Abstract
Nucleos(t)ide analogs (NA) suppress hepatitis B virus (HBV) replication and reduce the risk of hepatocellular carcinoma (HCC). However, NA cannot suppress carcinogenesis completely in patients with chronic hepatitis B. The aims of this study were to identify risk factors for HCC and develop a refined carcinogenesis prediction model. Patients receiving NA therapy (n = 1,183) were recruited retrospectively from the 16 hospitals. All patients had been receiving NA continuously for more than 1 year until the end of the follow-up. During a median follow-up of 4.9 (1.0–12.9) years, 52 (4.4%) patients developed HCC. A multivariate analysis revealed that male gender, older age, lower platelet counts at the baseline, and detectable HBV DNA during NA therapy were independent predictive factors of HCC development. The PAGE-B score was calculated by using these factors. 240 (20.3%), 661 (55.9%), and 282 (23.8%) patients were classified into low-, intermediate-, and high-risk groups, respectively. In the intermediate- and high-risk group, detectable HBV DNA was significantly associated with a higher risk of HCC development compared with continuously undetectable HBV DNA, respectively (HR 3.338; 95% CI 1.045–10.66/HR 3.191; 95% CI 1.543–6.597). PAGE-B–DNA, which is the combined PAGE-B and HBV DNA status, was valuable for a more refined stratification of PAGE-B.
Subject terms: Hepatitis, Hepatology, Liver cancer, Risk factors
Introduction
Hepatitis B virus (HBV) infection remains a major health threat. 257 million people worldwide are infected with HBV, which involves a risk of cirrhosis and hepatocellular carcinoma (HCC). This infection is responsible for more than 887,000 deaths annually1. Nucleos(t)ide analogs (NA) suppress HBV replication and reduce the risk of HCC and HBV-associated mortality2–7. Reports of NA therapy pertained mainly to entecavir (ETV), whereas the number of studies of tenofovir disoproxil fumarate (TDF) has increased in recent years8–11. Recently, tenofovir alafenamide (TAF), which was designed to exhibit greater plasma stability compared with TDF, was approved for clinical application. TAF is as effective as TDF and leads to continuous improvement in renal and bone safety in the treatment of patients with chronic hepatitis B (CHB)12–15. Reports of the reduction of the risk of HCC development induced by TAF are scarce, because of their short observation periods. NA cannot eliminate HBV from the host cells because of the persistence of HBV covalently closed circular DNA, which serves as the template for viral transcription16,17. Therefore, long-term treatment is necessary for HBV suppression.
As described above, there is some evidence of the reduction of the risk of HCC after treatment with NA; however, the authors did not mention the complete suppression of HCC. Several studies have addressed the risk of CHB patients received NA. Most reports pertain to age, gender, and platelet counts18–20. The PAGE-B score was generated based on these factors21,22. The PAGE-B score exhibits a high versatility and has been applied extensively worldwide. However, the number of patients in the intermediate PAGE-B group tends to be elevated. Recently, several studies reported that complete viral suppression reduced the risk of developing HCC23–25. Conversely, even low detectable levels of HBV DNA in the serum confer a risk of HCC development. The interaction between the host and the virus is very important for HBV pathogenesis. Therefore, we attempted to construct a prediction HCC model in CHB patients receiving NA therapy using a well-designed representative risk score of the host (PAGE-B) or of the virus (HBV DNA status).
The aims of this study were to develop a refined carcinogenesis prediction model based on conventional prediction models (PAGE-B and serum HBV DNA) in patients receiving NA therapy. Previously, we conducted a nationwide HBV cohort study26, which was developed further here. The risk factors for HCC among CHB patients were investigated via a multicenter (Japanese Red Cross Liver Study Group) analysis. Moreover, we assessed that the HBV DNA status on NA therapy discriminated the PAGE-B intermediate-risk group and the high-risk group.
Results
Baseline patient characteristics
All CHB patients received NA therapy continuously. Between October 2006 and August 2018, 1652 patients started to receive NA therapy (ETV/TDF/TAF = 1,010/270/372). Patients who were observed for less than 1 year, had a past HCC history, or exhibited HCC development within the first year of treatment were not included in this cohort. In total, 1,183 patients on NA therapy were included in the analyses. There were 700 naïve-treatment patients and 483 patients had prior history of treatment. The total cumulative number of patients previously treated by NA other than ETV/TDF/TAF were 381, and by interferon-based therapy 169. Among them, 703 (59.4%) patients were male; the mean age was 52.9 ± 12.9 years; 177 (14.9%) patients were diagnosed with cirrhosis; and 401 (33.8%) patients were hepatitis B e antigen (HBeAg) positive. The baseline serum data (platelet, albumin, bilirubin, alanine aminotransferase, alpha fetoprotein, creatinine, and serum HBV DNA levels) are described in Table 1. The median follow-up duration was 4.9 (1.0–12.9) years. Finally, 780 (65.9%), 206 (17.4%), and 197 (16.7%) patients received ETV, TDF, and TAF therapy, respectively.
