Abstract
The presence of tumor infiltrating lymphocytes (TILs) and tertiary lymphoid structures (TLSs) in tumor tissues are of great prognostic significance in several types of human cancer. The present study investigated the density of TILs and TLSs in gastric cancer (GC) tissues and their association with pathological parameters. Moreover, the clinical significance of follicular CD8+ cytotoxic T cells present within the germinal centers of the tumor-associated TLSs was investigated. Immunohistochemistry and H&E staining were used to examine the infiltration and distribution patterns of TILs, TLSs and germinal center (gc) CD8+ TILs in tumor tissues obtained from 63 patients with GC. The number of TILs, TLSs, combination of TILs and TLSs (TILs-TLSs) and gcCD8+ TILs were used to define tumoral immune parameters, and the prognostic value of these parameters was assessed. The analysis revealed that patients with GC with increased levels of TILs, TLSs, or gcCD8+ TILs exhibited improved overall survival. In addition, gcCD8+ TILs levels were significantly associated with patient age, histological grade and pTN stage. Increased levels of TILs-TLSs were positively associated with nerve invasion, tumor thrombus, nodal metastasis and histological grade. Multivariate Cox regression analysis revealed that TILs-TLSs and gcCD8+ TILs were independent prognostic factors. The data obtained in the present study demonstrated that high levels of tumoral immune parameters are important independent prognostic predictors for human GC. The results also suggested a possible role of gcCD8+ TILs in tumor immune surveillance.
Keywords: tumor infiltrating lymphocytes, tertiary lymphoid structures, follicular CD8+ T cells, gastric cancer
Introduction
Previous studies have, demonstrate that immune cell infiltration in human gastrointestinal cancers is associated with cancer progression and is a favorable prognostic predictor (1–5). Both cellular composition and organization of tumor infiltrating lymphocytes (TILs) are crucial for inhibiting cancer progression and are implicated in the success of cancer immunotherapy (1,2,6). Tertiary lymphoid structure (TLSs) are an important source of TILs, characterized by ectopic aggregated lymphocytes with high endothelial venules and have a similar function to secondary lymphoid organs (SLOs). The lymphocytes in TLSs have easy access to tumor antigens as TLSs are not encapsulated and embedded within the tumor microenvironment (5). The numbers of both TILs and TLSs within solid tumor tissues can be used for the assessment of tumor immune surveillance and are important prognostic factors for cancer (7–11).
Although TLSs are thought to be associated with anti-tumor immune responses (12–15), the functional role of their cellular components remains unclear. TLSs are divided into B- and T-zones, which consist of follicular dendritic cells (FDCs) and fibroblastic reticular cells (FRCs), respectively (16). TLSs can be further defined as primary and secondary TLSs, based on the absence or presence of a germinal center in B-cell follicles. Following stimulation by tumor antigens, B cells differentiate and form the germinal center, which is the site of B cell proliferation, class switching and somatic hyper mutation (17–19). CD4+ CXCR5+ T follicular helper (Tfh) cells are a subtype of CD4+ helper T cells, principally located in germinal centers, and play critical roles in recruiting, activating and regulating the germinal center (20–22). Previous studies suggested that Tfh cells mediate follicular and germinal center formation in TLSs (23,24). In recent studies, CXCR5+ CD8+ T cells were found within the lymphoid follicle, primarily within the germinal center, where they controlled viral replication during chronic HIV and lymphocytic choriomeningitis virus (LMCV) infection (25,26). The CD20+ B cells and CD8+ T cells co-localize in the tumor nest (20) and TLSs (21). This distribution pattern of follicular CD8+ T cells might be involved in antitumor immune responses.
The present study investigated follicular CD8+ T cells in the tumor tissues of patients with gastric cancer (GC). In addition, germinal center CD8+ (gcCD8+) TILs were quantified. The relationship between tumoral immune parameters such as TILs, TLSs, TILs-TLSs and gcCD8+ TILs and clinical pathological parameters was determined.
Materials and methods
Patients and tissue samples
The present study retrospectively analyzed data of patients admitted to the Department of Pathology, Third Affiliated Hospital of Soochow University between 2006 to 2008. The patients were enrolled according to the following criteria: i) Pathologically-confirmed diagnosis of primary GC (adenocarcinoma); ii) did not receive pre-operative chemotherapy or radiotherapy; iii) presence of adequate paraffin-embedded fixed tissue blocks; iv) at least one slide contained the invasive margin of the tumor; and v) availability of complete medical records and follow-up information. A total of 63 patients with GC were included in this present study. Tumor clinical and pathological staging system was based on the Eighth Edition of the Union for International Cancer Control/American Joint Committee on Cancer. Three slides contain cancer tissue were available for each patient, and a total of 189 slides were reviewed in the present study. Patient survival data were available until the end of November 2011. Patient clinical data are presented in Table I. The present study was approved by the Ethics Committee of Soochow University, and complied with the Declaration of Helsinki. Informed consent to use the tissue sample for scientific research was obtained from all patients.
Table I.
