Key Summary Points
| Recently it has been hypothesized that DPP4 inhibitors can have a beneficial effect on SARS-CoV-2 infection through immunoregulating activity. |
| Experimental study on streptozotocin-treated rats showed that liraglutide was able to stimulate the expression of pulmonary angiotensin converting enzyme 2 (ACE2) and angiotensin (1–7). |
| Liraglutide modulated different elements of the renin angiotensin system (RAS), significantly increasing ACE2 and Mas receptor (MasR) mRNA expression in pup lungs from food-restricted mothers. |
| Some action mechanisms support the hypothesis of a protective action of GLP-1R agonists, capable of mitigating a more serious clinical course among SARS-CoV-2-infected individuals with T2DM. |
Type 2 diabetes mellitus (T2DM) represents an important risk factor for a more severe evolution associated with higher lethality of the infection from the new coronavirus disease 2019 (COVID-19; caused by severe acute respiratory syndrome coronavirus 2, SARS-CoV-2), responsible for the current pandemic that originated from the epidemic which initially affected the Wuhan region in China in December 2019 [1]. SARS-CoV-2 uses as a receptor for the infection of respiratory epithelial cells—the angiotensin-converting enzyme 2 (ACE2) receptor [1]; this was also the case for SARS coronavirus (SARS-CoV), responsible for the epidemic that affected more than 8000 people mainly in Asia during the 2002–2003 period [2]. In contrast, Middle East respiratory syndrome (MERS) coronavirus, which caused (as of November 2019) 2494 confirmed cases of infection reported to the World Health Organization and 858 deaths [3], uses as a cellular receptor—the enzyme dipeptidyl peptidase 4 (DPP4) [4]. This enzyme is able to degrade glucagon-like peptide 1 (GLP-1), an enterohormone produced by the L cells of the ileum in response to the intestinal transit of glucose (so-called incretin effect) [5].
People with T2DM are frequently treated with orally delivered DPP4 inhibitors drugs (sitagliptin, vildagliptin, and saxagliptin with mimetic inhibition mechanisms, and alogliptin and linagliptin non-mimetic inhibitors) or GLP-1 receptor agonists (GLP-1 RAs) with daily (exenatide BID, lixisenatide, liraglutide) or weekly (semaglutide, exenatide LAR, dulaglutide) subcutaneous administration, or once-daily oral administration (semaglutide) [6, 7]. Recently it has been hypothesized that these two antidiabetic drug classes can have a beneficial effect on SARS-CoV-2 infection. DPP4 inhibitors seem to act through an immunoregulating activity by regulating M1/M2 macrophage polarization [8], whereas GLP-1 RAs have been considered excellent candidates for the treatment of patients with COVID-19 with or without T2DM owing to their multiple beneficial effects on excessive inflammation-induced acute lung injury [9].
Indeed, multiple preclinical studies performed in mice and rats with experimental induced lung injury demonstrated that GLP-1 RAs attenuate pulmonary inflammation, through inhibitory activity on cytokine release [10, 11], as a result of their interference with nuclear factor-kB signaling pathways [12].
In particular, an experimental study conducted on streptozotocin-treated rats showed that liraglutide was able to stimulate the expression of pulmonary ACE2 and angiotensin (1–7) [A(1–7)] and to increase the production of the lung surfactant proteins A and B (SP-A and SP-B) [13].
More recently a study conducted in an animal rat model showed that liraglutide significantly restored the SP-A mRNA expression in pup lungs from food-restricted mothers [14]. Moreover, liraglutide modulated different elements of the renin angiotensin system (RAS), significantly increasing ACE2 and Mas receptor (MasR) mRNA expression in pup lungs from food-restricted mothers [14].
Several studies have demonstrated the protective role of ACE2 in acute respiratory distress syndrome (ARDS) in many lung diseases partially as a consequence of restored A(1–7) production [15], and it has been suggested that ACE2 can favorably modulate the SARS-CoV infection [16].
In relation to SARS-CoV-2 infection, it has been hypothesized that the RAS dysregulation can be an important causative event leading to ARDS and multi-organ dysfunction [17].