Table 1.
Clinical characteristics of NA-treated patients at baseline.
| n = 1,183 | |
|---|---|
| Male gender (n, %) | 703 (59.4%) |
| Age (years) | 52.9 ± 12.9 |
| Cirrhosis (n, %) | 177 (14.9%) |
| Diabetes mellitus (n, %) | 121 (10.2%) |
| Platelet (× 109/l) | 182 ± 92 |
| Albumin (g/dl) | 4.2 ± 0.5 |
| Total bilirubin (mg/dl) | 0.9 ± 0.9 |
| Alanine aminotransferase (IU/l) | 114.4 ± 247.6 |
| Alpha fetoprotein (ng/ml) | 14.5 ± 59.4 |
| Creatinine (mg/dl) | 0.8 ± 0.6 |
| HBeAg positivity (n, %) | 401 (33.8%) |
| HBV DNA level (IU/ml) (n, %) | |
| < 2000 | 403 (34%) |
| 2000–200,000 | 195 (16.5%) |
| > 200,000 | 585 (49.5%) |
| Follow-up duration (years) | 4.9 (1.0–12.9) |
| Antiviral therapy (n, %) | |
| Entecavir | 780 (65.9%) |
| Tenofovir disoproxil fumarate | 206 (17.4%) |
| Tenofovir alafenamide | 197 (16.7%) |
NA nucleos(t)ide analogues, HBV DNA hepatitis B virus deoxyribonucleic acid, HBeAg HBV e antigen.
Cumulative incidence of HCC
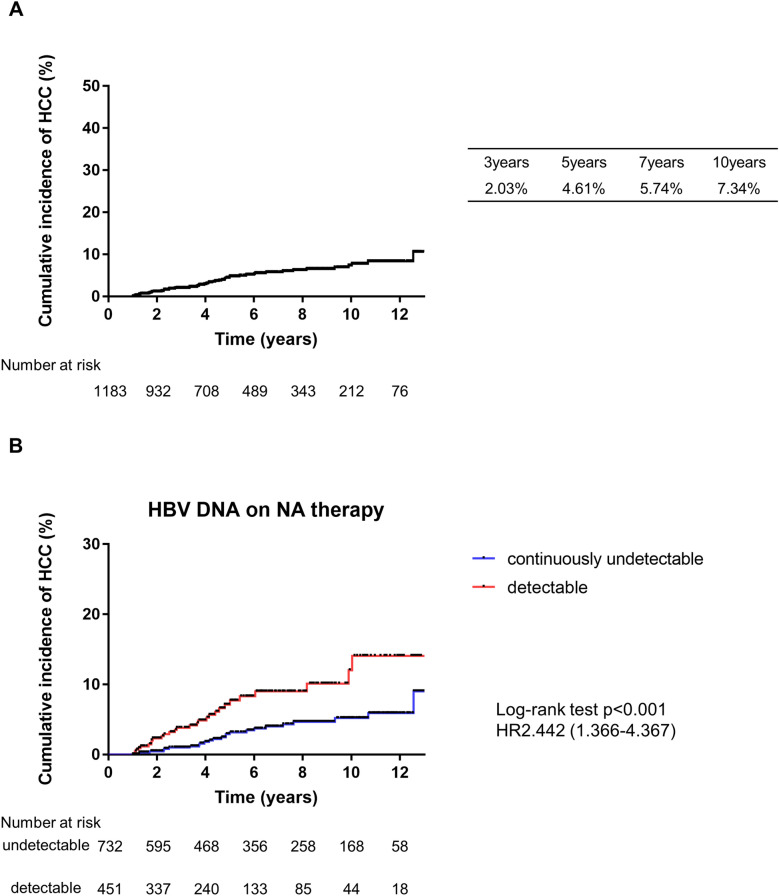
During the follow-up, 52 (4.39%) patients developed HCC as shown clinical characteristics at diagnosis in Table 2. The cumulative incidence rates of HCC at 3, 5, 7, and 10 years after ETV/TDF/TAF treatment were 2.03%, 4.61%, 5.74%, and 7.34%, respectively (Fig. 1A). There were 27 patients who died by almost non-liver related causes (other cancer 15, intracranial hemorrhage 1, pneumonia 2, heart failure 1, renal failure 1, liver related death 3, unknown 4).
Table 2.
Clinical characteristics of HCC patients at diagnosis.
| n = 52 | |
|---|---|
| Male gender (n, %) | 43 (82.7%) |
| Age (years) | 64.6 ± 9.5 |
| Cirrhosis (n, %) | 27 (51.9%) |
| Alanine aminotransferase (IU/l) | 28.5 ± 21.7 |
| Albumin (g/dl) | 4.2 ± 0.4 |
| Total bilirubin (mg/dl) | 1.0 ± 0.6 |
| BCLC stage 0/A/B/C/D (n) | 26/21/3/2/0 |
|
Treatment modalities (n) RFA/resection/TACE/sorafenib |
37/9/5/1 |
|
HBV DNA status (n) Continuously undetectable/detectable |
23/29 |
HCC hepatocellular carcinoma, BCLC Barcelona Clinic Liver Cancer, RFA radiofrequency ablation, TACE transarterial chemoembolization, HBV DNA hepatitis B virus deoxyribonucleic acid.