Details of parameters in the study cohort.
| Total study cohort | ||
|---|---|---|
| Parameters | n | % |
| Sex | ||
| Male | 49 | 77.8 |
| Female | 14 | 22.2 |
| Age, years | ||
| ≤50 | 8 | 12.7 |
| >50 | 55 | 87.3 |
| Tumor size | ||
| ≤5 cm | 39 | 61.9 |
| >5 cm | 24 | 38.1 |
| Nerve invasion | ||
| Yes | 25 | 39.7 |
| No | 38 | 60.3 |
| Tumor thrombus | ||
| Yes | 22 | 34.9 |
| No | 41 | 65.1 |
| Nodal metastasis | ||
| Yes | 36 | 57.1 |
| No | 27 | 42.9 |
| Histological grade | ||
| I–II | 30 | 47.6 |
| III | 33 | 52.4 |
| pTN stage | ||
| I | 23 | 36.5 |
| II | 13 | 20.6 |
| III | 27 | 42.9 |
| TILs | ||
| High | 44 | 69.8 |
| Low | 19 | 30.2 |
| TLSs | ||
| High | 43 | 68.3 |
| Low | 20 | 31.7 |
| TILs and TLSs | ||
| High | 32 | 50.8 |
| Low | 31 | 49.2 |
| gcCD8+ TILsa | ||
| High | 23 | 41.8 |
| Low | 32 | 58.2 |
gcCD8+ TILs were obtained in 55 patients. TILs, tumor infiltrating lymphocytes; TLSs, tertiary lymphoid structures.
Pathomorphological evaluation of TILs and TLSs
Cancer tissue was fixed in 10% (v/v) formalin and embedded in paraffin until use. The H&E stained slides of the resected GC tissues were reviewed and scored independently by two pathologists who were blinded to the clinical data and prognosis of the patients. The pathologists were trained in the pathomorphological evaluation of TILs and TLSs, and any problematic cases were discussed with them during subsequent scoring. The TILs and TLSs in the center of the tumor (CT) and the invasive margin (IM) were examined (11,27,28). The TILs scoring system incorporated two aspects: i) The number of infiltrating lymphocytes; score 0, no infiltration; score 1, mild infiltration; score 2, moderate infiltration; score 3, extensive infiltration (Fig. 1); and ii) the percentage of the tumor area containing TILs in the CT or IM. The CT-TILs and IM-TILs location scores were defined as the number of infiltrating lymphocytes multiplied by the percentage, and then, the final score was computed by summation of the CT- and IM-TILs scores. TLSs were also evaluated in the CT and IM by measuring two factors: The number of TLSs (aggregates of lymphocytes with and without germinal centers were counted) and the percentage, similar to the TILs, in the CT or IM (29,30). The CT-TLSs and IM-TLSs location scores were defined as the numbers multiplied by the percentage, and then, the final score of TLSs was computed by summation of the CT- and IM-TLS scores.
Figure 1.
Pathomorphological images of inflammatory cell reactions in gastric cancer. (A) Low and (B and C) high levels of tumor infiltrating lymphocytes. Magnification, ×200; scale bar, 50 µm. Tertiary lymphoid structures (marked with blue arrows) in different gastric anatomical layers: (D) In the mucosa, (E) in the submucosa and (F) in the muscularis propria. Magnification, ×100; scale bar, 100 µm.
Immunohistochemistry and evaluation of the gcCD8+ T cells
Formalin-fixed and paraffin-embedded (FFPE) tissues were cut into 4 µm thick consecutive sections deparaffinized in xylene and rehydrated in graded ethanol solutions. Monoclonal mouse antibody against human CD8 (MAB-0021, ready to use, Maixin Biotechnology Limited Corporation) was used to stain the T lymphocytes. The CD8 antigen was retrieved by boiling the slides in citrate buffer (10 mmol/l; pH 6.0) under high pressure for 2 min. Then, the sections were immersed in 3% hydrogen peroxide for 10 min to block endogenous peroxidase activity, rinsed three times in PBS, and then incubated with primary antibodies at 4°C overnight. The negative control was performed with PBS. The sections were incubated with horseradish peroxidase-labeled goat anti-mouse secondary antibody (ready to use, Maixin Biotechnology Limited Corporation). Diaminobenzene was used as the chromogen and hematoxylin as the nuclear counterstain. The scoring system for gcCD8+ TILs was assessed in every slide. The number of germinal centers and gcCD8+ TILs in each germinal center were counted. The average values were subsequently obtained.
Statistical analysis
Statistical analyses were performed using SPSS (version 24.0; IBM Corp.) and GraphPad Prism software (version 6; GraphPad Software, Inc.). Data were analyzed using the χ2 test, Kaplan-Meier method and Cox regression analysis as appropriate. All tests were two-sided and P<0.05 was considered to indicate a statistically significant difference.
Results
Relationship between tumoral immune parameters and clinical pathological parameters
The TILs, TLSs, and gcCD8+ TILs were defined as tumoral immune parameters in the present study (Tables I and II). In order to make the scoring system more facilitative to statistical analysis, these variables were converted into binary variables. TILs were sub-grouped by the TIL final score: TILhi 44 cases, score >0.45 [area under the receiver operating characteristic curve (AUC)=0.35; sensitivity, 82.1%; specificity, 50.0%], and TILlow 19 cases, score ≤0.45 (Table I; Fig. 1A-C). TLSs were sub-grouped by the TLS final score: TLShi 43 cases, score >0.42 (AUC=0.39; sensitivity, 79.5%; specificity, 50.0%), and TLSlow 20 cases, score ≤0.42 (Table I; Fig. 1D-F). Considering the combination of TILs and TLSs, the samples were divided into two groups. The TIL-TLShi group (32 cases) consisted of samples that scored as both TILhi and TLShi. The TIL-TLSlow group (31 cases) consisted of samples that scored as TILhi-TLSlow, TILlow-TLShi and TILlow-TLSlow (Table I). Based on the density of gcCD8+ TILs, 55 samples (specimens from eight cases were excluded due to absence of germinal centers) were divided into two groups: gcCD8hi TIL, 23 cases, score >6.93 (AUC=0.19; sensitivity, 59.5%; specificity, 94.4%; Fig. 2A-F), and gcCD8low TIL, 32 cases, score ≤6.93 (Table I; Fig. 2G-L).
