Abstract
Carcinoma embryonic antigen (CEA), osteopontin (OPN), and Dickkopf-1 (DKK1) expressed in serum are associated with hypoxia in tumor progression. However, the role of these proteins in the plasma of patients with non-small cell lung cancer (NSCLC) is poorly understood. The diagnostic values of CEA combined with OPN or DKK1 were compared in non-small cell lung cancer. This study investigated the diagnostic value of CEA combined with OPN and DKK1, respectively, in NSCLC. Eighty patients with NSCLC (NSCLC group) and 60 patients with benign lung diseases (benign lung disease group) admitted to Shandong Provincial Third Hospital from May 2014 to January 2015 were selected as the study subjects. In addition, 60 healthy subjects undergoing normal physical examination were selected as healthy control group. The OPN and DKK1 in serum of the two groups were detected by enzyme linked immunosorbent assay (ELISA), and the CEA expression was measured by Electrochemical Photometric method. The diagnostic value of CEA combined with OPN and DKK1, respectively, in NSCLC was analyzed. The expression of CEA, OPN, and DKK1 in serum of NSCLC group was significantly higher than that of healthy control group and benign lung disease group (P<0.05). The expression of CEA, OPN and DKK1 in serum of NSCLC patients was correlated with tumor diameter, lymph node metastasis, degree of pathological differentiation and clinical stage (P<0.05). ROC curve for diagnosis of NSCLC was drawn by further combination of serum CEA and OPN. The AUC of combined diagnosis of CEA and OPN for NSCLC was 0.920 (95% CI, 0.875–0.964), and the diagnostic sensitivity and specificity were 87.50 and 86.67%, respectively; the AUC of combined diagnosis of CEA and DKK1 for NSCLC was 0.912 (95% CI, 0.866–0.958), and the diagnostic sensitivity and specificity were 92.50 and 76.67%, respectively. CEA, OPN and DKK1 may be involved in the occurrence and progression of NSCLC and have good sensitivity and specificity in the diagnosis of NSCLC and may be new biomarkers for the diagnosis of NSCLC.
Keywords: CEA, OPN, DKK1, NSCLC, diagnostic value
Introduction
Lung cancer is a common malignant tumor and is the leading cause of cancer death worldwide; its incidence is increasing year by year (1). Non-small cell lung cancer (NSCLC) accounts for 85% of the total incidence of lung cancer; at present, the treatment of NSCLC is still a major clinical problem, but the treatment of early NSCLC has made great progress. The early NSCLC patients have a good prognosis after surgical resection, but the early clinical symptoms of NSCLC patients are not obvious, it is difficult to diagnose in time, and most NSCLC have progressed to the advanced stage on diagnoses, leading to the unsatisfactory overall survival rate (2,3).
For the diagnosis of early NSCLC, serum tumor marker detection is a commonly used method in clinical practice (3). Carcinoembryonic antigen (CEA) is a common tumor marker for non-small cell lung cancer, which exists in the digestive tract of normal embryos and is a good tumor marker for efficacy, development and prognosis of breast cancer, large intestine cancer and lung cancer (4,5). However, its sensitivity and specificity are not high, and its role in early diagnosis of lung cancer is not obvious (6). Ferreira et al (7,8) found that osteopontin (OPN) is closely related to the proliferation, infiltration, metastasis and prognosis of various tumor cells. Previous studies have shown that OPN plays an important role in the immune response and the development, invasion and metastasis of various malignant tumors (9–11). Cabiati et al (12) found that the expression of plasma and tissue OPN concentrations increase in liver cancer patients with clinical severity. They considered that OPN may be a useful starting point for prognostic and diagnostic markers of liver cancer, and that OPN overexpression is associated with an invasive phenotype of human NSCLC. Dickkopf-1 (DKK1) is a secreted glycoprotein that is abnormally expressed in rheumatic diseases such as ankylosing spondylitis and osteoarthritis and is involved in the regulation of bone formation. On the contrary, Rouanne et al (13) showe that the expression level of serum OPN is related to the pathological features of NSCLC and has some value in the diagnosis of NSCLC. DKK1 is a secretory glycoprotein and has been demonstrated to be expressed abnormally in colorectal cancer, gastric cancer, endometrial carcinoma and serum samples (14–16). It has been used as a new tumor marker and therapeutic target for esophageal and hepatic carcinoma (17). Kasoha et al (18) found that DKK1 is highly expressed in various tumor cell lines and can be used as a new biomarker (19). Yamabuki et al (17) found that the expression of DKK1 in lung cancer increased, which is an important indicator for the diagnosis and prognosis of lung cancer.
