Abstract
Cocoa and its products are rich sources of polyphenols such as flavanols. These compounds exert antioxidant and anti-inflammatory activities, accountable for cocoa health-promoting effects. However, cocoa polyphenols are poorly absorbed in the intestine, and most of them cannot reach the systemic circulation in their natural forms. Instead, their secondary bioactive metabolites are bioavailable, enter the circulation, reach the target organs, and exhibit their activities. In fact, once reaching the intestine, cocoa polyphenols interact bidirectionally with the gut microbiota. These compounds can modulate the composition of the gut microbiota exerting prebiotic mechanisms. They enhance the growth of beneficial gut bacteria, such as Lactobacillus and Bifidobacterium, while reducing the number of pathogenic ones, such as Clostridium perfringens. On the other hand, bioactive cocoa metabolites can enhance gut health, displaying anti-inflammatory activities, positively affecting immunity, and reducing the risk of various diseases. This review aims to summarize the available knowledge of the bidirectional interaction between cocoa polyphenols and gut microbiota with their various health outcomes.
Keywords: cocoa, polyphenols, flavanols, bioavailability, gut microbiota, human health
1. Introduction
Cocoa and its derivatives are abundantly consumed worldwide due to their pleasant taste and numerous functional effects. To date, several studies have demonstrated that consumption of cocoa and its products decreases the risk of cardiovascular diseases and metabolic disorders, positively affects the immune and nervous systems, prevents the risk of cancer, and shows systemic and intestinal anti-inflammatory activities [1]. Cocoa phytochemical constituents, particularly polyphenols, are strongly associated with health-promoting benefits. Cocoa polyphenols have effective antioxidant and anti-inflammatory properties, interacting with various relevant pathways associated with several health advantages. For instance, flavanol-rich cocoa and cocoa derivatives increase nitric oxide (NO) synthesis, augment flow-mediated dilation (FMD) and enhance microcirculation [2,3], induce vasodilation and reduce blood pressure [4], attenuate platelet aggregation and improve endothelial and vascular function [1,3,4,5,6,7,8].
Furthermore, cocoa flavanols regulate lipid synthesis and degradation and glucose homeostasis and reduce the risk of obesity-induced metabolic disorders due to an increase in the expression of Peroxisome Proliferator-Activated Receptors, specifically PPAR-γ [2,5,9,10]. Cocoa flavanols also exert neuroprotective properties and improve cognition, as they can cross the blood–brain barrier (BBB) into the brain [11]. Their neuroprotective actions can be direct by enhancing MAPK, ERK, PI3 signaling pathways related to an increase in brain-derived neurotrophic factor (BDNF) expression, which promotes neurogenesis and the growth of synaptic connection and neuronal viability. Indirectly, they can exert their action by improving endothelial function, increasing blood flow, and glucose supply to the brain [12,13]. In elderly and memory-declined subjects, cocoa flavanols have been shown to induce a positive modulation of cognitive performance and improvements in several cognitive domains [14].
In recent years, the scientific community has begun to study cocoa mechanisms of action, by considering the interaction between cocoa polyphenols and the gastrointestinal microbiota, and the subsequent health outcomes.
The gut microbiota is a complex and diverse community of trillions of microorganisms, having substantial effects on physiology, metabolism, and immunity of the host [15]. Among the many known factors, dietary patterns have a significant impact on the composition and function of the gut microbiota. The influence of diet on microbiota modulation is evident from breastfeeding to the introduction of solid foods throughout life [16,17]. Dietary polyphenols are also known to interact bidirectionally with the gut microbiota and selectively promote or inhibit microbial growth and proliferation [18]. The diversity and specificity of these microorganisms are essential for polyphenol metabolism to produce secondary bioactive metabolites that interact with human biochemical pathways [19].
Likewise, cocoa polyphenols appear to modulate microbial diversity by promoting the proliferation of some bacteria and inhibiting the potentially pathogenic ones, exerting prebiotic mechanisms [20,21,22]. Deciphering this bidirectional interaction allows us to understand the mechanism of action of cocoa polyphenols and to customize its dosages and type for human health.
Therefore, this review aims to summarize and examine the metabolism and bioavailability of cocoa polyphenols and their bidirectional interactions with gut microbiota, focusing on cocoa’s prebiotic properties, from the available experimental and clinical studies.
2. Cocoa Composition and Processing
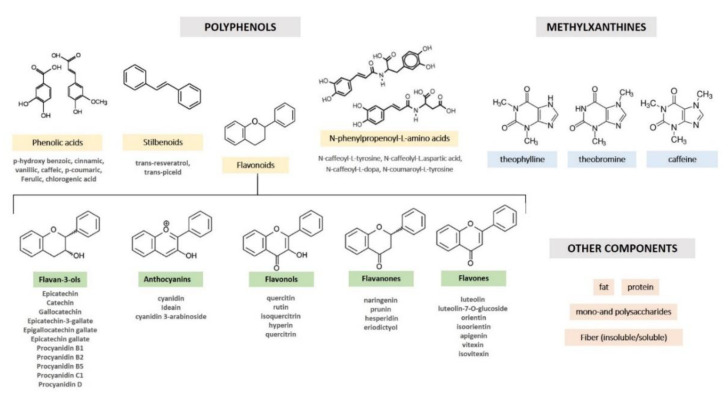
Botanically, cocoa (Theobroma cacao) is a typical evergreen tree belonging to the Sterculiaceae family, originally from tropics of Central and South America and then grown in the other parts of the world [23]. The plant has three main cultivars, with different phytochemical content and sensory properties: Criollo, the first cultivated group, Forastero, the hardier and the most prevalent group, and Trinitario, a cross-breed of both Criollo and Forastero groups [23,24]. Every year, millions of tons of cocoa are produced. The seeds of the fruits, cocoa beans, are then processed into cocoa powder, butter, and liquor [25]. Cocoa beans contain water, lipids, proteins, fibers, and many biologically active compounds [23]. In total, around 380 compounds have been identified in cocoa [1]. Among them, polyphenols and xanthine alkaloids are predominant, comprising nearly 14–20% of the bean weight [24]. Cocoa processing leads to a vast decrease in phytochemical concentration and changes their proportion [1]. In plants, phenolic compounds are involved in antioxidant activity, protection from environmental stressors such as UV radiation, microbial and fungal infection, and accordingly aiding the plant development [26].
Cocoa is especially abundant in flavanols, accounting for around 60% in non-fermented cocoa beans [27]. Cocoa flavanol comprises monomeric forms, (+)-catechin and (−)-epicatechin, and their oligomeric and polymeric forms, procyanidins. The types cocoa flavanols entail (−)–epicatechin, being the most abundant, (+)−catechin, procyanidin B1, and B2, and other flavanols at trace level, such as epigallocatechin, epigallocatechin-3-gallate, procyanidin B2-O-gallate, procyanidin B2-3,3-di-O-gallate, procyanidin B3, procyanidin B4, procyanidin B4-O-gallate, procyanidin C1, and procyanidin D (Table 1) [28]. Other minor polyphenolic compounds include flavones (luteolin, luteolin-7-O-glucoside, orientin, isoorientin, apigenin, vitexin, and isovitexin), flavanones (naringenin, prunin, hesperidin, and eriodictyol), flavonols (quercetin, quercetin-3-O-arabinoside, isoquercitrin, and hyperoside), anthocyanidins (cyanidin, 3-α-l-arabinosidyl cyanidin, 3-β-d-arabinosidyl cyanidin, 3-β-D-galactosidyl cyanidin), and certain phenolic acids (Figure 1) [29].
Table 1.