Table II.
Association between clinicopathological parameters and tumoral immune parameters.
| gcCD8+ TILs | TILs | TLSs | TILs-TLSs | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | Low | High | χ2 | P-value | Low | High | χ2 | P-value | Low | High | χ2 | P-value | Low | High | χ2 | P-value |
| Sex | ||||||||||||||||
| Male | 23 | 19 | 0.854 | 0.355 | 17 | 32 | 2.153 | 0.142 | 15 | 34 | 0.131 | 0.718 | 26 | 23 | 1.311 | 0.252 |
| Female | 9 | 4 | 2 | 12 | 5 | 9 | 5 | 9 | ||||||||
| Age (years) | ||||||||||||||||
| ≤50 | 6 | 0 | 4.841 | 0.028 | 4 | 4 | 1.713 | 0.191 | 3 | 5 | 0.140 | 0.708 | 5 | 3 | 0.648 | 0.421 |
| >50 | 26 | 23 | 15 | 40 | 17 | 38 | 26 | 29 | ||||||||
| Tumor size | ||||||||||||||||
| ≤5 cm | 18 | 17 | 1.804 | 0.179 | 7 | 32 | 7.246 | 0.007 | 12 | 27 | 0.045 | 0.832 | 16 | 23 | 2.741 | 0.098 |
| >5 cm | 14 | 6 | 12 | 12 | 8 | 16 | 15 | 9 | ||||||||
| Nerve invasion | ||||||||||||||||
| Yes | 12 | 8 | 0.043 | 0.836 | 14 | 11 | 13.140 | <0.001 | 10 | 15 | 1.303 | 0.254 | 18 | 7 | 8.616 | 0.003 |
| No | 20 | 15 | 5 | 33 | 10 | 28 | 13 | 25 | ||||||||
| Tumor thrombus | ||||||||||||||||
| Yes | 13 | 4 | 3.383 | 0.066 | 10 | 12 | 3.755 | 0.053 | 10 | 12 | 2.932 | 0.087 | 16 | 6 | 7.483 | 0.006 |
| No | 19 | 19 | 9 | 32 | 10 | 31 | 15 | 26 | ||||||||
| Nodal metastasis | ||||||||||||||||
| Yes | 20 | 9 | 2.932 | 0.087 | 16 | 20 | 8.139 | 0.004 | 12 | 24 | 0.098 | 0.755 | 22 | 14 | 4.763 | 0.029 |
| No | 12 | 14 | 3 | 24 | 8 | 19 | 9 | 18 | ||||||||
| Histological grade | ||||||||||||||||
| I–II | 11 | 16 | 6.631 | 0.010 | 5 | 25 | 4.950 | 0.026 | 8 | 22 | 0.682 | 0.409 | 10 | 20 | 5.773 | 0.016 |
| III | 21 | 7 | 14 | 19 | 12 | 21 | 21 | 12 | ||||||||
| pTN stage | ||||||||||||||||
| I | 9 | 13 | 6.053 | 0.048 | 2 | 21 | 8.072 | 0.018 | 6 | 17 | 0.642 | 0.725 | 7 | 16 | 5.399 | 0.067 |
| II | 6 | 5 | 5 | 8 | 5 | 8 | 7 | 6 | ||||||||
| III | 17 | 5 | 12 | 15 | 9 | 18 | 17 | 10 | ||||||||
Values in bold indicate P<0.05. TILs, tumor infiltrating lymphocytes; TLSs, tertiary lymphoid structures.
Figure 2.
Two cases of (A-F) gcCD8hi TILs and (G-L) gcCD8low TILs were presented. (A) Case 1 had the TLS with a follicle and germinal center (marked with blue arrow) in HE slides. (B) CD20 staining revealed B lymphocyte aggregation and the germinal center. (C) CD21 staining revealed the follicular dendritic cell network in the germinal center. (D) Enlarged images of the red square area revealed follicular CD8+ TILs around the germinal center. (E and F) gcCD8+ TILs were detected in the germinal center (red arrows). (G) TLS marked with a blue arrow were also observed in Case 2. (H-J) The same expression pattern of CD20, CD21 and CD8 was observed. (K-L) Conversely, there were no gcCD8+ TILs that infiltrated into the germinal center. (A-D and G-J) Magnification, ×100; scale bar, 100 µm. (E and K) Magnification, ×200; scale bar, 50 µm. (F and L) Magnification, ×400; scale bar, 20 µm. TLS, tertiary lymphoid structure; CD, cluster of differentiation; HE, hematoxylin and eosin; TILs, tumor infiltrating lymphocytes.
The TILs were significantly associated with the tumor size, nerve invasion, lymph nodal metastasis, histological grade and pTN stage (P=0.007, 0.001, 0.004, 0.026 and 0.018, respectively; Table II). The data showed that the TLSs were not significantly associated with clinical pathological parameters. The combination of TILs-TLSs was significantly associated with nerve invasion, tumor thrombus, lymph nodal metastasis and histological grade (P=0.003, 0.006, 0.029 and 0.016, respectively; Table II). The data also showed the gcCD8+ TILs was significantly associated with patient age, histological grade and pTN stage (P=0.028, 0.010 and 0.048, respectively; Table II). The higher levels of gcCD8+ TILs were associated with lower histological grade and lower pTN stage. These data suggested that the gcCD8+ TILs might be involved in antitumor immunity in GC.
