Abstract
Aims
Atrial fibrillation (AF) often starts as a paroxysmal self-terminating arrhythmia. Limited information is available on AF patterns and episode duration of paroxysmal AF. In paroxysmal AF patients, we longitudinally studied the temporal AF patterns, the association with clinical characteristics, and prevalence of AF progression.
Methods and results
In this interim analysis of the Reappraisal of AF: Interaction Between HyperCoagulability, Electrical Remodelling, and Vascular Destabilisation in the Progression of AF (RACE V) registry, 202 patients with paroxysmal AF were followed with continuous rhythm monitoring (implantable loop recorder or pacemaker) for 6 months. Mean age was 64 ± 9 years, 42% were women. Atrial fibrillation history was 2.1 (0.5–4.4) years, CHA2DS2-VASc 1.9 ± 1.3, 101 (50%) had hypertension, 69 (34%) heart failure. One-third had no AF during follow-up. Patients with long episodes (>12 hours) were often men with more comorbidities (heart failure, coronary artery disease, higher left ventricular mass). Patients with higher AF burden (>2.5%) were older with more comorbidities (worse renal function, higher calcium score, thicker intima media thickness). In 179 (89%) patients, 1-year rhythm follow-up was available. On a quarterly basis, average daily AF burden increased from 3.2% to 3.8%, 5.2%, and 6.1%. Compared to the first 6 months, 111 (62%) patients remained stable during the second 6 months, 39 (22%) showed progression to longer AF episodes, 8 (3%) developed persistent AF, and 29 (16%) patients showed AF regression.
Conclusions
In paroxysmal AF, temporal patterns differ suggesting that paroxysmal AF is not one entity. Atrial fibrillation burden is low and determined by number of comorbidities. Atrial fibrillation progression occurred in a substantial number.
Trial registration number
Clinicaltrials.gov identifier NCT02726698.
Keywords: Atrial fibrillation, Rhythm monitoring, Paroxysmal atrial fibrillation, Atrial fibrillation progression, Atrial fibrillation burden
What’s new?
We assessed temporal patterns of paroxysmal selfterminating atrial fibrillation (AF) by continuous rhythm monitoring in patients receiving a loop recorder for study purposes. Temporal AF patterns showed to be heterogeneous in terms of duration of AF episodes as well as AF burden.
Patients with longer episodes of paroxysmal AF had more underlying conditions.
Using continuous rhythm monitoring, AF progression within 1-year occurred in a substantial number of patients.
Continuous rhythm monitoring enables an improved characterization of patients with AF. Diverse patterns may reflect differences in underlying diseases and mechanisms, warranting personalized therapeutic interventions and patient-tailored therapies.
Introduction
Traditionally, atrial fibrillation (AF) is clinically categorized into paroxysmal, persistent, long-standing persistent, and permanent AF.1 Often starting as short-lasting, paroxysmal episodes, AF commonly progresses over time to persistent and permanent, non-selfterminating AF.2 Progression of AF is associated with a higher incidence of major adverse cardiovascular and cerebrovascular events.3,4 However, most data on AF and AF progression is obtained with intermittent rhythm monitoring, providing limited information on the total burden and the temporal pattern of AF.1,5,6 In the era of implantable loop recorders, it is now possible to assess progression of AF more precisely.7 These devices also provide information on the exact temporal AF pattern and number and duration of AF episodes, which may help explaining differences in underlying pathophysiological AF mechanisms and related clinical outcomes. Ultimately, this could aid to personalize AF therapy and may have clinical utility for the assessment of AF treatment response.
Recently, the first initiatives for an improved classification for paroxysmal AF have been proposed, based on single-lead electrocardiogram (ECG) monitoring.8 The Reappraisal of Atrial Fibrillation: Interaction Between HyperCoagulability, Electrical Remodelling, and Vascular Destabilisation in the Progression of Atrial Fibrillation (RACE V) registry aims to elucidate the factors associated with AF progression. At baseline, deep phenotyping of patients with paroxysmal self-terminating AF is performed. Exact longitudinal assessment of episode number and duration and AF burden is provided by continuous rhythm monitoring through an implantable loop recorder or pacemaker with the same AF detection algorithm. In the present interim analysis, we aim to longitudinally study AF temporal patterns, burden and short-term progression of AF and their association with clinical characteristics, in patients with paroxysmal self-terminating AF using continuous rhythm monitoring.
Methods
Patient population
The RACE V is an investigator-initiated, prospective, multicentre registry aiming to include 750 patients in multiple centres in The Netherlands. A total of 202 patients were included in five centres for the current interim analysis between June 2016 and December 2017. Inclusion criteria included patients aged >18 years with paroxysmal AF; a maximum history of 10 years since diagnosis at the moment of inclusion; a maximum CHA2DS2-VASc score of 5; and no other indication for anticoagulation drugs (e.g. mechanical valve prosthesis). Patients had to have at least two documented episodes of paroxysmal AF in the past year or one documented episode combined with at least two symptomatic episodes in the past year suspected to be AF. In patients with a Medtronic Advisa® pacemaker, atrial high rate episodes (AHRE) >190 beats per minute lasting >6 min were qualified as AF episodes. Patients with other types of pacemakers, defibrillators, or cardiac resynchronization therapy could not participate due to differences in AHRE algorithm or incompatibility with the type of home-monitoring. Patients with a history of persistent AF, currently treated with amiodarone, current pregnancy, or a life expectancy <2.5 years were not eligible to participate. Patients with AF caused exclusively by transient triggers (e.g. post-operative, due to infection) could also not participate, as well as patients with a previous pulmonary vein isolation (PVI), or intention to undergo PVI. The study was performed in concordance with the Declaration of Helsinki. The Institutional Review Board approved the protocol, and the study was registered at Clinicaltrials.gov (identifier NCT02726698). All centres approved the protocol and all patients gave written informed consent. Intended total follow-up duration is 2.5 years.
Study procedures
All patients received causal therapy for AF, as well as rate and rhythm control according to the European Society of Cardiology AF guidelines. At baseline, information on clinical characteristics, medical history, AF characteristics, symptomatology, and current medication were collected. Additionally, all patients underwent physical examination, a 12-lead ECG, echocardiography, cardiac computed tomography (CT), vascular assessment, and blood sampling. Quality of life, AF-related symptoms, and physical activity were assessed through questionnaires. After baseline, additional follow-up visits were planned at 1 and 2.5 years.
The cardiac CT was performed as a non-contrast ECG-gated scan with slice collimation of 0.6 mm and a tube voltage of 120 kV. Beta-blockers were administered in case of high heart rates to improve image quality. Cranial demarcation was placed at the aortic arch or higher and caudal demarcation was placed to include the whole heart. Automated coronary calcium scores (Agatston) were collected.