Figure 1.
Cumulative incidence of hepatocellular carcinoma. (A) All CHB patients received NA therapy. (B) HBV DNA status; 732 (61.8%) and 451 (38.2%) patients achieved continuously undetectable HBV DNA and detectable HBV DNA status, respectively. Patients who did or did not achieve continuously undetectable HBV DNA status (log-rank test, P < 0.001).
Factors associated with HCC development during NA therapy
In addition to the baseline characteristics that were used for the analysis of factors associated with HCC, we also focused on the HBV DNA status during NA therapy. We investigated the effects of these HBV DNA status in CHB patients on liver carcinogenesis. We found that 732 (61.8%) and 451 (38.2%) patients achieved continuously undetectable and detectable HBV DNA status, respectively. The detectable HBV DNA was significantly associated with a higher risk of HCC development compared with the continuously undetectable HBV DNA (HR 2.442; 95% CI 1.366–4.367; log-rank test, P < 0.001; Fig. 1B). For detail, the median serum HBV DNA in patients with detectable DNA were 2.5 (1.88, 3.25) Log IU/ml. The virological breakthrough was seen in 2 patients whose HBV resistant mutants were found. They all were rescued by adding TDF to ETV. There were 36 patients who never achieved undetectable HBV DNA and 141 patients who had virological relapse after complete suppression, respectively. However, these factors were not significantly different in the risk of HCC development.
27 patients achieved hepatitis B surface antigen (HBsAg) seroclearance and no patient developed HCC.
Next, we investigated the predictive factors of HCC using univariate and multivariate analysis. In the univariate analysis, male gender, older age, cirrhosis, lower platelet counts (< 150 × 109/l), lower albumin levels, higher bilirubin levels, the ALBI27 score (which is consisted albumin and bilirubin and useful for the evaluation of liver function), alcohol drinking, and detectable HBV DNA were significant predictive factors of HCC. In the multivariate analysis, male gender (HR 3.731; 95% CI 1.669–8.340; P = 0.001), older age (HR 1.06; 95% CI 1.033–1.088; P < 0.001), cirrhosis (HR 2.59; 95% CI 1.288–5.205; P = 0.007), lower platelet counts (HR 2.763; 95% CI 1.231–6.203; P = 0.013), and detectable HBV DNA (HR 3.234; 95% CI 1.732–6.035; P < 0.001) were independent predictive factors of HCC development during NA therapy (Table 3).
Table 3.
Cox proportional hazards regression analysis for factors associated with HCC.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Male gender | 3.362 | 1.639–6.898 | < 0.001 | 3.731 | 1.669–8.340 | 0.001 |
| Age | 1.068 | 1.038–1.085 | < 0.001 | 1.06 | 1.033–1.088 | < 0.001 |
| Cirrhosis | 5.334 | 3.092–9.201 | < 0.001 | 2.59 | 1.288–5.205 | 0.007 |
| Diabetes mellitus | 1.164 | 0.494–2.744 | 0.729 | |||
| Platelet (< 150 × 109/l) | 4.468 | 2.419–8.258 | < 0.001 | 2.763 | 1.231–6.203 | 0.013 |
| Albumin | 0.498 | 0.315–0.788 | 0.003 | 0.191 | 0.115–3.148 | 0.247 |
| Total bilirubin | 1.125 | 1.008–1.255 | 0.036 | 0.868 | 0.646–1.167 | 0.348 |
| ALBI score | 2.416 | 1.517–3.850 | 0.002 | 8.748 | 0.367–208.5 | 0.18 |
| Alanine aminotransferase | 0.999 | 0.998–1.001 | 0.455 | |||
| Creatinine | 1.208 | 0.787–1.855 | 0.386 | |||
| Positive HBeAg | 0.779 | 0.436–1.392 | 0.399 | |||
| Antiviral therapy ETV (vs. TDF/TAF) | 0.578 | 0.201–1.660 | 0.308 | |||
| Alcohol drinking | 3.058 | 1.550–6.031 | 0.001 | 2.141 | 0.989–4.464 | 0.061 |
| Detectable HBV DNA during NA therapy | 2.475 | 1.429–4.286 | 0.001 | 3.234 | 1.732–6.035 | < 0.001 |
HR hazards ratios, HCC hepatocellular carcinoma, ALBI Albumin-Bilirubin, HBeAg hepatitis B virus e antigen, ETV Entecavir, TDF Tenofovir disoproxil fumarate, TAF Tenofovir alafenamide, HBV DNA hepatitis B virus deoxyribonucleic acid, NA nucleos(t)ide analogues.