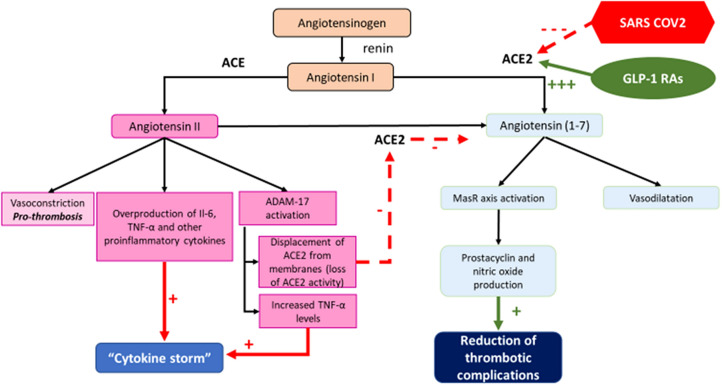
Both ACE and ACE2 are zinc metallic enzymes. ACE cleaves C-terminal dipeptide residues from susceptible substrates, originating in angiotensin II (AII) from angiotensin I with vasoconstrictor action mediated by AII receptor 1 (AT1R) activation [17]. ACE2 acts as a simple carboxypeptidase that can hydrolyze AII to A(1–7) which exerts numerous salutary and opposite effects to those of AII through an efficient binding with the G protein-coupled receptor MasR [18]. Therefore, the ACE2 → A(1–7) → MasR axis is counter-regulatory to the ACE → AII → AT1R axis [17]. Moreover, the ACE2 → A(1–7) → MasR axis activity has an important antithrombotic effect through prostacyclin and nitric oxide production [19] which opposes the pro-thrombotic effects of AII [20].
AII is able indeed to determine the overproduction of interleukin-6 (Il-6), tumor necrosis factor alpha (TNFα) and other pro-inflammatory cytokines [21]. Moreover it is noteworthy to consider that AII is also able to activate the disintegrin and metalloproteinase domain-containing protein 17 (ADAM17) Zn-dependent enzyme, which cleaves the membrane-anchored ACE2, thereby releasing a circulating form of ACE2 with loss of the catalytic activity of the remaining part of the membrane-anchored enzyme [22]. The endocytosed spike SARS-CoV-2 viral proteins stimulate ADAM17 activity, too [22]. Moreover, ADAM17 (also known as TNFα-converting enzyme) is able to mediate the extracellular domain shedding and activation of TNFα, which exhibits auto- and paracrine functionality [22]. TNFα activation of its tumor necrosis factor receptor represents a third pathway elevating ADAM17 activity, thus increasing ACE2 shedding and impaired production of A(1–7), with further RAS-mediated detrimental effects in a positive feedback cycle [22]. The enhanced AII and TNFα activation, along with systemic cytokines released as a result of SARS-CoV-2 infection, can indeed exacerbate the “cytokine storm” ultimately leading to ARDS [22] (Fig. 1).
Fig. 1.
Possible role of dysregulation of RAS during SARS-CoV-2 infection at lung level and potential beneficial effects of GLP-1 RAS therapy (see text for details)
Therefore the capacity of GLP-1 RAs to enhance the activity of the ACE2 → A(1–7) → MasR axis by directly stimulating ACE2 expression would contribute to reduce the progression of inflammatory and thrombotic processes frequently associated with the poor prognosis of SARS-CoV-2 infection [23], through the fostering of an antithrombotic and anti-inflammatory milieu.
It should also be considered that ACE2 is mainly expressed by type II pneumocytes, which represent the Sp-A- and SP-B-producing cells and the progenitor cells of the type I pneumocytes [17]. The damage of type II pneumocytes due to SARS-CoV-2 infection causes loss of lung surfactant, alveolar collapse, and impaired tissue repair capacity.
The aforementioned ability of GLP-1 RAs to induce the synthesis of the pulmonary surfactant proteins [13, 14], which exhibit anti-inflammatory and immune-modulating protective properties against bacterial and viral infections [24], can directly preserve the type II pneumocytes with consequently ARDS-preventing effect.
In addition the expression of ACE2 by intestinal enterocytes, where ACE2 has a RAS-independent function, regulating the intestinal amino acid homeostasis and the gut microbiome [25], can represent a second site of SARS-CoV-2 infection, with consequently intestinal barrier leakage and bloodstream invasion from endotoxin and other intestinal bacterial metabolites, exacerbating the multi-organ dysfunction and septic shock [22]. Therefore, the ability of GLP-1 RAs to restore the ACE2 → A(1–7) → MasR axis can exert a protective intestinal effect, with a further favorable effect on the clinical course of SARS-Cov-2 infection.
In conclusion, it is nowadays unclear whether GLP-1 RAs can play a role in modulating SARS-CoV-2 infection, but it is conceivable that administration of these drugs can exert a pulmonary protective effect, as it has been hypothesized regarding other therapeutic approaches to increase the ACE2 → A(1–7) → MasR axis activity [22, 26]. Particularly regarding as the possible role of AT1R blockers (ARBs), it has been hypothesized that chronic treatment with ARBs, already in place in patients infected with SARS-CoV-2, would stimulate greater ACE2 activity (ACE2 is the recognized viral receptor), and that this paradoxically would play a protective role against acute lung injury due to viral infection rather than promoting it [27].