Vascular assessment included pulse wave velocity (PWV) and intima media thickness (IMT) measurements. Pulse wave velocity was measured by SphygmoCor (Atcor Medical Blood Pressure Analysis System, Australia) or Complior (Alam Medical, France) at the carotid and femoral arteries. To determine aortic PWV, ≥20 consecutive pressure waveforms were collected at the carotid artery and the femoral artery. The system software calculated the wave transit time, using the R wave of the simultaneously recorded ECG. Distance between both measure points was determined and corrected by multiplying the distance by 0.8. The PWV was calculated by dividing the corrected distance by the wave transit time. Intima media thickness measurements were performed by ultrasound (Siemens Acuson S2000) with the Syncho US Workplace 3.5, Arterial Health Package for automated IMT measurement. Intima media thickness was bilaterally assessed in the common carotid artery, the carotid bifurcation, and internal carotid artery.
Rhythm follow-up
All patients had continuous rhythm monitoring to detect the exact AF burden (time spent in AF) and temporal AF pattern (the number and duration of AF episodes). Patients either received a Medtronic Reveal LINQ® implantable loop recorder subcutaneously merely for study purposes, or had a Medtronic Advisa® pacemaker implanted prior to inclusion. All patients received a home monitoring device (Medtronic Carelink®) in order to receive all data regarding cardiac arrhythmias on the Carelink Network®. Patients were instructed to perform at least weekly manual data transmissions to prevent potential data loss. AT/AF detection setting was set to AF detection only. Additional settings included a tachy-pause-brady data storage priority and balanced sensitivity for AF detection with nominal ectopy rejection for all patients. All episodes of AF ≥2 min were automatically detected. Episodes ≥182 beats per minute with a duration of ≥24 beats were automatically classified as tachycardia and if applicable, corrected to AF episodes. First, a dedicated service (Fysiologic, Amsterdam, The Netherlands) adjudicated all episodes. As a second assessment all episodes were independently adjudicated by two physicians (R.R.D.W.; Ö.E.) and corrected if needed. Information on changes in antiarrhythmic drug (AAD) therapy, electrical cardioversions (ECV) and PVI were collected during follow-up.
Atrial fibrillation patterns
Atrial fibrillation patterns during 6 months (183 days) were visualized by custom-made software using Microsoft Visual Basic. First day of monitoring started at mid-night the day after loop recorder implantation or at mid-night the day after inclusion in patients with a pacemaker. Atrial fibrillation patterns were independently adjudicated by four physicians (R.R.D.W.; Ö.E.; H.J.G.M.C.; I.C.V.G.), At first, all patients were divided into having no recurrence of AF or ≥1 episode of AF during follow-up. Secondly, patients with AF episodes were divided by into short AF episodes duration (<6 hours), intermediate (6–12 hours), and long AF episodes (>12 hours) by their longest AF episode during the first 6 months. Third, AF burden was calculated by the cumulative time in AF divided by the total follow-up time, expressed as a percentage. In addition, patients were divided according to degree of AF burden: low AF burden (>0–0.5%), intermediate (>0.5–.5%), and high AF burden (>2.5%). The aforementioned cut-offs for AF burden were used to create equally sized groups.
Atrial fibrillation progression was assessed in 179 patients in whom 1 year (366 days) of rhythm follow-up was available. These patients were also divided into no AF, short, intermediate, and long episodes according to the second 6 months of rhythm follow-up. Progression of AF was defined as deterioration of episode duration category (e.g. short to intermediate episodes) in the second 6 months, compared to the first 6 months. Regression was defined as episode category improvement (e.g. intermediate to short episodes). Daily averaged AF burden was calculated by the sum of the daily AF burden from all patients and divided by the number of patients. Patients with episodes >7 days were considered to have persistent AF.
Covariate definition
Covariate definitions that were used are shown in the Supplementary material online.
Statistical analysis
Data were presented as means ± standard deviation or median with interquartile range, depending on normality of the data. Categorical data were presented as numbers with percentages. Differences between patients with and without AF recurrence were tested by Student’s t-test, Mann–Whitney U test or Fisher’s exact (2 categories) or χ2 (>2 categories). Differences between different AF patterns were analysed by one-way ANOVA in normally distributed data, Kruskall–Wallis test in non-normally distributed data or χ2 in categorical data. Additional sensitivity analyses were performed excluding the following groups: (i) patients that underwent PVI or ECV during follow-up; (ii) patients that underwent PVI, ECV, or had changes in AAD therapy during follow-up; and (iii) patients that underwent PVI, ECV, or any AAD therapy during follow-up. Spearman’s correlation coefficient, including 95% confidence interval (CI), was calculated to assess the relation of AF burden and the duration of the longest AF episode. Changes in the duration of longest AF episode category in the first 6 months and second 6 months were visualised using a Sankey diagram. All analyses were performed by IBM SPSS Statistics for Windows version 23.0 (Armonk, New York, USA) and GraphPad Prism version 7.02 (GraphPad Software, La Jolla, USA). A P-value <0.05 was considered statistically significant.
Results
Patient characteristics
Baseline characteristics are shown in Table 1. Mean age was 64 ± 9 years, 85 (42%) were women, and median history of AF at baseline was 2.1 (0.5-4.4) years. The majority had an implantable loop recorder [185 (92%)]; 17 (8%) had a pacemaker. Patients with a pacemaker were older (72 ± 9 vs. 64 ± 9 years, P < 0.001) and had more comorbidities (2.9 ± 1.5 vs. 2.3 ± 1.3, P = 0.049).
Table 1.
