Abstract
Reliance on the honey bee as a surrogate organism for risk assessment performed on other bees is widely challenged due to differences in phenology, life history, and sensitivity to pesticides between bee species. Consequently, there is a need to develop validated methods for assessing toxicity in non-Apis bees including bumble bees. The usefulness of small-scale, queenless colonies, termed microcolonies, have not been fully investigated for hazard assessment. Using the insect growth regulator diflubenzuron as a reference toxicant, we monitored microcolony development from egg laying to drone emergence using the Eastern bumble bee Bombus impatiens (C.), a non-Apis species native to North America. Microcolonies were monitored following dietary exposure to diflubenzuron (nominal concentrations: 0.1, 1, 10, 100 and 1000 μg/L). Microcolony syrup and pollen consumption was significantly reduced by diflubenzuron exposure. Pupal cell production was also significantly decreased at the highest diflubenzuron concentration assessed. Ultimately, diflubenzuron inhibited drone production in a concentration-dependent manner and a 42-day 50% inhibitory concentration (IC50) was determined. None of the dietary concentrations of diflubenzuron tested affected adult worker survival, or average drone weight. These data strengthen the foundation for use of this methodology and provide valuable information for B. impatiens, however more work is required to better understand the utility of the bumble bee microcolony model for pesticide hazard assessment.
Keywords: bumble bee, microcolony, insect growth regulator, risk assessment, pesticide
Introduction
Native insects, including wild bees, are important pollinators of managed and natural landscapes. Populations of some species of wild bees are in serious decline (Kopec and Burd 2017) and in 2017 seven bee species were added to the list of federally Endangered/Threatened Species (Kennedy 2016, Gorman 2017). Population declines have been attributed to many interacting environmental factors, including poor nutrition, parasites, pathogens, and pesticides (Goulson 2010, Brittain and Potts 2011, Sanchez-Bayo and Goka 2014, PHTF 2015). The Federal, Insecticide, Fungicide, and Rodenticide Act, as amended most recently by the Food Quality Protection Act, requires that all pesticides be registered with the Environmental Protection Agency (EPA) and that new pesticides be subject to a risk assessment process for honey bees (Apis mellifera L.) and other bees (i.e. non-Apis bees) (EPA 2014).
Use of surrogate species for toxicity testing is a common practice rooted in practicality since testing all species is not feasible. The approach assumes that closely related species possess similar physiological attributes and will respond similarly to the same stressor (Banks et al. 2010, Wach et al. 2016). Honey bees serve a surrogate species for risk assessment for non-Apis bees; findings in honey bees are extrapolated to other species (EPA 2014). However, there are many notable differences in life histories and phenology between Apis and non-Apis bees, and the significance of those differences is not fully understood. Furthermore, there is increasing recognition of differences in pesticide sensitivity among species of bees (Brittain and Potts 2011, Cresswell et al. 2012, Arena and Sgolastra 2014, Stoner 2016, Thompson 2016, Heard et al. 2017). Consequently, reliance on the honey bee as a surrogate species for other bees has been challenged (Stoner 2016, Rortais et al. 2017), prompting the development of protocols specifically designed for assessing the effects of pesticides on non-Apis bees.
In order to examine differences in sensitivity between bee species, protocols specifically designed for assessing non-Apis bees, such as bumbles bees, are necessary. Recently, the Organization for Economic Co-operation and Development (OECD) finalized bumble bee-specific guidelines for testing adult bee acute oral and contact toxicity (OECD 2017b, a). However, laboratory methodologies for assessing chronic toxicity, including effects on bumble bee brood development, and for performing health assessments at the colony level, are still lacking. The attributes of bumble bee microcolonies may prove useful for filling some of these gaps since they can be used to conduct chronic exposure studies while assessing relevant lethal and sublethal effects of exposure under defined laboratory conditions.
Under conditions of confinement and in the absence of a true queen, bumble bee workers self-organize into a microcolony with a false queen that lays unfertilized eggs which give rise to drones (Free 1955). This experimental unit lends itself to hazard assessment since more colonies can be run simultaneously, fewer resources are required (e.g. mated queens not needed), and less experimental complexity is present (e.g. 5 workers instead of a full-sized, queenright colony). While a growing number of researchers are utilizing the microcolony model (Klinger et al. 2019), the majority of microcolony studies have been performed using the buff-tailed bumble bee (B. terrestris L.) (Dhadialla et al. 2005, Mommaerts et al. 2006, Mommaerts et al. 2009, Mommaerts et al. 2010, Gradish et al. 2012, Meeus et al. 2013), a species not ecologically relevant or commercially available in North America. Further, it is unknown whether B. terrestris reflects the sensitivity of other bumble bee species, and assay protocols designed specifically for B. terrestris may not be directly transferred to other species (Gradish et al. 2012). Thus, there is a need to develop protocols adapted for B. impatiens, a species relevant to North America, and more broadly standardize microcolony procedures (e.g., number of bees, pollen source, syrup diet formulation, age of bees, duration of experiment) (Klinger et al. 2019).