Furthermore, we performed a subgroup analysis with the HBV DNA status of cirrhosis and HBeAg. HBV DNA status during nucleos(t)ide analogues could significantly stratify the risk of HCC development in these subgroups (Supplementary Figure 1).
Particularly in treatment naive patients (n = 700), the cumulative incidence rate of HCC in patients who had higher pretreatment HBV DNA levels (> 4.0 log IU/ml) was significantly higher (HR 5.446; 95% CI 2.111–14.05; log-rank test, P = 0.0413) (Supplementary Figure 2A). As a predict factor for achievement continuously undetectable HBV DNA, serum HBV DNA 1 year after starting NA significantly related achievement continuously undetectable HBV DNA (P < 0.0001, Logistic regression). HBV DNA status 1 year after starting NA was also the significant risk factor of HCC development (HR 2.279; 95% CI 1.214–4.278; log-rank test, P = 0.0303) (Supplementary Figure 2B).
HBV DNA status stratifies the PAGE-B HCC predictive score
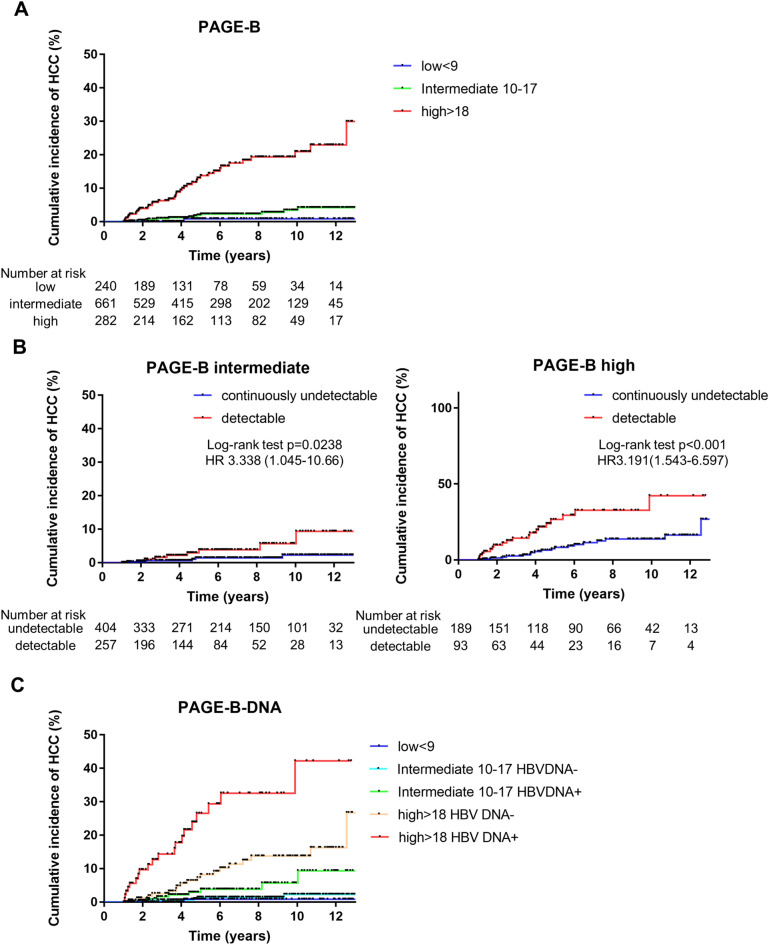
As described above, male gender, older age, and lower platelet counts were independent predictive factors of HCC development during NA therapy. The PAGE-B score was developed using these factors in CHB patients on antiviral therapy and exhibited a high concordance index value for the prediction of HCC development21,22. We validated the PAGE-B score in our cohort (Fig. 2A) and found that it was sufficiently useful.
Figure 2.
Cumulative incidence of hepatocellular carcinoma with PAGE-B score subdivided according to HBV DNA status on NA therapy. (A) Cumulative incidence rates of HCC based on PAGE-B. The PAGE-B score classified 240 (20.3%), 661 (55.9%), and 282 (23.8%) patients into the low-, intermediate-, and high-risk groups, respectively. (B) Patients who did or did not achieve a continuously undetectable HBV DNA status in intermediate- and high-risk PAGE-B groups. HBV DNA status classified the intermediate- and high-risk patients (based on PAGE-B) into subgroups (log-rank test, P = 0.0238, P < 0.001). (C) The PAGE-B score was stratified using the HBV DNA status.
We classified 240 (20.3%), 661 (55.9%), and 282 (23.8%) patients into low-, intermediate-, and high-risk groups, respectively. However, the majority of patients were in the intermediate-risk group. Therefore, we investigated whether HBV DNA status on NA therapy could subdivide the PAGE-B intermediate-risk group.