Baseline characteristics of the total population, and split on recurrence of AF during 6-month follow-up
| Characteristics | Total (N = 202) | No Recurrence of AF (N = 63) | Recurrence of AF (N = 139) | P-value (no recurrence vs. recurrence) |
|---|---|---|---|---|
| Age (years) | 64 ± 9 | 64 ± 10 | 64 ± 9 | 0.900 |
| Male sex | 117 (58%) | 36 (57%) | 81 (58%) | 0.880 |
| Total history AF (years) | 2.1 (0.5–4.4) | 1.9 (0.5–5.3) | 2.2 (0.6–4.5) | 0.574 |
| Heart failure | 69 (34%) | 21 (33%) | 48 (35%) | 0.868 |
| Hypertension | 101 (50%) | 40 (64%) | 61 (44%) | 0.010 |
| Diabetes mellitus | 19 (9%) | 8 (13%) | 11 (8%) | 0.304 |
| Coronary artery disease | 23 (11%) | 5 (8%) | 18 (13%) | 0.299 |
| Thrombo-embolic events | 22 (11%) | 9 (14%) | 13 (9%) | 0.297 |
| Chronic obstructive pulmonary disease | 11 (5%) | 3 (5%) | 8 (6%) | 0.773 |
| Number of comorbiditiesa | 2.3 ± 1.3 | 2.5 ± 1.3 | 2.3 ± 1.3 | 0.199 |
| CHA2DS2-VASc scoreb | 1.9 ± 1.3 | 2.2 ± 1.3 | 1.8 ± 1.3 | 0.030 |
| 0 | 25 (12%) | 4 (6%) | 21 (15%) | |
| 1 | 56 (28%) | 14 (22%) | 42 (30%) | |
| 2 | 60 (30%) | 25 (39%) | 35 (25%) | |
| 3 | 35 (17%) | 8 (12%) | 27 (19%) | |
| 4 | 18 (9%) | 7 (11%) | 11 (8%) | |
| 5 | 8 (4%) | 5 (8%) | 3 (2%) | |
| EHRA class | 0.143 | |||
| I | 22 (11%) | 9 (14%) | 13 (9%) | |
| IIa | 84 (42%) | 24 (38%) | 60 (43%) | |
| IIb | 78 (39%) | 28 (44%) | 50 (36%) | |
| III | 18 (9%) | 2 (3%) | 16 (12%) | |
| Height (cm) | 176 ± 10 | 176 ± 10 | 176 ± 11 | 0.873 |
| Weight (kg) | 86 ± 17 | 85 ± 15 | 86 ± 18 | 0.747 |
| BMI (kg/m2) | 28 ± 5 | 28 ± 5 | 28 ± 5 | 0.956 |
| Obesity (BMI > 30) | 50 (25%) | 17 (27%) | 33 (24%) | 0.621 |
| Waist circumference (cm) | 102 ± 13 | 103 ± 14 | 102 ± 13 | 0.692 |
| Blood pressure (mmHg) | ||||
| Systolic | 136 ± 18 | 135 ± 17 | 137 ± 19 | 0.458 |
| Diastolic | 81 ± 10 | 80 ± 11 | 81 ± 9 | 0.649 |
| NT-proBNP (pg/mL) | 50 (22–144) | 54 (27–123) | 48 (19–159) | 0.540 |
| Creatinine (µmol/L) | 82 (70–92) | 82 (70–92) | 82 (71–92) | 0.476 |
| eGFR (mL/min) | 80 (68–88) | 82 (68–91) | 79 (68–88) | 0.491 |
| Medications | ||||
| β-Blocker | 104 (52%) | 33 (52%) | 71 (51%)) | 0.864 |
| Verapamil/diltiazem | 31 (15%) | 9 (14%) | 22 (16%) | 0.778 |
| Class I antiarrhythmic drugs | 41 (20%) | 6 (10%) | 35 (25%) | 0.013 |
| Class III antiarrhythmic drugs | 11 (5%) | 3 (5%) | 8 (6%) | 1.000 |
| Digoxin | 2 (1%) | – | 2 (1.4%) | 0.339 |
| ACE-inhibitor | 43 (21%) | 18 (29%) | 25 (18%) | 0.089 |
| Angiotensin receptor blocker | 44 (22%) | 22 (35%) | 22 (16%) | 0.002 |
| Mineralocorticoid receptor antagonist | 3 (2%) | – | 3 (2%) | 0.240 |
| Statin | 80 (40%) | 27 (43%) | 53 (38%) | 0.524 |
| Diuretic | 33 (16%) | 17 (26%) | 16 (12%) | 0.006 |
| Anticoagulant | 0.079 | |||
| Vitamin K antagonist | 33 (16%) | 11 (17%) | 22 (16%) | |
| NOAC | 114 (56%) | 40 (63%) | 74 (53%) | |
| Echocardiographic variables | ||||
| Left atrial volume (mL) | 69 ± 23 | 62 ± 20 | 72 ± 24 | 0.013 |
| Left atrial volume index (mL/m2) | 35 ± 12 | 31 ± 10 | 37 ± 12 | 0.002 |
| Left ventricular ejection fraction (%) | 58 (55–60) | 58 (55–60) | 58 (55–60) | 0.661 |
| Left ventricular ejection fraction <45% | 4 (2%) | 2 (3%) | 2 (1%) | 0.412 |
| Left ventricular mass (g) | 162 ± 47 | 156 ± 45 | 164 ± 47 | 0.417 |
| Left ventricular mass index (g/m2) | 79 ± 18 | 77 ± 17 | 80 ± 19 | 0.468 |
| Left ventricular hypertrophy | 8 (4%) | 2 (3%) | 6 (4%) | 0.700 |
| CT | ||||
| Calcium score (Agatston) | 31 (0–227) | 41 (0–262) | 26 (0–216) | 0.733 |
| Vascular assessment | ||||
| IMT–CCA (mm) | 0.72 (0.63–0.87) | 0.71 (0.62–0.88) | 0.73 (0.64–0.87) | 0.418 |
| IMT–all segments (mm) | 0.73 (0.62–0.90) | 0.70 (0.60–0.94) | 0.74 (0.63–0.87) | 0.835 |
| Pulse wave velocity (m/s) | 8.3 (7.3–9.7) | 8.6 (7.2–9.7) | 8.1 (7.3–9.7) | 0.382 |
A P-value is given for the difference between recurrence vs. no recurrence of AF.
Data are presented as mean ± standard deviation, number of patients (%), or median (interquartile range).
ACE, angiotensin-converting enzyme; AF, atrial fibrillation; BMI, body mass index; CT, computed tomography; eGFR estimated glomerular filtration rate; EHRA, European Heart Rhythm Association class for symptoms; IMT, intima media thickness; NOAC, novel oral anticoagulation; NT-pro BNP, N-terminal pro-brain natriuretic peptide.
The number of comorbidities was calculated by awarding points for hypertension, heart failure, age >65 years, diabetes mellitus; coronary artery disease, BMI > 25kg/m2, moderate or severe mitral valve regurgitation and kidney dysfunction (eGFR < 60).
The CHA2DS2-VASc score assesses thrombo-embolic risk. C, congestive heart failure/LV dysfunction, H, hypertension; A2, age ≥75 years; D, diabetes mellitus; S2, stroke/transient ischaemic attack/systemic embolism; V, vascular disease; A, age 65–74 years; Sc, sex category (female sex).
Atrial fibrillation episodes
A total of 13 657 episodes of AF in 202 patients were automatically detected by the implanted device during follow-up of 183 days, of which 2231 (16%) episodes were adjudicated as false positive for AF, for example due to premature atrial beats, ventricular extra beats or artefacts. Most (93%) false positive episodes lasted ≤10 min. Forty episodes of AF were originally classified as tachycardia. After applying all corrections, 11 466 episodes of AF remained, with a median AF burden of 0.3% (0–2.1%, maximal burden 61.1%) during 6-months follow-up (total of 36 966 days of continuous day-to-day heart rhythm data for analysis). Of these 11 466 episodes, 11 456 (>99.9%) episodes were self-terminating. Ten episodes in eight patients were non-selfterminating AF. These patients underwent an ECV. Of these eight patients, two had short, three had intermediate, and three had long self-terminating AF episodes. Changes in AAD therapy were made in six patients (four patients started with flecainide; one patient started sotalol; and in one patient flecainide was stopped). Three patients underwent a PVI within 6 months (at Days 64, 162, and 171 of follow-up, respectively), of which one patient had episodes of intermediate duration; and two had long episodes.