In order to evaluate the usefulness of the microcolony methodology for B. impatiens for risk assessment, we considered several test system attributes, including its usefulness in assessing population-relevant endpoints, dose-dependent responses for key endpoints, repeatability, and efficiency. We chose the insect growth regulator (IGR) diflubenzuron as a reference toxicant because it primarily targets brood development (i.e. insect larval stages) and has low adult toxicity in bees (Barker and Taber III 1977, Emmett and Archer 1980, Mommaerts et al. 2006). This pesticide, commonly used to control moths, mosquitoes, mites (Hoying and Riedl 1980, EPA 1997, Costa and Tadei 2011), inhibits chitin synthesis and leads to larval death via molt failure (van Eck 1979, Hassan and Charnley 1987, Dhadialla et al. 2005, Merzendorfer 2006, Smagghe et al. 2007). Diflubenzuron is also a suggested reference toxicant for brood development assessments with honey bees (EPA 2018). Taken together, these characteristics make it a strong candidate reference toxicant for bumble bee microcolony nest productivity assessments. We chose a range of concentrations below that which would be expected to impact bumble bee worker survival (Mommaerts et al. 2006), so that microcolony development and productivity (i.e. brood development and drone production) would be the primary focus of this assessment.
In this work, we assessed this methodology by conducting 6-week microcolonies, from nest initiation to drone emergence, and investigated the effects of diflubenzuron on microcolony maturation and productivity. This work strengthens the foundation for use of this methodology for risk assessment. In addition, we identified knowledge gaps that need to be further addressed.
Materials and Methods
Chemicals and reagents
Diflubenzuron (purity ≥99.5%; CASRN 35367–38-5) and acetone were purchased from Sigma Aldrich (St. Louis, MO). Other chemicals used for this study include Honey-B-Healthy®, a feeding stimulant (Honey-B-Healthy Inc., Cumberland, MD), sorbic acid (Amresco, Dallas, TX), citric acid anhydrous (Fisher Scientific, Hampton, NH), pure cane sugar (Domino Foods, Inc) and distilled water (Gibco, Gaithersburg, MD).
Bumble bee stock
Newly emerged B. impatiens workers (Biobest®, Romulus, MI) were acclimated to the laboratory for 24 hrs prior to microcolony initiation. Workers were provisioned with 50/50 inverted sugar (50% glucose and 50% fructose) syrup diet prepared in distilled water containing 2.95 mM citric acid and 8.32 mM sorbic acid. Pollen paste (2.5 g pollen/1 mL 50/50 invert syrup) prepared from honey bee corbicular pollen (Brushy Mountain Bee Farm, Monrovia Falls, NC) was also provided. Bees were maintained in an environmental chamber (Percival, Perry, IA) in continuous darkness at 50 ± 5% relative humidity and 25 ± 0.5° C.
Microcolony initiation, pesticide treatment, and monitoring
Two cohorts of bumble bee workers were used to initiate microcolonies, and workers in each cohort originated from multiple queenright colonies. For each cohort, three microcolonies per treatment group were run (n = 6 total per treatment). The cohorts overlapped in time by 2 weeks. Two evaporation control microcolonies (no bees present) were run in parallel for the duration of both cohorts (10 weeks). To initiate microcolonies, workers were chilled on ice for 5 minutes, subdivided into groups of 5 bees, weighed, and then transferred to microcolony chambers. Chambers were food pans that measured 6.88 × 15.88 cm at the top, 13.97 × 12.07 cm at the bottom, and 10.16 cm in depth (1/6 size, Cambro, Camwear 64CW110). Pans were fitted with modified food pan lid (Cambro, Camwear 640CW110) with a white light blocking red acrylic insert and a perforated stainless-steel platform to separate bee waste from the nest (Fig. 1A). Upon initiation, microcolonies were provisioned with 3 g of pollen paste placed in the lid of a 35 mm x 10 mm disposable petri dish (Fig. 1A). Microcolonies were also equipped with a syrup feeder consisting of a 20 mL syringe (Medi-Dose-EPS, Ivyland, PA) with the end capped with a 1/8-inch access hole drilled at the 2 mL mark to eliminate the formation of an air pocket at the tip of the syringe that would prevent the bees from feeding. Diflubenzuron (0.1, 1, 10, 100, 1000 μg/L) formulated as an emulsion of the sugar syrup with 0.5% (v/v) Honey-B-Healthy® and 1% (v/v) acetone was delivered in syrup feeders. Syrup-only and vehicle control (1% acetone) microcolonies received an emulsion of sugar syrup with Honey-B-Healthy®. Five days after initiation, all microcolonies received an additional 2 g of pollen paste added directly to the nest initiation dish to stimulate nest building and egg laying. Microcolonies were fed 2 g of pollen paste in a separate feeding dish beginning one week after initiation and Monday, Wednesday, and Friday thereafter. Newly prepared sugar syrup was administered every Monday, Wednesday and Friday throughout the duration of the experiment. Syrup feeders and pollen dishes were weighed and replaced on feeding days to track food consumption. Bees and nests were moved to clean microcolony chambers during week three of the experiment. Microcolonies were monitored every Wednesday for adult worker mortality, appearance of egg chambers, brood masses, and pupal cells. Eclosed drones were removed from the microcolonies upon emergence and weighed. Progression of one randomly selected microcolony per treatment group was documented photographically using a digital camera (iPhone 6, Apple, Cupertino, CA). The experimental timeline is summarized in Figure 1B.
Figure 1. Microcolony configuration and experimental timeline.
(A) microcolony chambers composed of 1/6 size food pan, white light-blocking modified lid, and a perforated stainless-steel insert. Microcolony chambers were provisioned with a nest dish containing a pollen patty and a separate pollen feeding dish. (B) Experimental overview and timeline of key events and monitoring.
