Abstract
Vitamin D is responsible for regulation of calcium and phosphate metabolism and maintaining a healthy mineralized skeleton. It is also known as an immunomodulatory hormone. Experimental studies have shown that 1,25-dihydroxyvitamin D, the active form of vitamin D, exerts immunologic activities on multiple components of the innate and adaptive immune system as well as endothelial membrane stability. Association between low levels of serum 25-hydroxyvitamin D and increased risk of developing several immune-related diseases and disorders, including psoriasis, type 1 diabetes, multiple sclerosis, rheumatoid arthritis, tuberculosis, sepsis, respiratory infection, and COVID-19, has been observed. Accordingly, a number of clinical trials aiming to determine the efficacy of administration of vitamin D and its metabolites for treatment of these diseases have been conducted with variable outcomes. Interestingly, recent evidence suggests that some individuals might benefit from vitamin D more or less than others as high inter-individual difference in broad gene expression in human peripheral blood mononuclear cells in response to vitamin D supplementation has been observed. Although it is still debatable what level of serum 25-hydroxyvitamin D is optimal, it is advisable to increase vitamin D intake and have sensible sunlight exposure to maintain serum 25-hydroxyvitamin D at least 30 ng/mL (75 nmol/L), and preferably at 40–60 ng/mL (100–150 nmol/L) to achieve the optimal overall health benefits of vitamin D.
Keywords: vitamin D; immune function; 25-hydroxyvitamin D; 1,25-dihydroxyvitamin D; immunomodulation; autoimmune disorders; infectious diseases; lymphocytes; monocytes; macrophages; multiple sclerosis; type 1 diabetes; inflammation; endothelial membrane stability
1. Introduction
Vitamin D is classically known to regulate calcium and phosphate metabolism. It not only plays an essential role in maintaining healthy mineralized skeleton, but also is an immunomodulatory hormone [1,2]. Both the vitamin D receptor (VDR) and metabolizing enzymes are expressed by various types of immune cells including lymphocytes, monocytes, macrophages, and dendritic cells [3,4]. Experimental studies have shown that vitamin D has significant biologic activities on the innate and adaptive immune systems. Animal studies have demonstrated that administration of vitamin D or its metabolites leads to changes in the occurrence and progression of various immune-related diseases [1,5]. This supports the clinical and epidemiological data that link vitamin D with the incidence and severity of many disorders such as psoriasis, multiple sclerosis, rheumatoid arthritis, type 1 diabetes, and infectious diseases [2] (Figure 1). The purpose of the present review is to provide a high-level summary of the biologic effects of vitamin D on the immune system and the relationship between vitamin D and several types of immune-related diseases and conditions. This review also aims to give some perspective about the heterogeneity of evidence on the impact of vitamin D on prevention and treatment of immune-related diseases and to introduce the concept individual responsiveness to vitamin D as a potential explanation for such heterogeneity.
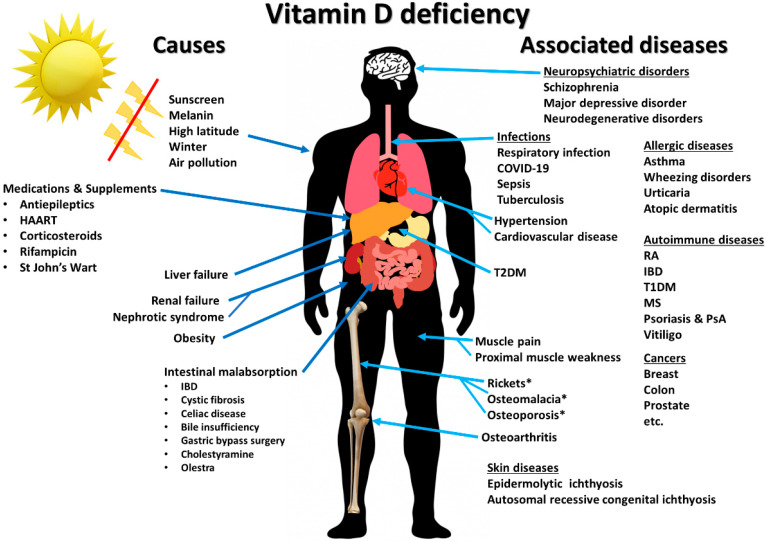
Figure 1.
Summary of causes of vitamin D deficiency and diseases and disorders associated with vitamin D deficiency. Abbreviations: HARRT: highly active antiretroviral therapy; IBD: inflammatory bowel diseases; MS: multiple sclerosis; PsA: psoriatic arthritis; T1DM: type 1 diabetes mellitus; T2DM: type 2 diabetes mellitus; RA: rheumatoid arthritis. Reproduced with permission from Holick MF, copyright 2020. ‘*’ denotes diseases that are direct consequences of vitamin D deficiency.
2. Physiology of Vitamin D
2.1. Sources, Synthesis, and Metabolism of Vitamin D
Human gets vitamin D from sunlight, diet, and supplements. There are two major forms of vitamin D: vitamin D2 and vitamin D3. Vitamin D2 is synthesized from ergosterol and found in yeast, sun dried and ultraviolet irradiated mushrooms, and plants. Vitamin D3 is synthesized endogenously from 7-dehydrocholesterol in the skin and found naturally in cod liver oil and oily fish. After entering the circulation, vitamin D (D represents either or both vitamin D2 and vitamin D3) is metabolized by the vitamin D-25-hydroxylase (CYP2R1) in the liver to 25-hydroxyvitamin D [25(OH)D]. 25(OH)D is further metabolized by the enzyme 25-hydroxyvitamin D-1α-hydroxylase (CYP27B1) to the active form, 1,25-dihydroxyvitamin D [1,25(OH)2D] [2,6]. 1,25(OH)2D exerts its physiologic functions in the target tissue by binding to the vitamin D receptor (VDR) in the nucleus where it leads to up- or down-regulation of a multitude of genes [7]. It should be noted that the main site of conversion of 25(OH)D into the systemically bioavailable 1,25(OH)2D is the kidneys. CYP27B1 is also expressed by many other tissues including activated macrophages, parathyroid glands, microglia, breast, colon, and keratinocytes where 1,25(OH)2D is produced and exerts its autocrine and paracrine functions [1,4,5].
2.2. Skeletal Effects of Vitamin D
1,25(OH)2D regulates calcium and phosphate homeostasis by acting on the small intestine, kidneys, and bone. It promotes bone mineralization passively by inducing intestinal calcium and phosphate absorption and renal tubular calcium reabsorption that help maintain an adequate calcium-phosphate product that crystallizes in the collagen matrix. 1,25(OH)2D also has direct effects on the bone, including inducing the expression of osteocalcin, the major non-collagenous protein in the skeleton, and stimulating receptor activator of nuclear factor kappa-B (RANK)-dependent bone resorption [2,6]. In addition, 1,25(OH)2D forms an endocrine system together with parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF23) to regulate calcium and phosphate homeostasis [8]. As a part of negative feedback loops, 1,25(OH)2D directly inhibits PTH production, leading to a decrease in bone resorption and increased urinary calcium excretion, and induces FGF23 production by the osteocytes, leading to an increase in urinary phosphate excretion [6,8,9].
