Abstract
Vasoactive intestinal peptide (VIP) is a neuropeptide with potent immunoregulatory properties. Reduced serum VIP levels and alterations in VIP receptors/signaling on immune cells have been associated with different inflammatory/autoimmune diseases. However, its role in autoimmune thyroid diseases (AITD) remains unknown. This study examined the interrelationship between VIP system, autoimmune background and thyroid hormones in peripheral immune cells in patients with AITD. Only Graves’ disease (GD) patients showed significantly lower serum VIP levels when compared to healthy subjects and to Hashimoto’s thyroiditis patients. Serum VIP levels were lower at the onset of GD, showing a significant negative correlation with thyroid hormone levels. The expression of VIP receptors, VPAC1 and VPAC2, was significantly upregulated in peripheral blood mononuclear cells (PBMC) from GD patients. There was an impairment of VIP signalling in these patients, probably attributable to a dysfunction of VPAC1 with preservation of VPAC2. The correlation between VPAC1 and thyroid hormone receptor expression in PBMC from healthy subjects was lost in GD patients. In summary, the VIP system is altered in peripheral immune cells of GD patients and this finding is associated with different thyroid hormone receptor patterns, showing a dynamic inter-regulation and a prominent role of VIP in this setting.
Subject terms: Immunology, Endocrinology
Introduction
Experimental data and clinical observations in animal models and humans point to an important role of interactions between neuroendocrine and immune systems for the maintenance of the overall homeostasis1,2. This bidirectional network is functionally supported by the presence of shared signalling molecules, including hormones, neuropeptides, cytokines, and their respective receptors. Thus, neuropeptides and hormones are able to modulate immune functions, and immune mediators are capable of affecting the endocrine system. Therefore, although the immune system itself has mechanisms of self-regulation, the neuroendocrine system is also involved in its control. Accordingly, impairment of mechanisms mediating such regulatory relationships has been linked with the development of autoimmune diseases3.
Thyroid hormones, triiodothyronine (T3) and thyroxine (T4), are implicated in key physiological processes, including development, differentiation, and regulation of metabolism4. Besides, accumulating evidence proves the impact of thyroid status on immune responses and inflammation, by directly affecting the functional activity of monocytes, macrophages, lymphocytes and natural killer3. Biological actions of thyroid hormones are mediated by classical signalling mechanisms initiated through binding to their corresponding nuclear receptors, TRα and TRβ, that directly modulate gene transcription5. In addition, recent investigations have described that they also act at the plasma membrane via integrin αvβ3, triggering signalling cascades that indirectly contribute to the regulation of gene expression4.
Thyroid autoimmune diseases (AITD) include a broad spectrum of disorders, with Graves’ disease (GD) and Hashimoto’s thyroiditis (HT) being the most frequently observed6–8. GD is characterized by the presence of serum autoantibodies directed against the thyrotropin (TSH) receptor (TRAb), which over-activate this receptor in thyrocytes, leading to thyroid hormone hyperproduction and unrestrained release. At the time of diagnosis, GD patients exhibit serum FT4 levels above the normal reference range and are classified as hyperthyroid patients. There are different treatment options available for GD such as antithyroid drugs, radioiodine and surgery (thyroidectomy), which may allow some patients to recover the normal FT4 levels, thus being classified as euthyroid GD patients. On the other hand, HT represents the archetype for T-cell-mediated degenerative diseases. It is characterized by a progressive autoimmune thyrocyte depletion, resulting in impaired thyroid hormone production and clinical hypothyroidism9.
Vasoactive intestinal peptide (VIP) is a homeostatic peptide secreted by nerve endings, endocrine and immune cells, with potent immunoregulatory and anti-inflammatory properties. The main signalling pathway mediating VIP effects is activation of adenylate cyclase (AC) through its specific Protein G coupled receptors, VPAC1 and VPAC210. The role of VIP in inflammatory disorders has been broadly reported. Specifically, exogenous VIP administration exhibits beneficial effects in murine models of inflammatory/autoimmune disorders, by reducing immune reactions and inducing anti-inflammatory mediators11,12. Moreover, in vitro studies on human cells have validated the ability of VIP to reshape both innate and adaptive immune responses. VIP impairs acquisition of the macrophage proinflammatory polarization profile13, and alters the Th1/Th2 balance in CD4 T cell differentiation in favour of Th2 cells, stimulating the acquisition of a Th17 non-pathogenic profile and inducing regulatory T cells (Treg)14. Furthermore, reduced serum VIP levels have been described in different inflammatory/autoimmune diseases, emerging as a potential prognostic biomarker in patients with early arthritis15,16 and early spondyloarthritis (SpA)17. In this sense, alterations in the expression and functionality of VIP receptors have also been observed in these autoimmune disorders, portraying an association with disease activity18,19.
Given the relationship between thyroid and immune function, our hypothesis was that both the autoimmune process and the alterations of thyroid hormone levels in AITD could be interrelated with changes in the VIP system, which, in turn, would modulate the immune response. Therefore, we first explored if serum VIP levels were altered in patients with GD or HT, and if these alterations were related to relevant clinical parameters or associated with different thyroid status. We then studied the expression and function of VIP receptors in peripheral blood mononuclear cells (PBMC). In addition, we characterized the expression pattern of thyroid hormone receptors in order to elucidate if alterations in either of these systems in PBMC could be involved in thyroid autoimmune diseases.
Results
Serum VIP levels are decreased in hyperthyroid GD patients
Given that a decreased expression of VIP has been reported in several autoimmune and inflammatory diseases, we first evaluated serum levels of VIP in GD and HT patients, in an attempt to explore its relevance in two clinically opposed thyroid autoimmune diseases. We also evaluated thyroid hormone levels and thyroid autoantibodies (Table 1a).
Table 1.