Data processing and statistical analysis
Syrup and pollen consumption values were corrected for evaporation prior to analysis. Differences in average worker weights, syrup consumption, and pollen consumption were compared for cohort 1 and cohort 2 microcolonies by Student’s t-tests in order to confirm appropriateness of combining cohorts for subsequent analyses. Syrup consumption, pollen consumption, and drone emergence were compared by Student’s t-tests for the syrup-only control and vehicle control groups to determine whether control groups were significantly different. Subsequent analyses combined cohort 1 and 2 and compared treatments to the vehicle control group. Differences between treatment groups and the control group were analyzed with One-way analysis of variance (ANOVA) and Dunn’s multiple comparison test when variances were no different. When variances were significantly different according to the Brown-Forsythe or Bartlett’s tests, a non-parametric test was used (Kruskal-Wallis with Dunnett’s multiple comparisons test). When differences between all groups were assessed, a One-way ANOVA with Tukey’s multiple comparisons test was used. The concentration of test article required to reduce drone production fifty percent (42-day IC50) was determined by nonlinear regression analysis (nonlinear model fit: log(concentration) vs normalized response with variable hill slope). Drone production was normalized to vehicle control emergence and expressed as percentages. Data are presented as mean ± standard deviation (SD) and statistical significance was defined as p<0.05. All statistical analyses were performed with GraphPad Prism® (v6; La Jolla, CA).
Results
Microcolony cohorts and controls
Cohort 1 and 2 were compared in order to determine whether significant differences between cohorts existed. There were no significant differences between cohort 1 and 2 for average initiating worker weights, total syrup consumption, or total pollen consumption for each treatment group (data not shown, Supplementary Table 1). As such, cohort 1 and cohort 2 were combined for further analyses. The global average worker weight was 0.178 ± 0.017 g.
Syrup-only and vehicle (1% acetone) controls were compared to determine whether vehicle exposure impacted microcolony progression. No significant differences were found for syrup consumption, pollen consumption, or average drone weights (data not shown, Supplementary Table 1). Further, no differences were observed in nest development or time to drone production. Thus, syrup-only controls were omitted from subsequent analysis and vehicle controls were used as the primary control group.
Microcolony initiation and development
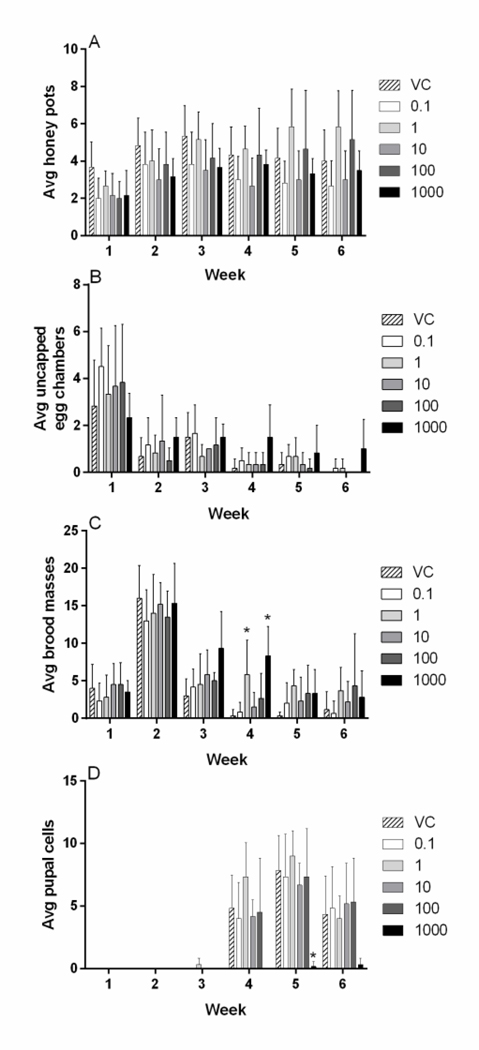
The average weight of initiating workers for each treatment group was compared, and no significant differences were found (data not shown, Supplementary Table 1). Microcolony nest development observations revealed all treatment groups initiated egg chamber and honey pot construction within the first week of the experiment (Figs. 2A, B). Brood masses were also detected by visual inspection within the first week in all treatment groups (Fig. 2C). Observed brood mass numbers were the highest during the second week of the experiment, after which they diminished in number through the subsequent weeks. Larval brood masses were significantly elevated in week 4 for 1.0 and 1000 μg/ diflubenzuron groups (Kruskal-Wallis statistic = 19.34, P = 0.0017, Fig. 2C). The presence of pupal cells occurred during weeks 3–6, following the peak week for brood masses. Pupal cell numbers were significantly decreased in the 1000 μg/L treatment group during week 5 (Kruskal-Wallis statistic = 16.9; P = 0.0046, Fig. 2D). Photographic documentation of representative microcolonies depict nest progression throughout the 6 weeks and qualitatively show reduced nest building activity with increasing concentrations of diflubenzuron (Fig. 3).
Figure 2. Indicators of nest development in B. impatiens microcolonies exposed to diflubenzuron.
(A) Average honey pots constructed by workers. (B) Average uncapped egg chambers present. (C) Average brood masses present. (D) Average pupal cells present. Microcolony observations were collected once per week for the duration of the experiment. Diflubenzuron concentrations are expressed in μg/L. Data shown as mean ± SD (n = 6). * denotes p<0.05.
Figure 3. Concentration-dependent inhibition of microcolony development.
