Abstract
People exposed to chronic stress age rapidly. The telomeres in their cells of all types shorten faster. Inflammation is another important feature of stress that, along with aging, accounts for the phenomenon of inflammaging. In addition to aging itself, inflammaging can contribute to the development of several pathologies, including atherosclerosis, diabetes, hypertension, and others. Oxidative stress is one of the main mechanisms related to stress. Oxidative stress is caused by the over-production of reactive oxygen species (ROS) that can damage various tissues. The main source of ROS is mitochondria. Being suppressed by mitochondrial mutations, mitophagy can aggravate the situation. In this case, the aging-specific pro-inflammatory changes are amplified. It happens because of the inability of cells to maintain the normal state of mitochondria. Macrophages are the crucial element of the innate immunity associated with the chronic inflammation and, subsequently, with the inflammaging. In this review, we focus on the therapy approaches potentially reducing the deleterious effects of oxidative stress. These include stimulation of mitophagy, activation of mitochondrial uncoupling, induction of the expression of the telomerase catalytic component gene, and use of antioxidants. Any method reducing oxidative stress should improve post-traumatic stress disorder.
Keywords: inflammaging, ROS, mitochondria, oxidative stress, mitophagy, uncoupling, antioxidants, macrophage
1. Psychological Stress and Aging
Psychological stress is considered to be an important risk factor for numerous diseases. The common feature of these pathologies is cellular senescence, which causes functional alterations and is associated with cancer and cardiovascular, neurodegenerative, and autoimmune disorders. All of these conditions are usually associated with whole-body aging, but, in the case of lasting severe stress, they can occur early in life [1,2].
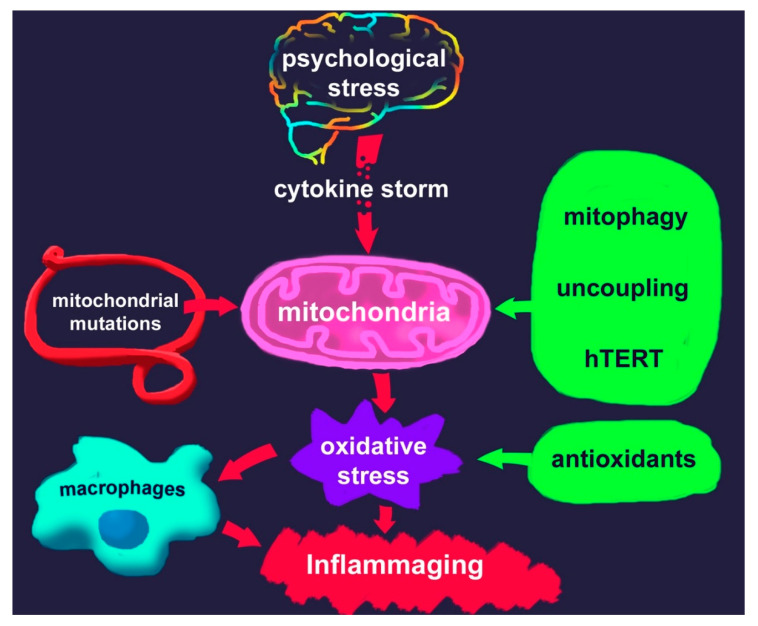
Numerous studies have shown a link between chronic psychological stress and mental disorders, such as major depressive disorder, and post-traumatic disorder (PTSD), as well as accelerated aging. This suggests the involvement of neural, physiologic, molecular, and genomic mechanisms. Chronic psychological stress is also believed to stimulate pro-inflammatory cytokines release (Figure 1) [3], thus triggering inflammation [4]. Accordingly, antidepressants were shown to reduce enhanced levels of cytokines [5].
Figure 1.
Scheme of the association between psychological stress and inflammaging through oxidative stress; potential interventions to reduce the resulting detrimental effects are listed on the right.
The hypothesis that oxidative stress stimulates inflammation is well known and is supported by a sufficient body of evidence. Based on it, oxidative stress was also suggested to trigger a crosstalk between the immune and the central neural systems within the human organism. This influence may have a crucial impact on emotional wellbeing [6].
Reactive oxygen species (ROS) overproduction can lead to cellular damage and macromolecule metabolism alterations that, in turn, are associated with the development of aging phenotypes [7]. Further investigations focused on the missing links between oxidative stress and telomere length. Initially, research was based on in vitro studies using cultured cells that demonstrated the association between oxidative stress inductors and premature senescence [8]. Epel et al. discovered the existence of the association between oxidative stress and accelerated telomeres shortening in humans in 2004 [9]. They analyzed the impact of psychological stress on pre-menopausal women. Although this study was small, it laid the groundwork for further analyses.
In vivo studies performed so far indicate that, in Parkinson’s disease [10] and diabetes type II [11], there is a clear association between selected biomarkers of oxidative stress-induced damage and the length of the telomeres, which was not observed in healthy individuals. In addition, Starr et al. demonstrated a possible association between two aging-related single-nucleotide polymorphisms (SNPs) of oxidative stress genes and telomere length [12].
A recent study examined the association between redox-state markers and telomere length in blood and vascular tissue in coronary artery disease. Tissue-specific inverse associations between superoxide production and telomere length were identified. However, no association was found between superoxide dismutase activity and telomere length in vascular and blood cells [13].
To sum up, the existing data indicate that the association between oxidative stress and telomere shortening may be more complicated than originally conceived. Differences in research data may be explained by clinical and ethnical diversity of the subjects and by inadequate choice of the tested biomarkers.
2. Cell Senescence
Speaking of aging, it is worth mentioning the cellular aging and senescence. Although the meaning of the term “cell senescence” seems obvious as it was first used to indicate the complex of processes that accompanies cell proliferation arrest in culture, it appears to be more complicated. Now it is clear that cell senescence is not just the exhaustion of cells proliferative potential, but it is a lasting arrest. Among the causes are various injuries, all kinds of stresses [14], a conflict of regulation during the activation of certain oncogenes, and even phenomena accompanying the wound process [15] and normal embryonic development [16].
Previously, Leonard Hayflick reported the limited proliferative potential of human cells, which later became known as the “Hayflick limit” [17]. He also suggested that the reaching of the Hayflick limit is related to in vivo aging. Later, the telomeric theory of aging was suggested, which explains the nature of the Hayflick limit. Further research in the field of cytogerontology revealed that the under-repair of telomeres is of great importance. It was shown that telomeric DNA loss increases under conditions of oxidative stress. Thus, it appeared that the free-radical theory could be combined with the telomeric theory, and under-repair was added to under-replication [18].