We observed that 404 (61.1%) and 257 (38.9%) patients achieved a continuously undetectable and a detectable HBV DNA, respectively. In the PAGE-B intermediate-risk group, the detectable HBV DNA was significantly associated with a higher risk of HCC development compared with the continuously undetectable HBV DNA (HR 3.338; 95% CI 1.045–10.66; log-rank test, P = 0.0238; Fig. 2B, left panel). Furthermore, in the PAGE-B high-risk group, the detectable HBV DNA was also significantly associated with a higher risk of HCC development (HR 3.191; 95% CI 1.543–6.597; log-rank test, P < 0.001; Fig. 2B, right panel). The cumulative incidence rate of HCC in the PAGE-B high-risk group with a continuously negative HBV DNA was significantly higher compared with that observed in the PAGE-B intermediate-risk with a detectable HBV DNA status (HR 2.369; 95% CI 1.098–5.111; log-rank test, P = 0.0352). A single case of HCC development was detected in the PAGE-B low-risk group. Thus, the low-risk group could show function as it was.
Based on these results, PAGE-B was significantly stratified when divided into five groups based on HBV DNA status (Fig. 2C). HBV DNA status on NA therapy was complementary to the PAGE-B score.
This PAGE-B–DNA prediction model is useful for the rigorous assessment of the cumulative incidence rate of HCC at 3, 5, 7, and 10 years in CHB patients who are on antiviral therapy (Table 4). The discriminative ability of our models was compared by Akaike's Information Criterion (AIC). AIC values were so small that better discriminative ability of predicting HCC development was shown in PAGE-B-DNA (Supplementary Table 1).
Table 4.
Cumulative incidence rate of HCC with PAGE-B-DNA prediction model stratifications at 3,5,7 and 10 years.
| PAGE-B-DNA (n = 1,183) | n, % | 3 years | 5 years | 7 years | 10 years |
|---|---|---|---|---|---|
| low (< 9) | 240 (20.3%) | 0% | 0.81% | 0.81% | 0.81% |
| intermediate (10–17) HBV DNA− | 404 (34.2%) | 0.59% | 1.39% | 1.39% | 2.27% |
| intermediate (10–17) HBV DNA+ | 257 (21.7%) | 1.56% | 2.89% | 3.82% | 5.71% |
| high (> 18) HBV DNA− | 189 (16.0%) | 2.53% | 8.21% | 11.30% | 13.73% |
| high (> 18) HBV DNA+ | 93 (7.8%) | 14.32% | 26.52% | 32.56% | 42.19% |
HCC hepatocellular carcinoma; PAGE-B platelets, age, gender-hepatitis B scores; HBV DNA hepatitis B virus deoxyribonucleic acid.
Discussion
This study provides the first evidence that HBV DNA status on NA therapy is useful for subdividing further the PAGE-B score. NA therapy suppress the risk of HCC and liver-related death. However, it did not mean that NA therapy in CHB patients suppressed HCC completely3. A report showed that surveillance leads to early detection of HCC and suppresses cancer-related death in patients with HBV28. Therefore, a simple and appreciate study aimed at evaluating the risk factors of HCC development during NA therapy is needed.
In our cohort, the cumulative incidence rates of HCC were 4.61% at 5 years and 7.34% at 10 years, which was in agreement with previous reports3–7 (Fig. 1A). Several previous studies addressed the HBV DNA status23–25. Here, male gender, old age, cirrhosis, lower platelet counts at the baseline, and HBV DNA during NA therapy were validated as significant factors of liver carcinogenesis in CHB patients (Fig. 1B, Table 3).
Many risk scores have been reported (CU-HCC, GAG-HCC, REACH-B, PAGE-B, mPAGE-B, etc.)23,29–32. Of them, PAGE-B was the most convenient score because of its high level of versatility21. As depicted in Fig. 2A, risk stratification was possible using PAGE-B. However, the number of intermediate-risk cases was particularly large, and the rate of cumulative incidence of the high-risk group was far from that of the intermediate-risk group. Therefore, subclassification was performed in these two groups. HBV DNA status significantly stratified the risk of HCC in both risk groups (Fig. 2B). Interestingly, the cumulative incidence rate of HCC in the PAGE-B high-risk group with a continuously negative HBV DNA status was significantly higher compared with the PAGE-B intermediate-risk group with a detectable HBV DNA status. It was suggested that the PAGE-B score was the main classifier, with HBV DNA status on NA therapy working complementary. These results showed that a more detailed risk assessment was possible (Fig. 2C, Table 4, Supplementary Table 1). This “PAGE-B–DNA” system was constructed using very simple parameters (age, gender, platelet counts, and HBV DNA) that can be measured during the clinical follow-up. This risk assessment exhibited a greater stratification and was highly useful. Moreover, the modified PAGE-B score, which was constructed by adding albumin level to the PAGE-B factors32, was also supported by subdivision using this complementary HBV DNA status on NA therapy (Supplementary Figure 3, Supplementary Tables 1, 2).