Atrial fibrillation patterns
During follow-up, 63 patients (31%) had no recurrence of AF (Table 1). Patients without AF recurrence had more often hypertension, a higher CHA2DS2-VASc score, smaller left atria, and were more often treated with ACE/ARB and diuretics. The estimated number of AF episodes the year before inclusion was not different from the ones with AF recurrence [9 (2–50) vs. 10 (4–50) episodes, P = 0.357].
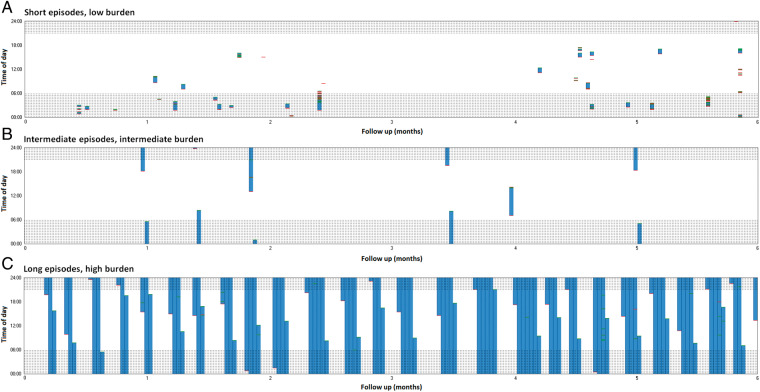
Forty-five (22%) patients had short, 38 (19%) had intermediate, and had 56 (28%) long episodes. Figure 1 shows examples of patients with short, intermediate, and long episodes. Patients with long episodes were more often men, had more often heart failure, coronary artery disease, a higher number of comorbidities, a larger waist circumference and higher left ventricular mass (Table 2). Several sensitivity analyses were performed, excluding patients with AAD therapy, ECV or PVI during follow-up (Supplementary material online). Although not all differences remained significant, similar trends were observed.
Figure 1.
Examples of patients with short (A), intermediate (B), and long (C) episodes during 6-month follow-up. Each day is represented by a bar. White means no AF is present, and blue represents ongoing episodes of AF. AF initiations are shown in red and AF terminations are shown in green. Shaded areas indicate nightly hours. The Y-axis is the time of day and the X-axis represents 6 months of follow-up. AF, atrial fibrillation.
Table 2.
Patients’ characteristics and longest AF episode duration during 6-month follow-up
| Characteristics | Short episodes (N = 45) | Intermediate episodes (N = 38) | Long episodes (N = 56) | P-value (between groups) |
|---|---|---|---|---|
| Age (years) | 63 ± 10 | 63 ± 10 | 67 ± 7 | 0.068 |
| Male sex | 17 (38%) | 23 (61%) | 41 (73%) | 0.002 |
| Total history AF (years) | 2.1 (0.7–4.5) | 1.8 (0.4–3.9) | 2.5 (0.6–4.8) | 0.384 |
| Heart failure | 9 (20%) | 16 (42%) | 23 (41%) | 0.044 |
| Hypertension | 20 (44%) | 13 (34%) | 28 (50%) | 0.317 |
| Diabetes | 3 (7%) | 1 (3%) | 7 (13%) | 0.205 |
| Coronary artery disease | 3 (7%) | 3 (8%) | 12 (21%) | 0.049 |
| Thrombo-embolic events | 6 (13%) | 3 (8%) | 4 (7%) | 0.533 |
| Chronic obstructive pulmonary disease | 2 (4%) | 2 (5%) | 4 (7%) | 0.836 |
| Number of comorbiditiesa | 1.9 ± 1.2 | 2.0 ± 1.2 | 2.7 ± 1.4 | 0.007 |
| CHA2DS2-VASc scoreb | 1.9 ± 1.1 | 1.6 ± 1.4 | 1.9 ± 1.2 | 0.407 |
| 0 | 4 (9%) | 11 (29%) | 6 (11%) | |
| 1 | 14 (31%) | 10 (26%) | 18 (32%) | |
| 2 | 14 (31%) | 7 (18%) | 14 (25%) | |
| 3 | 10 (22%) | 5 (13%) | 12 (21%) | |
| 4 | 2 (4%) | 4 (11%) | 5 (9%) | |
| 5 | 1 (2%) | 1 (3%) | 1 (2%) | |
| EHRA class | 0.965 | |||
| I | 4 (9%) | 3 (8%) | 6 (11%) | |
| IIa | 17 (38%) | 17 (45%) | 26 (46%) | |
| IIb | 22 (49%) | 15 (40%) | 13 (23%) | |
| III | 2 (4%) | 3 (8%) | 11 (20%) | |
| Height (cm) | 173 ± 10 | 176 ± 11 | 178 ± 11 | 0.057 |
| Weight (kg) | 82 ± 18 | 86 ± 16 | 90 ± 18 | 0.084 |
| BMI (kg/m2) | 27 ± 5 | 28 ± 4 | 28 ± 5 | 0.353 |
| Obesity (BMI > 30) | 9 (20%) | 8 (21%) | 16 (29%) | 0.543 |
| Waist circumference (cm) | 97 ± 13 | 101 ± 11 | 105 ± 13 | 0.010 |
| Blood pressure (mmHg) | ||||
| Systolic | 135 ± 18 | 139 ± 20 | 136 ± 18 | 0.675 |
| Diastolic | 80 ± 10 | 82 ± 10 | 81 ± 8 | 0.616 |
| NT-proBNP (pg/mL) | 33 (14–130) | 62 (23–165) | 48 (22–197) | 0.312 |
| Creatinine (µmol/L) | 76 (67–85) | 80 (71–92) | 86 (77–95) | 0.009 |
| eGFR (mL/min) | 83 (69–90) | 79 (66–86) | 76 (65–87) | 0.297 |
| Medications | ||||
| β-Blocker | 21 (47%) | 18 (47%) | 32 (57%) | 0.501 |
| Verapamil/diltiazem | 6 (13%) | 9 (24%) | 7 (13%) | 0.296 |
| Class I antiarrhythmic drugs | 17 (38%) | 11 (29%) | 7 (13%) | 0.012 |
| Class III antiarrhythmic drugs | 2 (4%) | 1 (3%) | 5 (9%) | 0.393 |
| Digoxin | 1 (2%) | – | 1 (2%) | 0.671 |
| ACE-inhibitor | 7 (16%) | 5 (13%) | 13 (23%) | 0.403 |
| Angiotensin receptor blocker | 5 (11%) | 5 (13%) | 12 (21%) | 0.321 |
| Mineralocorticoid receptor antagonist | – | 1 (3%) | 2 (4%) | 0.458 |
| Statin | 14 (31%) | 8 (21%) | 31 (55%) | 0.002 |
| Diuretic | 4 (9%) | 4 (11%) | 8 (15%) | 0.683 |
| Anticoagulant | 0.128 | |||
| Vitamin K antagonist | 5 (9%) | 5 (13%) | 12 (21%) | |
| NOAC | 24 (53%) | 18 (47%) | 32 (57%) | |
| Echocardiographic variables | ||||
| Left atrial volume (mL) | 71 ± 25 | 71 ± 23 | 72 ± 24 | 0.973 |
| Left atrial volume index (mL/m2) | 37 ± 13 | 37 ± 11 | 36 ± 11 | 0.850 |
| Left ventricular ejection fraction (%) | 58 (55–60) | 58 (55–62) | 58 (55–61) | 0.573 |
| Left ventricular ejection fraction < 45% | 1 (2%) | 1 (3%) | – | 0.498 |
| Left ventricular mass (g) | 141 ± 36 | 166 ± 36 | 178 ± 55 | 0.009 |
| Left ventricular mass index (g/m2) | 74 ± 14 | 78 ± 18 | 85 ± 21 | 0.046 |
| Left ventricular hypertrophy | 1 (2%) | 1 (3%) | 4 (7%) | 0.402 |
| CT | ||||
| Calcium score (Agatston) | 20 (0–149) | 6 (0–143) | 58 (1–299) | 0.090 |
| Vascular assessment | ||||
| IMT–CCA (mm) | 0.71 (0.62–0.82) | 0.72 (0.63–0.89) | 0.76 (0.69–0.89) | 0.192 |
| IMT–all segments (mm) | 0.71 (0.62–0.79) | 0.72 (0.62–0.87) | 0.77 (0.64–0.94) | 0.101 |
| Pulse wave velocity (m/s) | 7.9 (7.2–9.8) | 7.9 (7.4–10.0) | 8.3 (7.4–9.4) | 0.976 |
Data are presented as mean ± standard deviation, number of patients (%), or median (interquartile range).