Oxidative stress was demonstrated to stimulate the loss of telomeric DNA, and the processes resulting from telomeric DNA loss were shown to be similar to those occurring during cellular senescence [19]. A crucial role for mitochondria in the determination of telomere shortening rate was proposed due to their leading role in the ROS generation [20]. Moreover, a decrease in the number of mitochondria in cells was shown to lower the number of senescent cells in vivo [21].
It is essential to emphasize that there are several limitations in evaluating cell senescence by measuring telomere length. First, cell senescence can be local or generalized. By measuring the length of telomeres in blood cells, it is impossible to judge small local processes that occur in any specific pathologies, including, for example, pathologies limited to any region of the brain.
Second, changes in telomere length in blood cells can be transient [22]. The reason for this is unclear, but two possibilities can be assumed, i.e., the increase in telomerase activity that lengthens telomeres, or the exit from the acute stage of stress. The fact is that the maturation of hematopoietic stem cells to blood cells is long (when measured by the number of cell divisions). In conditions of acute need, the body triggers the proliferation of progenitor cells, not of stem cells. If this stimulus and resulting proliferation occur under stress conditions, then there is a noticeable shortening of the telomeres. At the end of stress, slowly, due to the proliferation of stem cells that were not affected (they very rarely divide and are located in a special hypoxic niche), telomere length can be restored.
The third limitation is that most measurements of telomere length are made via PCR. This is the easiest and cheapest way. At the same time, all the information collected concerns the average size of telomeres. However, cell senescence is determined by the shortest telomeres, which signal about DNA damage, leading to a stop in cell proliferation and initiating senescence. When analyzing telomere length using PCR, it is assumed by default that telomere size variance is the same in control and experimental samples.
3. Mitochondria and ROS Production
As mentioned above, oxidative stress is an important stimuli of the telomere shortening, which makes ROS and ROS-related enzymes a valuable part of the process of cellular senescence. ROS and reactive nitrogen species (RNS) normally participate in numerous redox reactions within the cell. However, the overproduction of ROS and RNS is associated with oxidative stress that can cause cellular damage, development of inflammation, and severe disorders. ROS also causes an irreversible progression of oxidative decay, promoting the impairment of physiological functions, increasing disease incidence, and reducing the life span [23].
Oxidative stress can be caused not only by the excessive production of ROS but also by insufficient activity of endogenous systems fighting against the oxidative attack [24,25].
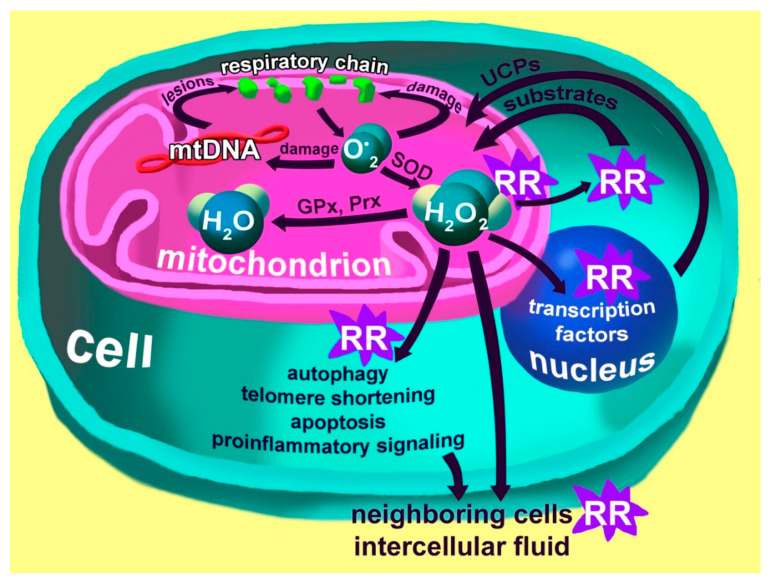
Normally, the greatest source of ROS production is the mitochondria as a result of the activity of the respiratory chain and oxidoreductases (Figure 2). ROS can also be generated by peroxisomes, endoplasmic reticulum, plasma membrane, cytosol, lysosomes, microsomes, and nuclear envelope [26].
Figure 2.
Generation of reactive oxygen species (ROS) by the mitochondrial respiratory chain. Superoxide anions generated by the respiratory chain damage the respiratory chain itself and the mitochondrial DNA, which leads to changes in the respiratory chain that increase the production of the superoxide anion. Superoxide anions also damage the mitochondrial membranes, causing lipid peroxidation and leading to changes in membrane properties. Special superoxide dismutase (SOD) enzymes convert superoxide anions into hydrogen peroxide, which is then converted to water by peroxiredoxins (Prx) and glutathione peroxidase (GPx). Hydrogen peroxide plays a major redox-regulating (RR) role in this system. In mitochondria, it changes the activity of enzymes, in the cytosol, it affects the delivery of substrates into the mitochondria, whereas, in the nucleus, it alters the activity of transcription factors, causing the response kernel, which is reflected in increased protein expression of uncouplers (UCPs). UCPs are transported to the mitochondria, where they reduce the proton potential of the inner membrane, thereby reducing the production of the superoxide anion. Excessive production of hydrogen peroxide in the cytoplasm triggers autophagy processes, causes shortening of telomeres, potentially starts the process of apoptosis and causes increased expression of pro-inflammatory factors that can affect neighboring cells and the whole organism [27].
Several circumstances are crucial for ROS generation in mitochondria. One of the most important factors is the potential of mitochondria inner membrane, the decrease of which results in a reduced generation of ROS. The two most obvious mechanisms of this process are a local decrease in oxygen concentration and a slowdown of electron transport. Uncoupling increases respiration, which reduces the local concentration of oxygen and therefore the production of ROS [28,29].
A close connection between the development of psychological stress and ROS production is underlined by changes in the activity of enzymes involved in ROS inactivation. ROS metabolism involves various genes, among which are glutathione S-transferase mu 1 and 2 (GSTM1 and GSTM2). Their transcripts are modified according to PTSD progression and are also contributed to a risk of PTSD development [30]. In addition, it has been shown that the expression level of thioredoxin reductase (TXNRD1) may be enhanced in PTSD patients compared to control individuals exposed to trauma [31].
The production of ROS by mitochondria depends on the activity of the uncoupling proteins. Various genetically programmed variants of these proteins are associated, on the one hand, with the intensity of oxidative stress and shortening of telomeres [32] and, on the other hand, with the overall life expectancy.