2.3. Healthy Serum Vitamin D and 25-Hydroxyvitamin D Levels
It is still controversial how much vitamin D is needed, how it should be given, i.e., daily versus weekly or monthly (bolus doses), and what level of serum 25(OH)D is optimal for immune health and overall health benefits [10,11]. It is also unknown whether maintenance of serum vitamin D itself has its own effect on modulating immune function. However, historical evidence suggests that our hunter gatherer forefathers maintained their circulating vitamin D levels in the range of 10–50 ng/mL (25–125 nmol/L). Indigenous populations such as Maasai herders and Hadza tribesmen were found to have serum 25(OH)D levels in the range of 40–60 ng/mL (100–150 nmol/L) [12,13,14]. These levels are consistent with those reported in populational studies to be associated with the lowest risk of several types of cancers, cardiovascular diseases, autoimmune diseases, and all-cause mortality [2,10,14,15]. To maintain these blood levels with minimal sunlight exposure, a person would require ingestion of 4000–6000 IUs of vitamin D daily, which would maintain serum vitamin D levels in the range of 20–40 ng/mL (50–100 nmol/L) and serum 25(OH)D levels in the range of 40–60 ng/mL (50–100 nmol/L) [11]. The recommended dosage for vitamin D intake by the Endocrine Society Guidelines on Vitamin D for treatment and prevention for vitamin D deficiency is shown in Table 1.
Table 1.
The recommended dosage for vitamin D intake in individuals who are at risk for vitamin D deficiency and dosage of vitamin therapy treatment for patients with vitamin D deficiency.
| Age Group | For Individuals at Risk for Vitamin D Deficiency | Treatment for Patients with Vitamin D Deficiency |
|
|---|---|---|---|
| Daily Requirement | Upper Limit | ||
| 0–1 years | 400–1000 IU | 2000 IU |
|
| 1–18 years | 600–1000 IU | 4000 IU |
|
| >18 years | 1500–2000 IU | 10,000 IU |
|
| Obese and malabsorptive patients | 4000–6000 IU | 10,000 IU |
|
Adapted from Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guidelines [11].
3. Effects of Vitamin D on Innate Immunity
3.1. Macrophages and Monocytes
Historical evidence that links vitamin D with innate immunity came from the reports in the mid-1800s and early 1900s prior to the antibiotic era that vitamin D3-rich cod liver oil and sunlight exposure were used for treatment of tuberculosis (TB) [16]. Later, subsequent studies have identified the explanation for the therapeutic effects of cod liver oil and sunlight. In the presence of infection, activated macrophages and monocytes, induced by toll-like receptor signaling and exposure to inflammatory cytokines such as interferon-Ƴ (IFN-Ƴ), strongly express CYP27B1 which converts 25(OH)D into 1,25(OH)2D [17]. Then, 1,25(OH)2D enhances antimicrobial activities of macrophages and monocytes in an autocrine fashion via VDR-RXR signaling, which, in turn, stimulates the production of endogenous antimicrobial cathelicidin LL-37 [1,2,5,17,18]. Cathelicidin acts against invading bacteria and fungi by destabilizing microbial membranes [19,20]. It also exhibits direct antiviral activities against many respiratory viruses by disrupting viral envelopes and altering viability of host target cells [20,21,22]. This process is especially robust in granulomatous inflammation such as TB, fungal infections, sarcoidosis, and some lymphomas. The macrophage production of 1,25(OH)2D not only is for the purpose of upregulating the production of cathelicidin LL-37 but also produces it so that it can exit the cell and influence nearby lymphocyte function [2] (Figure 2). However, an unintended consequence of this paracrine function is that the macrophages produce an excess amount of 1,25(OH)2D that enters the circulation and an unregulated fashion stimulates intestinal calcium absorption and bone calcium mobilization resulting in hypercalciuria and hypercalcemia [23,24]. This is more likely to occur when circulating levels of 25(OH)D are above 30 ng/mL (75 nmol/L) [11], which is the explanation for why patients with granulomatous disorders including sarcoidosis develop hypercalcemia during the summer [25].
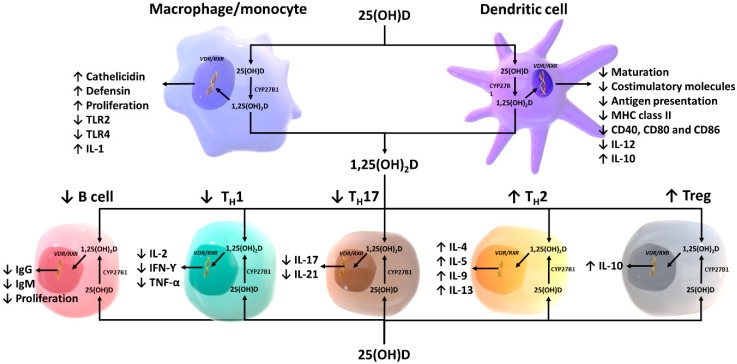
Figure 2.
Schematic representation of paracrine and intracrine function of vitamin D and its metabolites and actions of 1,25-dihydroxyvitamin D on the innate and adaptive immune systems. Abbreviation: 1,25(OH)2D: 1,25-dihydroxyvitamin D; 25(OH)D: 25-hydroxyvitamin D, IFN-Ƴ: interferon- Ƴ; IL: interleukin; MHC: membrane histocompatibility complex, TH1: T helper 1; TH2: T helper 2; TH17: T helper 17; Treg: regulatory T cell, TNF-α: Tumor necrosis factor- α; TLR2: toll-like receptor 2; TLR4: toll-like receptor 4. Reproduced with permission from Holick MF, copyright 2020.
3.2. Antigen-Presenting Cells and Natural Killer Cells
1,25(OH)2D modulates the differentiation and functions of antigen-presenting cells by inducing them to become more immature and tolerogenic, characterized by a decrease in the expression of major histocompatibility complex (MHC) class II and co-stimulatory molecules on the cell surface [26,27,28,29] (Figure 2). This results in a decrease in antigen presentation and production of interleukin-12 (IL-12), and an increase in production of IL-10, a tolerogenic cytokine [29,30,31]. 1,25(OH)2D is also shown to suppress the expression of toll-like receptors on the monocytes and inhibit the production of some inflammatory cytokines such as IL-2, IL-6, and IL-17 [5,32,33]. In addition, experimental studies have suggested that differentiation and function of natural killer (NK) cells can be modulated by 1,25(OH)2D treatment. However, whether 1,25(OH)2D induces or inhibits NK cell function is still unclear as data regarding the influence of 1,25(OH)2D on NK cells are inconsistent [34,35,36].
3.3. Endothelial Function and Vascular Permeability
A number of experimental studies have shown that vitamin D and its metabolites modulate endothelial function and vascular permeability via multiple genomic and extra-genomic pathways. For instance, Gibson et al. [37] demonstrated in the primary dermal human microvascular endothelial cell model that vitamin D3, 25(OH)D3 and 1,25(OH)2D3 non-genomically stabilized vascular endothelium, and that vitamin D3, normally circulating at about 100 times higher level than 1,25(OH)2D3, was at least 10 times more potent than 1,25(OH)2D3 and more than thousand times more potent than 25(OH)D3 in stabilizing the endothelium. Studies have also shown that 1,25(OH)2D3 is a transcriptional regulator of endothelial nitric oxide synthase (eNOS), causing up-regulation of eNOS gene expression and therefore increased endothelial production of nitric oxide [38,39]. Interestingly, Molinari et al. [40] observed that the effect of 1,25(OH)2D3 on endothelial nitric oxide production occurred most robustly within one minute after administrating the compound, implying that the action of 1,25(OH)2D3 was non-genomic. Activation of VDR by 1,25(OH)2D at the endothelial cell membrane is shown to increase eNOS activity via intracellular second messenger pathways including adenylyl cyclase/cyclic adenosine monophosphate (AC/cAMP) and inositol trisphosphate/diacylglycerol (IP3/DAG) pathways, which result in increased intracellular calcium concentration. Activation of VDR also activates eNOS via the phosphoinositide 3-kinase/protein kinase b (PI3K/Akt) pathway that triggers phosphorylation of serine-1779 on eNOS [41]. Furthermore, Cuenca et al. [42] showed in a uremic rat model using the immunofluorescence technique that 1,25(OH)2D2 promoted vascular endothelial-cadherin-based cell–cell junctions and inhibited F-actin stress fiber organization, thereby preventing the formation of endothelial intracellular gaps and attenuating endothelial damage in the presence of chronic kidney disease. Taken together, it is evident that vitamin D and its metabolites exert pleiotropic effects on the vascular endothelium that are protective against vascular dysfunction and tissue injury as a result of local and systemic inflammation.