There are many studies on the diagnostic value of single serum tumor markers in NSCLC, but the detection of single markers can cause missed diagnosis, misdiagnosis, and delay in treatment of patients. Therefore, the serum levels of CEA, OPN and DKK1 in patients with NSCLC, and patients with benign lung disease (NSCLC) as well as healthy people were compared and analyzed in this study, and the diagnostic value of combined detection for NSCLC was evaluated.
Patients and methods
General data
This was a retrospective study. A total of 200 hospitalized patients and healthy people in Shandong Provincial Third Hospital (Jinan, China) from May 2014 to January 2015 were selected, including 80 cases in NSCLC group, 60 cases in benign pulmonary lesions group and 60 healthy people in control group. There were 35 cases of tuberculosis, 36 cases of pulmonary infection, 8 cases of benign lung tumors in the benign pulmonary lesions group.
Inclusion criteria: All patients were diagnosed on the basis of clinical manifestations, imaging, pathological and laboratory examinations; the healthy control group was examined in the physical examination center of Shandong Provincial Third Hospital and the results were normal. Patients without other types of tumors, without heart, liver, kidney and other important organ diseases, and without family history of cancer were included.
Exclusion criteria: Pregnant patients; long-term bedridden patients; patients with thyroid and immune system diseases; patients with severe hypertension and diabetes; patients who had used glucocorticoids and antibiotics in the pprevious two weeks; patients with mental or cognitive impairment; patients with malignant tumors in other sites.
The study was approved by the Ethics Committee of Shandong Provincial Third Hospital. Signed informed consents were obtained from the patients and/or guardians.
Main instruments and reagents
Electrochemical luminescence analyzer (coase411, Roche); CEA Diagnostic Kit (IMG-80019, Imgenex); OPN Elisa Kit (KT-140900), DKK1 Elisa Kit (KT-1244) (both from Kamiya Biomedical Co.), Multifunctional Micro-hole Plate Reader (SpectraMaxiD5, Molecular Devices).
Test method
A 5-ml sample of fasting venous blood was collected from the study subjects of the three groups undergoing physical examination in the morning and placed in the vacuum collection of blood vessels for separating by centrifugation at 1,500 × g at 4°C for 10 min. The CEA in the separated serum was detected by electrochemiluminescence analysis. The indoor quality control was carried out and the results were controlled. The operation steps were strictly carried out according to the instructions of the kit. The serum OPN, DKK1 was detected by enzyme linked immunosorbent assay (ELISA). All kit components and samples were put at room temperature (18-25°C) in advance. Test reagent A and B were briefly rotated or centrifuged. Assay diluent was used to dilute A or B to working concentration (1:100). Twenty-microliters detergent concentrate (30X) was diluted with 580-ml deionized water or distilled water to prepare 600 ml detergent (1X), and 100 µl calibration product or sample was added to each well. The microplate was incubated at 37°C for 2 h, and a 100 µl prepared detection reagent A was added. The microplate was incubated at 37°C for 1 h, sucked 3 times and washed, and 100 µl prepared detection reagent B was added. The microplate was incubated at 37°C for 30 min, sucked and washed 5 times, and a 90 µl substrate solution was added. The microplate was incubated at 37°C for 15–25 min, and 50 µl stop solution was added. A multifunctional microplate reader was used to read 450 nm immediately. Three replicate wells were set per sample, and the experiment was repeated three times, 20% intra/inter plate variation was accepted.
Statistical methods
The SPSS 20.0 (IBM Corp.) statistical software was used to analyze the data. The categorical data was described by [n (%)], and analyzed by Chi-square test. The numerical data were expressed as the mean ± standard deviation, and analyzed by t-test. One-way analysis of variance was used for the comparison of multiple groups of count data, denoted by F. Subject operating characteristics (ROC) curves was used for evaluation of diagnostic efficacy of CEA, OPN and DKK1 for NSCLC. P<0.05 was considered as a statistically significant difference.