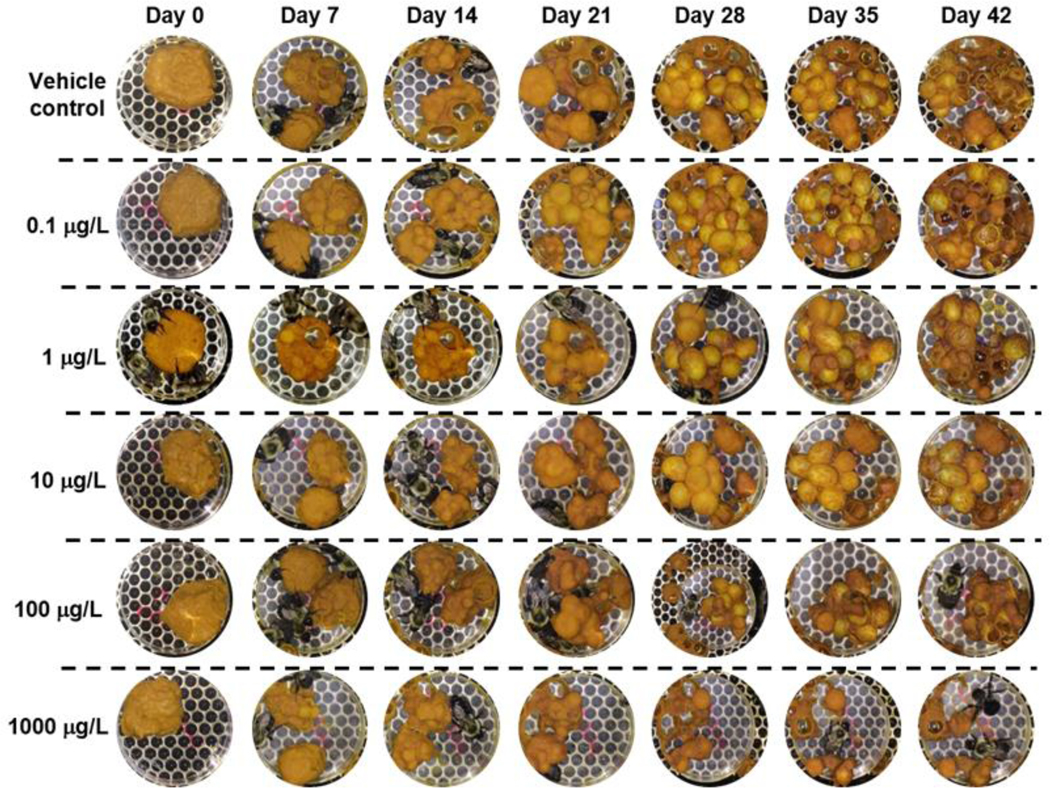
Microcolony development from nest initiation on day 0 to experimental termination on day 42 in the presence of diflubenzuron-containing sugar syrup captured photographically. Representative photos are shown from 1 of 6 microcolonies per treatment.
Compared to vehicle control, no significant adult mortality was detected in diflubenzuron-treated microcolonies (data not shown, Supplementary Table 1). No mortality occurred in the first three weeks, and the highest mortality observed was three deaths (out of 30 total bees in the treatment group) in the 1000 μg/L treatment group. No individual microcolony experienced more than one worker death. Dead bees were removed from the affected microcolonies and, to conserve our ability to make meaningful comparisons between experimental groups, were not replaced with new bees. Detailed mortality data are available in Supplementary Table 2.
Microcolony food consumption
Syrup and pollen consumption were tracked in order to establish consumption trends over time as well as expected ranges of consumption for B. impatiens microcolonies containing five workers. Syrup consumption was also used to evaluate the palatability of diflubenzuron-containing syrup.
Syrup consumption during the first week was no different between treatment groups, suggesting that syrup palatability was not impacted by the addition of diflubenzuron. Syrup consumption increased during the first three weeks of microcolony development for all treatment groups, and consumption reached maximum levels during weeks 3 and 4, which coincided with transition from brood mass to pupal cell formation within the developing nests (Figs. 2C, D; Fig. 4A). There were no significant differences in syrup consumption on a weekly basis between treatment groups apart from week 4, wherein 1000 μg/L microcolonies consumed significantly less syrup than the vehicle control group (F = 2.738; df = 5,30; P = 0.0374, Fig. 4A). Total syrup consumption across the entire experiment was significantly lower for 1,000 μg/L microcolonies as compared to vehicle controls (F = 3.422; df = 5,30; P = 0.0145, Fig. 4C).
Figure 4. Microcolony syrup and pollen consumption.
(A) Average syrup consumption by exposure group per week. (B) Average pollen consumption by exposure group per week. (C) Average syrup consumption for the full 6-week study for each exposure group. (D) Average pollen consumption for the full 6-week study for each exposure group. Tracking pollen consumption began during the second week of the experiment (Fig. 1B). All values have been corrected for evaporation. Diflubenzuron concentrations are expressed in μg/L. Data shown as mean ± SD (n = 6). * denotes p<0.05.
Pollen consumption followed a different trend through microcolony development, with pollen consumption dramatically increasing from week 2 to 3 before tapering off in subsequent weeks. This trend was observed for vehicle control, 0.1, 1, and 10 μg/L groups, and moderately so for 100 μg/L microcolonies. Both the 100 and 1000 μg/L groups consumed less pollen throughout the experiment. The 100 μg/L groups consumed less during week 2, 4, 5 and 6 (Table 1, Fig. 4B) and the 1000 μg/L group consumed significantly less pollen every week and did not display the same consumption trend as the other treatment groups (Table 1, Fig. 4B). All groups except 1000 μg/L consumed their highest levels of pollen during week 3 and 4. When total pollen consumption over the course of the experiment was assessed, both 100 and 1000 μg/L groups consumed significantly less pollen (F = 76.86; df = 5, 30; P < 0.0001, Fig. 4D). Detailed syrup and pollen consumption values are available in Supplementary Table 3 and 4, respectively.
Table 1.