Demographic and clinical characteristics of AITD patients and healthy donors. (a) Patients with different autoimmune thyroid diseases and healthy donors. (b) Subgroups of patients with Graves’ disease.
| a | HD (n = 49) |
HT (n = 78) |
GD (n = 144) |
P value |
|---|---|---|---|---|
| Age (years) | NA | 49 (35–55) | 49 (37–56) | 0.51 |
| Sex (female) | NA | 63 | 114 | 0.777 |
| FT4 (ng/dl) | NA | 1.17 (0.93–1.40) | 1.60 (1.14–2.55) | 0.000 |
| TSH (uU/ ml) | NA | 4.01 (2.09–6.67) | 0.01 (0.00–0.98) | 0.000 |
| TPOAb(UI/ml) | 20 (0–20) | 438 (174–771) | 155 (20–461) | 0.000 |
| TgAb (UI/ml) | 20 (0–20) | 336 (20–991) | 20 (20–557) | 0.002 |
| TRAb (U/l) | 0 (0–0) | 0.47 (0.19–0.69) | 2.70 (1.28–7.63) | 0.000 |
| VIP (pg/ml) | 364.11 (331.11–416.88) | 361.42 (309.28–424.03) | 334.24 (303.75–373.58) | 0.003 |
| b | Euthyroid GD (n = 36) | Hyperthyroid GD (n = 73) | Hypothyroid GD (n = 35) | P value |
|---|---|---|---|---|
| Sex (female) | 30 | 55 | 29 | 0.518 |
| Age (years) | 48 (40–54) | 47 (33–57) | 52 (33–60) | 0.773 |
| FT4 (ng/dl) | 1.12 (0.93–1.27) | 2.42 (1.95–3.36) | 1.19 (0.72–1.57) | 0.000 |
| TSH (uU/ ml) | 0.24 (0.01–1.94) | 0 (0–0.01) | 2.31 (0.5–10.16) | 0.000 |
| TPOAb (UI/ml) | 155 (20–459) | 165 (20–613) | 70 (20–300) | 0.297 |
| TgAb (UI/ml) | 20 (20–319) | 20 (20–649) | 20 (20–552) | 0.156 |
| TRAb (U/ml) | 2.39 (1.2–9.89) | 2.72 (1.58–8.0) | 3.21 (0.83–7.21) | 0.015 |
| VIP (pg/ml) | 346.07 (318.42–394.23) | 325.08 (295.73–351.29) | 353.63 (325.79–389.92) | 0.000 |
Values are given as the median and (interquartile range). HD (healthy donor), HT (Hashimoto’s thyroiditis), GD (Graves’ disease), FT4 (free thyroxine 4), TSH (thyroid-stimulating hormone), TPOAb (thyroid peroxidase antibody), TgAb (thyroglobulin antibody), TRAb (TSH-receptor antibody), VIP (vasoactive intestinal peptide), NA (not available). Normal reference values for hormones and autoantibodies are: FT4 (ng/dl): 0.93–1.7; TSH (uU/ml): 0.27–5; TPOAb (UI/ml) < 100; TgAb (UI/ml) < 344; TRAb (U/l) < 0.7. P values are shown for Chi-square test (categorical values) and Kruskal–Wallis test (continuous variables). For more details, see “Methods” section.
Regarding serum VIP levels, there were no differences between sexes and no significant correlation with age were observed. Likewise, no differences were detected between smokers and non-smokers, or in patients with or without family past medical history of autoimmune or thyroid disease. Interestingly, only GD patients showed significantly lower serum levels of VIP (median normalized: 334.24 pmol/ml) when compared to healthy subjects (364.11 pmol/ml) and with HT patients (361.42 pmol/ml) (Fig. 1a). Correlation and regression analysis were performed between VIP serum levels and relevant clinical parameters on each group of patients, including serum levels of FT4, TSH, TPOAb, TgAb and TRAb, age and sex. These analyses only revealed a significant negative correlation between VIP serum levels and FT4 levels in GD patients (B = − 7.709, P = 0.021), but no other significant relationships were found (data not shown).
Figure 1.
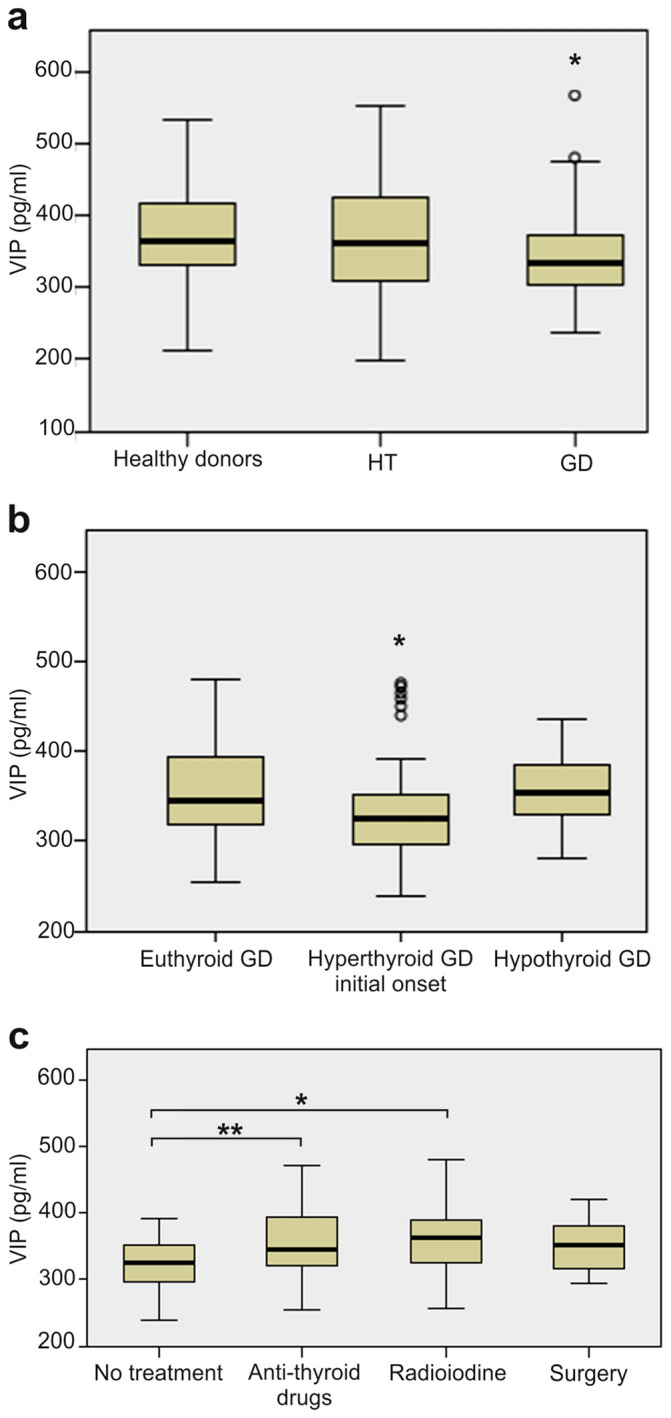
VIP serum levels in patients with different thyroid function status. (a) Association between VIP levels and thyroid status. ELISA determinations of VIP serum levels in 78 patients with Hashimoto’s thyroiditis (HT), 144 with Graves’ disease (GD), and 49 healthy donors (HD). (b) Association between VIP serum levels and thyroid function in patients with GD. ELISA determinations of VIP concentration in serum from 73 GD patients at the initial onset of the disease, 36 euthyroid GD patients controlled with antithyroid drugs and 35 GD hypothyroid patients due to treatment iatrogenesis. (c) Association between VIP levels and treatment groups in patients with GD. ELISA determinations of VIP concentration in serum from 73 GD patients with no treatment, 36 treated with anti-thyroid drugs, 21 treated with radioiodine and 14 that underwent surgery. Data are presented as the interquartile range (p75 upper edge of the box, p25 lower edge, p50 midline), p90 (line above the box), and p10 (line below the box) of serum VIP levels. Dots represent outliers. *P < 0.05; **P < 0.01.