Results
Study population
There was no significant difference in sex, smoking history, drinking history, baric index, residence, marital status, education degree, work status, inclusion criteria for benign lung disease, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) among the three groups (P>0.05). There were 37 males and 23 females, aged 30–79 years, with the average age of 64.37±5.57 years in the healthy group. In NSCLC group, 41 males and 39 females, aged 35–79 years, were included, with an average age of 61.39±7.41 years. The benign lung disease group had 34 males and 26 females, aged 32–80 years, and the average age was 64.02±4.98 years (Table I).
Table I.
General characteristics of the three groups [n (%)]/(mean ± SD).
| Characteristics | Healthy group (n=60) | Benign lung disease group (n=60) | NSCLC group (n=80) | F/t/χ2 | P-value |
|---|---|---|---|---|---|
| Sex | 1.525 | 0.466 | |||
| Male | 37 (61.67) | 34 (56.67) | 41 (51.25) | ||
| Female | 23 (38.33) | 26 (43.33) | 39 (48.75) | ||
| Age (years) | 64.37±5.57 | 64.02±4.98 | 61.39±7.41 | 4.922 | 0.008 |
| Smoking history | 0.954 | 0.621 | |||
| Yes | 28 (46.67) | 31 (51.67) | 44 (55.00) | ||
| No | 32 (53.33) | 29 (48.33) | 36 (45.00) | ||
| Drinking history | 0.486 | 0.784 | |||
| Yes | 38 (63.33) | 36 (60.00) | 46 (57.50) | ||
| No | 22 (36.67) | 24 (40.00) | 34 (42.50) | ||
| BMI (kg/m2) | 20.16±3.31 | 21.14±2.87 | 19.95±3.19 | 2.662 | 0.072 |
| Residence | 0.170 | 0.919 | |||
| Rural area | 35 (58.33) | 37 (61.67) | 47 (58.75) | ||
| Urban area | 25 (41.67) | 23 (38.33) | 33 (41.25) | ||
| Marital status | 0.147 | 0.929 | |||
| Unmarried | 29 (48.33) | 27 (45.00) | 38 (47.50) | ||
| Married | 31 (51.67) | 33 (55.00) | 42 (52.50) | ||
| Education degree | 0.144 | 0.930 | |||
| High school or below | 36 (60.00) | 38 (63.33) | 49 (61.25) | ||
| High school or above | 24 (40.00) | 22 (36.67) | 31 (38.75) | ||
| Work status | 0.3993 | 0.819 | |||
| No | 22 (36.67) | 25 (41.67) | 33 (41.25) | ||
| Yes | 38 (63.33) | 35 (58.33) | 47 (58.75) | ||
| AST (U/l) | 19.16±7.08 | 18.37±7.76 | 18.21±7.13 | 0.314 | 0.731 |
| ALT (U/l) | 22.37±9.53 | 21.65±10.18 | 21.93±9.47 | 0.084 | 0.919 |
χ2 test was used to analyze counting data, including data of sex, smoking history, drinking history, residence, marital status, education degree, and working status. One-way analysis of variance was used for the comparison of measurement data in multiple groups, including data of body mass index, AST and ALT. BM, body mass index; AST, aspartate aminotransferase; ALT, aspartate aminotransferase. χ2, statistic value of the χ2 test; F, statistic value of ANOVA; P-value, probability value.