Association between tumoral immune parameters and patient prognosis
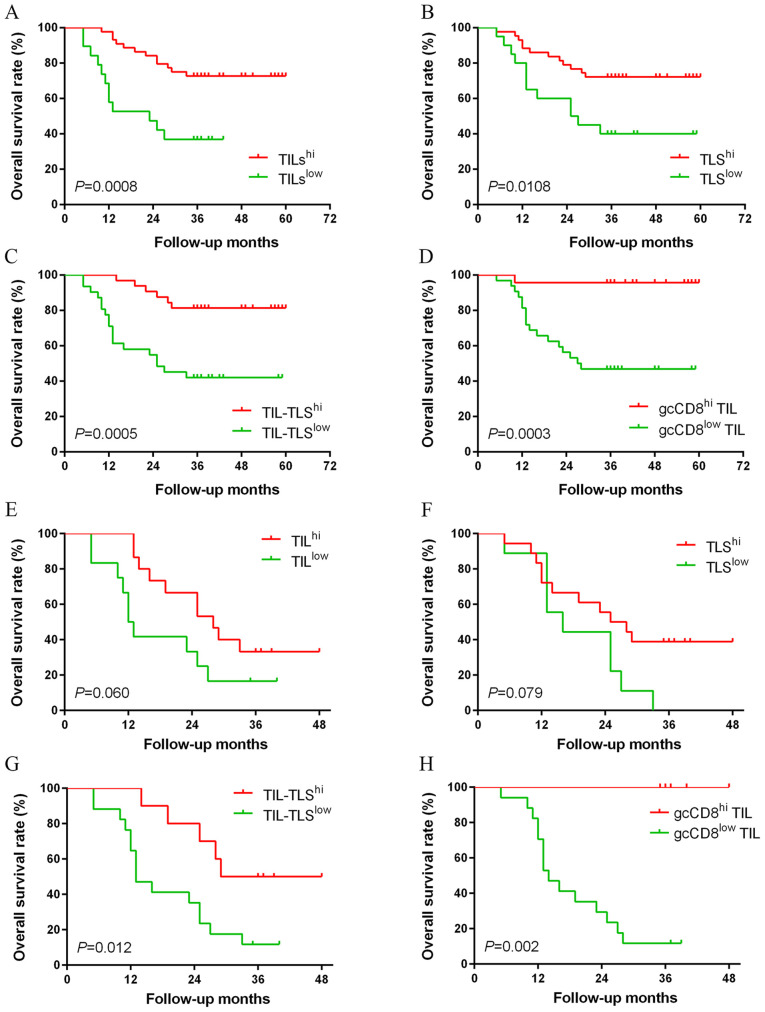
Kaplan-Meier survival analysis showed that the patients with TILhi, TLShi, TIL-TLShi or gcCD8hi TIL had favorable prognosis than those with lower levels (P=0.0008, 0.0108, 0.0005 and 0.0003, respectively; Fig. 3A-D). The prognostic value of tumoral immune parameters in patients with advanced GC (pTN III stage) was investigated. The patients in the TIL-TLShi and gcCD8hi TIL groups had an improved overall survival (OS) compared with patients in the TIL-TLSlow and gcCD8low TIL groups (P=0.012 and 0.002; Table III; Fig. 3G and H).
Figure 3.
Kaplan-Meier survival curves for tumoral immune parameters. Kaplan-Meier analysis of overall survival for 63 patients stratified by (A) TILs, (B) TLSs and (C) TILs-TLSs in gastric cancer, and for 55 patients stratified by (D) gcCD8+ TILs. In pTN III stage, there was no statistically significant difference between the (E) IL or (F) TLS levels and OS. However, patients with high levels of (G) TILs-TLSs and (H) gcCD8 TILs had an improved OS. TILs, tumor infiltrating lymphocytes; TLSs, tertiary lymphoid structures; OS, overall survival.
Table III.
Kaplan-Meier analysis of tumoral immune parameters.
| A, Tumoral immune parameters in all cases | |||||
|---|---|---|---|---|---|
| Parameters | Cases, n | Status of death | Mean OS, months | Log-Rank χ2 | P-value |
| TILs | |||||
| High | 44 | 12 | 49.25 | 11.198 | 0.001 |
| Low | 19 | 12 | 24.21 | ||
| TLSs | |||||
| High | 43 | 12 | 48.14 | 6.501 | 0.011 |
| Low | 20 | 12 | 33.40 | ||
| TILs-TLSs | |||||
| High | 32 | 6 | 53.03 | 12.148 | <0.001 |
| Low | 31 | 18 | 33.42 | ||
| gcCD8+ TILs | |||||
| High | 23 | 1 | 57.83 | 13.322 | <0.001 |
| Low | 32 | 17 | 36.16 | ||
| B, Tumoral immune parameters in pTN III stage cases | |||||
| Parameters | Cases, n | Status of death | Mean OS, months | Log-Rank χ2 | P-value |
| TILs | |||||
| High | 12 | 10 | 30.33 | 3.527 | 0.06 |
| Low | 15 | 10 | 18.58 | ||
| TLSs | |||||
| High | 18 | 11 | 19.11 | 3.089 | 0.079 |
| Low | 9 | 9 | 18.89 | ||
| TILs-TLSs | |||||
| High | 10 | 5 | 35.50 | 6.310 | 0.012 |
| Low | 17 | 15 | 19.00 | ||
| gcCD8+ TILs | |||||
| High | 5 | 0 | – | 9.334 | 0.002 |
| Low | 17 | 15 | 18.65 | ||
Values in bold indicate P<0.05. TILs, tumor infiltrating lymphocytes; TLSs, tertiary lymphoid structures.