Phytochemicals’ concentrations in cocoa powder.
| Phytochemical | Mg/g of Fresh Weight | Reference | |
|---|---|---|---|
| Flavanols | (−)-Epicatechin | 0.63- 3.3 | [37,38] |
| (+)-Catechin | 0.61–2.02 | [36,38] | |
| Procyanidin B1 (dimer) | 1.12 | [38] | |
| Procyanidin B2 (dimer) | 0.13- 2.62 | [37,38] | |
| Procyanidin C1 (trimer) | 0.05- 0.36 | [37] | |
| Cinnamtannin A2 (tetramer) | 0.31–0.56 | [37] | |
| Galactopyranosyl-ent-(−)-epicatechin (2alpha-->7, 4alpha->8)-(−)-epicatechin (Gal-EC-EC) | 0.02–0.07 | [37] | |
| Flavonols | Quercetin | 0.0032–0.2 | [39,40] |
| Isoquercitrin | 0.03334–0.04664 | [39] | |
| Quercetin-3-Arabinoside | 0.05258–0.09212 | [39] | |
| Quercetin 3-O-glucuronide | 0.00938–0.01512 | [39] | |
| Phenolic acids | |||
| Hydroxybenzoic acids | Benzoic acid | 0.00017–0.00097 | [41] |
| Hydroxycinnamic acids | Caffeoyl aspartate | 0.37 | [38] |
| Hydroxybenzaldehydes | Vanillin | 0.00014–0.00232 | [41] |
| Methylxanthines | Theobromine | 4.11–27.69 | [42,43] |
| Caffeine | 0.946–6.58 | [42,43] | |
| Others | Catechol | 0.0012 | [44] |
| Pyrogallol | 0.0018 | [44] |
Figure 1.
Nomenclature and chemical structures of cocoa phytocomplex.
Cocoa polyphenolic profile and concentration varies depending on the type of cultivar, quality of crop and cultivation site, geographic area, and the climate. Its content also considerably varies among different cocoa products, influenced by processing and manufacturing steps [23,24]. To estimate their variations, researchers examined the polyphenol profiles and antioxidant properties of different cocoa liquor samples from six different geographic places (Madagascar, Mexico, Ecuador, Venezuela, Sao Tome, and Ghana). They observed that similar types of polyphenol compounds are present, but their proportion and antioxidant activities are different. Among them, (−)-epicatechin ranged from 0.16 to 0.59 mg/g defatted cocoa liquorsDCL and (+)-catechin varied between 0.02 and 0.42 mg/g DCL. (−)-Gallocatechin was not found in Ghana and Venezuela cocoa liquors but in other samples ranged from 0.06 to 0.40 mg/g DCL. Likewise, (−)-epigallocatechin content estimated to vary between 0.15 and 0.42 mg/g DCL, although it was not detected in Mexico and Venezuela samples. Overall, total polyphenolic contents were in the following order: Madagascar > Mexico > Ecuador > Venezuela > Sao Tome > Ghana. They also observed that higher polyphenolic content corresponds to higher antioxidant activity, indicating the contributing role of polyphenols in the antioxidant property of cocoa [24].
The production of cocoa products from cocoa beans takes several steps, and its chemistry changes by each step of the processing. It starts with drying and a five-to-seven day of fermentation, which is carried out in specific containers with a temperature of 45–50 Celsius [25]. The beans are then broken to separate the nib from its shell and subsequently sterilized. This is followed by alkalization process by an alkali solution of potassium or sodium carbonate at a temperature of 80–100 Celsius [30]. Subsequently, the alkalized product is roasted and ground to reduce the nibs to liquor or pressed to separate the fat content from the powder and eventually to produce cocoa powder and butter [31]. These final products are widely used to make chocolate, candies, and other cocoa derivatives.
Fermentation is an essential step in cocoa bean processing, during which cocoa polyphenols are oxidized enzymatically by polyphenol oxidase or non-enzymatically; then, they are polymerized and bind with proteins, resulting in high-molecular compounds (tannins) with reduced solubility and astringency. Eventually, these alterations contribute to the final product color and flavor [32,33]. During this step, epicatechins and soluble polyphenols reduce by 10–20% due to oxidation and cocoa sweating. Anthocyanins, which gradually vanish during fermentation, are used as an index for determining the fermentation degree since they hydrolyze into anthocyanidins and polymerize with catechins to form tannins. Likewise, procyanidin level and fermentation degree are negatively associated, with their levels being reported to decrease three to five-fold once cocoa is fermented [34].
On the other hand, during fermentation, the total polyphenol amount is positively influenced by the presence of lactic acid bacteria, acetic acid bacteria, and yeasts. In contrast, molds and aerobic spores have the opposite effect. Notably, it is described that a more extended fermentation period leads to the growth of fungi and the production of aerobic spores, which in turn have a negative impact on polyphenol quantity and quality [32].
Considerable loss of cocoa polyphenols takes place during drying, alkalization, and roasting as well [24]. During drying, the polyphenol content is substantially reduced by enzymatic browning. For instance, a 2-day drying of cocoa beans causes a 50% reduction in epicatechin [34]. Depending on temperature and timing, the roasting step also results in reduced astringent taste due to the degradation of the polyphenols [31]. In a study, researchers observed that (−)-epicatechin and (+)-catechin enormously decreased once cocoa fermented, while the enantiomer, (−)-catechin, is formed. In the same manner, high-level roasting reduced (−)-epicatechin and (+)-catechin and increased (−)-catechin and alkalization led to a continuous decrease of the monomeric flavanols and a lesser loss of (−)-catechin [35].
In a study to determine the changes during cocoa processing, researchers noticed that roasting and alkalization of cocoa nibs had a major effect in altering the polyphenol content. Roasting resulted in a 27% loss of (−)-epicatechin, from 2.23 mg/g to 1.63 mg/g, while the (+)-catechin content increased from 0.28 mg/g to 0.33 mg/g, which was suggested to be due to its epimerization into (−)-catechin. Procyanidin B1 decreased by 57%, from 0.28 mg/g to 0.12 mg/g and procyanidin B2 declined by 30%, from 0.63 mg/g to 0.44 mg/g. Grounding of the roasted cocoa beans into cocoa liquor led to decrease in (−)-epicatechin by 25%. Subsequently, preparing cocoa baking chocolate further declined the (−)-epicatechin, (+)-catechin, procyanidin B1 and procyanidin B2 into 0.52 mg/g, 0.24 mg/g, 0.04 mg/g and 0.16 mg/g, respectively. Furthermore, alkalizing and drying the roasted and grounded cocoa beans reduced (−)-epicatechin procyanidin B1 by 64% and 60% to 0.49 mg/g and 0.05 mg/g, respectively. Procyanidin B2 decreased by 80% while catechin increased by 27% [36].
Therefore, depending on the type of cocoa processing, the polyphenol content varies considerably, and this must be considered for cocoa health outcomes (Table 2). Furthermore, information on these profile changes helps to find the optimal conditions to reduce the loss of the polyphenol content during processing.
Table 2.
(+)-catechin and (−)-epicatechin content of cocoa bean and its derivatives.
| Source | Quantity (mg/g) | Reference | |
|---|---|---|---|
| (+)-Catechin | (−)-Epicatechin | ||
| Cocoa bean | 0.28 | 2.23 | [33] |
| Roasted cocoa bean | 0.33 | 1.63 | [33] |
| Alkalised cocoa nibs | 0.26 | 0.58 | [33] |
| Alkalised cocoa powder | 0.60 | 0.88 | [33] |
| Dried alkalised cocoa nibs | 0.33 | 0.49 | [33] |
| Cocoa liquor | 0.34 | 1.22 | [33] |
| Cocoa liquor (Venezuela) | 0.14 | 0.74 | [37] |
| Cocoa liquor (Brazil) | 0.63 | 5.77 | [37] |
| Baking chocolate | 0.24 | 0.52 | [33] |
| Dark chocolate (Albert Heijn) | 0.1324 | 0.3274 | [45] |
| Dark chocolate (Verkade) | 0.1075 | 0.5025 | [45] |
| Milk chocolate | 0.05–0.12 | 0.18–0.24 | [36] |
| Milk chocolate (Albert Heijn) | 0.0383 | 0.1249 | [45] |
| Milk chocolate (Verkade) | 0.0269 | 0.1261 | [45] |
| Baking chips | 0.26–0.50 | 0.66–1.07 | [36] |
| Chocolate candy bar | 0.0217 | 0.0625 | [45] |
3. Cocoa Polyphenols Metabolism and Bioavailability
Polyphenols represent a large heterogeneous group of compounds that in plants are generally found as aglycones, esters, glycosides, and polymers. Once ingested, they are recognized by the human body as xenobiotics, and their absorption rate depends on the complexity of their chemical structures more than their concentration. Polyphenols generally have low bioaccessibility and bioavailability, and most of them cannot be absorbed in their natural forms. The metabolic fate of polyphenols is influenced by multiple factors: interpersonal differences in polyphenol metabolisms (genetic polymorphisms of metabolic enzymes, efflux pumps, and transporters, etc.), bidirectional interaction with the intestinal microbiota, and synergies or antagonism with other xenobiotics or nutrients [46]. All of these mechanisms modulate the polyphenol rate of absorption, distribution, metabolism, and excretion.