ACE, angiotensin-converting enzyme; AF, atrial fibrillation; BMI, body mass index; CT, computed tomography; eGFR, estimated glomerular filtration rate; EHRA, European Heart Rhythm Association class for symptoms; IMT, intima media thickness; NOAC, novel oral anticoagulation; NT-pro BNP, N-terminal pro-brain natriuretic peptide
The number of comorbidities was calculated by awarding points for hypertension, heart failure, age >65 years, diabetes mellitus; coronary artery disease, BMI > 25kg/m2, moderate or severe mitral valve regurgitation and kidney dysfunction (eGFR < 60).
The CHA2DS2-VASc score assesses thrombo-embolic risk. C, congestive heart failure/LV dysfunction, H, hypertension; A2, age ≥75 years; D, diabetes mellitus; S2, stroke/transient ischaemic attack/systemic embolism; V, vascular disease; A, age 65–74 years; Sc, sex category (female sex).
Fifty (25%) patients had low; 44 (22%) had intermediate; and 45 (22%) had high AF burden (Table 3). Patients with high AF burden were older, had a higher number of comorbidities, a higher CHA2DS2-VASc score, a lower eGFR, higher coronary calcium score and a larger IMT. Figure 2 shows the correlation between AF burden and the duration of the longest AF episode [Spearman’s ρ 0.917 (95% CI 0.891–0.937), P < 0.001]. Only four patients had long episodes accompanied by a low AF burden and two had short episodes with a high AF burden (Supplementary material online, Figure S1). Examples of different AF burdens are shown in Supplementary material online, Figure S2.
Table 3.
Patients’ characteristics and AF burden during 6 months
| Characteristics | Low AF burden (N = 50) | Intermediate AF burden (N = 44) | High AF burden (N = 45) | P-value (between groups) |
|---|---|---|---|---|
| Age (years) | 62 ± 10 | 64 ± 8 | 68 ± 8.8 | 0.005 |
| Male sex | 24 (48%) | 29 (66%) | 28 (62%) | 0.173 |
| Total history AF (years) | 1.8 (0.6–4.8) | 2.9 (0.9–4.6) | 1.9 (0.3–4.0) | 0.530 |
| Heart failure | 13 (26%) | 17 (39%) | 18 (40%) | 0.282 |
| Hypertension | 21 (42%) | 21 (48%) | 19 (42%) | 0.824 |
| Diabetes | 3 (6%) | 1 (2%) | 7 (16%) | 0.056 |
| Coronary artery disease | 3 (6%) | 5 (11%) | 10 (22%) | 0.059 |
| Thrombo-embolic events | 5 (10%) | 3 (7%) | 5 (11%) | 0.770 |
| Chronic obstructive pulmonary disease | 2 (4%) | 3 (7%) | 3 (7%) | 0.801 |
| Number of comorbiditiesa | 1.9 ± 1.2 | 2.3 ± 1.1 | 2.2 ± 1.3 | 0.032 |
| CHA2DS2-VASc scoreb | 1.6 ± 1.1 | 1.6 ± 1.3 | 2.2 ± 1.3 | 0.019 |
| 0 | 9 (18%) | 8 (18%) | 4 (9%) | |
| 1 | 14 (28%) | 18 (41%) | 10 (22%) | |
| 2 | 16 (32%) | 8 (18%) | 11 (24%) | |
| 3 | 9 (18%) | 6 (14%) | 12 (27%) | |
| 4 | 2 (4%) | 2 (5%) | 7 (16%) | |
| 5 | – | 2 (5%) | 1 (2%) | |
| EHRA class | 0.017 | |||
| I | 3 (6%) | 3 (7%) | 7 (16%) | |
| IIa | 21 (42%) | 15 (34%) | 24 (53%) | |
| IIb | 24 (48%) | 18 (41%) | 8 (18%) | |
| III | 2 (4%) | 8 (18%) | 6 (13%) | |
| Height (cm) | 176 ± 10 | 177 ± 11 | 177 ± 11 | 0.785 |
| Weight (kg) | 85 ± 18 | 90 ± 18 | 84 ± 17 | 0.280 |
| BMI (kg/m2) | 28 ± 5 | 29 ± 5 | 27 ± 5 | 0.253 |
| Obesity (BMI > 30) | 13 (26%) | 11 (25%) | 9 (20%) | 0.768 |
| Waist circumference (cm) | 100 ± 14 | 103 ± 12 | 102 ± 13 | 0.486 |
| Blood pressure (mmHg) | ||||
| Systolic | 137 ± 18 | 139 ± 19 | 135 ± 19 | 0.631 |
| Diastolic | 82 ± 9 | 84 ± 10 | 78 ± 8 | 0.012 |
| NT-proBNP (pg/mL) | 32 (12–127) | 46 (22–125) | 53 (24–214) | 0.101 |
| Creatinine (µmol/L) | 79 (68–89) | 83 (74–87) | 83 (73–100) | 0.056 |
| eGFR (mL/min) | 85 (71–90) | 79 (73–87) | 73 (62–86) | 0.020 |
| Medications | ||||
| β-Blocker | 25 (50%) | 21 (48%) | 21 (56%) | 0.748 |
| Verapamil/diltiazem | 6 (12%) | 10 (23%) | 6 (13%) | 0.312 |
| Class I antiarrhythmic drugs | 19 (38%) | 11 (25%) | 5 (11%) | 0.011 |
| Class III antiarrhythmic drugs | 1 (2%) | 3 (7%) | 4 (9%) | 0.332 |
| Digoxin | – | 1 (2%) | 1 (2%) | 0.565 |
| ACE-inhibitor | 8 (16%) | 6 (14%) | 11 (24%) | 0.373 |
| Angiotensin receptor blocker | 6 (12%) | 7 (16%) | 9 (20%) | 0.566 |