In the univariate Cox regression analysis (analysisa), smaller tumor size, lower histological grade, absence of tumor thrombus or lymph nodal metastasis, lower pTN stage and higher levels of tumoral immune parameters were favorable prognostic factors for OS (Table IV). Considering the possible interference among TILs, TLSs and TILs-TLSs, we did a cohort of multivariate Cox regression analysis. Multivariate Cox regression analysisb (based on the clinicopathological feature, TILs, TLSs and gcCD8+ TILs) revealed that the gcCD8+ TILs were the only independent prognostic factor for OS (HR=0.087; 95% CI: 0.011–0.692; P=0.021; Table IV). Multivariate Cox regression analysisc (based on the clinicopathological feature, TILs-TLSs and gcCD8+ TILs) demonstrated that TILs-TLSs and gcCD8+ TILs were independent prognostic factors (HR=0.247, 95% CI: 0.069–0.882, P=0.031; HR=0.067, 95% CI: 0.008–0.561, P=0.013, respectively). Moreover, the multivariate Cox regression analysisd was designed to determine the association between tumoral immune parameters (TILs, TLSs and gcCD8+ TILs). The result showed TILs and gcCD8+ TILs could be used as independent prognostic factors (HR=0.322, 95% CI: 0.124–0.835, P=0.020; HR=0.059, 95% CI: 0.008–0.451, P=0.006, respectively). The tumoral immune parameters exhibited an HR <1 in the Cox regression analysis, suggesting that may protect against tumor progression.
Table IV.
Univariate and multivariate Cox regression analyses of clinicopathological parameters and tumoral immune parameters.
| A, Univariate analysis | ||||
|---|---|---|---|---|
| Variables | Unfavorable/favorable | HR | 95% CI | P-value |
| Sex | Male/Female | 0.863 | 0.343–2.176 | 0.755 |
| Age, years | ≤50/>50 | 0.747 | 0.255–2.187 | 0.594 |
| Tumor size, cm | ≤5/>5 | 3.643 | 1.587–8.363 | 0.002 |
| Histological grade | I–II/III | 4.624 | 1.722–12.421 | 0.002 |
| Neural Invasion | No/Yes | 2.168 | 0.969–4.847 | 0.060 |
| Tumor thrombus | No/Yes | 6.619 | 2.716–16.134 | 0.001 |
| Lymphatic metastasis | No/Yes | 11.528 | 2.702–49.184 | 0.001 |
| pTN stage | I–II/III | 18.732 | 2.525–138.976 | 0.004 |
| TILs | High/Low | 0.279 | 0.125–0.623 | 0.002 |
| TLSs | High/Low | 0.370 | 0.166–0.825 | 0.015 |
| gcCD8+ TILs | High/Low | 0.062 | 0.008–0.470 | 0.007 |
| TILs-TLSs | High/Low | 0.376 | 0.222–0.636 | 0.000 |
| B, Multivariate Cox regression analysis | ||||
| Variables | Unfavorable/favorable | HR | 95% CI | P-value |
| Tumor size, cm | ≤5/>5 | 0.896 | 0.198–4.046 | 0.886 |
| Histological grade | I–II/III | 1.009 | 0.277–3.671 | 0.990 |
| Tumor thrombus | No/Yes | 2.227 | 0.482–10.748 | 0.299 |
| Lymphatic metastasis | No/Yes | 1.251 | 0.135–11.560 | 0.844 |
| pTN stage | I–II/III | 5.594 | 0.336–93.237 | 0.230 |
| TILs | High/Low | 0.503 | 0.169–1.492 | 0.215 |
| TLSs | High/Low | 0.609 | 0.203–1.831 | 0.377 |
| gcCD8+ TILs | High/Low | 0.087 | 0.011–0.692 | 0.021 |
| C, Multivariate Cox regression analysis | ||||
| Variables | Unfavorable/favorable | HR | 95% CI | P-value |
| Tumor size, cm | ≤5/>5 | 1.208 | 0.273–5.355 | 0.803 |
| Histological grade | I–II/III | 0.806 | 0.208–3.116 | 0.754 |
| Tumor thrombus | No/Yes | 1.742 | 0.381–7.970 | 0.474 |
| Lymphatic metastasis | No/Yes | 0.980 | 0.103–9.296 | 0.986 |
| pTN stage | I–II/III | 7.159 | 0.419–122.410 | 0.174 |
| gcCD8+ TILs | High/Low | 0.067 | 0.008–0.561 | 0.013 |
| TILs-TLSs | High/Low | 0.247 | 0.069–0.882 | 0.031 |
| D, Multivariate Cox regression analysis | ||||
| Variables | Unfavorable/favorable | HR | 95% CI | P-value |
| TILs | High/Low | 0.322 | 0.124–0.835 | 0.020 |
| TLSs | High/Low | 0.396 | 0.148–1.056 | 0.064 |
| gcCD8+ TILs | High/Low | 0.059 | 0.008–0.451 | 0.006 |
Values in bold indicate P<0.05. TILs, tumor infiltrating lymphocytes; TLSs, tertiary lymphoid structures; HR, hazard ratio.
Discussion
The present study demostrated that patients with GC with higher levels of TILs, or TLSs, or gcCD8+ TILs had an improved OS. Multivariate Cox regression analysis revealed that TILs-TLSs and gcCD8+ TILs were independent prognostic factors. In addition, gcCD8+ TILs were significantly associated with patient age, histological grade and pTN stage. Higher levels of TILs-TLSs was positively associated with the nerve invasion, tumor thrombus, lymph nodal metastasis and histological grade. The data obtained in the present study suggested that high levels of tumoral immune parameters were associated with improved prognosis in patients with GC.