Statistical results of pollen consumption and drone emergence.
| Comparison | Treatment (μg/L Diflubenzuron) | Result |
|---|---|---|
| Pollen consumption | ||
| Week 2b | VC, 0.1, 1, 10, 100, 1000 | F = 20.57; df = 5,30; P < 0.0001 |
| Week 3a | VC, 0.1, 1, 10, 100, 1000 | Kruskal-Wallis statistic = 24.01; P = 0.0002 |
| Week 4b | VC, 0.1, 1, 10, 100, 1000 | F = 32.22; df = 5,30; P < 0.0001 |
| Week 5a | VC, 0.1, 1, 10, 100, 1000 | Kruskal-Wallis statistic = 24.46; P = 0.0002 |
| Week 6b | VC, 0.1, 1, 10, 100, 1000 | F = 18.27; df = 5,30; P < 0.0001 |
| Avg drone emergence | ||
| Day 37a | VC, 0.1, 1, 10, 100, 1000 | Kruskal-Wallis statistic = 19.80; P = 0.0014 |
| Day 40a | VC, 0.1, 1, 10, 100, 1000 | Kruskal-Wallis statistic = 25.50; P = 0.0001 |
| Day 42a | VC, 0.1, 1, 10, 100, 1000 | Kruskal-Wallis statistic = 25.40; P = 0.0001 |
Kruskal-Wallis with Dunn’s multiple comparisons test;
One-way ANOVA with Dunnett’s multiple comparisons test. VC = Vehicle control.
Microcolony drone production
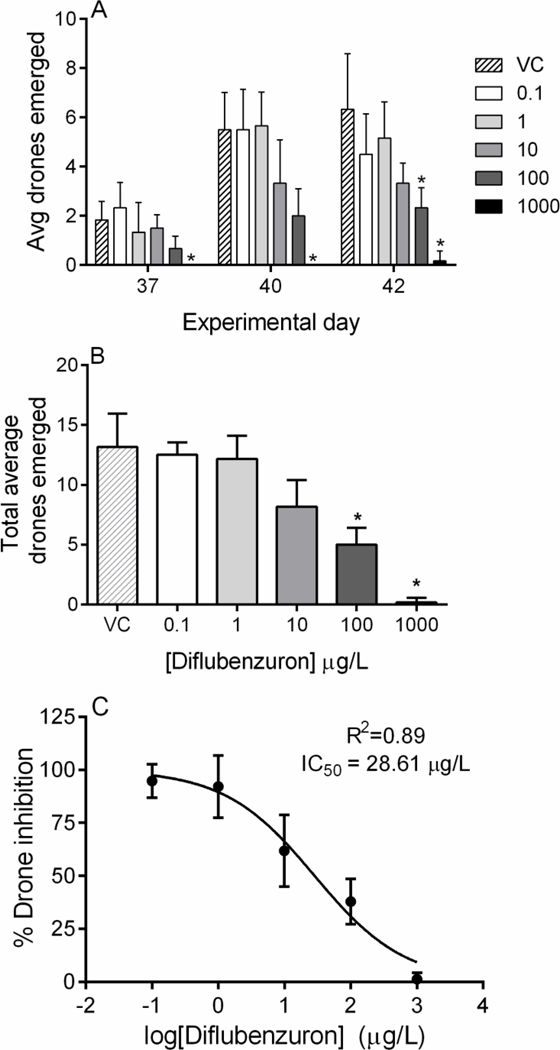
Drone emergence began on day 37 and continued until termination of the experiment. Drones were removed from microcolonies on day 37, 40, and 42, corresponding to replacement of syringe and pollen dishes. Average drone emergence was significantly lower for 100 μg/L microcolonies on day 42 and was significantly lower on all days for the 1000 μg/L microcolonies (Table 1, Fig. 5A). When considering total drones emerged, 100 and 1000 μg/L produced significantly fewer drones than the vehicle control group (Kruskal-Wallis statistic = 28.97; P < 0.0001, Fig. 5B). Drone production was inhibited by diflubenzuron in a concentration-dependent manner, thus an IC50 was calculated by analyzing the percent by which drone production was inhibited. The 42-day IC50 for drone production was 28.61 ± 14.41 μg/L (non-linear fit model, df = 28; R2 = 0.89, Fig. 5C). Finally, we assessed whether drone weights were impacted by diflubenzuron treatment and found no significant differences in average drone weights in treatment groups compared to vehicle controls (data not shown, F = 0.3497; df = 4, 29; P = 0.8417), however, the 1000 μg/L group was omitted from this analysis since only one drone emerged from those microcolonies. No morphological abnormalities were identified in drones from any group. Detailed drone emergence data are available in Supplementary Table 5.
Figure 5. Influence of diflubenzuron on drone production.
Drones were collected from each microcolony and counted as they emerged. (A) Average drone emergence on day 37, 40, and 42 by diflubenzuron concentration (μg/L). (B) Average drone emergence from microcolonies for the entire experiment. (C) Drone inhibition (normalized to vehicle control) due to diflubenzuron exposure. The IC50 was calculated to be 28.61 ± 14.41 μg/L. Diflubenzuron concentrations are expressed in μg/L. Data shown as mean ± SD (n = 6). * denotes p < 0.05.
Discussion
Here we assessed the impact of diflubenzuron exposure on B. impatiens microcolony initiation, development, and productivity. We found that diflubenzuron exposure decreased microcolony syrup and pollen consumption, and dramatically reduced nest building activities at high concentrations. Pupal cell construction, and subsequently drone production, were significantly reduced at high concentrations. We observed that drone production was reduced in a concentration-dependent manner and the 42-day IC50 was calculated to be 28.61 μg/L diflubenzuron. This study also provided useful information regarding the timeline associated with microcolony development for B. impatiens.