4. The Special Role of Mitochondria and Macrophages in the Development of Chronic Inflammation
As depicted in Figure 2, mitochondria are the main producers of ROS, but, at the same time, they can also be harmed by an increased ROS concentration. There are a few possible scenarios. A defect in mitochondria is another potential consequence of ROS influence. It inhibits the utilization of the defective mitochondria preventing mitophagy or the mitochondrial fission process, which often precedes mitophagy. These mitochondria become a permanent source of ROS over time, and the cell undergoes accelerated aging and acquires senescence-associated secretory phenotype (SASP). At some point, the mitochondria are destroyed. In case they cannot be utilized properly (mitophagy), the innate immune system, in concert with inflammatory components, recognizes the mitochondrial DNA as an object of attack. It is believed that mitochondria can stimulate the innate immune system because of their bacterial origin. If such events occur with the macrophage, it becomes a permanent source of pro-inflammatory factors; the process increases over time and cannot be stopped. Such macrophage reactions can contribute to the development of atherosclerosis, osteoporosis, and neurodegenerative processes. Genetic analysis of mitochondrial DNA, in addition to conventional genetic analysis, can be useful for patients suffering from stress disorders to identify those most vulnerable to oxidative stress.
5. Oxidative Stress and the Pro-Inflammatory Phenotype of Immune Cells
The immune system is not an exception regarding cell senescence. As supported by the majority of experimental data, immunosenescence involves the deterioration of the innate and adaptive immune response efficacy, although the elements of the immune system are altered with aging in different ways.
Several features accompany the senescence of immune cells, among which are the termination of proliferation, telomeres shortening, morphological changes, activity of the SA-βGal enzyme, and also SASP. SASP is associated with the ability to secrete pro-inflammatory cytokines [33].
5.1. Innate Immunity
Many age-related alterations in neutrophil function have been described. Functions, such as the production of free radicals, intracellular killing, apoptosis, and chemotaxis, decline. In addition, the release of pro-inflammatory cytokines is enhanced, and this may promote inflammaging. Notably, adhesion and phagocytosis appear not to change with aging [34].
Decreased level of major histocompatibility complex (MHC) class II human leukocyte antigen DR-isotype (HLA-DR) molecules and decreased activation ability of macrophages were observed as a result of aging [35]. The generation of reactive nitrogen and oxygen intermediate products, phagocytosis ability, and respiratory burst also decrease, which indicates the weakening of the microbicidal activity of macrophages [36]. In addition, the minor population of CD14+CD16+ monocytes were observed to be significantly enhanced in elderly subjects. Although the number of membrane receptors responsible for cell activation does not decrease during aging, signaling pathways are affected [37]. These include phosphoinositide 3-kinases (PI3K), mitogen-activated protein kinase (MAPK), the Janus kinase-signal transducer and activator of transcription (Jak/STAT) pathways, and others [38].
The changes in Toll-like receptor (TLR) function deserve particular attention [38]. The lowering of TLR-1 expression and a subsequent decrease in the levels of CD80 and CD86 expression is accompanied by a decrease of cytokines release. Conversely, the stimulation of TLR4 in CD14+CD16+ inflammatory monocytes was shown to enhance cytokine generation, which can be explained by the persistent activity of NF-κB [37].
Aging affects the expression of surface receptors in natural killer (NK) cells. The expression of cytotoxicity-activating receptors was shown to be decreased and, consequently, the cytotoxic potential of single cells was reduced. The total number of NK cells increases with aging, and this may have a compensatory value. The proportion of different phenotypes of NK cells is also affected. Thus, the proportion of CD56bright NK cells decreases, and the proportion of CD56-NK cells increases [39]. Notably, antibody-dependent cellular cytotoxicity capacity, CD16 expression, and some other parameters are not influenced by aging.
5.2. Adaptive Immunity
Phenotypes and functions of both T and B cells are more vulnerable to aging than those of the cellular components of innate immunity. This makes age-related detrimental changes more frequent in the adaptive immune response [40]. OCTO and NONA studies tracked persistent changes in the immune response related to aging. The aim of these studies was to cluster immune parameters, such as poor T cell proliferation, low B cell number associated with the so-called immune risk phenotype (IRP), inversion of the CD4/CD8 ratio, expansion of late differentiated CD8+ T cells, and cytomegalovirus (CMV) seropositivity [41]. Unfortunately, the link between IRP and increased mortality has not been replicated.
Decreased avidity and quantitative decrease of the antibody response were demonstrated in elderly subjects, along with decreased efficacy. This was found to be the consequence of the limited repertoire diversity of memory B cells [42].
Concerning T cells, we are facing phenomena called memory inflation, which result from naïve T cell activation following exposure to antigens and insufficient renewal of the naïve cellular pool throughout the lifespan [43].
The long-lasting antigenic stimulation causes the accumulation of memory cells according to the antigens to which the cells were previously exposed, in which effectiveness decreases with time [44]. This causes the progressive filling of the immune space and reduces memory cell functions, such as clonal expansion and IL-2 production. Alternatively, it increases interferon-gamma (IFNγ) that has cytotoxic functions and accumulates in inflammatory lesions. All these modulations are associated with changes in CD28 receptor signaling and other signaling pathways [45]. To compensate for the insufficient production of naïve T cells due to thymic involution, already existing naïve cells undergo peripheral homeostatic proliferation. T regulatory cells may also be affected both quantitatively and qualitatively, which causes important alterations in the host immune response [46]. PTSD is an example of the disease that involves oxidative stress. Oxidative stress affects immune cells, favoring their pro-inflammatory phenotype. PTSD is also characterized by a phenomenon called oxi-inflamm-aging or inflammaging [47].
6. Inflammaging
During the aging process, several cytokine-related effects are observed. In particular, anti-inflammatory cytokines levels decrease, while the levels of pro-inflammatory cytokines (interleukin-1 (IL-1), IL-6, tumor necrosis factor alpha (TNF-α), and others) grow. The complex of these changes in combination with alterations of the innate immune response was termed “inflammaging”. The state of inflammaging is characterized by subclinical inflammation caused by alterations in immune responses. Serum tests of aging subjects revealed only a small increase in the levels of pro-inflammatory cytokines [48].
Inflammaging is now considered the consequence of immunosenescence, which implies the inappropriate response of adaptive immunity to pathogens exposure and other types of chronic stress in aging subjects [49].
7. Potential Approaches to Reduce Oxidative Stress in Stress Disorders
7.1. Potential Anti-Inflammatory Treatment Strategies
Today, only a few drugs with the properties of selective inhibitors of serotonin reuptake (SSRIs) are approved by US Food and Drug Administration and other organizations for the therapy of stress-related disorders. Their effectiveness is limited [50].
The effectiveness of cyclooxygenase 2 inhibitors is high in animal models, but, in the case of human disorders, it remains to be determined [51].
Cytokine-blocking antibodies have not been tested. To inhibit cytokines, monoclonal antibodies are often used for the treatment of malignancy and autoimmune disorders. Many compounds of this class are approved, and several of them were shown to be effective as the therapeutic approach for the depression. Among such agents are infliximab, adalimumab (anti-TNF-α antibodies), and tocilizumab (anti-IL-6 receptor antibody).