This study was valuable because it included the host factor together with a viral factor, i.e., HBV DNA status, during therapy. The intervention to viral factors may be possible, though intervention to host factors is difficult. Several approaches (switching NA, interferon sequential therapy, new therapeutic agents, etc.) are considered to achieve continuously undetectable HBV DNA. The further interventions to virus might yield better results for CHB patients during NA therapy. A few articles reported the risk factors for HCC development during NA therapy in Japan26,33–35. Therefore, such a Japanese nationwide multicenter analysis was very valuable.
However, this study had several limitations. First, this was a retrospective study in which the exclusion of unidentified biases was impossible. Second, regarding HBV DNA status on NA therapy, we were unable to determine why the HBV DNA was detectable. If possible, virus resistance mutations and patients’ adherence to medication should be examined. Lack of data and serum samples precluded the performance of such analyzes here. Several characteristics were available for the CHB patients included in this cohort; i.e., this study was based on real-world data and seem to be useful for actual clinical practice. External validation is needed for implementing prediction models in clinical practice. Therefore, further study including the external validation cohort is expected.
In conclusion, HBV DNA status was useful for stratifying HCC risk in CHB patients on NA therapy. Furthermore, the PAGE-B-DNA system, which combined PAGE-B with HBV DNA status, was valuable because it provided a more detailed stratification compared with PAGE-B alone. The application of PAGE-B-DNA to appreciate surveillance may lead to the early detection and treatment of HCC, thus helping to improve the prognosis of CHB patients.
Method
Patients
Patients receiving NA therapy (n = 1652) were recruited retrospectively from the 16 hospitals that are part of the Japanese Red Cross Liver Study Group. All patients were HBsAg positive for more than 6 months and serum HBV DNA positive before NA therapy. No patients had coinfection with the hepatitis C virus or human immunodeficiency virus.
469 patients were excluded because of a short follow-up duration (less than 1 year) or the development of HCC before NA therapy or within 1 year of NA therapy onset. Finally, 1,183 patients were included in the analyses.
All patients received NA therapy continuously for more than 1 year until the end of the follow-up. The study was approved by the institutional review board of each hospital (e.g. Musashino Red Cross Hospital Ethics Review Committee), in accordance with the Declaration of Helsinki. The written informed consent was obtained from all patients.
Clinical evaluation and follow-up
The age and gender of the patients were recorded at the time of study entry. HBeAg and anti-HBe antibodies (HBeAb) were determined using commercially available enzyme immunoassay kits at each hospital. The quantitative measurement of HBV DNA and HBsAg was performed using real-time PCR (COBAS 6800/8800 system, TaqMan HBV assay; Roche) and a Chemiluminescence Immunoassay (CLIA; Abbott Japan), respectively. Cirrhosis was assessed based on the presence of clinical, radiological, endoscopic, and laboratory evidence (platelet count, < 100 × 109/l, and a blunted, nodular liver edge accompanied by splenomegaly > 12 cm) or clinical symptoms of portal hypertension, such as ascites, esophageal or gastric varices, and hepatic encephalopathy.
Ultrasonography and blood tests, including assays of tumor markers, were performed every 3–6 months for HCC surveillance. When tumor marker levels rose abnormally and/or abdominal ultrasonography suggested a lesion that was suspicious for HCC, contrast-enhanced computed tomography, magnetic resonance imaging, or angiography was performed. HCC was diagnosed in tumors that displayed vascular enhancement at the early phase and washout at the later phase, according to the guidelines of the American Association for the Study of Liver Diseases36, the European Association for the Study of the Liver37, and the Japan Society of Hepatology38. Tumor biopsy was used to support diagnoses. The primary outcome was HCC development in CHB patients on NA therapy.
HBV DNA status
In many cases, NA treatment causes HBV DNA negative. There are two possible clinical courses. Therefore, we defined HBV DNA status on NA therapy as described below.
Continuously undetectable HBV DNA
The serum HBV DNA was quantified by real-time PCR (using the COBAS 6800/8800 system mainly) with a detection range of 1.0–9.0 Log IU/ml. The lower limit was 10 IU/ml. A continuously undetectable status was defined as an undetectable level of serum HBV DNA that was maintained up to the last observation, excluded detectable under 10 IU/ml on NA therapy.
Detectable HBV DNA
A detectable status was defined as a literally detectable serum HBV DNA and included occasionally detected HBV DNA among undetectable cases during the follow-up.
Statistical analyzes
Cumulative incidences of HCC development curves were prepared using the Kaplan–Meier method. The cumulative incidence curves were compared using the log-rank Mantel–Cox test. The factors associated with HCC development were analyzed using a Cox proportional hazards model. Significance was set at P < 0.05. The GraphPad Prism software (GraphPad Software, San Diego, CA, USA) and EZR (Saitama Medical Center, Jichi Medical University, Shimotsuke, Japan) were used to analyze statistical significance.