Cancer progression is a multi-step process that involves genetic, epigenetic, as well as histopathological change (3,31–33). Infiltrating immune cells play an important role in preventing or promoting cancer progression (34). Previous studies have demonstrated that high levels of TILs may inhibit the progression of breast and colorectal cancer (8,31,35). The present study revealed that a high level of TILs was associated with improved prognosis in patient with GC. Univariate and multivariate Cox regression analyses revealed that TILs could be used as an independent favorable prognostic factor.
Previous studies demonstrated that TLSs are required for the development of the T- and B-cell mediated immune response against human cancer and various other diseases (25,26,31,36,37). TLSs have been previously described in several types of cancer, such as lung cancer and breast cancer, and are associated with patient prognosis (10,17,29). TLSs are organization by heterotopic lymphoid tissues, and exhibit similar organization and functionality to SLOs. The basic components of TLSs include the T-zone, comprising of T cells and FRCs, and the B-zone, comprising of B cells and FDCs. TLSs are involved in establishing a systemic memory response to protect patients against circulating metastatic cancer cells, therefor inhibiting tumor recurrence for several years (8,31). The present study revealed that the high levels of TLSs were associated with improved outcome in patients with GC, and could therefor be used as an independent prognostic factor. The combination of TILs and TLSs is superior to TILs or TLSs alone for predicting survival. In advanced cancer, the high levels of TILs-TLSs were associated with improved OS. Based on the results obtained in the present study, TLSs are an important niche in which tumor antigen specific T and B cells are generated.
Despite the association of gcCD8+ T cells with improved prognosis, the role of gcCD8+ T cells in antitumor immune responses and germinal center B cell responses remains unknow. Previous studies investigating chronic infection with LMCV and HIV revealed that there are two subsets of CD8+ effector T cells, namely CXCR5+ PD-1+ CD8+ T and CXCR5− PD-1+ CD8+ T cells (26,38). The CXCR5+ subset exhibits stem cell-like properties and is localized in the B cell follicle and germinal center during chronic infection. The CXCR5+ subset undergo self-renewal and can differentiate into CXCR5− CD8+ T cells. The de novo converted CXCR5− subset exhibits increased cytotoxicity and removes virus infected cells. Hornquist et al (39) studied the role of CD8+ T cells in the regulation of gut mucosal immune responses and showed that CD8+ T cells inhibited local B cell responses. By contrast, B cells and plasma cells have been shown to impede T cell-mediated antitumor immune responses (40–42). Based on the aforementioned findings, gcCD8+ T cells are likely to promote cell-mediate antitumor immune responses and inhibit humoral immunity. A diagrammatic representation based on two hypotheses is presented in Fig. S1. Based on the results obtained from studies investigating chronic HIV or LMCV infection (43,44), it is hypothesized that PD1+ CD8+ TILs may be further divided into CXCR5+ and CXCR5− subsets, which are regulated by the inhibitor of DNA binding 2 (ID2)/transcription factor E2-α axis. The CXCR5+ subset located in the B cell follicle and the germinal center. The follicular CXCR5+ CD8+ T cells subset can undergo proliferation and differentiate into the CXCR5− subset following ID2 upregulation. 2. According to the studies by Hornquist et al (39) and Li et al (44), germinal center CXCR5+ CD8+ T cells can exert a suppressive effect on germinal B cell responses and inhibit the generation of plasma cells. Some mechanisms in these hypotheses were demonstrated. Elimination of immunosuppressive B cells expressing IgA, IL-10 and PD-L1 allows CD8+ cytotoxic T cells eradication of oxaliplatin-resistance prostate cancer (40). The expression of CXCR5 on CD8+ T cells was necessary for T cells infiltrating into B-cells follicular (43). The present study revealed that the phenotype of TILs, TLSs and gcCD8+ TILs exhibited significant heterogeneity in patients with GC. TILs, TLSs and gcCD8+ TILs may be had the potential function associated with GC immunotherapy. Further investigation is required to validate the hypotheses proposed in the present study.
Supplementary Material
Acknowledgements
Not applicable.
Glossary
Abbreviations
- TILs
tumor infiltrating lymphocytes
- TLSs
tertiary lymphoid structures
- GC
gastric cancer
- Tfc
follicular CD8+ cytotoxic T cells
- gcCD8+ TILs
germinal center CD8+ TILs
- SLO
secondary lymphoid organs
- TME
tumor microenvironment
- FDC
follicular dendritic cell
- FRC
fibroblastic reticular cell
- CT
center of the tumor
- IM
invasive margin
- pTN
pathological tumor and lymph node
- AUC
area under the receiver operating charatcteristic curve
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 31701111), Key R&D Project of Science and Technology Department of Jiangsu Province (BE2015633). Changzhou Health and Family Planning Commission Youth Talent Science and Technology Project (QN201709). This work was also supported in part by Roswell Park Cancer Institute/University of Pittsburgh Cancer Institute Ovarian Cancer Specialized Programs of Research Excellence Grants (P50CA159981).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