Several studies on the use of glucocorticoids have shown some effectiveness [52].
Angiotensin-converting enzyme inhibitors have anti-inflammatory properties and were shown to reduce PTSD symptoms [53].
Beneficial effects on the stress-related disorders, such as PTSD, were described as the result of a healthy lifestyle. This includes a healthy diet and physical activity. However, these results are unclear due to the unaccounted differences in sampling [54,55]. Intriguingly, there is increasing evidence that physical activity and exercise can reduce inflammation [56].
It should be noted that inflammation is the evolutionary conserved response of the body aimed at restoring the status quo. It is a “good” reaction. However, sometimes this reaction does not achieve its goal. For example, a too intense inflammation has great destructive power and damages organs and tissues, whereas a too weak inflammation does not reach its goal and can turn into a chronic state. Direct artificial suppression of inflammatory processes, especially in the case of unclear pathophysiological mechanisms, can harm the course of a disease. In the case of PTSD, it is only known that the disease occurs due to an incorrect reaction of the body to stress.
A recent study encourages us to take a fresh look at the possible ways of PTSD development [57]. A statistically significant association of a weak inflammatory response in the acute phase of trauma with subsequent development of PTSD was found. This investigation once again highlights the fact that applying anti-inflammatory therapy in the case of PTSD can be dangerous.
Serious changes in the concentration of various cytokines occur during the development of PTSD (cytokine storm; see Figure 1). These changes generally lead to increased production of reactive oxygen species in various cell types, including cells of the immune system, vascular wall, and nervous system. The entire body is affected by oxidative stress and begins to age rapidly. If it is impossible to target inflammation, it is possible to reduce oxidative stress. Notably, anti-aging measures (regarding lifestyle, diet, exercise, etc.; see above) also have a positive effect on PTSD symptoms.
7.2. Activators of Mitophagy
Autophagy is directed at digesting useless components and those that work improperly in cells. The special type of autophagy aimed at the degradation of damaged or dysfunctional mitochondria is called mitophagy. Activation of mitophagy is one of the ways to reduce the oxidative stress due to mitochondria activity in oxidative stress-associated ROS production [58].
Pink1, a PTEN-induced kinase, is attracted to the mitochondrial membrane in response to the loss of membrane potential that is associated with mitochondrial damage. There, Pink1 phosphorylates and activates an ubiquitin ligase called parkin, which, in turn, recruits the autophagy adaptor protein p62. This causes the encapsulation of mitochondria in autophagosomes with the participation of LC3 [59].
Cellular ROS production was shown to increase in response to the knockout of Pink1 and other mitophagy-related genes. This suggested that ROS are generated by mitochondria that escaped mitophagy [60]. Resveratrol, a sirtuin 1 (SIRT1) activator, was reported to stimulate mitophagy and thus to reduce oxidative stress in mdx mice [61].
Activation of sirtuin-3 (SIRT-3) was reported to have a positive impact on the stimulation of mitophagy [62]. Among SIRT3 activators, dihydromyricetin and honokiol were tested in various conditions related to mitochondrial functional impairments. Dihydromyricetin was shown to suppress chondrocyte degeneration in osteoarthritis [63]. Honokiol was reported to activate SIRT3 in a study dedicated to the treatment of intervertebral disc degeneration [64].
7.3. Uncouplers
Uncouplers increase the permeability to protons of the inner mitochondrial membrane, which leads to a decrease in the proton gradient. The significant decrease in the proton gradient complicates ATP generation mediated by complex V, located in the inner membrane of mitochondria, which uses the proton gradient. Thus, energy is consumed as heat, which results in the separation of oxidation and phosphorylation.
Uncouplers were shown to suppress the generation of ROS [65]. An increase in uncouplers production was reported to be stimulated by ROS generation. ROS production, in turn, can be lowered by decreasing the proton gradient in response to uncouplers [66].
The function of UCP1, the thermogenin protein from brown fat, is quite well established. Thermogenesis reduces cold-induced ROS production in the presence of oxidative modification of UCP1 at the cysteine residue 253. Thermogenesis can be blocked by the mitochondrial antioxidant MitoQ or by the restoration of the redox potential with N-acetylcysteine [67].
Dinitrophenol (DNP) and niclosamide are uncouplers that work as weak acids, providing an H+ source for the lowering of the mitochondrial membrane potential [68]. Interestingly, DNP has been known for more than 120 years and prescribed as an anti-obesity drug [69].
DNP has been shown to increase the lifespan of mice. At the same time, an increase in tissue respiration, weight loss, and a decrease in plasma glucose and triglycerides were observed [70]. DNP also caused an increase in the average lifespan of flies, without affecting the maximum value [71].
7.4. hTERT Activators
Since the stress is accompanied by accelerated aging, telomerase induction should reduce the effects of aging by lengthening the telomeres.
Telomerase has numerous functions within the cell besides telomere stabilization, but the function of the catalytic subunit hTERT in mitochondria is not clear yet. However, recent studies provide evidence of the participation of hTERT in the reduction of oxidative stress-induced damage [72]. Reduced telomerase activity can be restored by various mechanisms. PI3K/Akt, MAPK/ERK1/2, and the Wnt/β-catenin pathways were shown to upregulate hTERT expression and/or activity [73].
Cycloastragenol, a telomerase activator, was reported to transiently activate telomerase in T-lymphocytes, neonatal keratinocytes, and fibroblasts [74]. This substance received the commercial name TA-65 and has been sold as a food supplement since 2013. It works through the activation of the ERK pathway, enhancing the expression of telomerase. Telomeres lengthening induced by cycloastragenol was not accompanied by an increase in cancer incidence [75]. However, not many studies have evaluated the positive impact of this drug, and more detailed investigations are needed.
Resveratrol was shown to activate telomerase in addition to mitophagy. It also seems to act through the activation of SIRT1 [76]. Despite the well-known antioxidant properties, resveratrol can have also prooxidant effect. This was proven in various researches that revealed different properties of resveratrol depending on the cell type and concentration [77,78]. Moreover, resveratrol was demonstrated to have apoptosis-inducing properties in the melanoma cell line, A375SM. It is implemented through the stimulation of ROS generation [79]. The co-transfection of bone marrow mesenchymal stem cells of rats with telomerase and nerve growth factor displayed a positive impact on learning and memory [80].
7.5. Antioxidants
The use of mitochondria-targeted antioxidants is probably the most obvious approach to reduce ROS production. It turned out that these antioxidants, for example, SkQ1, can marginally increase the lifespan of mice and lower the severity of many aging-associated pathologies, although MitoQ was demonstrated to increase the proliferative potential of cells only in the presence of minor oxidative stress [81].