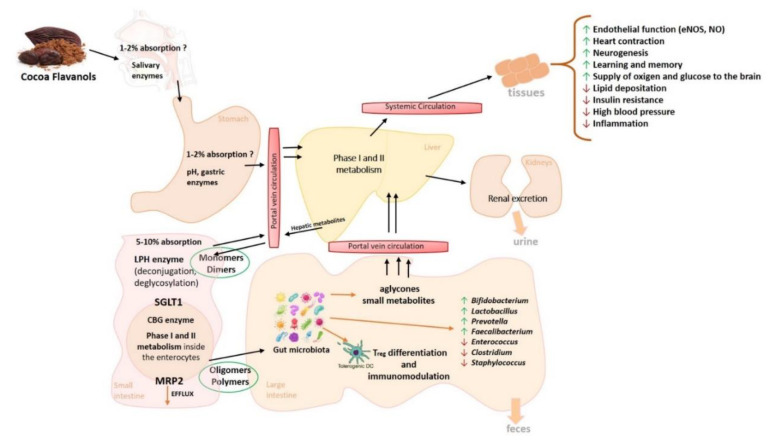
Instead, the type of metabolism strongly affects the fate of polyphenols in the human body. In particular, once ingested, polyphenols need to be extensively modified by hydrolyzation, conjugation, and microbial degradation into their secondary metabolites that are bioactive and bioavailable [47]. Colonic microbial biotransformation of polyphenols seems to be the most effective way to produce small bioavailable secondary metabolitesable to enter the circulation, reach the target organs, and exhibit their activities [47,48]. It is estimated that only 5–10% of polyphenols are absorbed in the small intestine; the remaining 90–95% reach the colon, where they undergo fermentation processes by the intestinal microbiota into their metabolites with various physiological consequences that influence intestinal ecology and human health (Figure 2) [49,50].
Figure 2.
Bioavailability and properties of cocoa polyphenols.
Cocoa polyphenols’ metabolism begins in the oral cavity. In saliva, flavonoid glucosides are converted into aglycones and subsequently into other bioactive compounds that are absorbable by oral epithelium. Once in the stomach, oligomeric polyphenols are transformed into their monomeric units [51]. Cocoa polyphenol metabolites absorbed in the small intestine reach the liver via the portal circulation, and they undergo phase I and II biotransformation reactions, producing new metabolites. Phase I enteric and hepatic biotransformation involves oxidation, reduction, and hydrolysis of the metabolites. The following Phase II involves their conjugation that leads to the formation of [46,52] water-soluble conjugated metabolites (glucuronide, sulfate, and methyl derivatives), rapidly released into the systemic circulation for subsequent release in the target organs [53,54]. Therefore, these various metabolites reach their plasma peak nearly 2 h following oral intake [27,49]. Several sulfates, glucuronide, and methyl metabolites of epicatechin have been identified in the plasma and urine after ingestion of 100 g of dark chocolate [55].
The remaining glycosides, esters, and polymers require hydroxylation by small intestinal enzymes, or they reach the colon and undergo extensive metabolism by the intestinal microbiota [46,56]. As a result, two main metabolites, phenyl-γ-valerolactone and phenylvaleric acid, can be found in the urine after 5–10 h from oral intake of cocoa or its products, and they represent two main cocoa metabolites to be potentially used as a marker of cocoa polyphenol biotransformation [57].
The biotransformation of (−)–epicatechin and its procyanidin B2 dimer by human fecal microbiota has been assessed in vitro. No more than 10% of procyanidin B2 was converted to epicatechin by cleavage of the inter-flavanic bond. Some phenolic acid catabolites have been shown for both substrates. The dominant catabolites detected were 5-(3’-hydroxy phenyl) valeric (9), 3–(3’-hydroxyphenyl) propionic (10) acid and phenylacetic acid [58].
Cocoa epicatechin availability is strongly influenced by the intestinal microbiota. In a recent randomized, blinded crossover study on healthy men and women, the bioavailability and metabolites of polyphenols contained in two soluble cocoa products were evaluated: a standard and a flavanol-rich product [59]. Among the multiple metabolites, some phase II derivatives of epicatechin were consistent with the first absorption of cocoa flavanols in the small intestine. However, the predominant group of identified metabolites corresponded to those formed in the colon, such as hydroxyphenyl-γ-valerolatones and phenylvaleric acid. In the urine 5- (hydroxyphenyl)–γ-valerolactone 30-sulfate has been detected at high concentrations and could be used as a biomarker for the intake of flavanol-rich foods [59].
Recently, to increase cocoa polyphenol bioavailability, different cocoa-based interventions have been developed [60,61]. In food, microencapsulation of cocoa polyphenols in cocoa and hazelnut creams or the addition of bioactive components such as dietary fiber and other polyphenols in new soluble cocoa products are described [61]. In most studies, however, it has been shown and partially confirmed that flavanols and their circulating metabolites mediate the health benefits of cocoa consumption underlying the significant importance of cocoa flavanol metabolism by the intestinal microbiota.
In oral cavity, flavonoids glucosides are converted into aglycones and subsequently absorbed. In the stomach, some polyphenols undergo a first reduction into monomeric units [51]. Then, in the small intestine, a small amount of polyphenols are absorbed, mainly after de-conjugation reactions such as de-glycosylation. Aglycones can be absorbed in the small intestine, whereas glycosides, esters, and polymers typically require a first hydroxylation by the small intestinal enzymes or they reach colon microflora [46,56] and undergo microbial degradation. In addition, polyphenols absorbed in the upper part of the gastrointestinal tract—metabolized by the liver and excreted in the bile or directly extruded by the efflux pumps of the small intestine enterocytes—reach the colon and experience microbial fermentation or fecal elimination [52].
Polyphenol glycosides are hydrolyzed by two main enzymatic mechanisms in the small intestine: Lactase phenylin hydrolase (LPH) and cytosolic β-glucosidase (CBG). Once absorbed in the small intestine, residual polyphenolic compounds undergo Phase I enteric and hepatic biotransformation (oxidation, reduction, and hydrolysis) and, subsequently, Phase II reactions (conjugation) [46,52]. These transformations generate water-soluble conjugated metabolites (glucuronide, sulfate, and methyl derivatives), are released into the systemic circulation, and reach the target organs [53,54]. In the large intestine, microbial enzymes influence the 90–95% unabsorbed polyphenols and sequentially produce bioactive metabolites with various physiological consequences that influence intestinal ecology and affect human health [62,63]. Legend: MRP2 (Multidrug Resistance Associated Protein 2), SGLT1 (sodium-dependent glucose transporter 1); LPH (lactase phenylin hydrolase); CBG (cytosolic β-glucosidase).
4. Cocoa Polyphenols and Gut Microbiota
There is ongoing research to reveal how cocoa polyphenols shape gut microbiota and what effects the microorganisms, in turn, have on polyphenol metabolism and human health. Recent studies argue that unmodified dietary polyphenols, as well as part of the aglyconic metabolites, may alter the microflora community by exhibiting prebiotic effects and selective antimicrobial action against pathogenic intestinal microbes [47,64,65].