QL, DZ and WH designed the study and wrote the manuscript. DZ, TC, ZY, XG and LC performed all of the experiments. XZ and BX performed the statistical analysis. BL and JJ conceived and designed the study, guided the experiments and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Soochow University, and complied with the Declaration of Helsinki. Informed consent to use the tissue sample for scientific research was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chen LJ, Sun J, Wu HY, Zhou SM, Tan Y, Tan M, Shan BE, Lu BF, Zhang XG. B7-H4 expression associates with cancer progression and predicts patient's survival in human esophageal squamous cell carcinoma. Cancer Immunol Immunother. 2011;60:1047–1055. doi: 10.1007/s00262-011-1017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen LJ, Zheng X, Shen YP, Zhu YB, Li Q, Chen J, Xia R, Zhou SM, Wu CP, Zhang XG, et al. Higher numbers of T-bet(+) intratumoral lymphoid cells correlate with better survival in gastric cancer. Cancer Immunol Immunother. 2013;62:553–561. doi: 10.1007/s00262-012-1358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Y, Chen L, Xu B, Xiong Y, Yang M, Rui X, Shi L, Wu C, Jiang J, Lu B. Higher numbers of T-Bet+ tumor-infiltrating lymphocytes associate with better survival in human epithelial ovarian cancer. Cell Physiol Biochem. 2017;41:475–483. doi: 10.1159/000456600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu Y, Ju S, Chen E, Dai S, Li C, Morel P, Liu L, Zhang X, Lu B. T-bet and eomesodermin are required for T cell-mediated antitumor immune responses. J Immunol. 2010;185:3174–3183. doi: 10.4049/jimmunol.1000749. [DOI] [PubMed] [Google Scholar]
- 5.Pimenta EM, Barnes BJ. Role of tertiary lymphoid structures (TLS) in anti-tumor immunity: Potential tumor-induced cytokines/chemokines that regulate TLS formation in epithelial-derived cancers. Cancers (Basel) 2014;6:969–997. doi: 10.3390/cancers6020969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singer M, Wang C, Cong L, Marjanovic ND, Kowalczyk MS, Zhang H, Nyman J, Sakuishi K, Kurtulus S, Gennert D, et al. A distinct gene module for dysfunction uncoupled from activation in tumor-infiltrating T cells. Cell. 2016;166:1500–1511.e9. doi: 10.1016/j.cell.2016.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26:4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 8.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 9.Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 10.Goc J, Fridman WH, Sautès-Fridman C, Dieu-Nosjean MC. Characteristics of tertiary lymphoid structures in primary cancers. Oncoimmunology. 2013;2:e26836. doi: 10.4161/onci.26836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang D, He W, Wu C, Tan Y, He Y, Xu B, Chen L, Li Q, Jiang J. Scoring system for tumor-infiltrating lymphocytes and its prognostic value for gastric cancer. Front Immunol. 2019;10:71. doi: 10.3389/fimmu.2019.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinet L, Filleron T, Le Guellec S, Rochaix P, Garrido I, Girard JP. High endothelial venule blood vessels for tumor-infiltrating lymphocytes are associated with lymphotoxin beta-producing dendritic cells in human breast cancer. J Immunol. 2013;191:2001–2008. doi: 10.4049/jimmunol.1300872. [DOI] [PubMed] [Google Scholar]
- 13.Cipponi A, Mercier M, Seremet T, Baurain JF, Théate I, van den Oord J, Stas M, Boon T, Coulie PG, van Baren N. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res. 2012;72:3997–4007. doi: 10.1158/0008-5472.CAN-12-1377. [DOI] [PubMed] [Google Scholar]
- 14.de Chaisemartin L, Goc J, Damotte D, Validire P, Magdeleinat P, Alifano M, Cremer I, Fridman WH, Sautès-Fridman C, Dieu-Nosjean MC. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res. 2011;71:6391–6399. doi: 10.1158/0008-5472.CAN-11-0952. [DOI] [PubMed] [Google Scholar]
- 15.Bergomas F, Grizzi F, Doni A, Pesce S, Laghi L, Allavena P, Mantovani A, Marchesi F. Tertiary intratumor lymphoid tissue in colo-rectal cancer. Cancers (Basel) 2011;4:1–10. doi: 10.3390/cancers4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joshi NS, Akama-Garren EH, Lu Y, Lee DY, Chang GP, Li A, DuPage M, Tammela T, Kerper NR, Farago AF, et al. Regulatory T cells in tumor-Associated tertiary lymphoid structures suppress anti-tumor T cell responses. Immunity. 2015;43:579–590. doi: 10.1016/j.immuni.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goc J, Germain C, Vo-Bourgais TK, Lupo A, Klein C, Knockaert S, de Chaisemartin L, Ouakrim H, Becht E, Alifano M, et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 2014;74:705–715. doi: 10.1158/0008-5472.CAN-13-1342. [DOI] [PubMed] [Google Scholar]
- 18.Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, Ravoet M, Le Buanec H, Sibille C, Manfouo-Foutsop G, et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. 2013;123:2873–2892. doi: 10.1172/JCI67428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottlin EB, Bentley RC, Campa MJ, Pisetsky DS, Herndon JE, II, Patz EF., Jr The association of intratumoral germinal centers with early-stage non-small cell lung cancer. J Thorac Oncol. 2011;6:1687–1690. doi: 10.1097/JTO.0b013e3182217bec. [DOI] [PubMed] [Google Scholar]