Unfortunately, numerous studies revealed no effect of antioxidants on cell proliferation in culture. This makes the understanding of the underlying processes controversial. However, the recent work of Liao et al. demonstrated the inhibition of cell senescence in adipose tissue-derived mesenchymal stem cells (ADSCs) by reduced glutathione and melatonin [82].
These contradictions reflect the limitations of the free-radical theory of aging. Cell culture is a kind of pure isolated system that does not provide all stress stimuli normally present within a living organism. This is proven by the significant enhancement of the lifespan of mice who were held in normal vivarium conditions, in contrast to what observed from mice kept in a pathogen-free vivarium, in which antioxidants showed no effect [83].
The most beneficially seems to be the diet with natural antioxidants, which have the potential to reduce the oxidative stress and thus to decrease an inflammation rate.
8. Conclusions
The ideal treatment for stress-related disorders has to be based on the knowledge of the mechanisms causing each disorder operating at the nervous system level. It should aim at “erasing” the memory of an event, or at least at blocking newly formed, easily aroused connections between brain structures. Research in this direction is underway, but it is still far from practical implementation.
Under these conditions, it is possible to try blocking the negative consequences of these diseases development. The main pathogenetic factor that can be involved in is oxidative stress, which is associated with inflammation and occurs as a result of psychological stress.
The stress response is an adaptive mechanism that allows mobilizing all the resources of the body to fight a temporary threat. When the process becomes chronic, the stress associated with adaptive mechanisms becomes destructive. Glucocorticoid receptors are present in most cells of the body, so the stress response is very generalized. Cells receive a huge number of signals that significantly affect their physiology. In the case of immune system cells, this process is even more pronounced. Cellular responses to such essential signals are almost always associated with redox regulation, which involves mitochondria as generators of the oxidative reaction components. As a result, the entire body falls under conditions of oxidative stress. Conditions are created for accelerated aging and the development of a wide variety of pathologies associated with aging. During the development of such pathologies, senescent cells arise, which, in turn, acquire SASP and begin to secrete pro-inflammatory components into the surrounding space. Thus, the overwhelming effect on oxidative stress can break this vicious circle and reduce the deleterious effects of psychological stress.
In principle, two strategies are possible to control oxidative stress: the inactivation of already formed ROS and RNS or the reduction of their formation. The second option is preferable. In this view, antioxidants are less preferable than therapies reducing ROS production.
Reducing ROS production can be achieved by removing dysfunctional mitochondria, which become autonomous sources of ROS, promoting damage to cells and their environment. This can be achieved by enhancing mitophagy, i.e., by delivering such dysfunctional mitochondria to lysosomes for complete disposal. Unfortunately, defective mitochondria that produce excess ROS can maintain a high membrane potential for a long time and thus be inaccessible to the natural mechanism of mitophagy targeted at removing mitochondria with very low membrane potential.
Only in the 21st century has it become clear that, in response to oxidative stress, the protein component of telomerase (hTERT) migrates from the cell nucleus to the mitochondria and increases cell survival. The mechanism of this process is still unclear, but it is very tempting to induce hTERT expression in cells to resist oxidative stress. Unfortunately, natural selection has very significantly suppressed hTERT expression in human cells, and 30 years of attempts to stimulate hTERT expression have yielded only minor results. From a formal point of view, hTERT is an oncogene that can cause tumor growth of already altered, precancerous cells.
The molecules most suitable for practical use are uncouplers. Their effectiveness against oxidative stress is well proven; however, it is necessary to develop compounds with reduced and prolonged tissue-specific activity. Possible candidates may be free fatty acids, which are natural uncouplers. In addition to reducing oxidative stress, uncouplers can reduce weight and lower the plasma concentration of glucose and triglycerides, thereby reducing the likelihood of developing metabolic syndrome, type 2 diabetes, and atherosclerosis, which are diseases of our modern civilization.
Author Contributions
Writing—original draft preparation, A.V.P. and Y.E.Y.; writing—review and editing, A.N.O., I.A.S. and N.G.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Science Foundation (Grant # 19-15-00297).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Sahin E., Colla S., Liesa M., Moslehi J., Müller F.L., Guo M., Cooper M., Kotton D., Fabian A.J., Walkey C., et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Donovan A., Tomiyama A.J., Lin J., Puterman E., Adler N.E., Kemeny M., Wolkowitz O.M., Blackburn E.H., Epel E.S. Stress appraisals and cellular aging: A key role for anticipatory threat in the relationship between psychological stress and telomere length. Brain Behav. Immun. 2012;26:573–579. doi: 10.1016/j.bbi.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toft H., Bramness J.G., Lien L., Abebe D.S., Wampold B.E., Tilden T., Hestad K., Neupane S.P. PTSD patients show increasing cytokine levels during treatment despite reduced psychological distress. Neuropsychiatr. Dis. Treat. 2018;14:2367–2378. doi: 10.2147/NDT.S173659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hori H., Kim Y. Inflammation and post-traumatic stress disorder. Psychiatry Clin. Neurosci. 2019;73:143–153. doi: 10.1111/pcn.12820. [DOI] [PubMed] [Google Scholar]
- 5.Basterzi A.D., Aydemir C., Kisa C., Aksaray S., Tuzer V., Yazici K., Göka E. IL-6 levels decrease with SSRI treatment in patients with major depression. Hum. Psychopharmacol. 2005;20:473–476. doi: 10.1002/hup.717. [DOI] [PubMed] [Google Scholar]