Out of the many gut microbial species, Escherichia coli, Bifidobacterium sp., Lactobacillus sp., Bacteroides sp., and Eubacterium sp. are shown to be mostly responsible for the cocoa’s polyphenol metabolism (Figure 2) [18]. Colonic microflora transform cocoa polyphenols into small bioactive compounds, which have the ability to influence the intestinal ecology and to be better absorbed into the systemic circulation instead of aglycones produced in the upper part of the gastrointestinal tract [47]. Polyphenol supplementation enhances the growth of beneficial gut bacteria, such as Lactobacillus and Bifidobacterium, while reduces the number of Clostridium pathogenic species, for instance Clostridium perfringens [66].
The two-way interaction between cocoa polyphenols and intestinal microbiota with the health benefits related to their prebiotic properties is discussed in the following paragraphs and summarized in Table 3.
Table 3.
The effect of cocoa and its derivatives on the growth of gut bacteria from in vitro, in vivo and clinical studies.
| Type of Cocoa | Gut Bacteria | Growth Rate | Health Outcome | Reference | |||
|---|---|---|---|---|---|---|---|
| Phylum | Genus | Species | |||||
| In vitro studies | Water-insoluble cocoa fraction (WICF) of alkali-treated commercial cocoa powder. | Actinobacteria | Bifidobacterium | Increased | Exertion of antioxidant action by insoluble polyphenols | [68] | |
| Firmicutes | Lactobacillus | ||||||
| Commercial, non-alkali treated, defatted cocoa powder | Firmicutes | Lactobacillus | casei | Increased | - Diminished adherence and invasion ability of pathogenic gut bacteria | [69] | |
| Lactobacillus | rhamnosus | ||||||
| Lactobacillus | plantarum | ||||||
| Lactobacillus | acidophilus | ||||||
| Bacillus | subtilis | ||||||
| Enterococcus | faecalis | ||||||
| Streptococcus | thermophilus | ||||||
| Proteobacteria | Escherichia | coli O157:H7 | Decreased | ||||
| Salmonella | Typhimurium | ||||||
| Firmicutes | Listeria | monocytogenes | |||||
| In vivo studies | Natural Forastero cocoa powder | Bacteroidetes | Bacteroides | Decreased | - Lower body weight gain - Downregulated colon TLR2 and TLR7 and TLR9 - Reduced intestinal IgA concentration - Prevented age-related percentage increase of IgA-coating bacteria |
[70] | |
| Firmicutes | Staphylococcus | ||||||
| Clostridium | |||||||
| Cocoa husks | Bacteroidetes | Bacteroides−Prevotella | Increased | - Increased fibrous fractions of the diet, mainly ADL | [71] | ||
| Faecalibacterium | prausnitzii | ||||||
| Firmicutes | Lactobacillus−Enterococcus | Decreased | |||||
| Clostridium | histolyticum | ||||||
| Flavanol-enriched cocoa powder (Acticoa) | Firmicutes | Lactobacillus | Increased | - Decreased TNF-a, TLR2, TLR4 and TLR9 expression of intestinal tissues | [72] | ||
| Actinobacteria | Bifidobacterium | ||||||
| Natural Forastero cocoa powder | Firmicutes | Blautia | Hansenii wexleare others | Increased | - Reduced body weight - Decreased glycaemia, insulinaemia and HbA1c - Lower insulin resistance state and enhanced beta-cell function - Improved glucose tolerance - Greater crypt depth - Promoted colonocyte proliferation and apoptosis - Elevated mucin glycoprotein Increased Zonula occludens-1 (ZO-1) proteins - Partially dropped level of TNFα, IL-6, MCP-1 and CD45 lin the colonic mucosa |
[73] | |
| Bacteroidetes | Flavobacterium | ||||||
| Deferribacteres | |||||||
| Cyanobacteries | |||||||
| Firmicutes | Enterococcus | Decreased | |||||
| Lactobacillus | |||||||
| Bacteroidetes | Parabacteroides | Goldsteinii distasonis others | |||||
| Proteobacteria | Sutterella | ||||||
| Cocoa powder | Firmicutes | Lactobacillus | reuteri | Increased | - Lower intestinal IgA concentration | [74] | |
| Anaerostipes | |||||||
| Bacteroidetes | Prevotella | ||||||
| Bacteroides | uniformis | ||||||
| Tenericutes | |||||||
| Cyanobacteria | |||||||
| Firmicutes | Ruminococcus | flavefaciens | Decreased | ||||
| Holdemania | |||||||
| Proteobacteria | |||||||
| Natural Forastero cocoa powder | Firmicutes | Butyrivibrio | Increased | - Lower body weight gain - Higher SCFA (acetic, propionic, butyric and formic acids) - Smaller percentages of fecal IgA-coated bacteria |
[77] | ||
| Bacteroidetes | Prevotella | ||||||
| Cyanobacteria | |||||||
| Firmicutes | Anaerotruncus | Decreased | |||||
| Bacteroidetes | Bacteroides | acidifaciens | |||||
| Human studies | Dairy-based cocoa-beverage mixes (standardized flavanol content) | Actinobacteria | Bifidobacterium | Increased | - Decreased total cholesterol concentrations - Reduced plasma triacylglycerol concentrations - Lowered Plasma CRP concentrations |
[65] | |
| Firmicutes | Lactobacillus | ||||||
| Enterococcus | |||||||
| Eubacterium | rectale | ||||||
| Clostridium | coccoides | ||||||
| Firmicutes | Clostridium | histolyticum | Decreased | ||||
| Dark chocolate made from Trinitario cocoa beans | Firmicutes | Lactobacillus | Increased | - Diminished Inflammatory Oxidative Damage (IOD) - Reduced LDL-Px - Increased lipoprotein O2 - Reduced corneocyte exfoliation |
[82] | ||
4.1. In Vitro and In Vivo Studies
Several in vitro and in vivo studies underlying cocoa polyphenol interactions with the gut microbiota have been conducted. Flavanols positively influence the growth rate of Bifidobacterium and Lactobacillus but reduce the number of Clostridium histolyticum, as demonstrated by Tzounis et al., when they incubated fecal bacteria with flavanol. They also noted that flavanol does not have any considerable effect on the growth of Clostridium coccoides and Eubacterium rectale. Further, over the incubation period, a decrease of flavanol forms was observed, indicating their degradation by fecal microbiota [67].
Fogliano et al. examined the in vitro digestion of water-insoluble cocoa fraction (WICF) and its fermentation by gut enzymes and human colonic models [68]. WICF exerted prebiotic activities, by forming, in the last section of the gastrointestinal tract, a high concentration of 3-hydroxyphenylpropionic acid (3-HPP) as a result of flavanol bacterial biotransformation into phenolic acids [68].
The addition of cocoa powder in dairy products stimulates the growth of probiotic bacteria, without increasing the risk of cross-contamination with enteric pathogens. As shown in an in vitro study, where the addition of 3% cocoa powder significantly stimulated the abundance of beneficial bacteria, including Lactobacillus and other bacteria residing in milk. In contrast, the growth of three main food pathogens, Escherichia coli enterohemorrhagic O157: H7 (EHEC), Salmonella typhimurium, and Listeria monocytogenes were significantly inhibited within 9 h of ingestion. Furthermore, cocoa powder reduced the adhesion and invasion of these pathogens in a dose-dependent manner [69].
Cocoa-enriched food considerably decreases the fecal proportion of Bacteroides, Clostridium, and Staphylococcus genera in rodents and positively modulate the intestinal immune system, by altering colon toll-like receptors (TRR) pattern [70]. Furthermore, cocoa husks exert its positive effect on gut microflora by improving the relative proportion of different gut microbial phyla, as observed by fecal analysis of cocoa-husk fed pig models, in which a decrease in the abundance of Firmicutes (Lactobacillus−Enterococcus group and Clostridium histolyticum) and an increase in Bacteroidetes (Prevotella group and Faecalibacterium prausnitzii) population was reported. The production of short-chain fatty acids (SCFA) by the gut microbial community showed positive health effects, leading to a reduced risk of various intestinal inflammatory diseases [71]. In the same way, cocoa powder alters gut microbial metabolites and increases the growth of Lactobacillus and Bifidobacterium groups in pigs [72].