3.4. Intestinal Epithelium and Paneth Cells
Multiple studies have shown that vitamin D plays a role in maintaining gut integrity and intestinal homeostasis between host and gut microbiota. Vitamin D signaling is shown to increase the viability of intestinal epithelial cells and and alleviate intestinal epithelial damages from bacterial lipopolysaccharide [43,44]. It promotes mucosal barrier function by enhancing the expression of intracellular pathogen recognition proteins and epithelial membrane junction proteins [45,46]. Moreover, 1,25(OH)2D induces the production and secretion of antimicrobial peptides by the intestinal epithelial cells, Paneth cells, and intraepithelial lymphocytes [47,48]. These result in limitation of gut bacterial translocation into the interstitium and maintenance of intestinal homeostasis, which are believed to involve in the pathogenesis of multiple autoinflammatory and metabolic disorders [49,50].
4. Effects of Vitamin D on Adaptive Immunity
4.1. T lymphocytes
It was originally observed that a human monocyte cell line U937, clonal human T cells, and peripheral human blood mononuclear cells express VDR [51,52]. Further analysis revealed that peripheral blood monocytes express VDR but that resting T lymphocytes did not. When resting T lymphocytes were stimulated with phytohemagglutinin, they became activated and with the activation, T cells expressed the VDR [51,52]. Moreover, activated T lymphocytes are known to express CYP27B1 that mediates local conversion of 25(OH)D into 1,25(OH)2D which is believed to stimulate intracrine activation of VDR [53,54] (Figure 2).
In general, 1,25(OH)2D produced locally by monocytes/macrophages results in a dramatic shift of immune status from proinflammatory state to tolerogenic state. 1,25(OH)2D suppresses the proliferation of T lymphocytes, and modulates cytokine production and differentiation with diverse effects on different subgroups of T lymphocytes [55]. It promotes a shift from TH1 and TH17 to TH2 immune profile by suppressing the expression of TH1 (IL-2, IFN-Ƴ, TNF-α) and TH17 (IL-17, IL-21) cytokines while inducing the expression of TH2 cytokines (IL-4, IL-5, IL-9, IL-13) [56,57,58]. 1,25(OH)2D can also promote differentiation of regulatory T cells (Treg) both directly and indirectly via its interaction with antigen-presenting cells, resulting in a suppression of proinflammatory state [59,60]. This is believed to be one of the explanations by which vitamin D could exert protective effects against autoimmune diseases.
Similar to T helper cells, cytotoxic T lymphocytes (CTL) express both CYP27B1 and VDR, and upregulation of VDR can be observed in response to infection as well as mitogen stimulation, suggesting a coordinate regulation of VDR signaling pathway and CTL responses [61,62]. Studies have shown that decreased CD4/CD8 ratio, an indicator of increased immune activation, was associated with low levels of 25(OH)D [63] and that giving 5000–10,000 IUs of vitamin D3 was associated with an increase in CD4/CD8 ratio, reflecting immune suppression [64,65]. However, little is known about the direct influence of vitamin D on CTL. The effects of 1,25(OH)2D on differentiation, proliferation, and functions of CTL are likely mediated by both direct intracrine activation of VDR and alteration of cytokines signaling via T helper cells and antigen-presenting cells [55,62].
4.2. B lymphocytes
Inactive B lymphocytes have no VDR, and only when they become activated to proliferate by mitogens do they upregulate their VDR expression [66] (Figure 2). Initially, it was found that 1,25(OH)2D inhibited immunoglobulin synthesis and therefore could potentially be detrimental to the immune system. However, a variety of studies have demonstrated that just as 1,25(OH)2D modulates T cell function so too does it regulate B-cell activity. When in a hyperactive state, 1,25(OH)2D appears to dampen the immunoglobulin immune response by a variety of mechanisms. It inhibits plasma cell formation and inducing apoptosis of both activated B cells and plasma cells [67,68]. It also inhibits cytokine-mediated B-cell activation by acting on T-helper cells, directly promotes B-cell anti-inflammatory cytokines production (IL-10, CCR10) and suppresses the differentiation from mature B cells to plasma cells and class-switched memory B cells [66,69,70]. It is believed that, by controlling B-cell activity and B-cell transformation into the plasma cells, 1,25(OH)2D helps reduce autoantibody production, thereby reducing risk for antibody-mediated autoimmune disorders such as systemic lupus erythematosus [66,71]. It has also been suggested that lymphocytes also have the capacity to generate 1,25(OH)2D. What is known is that only B lymphocytes can express CYP27B1 and that 25(OH)D in vitro at 25 times the level of 1,25(OH)2D can cause similar biologic responses as 1,25(OH)2D in B lymphocytes [66]. However, there has not been any direct demonstration that B lymphocytes can produce 1,25(OH)2D.
5. Vitamin D and Immune-Related Diseases
5.1. Psoriasis
1,25(OH)2D was well-documented to be a very effective hormone in inhibiting proliferation and inducing terminal differentiation of a wide variety of cultured malignant cells including prostate cancer, colon cancer, breast cancer, and leukemic cells [2]. However, 1,25(OH)2D and its analogs have never been successfully developed for treating any cancer. An attempt was made to treat preleukemia; however, not only did 1,25(OH)2D3 cause undesirable hypercalcemia, but also ultimately the leukemic cells became resistant to the antiproliferative activity [72]. Psoriasis, however, is a non-malignant hyperproliferative disorder of the skin that is a chronic inflammatory disorder affecting 2–3% of global population [73]. It has been well documented that the epidermis is the major site for the cutaneous production of vitamin D3 [74]. It was also recognized that keratinocytes express VDR and 1,25(OH)2D was extremely effective at physiologic concentrations not only in inhibiting cultured keratinocyte proliferation, but also in inducing their terminal differentiation [75,76]. These observations gave rise to the idea that 1,25(OH)2D could be developed for treating this hyperproliferative skin disorder [77].
It had been previously observed that cultured dermal fibroblasts from psoriatic patients had a partial resistance to the antiproliferative activity of 1,25(OH)2D [78]. Further studies revealed that only about 20% of patients with psoriasis had fibroblasts with a partial resistance to the antiproliferative activity of 1,25(OH)2D3. It was observed that cultured keratinocytes from psoriasis patients responded to the antiproliferative and pro-differentiating activity of 1,25(OH)2D3 as well as cultured keratinocytes obtained from neonatal foreskins [79]. This led to a pilot study to evaluate topical and oral 1,25(OH)2D3 to determine its clinical effectiveness [79]. However, there was concern about the hypercalcemic potential of clinically using 1,25(OH)2D3. This prompted the development of an analog of 1,25(OH)2D3 that was considered to have less calcemic activity. This analog, calcipotriene (50 mcg/g), was found to be reasonably effective when topically applied for treating psoriasis [80]. No significant hypercalcemia was observed unless large body surface was treated. However, the major side effect was skin irritation that occurred mainly on the face and therefore it was not recommended for treating psoriasis on the face and other sensitive skin areas [80]. 1,25(OH)2D3 was demonstrated to be effective at 15 mcg/g and also was found to be safe and effective and did not cause skin irritation. 1,25(OH)2D3 was finally developed as a topical treatment and formulated as 3 mcg/g [77]. Other analogs have since been developed and are available for treating psoriasis skin lesions that usually affect less than 10% of the body surface. Oral 1,25(OH)2D3 was also found to be safe and effective but was not developed as a pharmaceutical because of concerns about hypercalcemia.