To further examine the altered levels of VIP found in the group of GD patients, VIP serum concentrations were analysed in relation to the thyroid hormone status of these patients. VIP levels were only decreased in recently diagnosed hyperthyroid GD patients but not in euthyroid or hypothyroid patients after therapy (Table 1b, Fig. 1b). Specifically, patients who had not received any prior treatment for hyperthyroidism had the lowest median serum VIP levels (325.08 pg/ml) compared to patients who had received anti-thyroid drugs (345.01 pg/ml, P = 0.006) or radioiodine (361.92 pg/ml, P = 0.024). The number of patients that had undergone previous surgery was lower, but we also observed a trend for higher VIP levels (350.65 pg/ml) than treatment-naïve patients (P = 0.065). Interestingly, we did not find significant differences in serum VIP levels between the three treatment groups (Fig. 1c). Therefore, our results revealed that diminished VIP levels in GD patients are only associated with the hyperthyroid status.
Increased expression and altered function of VIP receptors, VPAC1 and VPAC2, in PBMC from GD patients
Once confirmed that serum VIP levels were only reduced in hyperthyroid GD patients and given that we hypothesized that the alterations of thyroid hormone system might be interrelated with the VIP axis, we next examined the expression and functionality of VIP receptors in PBMC from both euthyroid and hyperthyroid GD patients.
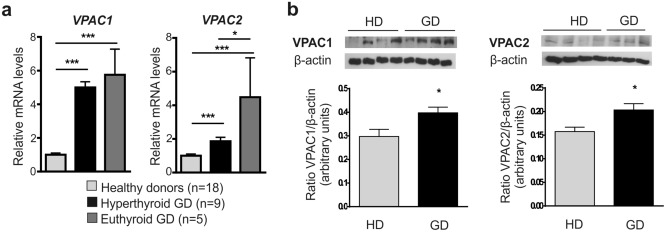
We found that VPAC1 and VPAC2 transcripts levels were significantly upregulated in GD patients, in both hyperthyroid and euthyroid status when compared with healthy donors (Fig. 2a). Gene expression of VPAC1 was similarly high in both GD groups, whereas increase for VPAC2 mRNA expression was more pronounced in patients with normal thyroid status.
Figure 2.
Characterization of VIP receptors in PBMC from healthy donors and from both hyperthyroid and euthyroid GD patients. (a) VPAC1 and VPAC2 mRNA expression levels in PBMC were determined by real-time PCR. Results are expressed as relative mRNA expression (relative to GAPDH mRNA levels) and referred to the expression level in healthy donors. Determinations were done in triplicate, and means and SEM are shown. (b) Protein levels of VPAC1 and VPAC2 in lysates of PBMC were measured by Western blotting. Protein bands were analyzed by densitometric analysis and normalized against the intensity of β-actin. Results are the mean ± SEM of at least 3 experiments and representative pictures are shown. *P < 0.05; **P < 0.01; ***P < 0.001.
We then assessed protein expression of VIP receptors by means of Western blot. Results showed that VPAC1 and VPAC2 protein levels were significantly higher in PBMC from GD patients compared to healthy donors (Fig. 2b), in agreement with mRNA results. We then explored if these changes in receptor expression entailed an alteration in their function. As AC signalling pathway is the major transduction route mediating VIP effects, intracellular cAMP accumulation in PBMC was measured following stimulation with VIP or VPAC selective agonists (Table 2). We found that cAMP production elicited by VIP was significantly reduced in PBMC from hyperthyroid GD patients compared with healthy donors, whereas PBMC from euthyroid GD patients displayed a non-significant decrease in the VIP potency to stimulate AC activity. VPAC1 agonist-induction of intracellular cAMP was significantly decreased in all GD patients, being almost abolished in the euthyroid group. Conversely, VPAC2 agonist maintained its functional capacity to induce cAMP accumulation and showed an improved ability in euthyroid patients, reaching levels comparable to those induced by VIP in PBMC from healthy donors. Collectively, our findings showed an increased expression of VPAC1 and VPAC2 receptors in PBMC from GD patients. Hyperthyroid GD patients exhibited an impairment in VIP signalling not found in the euthyroid group which displayed a higher expression and improved functionality of VPAC2 receptors.
Table 2.
Effect of VIP, VPAC1 agonist, and VPAC2 agonist on intracellular cAMP levels in PBMC. Intracellular cAMP levels were determined by ELISA in PBMC unstimulated or stimulated for 60 min with VIP (10 nM), VPAC1 agonist (10 nM), or VPAC2 agonist (10 nM). Determinations were done in duplicate.
| Healthy donors (n = 18) |
Hyperthyroid GD (n = 9) |
Euthyroid GD (n = 5) |
|
|---|---|---|---|
| Basal | 0.38 ± 0.09 | 0.24 ± 0.05 | 0.31 ± 0.23 |
| VIP, 10 nM | 1.34 ± 0.22 | 0.42 ± 0.85* | 0.51 ± 0.09 |
| VPAC1 agonist, 10 nM | 1.76 ± 0.25 | 0.47 ± 0.05** | 0.05 ± 0.04*** |
| VPAC2 agonist, 10 nM | 0.97 ± 0.18 | 0.97 ± 0.23 | 1.6 ± 0.41 |
Data are expressed as pmol cAMP/mg protein, and means and SEM are shown. P values less than 0.05 were considered significant (*P < 0.05; **P < 0.001; ***P < 0.001), versus healthy donors. For more details, see “Methods” section.