| Mineralocorticoid receptor antagonist | – | 1 (2%) | 2 (4%) | 0.330 |
| Statin | 13 (26%) | 19 (43%) | 21 (47%) | 0.083 |
| Diuretic | 4 (8%) | 7 (16%) | 5 (11%) | 0.485 |
| Anticoagulant | 0.025 | |||
| Vitamin K antagonist | 5 (10%) | 7 (16%) | 10 (22%) | |
| NOAC | 26 (52%) | 20 (45%) | 28 (62%) | |
| Echocardiographic variables | ||||
| Left atrial volume (mL) | 73 ± 26 | 71 ± 24 | 71 ± 22 | 0.927 |
| Left atrial volume index (mL/m2) | 37 ± 12 | 37 ± 13 | 37 ± 10 | 0.989 |
| Left ventricular ejection fraction (%) | 58 (55–60) | 58 (55–61) | 58 (58–61) | 0.333 |
| Left ventricular ejection fraction <45% | 1 (2%) | 1 (2%) | – | 0.611 |
| Left ventricular mass (g) | 152 ± 38 | 177 ± 52 | 165 ± 50 | 0.151 |
| Left ventricular mass index (g/m2) | 73 ± 17 | 84 ± 19 | 83 ± 20 | 0.054 |
| Left ventricular hypertrophy | 1 (2%) | 2 (5%) | 3 (7%) | 0.533 |
| CT | ||||
| Calcium score (Agatston) | 21 (0–161) | 6 (0–96) | 94 (16–360) | 0.012 |
| Vascular assessment | ||||
| IMT–CCA (mm) | 0.71 (0.61–0.80) | 0.74 (0.67–0.91) | 0.78 (0.68–0.91) | 0.029 |
| IMT–all segments (mm) | 0.72 (0.61–0.81) | 0.72 (0.62–0.86) | 0.78 (0.64–0.95) | 0.104 |
| Pulse wave velocity (m/s) | 8.0 (7.2–9.9) | 8.3 (7.5–9.7) | 8.1 (7.3–9.4) | 0.903 |
Data are presented as mean ± standard deviation, number of patients (%), or median (interquartile range).
ACE, angiotensin-converting enzyme; AF, atrial fibrillation; BMI, body mass index; CT, computed tomography; eGFR, estimated glomerular filtration rate; EHRA, European Heart Rhythm Association class for symptoms; NOAC, novel oral anticoagulation; NT-pro BNP, N-terminal pro-brain natriuretic peptide.
The number of comorbidities was calculated by awarding points for hypertension, heart failure, age >65 years, diabetes mellitus; coronary artery disease, BMI > 25kg/m2, moderate or severe mitral valve regurgitation and kidney dysfunction (eGFR < 60).
The CHA2DS2-VASc score assesses thrombo-embolic risk. C, congestive heart failure/LV dysfunction, H, hypertension; A2, age ≥75 years; D, diabetes mellitus; S2, stroke/transient ischaemic attack/systemic embolism; V, vascular disease; A, age 65–74 years; Sc, sex category (female sex).
Figure 2.
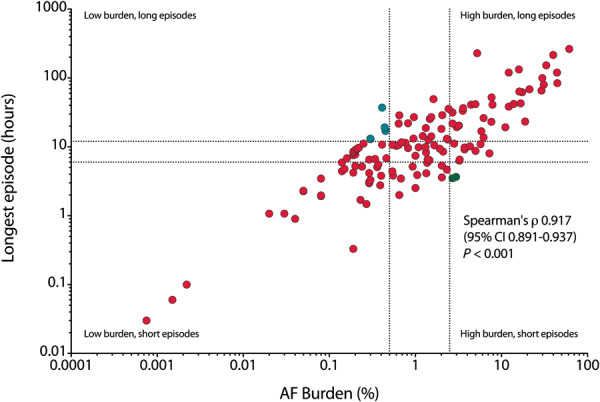
Scatterplot showing a high rate of agreeability between the AF burden (X-axis) and the duration of the longest AF episode (Y-axis), both on logarithmic scales. Data shown for 139 patients with AF recurrence during 6-month follow-up. In turquoise, four patients are identified with long AF episodes, with low AF burden. In green, two patients are identified with short episodes, and high AF burden. AF, atrial fibrillation; CI, confidence interval.
Atrial fibrillation progression
In 179 (89%) patients, 1-year rhythm follow-up was available. Figure 3 shows the categorization of patients based on the longest AF episode duration during the first 6 months as compared to the second 6 months. During follow-up, 111 (62%) patients remained in the same category, 39 (22%) had progression, and 29 (16%) had regression. Twenty-nine (74%) out of 39 patients with progression only progressed to the next category (e.g. short to intermediate or intermediate to long episodes). Eight patients developed persistent AF. A total of five patients started AAD, three showed AF regression, and two remained in the same category. Six patients underwent PVI (three within the first 6 months, three in the second 6 months) three showed regression, two remained in the same category, and one patient developed persistent AF and subsequently underwent PVI. On a quarterly basis, average daily AF burden increased from 3.2% to 3.8%, 5.2%, and 6.1%. When only selecting episodes >1 hours (instead of all episodes ≥2 min) the numbers remained similar: 3.1% to 3.6%, 5.0%, and 5.9%.
Figure 3.
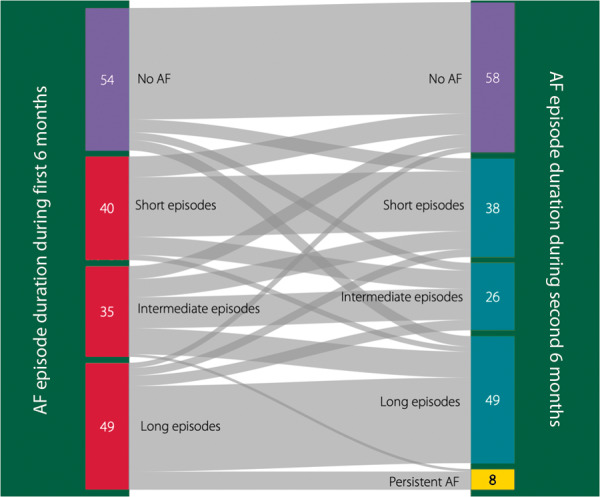
Sankey diagram illustrating the categorization based on the longest AF episode during the first 6 months on the left, and the second 6 months on the right. AF, atrial fibrillation.