It is now recognized that there is a strong immunologic component to psoriasis. The hyperproliferation of keratinocytes is associated with TH1, TH17, and TH22 inflammatory response to self-antigen [81]. 1,25(OH)2D exerts inhibitory effects against the inflammatory activity associated with psoriasis not only by suppressing dendritic cell’s differentiation, chemotaxis, and antigen presentation, but also by inhibiting the production of several pro-inflammatory cytokines such as IL-1β, IL-6, IL-8, and TNF-α [82].
Vitamin D deficiency, defined by serum 25(OH)D <20 ng/mL (50 nmol/L), has been reported as one of the independent risk factors for psoriasis. Multiple observational studies found a higher prevalence of vitamin D deficiency among patients with psoriasis compared with the general population, even after adjusting for confounders in a multivariate analysis [82,83]. The likely explanation is that patients with psoriasis are less likely to expose their skin to sunlight which is a major source of vitamin D. However, correcting vitamin D deficiency may be beneficial.
Multiple clinical trials have attempted to investigate the influence of different forms of vitamin D supplementation on treatment of psoriasis, yielding varying results. A six-month study of psoriasis patients who received 60,000 IUs of vitamin D2 once every 2 weeks not only showed significant improvement in their PASI (psoriasis area and severity index) score but also demonstrated a direct association with improvement in blood levels of 25(OH)D [84]. Another pilot study giving daily 35,000 IUs of vitamin D3 to 9 patients with psoriasis and 16 patients with vitiligo who were vitamin D-deficient or insufficient for six months demonstrated a significant improvement in psoriasis area and severity index (PASI) in all psoriatic patients and 25–75% repigmentation in 14 of 16 vitiligo patients, without development of any complications suggestive of vitamin D toxicity including hypercalcemia, kidney stones, nephrocalcinosis, or hypercalciuria [85]. On the other hand, a one-year study giving 100,000 IUs per month of vitamin D3 or placebo to psoriatic patients for one year did not demonstrate a direct benefit of vitamin D supplementation on disease activity [86]. The study however revealed that the severity of psoriasis was less for those with higher blood levels of 25(OH)D [86]. Based on the evidence from these studies, it is advisable to test and treat patients with psoriasis for vitamin D deficiency in order to maintain their serum 25(OH)D levels in a preferred range of 40–60 ng/mL (100–150 nmol/L) [11].
In addition, oral administration of 1,25(OH)2D3 has been shown to have some therapeutic effects on psoriasis. A long-term follow-up study demonstrated the efficacy and safety of oral calcitriol as a potential treatment of psoriasis. Of the 85 patients included in that study that received oral calcitriol for 36 months, 88.0% had some improvement in their disease, whereas 26.5%, 26.3%, and 25.3% had complete, moderate, and slight improvement in their disease, respectively. Serum calcium concentrations and 24-h urinary calcium excretion increased by 3.9% and 148.2%, respectively, but were not outside the normal range. Bone mineral density of these patients remained unchanged. A very important consideration for the use of orally administration is the 1,25(OH)2D3 dosing technique. To avoid its effects on enhancing dietary calcium absorption, it is very important to provide 1,25(OH)2D3 at night time. Perez et al. [87] showed that as a result of this dosing technique, doses of 2–4 µg/night of 1,25(OH)2D3 were well tolerated by psoriatic patients. Ezquerra et al. demonstrated that a combination of acitretin and oral 1,25(OH)2D3 resulted in a faster reduction of PASI score in patients of chronic plaque psoriasis than acitretin alone [88]. Given the small sample sizes in each study, more studies with a larger number of participants are warranted to demonstrate the efficacy of oral 1,25(OH)2D for treatment of psoriasis.
5.2. Type 1 Diabetes
Finland has the highest incidence of type 1 diabetes in the world [89]. Many theories have been proposed to explain the epidemic such as exposure to certain toxic substances [90] and coxsackievirus B infections that trigger the development of autoimmunity [91]. It is however likely that this can be explained by the lack of sunlight exposure resulting in a high rate of vitamin D deficiency in Finnish population especially in northern Finland. Finns, similar Norwegians living in the far north, are unable to produce vitamin D3 in their skin from sun exposure for more than half a year during the winter and early spring and late fall [13,92,93]. This is also supported by the previous report that type 1 diabetes is more common in countries with high latitude and short daytime period [94].
Although not completely understood, type 1 diabetes is believed to be mediated by the development of by autoantibody as well as autoreactive TH1 and CTL, causing immune-associated destruction of insulin-producing pancreatic β cells [95,96]. Administration of 1,25(OH)2D3 was found to enhance Treg and inhibit TH1, leading to a reduction in the incidence of type 1 diabetes in the non-obese diabetic mouse model [60]. In addition, 1,25(OH)2D3 promoted insulin secretion directly by its interaction with VDR in the pancreatic β cells [97]. These mechanisms help explain how vitamin D has a potential protective and therapeutic role in reducing risk for developing type 1 diabetes and support observational studies that increasing vitamin D intake for children was associated with a lower risk for developing type 1 diabetes [98].
The EURODIAB multicenter case-control study in 825 type 1 diabetes patients and 2335 controls revealed that vitamin D supplementation of any dose was associated with a reduced risk of type 1 diabetes (odds ratio, 0.67; 95%CI: 0.53–0.86) [98]. A Finnish cohort study of 10,366 children showed that those who were given daily 2000 IUs of vitamin D3 during their first year of life had a 88% reduction in the risk of developing type 1 diabetes (relative risk, 0.22; 95% CI: 0.05–0.89) [99].
Evidence from randomized controlled trials (RCTs) has suggested that vitamin D supplementation in the form of 1α-hydroxyvitamin D3 [1α(OH)D3] and vitamin D3 appeared to be of benefit in the treatment of type 1 diabetes. These treated patients had a significant reduction in daily insulin dose as well as an increase in fasting and stimulated C-peptide levels [100,101,102,103]. On the other hand, studies that evaluated the benefit of maternal vitamin D supplement for prevention of type 1 diabetes in offspring were unable to demonstrate the association [104]. This is not unexpected especially if this autoimmune disorder is initiated during childhood and not in utero.
In summary, increasing vitamin D intake in early childhood to maintain serum 25(OH)D in the optimal range seems to be protective against the development of type 1 diabetes. Supplementation of vitamin D, although not curative, tends to help control the disease activity. However, there is still no evidence for the long-term effects of vitamin D supplementation on morbidities and mortalities in type 1 diabetes patients.
5.3. Multiple Sclerosis
The prevalence of multiple sclerosis (MS) is higher in countries with higher latitudes where people are more susceptible to vitamin D deficiency similar to what has been observed in type 1 diabetes [105]. Living below the latitude of 35° for the first 10 years of life is associated with approximately 50% lower risk of developing MS [106]. Munger et al. reported in a prospective nested case-control study of 148 MS patients and 296 controls that the risk of MS decreased by 41% for every 20 ng/mL (50 nmol/L) increase in serum 25(OH)D levels above 24 ng/mL (60 nmol/L) (odds ratio, 0.59; 95% CI, 0.36–0.97) [107]. The same group also showed that women who ingested more than 400 IUs of vitamin D per day had a 41% decreased risk of developing MS [108]. It is therefore believed that vitamin D deficiency plays a role in the development of dysregulated T helper cells, CTL, NK cells, B cells resulting in autoinflammation of the central nervous system that damages neurons and oligodendrocytes seen in MS [109,110].
Individuals carrying certain human leukocyte antigen (HLA) alleles such as HLA-DRB1*1501 have a significantly higher risk of developing MS [111]. Interestingly, vitamin D response elements have been identified in the promoter region of the HLA-DRB1 gene, and its expression can be altered by activation of VDR by 1,25(OH)2D, strengthening the link between vitamin D and MS [112,113].