Supplementary information
Acknowledgements
The authors appreciate the cooperation of the following members of the Japanese Red Cross Liver Study Group: Dr. T. Tamada, Takatsuki Red Cross Hospital; Dr. M. Fujii, Kobe Red Cross Hospital; Dr. R. Narita, Oita Red Cross Hospital; Dr. A. Mitsuda, Tottori Red Cross Hospital; Dr. T. Sato, Nasu Red Cross Hospital; Dr. T. Soda, Fukuoka Red Cross Hospital; Dr. M. Uebayashi, Kitami Red Cross Hospital; Dr. K. Okada, Toyama Red Cross Hospital; Dr. R. Ogawa, Takamatsu Red Cross Hospital; Dr K. Furuta, Masuda Red Cross Hospital, and Ms. S. Kobori, Japanese Red Cross Liver Study Group office. The study was funded by the Ministry of Health, Labor and Welfare in Japan and Japan Agency for Medical Research and Development (JP19fk0210025h0003).
Author contributions
Study concept and design: S. Kaneko, M. Kurosaki, N.I. Acquisition of data: S. Kaneko, K.J., H.M., M. Kondo, Y.K., Y.U., H. Kimura, K.T, H.Yagisawa, A.K., H. Kobashi, T. Akahane, N.T., S. Kirino, T. Abe, H. Yoshida, T.M., and C.H. Analysis and interpretation of data: S. Kaneko and M. Kurosaki. Drafting of the manuscript: S. Kaneko and M. Kurosaki. Statistical analysis: S. Kaneko, M. Kurosaki, N.T., and S. Kirino. Study supervision: N.I. Final approval: All of the authors. Agreement to be accountable for all aspects of the work: All of the authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-69522-w.
References
- 1.WHO. Media Centre, Hepatitis B, 2015. Update. https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-b.
- 2.Liaw YF, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N. Engl. J. Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 3.Papatheodoridis GV, Chan HL, Hansen BE, Janssen HL, Lampertico P. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J. Hepatol. 2015;62:956–967. doi: 10.1016/j.jhep.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Hosaka T, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98–107. doi: 10.1002/hep.26180. [DOI] [PubMed] [Google Scholar]
- 5.Kumada T, et al. Effect of nucleos(t)ide analogue therapy on hepatocarcinogenesis in chronic hepatitis B patients: a propensity score analysis. J. Hepatol. 2013;58:427–433. doi: 10.1016/j.jhep.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 6.Wu CY, et al. Association of nucleos(t)ide analogue therapy with reduced risk of hepatocellular carcinoma in patients with chronic hepatitis B: a nationwide cohort study. Gastroenterology. 2014;147:143–151.e145. doi: 10.1053/j.gastro.2014.03.048. [DOI] [PubMed] [Google Scholar]
- 7.Wong GL, et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology. 2013;58:1537–1547. doi: 10.1002/hep.26301. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen MH, et al. Reduced incidence of hepatocellular carcinoma in cirrhotic and noncirrhotic patients with chronic hepatitis B treated with tenofovir—a propensity score-matched study. J. Infect. Dis. 2019;219:10–18. doi: 10.1093/infdis/jiy391. [DOI] [PubMed] [Google Scholar]
- 9.Choi J, et al. Risk of hepatocellular carcinoma in patients treated with entecavir vs tenofovir for chronic hepatitis B: a Korean Nationwide Cohort Study. JAMA Oncol. 2019;5:30–36. doi: 10.1001/jamaoncol.2018.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SU, et al. A multicenter study of entecavir vs. tenofovir on prognosis of treatment-naive chronic hepatitis B in South Korea. J. Hepatol. 2019 doi: 10.1016/j.jhep.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Cheuk-Fung Yip T, et al. Tenofovir is associated with lower risk of hepatocellular carcinoma than entecavir in patients with chronic HBV infection in China. Gastroenterology. 2019 doi: 10.1053/j.gastro.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal K, et al. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection. J. Hepatol. 2018;68:672–681. doi: 10.1016/j.jhep.2017.11.039. [DOI] [PubMed] [Google Scholar]
- 13.Chan HL, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol. Hepatol. 2016;1:185–195. doi: 10.1016/s2468-1253(16)30024-3. [DOI] [PubMed] [Google Scholar]
- 14.Buti M, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol. Hepatol. 2016;1:196–206. doi: 10.1016/s2468-1253(16)30107-8. [DOI] [PubMed] [Google Scholar]
- 15.Kaneko S, et al. Tenofovir alafenamide for hepatitis B virus infection including switching therapy from tenofovir disoproxil fumarate. J. Gastroenterol. Hepatol. 2019 doi: 10.1111/jgh.14686. [DOI] [PubMed] [Google Scholar]
- 16.Protzer U, Maini MK, Knolle PA. Living in the liver: hepatic infections. Nat. Rev. Immunol. 2012;12:201–213. doi: 10.1038/nri3169. [DOI] [PubMed] [Google Scholar]