Expression of serum CEA, OPN, DKK1 of the three groups
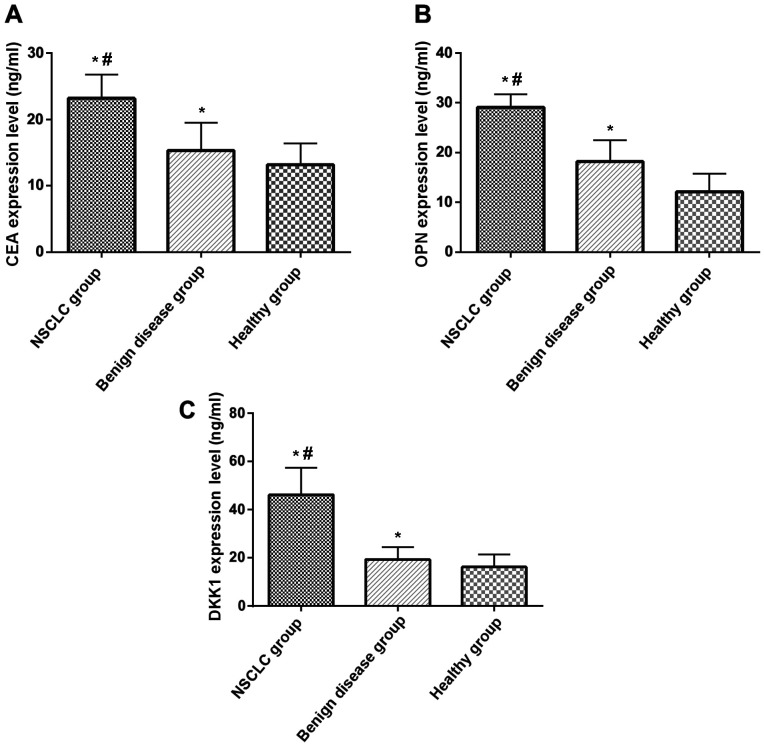
Expression of CEA, OPN and DKK1 between the three groups were compared by one-way analysis of variance, indicated by F. The expression levels of serum CEA, OPN and DKK1 in NSCLC patients were significantly higher than those in healthy control group and benign lung disease group (P<0.05). The expression levels of serum CEA, OPN and DKK1 in patients with benign lung disease group were significantly higher than that in healthy control group (P<0.05) (Table II and Fig. 1).
Table II.
Comparison of serum CEA, OPN, DKK1 levels among three groups (mean ± SD).
| Group | n | CEA (ng/ml) | OPN (ng/ml) | DKK1 (ng/ml) |
|---|---|---|---|---|
| NSCLC group | 80 | 23.18±3.59a,b | 29.13±2.57a,b | 46.13±11.21a,b |
| Benign lung disease group | 60 | 15.32±4.17a | 18.17±4.31a | 19.29±5.21a |
| Healthy control group | 60 | 13.15±3.21 | 12.08±3.63 | 16.24±5.14 |
| F-value | 147.900 | 449.800 | 292.800 | |
| P-value | <0.001 | <0.001 | <0.001 |
P<0.05, compared with healthy control group
P<0.05, compared with benign lung disease group. F-value, statistical value of ANOVA; P-value, probability value. CEA, carcinoma embryonic antigen; OPN, osteopontin; DKK1, Dickkopf-1; NSCLC, non-small cell lung cancer.
Figure 1.
Expression of CEA, OPN and DKK1 in NSCLC group, benign lung disease group and healthy control group. (A) Comparison of CEA expression among NSCLC group, benign lung disease group and healthy control group; (B) comparison of OPN expression among NSCLC group, benign lung disease group and healthy control group; (C) comparison of DKK1 expression among NSCLC group, benign lung disease group and healthy control group. *P<0.05, compared with healthy control group; #P<0.05, compared with benign lung disease group. CEA, carcinoma embryonic antigen; OPN, osteopontin; DKK1, Dickkopf-1; NSCLC, non-small cell lung cancer.
Relationship between the expression of serum CEA, OPN, DKK1 and clinicopathological parameters of NSCLC
There was no significant difference among the expression level of CEA, OPN, DKK1 and the sex, age, smoking history and drinking history in NSCLC patients (P>0.05); but there were significant differences between the expression level and clinical stage, tumor diameter, lymphatic metastasis, pathological differentiation degree (P<0.05) (Table III)(20).
Table III.
Relationship between expression levels of serum CEA, OPN and DKK1 and clinicopathological features of NSCLC patients (mean ± SD).