It is conceivable that many actions of 1,25(OH)2D on the immune system are similar to mechanisms described for interferon-beta, an immunomodulatory agent used for treatment of MS, implying the possible therapeutic role of vitamin D in MS. Although the results from existing RCTs are conflicting, some studies showed the significant benefits of high-dose vitamin D supplement (up to 14,000 IUs/day) alone or as an add-on therapy in decreasing relapse rate and improving inflammatory markers and MRI findings in MS patients [114]. Most of the existing clinical trials included relatively small number of patients, and dosages of vitamin D given for treatment vary significantly across the studies.
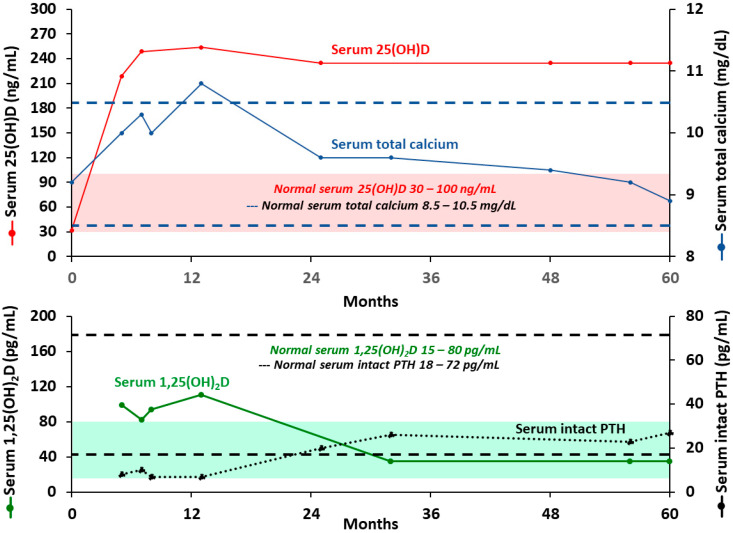
Coimbra’s clinical research program in Brazil has been conducting studies with extremely high doses of vitamin D3 for treating a variety of autoimmune disorders including among others psoriasis, vitiligo, and multiple sclerosis [85]. The authors’ (MFH) personal clinical experience that treatment with a very high dose of vitamin D supplementation (50,000 IUs/day or 1000 IUs/kg/day) to increase serum 25(OH)D level to 200–300 ng/mL (500–750 nmol/L) was found to be remarkably effective in controlling and/or improving symptoms and improving MRI findings in five MS patients who either failed to respond to or refused conventional MS therapy. The risk of hypercalcemia and hypercalciuria was minimized by advising the patients to strictly follow a zero calcium diet. This required complete elimination of all dairy products and any other foods that contain significant amounts of calcium. A 52-year-old female MS patient was treated with 40,000 IUs/day (1000 IUs/kg/day) of vitamin D3 for five years which was found to improve her neurological symptoms. She was advised to avoid all dietary sources of calcium. Serum 25(OH)D was maintained at approximately 250 ng/mL (625 nmol/L) and her total calcium levels transiently increased at approximately 12 months. A review of her diet had revealed that she was eating some vegetables that contained a significant amount of calcium. This supports that calcium was eliminated from her diet and her serum calcium returned to normal levels and have been maintained in a normal range for 5 years. During the first year, serum PTH levels were in the low normal range and serum 1,25(OH)2D levels were above the normal range. After the change in her diet both returned into the normal range and have been maintained in the normal range (Figure 3). No hypercalciuria, kidney stones, or nephrocalcinosis was observed. Figure 4 demonstrates changes in serum levels of calcium and calciotropic hormones in a 32-year-old male who refused conventional therapy and was given 54,000 IUs vitamin D3 daily. He was able to rapidly increase his circulating level of 25(OH)D and reach a plateau of approximately 250 ng/mL (625 nmol/L) within 2 months. This has been maintained for 4 months and the serum PTH and 1,25(OH)2D remained normal as well as his 24-h urine calcium excretion.
Figure 3.
Biochemical measurements of a 52-year-old multiple sclerosis female who received 40,000 international units per day of vitamin D3 for 5 years. Abbreviation: 1,25(OH)2D: 1,25-dihydroxyvitamin D; 25(OH)D: 25-hydroxyvitamin D; PTH: parathyroid hormone. Note: Red solid line represents serum 25-hydroxyvitamin D levels. Blue solid line represents serum total calcium levels. Green solid line represents serum 1,25-dihydroxyvitamin D levels (15–80 pg/mL). Black dotted line represents serum intact parathyroid hormone levels (18–72 pg/mL). Red highlight represents normal range for serum 25-hydroxytamin D (30–100 ng/mL/75–250 nmol/L). Green highlight represents normal range for serum 1,25-dihydroxyvitamin D (15–80 pg/mL). Blue dashed line represents normal range for serum total calcium (8.5–10.5 mg/dL). Black dashed line represents normal range for serum intact parathyroid hormone (18–72 pg/mL). Reproduced with permission from Holick MF, copyright 2020.
Figure 4.
Biochemical measurements of a 32-year-old multiple sclerosis male who received 54,000 international units per day of vitamin D3 for 4 months. Abbreviation: 1,25(OH)2D: 1,25-dihydroxyvitamin D; 25(OH)D: 25-hydroxyvitamin D; PTH: parathyroid hormone. Note: Red solid line represents serum 25-hydroxyvitamin D levels. Blue solid line represents serum total calcium levels. Red highlight represents normal range for serum 25-hydroxytamin D (30–100 ng/mL/75–250 nmol/L). Blue dashed line represents normal range for serum total calcium (8.5–10.5 mg/dL). Normal range for serum intact parathyroid hormone is 10–72 pg/mL. Normal range for serum 1,25-dihydroxyvitamin D is 15–80 pg/mL. Reproduced with permission from Holick MF, copyright 2020.
To date, there is no RCT that investigate the efficacy and safety of this massive dose of vitamin D supplement (1000 IUs/kg/day) for MS treatment. Supplementation of lower doses of vitamin D (up to 14,000 IUs/day) appears to have some benefits in controlling the disease activity although limited evidence. What is known is that maintaining sufficient vitamin D intake and serum 25(OH)D level in a healthy range may reduce the risk of developing MS. Further studies are required before this treatment strategy can be implemented in the general clinical practice.
5.4. Inflammatory Bowel Diseases
It is well recognized that patients with inflammatory bowel diseases (IBD) are more prone to vitamin D deficiency, causing them to have a higher risk of osteomalacia, osteoporosis, and fragility fractures [115,116,117,118]. This is because they are unable to efficiently form micelles and chylomicron to absorb vitamin D in their gastrointestinal tract [115,116]. Therefore, these patients should be screened for vitamin D deficiency and treated with a higher dose of vitamin D to achieve a normal serum 25(OH)D level of at least 30 ng/mL (75 nmol/L) [11]. Emerging evidence suggests that the relationship between vitamin D status and IBD could be bidirectional. Data from two prospective Nurses’ Health Studies showed that nurses living in lower latitudes had a consistently lower risk of developing IBD than those in higher latitudes [119]. These observations were supported by a prospective cohort study of 72,719 women enrolled in the Nurses’ Health Study showing that the highest quartile of predicted serum levels of 25(OH)D was associated with 46% reduced risk of Crohn’s disease (CD) and 35% reduced risk of ulcerative colitis (UC) [120].
The pathogenesis of IBD involves a combination of dysfunctional innate and adaptive immunity, defective intestinal epithelial barrier, and imbalanced intestinal microbiota, causing chronic relapsing inflammatory disorder of the intestine [121,122]. CD is thought to be mainly driven by a TH1 response, while UC is associated with a TH2 response [122,123]. TH17 cells are also involved in the inflammatory response in both CD and UC [122,124]. Multiple studies reported that 1,25(OH)2D3 not only modulates T cell activity by promoting Treg and inhibiting TH1 and TH17 responses, but also maintains integrity of the intestinal mucosal barrier by enhancing the expression of epithelial membrane junction proteins and intracellular pathogen recognition proteins, and inducing the production of antibacterial substances such as angiogenin, cathelicidin, and defensin by the intestinal epithelial cells, Paneth cells, and intraepithelial lymphocytes [45,46,47,125,126].