In an animal model of diabetes, Zucker diabetic fatty (ZDF) rats, cocoa-rich diet modifies diabetes-induced gut microbial changes to that of lean rats. It increases the relative abundance of acetate-producing bacteria, Blautia particularly, while decreases lactate-producing bacteria, mainly Enterococcus and Lactobacillus genera. In ZDF rat colon, cocoa elevates the levels of the tight junction protein Zonula occludens-1 (ZO-1) and mucin glycoprotein and reduces the expression of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and monocyte chemoattractant protein 1 (MCP-1). Furthermore, the biochemical parameters related to glucose homeostasis and intestinal integrity are improved [73].
Similar effects were reported in orally sensitized animal models by Camps-Bossacoma et al., in which cocoa consumption altered microbiota pattern by inducing the number of Tenericutes and Cyanobacteria phyla while reducing Firmicutes and Proteobacteria phyla. Although a cocoa diet did not influence the amount of Bacteroidetes phylum, it increased some families from this phylum, for example, Prevotella genus and Bacteroides uniformis. These results could be associated with cocoa’s polyphenol content since they are increased in humans who consume red wine polyphenols daily, and Prevotella is abundant in people consuming plant-rich diets [74]. In addition, cocoa-fed animals had an increase in the relative abundance of Lactobacillus reuteri, known for its immunomodulatory properties on Th1/Th2 regulation. This could explain the attenuation of Th2 responses induced by cocoa consumption [75] and the regulation of immunological tolerance through stimulation of Treg cells [74,75].
Cocoa polyphenols are not exclusively responsible for gut microflora alterations; other phytochemicals, such as theobromine, influence and modify gut microbiota, as well. As have been examined in rodents, cocoa containing diet that is rich in theobromine and fiber has more modulatory effects on gut microbiota than polyphenols [76]. Similar results were seen in a two-week animal-model study, in which the intake of cocoa or cocoa’s theobromine alone, lowered the amount of E. coli in fecal samples compared to a standard diet, indicating the role of cocoa components in reducing the abundance of gram-negative bacteria. In the same manner, the ingestion of theobromine, but not total cocoa, led to a decrease in Bifidobacterium spp., Streptococcus spp., Clostridium histolyticum, and Clostridium perfingens. These studies suggest that the different outcomes might be a consequence of a counteracting or enhancing association between different cocoa components, namely, polyphenols and fibers, with theobromine [77].
4.2. Clinical Studies
In humans, it seems challenging to determine the exact effects of cocoa polyphenols on the gut. Firstly, because of the low number of clinical trials, to date. Secondly, due to the complex relationship between gut microbes and polyphenols that are not only influenced by each other but also by other factors, such as interpersonal differences in gut microflora, polyphenol concentration, food matrix, and the presence of other nutrients [49,78].
The effect of food matrix has been observed in a twenty-one-participant trial, where the matrix in which the cocoa powder was dissolved, significantly increased or decreased urine concentration of different phenolic acids that are a part of gut microbial degradation pathways of flavanols. Colonic microbial metabolism of cocoa’s flavanol seemed to be affected by the consumption of cocoa with milk, but not water [79], indicating that cocoa polyphenols lead to different biological effects in different conditions.
Among the gut microorganisms, Bifidobacterium and Lactobacillus are examples of bacteria that promote human wellbeing [80]. These bacteria, along with some others, metabolize cocoa polyphenols and their growth would be mutually affected by them. The bifidogenic effect, enhancement of Bifidobacteria growth, by cocoa polyphenols is primarily due to their ability to create a redox environment in the gut that positively selects those bacteria. In a healthy gut, these polyphenols largely lead to beneficiary consequences, and in an unhealthy gut, they cause an improvement of the microbiota composition [81].
Consumption of cocoa flavanol-based food increases the number of Lactobacilli and Bifidobacteria in humans. Subsequently, the presence of these bacteria influences immunological tolerance by promoting the differentiation of the Treg cells that produce Il-10. It is suggested that cocoa flavanols might contribute as prebiotics to maintain this immunomodulation process by interfering with the intestinal microbiota [65]. Additionally, the alteration of the total number of fecal bifidobacteria and lactobacilli is associated with decreased concentration of C-reactive protein (CRP). Then, reduced CRP is linked to lower cardiovascular risk issues [18].
The aforementioned effects of cocoa flavanols were reported by Tzounis et al., in which a four-week consumption of flavonol-enriched cocoa drinks significantly enhanced the growth of Bifidobacterium and Lactobacillus groups but decreased the number of pathogenic bacteria, for example, Clostridium histolyticum. Similarly, the development of Clostridium perfringens was prevented, which is a bacterium with a possible role in the development of colon cancer and contributes to the risk of intestinal inflammatory disease. The findings also showed that the alteration of gut microbiota is linked to a variation in the triglycerides concentration in the blood [18].
A recent clinical trial investigated the prebiotic potential of dark chocolate derived from Trinitario cocoa beans, with or without lycopene incorporated in its matrix, in healthy individuals with moderate obesity. A 10 g bar of dark chocolate was quantified to contain 1.5 mg of catechins, 6.6 mg of epicatechins, 1.9 mg of dimer-B2, 7.5 mg of caffeine, 75 mg of theobromine, 75 µg of phenylethylamine, 55 µg of serotonin, and ≤ 0.1 µg of resveratrol. The results showed that dark chocolate consumption, leads to a decrease in Bacteroidetes abundance but an increase in the relative abundance of Lactobacillus; a reduction of liver associated blood markers of oxidative damage and inflammation and a dose-dependent change in the gut microbiota profile, blood, liver metabolism, skeletal muscle, and skin parameters were noted [82].
5. Conclusions and Future Perspectives
Cocoa and its products have been demonstrated to have many health benefits, primarily due to their polyphenol content. The polyphenolic profile varies between different cocoa cultivars, growing conditions, geographic areas, and processing steps. The bioavailability and health outcomes of cocoa polyphenols depend on their chemical structures and concentration, the host-related factors, and their interaction with other nutrients and the food matrix. Likewise, there might be a counteracting or enhancing association between different cocoa’s components, such as between polyphenols and fibers with theobromine. Many cocoa polyphenols reach the colon, where they are degraded into their smaller metabolites by gut microbiota. Together with their metabolites, these compounds shape the microbial population. Several experimental and clinical studies have reported the prebiotic effects of cocoa, in which they enhance the growth of beneficial bacteria such as Lactobacillus and Bifidobacterium but reduce the number of harmful ones, namely certain species of Clostridium genus. Finally, cocoa consumption health-promoting effects have been observed in numerous studies, primarily through their antioxidant and anti-inflammatory activities. However, the mechanisms are yet to be fully characterized. Therefore, more studies are necessary to understand cocoa polyphenols and the gut bacteria interplay fully and to determine conclusive evidence about the impacts on human health.
Acknowledgments
We thank group members of Solgar Italia Multrinutrient S.p.A. for their thorough review and helpful discussions during the preparation of this manuscript and for their help in elaborating on the search strategy. We also thank Dr. Luca Benotti for the precious graphic support for figures realization.