| Clinicopathological features | n | CEA (ng/ml) | t/F | P-value | OPN (ng/ml) | t value | P-value | DKK1 (ng/ml) | t value | P-value |
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | 0.711 | 0.480 | 1.322 | 0.190 | 0.278 | 0.782 | ||||
| Male | 41 | 22.97±4.17 | 29.12±3.12 | 45.59±10.57 | ||||||
| Female | 39 | 23.57±3.32 | 28.27±2.59 | 46.27±11.32 | ||||||
| Age (years) | 1.181 | 0.241 | 0.981 | 0.330 | 0.677 | 0.501 | ||||
| <60 | 34 | 22.68±2.98 | 30.12±3.23 | 46.28±9.89 | ||||||
| ≥60 | 46 | 23.58±3.63 | 29.35±3.64 | 44.63±11.39 | ||||||
| Smoking history | 1.679 | 0.097 | 1.899 | 0.061 | 0.693 | 0.490 | ||||
| Yes | 44 | 24.87±3.98 | 29.34±2.6 | 46.74±10.64 | ||||||
| No | 36 | 23.56±2.72 | 28.09±2.89 | 44.43±11.15 | ||||||
| Drinking history | 0.864 | 0.390 | 1.117 | 0.267 | 0.062 | 0.951 | ||||
| Yes | 45 | 23.18±2.53 | 30.57±3.11 | 45.64±10.39 | ||||||
| No | 34 | 22.59±3.54 | 29.85±2.54 | 45.78±9.58 | ||||||
| TNM stage | 4.744 | <0.001 | 8.179 | <0.001 | 3.805 | <0.001 | ||||
| Stage I–II | 47 | 22.37±3.80 | 28.34±2.39 | 43.02±9.78 | ||||||
| Stage III–IV | 33 | 25.98±2.57 | 33.38±3.12 | 51.98±12.16 | ||||||
| Tumor diameter | 3.986 | <0.001 | 7.596 | <0.001 | 3.455 | <0.001 | ||||
| ≤3 cm | 33 | 21.87±3.98 | 28.21±3.64 | 43.23±9.52 | ||||||
| >3 cm | 47 | 24.93±2.89 | 32.57±1.27 | 51.86±12.12 | ||||||
| Lymphatic metastasis | 4.396 | <0.001 | 9.361 | <0.001 | 3.596 | <0.001 | ||||
| Yes | 38 | 24.31±3.38 | 33.78±3.53 | 51.83±9.85 | ||||||
| No | 42 | 21.07±3.21 | 28.27±1.38 | 43.15±11.56 | ||||||
| Pathological differentiation degree | 3.981 | <0.001 | 7.824 | <0.001 | 3.475 | <0.001 | ||||
| Low | 49 | 25.39±3.77 | 29.78±2.90 | 51.95±10.36 | ||||||
| High-middle | 31 | 22.31±2.61 | 34.21±1.54 | 43.76±9.37 | ||||||
| Pathological type | 3.03 | 0.054 | 2.974 | 0.057 | 0.213 | 0.808 | ||||
| Squamous cell carcinoma | 27 | 24.14±2.56 | 31±3.17 | 45.15±10.17 | ||||||
| Adenocarcinoma | 33 | 23.19±3.21 | 29±3.28 | 46.36±9.51 | ||||||
| Large cell carcinoma | 20 | 25.18±2.67 | 30±2.95 | 44.71±9.32 |
TNM staging is based on the AJCC standard (20). CEA, carcinoma embryonic antigen; OPN, osteopontin; DKK1, Dickkopf-1; NSCLC, non-small cell lung cancer. t, statistic value of t-test; P-value, probability value.
Diagnostic value of serum CEA, OPN and DKK1 in NSCLC
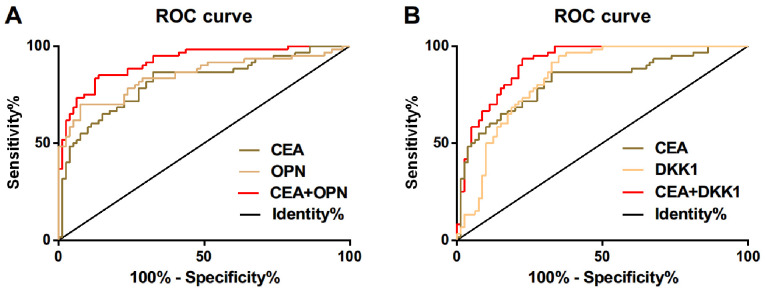
ROC curve of serum CEA, OPN and DKK1 expression for diagnosis of NSCLC was drawn. The AUC of serum CEA in diagnosis of NSCLC was 0.818, and the diagnostic sensitivity and specificity were 86.25 and 70.00%, respectively; the AUC of serum OPN in diagnosis of NSCLC was 0.847, and the diagnostic sensitivity and specificity were 71.61 and 91.25%, respectively; the AUC of serum DKK1 in diagnosis of NSCLC was 0.838, and the diagnostic sensitivity and specificity were 92.5 and 65%, respectively. ROC curve for diagnosis of NSCLC was drawn by further combination of serum CEA and OPN. The AUC of combined diagnosis of CEA and OPN for NSCLC was 0.920, and the diagnostic sensitivity and specificity were 87.50 and 86.67%, respectively; but the diagnostic sensitivity and specificity of combined diagnosis of CEA and DKK1 for NSCLC were 92.50 and 76.67%, respectively (Table IV and Fig. 2).