A meta-analysis of 18 RCTs demonstrated that vitamin D supplementation in patients with IBD is associated with decreased relapse rate, supporting a therapeutic role of vitamin D as an adjunctive treatment of IBD [127]. Moreover, a recent pilot clinical trial showed that giving 380,000 IU of orally administered 25(OH)D supplementation to patients with CD increased the abundance of potential beneficial bacterial strains. [128]. Although this observation remains to be confirmed by a larger clinical trial, a RCT of healthy adults who were vitamin D deficient and received either 600, 4000, or 10,000 IUs daily for 6 months had a similar dose-dependent improvement in their gut microbiota towards genera associated with increased expansion of Treg and lower inflammatory burden [129]. Thus, improvement in the vitamin D status of patients with IBD is warranted not only for modulating the immune response but also improving their gut microbiota.
In summary, patients with IBD could not effectively absorb vitamin D and therefore require a 2–3 times higher dose of vitamin D supplementation to achieve normal serum 25(OH)D levels. Adequate supplementation of vitamin D in IBD is not only required to reduce the risk of osteoporosis, osteomalacia, and fragility fracture, but also considered as an adjunctive immunomodulatory agent that has been shown to improve the disease activity.
5.5. Rheumatoid Arthritis
Low levels of serum 25(OH)D have been shown in multiple studies to be associated with an increased risk of RA [130,131]. Merlino et al. [130] showed in a prospective cohort study that women with highest tertile of vitamin D intake had a lower risk for developing RA by 33% compared with the lowest tertile. Some studies also reported the association between low serum 25(OH)D levels and higher disease activity in RA patients [131,132,133]. Although the association could simply be explained the fact that these patients tend to have limited physical outdoor activities and sunlight exposure, vitamin D and its metabolites are believed to have a therapeutic activity against RA based on the immunologic activities of 1,25(OH)2D that suppress TH1 and TH17 responses and enhance Treg activity [134]. Overexpression of TH1 and TH17 as well as dysfunctional Treg play a crucial role in triggering chronic synovial inflammation and symmetrical polyarthritis seen in RA [135,136,137].
The efficacy of vitamin D and its metabolites as an adjunctive treatment for RA is heterogeneous across the clinical trials. Gopinath et al. [138] demonstrated in a RCT that giving 500 IUs of vitamin D3 daily along with disease-modifying anti-rheumatic drugs (DMARDs) and calcium to RA patients lead to a significantly higher pain relief than those receiving DMARDs and calcium alone. Another study by Li et al. [139] that randomized RA patients to receive 22-oxa-1,25(OH)2D3 or 1,25(OH)2D3 or placebo observed significantly decreased swollen joints and improved Health Assessment Questionnaire Disease Activity Index scores in those receiving 22-oxa-1,25(OH)2D and those receiving 1,25(OH)2D compared with the placebo group. However, the doses of 1,25(OH)2D3 and 22-oxa-1,25(OH)2D3 used in the study were reported to be 1250 µg per week, which was about 1000 times higher than the therapeutic dose of these compounds and would likely cause toxicity, thus indicating that there might be some type of error, possibly a decimal point error [139]. Other studies that used vitamin D2 [140], vitamin D3 [141,142], or 1α(OH)D3 [143] in the treatment arm failed to demonstrate the efficacy of the intervention.
Taken together, there is convincing evidence that increasing vitamin D intake to maintain serum 25(OH)D levels in a preferred range of 40–60 ng/mL (100–150 nmol/L) may reduce the risk of RA. However, there is still not enough evidence to justify whether vitamin D supplementation in any form can improve the outcomes of RA.
5.6. Tuberculosis
In the early 1900s, Finsen made an enlightening observation that exposure to sunlight dramatically improved cutaneous TB infection (lupus vulgaris) and received the Nobel Prize in 1903 for his discovery. This resulted in the use of solariums as an effective treatment for TB [13]. Nowadays, tuberculosis continues to be a major public health problem that is a leading cause of morbidity and mortality in many developing countries [144]. Latent TB is the condition in which host immune response is able to form granuloma to engulf the mycobacterium in an attempt to control its proliferation. Once the granuloma fails to limit mycobacterial proliferation, patients become symptomatic and are diagnosed with active TB [145]. Vitamin D plays an essential role in combating TB infection. Activated macrophages and monocytes in response to antigen exposure locally produce 1,25(OH)2D which then induces the production of cathelicidin, an antibacterial peptide responsible for killing infectious agents like Mycobacterium tuberculosis [1,2,5,17].
Multiple studies have reported low levels of serum 25(OH)D in patients with active TB. In a nested case-control study by Aibana et al., individuals with low serum 25(OH)D levels had a 63% increased odds of developing active TB [146]. A subsequent meta-analysis by the same group including data from seven studies showed significantly (48%) higher odds of developing TB in the vitamin D-deficient group [146]. The association between vitamin D deficiency and TB is thought to be bidirectional [146,147]. In the presence of overt granulomatous inflammation, the increase in circulating 1,25(OH)2D produced by activated macrophages and monocytes results in up-regulation of the expression of CYP24A1 encoding the enzyme 25-hydroxyvitamin D-24-hydroxylase, which, in turn, converts 25(OH)D and 1,25(OH)2D into a water-soluble inactive carboxylic acids [2,6,148]. Moreover, serum 25(OH)D levels can be affected by anti-tuberculosis and concurrent medications such as antiretroviral drugs which are commonly used for treatment of comorbid HIV infection [2,149]. On the other hand, the association could be explained by the insufficient amount of 25(OH)D substrate required for the conversion into 1,25(OH)2D to stimulate granulomatous immune response against the invading organisms.
To date, the efficacy of vitamin D supplementation as an adjunctive treatment for TB remains unclear as some RCTs demonstrated the impact of vitamin D on improving treatment outcomes such as smear conversion rate [150], culture conversion rate [151], time to sputum culture conversion [152], and improvement of clinical and radiographic findings [153], while others did not [154,155,156,157].
To sum up, vitamin D is essential for host inflammatory response to TB. Vitamin D deficiency is associated with an increased risk for developing active TB infection. However, whether supplementation of vitamin D can improve treatment outcomes for TB is still to be clarified due to differences in the results across the clinical trials.
5.7. Sepsis and Critical Illness
Sepsis, a systemic inflammatory host response to a microbial pathogen, is a major cause of death among hospitalized patients in the intensive care unit (ICU) [158]. Multiple observational studies reported the link between low level of serum 25(OH)D and the occurrence of sepsis, as well as increased morbidity, mortality, and prolonged length of stay in the ICU in septic and critically ill patients [159,160]. The relationship could be explained by the effects of 1,25(OH)2D which prevents overexpression of inflammatory cytokines and promotes anti-bacterial responses in innate immunity [1,5,161]. In addition, vitamin D3 and its metabolites can exert non-genomic actions on endothelial cells to prevent vascular leakage, which theoretically could be life-saving in septic shock [37]. It is also possible that low serum 25(OH)D levels in sepsis and critical illness could be caused by extravascular leakage of vitamin D-binding protein and increased 25-hydroxyvitamin D-24-hydroxylase activity due to systemic inflammation [162,163].