Moreover the current position statements of the Council of Hypertension of the European Society of Cardiology (www.escardio.org) clarify that ACE inhibitors and ARBs should be continued during SARS-Cov-2 infection [11]. Two trials of losartan as additional treatment for SARS-CoV-2 infection in hospitalized (NCT04312009) or non-hospitalized (NCT04311177) patients have been announced and it is hoped that the results of these trials will answer this question [17].
Nevertheless, regarding the protective role of these antihypertensive drugs, it is noteworthy to consider that they lack any action on the synthesis of pulmonary surfactant proteins and lack direct inhibitory activity on the synthesis of pro-inflammatory cytokines, exerting anti-inflammatory properties through the inhibition of unbalanced AT1R activation [28]. It is therefore conceivable that the GLP-1 RAs can exert an adjunctive anti-inflammatory and anti-thrombotic activity beyond the enhancing effect of ACE2 → A(1–7) → MasR axis activity.
To better explore this issue the percentage of people with T2DM treated with GLP-1 RAs in the general population should be compared with that of inpatients with T2DM presenting serious symptoms of SARS-CoV-2 infection, as already suggested [9, 11]. If the latter percentage turned out to be significantly smaller, this would support the hypothesis in favor of a protective action of GLP-1 RAs, capable of mitigating a more serious clinical course among SARS-CoV-2-infected individuals with T2DM. As shown in Fig. 1, SARS-CoV-2 depresses ACE2 activity, thus imbalancing the RAS towards predominantly ACE activity and ADAM17 activation. This leads to a pro-thrombotic milieu and overproduction of pro-inflammatory cytokines that, along with systemic cytokines released as a result of SARS-CoV-2 infection, can exacerbate the “cytokine storm”. The cited preclinical studies have shown that GLP-1 RAs can significantly increase ACE2 and MasR mRNA expression at pulmonary level [13, 14]. Thus, increased ACE2 levels could shift the RAS toward A(1–7) production exerting two different protective effect: increased prostacyclin and nitric oxide levels, which could reduce the thrombotic complications of SARS-Cov-2 infection, and reduced AT1R activation counteracting its detrimental effects.
Finally, it is conceivable that the rebalancing of ACE2 → A(1–7) → MasR axis activity through ACE2 expression enhancement can at least partially account for the well-documented anti-atherosclerotic and cardiovascular protective effects of GLP-1 RAs [29]. These ACE2-mediated favorable effects are entirely insulin-independent and may be of particular relevance in those pathological conditions of reduced ACE2 production and RAS dysregulation like T2DM. In addition, it is conceivable that the documented renal protective effects of GLP-1 RAs [30] may also be at least partially mediated by the stimulation of ACE2 and A(1–7) expression. Further clinical and experimental studies are therefore warranted in the near future to demonstrate the importance of ACE2 in mediating the favorable multi-organ extraglycemic effects of GLP-1 RAs.
This study was conducted in conformance with good clinical practice standards. No human or animal data are present in the paper for which compliance with the ethical guidelines of the Helsinki Declaration and subsequent versions are not applicable.
Acknowledgements
Special thanks are due to doctor Paola Murano of Nefrocenter Research and Nyx Start-up, Naples, Italy, for the continuous logistic support.
Funding
No Rapid Service Fee was received by the journal for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published: Vincenzo Maria Monda, Felice Strollo, Francesca Porcellati and Sandro Gentile. The authors declare that the text is original and has not been submitted to another journal at the same time.
Authorship Contributions
VMM created the paper and wrote it. SG and FS critically revised and approved the paper. FP contributed with a critical reading of the text and with the revision of the pharmacological aspects of the paper. All authors approved the final text.
Disclosures
Vincenzo M. Monda, Francesca Porcellati and Felice Strollo have nothing to disclose. Sandro Gentile is a member of the journal’s editorial board.
Compliance with Ethics Guidelines
This study was conducted in conformance with good clinical practice standards. No human or animal data are present in the paper for which compliance with the ethical guidelines of the Helsinki Declaration and subsequent versions are not applicable.
Data Availability
No data obtained directly from the authors appear in the text.
Footnotes
Digital Features
To view digital features for this article go to 10.6084/m9.figshare.12722258.
References
- 1.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan-Yeung M, Xu RH. SARS: epidemiology. Respirology. 2003;8(suppl):S9–14. doi: 10.1046/j.1440-1843.2003.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO Middle East respiratory syndrome coronavirus (MERS-CoV). https://www.who.int/emergencies/mers-cov/en/. Accessed 16 Jan 2018.