Thyroid hormone receptors are increased in PBMC from GD patients and its expression is related to the VIP system
Given that PBMC from both hyperthyroid and euthyroid GD patients exhibited a variation in VIP receptors, we next assessed whether thyroid hormone receptors were also altered. To that end, we examined the gene expression of the nuclear thyroid hormone receptors TRα and TRβ (THRA and THRB, respectively), and the plasma membrane integrin αvβ3 (codified by ITGAV and ITGB3 genes) were examined in PBMC from patients with GD and healthy donors. Then, we studied its relationship with the VIP axis.
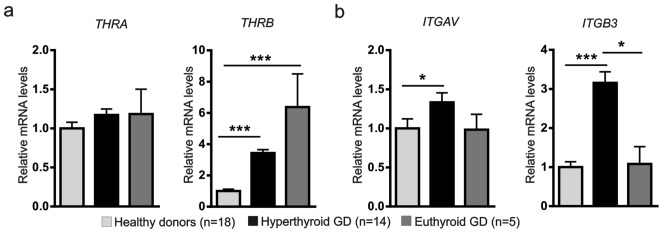
Transcript levels of thyroid hormone receptors, with the exception of THRA, were significantly increased in hyperthyroid GD patients compared to healthy donors (Fig. 3a, b).
Figure 3.
Gene expression of nuclear thyroid hormone receptors, TRα and TRβ, and plasma membrane integrin αvβ3 in PBMC from healthy donors and from both hyperthyroid and euthyroid GD patients. The mRNA expression levels of (a) THRA and THRB, and (b) ITGAV and ITGB3 in PBMC were determined by real-time PCR. Results are expressed as relative mRNA expression (relative to GAPDH mRNA levels) and referred to the expression level in healthy donors. Determinations were done in triplicate, and means and SEM are shown. *P < 0.05; ***P < 0.001.
When we explored the possible relationship between the altered expression patterns of receptors for VIP and for thyroid hormone, a significant correlation was found between the gene expression of VPAC1 and all genes encoding thyroid hormone receptors in PBMC from healthy donors (Table 3). Specifically, there were positive correlations between mRNA expression levels of VPAC1, the nuclear receptors THRA, THRB and the ITGAV subunit of integrin, whereas a negative correlation was found with the subunit ITGB3. Conversely, none of these correlations were found in hyperthyroid GD patients, whereas euthyroid GD patients retained positive correlations with THRA and ITGAV while recovered the negativity in the correlation with ITGB3 subunit, although not statistically significant (Table 3). Regarding VPAC2, only a significant positive correlation for ITGAV was found in healthy subjects, which was not observed in GD (Table 3). Hence, our results showed an imbalance in the expression pattern of VIP receptors and thyroid hormone receptors in PBMC from GD patients. The relationship observed between gene expression profiles of both types of receptors in healthy subjects was lost in the hyperthyroid group, whereas was partially retained in euthyroid patients.
Table 3.
Correlation between gene expression of the receptors for VIP and for thyroid hormone in PBMC. Spearman’s correlation test performed with the relative mRNA expression levels of VPAC1 or VPAC2 and Thyroid hormone receptors.
| Healthy donors (n = 18) |
Hyperthyroid GD (n = 9) |
Euthyroid GD (n = 5) |
||||
|---|---|---|---|---|---|---|
| VPAC1 | VPAC2 | VPAC1 | VPAC2 | VPAC1 | VPAC2 | |
| THRA | 0.3609** | − 0.0329 | 0.3059 | 0.4011* | 0.8464*** | 0.2714 |
| THRB | 0.4597*** | 0.4152 | 0.1068 | 0.232 | − 0.3107 | − 0.0964 |
| ITGAV | 0.4911*** | 0.6923*** | 0.1148 | 0.3553 | 0.6321* | 0.0678 |
| ITGB3 | − 0.4638*** | − 0.2129 | 0.2637 | 0.243 | − 0.0514 | − 0.1429 |
Spearman’s correlation coefficients (rho) are shown, and statistical signification is marked as *P < 0.05; **P < 0.001; ***P < 0.001. For more details, see “Methods” section.
Discussion
A complex network of immune-neuroendocrine regulatory interactions, mediated by hormones, neuropeptides and other signalling molecules, is crucial in the maintenance of homeostasis1. In this regard, potent anti-inflammatory and immunomodulatory effects of VIP have been demonstrated in several inflammatory/autoimmune diseases, where reduced serum levels of this neuropeptide and alterations in its signalling pathway in immune cells have been described10,11,20. Thyroid hormone levels, on their part, exhibit a positive correlation with markers of inflammation and immune activation, and thus, they may exacerbate the alterations of VIP axis21. However, the potential involvement of VIP in the particular case of autoimmune thyroid disease, which represent a model of autoimmunity and inflammation with abnormal thyroid hormones levels22 is not fully understood.
In the present study, we describe that VIP serum levels are significantly reduced in GD patients at the onset of the disease, when thyroid hormone levels are elevated (hyperthyroid status). On the contrary, no significant variations of VIP levels were observed in GD patients with normal thyroid status (euthyroid patients) or iatrogenic hypothyroidism. Levels of VIP were normalized in euthyroid GD patients independently of the treatment used to control hyperthyroidism, including antithyroid drugs, radioiodine or surgery. Accordingly, we also observed a negative correlation between VIP and FT4 serum levels in GD patients, whereas no significant relationships were found with other clinical parameters, including TSH, TgAb, TPOAb, and TRAb. It is worthy to note that variations in these clinical parameters are considered not specific for GD since can also be found in other AITDs and even in healthy subjects. However, thyroid hormone status of GD patients is evaluated on the basis of serum FT4 levels. Therefore, hyperthyroid GD patients show FT4 levels above the normal reference range whereas the euthyroid group has normal or near-normal hormonal values. In other words, our results convey that patients with an ongoing autoimmune thyroid hyperactivity exhibit significantly lower serum levels of VIP, suggesting that the increased serum FT4 levels could be a contributing factor to the decreased VIP levels in hyperthyroid GD patients. Our hypothesis is in agreement with previous reports in murine models which demonstrated modulatory effects of thyroid hormone on VIP mRNA levels in different adult brain areas23 and an upregulation of VIP content in the anterior pituitary gland under hypothyroid conditions24–26. Besides, our results link GD to other inflammatory/autoimmune diseases where VIP serum levels are decreased, such as juvenile idiopathic arthritis (JIA)27, early arthritis15, SpA17, osteoarthritis28 and asthma29. Moreover, the fact that only the hyperthyroid subgroup with recent onset GD showed reduced VIP levels, suggests an association between low VIP levels and disease activity, as it has also been previously demonstrated in patients with early arthritis15,16, SpA17, Chagas cardiomyopathy30 and JIA27. In this regard, GD activity has been associated with high levels of pathogenic Th17 and impaired Treg response31–33. These data would indeed be in accordance with the low VIP levels found at the onset of GD, given that VIP is able to promote Treg responses in several autoimmune diseases34,35, and induces a non-pathogenic phenotype in in vitro differentiated Th17 cells36. Therefore, it is worth speculating that GD might represent an additional autoimmune disease in which a disruption of VIP-mediated immune homeostasis could be linked to clinical outcome. Nevertheless, longitudinal studies in larger population samples would be useful to verify the potential role of VIP as a biomarker of GD activity.