Previous research with the European bumble bee B. terrestris has shown that diflubenzuron consumed by egg-laying workers can be transported to ovaries and into eggs (Mommaerts et al. 2006). Since diflubenzuron-containing syrup was available to workers prior to egg laying in the present study, exposure to diflubenzuron may have begun in the egg stage within the microcolonies. However, we observed that early stage brood development (uncapped egg chambers and brood masses) was no different between treatment groups, suggesting that egg laying rates and egg hatching rates were largely unaffected by the exposure concentrations used here.
The differences observed in syrup and pollen consumption between groups was likely related to larval mortality within microcolonies receiving high concentrations of diflubenzuron. Since bumble bee workers feed larvae a mixture of pollen and syrup (Goulson 2010), larval mortality will decrease food demands for the entire microcolony. Bumble bee workers also consume some of the food provisions themselves, allocate some to storage pots, and incorporate a portion into the structure of the nest. As such, we could not determine a diflubenzuron dose to the workers or brood.
The drone production IC50 determined here, 28.61 μg/L, is lower than a comparable measurement found for B. terrestris exposed to diflubenzuron. Mommaerts and colleagues (2006) studied microcolonies for 11 weeks and exposed workers to diflubenzuron orally via sugar water. They found the IC50 for drone production to be 320 μg/L, over 10-fold higher than the IC50 found here. This divergence may be due to differences in body size between the two species since reported average body weights for B. terrestris (Mommaerts et al. 2011, Gosterit et al. 2016, Thompson 2016) and B. impatiens (Cnaani et al. 2002, Scholer and Krischik 2014) workers indicate that B. terrestris is a larger species. Therefore, to facilitate cross species comparisons of pesticide sensitivity, body weight should be collected and reported (Thompson 2016). The observed differences in diflubenzuron sensitivity may also be due to physiological differences, or other departures in study design. Our IC50 value reflects the quantity of diflubenzuron transferred to the entire microcolony as a unit given the challenges in determining a precise dose to the brood. Defining the colony as the experimental unit for pesticide studies involving honey bees is typical due to similar challenges (OECD 2014). To obtain a detailed understanding of the dose experienced by the workers and brood, analytical studies could be conducted to reveal the concentrations and fate of test substances in microcolonies.
A high concentration (1000 μg/L) of diflubenzuron resulted in B. impatiens larval mortality, as inferred by the reductions in food utilization, paucity of pupal cell construction, and subsequent reductions in drone emergence in those microcolonies. Interestingly, in mid-range diflubenzuron treatment groups (10 and 100 μg/L), pupal cell number was no different than low dose and vehicle control microcolonies, despite reductions in overall drone production. This suggests that either prepupal, pupal mortality or delayed development may have occurred at these concentrations. Since worker mortality was low, and only occurred in the final weeks of the experiment, worker mortality did not influence egg-laying in the critical early stages of microcolony development.
Other researchers have investigated the effects of pesticides on microcolony development with B. terrestris (Mommaerts et al. 2006, Smagghe et al. 2007, Besard et al. 2010, Laycock et al. 2012, Elston et al. 2013, Smagghe et al. 2013, Laycock et al. 2014) and B. impatiens (Gradish et al. 2012), however significantly more work is needed to determine whether there are meaningful differences between these two bumble bee species. In general, there are very few studies directly comparing the pesticide sensitivity of different bumble bee species (Wu et al. 2010, Baron et al. 2017). This further highlights the need for microcolony method standardization, since these two species are only available in geographically distinct areas.
A valuable aspect of this dataset is that it begins the process of establishing validity criteria for B. impatiens microcolonies of this worker composition and duration for the purpose of producing data to support risk assessment. The syrup and pollen consumption, drone production, and mortality data collected here all contribute to this end. Ideally, values for these endpoints would be repeated and verified by other researchers working with B. impatiens. Further, this data set demonstrates that the microcolony model is a useful test system in that it can effectively be used to assess population relevant endpoints (e.g. brood development and subsequent eclosion). We also show that this system can be used to generate dose-dependent responses to key endpoints, which in the present study was drone production. And, while repeatability of this system needs to be verified with further experiments, the microcolony model has efficiency benefits over queenright colony assays since mated queens are not needed. Overall, we show that the microcolony model meets the requirements of a useful test system for the purposes of risk assessment.
Our data and other published reports suggest that microcolonies are a useful tool for assessing lethal and sublethal exposure and effects of pesticides on Bombus species (Klinger et al. 2019). As the first published investigation of the effects of diflubenzuron on B. impatiens, this study contributes to the existing knowledgebase for B. impatiens microcolonies. Additional studies are necessary to establish diflubenzuron as a reference toxicant for use in this model and would ideally be conducted in a ring-test and include verification of nominal concentrations. Other future studies should be conducted that evaluate: 1) different routes of exposure; 2) the timing of exposure (e.g. at time of microcolony initiation vs. after first oviposition); and 3) pesticide distribution within the microcolony. While more work is required to advance this model for use in risk assessment, the data presented here strengthen the foundation supporting its use.
Supplementary Material
Acknowledgments –
The authors thank Dr. G. M. Lehmann, Dr. R. Koethe and Dr. Weitekamp for thoughtful and critical review of this manuscript. We are also grateful to Dr. T. Steeger, Dr. D. Schmehl, A. Krueger, Dr. J. Strange, Dr. D. Cox-Foster and Dr. A. Gradish for their generosity and guidance. Thanks to Ms. J. Kalinowski for technical support and Mr. E. Puckett for manufacturing our microcolony chambers. We also thank the EPA Region 1 Regional Science Council for supporting the research effort. This project was supported in part by an appointment to the Research Participation Program at the Office of Research and Development, U.S. Environmental Protection Agency, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and EPA.