A number of RCTs have been conducted in order to investigate the impact of vitamin D on clinical and biochemical outcomes in sepsis and critically ill patients, yielding heterogeneous results. In a pilot clinical trial, giving a single enteral dose of 400,000 IUs of vitamin D3 to sepsis patients compared with placebo was shown to increase serum cathelicidin [164,165]. Whereas, the same effect was not observed when giving intravenous 2 µg of 1,25(OH)2D3 to patients with severe sepsis or septic shock [166]. A RCT gave enterally 540,000 IUs of vitamin D3 followed by monthly maintenance doses of 90,000 IU for 5 months to vitamin D-deficient, defined by serum 25(OH)D <20 ng/mL (50 nmol/L), or placebo to 475 critically ill patients and observed a significant decrease in hospital mortality in a subgroup of 200 patients with severe vitamin D deficiency (serum 25(OH)D <12 ng/mL/30 nmol/L, hazard ratio 0.56; 95% CI: 0.35–0.90) [167]. In a post-hoc analysis after excluding patients who died or were discharged within 7 days after study inclusion, vitamin D supplementation was associated with a decrease in 28-day mortality in the remaining 410 patients (odds ratio 0.58; 95% CI: 0.35–0.97) [168]. Another pilot study that gave a single dose of enteral 500,000 IUs or 250,000 IUs of vitamin D3 or placebo to 31 vitamin D-deficient mechanically ventilated ICU patients observed a decrease in hospital length of stay in the vitamin D groups compared to the placebo group [169]. Nevertheless, in a larger clinical trial that gave a single dose of enteral 540,000 IUs of vitamin D3 or placebo to 1360 critically ill patients, the impact of vitamin D3 administration on mortality and other clinical outcomes was not observed [170].
It can be concluded based on the current evidence that critically ill patients have a very high risk for vitamin D deficiency and therefore should be screened and treated for this condition. In fact, some studies have demonstrated a potential benefit on hospital outcomes in this group of patients. However, inpatient supplementation of vitamin D is still not universally accepted given the inconsistent results from clinical trials.
5.8. Respiratory Viral Infection and COVID-19
It is known that the outbreak of influenza infection is periodic and usually occurs during the wintertime at higher latitudes but is sporadic throughout the year in the tropical area [171]. One of the proposed explanations is that the seasonal outbreak could be due to a seasonal variation in circulating levels of 25(OH)D which reaches the lowest levels in the winter [172]. Several studies have supported this hypothesis as they reported the independent association between low level of serum 25(OH)D and incidence and severity of respiratory tract infection in children and adults [173,174]. A prospective cohort study in healthy adults living in New England showed a two-fold reduction in the risk of developing acute respiratory tract infection (ARI) in those with serum 25(OH)D levels of 38 ng/mL (95 nmol/L) or more [175]. A case-control study in children aged less than 2 years reported that children requiring hospitalization for ARI had significantly 1.7-times higher odds of vitamin D deficiency as compared to those with mild ARI [174]. This indicates the protective effects of sufficient vitamin D status against respiratory viral infection. Respiratory viruses enter the respiratory epithelium via the specific entry receptors where it causes cellular and tissue damages and triggers innate and adaptive immune responses, which then result in airway and systemic inflammation and, in severe cases, life-threatening sepsis or acute respiratory distress syndrome [176,177]. 1,25(OH)2D exerts anti-viral activities and modulates inflammatory response to viral infection by stimulating cathelicidin release, modulation of toll-like receptor expression and NK cells function, as well as suppressing overexpression of proinflammatory cytokines [178]. A recent meta-analysis of 25 RCTs showed that supplementation of vitamin D2 or D3 can protect against the development of acute respiratory tract infection compared with placebo (odds ratio 0.88; 95% CI: 0.81–0.96) [179].
The rise of the COVID-19 pandemic, the out-of-proportion rate of symptomatic infection, morbidity and mortality observed in African American and obese individuals suggests the possible impact of vitamin D on host response and susceptibility to the infection as obese and Black individuals are known to have an elevated risk for vitamin D deficiency [2,180,181]. Apart from the immunomodulatory and anti-viral effects, 1,25(OH)2D acts specifically as a modulator of the renin–angiotensin pathway and down-regulates the expression of angiotensin converting enzyme-2 expression, which serves as the host cell receptor that mediates infection by SARS-CoV-2 [182]. It is therefore proposed that supplementation of vitamin D can reduce the risk and severity of COVID-19 infection [183,184].
Although the efficacy of vitamin D is still unclear as the results of ongoing clinical trials are still pending, it is advisable that one should maintain adequate vitamin D intake to achieve the desirable serum 25(OH)D level of 40–60 ng/mL (100–150 nmol/L) in order to minimize the risk and severity of COVID-19 infection. It is well documented that worldwide on average approximately 40% of children and adults have circulating levels of 25(OH)D <20 ng/mL (50 nmol/L) and approximately 60% <30 ng/mL (75 nmol/L) [185]. Thus, patients presenting to the hospital with COVID-19 are likely to have vitamin D deficiency or insufficiency. It is therefore reasonable to institute as a standard of care to give at least one single dose of 50,000 of vitamin D to all COVID-19 patients as soon as possible after being hospitalized. For patients who are intubated and are being fed by a G-tube, they should be treated with a liquid form of vitamin D. Drisdol is a pediatric liquid vitamin D2 formulation that contains 8000 IUs per mL that can be given daily to these patients to treat vitamin D deficiency.
6. The Concept of Individual Responsiveness to Vitamin D
Despite the promising experimental data indicative of immunomodulatory effects of vitamin D supported by the observed association between low serum 25(OH)D level and multiple immune-related diseases, there is marked discrepancy in the results among clinical trials to determine the impact of vitamin D for treatment and prevention of most disorders. This could be explained by differences in dosage, form (vitamin D or 1,25(OH)2D or other metabolites and analogs), route of administration (oral, injection), patients characteristics including baseline levels of 25(OH)D, and outcome measurement. One of the major issues that could underpower the RCTs is that many of them were not truly controlled since control subjects were still permitted to take a certain amount of vitamin D up to a certain limit, usually 600 or 800 IUs daily [10]. It has been shown that even 600 IUs of vitamin D daily can have a significant effect on gene expression in immune cells [186].