Considering the hypothesis of an alteration of the VIP axis on immune cells, we characterized VIP receptors in PBMC from patients with GD and related these findings to thyroid status. Our findings showed an upregulation of VPAC1 and VPAC2 in GD patients as a group, in accordance with the dynamic regulation of both receptors reported in several autoimmune diseases, such as in PBMC and T cells from early arthritis patients14,18,in monocytes from rheumatoid arthritis (RA) patients37,in CD4+ T cells from multiple sclerosis (MS) patients38, and in monocytes from Sjögren’s syndrome (SS) patients39. Despite the higher expression of VPAC receptors, VIP-stimulated signalling through VPAC1 receptor was significantly impaired in both hyperthyroid and euthyroid GD patients, whereas VPAC2 preserved its functional capacity. Interestingly, a remarkable increase in VPAC2 transcript levels was observed in PBMC from euthyroid GD patients compared to the hyperthyroid group. Therefore, the enhanced expression of VPAC2 could be considered as a mechanism that attempts to counterbalance the VPAC1 dysfunction by upregulating the expression of the functional receptor. Furthermore, VPAC2 agonist also displayed an improved ability to increase intracellular cAMP in euthyroid GD PBMC compared with healthy donors, suggesting a reinforcement of VPAC2 mediated signalling. This compensatory mechanism through VPAC2 would not be operating in the hyperthyroid group, which exhibited a impairment in VIP signalling. These data are in agreement with the role of VPAC2 in mediating VIP anti-inflammatory effects in synovial fibroblasts from RA patients that showed a reduced expression of VPAC140. Moreover, our results point that, also in GD, VPAC2 expression is probably related to cellular activation and/or pathological conditions19. In this sense, VPAC2 also become the dominant receptor in PBMC, activated memory Th cells and Th17-polarized cells from early arthritis patients14,18. Moreover monocytes from SS patients exhibit higher expression of VPAC2, which is absent in healthy donors monocytes39, and activated CD4+ T cells isolated from patients with MS also show a remarkable increase in the expression of VPAC238.
Regarding thyroid hormone receptors, as far as we know, our results reveal for the first time an altered expression pattern of these receptors in PBMC from GD patients. Transcript levels of the nuclear TRβ receptor were significantly upregulated in all GD patients, whereas plasma membrane receptor αvβ3 was only increased in hyperthyroid GD patients. When we examined the interplay between the systems involving VIP and thyroid hormones in healthy subjects, we found a positive correlation between the relative expression of VPAC1 and thyroid hormone receptors mRNA, with the exception of the integrin β3 subunit which exhibited a negative correlation. Such negative relationship may reflect a limiting interaction between expression of VPAC1 and ITGB3, by which the presence of the integrin heterodimer on the plasma membrane might be regulated. Interestingly, all correlations were lost in hyperthyroid GD patients, whereas the euthyroid group recovered the positive correlations with THRA and ITGAV genes, and also, the negativity in the correlation with ITGB3, although not statistically significant. Therefore, the restoration of such immune-neuroendocrine interactions in patients with normal thyroid status after medical treatment could be interpreted as an additional evidence of the direct relationship between these systems1, suggesting the existence of reciprocal regulatory mechanism.
In summary, our findings of reduced serum VIP levels and dysfunctional VPAC signalling in PBMC from hyperthyroid GD patients, suggest the existence of a dynamic connection between the neuroendocrine and immune systems and a prominent role of VIP in this setting (Fig. 4). Although further studies are needed to corroborate this hypothesis, our results represent an initial step to unravel the neuroendocrine-immune regulatory interactions in the specific setting of GD, which could certainly open new opportunities for therapeutic intervention.
Figure 4.
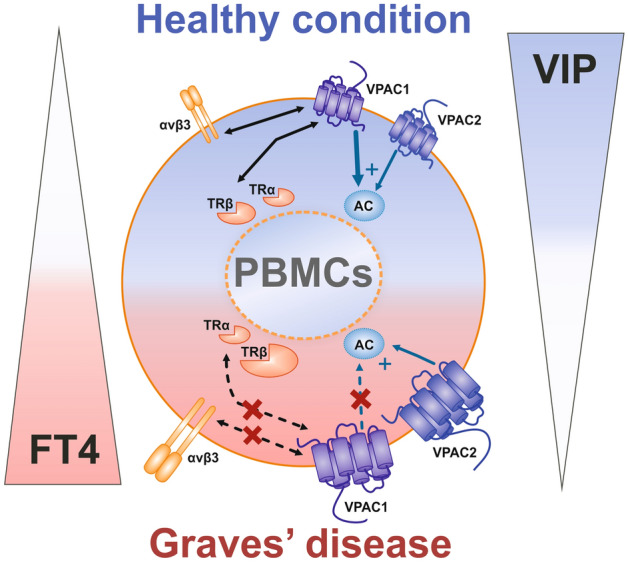
Graphical schematic representation for the proposed inter-relationship between thyroid hormone and VIP system in GD patients. VIP serum levels are significantly lower and negatively correlated with FT4 in hyperthyroid GD patients. Transcript levels of VIP receptors (VPAC1 and VPAC2) and thyroid hormone receptors (nuclear TRβ and plasma membrane αvβ3) are increased in GD patients compared to healthy donors, with the exception of TRα. GD patients show an impairment of VIP signalling through VPAC1 (dotted blue arrows), whereas VPAC2 maintains its capacity to stimulate AC activity (solid blue arrows). There is a correlation between the relative expression of VPAC1 and thyroid hormone receptors in healthy donors (solid black arrows) which is lost in hyperthyroid GD patients (dotted black arrows), suggesting the existence of interactions between both systems in this group of patients. (PBMC peripheral blood mononuclear cell, FT4 free thyroxine 4, AC adenylate cyclase. Blue arrows indicate the contribution of each VPAC receptor signalling to AC activation. Bi-directional black arrows represent correlation between VPAC1 and thyroid hormone receptors).