Footnotes
Disclaimer: This article has been reviewed by the U.S. Environmental Protection Agency and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency or of the US Federal Government, nor does the mention of trade names or commercial products constitute endorsement or recommendations for use of those products. The authors report no financial or other conflicts of interest. The authors alone are responsible for the content and writing of this article.
References:
- Arena M, and Sgolastra F. 2014. A meta-analysis comparing the sensitivity of bees to pesticides. Ecotoxicology 23: 324–334. [DOI] [PubMed] [Google Scholar]
- Banks JE, Ackleh AS, and Stark JD. 2010. The use of surrogate species in risk assessment: Using life history data to safeguard against false negatives. Risk. Anal. 30: 175–182. [DOI] [PubMed] [Google Scholar]
- Barker RJ, and Taber III S. 1977. Effects of diflubenzuron fed to caged honey bees. Environ. Entomol. 6: 167–168. [Google Scholar]
- Baron GL, Raine NE, and Brown MJF. 2017. General and species-specific impacts of a neonicotinoid insecticide on the ovary development and feeding of wild bumblebee queens. Proc. R. Soc. Ser. B-Bio. 284: 20170123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besard L, Mommaerts V, Vandeven J, Cuvelier X, Sterk G, and Smagghe G. 2010. Compatibility of traditional and novel acaricides with bumblebees (Bombus terrestris): a first laboratory assessment of toxicity and sublethal effects. Pest. Manag. Sci. 66: 786–793. [DOI] [PubMed] [Google Scholar]
- Brittain C, and Potts SG. 2011. The potential impacts of insecticides on the life-history traits of bees and the consequences for pollination. Basic. Appl. Ecol. 12: 321–331. [Google Scholar]
- Cnaani J, Schmid-Hempel R, and Schmidt JO. 2002. Colony development, larval development and worker reproduction in Bombus impatiens Cresson. Insect. Soc. 49: 164–170. [Google Scholar]
- Costa FM, and Tadei WP. 2011. Laboratory toxicity evaluation of Diflubenzuron, a chitin-synthesis inhibitor, against Anopheles darlingi (Diptera, Culicidae). J. Res. Biol. 6: 444–450. [Google Scholar]
- Cresswell JE, Page CJ, Uygun MB, Holmbergh M, Li Y, Wheeler JG, Laycock I, Pook CJ, de Ibarra NH, Smirnoff N, and Tyler CR. 2012. Differential sensitivity of honey bees and bumble bees to a dietary insecticide (imidacloprid). Zoology 115: 365–371. [DOI] [PubMed] [Google Scholar]
- Dhadialla TS, Retnakaran A, and Smagghe G. 2005. Insect growth- and development-disrupting insecticides, vol. 6, Pergamon Press, New York. [Google Scholar]
- Elston C, Thompson HM, and Walters KFA. 2013. Sub-lethal effects of thiamethoxam, a neonicotinoid pesticide, and propiconazole, a DMI fungicide, on colony initiation in bumblebee (Bombus terrestris) microcolonies. Apidologie 44: 563–574. [Google Scholar]
- Emmett BJ, and Archer BM. 1980. The toxicity of diflubenzuron to honey bee (Apis mellifera L.) colonies in applie orchards. Plant Pathol. 29: 177–183. [Google Scholar]
- EPA. 1997. Diflubenzuron. United States Environmental Protection Agency, Prevention, Pesticides, and Toxic Substances. [Google Scholar]
- EPA. 2014. Guidance for assessing pesticide risks to bees, pp. 1–59. United States Environmental Protection Agency. [Google Scholar]
- EPA. 2018. Honeybee Toxicity Testing Frequently Asked Questions. https://www.epa.gov/sites/production/files/2018-08/documents/pollinator-faq.pdf.
- Free JB 1955. The division of labour within bumblebee colonies. Insect. Soc. 2: 195–212. [Google Scholar]
- Gorman S 2017. U.S. lists a bumble bee species as endangered for the first time, Scientific American. [Google Scholar]
- Gosterit A, Koskan O, and Gurel F. 2016. The relationship of weight and ovarian development in Bombus terrestris L. workers under different social conditions. J. Apic. Sci. 60: 51–57. [Google Scholar]
- Goulson D 2010. Bumblebees: behaviour, ecology, and conservation., Oxford University Press, Oxford, UK. [Google Scholar]
- Gradish AE, Scott-Dupree CD, Frewin AJ, and Cutler GC. 2012. Lethal and sublethal effects of some insecticides recommended for wild blueberry on the pollinator Bombus impatiens. Can. Entomol. 144: 478–486. [Google Scholar]
- Hassan AEM, and Charnley AK. 1987. The effect of Dimlin on the ultrastructure of the integument of Manduca sexta. J. Insect. Physiol. 33: 669–676. [Google Scholar]
- Heard MS, Baas J, Dorne JL, Lahive E, Robinson AG, Rortais A, Spurgeon DJ, Svendsen C, and Hesketh H. 2017. Comparative toxicity of pesticides and environmental contaminants in bees: Are honey bees a useful proxy for wild bee species? Sci. Total. Environ. 578: 357–365. [DOI] [PubMed] [Google Scholar]
- Hoying SA, and Riedl H. 1980. Susceptibility of the codling moth to diflubenzuron. J. Econ. Entomol. 73: 556–560. [Google Scholar]
- Kennedy M 2016. Bees added to U.S. endangered species list for 1st time. https://www.npr.org/sections/thetwo-way/2016/10/03/496402620/bee-species-added-to-u-s-endangered-species-list-for-1st-time.