Discussion
In this interim analysis of the RACE V registry, we longitudinally investigated temporal patterns, burden, and progression of paroxysmal AF and the association with clinical characteristics using continuous rhythm monitoring. We found that: (i) paroxysmal self-terminating AF has very heterogeneous temporal arrhythmia patterns, both in respect to episode duration and AF burden; (ii) patients with longer AF episodes and higher AF burden had more severe underlying comorbidities; and (iii) modest AF progression within 1-year occurred in a substantial number of patients.
Quantifying paroxysmal atrial fibrillation
The currently used clinical AF classification poorly reflects the temporal pattern and burden of AF.7−9 Patients classified in the same clinical AF category may be inherently heterogeneous in terms of temporal AF persistence and AF burden. We show that patients either had short episodes of AF, or only longer AF episodes with a strong correlation between duration of the self-terminating AF episodes and AF burden. In general, in our population AF burden was low. Only 10 episodes were electrically cardioverted, all other episodes remained self-terminating during follow-up.
Recently, it was acknowledged that an important knowledge gap includes understanding the best parameter for AF quantification, as well as the relation of the amount of AF and stroke and other major adverse cardiovascular events.10To improve classification for paroxysmal AF, Wineinger et al. proposed the ‘staccato’ AF subtype (frequent and short episodes of paroxysmal AF), and the ‘legato’ AF subtype (infrequent and long episodes of paroxysmal AF), based on single-lead ECG monitoring for a mean of 11 days in 13 000 patients.8 Unfortunately, no exact cut-off values for this classification were provided nor were AF patterns correlated to clinical characteristics. In general, intermittent short-term monitoring precludes optimal classification of the type of AF. Continuous ECG monitoring can provide new evidence for the heterogeneity of AF.11 Our study extends these findings by proposing three patterns of paroxysmal self-terminating AF based on the duration of the longest episode. Yet, these cut-off values will need to be validated in future studies.
Comorbidities and atrial fibrillation
The duration of AF episodes may reflect the severity of the atrial cardiomyopathy which itself relates to the presence of risk factors and comorbidities next to AF burden.12 Our data support this concept by showing differences in clinical and echocardiographic characteristics within the three groups of AF episode duration and of AF burden, with more comorbidities in patients with longer AF episodes and a higher AF burden. Episode duration might reflect the severity of the substrate, while the number of shorter AF episodes may be mainly driven by the amount of triggers. The latter may explain the lack of association between very short episodes and cardiovascular outcome.13 Yet, little is known on the exact cut-offs in terms of episode duration to distinguish both entities.
No recurrence of atrial fibrillation
Despite selecting patients with at least two episodes of AF in the past year, approximately one third did not show any recurrent AF episode during the first 6 months of follow-up. The lower left atrial volume in these patients might indicate less severe structural remodelling. On the other hand, the number of comorbidities in patients in this group was similar to that in patients with recurrences. The higher proportion of hypertension and higher CHA2DS2-VASc score in these patients at baseline together with higher rates of antihypertensive drugs (ARBs and diuretics) may indicate that comorbidities were more frequently diagnosed and potentially treated more appropriately. As has been shown by The Routine vs. Aggressive risk factor driven upstream rhythm Control for prevention of Early atrial fibrillation in heart failure (RACE 3) trial and The AggRessive Risk factor rEduction STudy for Atrial Fibrillation (ARREST-AF), risk factor management improves sinus rhythm maintenance in patients with AF.14,15
Atrial fibrillation progression
A recent meta-analysis on AF progression showed a pooled incidence of AF progression of 8.1 per 100 patient-years of follow-up.16This meta-analysis, however, was hampered by differences in follow-up duration and patient characteristics between the respective studies, and the use of intermitted rhythm monitoring. Continuous rhythm monitoring may better define AF progression.11 The relevance of AF progression is that it is associated with worse clinical outcome. In clinical AF, De Vos et al. showed that progression from selfterminating to non-selfterminating AF had prognostic clinical value, which was supported by other studies.1,13 Also in subclinical AF prolongation of duration of AF episodes from ≤24 to >24 hours was associated with more strokes and more heart failure hospitalizations.4,17 In a recent high risk elderly population without known AF, the median AF burden was 0.13% during 40 months of follow-up. Progression to 24-hour episodes occurred in 33 of 590 patients (5.6%) indicating that in these high risk patients AF burden was low, and progression was limited.11 In our cohort with lower risk paroxysmal AF patients, we observed a small increase in averaged AF burden throughout follow-up, and found that 22% of patients showed AF episode prolongation during 1 year. This indicates that AF progression is a slow and subtle process. Interestingly, we also found a significant number of patients with AF regression, which has also been shown previously.18 Factors such as changes in antiarrhythmic therapy, which only occurred in a few patients, or more intense risk factor control may partially explain this interesting observation.14,18
Clinical implications
Continuous rhythm monitoring enables an improved characterization of patients with AF. Diverse patterns may reflect differences in underlying diseases and mechanisms, warranting personalized therapeutic interventions and patient tailored therapies. More details on AF patterns may aid in selecting patients for specific AAD, PVI or other therapies. Additionally, since AF classification is dynamic, observed longitudinal changes in AF patterns may have clinical utility for assessing the progression of the underlying substrate as well as monitoring response to therapeutic interventions. Clinical implications may be tested by extended follow-up in the total population of the RACE V registry.
Strengths and limitations
Strengths include the well-phenotyped cohort and availability of continuous rhythm monitoring, for the vast majority in patients without implanted cardiac devices prior to study enrolment. In contrast, most other studies with continuous rhythm monitoring were performed in patients with a pacemaker or defibrillator.1,7,11,17
Limitations include the modest sample size, the limited follow-up time and the observational nature. At this point it is not yet possible to show any prognostic value of the different paroxysmal AF entities, nor were the exact cut-offs for AF episode duration validated. Inevitably, start and changes of treatment during follow-up will interfere with the natural course of AF, but are inherent to the nature of the arrhythmia. To correct for this, sensitivity analyses were performed that showed similar results, although not all differences remained significant, most likely due to a lack of power. And finally, we acknowledge the differences in detections of different signals between the LinQ and pacemaker patients. Nevertheless, AF detection in Medtronic pacemakers and with the LinQ has been shown to be very reliable.19,20
Conclusion
Our main findings of this interim analysis include that paroxysmal selfterminating AF is a very heterogeneous arrhythmia: one third of the patients did not show any recurrence during 6 months of follow-up. In those with recurrences, AF patterns varied considerably both with respect to duration of AF episodes as well as AF burden. Patients with longer episodes and higher AF burden had more severe underlying comorbidities. Finally, AF progression occurred only in a minority of patients.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Acknowledgements
We acknowledge the support from the Netherlands Cardiovascular Research Initiative: an initiative with support of the Dutch Heart Foundation, CVON 2014–9: Reappraisal of Atrial Fibrillation: interaction between hyperCoagulability, Electrical remodelling, and Vascular destabilisation in the progression of AF (RACE V), and grant support from Medtronic to the institution.