Methods
Study population
We evaluated 222 patients (177 women, 79.7%) with AIDT: 78 with HT and 144 with GD. Diagnosis was established on commonly accepted clinical and laboratory criteria41. Specifically, diagnosis of HT was made when TSH levels were above the upper limit of normal (> 5 uU/ml), with or without low serum free T4 (FT4), and positive antibodies against thyroperoxidase (TPOAb > 100 U/ml) and/or thyroglobulin (TgAb > 344 U/ml). GD, on its part, was diagnosed when TSH levels were below the lower limit of normal (< 0.27 uU/ml), with or without elevated FT4 (> 1.7 ng/dl) and positive TSH Receptor antibodies (TRAb > 1U/ml). Another 49 healthy subjects, matched for age and sex, were included as controls. Complete clinical and demographic data were collected for all patients, including age, sex, history of tobacco, history of other autoimmune diseases, presence of thyroid diseases in other members of the family, goiter, orbital alterations, and previous therapies (anti-thyroid drugs, radioiodine, surgery or levothyroxine). No patient had been previously treated with steroids. 47 patients (21.2%) acknowledged a smoking habit and 60 patients (27.0%) recalled some sort of past family medical history regarding thyroid disease.
Clinical status at the time of blood sampling was evaluated in each of the 144 patients with GD and categorized as follows. We considered patients as “hyperthyroid GD, initial onset” when blood was collected at the time diagnosis and FT4 levels were above the normal reference limit (1.7 ng/ml); “euthyroid GD”, when patients were on anti-thyroid drugs and had normal or near-normal hormonal values; and “hypothyroid GD” for patients who developed hypothyroidism as a consequence of overtreatment with antithyroid drugs, or after therapy with radioactive iodine or surgery if they were not yet adequately replaced with thyroid hormone. Regarding treatments received by GD patients: 36 patients (25.0%) had received anti-thyroid drugs, 21 (14.6%) radioiodine and 14 (9.7%) had undergone surgery. The remaining 73 patients (50.7%) were naïve to any thyroid-directed treatment.
An informed consent was obtained from all patients participating in the study.
All the procedures were reviewed and approved by the Research Ethics Committee of Instituto de Investigación Sanitaria La Princesa (Madrid, Spain), and were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All patients signed an informed consent form before sampling.
Clinical, hormonal and autoantibody evaluation
Serum levels of FT4, TSH and TgAb, TPOAb, and TRAb were analyzed for each patient. FT4 levels were measured by radioimmunoassay (RIA) with Amerlex FT4 RIA kit (Trinity Biotech); plasma TSH was determined by a highly sensitive radioimmunometric assay Diagnost hTSH (Boehring Co); levels of TRAb were measured by enzyme-linked immunosorbent assay (ELISA) (DRG Instruments GmbH); levels of TgAb and TPOAb were assessed by the immunoradiometric assays ImmunoCAP Thyroglobulin and Immuno-CAP Thyroid Peroxidase kits (Phadia AB).
Evaluation of levels of vasoactive intestinal peptide
We assessed levels of VIP using a commercially available competitive ELISA kit (Phoenix Pharmaceuticals), as previously described17. Serum samples were freeze-dried and dissolved in ELISA buffer (2:1). Levels of VIP were determined applying the corresponding dilution factor. Samples from each patient were assayed twice. The minimum detectable concentration was 0.12 ng/ml, with an intra-assay and inter-assay variation of ≤ 5 and 15%, respectively.
Human peripheral blood mononuclear cells
PBMC from healthy donors, hyperthyroid GD patients and euthyroid GD patients were isolated from heparinized peripheral blood by density gradient centrifugation on Ficoll-Hypaque (Sigma Aldrich).
RNA extraction, cDNA synthesis and real-time polymerase chain reaction (PCR)
As previously described in Carrion et al.13, total RNA was extracted using TriReagent method (Sigma Aldrich). RNA quantity and purity were measured on a NanoDrop and 2 µg were used for cDNA synthesis using a High Capacity cDNA Reverse Transcription Kit (Life Technologies). Real-time PCR analysis for all target genes and one house keeping gene (GADPH) were performed using TaqMan Gene Expression Master Mix (Life Technologies), with manufacturer-predesigned primers. Assays were made in triplicate, and results were normalized according to the expression levels of GADPH. Results were obtained using the 2-ΔΔCt method for quantification.
Western blot analysis of VPAC1 and VPAC2 receptors
As previously described in Carrion et al.13 PBMC proteins were extracted in ice-cold radioimmunoprecipitation assay buffer (50 mM Tris–HCl pH 7.5, 150 mM NaCl, 30 mM NaF, 5 mM EDTA, 1% Triton X-100, 1% NP-40, 0.1% SDS, 1 mM DTT, 1 mM sodium orthovanadate, protease inhibitor cocktail). 30 μg and 40 μg protein extracts, for VPAC1 and VPAC2 respectively, were subjected to 10% SDS-PAGE and transferred to PVDF membranes (BioRad). After blocking, membranes were incubated overnight at 4ºC with rabbit polyclonal anti-human VPAC1 (1: 15,000; Thermo Scientific) and mouse monoclonal anti-human VPAC2 (1: 1000; Abnova Corporation). Mouse anti β-actin (1:20,000, Sigma-Aldrich), was used as a loading control. Horseradish peroxidase conjugated secondary antibody (1:10,000, Santa Cruz Biotechnology) was used. Proteins were detected using Western Blotting Luminol reagent (Santa Cruz Biotechnology), analyzed using the Bio-Rad Quantity One program and normalized against β-actin.
Determination of intracellular cAMP concentrations
Levels of cAMP were determined by an ELISA kit (ADI-900-066, Enzo Life Sciences). PBMC obtained from 18 healthy subjects, 9 hyperthyroid GD patients and 5 euthyroid GD patients were cultured in RPMI-1640-GlutaMAX media (Life Technologies) supplemented with 10% FBS (Lonza,) plus 1% penicillin/streptomycin. After cells were treated with 10 nM VIP (Polypeptide Group), VPAC1 agonist [Lys15Arg16Leu27-VIP (1-7)-GRF (8-27)], or VPAC2 agonist (RO 25-1553) (Bachem) for 60 min, the reaction was terminated by aspiration of the growth medium and addition of 0.1 N HCl. The concentrations of cAMP in the cell lysates were measured according to the manufacturer’s instructions. Protein concentration was determined using a QuantiPro BCA Assay Kit (Sigma- Aldrich).