Author Contributions
Conceptualization, V.S., G.S.; writing—original draft preparation, V.S., S.A., L.M.; writing—review and editing, V.S., G.S., S.D., A.P.; supervision, V.S, S.D., G.S.; funding acquisition, V.S.; G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Andújar I., Recio M.C., Giner R.M., Ríos J.L. Cocoa Polyphenols and Their Potential Benefits for Human Health. Oxidative Med. Cell. Longev. 2012;2012:906252. doi: 10.1155/2012/906252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fraga C.G., Litterio M.C., Prince P.D., Calabró V., Piotrkowski B., Galleano M. Cocoa flavanols: Effects on Vascular Nitric Oxide and Blood Pressure. J. Clin. Biochem. Nutr. 2011;48:63–67. doi: 10.3164/jcbn.11-010FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schroeter H., Heiss C., Balzer J., Kleinbongard P., Keen C.L., Hollenberg N.K., Sies H., Kwik-Uribe C., Schmitz H.H., Kelm M. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci USA. 2006;103:1024–1029. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taubert D., Roesen R., Lehmann C., Jung N., Schömig E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: A randomized controlled trial. JAMA. 2007;298:49–60. doi: 10.1001/jama.298.1.49. [DOI] [PubMed] [Google Scholar]
- 5.Magrone T., Russo M.A., Jirillo E. Cocoa and dark chocolate polyphenols: From biology to clinical Applications. Front. Immunol. 2017;8:677. doi: 10.3389/fimmu.2017.00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scapagnini G., Davinelli S., Di Renzo L., De Lorenzo A., Olarte H.H., Micali G., Cicero A.F., Gonzalez S. Cocoa bioactive compounds: Significance and potential for the maintenance of skin health. Nutrients. 2014;6:3202–3213. doi: 10.3390/nu6083202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davinelli S., Corbi G., Righetti S., Sears B., Olarte H.H., Grassi D., Scapagnini G. Cardioprotection by cocoa polyphenols and ω-3 fatty acids: A disease-prevention perspective on aging-associated cardiovascular risk. J. Med. Food. 2018;21:1060–1069. doi: 10.1089/jmf.2018.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calzavara-Pinton P., Calzavara-Pinton I., Arisi M., Rossi M.T., Scapagnini G., Davinelli S., Venturini M. Cutaneous Photoprotective Activity of a Short-term Ingestion of High-Flavanol Cocoa: A Nutritional Intervention Study. Photochem. Photobiol. 2019;95:1029–1034. doi: 10.1111/php.13087. [DOI] [PubMed] [Google Scholar]
- 9.Ali F., Ismail A., Kersten S. Molecular mechanisms underlying the potential antiobesity-related diseases effect of cocoa polyphenols. Mol. Nutr. Food Res. 2014;58:33–48. doi: 10.1002/mnfr.201300277. [DOI] [PubMed] [Google Scholar]
- 10.Davinelli S., Corbi G., Zarrelli A., Arisi M., Calzavara-Pinton P., Grassi D., De Vivo I., Scapagnini G. Short-term supplementation with flavanol-rich cocoa improves lipid profile, antioxidant status and positively influences the AA/EPA ratio in healthy subjects. J. Nutr. Biochem. 2018;61:33–39. doi: 10.1016/j.jnutbio.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Nehlig A. The neuroprotective effects of cocoa flavanol and its influence on cognitive performance. Br. J. Clin. Pharmacol. 2013;75:716–727. doi: 10.1111/j.1365-2125.2012.04378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Socci V., Tempesta D., Desideri G., De Gennaro L., Ferrara M. Enhancing Human Cognition with Cocoa Flavonoids. Front. Nutr. 2017;4:19. doi: 10.3389/fnut.2017.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sokolov A.N., Pavlova M.A., Klosterhalfen S., Enck P. Chocolate and the brain: Neurobiological impact of cocoa flavanols on cognition and behavior. Neurosci. Biobehav. Rev. 2013;37:2445–2453. doi: 10.1016/j.neubiorev.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Mastroiacovo D., Kwik-Uribe C., Grassi D., Necozione S., Raffaele A., Pistacchio L., Righetti R., Bocale R., Lechiara M.C., Marini C., et al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: The Cocoa, Cognition, and Aging (CoCoA) Study—A randomized controlled trial. Am. J. Clin. Nutr. 2015;101:538–548. doi: 10.3945/ajcn.114.092189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guinane C.M., Cotter P.D. Role of the gut microbiota in health and chronic gastrointestinal disease: Understanding a hidden metabolic organ. Ther. Adv. Gastroenterol. 2013;6:295–308. doi: 10.1177/1756283X13482996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.David L.A., Maurice C.F., Rachel N. Carmody,1 David B. Gootenberg,1 Julie E. Button,1 Benjamin E. Wolfe,1 Alisha V. Ling,3 A. Sloan Devlin,4 Yug Varma,4 Michael A. Fischbach,4 Sudha B. Biddinger,3 Rachel J. Dutton,1 and Peter J. Turnbaugh1,*Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daien C.I., Pinget G.V., Tan J.K., Macia L. Detrimental impact of microbiota-accessible carbohydrate-deprived diet on gut and immune homeostasis: An overview. Front. Immunol. 2017;8:548. doi: 10.3389/fimmu.2017.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardona F., Andrés-Lacueva C., Tulipania S., Tinahones F.J., Queipo-Ortuño M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013;24:1415–1422. doi: 10.1016/j.jnutbio.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Blumberg J.B., Ding E.L., Dixon R., Pasinetti G.M., Villarreal F. The science of cocoa flavanols: Bioavailability, emerging evidence, and proposed mechanisms. Adv. Nutr. 2014;5:547–549. doi: 10.3945/an.114.006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Del Rio D., Costa L.G., Lean M.E.J., Crozier A. Polyphenols and health: What compounds are involved? Nutr. Metab. Cardiovasc. Dis. 2010;20:1–6. doi: 10.1016/j.numecd.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D., et al. Expert consensus document: The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 22.Sonnenburg E.D., Sonnenburg J.L. Starving our microbial self: The deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014;20:779–786. doi: 10.1016/j.cmet.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colombo M.L., Pinorini-Godly M.T., Conti A. Chocolate and Health. Springer Milan; Milano, Italy: 2012. Botany and Pharmacognosy of the Cacao Tree; pp. 41–62. [Google Scholar]
- 24.Redovnikovic I.R., Delonga K., Mazor S., Verica D.U., Caric M., Vorkapic-Furac J. Polyphenolic Content and Composition and Antioxidative Activity of Different Cocoa Liquors. Czech J. Food Sci. 2009;27:330–337. doi: 10.17221/119/2008-CJFS. [DOI] [Google Scholar]
- 25.Schwan R.F., Wheals A.E. The microbiology of cocoa fermentation and its role in chocolate quality. Crit. Rev. food Sci. Nutr. 2004;44:205–221. doi: 10.1080/10408690490464104. [DOI] [PubMed] [Google Scholar]
- 26.Close D.C., McArthur C. Rethinking the role of many plant phenolics – protection from photodamage not herbivores? Oikos. 2002;99:166–172. doi: 10.1034/j.1600-0706.2002.990117.x. [DOI] [Google Scholar]
- 27.Flores M.E.J. Cocoa Flavanols: Natural Agents with Attenuating Effects on Metabolic Syndrome Risk Factors. Nutrients. 2019;11:751. doi: 10.3390/nu11040751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borchers A.T., Keen C.L., Hannum S.M., Gershwin M.E. Cocoa and chocolate: Composition, bioavailability, and health implications. J. Med. Food. 2000;3:77–105. doi: 10.1089/109662000416285. [DOI] [Google Scholar]