Table IV.
Diagnostic value of serum CEA, OPN and DKK1 in NSCLC.
| Diadynamic criteria | AUC | 95% CI | Standard error | Cut-off value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| CEA | 0.818 | 0.745–0.891 | 0.037 | 21.34 (ng/ml) | 86.25 | 70.00 |
| OPN | 0.847 | 0.777–0.917 | 0.036 | 23.31 (ng/ml) | 71.61 | 91.25 |
| DKK1 | 0.838 | 0.772–0.904 | 0.033 | 43.39 (ng/ml) | 92.50 | 65.00 |
| CEA+OPN | 0.920 | 0.875–0.964 | 0.023 | 0.477 | 87.50 | 86.67 |
| CEA+DKK1 | 0.912 | 0.866–0.958 | 0.023 | 0.332 | 92.50 | 76.67 |
AUC, area under curve; 95% CI, 95% confidence interval; CEA, carcinoma embryonic antigen; OPN, osteopontin; DKK1, Dickkopf-1; NSCLC, non-small cell lung cancer.
Figure 2.
ROC curve for serum CEA, OPN and DKK1 expression in the diagnosis of NSCLC. (A) ROC curve of serum CEA and OPN expression in diagnosis of NSCLC; (B) ROC curve of serum CEA and DKK1 expression in diagnosis of NSCLC. CEA, carcinoma embryonic antigen; OPN, osteopontin; DKK1, Dickkopf-1; NSCLC, non-small cell lung cancer.
Discussion
The levels of CEA, OPN and DKK1 in serum of patients with non-small cell lung cancer, patients with benign lung disease and healthy controls were compared. The results of this study showed that the level of serum CEA, OPN and DKK1 in NSCLC patients was significantly higher than that in benign disease group and healthy control group. The serum level of CEA, OPN and DKK1 was correlated with tumor diameter, lymph node metastasis, pathological differentiation and clinical stage, suggesting that CEA, OPN and DKK1 may be involved in the occurrence and progression of NSCLC. Lei et al (21) found that the increase of CEA secretion in respiratory tract is related to the malignant pathological changes of respiratory system, which may lead to the increase of CEA expression level. Lin et al (22) used immunohistochemistry to detect the expression of OPN in 146 patients with lung cancer, and concluded that OPN expression was an independent prognostic factor of NSCLC, and was significantly correlated with lymph node metastasis, TNM staging and pathological types of patients with lung cancer. Sheng et al (23) found that the expression of serum DKK1 in patients with lung cancer was significantly higher than that in patients with benign lung tumor and healthy subjects by detecting serum DKK1 in healthy people and patients with lung cancer and benign lung tumor diseases. This suggests that CEA, OPN and DKK1 may be involved in the occurrence, progression, migration and transfer of NSCLC, and these three may be the biomarkers of NSCLC. Our studies showed that the sensitivity and specificity of serum CEA in the diagnosis of NSCLC were 86.25 and 70.00%, respectively; the sensitivity and specificity of serum OPN in the diagnosis of NSCLC were 71.61 and 91.25%, respectively; the sensitivity and specificity of serum DKK1 in the diagnosis of NSCLC were 92.5 and 65%, respectively; the sensitivity and specificity of further combination of serum CEA and OPN were 87.50 and 86.67%, respectively in the diagnosis of NSCLC; the sensitivity and specificity of the combination of serum CEA and DKK1 in the diagnosis of NSCLC were 92.50 and 76.67%, respectively. These results suggest that these three methods can be used as biological markers for the diagnosis of NSCLC. The study by Ma et al (24) evaluated the value of CEA, the combination of cytokeratin 19 fragment (CYFRA21-1) and CA125 in the clinical diagnosis of non-small cell lung cancer NSCLC, and they found that the combined detection of these three tumor markers can greatly improve the diagnostic sensitivity to NSCLC. This indicates that combined detection can improve the sensitivity of diagnosis of NSCLC and promote the sensitivity and accuracy of diagnosis.