- 4.Raj VS, Mou H, Smits SL, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495(7440):251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5(5):262–269. doi: 10.1038/nrendo.2009.48. [DOI] [PubMed] [Google Scholar]
- 6.Martin JH, Deacon CF, Gorrell MD, Prins JB. Incretin-based therapies—review of the physiology, pharmacology and emerging clinical experience. Intern Med J. 2011;41(4):299–307. doi: 10.1111/j.1445-5994.2011.02439.x. [DOI] [PubMed] [Google Scholar]
- 7.Hedrington MS, Davis SN. Oral semaglutide for the treatment of type 2 diabetes. Expert Opin Pharmacother. 2019;20(2):133–141. doi: 10.1080/14656566.2018.155225. [DOI] [PubMed] [Google Scholar]
- 8.Iacobellis G. COVID-19 and diabetes: can DPP-IV inhibition play a role? Diabetes Res Clin Pract. 2020;162:108125. doi: 10.1016/j.diabres.2020.108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tianru J. Letter to the editor: Comment on GLP-1-based drugs and COVID-19 treatment. Acta Pharm Sin B. 2020 doi: 10.1016/j.apsb.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drucker DJ. Coronavirus infections and type 2 diabetes-shared pathways with therapeutic implications. Endocr Rev. 2020;41(3):bnaa011. doi: 10.1210/endrev/bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mirabelli M, Chiefari E, Puccio L, Foti DP, Brunetti A. Potential benefits and harms of novel antidiabetic drugs during COVID-19 crisis. Int J Environ Res Public Health. 2020;17(10):3664. doi: 10.3390/ijerph17103664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YS, Jun HS. Anti-inflammatory effects of GLP-1-based therapies beyond glucose control. Mediators Inflamm. 2016;2016:3094642. doi: 10.1155/2016/3094642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romaní-Pérez M, Outeiriño-Iglesias V, Moya CM, et al. Activation of the GLP-1 receptor by liraglutide increases ACE2 expression, reversing right ventricle hypertrophy, and improving the production of SP-A and SP-B in the lungs of type 1 diabetes rats. Endocrinology. 2015;156(10):3559–3569. doi: 10.1210/en.2014-1685. [DOI] [PubMed] [Google Scholar]
- 14.Fandiño J, Vaz AA, Toba L, et al. Liraglutide enhances the activity of the ACE-2/Ang(1–7)/Mas receptor pathway in lungs of male pups from food-restricted mothers and prevents the reduction of SP-A. Int J Endocrinol. 2018;2018:6920620. doi: 10.1155/2018/6920620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wösten-van Asperen RM, Lutter R, Specht PA, et al. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1–7) or an angiotensin II receptor antagonist. J Pathol. 2011;225(4):618–627. doi: 10.1002/path.2987. [DOI] [PubMed] [Google Scholar]
- 16.Kuba K, Imai Y, Rao S, Jiang C, Penninger JM. Lessons from SARS: control of acute lung failure by the SARS receptor ACE2. J Mol Med (Berl) 2006;84(10):814–820. doi: 10.1007/s00109-006-0094-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020 doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santos RAS, Sampaio WO, Alzamora AC, et al. The ACE2/Angiotensin-(1–7)/MAS axis of the renin-angiotensin system: focus on angiotensin-(1–7) Physiol Rev. 2018;98(1):505–553. doi: 10.1152/physrev.00023.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang C, Stavrou E, Schmaier AA, et al. Angiotensin 1–7 and Mas decrease thrombosis in Bdkrb2-/- mice by increasing NO and prostacyclin to reduce platelet spreading and glycoprotein VI activation. Blood. 2013;121(15):3023–3032. doi: 10.1182/blood-2012-09-459156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Celi A, Cianchetti S, Dell'Omo G, Pedrinelli R. Angiotensin II, tissue factor and the thrombotic paradox of hypertension. Expert Rev Cardiovasc Ther. 2010;8(12):1723–1729. doi: 10.1586/erc.10.161. [DOI] [PubMed] [Google Scholar]
- 21.Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2(7):247–257. doi: 10.1002/emmm.201000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gheblawi M, Wang K, Viveiros A, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126(10):1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5(1):58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto T, Perlot T, Rehman A, et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487(7408):477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020 doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benicky J, Sánchez-Lemus E, Pavel J, Saavedra JM. Anti-inflammatory effects of angiotensin receptor blockers in the brain and the periphery. Cell Mol Neurobiol. 2009;29(6–7):781–792. doi: 10.1007/s10571-009-9368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776–785. doi: 10.1016/S2213-8587(19)30249-9. [DOI] [PubMed] [Google Scholar]
- 30.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019;394(10193):131–138. doi: 10.1016/S0140-6736(19)31150-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data obtained directly from the authors appear in the text.