Statistical analysis
Descriptive results are given as mean ± standard deviation and median (interquartile range) for normally- and not-normally-distributed continuous variables, respectively. Categorical variables were summarized as frequencies and percentages.
In order to overcome the batch effect, which is intrinsic to ELISA determinations, the results obtained for VIP levels were homogenized. Association of VIP levels with patients’ characteristics was assessed using two-sided analysis of variance (Kruskal–Wallis or U-Mann Whitney tests, as required), general linear models, logistic and linear regression analysis and bivariate correlations (Spearman).
All statistical analyses were performed using SPSS version 21.0 (IBM SPSS Statistics Inc.). The P values were two-sided and statistical significance was considered when P < 0.05. Significance of results was analysed using the GraphPad Prism software version 6 (Graphpad Software Inc.). Data were subjected to normality test (Kolmogórov-Smirnov test) and equal variance test (F-test). Mann Whitney or Kruskal–Wallis test was used in intergroup comparison between not-normally-distributed continuous variables. P values less than 0.05 were considered significant (*P < 0.05; **P < 0.01; ***P < 0.001). Results are expressed as the mean ± standard error of the mean (SEM).
Acknowledgements
This work has been supported by Instituto de Salud Carlos III, Spain, cofinanced by FEDER, European Union: RETICS program, Red de Investigación en Inflamación y Enfermedades Reumáticas (RD16/0012/0008, PI17/00027, PI16-02091, PIE13-0004) and from Consejería de Educación, Juventud y Deporte, Comunidad de Madrid: B2017/BMD3724.
Author contributions
M.C., A.M.R.-L., I.V.S., R.M.-H., A.S.-S., D.C., Y.J., I.G.-A., R.P.G, M.M. contributed to the study conception and design. Material preparation, data collection and analysis were performed by M.C., A.M.R.-L., R.M., A.S.-S. and D.C. The first draft of the manuscript was written by M.C. and A.M.R.-L. All authors reviewed and edited previous versions of the manuscript. M.C., A.M.R.-L., I.V.S., R.M.-H., A.S.-S., D.C., Y.J., I.G.-A., R.P.G., M.M. read and approved the final manuscript.
Data availability
All relevant data are within the paper. The datasets generated and/or analysed during the current study are not publicly available due to the confidential nature of the clinical data but are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: M. Carrión and A. M. Ramos-Leví.
These authors jointly supervised this work: Mónica Marazuela and Rosa P. Gomariz.
References
- 1.Del Rey A, Besedovsky HO. Immune-neuro-endocrine reflexes, circuits, and networks: physiologic and evolutionary implications. Front. Horm. Res. 2017;48:1–18. doi: 10.1159/000452902. [DOI] [PubMed] [Google Scholar]
- 2.Ganea DA, Dines M, Basu S, Lamprecht R. The membrane proximal region of AMPA receptors in lateral amygdala is essential for fear memory formation. Neuropsychopharmacology. 2015;40:2727–2735. doi: 10.1038/npp.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jara EL, et al. Modulating the function of the immune system by thyroid hormones and thyrotropin. Immunol. Lett. 2017;184:76–83. doi: 10.1016/j.imlet.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Davis PJ, Goglia F, Leonard JL. Nongenomic actions of thyroid hormone. Nat. Rev. Endocrinol. 2016;12:111–121. doi: 10.1038/nrendo.2015.205. [DOI] [PubMed] [Google Scholar]
- 5.Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014;94:355–382. doi: 10.1152/physrev.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Struja T, et al. Is Graves' disease a primary immunodeficiency? New immunological perspectives on an endocrine disease. BMC Med. 2017;15:174. doi: 10.1186/s12916-017-0939-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genovese BM, Noureldine SI, Gleeson EM, Tufano RP, Kandil E. What is the best definitive treatment for Graves' disease? A systematic review of the existing literature. Ann. Surg. Oncol. 2013;20:660–667. doi: 10.1245/s10434-012-2606-x. [DOI] [PubMed] [Google Scholar]
- 8.Brent GA. Mechanisms of thyroid hormone action. J. Clin. Investig. 2012;122:3035–3043. doi: 10.1172/JCI60047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonelli A, Ferrari SM, Corrado A, Di Domenicantonio A, Fallahi P. Autoimmune thyroid disorders. Autoimmun. Rev. 2015;14:174–180. doi: 10.1016/j.autrev.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Ganea D, Hooper KM, Kong W. The neuropeptide vasoactive intestinal peptide: direct effects on immune cells and involvement in inflammatory and autoimmune diseases. Acta Physiol. (Oxf.) 2015;213:442–452. doi: 10.1111/apha.12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomariz RP, Martinez C, Abad C, Leceta J, Delgado M. Immunology of VIP: a review and therapeutical perspectives. Curr. Pharm. Des. 2001;7:89–111. doi: 10.2174/1381612013398374. [DOI] [PubMed] [Google Scholar]
- 12.Gomariz RP, et al. VIP-PACAP system in immunity: new insights for multitarget therapy. Ann. N. Y. Acad. Sci. 2006;1070:51–74. doi: 10.1196/annals.1317.031. [DOI] [PubMed] [Google Scholar]
- 13.Carrion M, et al. VIP impairs acquisition of the macrophage proinflammatory polarization profile. J. Leukoc. Biol. 2016;100:1385–1393. doi: 10.1189/jlb.3A0116-032RR. [DOI] [PubMed] [Google Scholar]
- 14.Jimeno R, et al. Th17 polarization of memory Th cells in early arthritis: the vasoactive intestinal peptide effect. J. Leukoc. Biol. 2015;98:257–269. doi: 10.1189/jlb.3A0714-327R. [DOI] [PubMed] [Google Scholar]