In the present study, the serum levels of CEA, OPN and DKK1 in patients with NSCLC, benign pulmonary disease and normal controls were compared and analyzed, and the diagnostic value of combined detection for NSCLC was discussed. There are some limitations in this study, because of the retrospective collection of patient data, the data obtained sometimes inevitably interfere with subjective factors. These markers were not observed in terms of curative effect and prognosis. Thus, further study is required.
In conclusion, CEA, OPN and DKK1 may be involved in the occurrence and development of NSCLC and have good sensitivity and specificity in the diagnosis of NSCLC. Combined detection has high diagnostic value in the diagnosis of NSCLC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
JS and XC conceived and designed the study, and drafted the manuscript. JS, XC and YW collected, analyzed and interpreted the experiment data, and revised the manuscript critically for important intellectual content. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Shandong Provincial Third Hospital (Jinan, China). Signed informed consents were obtained from the patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Zhu W, Zhou K, Zha Y, Chen D, He J, Ma H, Liu X, Le H, Zhang Y. Diagnostic value of serum miR-182, miR-183, miR-210, and miR-126 levels in patients with early-stage non-small cell lung cancer. PLoS One. 2016;11:e0153046. doi: 10.1371/journal.pone.0153046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scagliotti GV, Fossati R, Torri V, Crinò L, Giaccone G, Silvano G, Martelli M, Clerici M, Cognetti F, Tonato M, Adjuvant Lung Project Italy/European Organisation for Research Treatment of Cancer - Lung Cancer Cooperative Group Investigators Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small cell lung cancer. J Natl Cancer Inst. 2003;95:1453–1461. doi: 10.1093/jnci/djg059. [DOI] [PubMed] [Google Scholar]
- 4.Verberne CJ, Wiggers T, Grossmann I, de Bock GH, Vermeulen KM. Cost-effectiveness of a carcinoembryonic antigen (CEA) based follow-up programme for colorectal cancer (the CEA Watch trial) Colorectal Dis. 2016;18:O91–O96. doi: 10.1111/codi.13273. [DOI] [PubMed] [Google Scholar]
- 5.Wang W, Xu X, Tian B, Wang Y, Du L, Sun T, Shi Y, Zhao X, Jing J. The diagnostic value of serum tumor markers CEA, CA19-9, CA125, CA15-3, and TPS in metastatic breast cancer. Clin Chim Acta. 2017;470:51–55. doi: 10.1016/j.cca.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 6.Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer. 2012;76:138–143. doi: 10.1016/j.lungcan.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira LB, Lima RT, da Fonseca Bastos AC, Silva AM, Tavares C, Pestana A, Rios E, Eloy C, Sobrinho-Simões M, Etel Gimba RP, et al. OPNa variant expression is associated with matrix mineralization in thyroid cancer cell lines. Cancer Res. 2018 Jul 1; doi: 10.3390/ijms19102990. (Epub ahead of print). doi: 10.1158/1538-7445.AM2018-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan Y, Cai W, Li S, Zhang L. Proceedings of 2018 International Conference on Biomedical Engineering, Machinery and Earth Science (BEMES 2018) Francis Academic Press; UK: 2018. Effect of Ubumex combined with AC-T sequential chemotherapy on serum T cell subsets. OPN levels and quality of life in patients with triple negative breast cancer. [Google Scholar]
- 9.Byeon H, Lee SD, Hong EK, Lee DE, Kim BH, Seo Y, Joo J, Han SS, Kim SH, Park SJ, et al. Long-term prognostic impact of osteopontin and Dickkopf-related protein 1 in patients with hepatocellular carcinoma after hepatectomy. Pathol Res Pract. 2018;214:814–820. doi: 10.1016/j.prp.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Clemente N, Raineri D, Cappellano G, Boggio E, Favero F, Soluri MF, Dianzani C, Comi C, Dianzani U, Chiocchetti A. Osteopontin bridging innate and adaptive immunity in autoimmune diseases. J Immunol Res. 2016;2016:7675437. doi: 10.1155/2016/7675437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin H, Wang R, Wei G, Wang H, Pan G, Hu R, Wei Y, Tang R, Wang J. Overexpression of osteopontin promotes cell proliferation and migration in human nasopharyngeal carcinoma and is associated with poor prognosis. Eur Arch Otorhinolaryngol. 2018;275:525–534. doi: 10.1007/s00405-017-4827-x. [DOI] [PubMed] [Google Scholar]