- 20.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 21.Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res. 2010;70:8368–8377. doi: 10.1158/0008-5472.CAN-10-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bos R, Marquardt KL, Cheung J, Sherman LA. Functional differences between low- and high-affinity CD8(+) T cells in the tumor environment. Oncoimmunology. 2012;1:1239–1247. doi: 10.4161/onci.21285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroenke MA, Eto D, Locci M, Cho M, Davidson T, Haddad EK, Crotty S. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. J Immunol. 2012;188:3734–3744. doi: 10.4049/jimmunol.1103246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang C, Hillsamer P, Kim CH. Phenotype, effector function, and tissue localization of PD-1-expressing human follicular helper T cell subsets. BMC Immunol. 2011;12:53. doi: 10.1186/1471-2172-12-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petrovas C, Ferrando-Martinez S, Gerner MY, Casazza JP, Pegu A, Deleage C, Cooper A, Hataye J, Andrews S, Ambrozak D, et al. Follicular CD8 T cells accumulate in HIV infection and can kill infected cells in vitro via bispecific antibodies. Science translational medicine. 2017;9:eaag2285. doi: 10.1126/scitranslmed.aag2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He R, Hou S, Liu C, Zhang A, Bai Q, Han M, Yang Y, Wei G, Shen T, Yang X, et al. Follicular CXCR5- expressing CD8(+) T cells curtail chronic viral infection. Nature. 2016;537:412–428. doi: 10.1038/nature19317. [DOI] [PubMed] [Google Scholar]
- 27.Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. 2014;232:199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hennequin A, Derangère V, Boidot R, Apetoh L, Vincent J, Orry D, Fraisse J, Causeret S, Martin F, Arnould L, et al. Tumor infiltration by Tbet+ effector T cells and CD20+ B cells is associated with survival in gastric cancer patients. Oncoimmunology. 2016;5:e1054598. doi: 10.1080/2162402X.2015.1054598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee HJ, Park IA, Song IH, Shin SJ, Kim JY, Yu JH, Gong G. Tertiary lymphoid structures: Prognostic significance and relationship with tumour-infiltrating lymphocytes in triple-negative breast cancer. J Clin Pathol. 2016;69:422–430. doi: 10.1136/jclinpath-2015-203089. [DOI] [PubMed] [Google Scholar]
- 30.Carney JA. Gastric mucosal lymphoid follicles: Histology, distribution, frequency, and etiologic features. Am J Surg Pathol. 2010;34:1019–1024. doi: 10.1097/PAS.0b013e3181e1acb0. [DOI] [PubMed] [Google Scholar]
- 31.Dieu-Nosjean MC, Giraldo NA, Kaplon H, Germain C, Fridman WH, Sautes-Fridman C. Tertiary lymphoid structures, drivers of the anti-tumor responses in human cancers. Immunol Rev. 2016;271:260–275. doi: 10.1111/imr.12405. [DOI] [PubMed] [Google Scholar]
- 32.Geng Y, Shao Y, He W, Hu W, Xu Y, Chen J, Wu C, Jiang J. Prognostic role of tumor-infiltrating lymphocytes in lung cancer: A meta-analysis. Cell Physiol Biochem. 2015;37:1560–1571. doi: 10.1159/000438523. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Chen L, Xiong Y, Zheng X, Xie Q, Zhou Q, Shi L, Wu C, Jiang J, Wang H. Knockdown of PD-L1 in human gastric cancer cells inhibits tumor progression and improves the cytotoxic sensitivity to CIK therapy. Cell Physiol Biochem. 2017;41:907–920. doi: 10.1159/000460504. [DOI] [PubMed] [Google Scholar]
- 34.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 35.Lee HJ, Kim JY, Park IA, Song IH, Yu JH, Ahn JH, Gong G. Prognostic significance of tumor-infiltrating lymphocytes and the tertiary lymphoid structures in HER2-positive breast cancer treated with adjuvant trastuzumab. Am J Clin Pathol. 2015;144:278–288. doi: 10.1309/AJCPIXUYDVZ0RZ3G. [DOI] [PubMed] [Google Scholar]
- 36.Gu-Trantien C, Migliori E, Buisseret L, de Wind A, Brohée S, Garaud S, Noël G, Dang Chi VL, Lodewyckx JN, Naveaux C, et al. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight. 2017;2:e91487. doi: 10.1172/jci.insight.91487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schweiger T, Berghoff AS, Glogner C, Glueck O, Rajky O, Traxler D, Birner P, Preusser M, Klepetko W, Hoetzenecker K. Tumor-infiltrating lymphocyte subsets and tertiary lymphoid structures in pulmonary metastases from colorectal cancer. Clin Exp Metastasis. 2016;33:727–739. doi: 10.1007/s10585-016-9813-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537:417–421. doi: 10.1038/nature19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hornquist E, Grdic D, Mak T, Lycke N. CD8-deficient mice exhibit augmented mucosal immune responses and intact adjuvant effects to cholera toxin. Immunology. 1996;87:220–229. doi: 10.1046/j.1365-2567.1996.473536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shalapour S, Font-Burgada J, Di Caro G, Zhong Z, Sanchez-Lopez E, Dhar D, Willimsky G, Ammirante M, Strasner A, Hansel DE, et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature. 2015;521:94–98. doi: 10.1038/nature14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shalapour S, Lin XJ, Bastian IN, Brain J, Burt AD, Aksenov AA, Vrbanac AF, Li W, Perkins A, Matsutani T, et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature. 2017;551:340–345. doi: 10.1038/nature24302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lampropoulou V, Hoehlig K, Roch T, Neves P, Calderón Gómez E, Sweenie CH, Hao Y, Freitas AA, Steinhoff U, Anderton SM, Fillatreau S. TLR-activated B cells suppress T cell-mediated autoimmunity. J Immunol. 2008;180:4763–4773. doi: 10.4049/jimmunol.180.7.4763. [DOI] [PubMed] [Google Scholar]
- 43.Ayala VI, Deleage C, Trivett MT, Jain S, Coren LV, Breed MW, Kramer JA, Thomas JA, Estes JD, Lifson JD, Ott DE. CXCR5-dependent entry of CD8 T cells into rhesus macaque B-Cell follicles achieved through T-cell engineering. J Virol. 2017;91 doi: 10.1128/JVI.02507-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li S, Folkvord JM, Rakasz EG, Abdelaal HM, Wagstaff RK, Kovacs KJ, Kim HO, Sawahata R, MaWhinney S, Masopust D, et al. Simian immunodeficiency virus-producing cells in follicles are partially suppressed by CD8+ cells in vivo. J Virol. 2016;90:11168–11180. doi: 10.1128/JVI.01332-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.