- 15.Martinez C, et al. Serum levels of vasoactive intestinal peptide as a prognostic marker in early arthritis. PLoS ONE. 2014;9:e85248. doi: 10.1371/journal.pone.0085248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seoane IV, et al. Vasoactive intestinal peptide gene polymorphisms, associated with its serum levels, predict treatment requirements in early rheumatoid arthritis. Sci. Rep. 2018;8:2035. doi: 10.1038/s41598-018-20400-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seoane IV, et al. Vasoactive intestinal peptide in early spondyloarthritis: low serum levels as a potential biomarker for disease severity. J. Mol. Neurosci. MN. 2015;56:577–584. doi: 10.1007/s12031-015-0517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seoane IV, et al. Clinical relevance of VPAC1 receptor expression in early arthritis: association with IL-6 and disease activity. PLoS ONE. 2016;11:e0149141. doi: 10.1371/journal.pone.0149141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villanueva-Romero R, et al. The anti-inflammatory mediator, vasoactive intestinal peptide, modulates the differentiation and function of Th subsets in rheumatoid arthritis. J. Immunol. Res. 2018;2018:6043710. doi: 10.1155/2018/6043710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delgado M, et al. Vasoactive intestinal peptide in the immune system: potential therapeutic role in inflammatory and autoimmune diseases. J. Mol. Med. 2002;80:16–24. doi: 10.1007/s00109-001-0291-5. [DOI] [PubMed] [Google Scholar]
- 21.Cremaschi GA, Cayrol F, Sterle HA, Diaz Flaque MC, Barreiro Arcos ML. Thyroid hormones and their membrane receptors as therapeutic targets for T cell lymphomas. Pharmacol. Res. 2016;109:55–63. doi: 10.1016/j.phrs.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Menconi F, Marcocci C, Marino M. Diagnosis and classification of Graves' disease. Autoimmun. Rev. 2014;13:398–402. doi: 10.1016/j.autrev.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Giardino L, Ceccatelli S, Zanni M, Hökfelt T, Calzà L. Regulation of VIP mRNA expression by thyroid hormone in different brain areas of adult rats. Mol. Brain Res. 1994;27:87–94. doi: 10.1016/0169-328X(94)90188-0. [DOI] [PubMed] [Google Scholar]
- 24.Toni R, Mosca S, Ruggeri F, Valmori A, Orlandi G, Toni G, Lechan RM, Vazzani P. Effect of hypothyroidism on vasoactive intestinal peptide-immunoreactive neurons in forebrain-neurohypophysial nuclei of the rat brain. Brain Res. 1995;682:101–105. doi: 10.1016/0006-8993(95)00340-V. [DOI] [PubMed] [Google Scholar]
- 25.Segerson TP, Lam KS, Cacicedo L, Minamitani N, Fink JS, Lechan RM, Reichlin S. Thyroid hormone regulates vasoactive intestinal peptide (VIP) mRNA levels in the rat anterior pituitary gland. Endocrinology. 1989;125:2221–2223. doi: 10.1210/endo-125-4-2221. [DOI] [PubMed] [Google Scholar]
- 26.Michalkiewick M, Suzuki M. Adenohypophyseal vasoactive intestinal peptide and neuropeptide Y responses to hypothyroidism are abolished after anterolateral deafferentation of the hypothalamus. Neuroendocrinology. 1994;59:85–91. doi: 10.1159/000126643. [DOI] [PubMed] [Google Scholar]
- 27.El-Sayed ZA, et al. Cardiovascular autonomic function assessed by autonomic function tests and serum autonomic neuropeptides in Egyptian children and adolescents with rheumatic diseases. Rheumatology (Oxford, England) 2009;48:843–848. doi: 10.1093/rheumatology/kep134. [DOI] [PubMed] [Google Scholar]
- 28.Grassel S, Muschter D. Do neuroendocrine peptides and their receptors qualify as novel therapeutic targets in osteoarthritis? Int. J. Mol. Sci. 2018 doi: 10.3390/ijms19020367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olopade CO, Yu J, Abubaker J, Mensah E, Paul S. Catalytic hydrolysis of VIP in pregnant women with asthma. J. Asthma. 2006;43:429–432. doi: 10.1080/02770900600710730. [DOI] [PubMed] [Google Scholar]
- 30.Correa MV, et al. Low levels of vasoactive intestinal peptide are associated with Chagas disease cardiomyopathy. Hum. Immunol. 2013;74:1375–1381. doi: 10.1016/j.humimm.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 31.Vitales-Noyola M, et al. Pathogenic Th17 and Th22 cells are increased in patients with autoimmune thyroid disorders. Endocrine. 2017;57:409–417. doi: 10.1007/s12020-017-1361-y. [DOI] [PubMed] [Google Scholar]
- 32.Peng D, Xu B, Wang Y, Guo H, Jiang Y. A high frequency of circulating th22 and th17 cells in patients with new onset graves' disease. PLoS ONE. 2013;8:e68446. doi: 10.1371/journal.pone.0068446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramos-Levi AM, Marazuela M. Pathogenesis of thyroid autoimmune disease: the role of cellular mechanisms. Endocrinol. Nutr. 2016;63:421–429. doi: 10.1016/j.endonu.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Leceta J, et al. Vasoactive intestinal peptide regulates Th17 function in autoimmune inflammation. NeuroImmunoModulation. 2007;14:134–138. doi: 10.1159/000110636. [DOI] [PubMed] [Google Scholar]
- 35.Gutierrez-Canas I, et al. Immunoregulatory properties of vasoactive intestinal peptide in human T cell subsets: implications for rheumatoid arthritis. Brain Behav. Immun. 2008;22:312–317. doi: 10.1016/j.bbi.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Jimeno R, et al. The pathogenic Th profile of human activated memory Th cells in early rheumatoid arthritis can be modulated by VIP. J. Mol. Med. 2015;93:457–467. doi: 10.1007/s00109-014-1232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delgado M, et al. Genetic association of vasoactive intestinal peptide receptor with rheumatoid arthritis: altered expression and signal in immune cells. Arthritis Rheum. 2008;58:1010–1019. doi: 10.1002/art.23482. [DOI] [PubMed] [Google Scholar]
- 38.Sun W, Hong J, Zang YC, Liu X, Zhang JZ. Altered expression of vasoactive intestinal peptide receptors in T lymphocytes and aberrant Th1 immunity in multiple sclerosis. Int. Immunol. 2006;18:1691–1700. doi: 10.1093/intimm/dxl103. [DOI] [PubMed] [Google Scholar]
- 39.Hauk V, et al. Monocytes from Sjogren's syndrome patients display increased vasoactive intestinal peptide receptor 2 expression and impaired apoptotic cell phagocytosis. Clin. Exp. Immunol. 2014;177:662–670. doi: 10.1111/cei.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Juarranz Y, et al. Differential expression of vasoactive intestinal peptide and its functional receptors in human osteoarthritic and rheumatoid synovial fibroblasts. Arthritis Rheum. 2008;58:1086–1095. doi: 10.1002/art.23403. [DOI] [PubMed] [Google Scholar]
- 41.Weetman AP. Autoimmune thyroid disease. Autoimmunity. 2004;37:337–340. doi: 10.1080/08916930410001705394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper. The datasets generated and/or analysed during the current study are not publicly available due to the confidential nature of the clinical data but are available from the corresponding author on reasonable request.