- 12.Cabiati M, Gaggini M, Cesare MM, Caselli C, De Simone P, Filipponi F, Basta G, Gastaldelli A, Del Ry S. Osteopontin in hepatocellular carcinoma: A possible biomarker for diagnosis and follow-up. Cytokine. 2017;99:59–65. doi: 10.1016/j.cyto.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Rouanne M, Adam J, Goubar A, Robin A, Ohana C, Louvet E, Cormier J, Mercier O, Dorfmüller P, Fattal S, et al. Osteopontin and thrombospondin-1 play opposite roles in promoting tumor aggressiveness of primary resected non-small cell lung cancer. BMC Cancer. 2016;16:483. doi: 10.1186/s12885-016-2541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jumpertz S, Hennes T, Asare Y, Schütz AK, Bernhagen J. CSN5/JAB1 suppresses the WNT inhibitor DKK1 in colorectal cancer cells. Cell Signal. 2017;34:38–46. doi: 10.1016/j.cellsig.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Jia X, Li N, Peng C, Deng Y, Wang J, Deng M, Lu M, Yin J, Zheng G, Liu H, et al. miR-493 mediated DKK1 down-regulation confers proliferation, invasion and chemo-resistance in gastric cancer. Oncotarget. 2016;7:7044–7054. doi: 10.18632/oncotarget.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yi N, Liao QP, Li T, Xiong Y. Novel expression profiles and invasiveness-related biology function of DKK1 in endometrial carcinoma. Oncol Rep. 2009;21:1421–1427. doi: 10.3892/or_00000370. [DOI] [PubMed] [Google Scholar]
- 17.Yamabuki T, Takano A, Hayama S, Ishikawa N, Kato T, Miyamoto M, Ito T, Ito H, Miyagi Y, Nakayama H, et al. Dikkopf-1 as a novel serologic and prognostic biomarker for lung and esophageal carcinomas. Cancer Res. 2007;67:2517–2525. doi: 10.1158/0008-5472.CAN-06-3369. [DOI] [PubMed] [Google Scholar]
- 18.Kasoha M, Bohle RM, Seibold A, Gerlinger C, Juhasz-Böss I, Solomayer EF. Dickkopf-1 (Dkk1) protein expression in breast cancer with special reference to bone metastases. Clin Exp Metastasis. 2018;35:763–775. doi: 10.1007/s10585-018-9937-3. [DOI] [PubMed] [Google Scholar]
- 19.Sakabe T, Azumi J, Umekita Y, Toriguchi K, Hatano E, Hirooka Y, Shiota G. Expression of cancer stem cell-associated DKK1 mRNA serves as prognostic marker for hepatocellular carcinoma. Anticancer Res. 2017;37:4881–4888. doi: 10.21873/anticanres.11897. [DOI] [PubMed] [Google Scholar]
- 20.Edge SB, Byrd DR, Carducci MA, Compton CC, Fritz AG, Greene FL, Trotti A, editors. Springer; New York, NY: 2010. AJCC Cancer Staging Manual. [Google Scholar]
- 21.Lei L, Chen Q, Wang Z, Han N, Chen B, Qin J, Lu HY. Usefulness of carcinoembryonic antigen in the diagnosis of small cell lung cancer combined with adenocarcinoma. Advances in Clinical and Experimental Medicine: Official Organ Wroclaw Medical University. 2017;26:1091–1094. doi: 10.17219/acem/66372. [DOI] [PubMed] [Google Scholar]
- 22.Lin Q, Guo L, Lin G, Chen Z, Chen T, Lin J, Zhang B, Gu X. Clinical and prognostic significance of OPN and VEGF expression in patients with non-small-cell lung cancer. Cancer Epidemiol. 2015;39:539–544. doi: 10.1016/j.canep.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Sheng SL, Huang G, Yu B, Qin WX. Clinical significance and prognostic value of serum Dickkopf-1 concentrations in patients with lung cancer. Clin Chem. 2009;55:1656–1664. doi: 10.1373/clinchem.2009.125641. [DOI] [PubMed] [Google Scholar]
- 24.Ma L, Xie XW, Wang HY, Ma LY, Wen ZG. Clinical evaluation of tumor markers for diagnosis in patients with non-small cell lung cancer in China. Asian Pac J Cancer Prev. 2015;16:4891–4894. doi: 10.7314/APJCP.2015.16.12.4891. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.