- 6.Salim S. Oxidative stress: A potential link between emotional wellbeing and immune response. Curr. Opin. Pharmacol. 2016;29:70–76. doi: 10.1016/j.coph.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 7.El Assar M., Angulo J., Carnicero J.A., Walter S., García-García F.J., López-Hernández E., Sánchez-Puelles J.M., Rodríguez-Mañas L. Frailty Is Associated With Lower Expression of Genes Involved in Cellular Response to Stress: Results From the Toledo Study for Healthy Aging. J. Am. Med. Dir. Assoc. 2017;18 doi: 10.1016/j.jamda.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 8.Erusalimsky J.D. Oxidative stress, telomeres and cellular senescence: What non-drug interventions might break the link? [published online ahead of print, 2020 Feb 13] Free Radic. Biol. Med. 2020 doi: 10.1016/j.freeradbiomed.2020.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Epel E.S., Blackburn E.H., Lin J., Dhabhar F.S., Adler N.E., Morrow J.D., Cawthon R.M. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. USA. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watfa G., Dragonas C., Brosche T., Dittrich R., Sieber C.C., Alecu C., Benetos A., Nzietchueng R. Study of telomere length and different markers of oxidative stress in patients with Parkinson’s disease. J. Nutr. Health Aging. 2011;15:277–281. doi: 10.1007/s12603-010-0275-7. [DOI] [PubMed] [Google Scholar]
- 11.Salpea K.D., Talmud P.J., Cooper J.A., Maubaret C.G., Stephens J.W., Abelak K., Humphries S.E. Association of telomere length with type 2 diabetes, oxidative stress and UCP2 gene variation. Atherosclerosis. 2010;209:42–50. doi: 10.1016/j.atherosclerosis.2009.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starr J.M., Shiels P.G., Harris S.E., Pattie A., Pearce M.S., Relton C.L., Deary I.J. Oxidative stress, telomere length and biomarkers of physical aging in a cohort aged 79 years from the 1932 Scottish Mental Survey. Mech. Ageing Dev. 2008;129:745–751. doi: 10.1016/j.mad.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Margaritis M., Sanna F., Lazaros G., Akoumianakis I., Patel S., Antonopoulos A.S., Duke C., Herdman L., Psarros C., Oikonomou E.K., et al. Predictive value of telomere length on outcome following acute myocardial infarction: Evidence for contrasting effects of vascular vs. blood oxidative stress. Eur. Heart J. 2017;38:3094–3104. doi: 10.1093/eurheartj/ehx177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Achuthan S., Santhoshkumar T.R., Prabhakar J., Nair S.A., Pillai M.R. Drug-induced senescence generates chemoresistant stemlike cells with low reactive oxygen species. J. Biol. Chem. 2011;286:37813–37829. doi: 10.1074/jbc.M110.200675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demaria M., Ohtani N., Youssef S.A., Rodier F., Toussaint W., Mitchell J.R., Laberge R.M., Vijg J., Van Steeg H., Dollé M.E., et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell. 2014;31:722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Storer M., Mas A., Robert-Moreno A., Pecoraro M., Ortells M.C., Di Giacomo V., Yosef R., Pilpel N., Krizhanovsky V., Sharpe J., et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell. 2013;155:1119–1130. doi: 10.1016/j.cell.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 17.Hayflick L., Moorhead P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 18.Olovnikov A.M. The role of incomplete terminal repair of chromosomal DNA in the aging of neurons and postmitotic organisms. Biol. Bull. 1995:504. [PubMed] [Google Scholar]
- 19.Hewitt G., Jurk D., Marques F.D., Correia-Melo C., Hardy T., Gackowska A., Anderson R., Taschuk M., Mann J., Passos J.F. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat. Commun. 2012;3:708. doi: 10.1038/ncomms1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Passos J.F., Saretzki G., Ahmed S., Nelson G., Richter T., Peters H., Wappler I., Birket M.J., Harold G., Schaeuble K., et al. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol. 2007;5:e110. doi: 10.1371/journal.pbio.0050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Correia-Melo C., Marques F.D., Anderson R., Hewitt G., Hewitt R., Cole J., Carroll B.M., Miwa S., Birch J., Merz A., et al. Mitochondria are required for pro-ageing features of the senescent phenotype. EMBO J. 2016;35:724–742. doi: 10.15252/embj.201592862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verhoeven J.E., Revesz D., Wolkowitz O.M., Penninx B.W.J.H. Cellular aging in depression: Permanent imprint or reversible process? Bioessays. 2014;36:968–978. doi: 10.1002/bies.201400068. [DOI] [PubMed] [Google Scholar]
- 23.Liguori I., Russo G., Curcio F., Bulli G., Aran L., Della-Morte D., Gargiulo G., Testa G., Cacciatore F., Bonaduce D., et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller M.W., Alex P., Lin A.P., Wolf E.J., Miller D.R. Oxidative Stress, Inflammation, and Neuroprogression in Chronic PTSD. Harv. Rev. Psychiatry. 2018;26:57–69. doi: 10.1097/HRP.0000000000000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pisoschi A.M., Pop A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 26.Roy J., Galano J.M., Durand T., Le Guennec J.Y., Lee J.C. Physiological role of reactive oxygen species as promoters of natural defenses. FASEB J. 2017;31:3729–3745. doi: 10.1096/fj.201700170R. [DOI] [PubMed] [Google Scholar]
- 27.Forrester S.J., Kikuchi D.S., Hernandes M.S., Xu Q., Griendling K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018;122:877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kowaltowski A.J., De Souza-Pinto N.C., Castilho R.F., Vercesi A.E. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 2009;47:333–343. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tylee D.S., Chandler S.D., Nievergelt C.M., Liu X., Pazol J., Woelk C.H., Lohr J.B., Kremen W.S., Baker D.G., Glatt S.J., et al. Resiliency Study Investigators. Blood-based gene-expression biomarkers of post-traumatic stress disorder among deployed marines: A pilot study. Psychoneuroendocrinology. 2015;51:472–494. doi: 10.1016/j.psyneuen.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Logue M.W., Smith A.K., Baldwin C., Wolf E.J., Guffanti G., Ratanatharathorn A., Stone A., Schichman S.A., Humphries D., Binder E.B., et al. An analysis of gene expression in PTSD implicates genes involved in the glucocorticoid receptor pathway and neural responses to stress. Psychoneuroendocrinology. 2015;57:1–13. doi: 10.1016/j.psyneuen.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rose G., Crocco P., De Rango F., Montesanto A., Passarino G. Further support to the uncoupling-to survive theory: The genetic variation of human UCPgenes is associated with longevity. PLoS ONE. 2011;6:e29650. doi: 10.1371/journal.pone.0029650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olivieri F., Albertini M.C., Orciani M., Ceka A., Cricca M., Procopio A.D., Bonafè M. DNA damage response (DDR) and senescence: Shuttled inflamma-miRNAs on the stage of inflamm-aging. Oncotarget. 