogenic diet for medically intractable epilepsy. Epilepsia Open. 2017;2(3):317-321. doi: 10.1002/epi4.12058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.IJff DM, Postulart D, Lambrechts DAJE, et al. Cognitive and behavioral impact of the ketogenic diet in children and adolescents with refractory epilepsy: a randomized controlled trial. Epilepsy Behav. 2016;60:153-157. doi: 10.1016/j.yebeh.2016.04.033 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Data Sharing Statement