- Klinger EG, Camp AA, Strange JP, Cox-Foster D, and Lehmann DM. 2019. Bombus (Hymenoptera: Apidae) Microcolonies as a Tool for Biological Understanding and Pesticide Risk Assessment. Environ. Entomol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec K, and Burd LA. 2017. Pollinators in peril: A systematic status review of North American and Hawaiian native bees, pp. 1–14. Center for Biological Diversity. [Google Scholar]
- Laycock I, Lenthall KM, Barratt AT, and Cresswell JE. 2012. Effects of imidacloprid, a neonicotinoid pesticide, on reproduction in worker bumble bees (Bombus terrestris). Ecotoxicology 21: 1937–1945. [DOI] [PubMed] [Google Scholar]
- Laycock I, Cotterell KC, O’Shea-Wheller TA, and Cresswell JE. 2014. Effects of the neonicotinoid pesticide thiamethoxam at field-realistic levels on microcolonies of Bombus terrestris worker bumble bees. Ecotoxicol. Environ. Saf. 100: 153–158. [DOI] [PubMed] [Google Scholar]
- Meeus I, Mommaerts V, Billiet A, Mosallanejad H, Van de Wiele T, Wäckers F, and Smagghe G. 2013. Assessment of mutualism between Bombus terrestris and its microbiota by use of microcolonies. Apidologie 44: 708–719. [Google Scholar]
- Merzendorfer H 2006. Insect chitin synthases: a review. J Comp Physiol B 176: 1–15. [DOI] [PubMed] [Google Scholar]
- Mommaerts V, Sterk G, and Smagghe G. 2006. Hazards and uptake of chitin synthesis inhibitors in bumblebees Bombus terrestris. Pest. Manag. Sci. 62: 752–758. [DOI] [PubMed] [Google Scholar]
- Mommaerts V, Sterk G, and Smagghe G. 2010. Bumblebees and neonicotinoids: a bioassay to evaluate sublethal effects on foraging behavior. Proceedings of the Netherlands Entomological Society Meeting 21: 19–127. [Google Scholar]
- Mommaerts V, Reynders S, Boulet J, Besard L, Sterk G, and Smagghe G. 2009. Risk assessment for side-effects of neonicotinoids against bumblebees with and without impairing foraging behavior. Ecotoxicology 19: 207. [DOI] [PubMed] [Google Scholar]
- Mommaerts V, Hagenaars A, Meyer J, De Coen W, Swevers L, Mosallanejad H, and Smagghe G. 2011. Impact of a perfluorinated organic compound PFOS on the terrestrial pollinator Bombus terrestris (Insecta, Hymenoptera). Ecotoxicology 20: 447–456. [DOI] [PubMed] [Google Scholar]
- OECD. 2014. Guidance Document on the Honey Bee (Apis Mellifera L.) Brood Test Under Semi-Field Conditions. [Google Scholar]
- OECD. Test No. 247: Bumblebee, Acute Oral Toxicity Test 2017a [Google Scholar]
- OECD. Test No. 246: Bumblebee, Acute Contact Toxicity Test 2017b [Google Scholar]
- PHTF. 2015. National strategy to promote the health of honey bees and other pollinators, pp. 1–64, Pollinator Health Task Force. [Google Scholar]
- Rortais A, Arnold G, Dorne JL, More SJ, Sperandio G, Streissl F, Szentes C, and Verdonck F. 2017. Risk assessment of pesticides and other stressors in bees: Principles, data gaps and perspectives from the European Food Safety Authority. Sci. Total. Environ. 587–588: 524–537. [DOI] [PubMed] [Google Scholar]
- Sanchez-Bayo F, and Goka K. 2014. Pesticide residues and bees - A risk assessment. Plos One 9: e94482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholer J, and Krischik V. 2014. Chronic exposure of imidacloprid and clothianidin reduce queen survival, foraging, and nectar storing in colonies of Bombus impatiens. PLoS One 9: e91573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smagghe G, Deknopper J, Meeus I, and Mommaerts V. 2013. Dietary chlorantraniliprole suppresses reproduction in worker bumblebees. Pest. Manag. Sci. 69: 787–791. [DOI] [PubMed] [Google Scholar]
- Smagghe G, S Reynders S, Maurissen I, Boulet J, Cuvelier X, Dewulf E, Put K, Jans C, Sterk G, and Mommaerts V. 2007. Analysis of side effects of Diflubenzuron and Tebufenozide in pollinating bumblebees Bombus terrestris. Rev. Sci. Technol. 16: 39–48. [Google Scholar]
- Stoner KA 2016. Current pesticide risk assessment protocols do not adequately address differences between honey bees (Apis mellifera) and bumble bees (Bombus spp.). Front. Environ. Sci 4. [Google Scholar]
- Thompson H 2016. Extrapolation of acute toxicity across bee species. Integr. Environ. Asses. 12: 622–626. [DOI] [PubMed] [Google Scholar]
- van Eck WH 1979. Mode of action of two benzyulphenyl ureas as inhibitors of chitin sythesis in insects. Insect Biochem. 9: 295–300. [Google Scholar]
- Wach M, Hellmich RL, Layton R, Romeis J, and Gadaleta PG. 2016. Dynamic role and importance of surrogate species for assessing potential adverse environmental impacts of genetically engineered insect-resistant plants on non-target organisms. Transgenic Res. 25: 499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Li JL, Peng WJ, and Hu FL. 2010. Sensitivities of three bumblebee species to four pesticides applied commonly in greenhouses in China. Insect Sci. 17: 67–72. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.