2015;6:35509–35521. doi: 10.18632/oncotarget.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baëhl S., Garneau H., Le Page A., Lorrain D., Viens I., Svotelis A., Lord J.M., Phillips A.C., Cabana F., Larbi A., et al. Altered neutrophil functions in elderly patients during a 6-month follow-up period after a hip fracture. Exp. Gerontol. 2015;65:58–68. doi: 10.1016/j.exger.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Linton P.J., Thoman M.L. Immunosenescence in monocytes, macrophages, and dendritic cells: Lessons learned from the lung and heart. Immunol. Lett. 2014;162:290–297. doi: 10.1016/j.imlet.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fulop T., Larbi A., Dupuis G., Le Page A., Frost E.H., Cohen A.A., Witkowski J.M., Franceschi C. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2018;8:1960. doi: 10.3389/fimmu.2017.01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaw A.C., Goldstein D.R., Montgomery R.R. Age-dependent dysregulation of innate immunity. Nat. Rev. Immunol. 2013;13:875–887. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Saux S., Weyand C.M., Goronzy J.J. Mechanisms of immunosenescence: Lessons from models of accelerated immune aging. Ann. N. Y. Acad. Sci. 2012;1247:69–82. doi: 10.1111/j.1749-6632.2011.06297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gounder S.S., Abdullah B.J.J., Radzuanb N.E.I.B.M., Zain F.D.B.M., Sait N.B.M., Chua C., Subramani B. Effect of Aging on NK Cell Population and Their Proliferation at Ex Vivo Culture Condition. Anal. Cell. Pathol. 2018;2018:7871814. doi: 10.1155/2018/7871814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nilsson B.O., Ernerudh J., Johansson B., Evrin P.E., Löfgren S., Ferguson F.G., Wikby A. Morbidity does not influence the T-cell immune risk phenotype in the elderly: Findings in the Swedish NONA Immune Study using sample selection protocols. Mech. Ageing Dev. 2003;124:469–476. doi: 10.1016/S0047-6374(03)00024-1. [DOI] [PubMed] [Google Scholar]
- 41.Olsson J., Wikby A., Johansson B., Löfgren S., Nilsson B.O., Ferguson F.G. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: The Swedish longitudinal OCTO immune study. Mech. Ageing Dev. 2000;121:187–201. doi: 10.1016/S0047-6374(00)00210-4. [DOI] [PubMed] [Google Scholar]
- 42.Tabibian-Keissar H., Hazanov L., Schiby G., Rosenthal N., Rakovsky A., Michaeli M., Shahaf G.L., Pickman Y., Rosenblatt K., Melamed D., et al. Aging affects B-cell antigen receptor repertoire diversity in primary and secondary lymphoid tissues. Eur. J. Immunol. 2016;46:480–492. doi: 10.1002/eji.201545586. [DOI] [PubMed] [Google Scholar]
- 43.Appay V., Sauce D. Naive T cells: The crux of cellular immune aging? Exp. Gerontol. 2014;54:90–93. doi: 10.1016/j.exger.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Larbi A., Fulop T. From “truly naïve” to “exhausted senescent” T cells: When markers predict functionality. Cytom. A. 2014;85:25–35. doi: 10.1002/cyto.a.22351. [DOI] [PubMed] [Google Scholar]
- 45.Dock J.N., Effros R.B. Role of CD8 T Cell Replicative Senescence in Human Aging and in HIV-mediated Immunosenescence. Aging Dis. 2011;2:382–397. [PMC free article] [PubMed] [Google Scholar]
- 46.Fessler J., Ficjan A., Duftner C., Dejaco C. The impact of aging on regulatory T-cells. Front. Immunol. 2013;4:231. doi: 10.3389/fimmu.2013.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De la Fuente M., Miquel J. An update of the oxidation-inflammation theory of aging: The involvement of the immune system in oxi-inflamm-aging. Curr. Pharm. Des. 2009;15:3003–3026. doi: 10.2174/138161209789058110. [DOI] [PubMed] [Google Scholar]
- 48.Fülöp T., Larbi A., Witkowski J.M. Human Inflammaging. Gerontology. 2019;65:495–504. doi: 10.1159/000497375. [DOI] [PubMed] [Google Scholar]
- 49.Goronzy J.J., Weyand C.M. Successful and Maladaptive T Cell Aging. Immunity. 2017;46:364–378. doi: 10.1016/j.immuni.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feduccia A.A., Jerome L., Yazar-Klosinski B., Emerson A., Mithoefer M.M., Doblin R. Breakthrough for trauma treatment: Safety and efficacy of MDMA-assisted psychotherapy compared to Paroxetine and Sertraline. Front. Psychiatry. 2019;10:650. doi: 10.3389/fpsyt.2019.00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee B., Sur B., Yeom M., Shim I., Lee H., Hahm D.H. Effects of systemic administration of ibuprofen on stress response in a rat model of post-traumatic stress disorder. Korean J. Physiol. Pharmacol. 2016;20:357–366. doi: 10.4196/kjpp.2016.20.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yehuda R., Golier J.A., Kaufman S. Circadian rhythm of salivary cortisol in Holocaust survivors with and without PTSD. Am. J. Psychiatry. 2005;162:998–1000. doi: 10.1176/appi.ajp.162.5.998. [DOI] [PubMed] [Google Scholar]
- 53.Khoury N.M., Marvar P.J., Gillespie C.F., Wingo A., Schwartz A., Bradley B., Kramer M., Ressler K.J. The renin-angiotensin pathway in posttraumatic stress disorder: Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with fewer traumatic stress symptoms. J. Clin. Psychiatry. 2012;73:849–855. doi: 10.4088/JCP.11m07316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Narita-Ohtaki R., Hori H., Itoh M., Lin M., Niwa M., Ino K., Imai R., Ogawa S., Sekiguchi A., Matsui M., et al. Cognitive function in Japanese women with posttraumatic stress disorder: Association with exercise habits. J. Affect. Disord. 2018;236:306–312. doi: 10.1016/j.jad.2018.02.061. [DOI] [PubMed] [Google Scholar]
- 55.Uemura M., Ohira T., Yasumura S., Otsuru A., Maeda M., Harigane M., Horikoshi N., Suzuki Y., Yabe H., Takahashi H., et al. Association between psychological distress and dietary intake among evacuees after the Great East Japan Earthquake in a cross-sectional study: The Fukushima Health Management Survey. BMJ Open. 2016;6:e011534. doi: 10.1136/bmjopen-2016-011534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bower J.E., Irwin M.R. Mind-body therapies and control of inflammatory biology: A descriptive review. Brain Behav. Immun. 2016;51:1–11. doi: 10.1016/j.bbi.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Michopoulos V., Beurel E., Gould F., Dhabhar F.S., Schultebraucks K., Galatzer-Levy I., Rothbaum B.O., Ressler K.J., Nemeroff C.B. Association of Prospective Risk for Chronic PTSD Symptoms with Low TNFα and IFNγ Concentrations in the Immediate Aftermath of Trauma Exposure. Am. J. Psychiatry. 2020;177:58–65. doi: 10.1176/appi.ajp.2019.19010039. [DOI] [PubMed] [Google Scholar]