- 17.Zoulim F. Hepatitis B virus resistance to antiviral drugs: where are we going? Liver Int. 2011;31(Suppl 1):111–116. doi: 10.1111/j.1478-3231.2010.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papatheodoridis GV, et al. Incidence and predictors of hepatocellular carcinoma in Caucasian chronic hepatitis B patients receiving entecavir or tenofovir. J. Hepatol. 2015;62:363–370. doi: 10.1016/j.jhep.2014.08.045. [DOI] [PubMed] [Google Scholar]
- 19.Chen CH, et al. Prediction model of hepatocellular carcinoma risk in Asian patients with chronic hepatitis B treated with entecavir. Oncotarget. 2017;8:92431–92441. doi: 10.18632/oncotarget.21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arends P, et al. Entecavir treatment does not eliminate the risk of hepatocellular carcinoma in chronic hepatitis B: limited role for risk scores in Caucasians. Gut. 2015;64:1289–1295. doi: 10.1136/gutjnl-2014-307023. [DOI] [PubMed] [Google Scholar]
- 21.Papatheodoridis G, et al. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J. Hepatol. 2016;64:800–806. doi: 10.1016/j.jhep.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 22.Kim MN, et al. Validation of PAGE-B model in Asian chronic hepatitis B patients receiving entecavir or tenofovir. Liver Int. 2017;37:1788–1795. doi: 10.1111/liv.13450. [DOI] [PubMed] [Google Scholar]
- 23.Wong GL, et al. Accuracy of risk scores for patients with chronic hepatitis B receiving entecavir treatment. Gastroenterology. 2013;144:933–944. doi: 10.1053/j.gastro.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Kim JH, et al. Low-level viremia and the increased risk of hepatocellular carcinoma in patients receiving entecavir treatment. Hepatology. 2017;66:335–343. doi: 10.1002/hep.28916. [DOI] [PubMed] [Google Scholar]
- 25.Yip TC, et al. HBsAg seroclearance further reduces hepatocellular carcinoma risk after complete viral suppression with nucleos(t)ide analogues. J. Hepatol. 2019;70:361–370. doi: 10.1016/j.jhep.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Orito E, et al. Risk of hepatocellular carcinoma in cirrhotic hepatitis B virus patients during nucleoside/nucleotide analog therapy. Hepatol. Res. 2015;45:872–879. doi: 10.1111/hepr.12427. [DOI] [PubMed] [Google Scholar]
- 27.Johnson PJ, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015;33:550–558. doi: 10.1200/jco.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2004;130:417–422. doi: 10.1007/s00432-004-0552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuen MF, et al. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J. Hepatol. 2009;50:80–88. doi: 10.1016/j.jhep.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 30.Wong VW, et al. Clinical scoring system to predict hepatocellular carcinoma in chronic hepatitis B carriers. J. Clin. Oncol. 2010;28:1660–1665. doi: 10.1200/jco.2009.26.2675. [DOI] [PubMed] [Google Scholar]
- 31.Yang HI, et al. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. Lancet Oncol. 2011;12:568–574. doi: 10.1016/s1470-2045(11)70077-8. [DOI] [PubMed] [Google Scholar]
- 32.Kim JH, et al. Modified PAGE-B score predicts the risk of hepatocellular carcinoma in Asians with chronic hepatitis B on antiviral therapy. J. Hepatol. 2018;69:1066–1073. doi: 10.1016/j.jhep.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 33.Ando Y, et al. Cumulative incidence and risk factors for the development of hepatocellular carcinoma in patients with chronic hepatitis B who achieved sustained disappearance of viremia by nucleos(t)ide analog treatment. Hepatol. Res. 2018;48:E240–e251. doi: 10.1111/hepr.12976. [DOI] [PubMed] [Google Scholar]
- 34.Hosaka T, et al. Impact of hepatitis B core-related antigen on the incidence of hepatocellular carcinoma in patients treated with nucleos(t)ide analogues. Aliment. Pharmacol. Ther. 2019;49:457–471. doi: 10.1111/apt.15108. [DOI] [PubMed] [Google Scholar]
- 35.Shinkai N, et al. High levels of serum Mac-2-binding protein glycosylation isomer (M2BPGi) predict the development of hepatocellular carcinoma in hepatitis B patients treated with nucleot(s)ide analogues. J. Gastroenterol. 2018;53:883–889. doi: 10.1007/s00535-017-1424-0. [DOI] [PubMed] [Google Scholar]
- 36.Heimbach JK, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 37.EASL clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol.69, 182–236. 10.1016/j.jhep.2018.03.019 (2018). [DOI] [PubMed]
- 38.Kokudo N, et al. Clinical practice guidelines for hepatocellular carcinoma: the Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol. Res. 2019;49:1109–1113. doi: 10.1111/hepr.13411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.