- 29.Martín M.A., Ramos S. Cocoa polyphenols in oxidative stress: Potential health implications. J. Funct. Foods. 2016;27:570–588. doi: 10.1016/j.jff.2016.10.008. [DOI] [Google Scholar]
- 30.Miller K.B., Hurst W.J., Payne M.J., Stuart D.A., Apgar J., Sweigart D.S., Ou B. Impact of alkalization on the antioxidant and flavanol content of commercial cocoa powders. J. Agric. Food Chem. 2008;56:8527–8533. doi: 10.1021/jf801670p. [DOI] [PubMed] [Google Scholar]
- 31.Rocha I.S., de Santana L.R.R., Soares S.E., Bispo E.S. Effect of the roasting temperature and time of cocoa beans on the sensory characteristics and acceptability of chocolate. Food Sci. Technol. 2017;37:522–530. doi: 10.1590/1678-457x.16416. [DOI] [Google Scholar]
- 32.Giacometti J., Jolić S.M., Josić D. Chapter 73—Cocoa Processing and Impact on Composition. In: Preedy V., editor. Processing and Impact on Active Components in Food. Academic Press; San Diego, CA, USA: 2015. pp. 605–612. [Google Scholar]
- 33.Jolic S., Redovniković I.R., Marković K., Šipušić D.I., Delonga K. Changes of phenolic compounds and antioxidant capacity in cocoa beans processing. Int. J. Food Sci. Technol. 2011;46:1793–1800. doi: 10.1111/j.1365-2621.2011.02670.x. [DOI] [Google Scholar]
- 34.Wollgast J., Anklam E. Review on polyphenols in theobroma cacao: Changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res. Int. 2000;33:423–447. doi: 10.1016/S0963-9969(00)00068-5. [DOI] [Google Scholar]
- 35.Hurst W.J., Krake S.H., Bergmeier S.C., Payne M.J., Miller K.B., Stuart D.A. Impact of fermentation, drying, roasting and Dutch processing on flavan-3-ol stereochemistry in cacao beans and cocoa ingredients. Chem. Cent. J. 2011;5:53. doi: 10.1186/1752-153X-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller K.B., Stuart D.A., Smith N.L., Lee C.Y., McHale N.L., Flanagan J.A., Ou B., Hurst W.J. Antioxidant activity and polyphenol and procyanidin contents of selected commercially available cocoa-containing and chocolate products in the United States. J. Agric. Food Chem. 2006;54:4062–4068. doi: 10.1021/jf060290o. [DOI] [PubMed] [Google Scholar]
- 37.Natsume M., Osakabe N., Yamagishi M., Takizawa T., Nakamura T., Miyatake H., Hatano T., Yoshida T. Analyses of polyphenols in cacao liquor, cocoa, and chocolate by normal-phase and reversed-phase HPLC. Biosci. Biotechnol. Biochem. 2000;64:2581–2587. doi: 10.1271/bbb.64.2581. [DOI] [PubMed] [Google Scholar]
- 38.Tomas-Barberan F.A., Cienfuegos-jovellanos E., Marìn A., Muguerza B., Gil-Izquierdo A., Cerda B., Zafrilla P., Morillas J., Mulero J., Ibarra A., et al. A new process to develop a cocoa powder with higher flavonoid monomer content and enhanced bioavailability in healthy humans. J. Agric. Food Chem. 2007;55:3926–3935. doi: 10.1021/jf070121j. [DOI] [PubMed] [Google Scholar]
- 39.Andres-Lacueva C., Monagas M., Khan N., Izquierdo-Pulido M., Urpi-Sarda M., Permanyer J., Lamuela-Raventós R.M. Flavanol and flavonol contents of cocoa powder products: Influence of the manufacturing process. J. Agric. Food Chem. 2008;56:3111–3117. doi: 10.1021/jf0728754. [DOI] [PubMed] [Google Scholar]
- 40.Larson A., Symons J., Jalili T. Therapeutic Potential of Quercetin to Decrease Blood Pressure: Review of Efficacy and Mechanisms. Adv. Nutr. (Bethesda, Md.) 2012;3:39–46. doi: 10.3945/an.111.001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krings U., Zelena K., Wu S., Berger R.G. Thin-layer high-vacuum distillation to isolate volatile flavour compounds of cocoa powder. Eur. Food Res. Technol. 2006;223:675–681. doi: 10.1007/s00217-006-0252-x. [DOI] [Google Scholar]
- 42.Li Y., Feng Y., Zhu S., Luo C., Ma J., Zhong F. The effect of alkalization on the bioactive and flavor related components in commercial cocoa powder. J. Food Compos. Anal. 2012;25:17–23. doi: 10.1016/j.jfca.2011.04.010. [DOI] [Google Scholar]
- 43.Ramli N., Yatim A., Hok H. HPLC Determination of Methylxanthines and Polyphenols Levels In Cocoa and Chocolate Products. Mal. J. Anal. Sci. 2001;7:377–386. [Google Scholar]
- 44.Lang R., Mueller C., Hofmann T. Development of a stable isotope dilution analysis with liquid chromatography-tandem mass spectrometry detection for the quantitative analysis of di- and trihydroxybenzenes in foods and model systems. J. Agric. Food Chem. 2006;54:5755–5762. doi: 10.1021/jf061118n. [DOI] [PubMed] [Google Scholar]
- 45.Arts I.C., van de Putte B., Hollman P.C. Catechin contents of foods commonly consumed in The Netherlands. 1. Fruits, vegetables, staple foods, and processed foods. J. Agric. Food Chem. 2000;48:1746–1751. doi: 10.1021/jf000025h. [DOI] [PubMed] [Google Scholar]
- 46.D’Archivio M., Filesi C., Varì R., Scazzocchio B., Masella R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010;11:1321–1342. doi: 10.3390/ijms11041321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rechner A.R., Smith M.A., Kuhnle G., Gibson G.R., Debnam E.S., Srai S.K.S., Moore K.P., Rice-Evans C.A. Colonic metabolism of dietary polyphenols: Influence of structure on microbial fermentation products. Free Radic. Biol. Med. 2004;36:212–225. doi: 10.1016/j.freeradbiomed.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 48.D’Archivio M., Filesi C., Di benedetto R., Gargiulo R., Giovannini C., Masella R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super Sanita. 2007;43:348–361. [PubMed] [Google Scholar]
- 49.Oracz J., Nebesny E., Zyzelewicz D., Budryn G., Luzak B. Bioavailability and metabolism of selected cocoa bioactive compounds: A comprehensive review. Crit. Rev. food Sci. Nutr. 2020;60:1947–1985. doi: 10.1080/10408398.2019.1619160. [DOI] [PubMed] [Google Scholar]
- 50.Sorrenti V., Fortinguerra S., Caudullo G., Buriani A. Deciphering the Role of Polyphenols in Sports Performance: From Nutritional Genomics to the Gut Microbiota toward Phytonutritional Epigenomics. Nutrients. 2020;12:1265. doi: 10.3390/nu12051265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Metere A., Giacomelli L. Absorption, metabolism and protective role of fruits and vegetables polyphenols against gastric cancer. Eur. Rev. Med. Pharmacol. Sci. 2017;21:5850–5858. doi: 10.26355/eurrev_201712_14034. [DOI] [PubMed] [Google Scholar]
- 52.Williamson G., Manach C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 93 bioavailability studies. Am. J. Clin. Nutr. 2005;81(Suppl. 1):230S–242S. doi: 10.1093/ajcn/81.1.243S. [DOI] [PubMed] [Google Scholar]
- 53.Luca S.V., Macovei I., Bujor A., Miron A., Skalicka-Woźniak K., Aprotosoaie A.C., Trifan A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020;60:626–659. doi: 10.1080/10408398.2018.1546669. [DOI] [PubMed] [Google Scholar]
- 54.Scalbert A., Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130(Suppl. 8S):2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 55.Montagnana M., Danese E., Angelino D., Mena P., Rosi A., Benati M., Gelati M., Salvagno G.L., Favaloro E.J., Del Rio D., et al. Dark chocolate modulates platelet function with a mechanism mediated by flavan-3-ol metabolites. Medicine (Baltimore) 2018;97:e13432. doi: 10.1097/MD.0000000000013432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kay C.D., Mazza G., Holub B.J., Wang J. Anthocyanin metabolites in human urine and serum. Br. J. Nutr. 2004;91:933–942. doi: 10.1079/BJN20041126. [DOI] [PubMed] [Google Scholar]