- 58.Galluzzi L., Baehrecke E.H., Ballabio A., Boya P., Bravo-San Pedro J.M., Cecconi F., Choi A.M., Chu C.T., Codogno P., Colombo M.I., et al. Molecular definitions of autophagy and related processes. EMBO J. 2017;36:1811–1836. doi: 10.15252/embj.201796697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen T.N., Padman B.S., Lazarou M. Deciphering the Molecular Signals of PINK1/Parkin Mitophagy. Trends Cell Biol. 2016;26:733–744. doi: 10.1016/j.tcb.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 60.Lee J., Giordano S., Zhang J. Autophagy, mitochondria and oxidative stress: Cross-talk and redox signalling. Biochem. J. 2012;441:523–540. doi: 10.1042/BJ20111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sebori R., Kuno A., Hosoda R., Hayashi T., Horio Y. Resveratrol Decreases Oxidative Stress by Restoring Mitophagy and Improves the Pathophysiology of Dystrophin-Deficient mdx Mice. Oxid. Med. Cell Longev. 2018;2018:9179270. doi: 10.1155/2018/9179270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vernucci E., Tomino C., Molinari F., Limongi D., Aventaggiato M., Sansone L., Tafani M., Russo M.A. Mitophagy and Oxidative Stress in Cancer and Aging: Focus on Sirtuins and Nanomaterials. Oxid. Med. Cell Longev. 2019;2019:6387357. doi: 10.1155/2019/6387357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang J., Wang K., Huang C., Lin D., Zhou Y., Wu Y., Tian N., Fan P., Pan X., Xu D., et al. SIRT3 Activation by Dihydromyricetin Suppresses Chondrocytes Degeneration via Maintaining Mitochondrial Homeostasis. Int. J. Biol. Sci. 2018;14:1873–1882. doi: 10.7150/ijbs.27746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang J., Nisar M., Huang C., Pan X., Lin D., Zheng G., Jin H., Chen D., Tian N., Huang Q., et al. Small molecule natural compound agonist of SIRT3 as a therapeutic target for the treatment of intervertebral disc degeneration. Exp. Mol. Med. 2018;50:146. doi: 10.1038/s12276-018-0173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miwa S., Brand M.D. Mitochondrial matrix reactive oxygen species production is very sensitive to mild uncoupling. Biochem. Soc. Trans. 2003;31:1300–1301. doi: 10.1042/bst0311300. [DOI] [PubMed] [Google Scholar]
- 66.Liu D., Chan S.L., De Souza-Pinto N.C., Slevin J.R., Wersto R.P., Zhan M., Mustafa K., De Cabo R., Mattson M.P. Mitochondrial UCP4 mediates an adaptive shift in energy metabolism and increases the resistance of neurons to metabolic and oxidative stress. Neuromol. Med. 2006;8:389–414. doi: 10.1385/NMM:8:3:389. [DOI] [PubMed] [Google Scholar]
- 67.Chouchani E.T., Kazak L., Jedrychowski M.P., Lu G.Z., Erickson B.K., Szpyt J., Pierce K.A., Laznik-Bogoslavski D., Vetrivelan R., Clish C.B., et al. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature. 2016;532:112–116. doi: 10.1038/nature17399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tao H., Zhang Y., Zeng X., Shulman G.I., Jin S. Niclosamide ethanolamine-induced mild mitochondrial uncoupling improves diabetic symptoms in mice. Nat. Med. 2014;20:1263–1269. doi: 10.1038/nm.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Geisler J.G. 2,4 Dinitrophenol as Medicine. Cells. 2019;8:280. doi: 10.3390/cells8030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Caldeira da Silva C.C., Cerqueira F.M., Barbosa L.F., Medeiros M.H., Kowaltowski A.J. Mild mitochondrial uncoupling in mice affects energy metabolism, redox balance and longevity. Aging Cell. 2008;7:552–560. doi: 10.1111/j.1474-9726.2008.00407.x. [DOI] [PubMed] [Google Scholar]
- 71.Padalko V.I. Uncoupler of oxidative phosphorylation prolongs the lifespan of Drosophila. Biochemistry. 2005;70:986–989. doi: 10.1007/s10541-005-0213-1. [DOI] [PubMed] [Google Scholar]
- 72.Zheng Q., Huang J., Wang G. Mitochondria, Telomeres and Telomerase Subunits. Front. Cell Dev. Biol. 2019;7:274. doi: 10.3389/fcell.2019.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jäger K., Walter M. Therapeutic Targeting of Telomerase. Genes. 2016;7:39. doi: 10.3390/genes7070039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fauce S.R., Jamieson B.D., Chin A.C., Mitsuyasu R.T., Parish S.T., Ng H.L., Kitchen C.M., Yang O.O., Harley C.B., Effros R.B. Telomerase-based pharmacologic enhancement of antiviral function of human CD8+ T lymphocytes. J. Immunol. 2008;181:7400–7406. doi: 10.4049/jimmunol.181.10.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bernardes de Jesus B., Schneeberger K., Vera E., Tejera A., Harley C.B., Blasco M.A. The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. Aging Cell. 2011;10:604–621. doi: 10.1111/j.1474-9726.2011.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sprouse A.A., Steding C.E., Herbert B.S. Pharmaceutical regulation of telomerase and its clinical potential. J. Cell. Mol. Med. 2012;16:1–7. doi: 10.1111/j.1582-4934.2011.01460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heiss E.H., Schilder Y.D., Dirsch V.M. Chronic treatment with resveratrol induces redox stress- and ataxia telangiectasia-mutated (ATM)-dependent senescence in p53-positive cancer cells. J. Biol. Chem. 2007;282:26759–26766. doi: 10.1074/jbc.M703229200. [DOI] [PubMed] [Google Scholar]
- 78.De la Lastra C.A., Villegas I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 2007;35:1156–1160. doi: 10.1042/BST0351156. [DOI] [PubMed] [Google Scholar]
- 79.Singh A., Sati S., Mishra R. Resveratrol: Antioxidant-prooxidant. Int. J. Technol. Res. Sci. 2016;1:106–112. [Google Scholar]
- 80.Wang F., Chang G., Geng X. NGF and TERT co-transfected BMSCs improve the restoration of cognitive impairment in vascular dementia rats. PLoS ONE. 2014;9:e98774. doi: 10.1371/journal.pone.0098774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saretzki G., Murphy M.P., Von Zglinicki T. MitoQ counteracts telomere shortening and elongates lifespan of fibroblasts under mild oxidative stress. Aging Cell. 2003;2:141–143. doi: 10.1046/j.1474-9728.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- 82.Liao N., Shi Y., Zhang C., Zheng Y., Wang Y., Zhao B., Zeng Y., Liu X., Liu J. Antioxidants inhibit cell senescence and preserve stemness of adipose tissue-derived stem cells by reducing ROS generation during long-term in vitro expansion. Stem Cell Res. Ther. 2019;10:306. doi: 10.1186/s13287-019-1404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anisimov V.N., Egorov M.V., Krasilshchikova M.S., Lyamzaev K.G., Manskikh V.N., Moshkin M.P., Novikov E.A., Popovich I.G., Rogovin K.A., Shabalina I.G., et al. Effects of the mitochondria-targeted antioxidant SkQ1 on lifespan of rodents. Aging. 2011;3:1110–1119. doi: 10.18632/aging.100404. [DOI] [PMC free article] [PubMed] [Google Scholar]