Conflict of interest: R.G.T. reports grants and personal fees from Boehringer Ingelheim, personal fees from BMS/Pfizer, personal fees from Bayer, grants from Medtronic, grants from St Jude Medical, outside the submitted work; In addition, R.G.T. has a patent as a co-inventor of the MyDiagnostick issued. M.E.W.H. reports personal fees from Medtronic, outside the submitted work. J.R.D.G reports grants from Abbott, grants and personal fees from Atricure, grants from Boston Scientific, grants and personal fees from Medtronic, grants and personal fees from Bayer, personal fees from Daiichi Sankyo, personal fees from Johnson&Johnson, personal fees from Novartis, personal fees from Servier, outside the submitted work. C.O.S.S. and M.d.M. report they are employees of Medtronic. U.S. reports grants from Dutch Heart Foundation, during the conduct of the study; personal fees from Johnson & Johnson, grants from Roche, grants from EP solutions, other from YourRhythmics BV, outside the submitted work; In addition, U.S. has a patent Non-invasive classification of AF issued, and a patent Biomarkers for AF pending. J.G.L.M.L. reports personal fees from Medtronic, outside the submitted work. H.J.G.M.C. reports Grant to support the present work, from the Netherlands Cardiovascular Research Initiative: an initiative with support of the Dutch Heart Foundation, CVON 2014–9: Reappraisal of Atrial Fibrillation: interaction between hyperCoagulability, Electrical remodelling, and Vascular destabilisation in the progression of AF (RACE V). All other authors have nothing to declare.
References
- 1. De Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen RJ. et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol 2010;55:725–31. [DOI] [PubMed] [Google Scholar]
- 2. Nattel S, Guasch E, Savelieva I, Cosio FG, Valverde I, Halperin JL. et al. Early management of atrial fibrillation to prevent cardiovascular complications. Eur Heart J 2014;35:1448–56. [DOI] [PubMed] [Google Scholar]
- 3. Steinberg BA, Hellkamp AS, Lokhnygina Y, Patel MR, Breithardt G, Hankey GJ, et al. ; ROCKET-AF Steering Committee and Investigators. Higher risk of death and stroke in patients with persistent vs. paroxysmal atrial fibrillation: results from the ROCKET-AF Trial. Eur Heart J 2015;36:288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wong JA, Conen D, Van Gelder IC, McIntyre WF, Crijns HJ, Wang J. et al. Progression of device-detected subclinical atrial fibrillation and the risk of heart failure. J Am Coll Cardiol 2018;71:2603–11. [DOI] [PubMed] [Google Scholar]
- 5. Vanassche T, Lauw MN, Eikelboom JW, Healey JS, Hart RG, Alings M, Avezum A. et al. Risk of ischaemic stroke according to pattern of atrial fibrillation: analysis of 6563 aspirin-treated patients in ACTIVE-A and AVERROES. Eur Heart J 2015;36:281–7a. [DOI] [PubMed] [Google Scholar]
- 6. De With RR, Marcos EG, Van Gelder IC, Rienstra M.. Atrial fibrillation progression and outcome in patients with young-onset atrial fibrillation. Europace 2018;20:1750–7. [DOI] [PubMed] [Google Scholar]
- 7. Charitos EI, Stierle U, Ziegler PD, Baldewig M, Robinson DR, Sievers HH, Hanke T.. A comprehensive evaluation of rhythm monitoring strategies for the detection of atrial fibrillation recurrence: insights from 647 continuously monitored patients and implications for monitoring after therapeutic interventions. Circulation 2012;126:806–14. [DOI] [PubMed] [Google Scholar]
- 8. Wineinger NE, Barrett PM, Zhang Y, Irfanullah I, Muse ED, Steinhubl SR. et al. Identification of paroxysmal atrial fibrillation subtypes in over 13,000 individuals. Heart Rhythm 2019;16:26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De With RR, Marcos EG, Dudink E, Spronk HM, Crijns H, Rienstra M. et al. Atrial fibrillation progression risk factors and associated cardiovascular outcome in well-phenotyped patients: data from the AF-RISK study. Europace 2020;22:352–60. [DOI] [PubMed] [Google Scholar]
- 10. Chen LY, Chung MK, Allen LA, Ezekowitz M, Furie KL, McCabe P, et al. ; American Heart Association Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Quality of Care and Outcomes Research, and Stroke Council. Atrial fibrillation burden: moving beyond atrial fibrillation as a binary entity: a scientific statement from the American Heart Association. Circulation 2018;137:e623–e644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diederichsen SZ, Haugan KJ, Brandes A, Lanng MB, Graff C, Krieger D. et al. Natural history of subclinical atrial fibrillation detected by implanted loop recorders. J Am Coll Cardiol 2019;74:2771–81. [DOI] [PubMed] [Google Scholar]
- 12. Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA. et al. Document Reviewers. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace 2016;18:1455–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Steinberg BA, Piccini JP.. When low-risk atrial fibrillation is not so low risk: beast of burden. JAMA Cardiol 2018;3:558–60. [DOI] [PubMed] [Google Scholar]
- 14. Rienstra M, Hobbelt AH, Alings M, Tijssen JGP, Smit MD, Brugemann J, Geelhoed B, Tieleman RG, Hillege HL, Tukkie R, Van Veldhuisen DJ, Crijns H, Van Gelder IC; RACE 3 Investigators. Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: results of the RACE 3 trial. Eur Heart J 2018;39:2987–96. [DOI] [PubMed] [Google Scholar]
- 15. Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX. et al. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol 2015;65:2159–69. [DOI] [PubMed] [Google Scholar]
- 16. Blum S, Meyre P, Aeschbacher S, Berger S, Auberson C, Briel M. et al. Incidence and predictors of atrial fibrillation progression: a systematic review and meta-analysis. Heart Rhythm 2019;16:502–10. [DOI] [PubMed] [Google Scholar]
- 17. Van Gelder IC, Healey JS, Crijns H, Wang J, Hohnloser SH, Gold MR. et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J 2017;38:1339–44. [DOI] [PubMed] [Google Scholar]
- 18. Middeldorp ME, Pathak RK, Meredith M, Mehta AB, Elliott AD, Mahajan R. et al. PREVEntion and regReSsive Effect of weight-loss and risk factor modification on Atrial Fibrillation: the REVERSE-AF study. Europace 2018;20:1929–35. [DOI] [PubMed] [Google Scholar]
- 19. Sanders P, Purerfellner H, Pokushalov E, Sarkar S, Di Bacco M, Maus B. et al. ; Reveal LINQ Usability Investigators. Performance of a new atrial fibrillation detection algorithm in a miniaturized insertable cardiac monitor: results from the Reveal LINQ Usability Study. Heart Rhythm 2016;13:1425–30. [DOI] [PubMed] [Google Scholar]
- 20. Passman RS, Weinberg KM, Freher M, Denes P, Schaechter A, Goldberger JJ. et al. Accuracy of mode switch algorithms for detection of atrial tachyarrhythmias. J Cardiovasc Electrophysiol 2004;15:773–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.