- 57.Mena P., Bresciani L., Brindani N., Ludwig I.A., Pereira-Caro G., Angelino D., Llorach R., Calani L., Brighenti F., Clifford M.N., et al. Phenyl-γ-valerolactones and phenylvaleric acids, the main colonic metabolites of flavan-3-ols: Synthesis, analysis, bioavailability, and bioactivity. Nat. Prod. Rep. 2019;36:714–752. doi: 10.1039/C8NP00062J. [DOI] [PubMed] [Google Scholar]
- 58.Stoupi S., Williamson G., Drynan J.W., Barron D., Clifford M.N. A comparison of the in vitro biotransformation of (-)-epicatechin and procyanidin B2 by human faecal microbiota. Mol. Nutr. Food Res. 2010;54:747–759. doi: 10.1002/mnfr.200900123. [DOI] [PubMed] [Google Scholar]
- 59.Gómez-Juaristi M., Sarria B., Martínez-López S., Bravo Clemente L., Mateos R. Flavanol Bioavailability in Two Cocoa Products with Different Phenolic Content. A Comparative Study in Humans. Nutrients. 2019;11:1441. doi: 10.3390/nu11071441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petyaev I.M., Dovgalevsky P.Y., Chalyk N.E., Klochkov V., Kyle N.H. Reduction in blood pressure and serum lipids by lycosome formulation of dark chocolate and lycopene in prehypertension. Food Sci. Nutr. 2014;2:744–750. doi: 10.1002/fsn3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vitaglione P., Lumaga R.B., Ferracane R., Sellitto S., Morelló J.R., Miranda J.R., Shimoni E., Fogliano V. Human bioavailability of flavanols and phenolic acids from cocoa-nut creams enriched with free or microencapsulated cocoa polyphenols. Br. J. Nutr. 2013;109:1832–1843. doi: 10.1017/S0007114512003881. [DOI] [PubMed] [Google Scholar]
- 62.Kawabata K., Yoshioka Y., Terao J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules. 2019;24:370. doi: 10.3390/molecules24020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Q., Liang Q., Balakrishnan B., Belobrajdic D.P., Feng Q.-J., Zhang W. Role of Dietary Nutrients in the Modulation of Gut Microbiota: A Narrative Review. Nutrients. 2020;12:381. doi: 10.3390/nu12020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumar Singh A., Cabral C., Kumar R., Ganguly R., Kumar Rana H., Gupta A., Rosaria Lauro M., Carbone C., Reis F., Pandey A.K. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients. 2019;11:2216. doi: 10.3390/nu11092216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tzounis X., Rodriguez-Mateos A., Vulevic J., Gibson G.R., Kwik-Uribe C., Spencer J.P.E. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011;93:62–72. doi: 10.3945/ajcn.110.000075. [DOI] [PubMed] [Google Scholar]
- 66.Ma G., Chen Y. Polyphenol supplementation benefits human health via gut microbiota: A systematic review via meta-analysis. J. Funct. Foods. 2020;66:103829. doi: 10.1016/j.jff.2020.103829. [DOI] [Google Scholar]
- 67.Tzounis X., Vulevic J., Kuhnle G.G.C., George T., Leonczak J., Gibson G.R., Kwik-Uribe C., Spencer J.P.E. Flavanol monomer-induced changes to the human faecal microflora. Br. J. Nutr. 2008;99:782–792. doi: 10.1017/S0007114507853384. [DOI] [PubMed] [Google Scholar]
- 68.Fogliano V., Corollaro M.L., Vitaglione P., Napolitano A., Ferracane R., Travaglia F., Arlorio M., Costabile A., Klinder A., Gibson G. In vitro bioaccessibility and gut biotransformation of polyphenols present in the water-insoluble cocoa fraction. Mol. Nutr. Food Res. 2011;55(Suppl. 1):S44–S55. doi: 10.1002/mnfr.201000360. [DOI] [PubMed] [Google Scholar]
- 69.Peng M., Aryal U., Cooper B., Biswas D. Metabolites produced during the growth of probiotics in cocoa supplementation and the limited role of cocoa in host-enteric bacterial pathogen interactions. Food Control. 2015;53:124–133. doi: 10.1016/j.foodcont.2015.01.014. [DOI] [Google Scholar]
- 70.Massot-Cladera M., Pérez-Berezo T., Franch A., Castell M., Pérez-Cano F.J. Cocoa modulatory effect on rat faecal microbiota and colonic crosstalk. Arch. Biochem. Biophys. 2012;527:105–112. doi: 10.1016/j.abb.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 71.Magistrelli D., Zanchi R., Malagutti L., Galassi G., Canzi E., Rosi F. Effects of Cocoa Husk Feeding on the Composition of Swine Intestinal Microbiota. J. Agric. Food Chem. 2016;64:2046–2052. doi: 10.1021/acs.jafc.5b05732. [DOI] [PubMed] [Google Scholar]
- 72.Jang S., Sun J., Chen P., Lakshman S., Molokin A., Harnly J.M., Vinyard B.T., Urban J.F., Davis C.D., Solano-Aguilar G. Flavanol-Enriched Cocoa Powder Alters the Intestinal Microbiota, Tissue and Fluid Metabolite Profiles, and Intestinal Gene Expression in Pigs. J. Nutr. 2016;146:673–680. doi: 10.3945/jn.115.222968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Álvarez-Cilleros D., Ramos S., López-Oliva M.E., Escrivá F., Álvarez C., Fernández-Millán E., Martín M.A. Cocoa diet modulates gut microbiota composition and improves intestinal health in Zucker diabetic rats. Food Res. Int. 2020;132:109058. doi: 10.1016/j.foodres.2020.109058. [DOI] [PubMed] [Google Scholar]
- 74.Camps-Bossacoma M., Pérez-Cano F.J., Franch A., Castell M. Gut Microbiota in a Rat Oral Sensitization Model: Effect of a Cocoa-Enriched Diet. Oxidative Med. Cell. Longev. 2017;2017:7417505. doi: 10.1155/2017/7417505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abril-Gil M., Pérez-Cano F.J., Franch A., Castell M. Effect of a cocoa-enriched diet on immune response and anaphylaxis in a food allergy model in Brown Norway rats. J. Nutr. Biochem. 2016;27:317–326. doi: 10.1016/j.jnutbio.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 76.Massot-Cladera M., Abril-Gil M., Torres S., Franch A., Castell M., Pérez-Cano F.J. Impact of cocoa polyphenol extracts on the immune system and microbiota in two strains of young rats. Br. J. Nutr. 2014;112:1944–1954. doi: 10.1017/S0007114514003080. [DOI] [PubMed] [Google Scholar]
- 77.Martín-Peláez S., Camps-Bossacoma M., Massot-Cladera M., Rigo-Adrover M., Franch A., Pérez-Cano F.J., Castell M. Effect of cocoa’s theobromine on intestinal microbiota of rats. Mol. Nutr. Food Res. 2017;61 doi: 10.1002/mnfr.201700238. [DOI] [PubMed] [Google Scholar]
- 78.Castell M., Saldaña-Ruíz S., Rodríguez-Lagunas M.J., Franch À., Pérez-Cano F.J. Second International Congress on Chocolate and Cocoa in Medicine Held in Barcelona, Spain, 25–26th September 2015. Nutrients. 2015;7:9785–9803. doi: 10.3390/nu7125502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Urpi-Sarda M., Llorach R., Khan N., Monagas M., Rotches-Ribalta M., Lamuela-Raventos R., Estruch R., Tinahones F.J., Andres-Lacueva C. Effect of milk on the urinary excretion of microbial phenolic acids after cocoa powder consumption in humans. J. Agric. Food Chem. 2010;58:4706–4711. doi: 10.1021/jf904440h. [DOI] [PubMed] [Google Scholar]
- 80.Orrhage K., Nord C.E. Bifidobacteria and lactobacilli in human health. Drugs Exp. Clin. Res. 2000;26:95–111. [PubMed] [Google Scholar]
- 81.Lavefve L., Howard L., Carbonero F. Berry polyphenols metabolism and impact on human gut microbiota and health. Food Funct. 2019;11:45–65. doi: 10.1039/C9FO01634A. [DOI] [PubMed] [Google Scholar]
- 82.Wiese M., Bashmakov Y., Chalyk N., Nielsen D.S., Krych Ł., Kot W., Klochkov V., Pristensky D., Bandaletova T., Chernyshova M., et al. Prebiotic Effect of Lycopene and Dark Chocolate on Gut Microbiome with Systemic Changes in Liver Metabolism, Skeletal Muscles and Skin in Moderately Obese Persons. Biomed. Res. Int. 2019;2019:4625279. doi: 10.1155/2019/4625279. [DOI] [PMC free article] [PubMed] [Google Scholar]