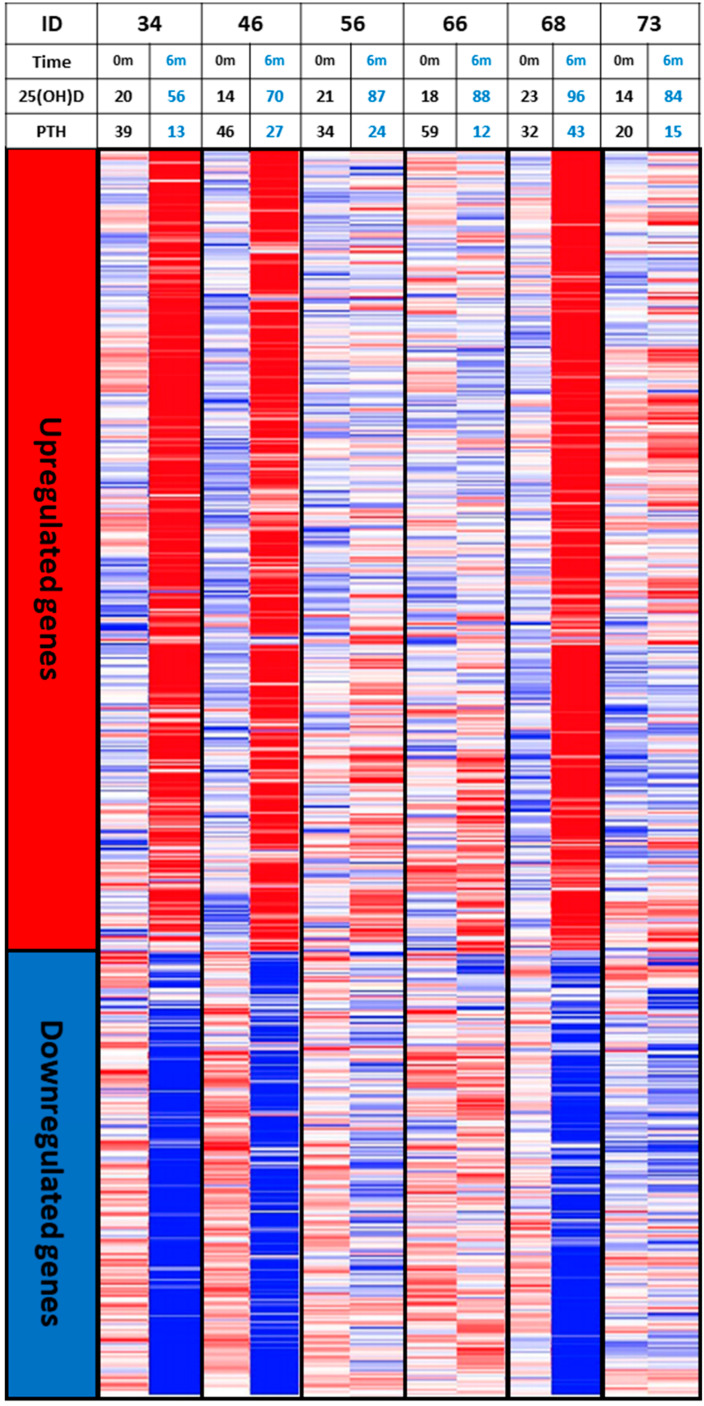
Equally important if not more is the notion that some individuals might be able to benefit from vitamin D more or less than others as high inter-individual difference in broad gene expression in human peripheral blood mononuclear cells (PBMCs) in response to vitamin D supplementation has been documented [186,187,188]. Carlberg et al. gave daily 3200 IUs of vitamin D3 to 71 prediabetic patients for 5 months and found robust changes in total gene expression in PBMCs only in about half of the subjects [187]. In a more recent clinical trial by Shirvani et al. [186], healthy adults who were vitamin D deficient and who received this same dose of vitamin D and raised their blood levels of 25(OH)D to the same degree showed marked differences in the level of expression of the same genes. This is dramatically illustrated in Figure 5 which shows that 60% of the healthy vitamin D deficient adults who received 10,000 IUs daily for 6 months had a robust response in gene expression compared to the other 40% who had minimum to modest responses even though these subjects raised their blood levels of 25(OH)D in the same range of 60–90 ng/mL (150–225 nmol/L) [186]. In addition, different patterns of serum metabolomic profile were also observed between the subjects with robust and minimum to modest responses in gene expression [186,189]. It is therefore possible that the effect of vitamin D supplementation on health outcomes at the populational level is diluted due to the possibility that some individuals might benefit from vitamin D differently from others. This may help explain the null results reported by some large RCTs aiming to investigate effects of vitamin D supplementation on non-skeletal outcomes [190,191].
Figure 5.
Heatmaps of vitamin D responsive genes whose expression response variation in 6 vitamin D-deficient subjects taking 10,000 international units per day of vitamin D3 for 6 months showing that 3 subjects had a robust response in gene expression compared to the other 3 subjects who had minimum to modest responses even though these subjects raised their blood levels of 25(OH)D in the same range of ~60–90 ng/mL (150–225 nmol/L). Abbreviation: 0m: 0 month; 6m: 6 months; 25(OH)D: 25-hydroxyvitamin D; PTH: parathyroid hormone. Reproduced with permission from Holick MF, copyright 2019.
What is responsible for determining individual responsiveness to vitamin D supplementation is still undetermined and requires further investigation. However, there are some potential genetic variations in vitamin D related pathways that might play a role and should be put into perspective. First, it has been observed that certain polymorphisms in the vitamin D receptor (VDR) gene are associated with individual risk of cardiovascular diseases, cancer, and some autoimmune disorders independent of serum 25(OH)D levels [192,193,194,195,196]. Second, only tissues that express the megalin/cubilin complex such as the kidney are able to uptake DBP-bound-25(OH)D, while most tissues can utilize only the free level [197]. Genetic polymorphisms in the vitamin D-binding protein (DBP) (GC) gene was shown to influence the ratio between circulating total and free 25(OH)D [197]. Finally, it has been demonstrated that the variations of vitamin D responsive elements (VDRE) located in the promotor regions of VDR target genes could significantly affect the binding affinity to VDR–RXR complex, resulting in varying expression of the target gene in response to VDR signaling [112,113,198]. Thus, it is possible that individuals carrying different polymorphisms of VDR or GC genes among others or VDRE in the target genes may have varying genomic responses to the comparable levels of total serum 25(OH)D and therefore might benefit from vitamin D differently.
7. Conclusions
Vitamin D plays an essential undisputed role in the maintenance of calcium, phosphate, and bone metabolism. There is compelling evidence that immune cells convert 25(OH)D to 1,25(OH)2D in an unregulated manner and are dependent on the circulating levels of 25(OH)D to be at least 30 ng/mL (75 nmol/L) [4,17,18]. Once a 1,25(OH)2D is produced, it acts in an autocrine and paracrine fashion to modulate the innate and adaptive immune systems. There is also some evidence that vitamin D itself may modulate immune function in a non-genomic manner by stabilizing endothelial membranes [36]. Most of the evidence, to date, suggests that maintenance of a healthy vitamin D status is important for modulating the body’s immune function. Low serum levels of 25(OH)D are associated with multiple immune-related diseases including autoimmune disorders and infectious diseases. There is less convincing evidence that vitamin D is an effective treatment strategy for autoimmune diseases and infectious diseases with a few exceptions documented in this review. Whether vitamin D therapy is effective as an adjunctive immunomodulatory agent for treatment of most diseases it is still controversial based on heterogeneous findings from the clinical trials.
National and international programs should be instituted to educate the public about the health benefits of vitamin D and policies to fortify commonly consumed foods with vitamin D to reduce the risk of vitamin D deficiency during pregnancy, childhood, and in young and middle-aged adults when autoimmune disorders are most prevalent. In addition, improvement in vitamin D status from birth until death may help reduce the risk of infectious diseases such as influenza and COVID-19 that can have devastating consequences especially for the elderly. However, more investigation is needed to determine who would most benefit from vitamin D, and how much vitamin D is required for its maximum health benefit based on their individual vitamin D responsive profile. It is also unknown whether giving 1,25(OH)2D3 or one of its analogs is a reasonable approach for treating autoimmune disorders and infectious diseases. Blood levels of 1,25(OH)2D3 are tightly controlled and for good reason, i.e., any significant increase in circulating levels of 1,25(OH)2D will result in an increase in intestinal calcium absorption and, when uncontrolled, this causes hypercalciuria and ultimately hypercalcemia. It is more likely that the endogenous production of 1,25(OH)2D in the immune cells including monocytes and macrophages is what is required for vitamin D to have its immunomodulatory functions.
Although most of the biologic effects of vitamin D have been related to its active metabolite there continues to be intriguing evidence that vitamin D itself may have its own biologic actions independent of its metabolism. Our hunter gatherer forefathers likely maintained serum vitamin D levels in the range of 10–50 ng/mL (25–125 nmol/L). This is supported by the observation that Maasai herders and Hadza tribesmen maintained serum 25(OH)D in the range of 40–60 ng/mL (100–150 nmol/L) [12,13]. To maintain these blood levels, a person would require ingesting approximately 4000–6000 IUs daily. This would therefore maintain circulating levels of vitamin D in the range of 20–40 ng/mL (50–100 nmol/L). The observation that in vitro vitamin D3 was much more effective than either 25(OH)D3 or 1,25(OH)2D3 in stabilizing endothelial membranes thereby reducing inflammation may help explain the interesting clinical observations that extremely high doses of vitamin D have been effective in treating or at least reducing symptoms of some autoimmune disorders including psoriasis, vitiligo, and multiple sclerosis [37,85]. The observation that children with congenital autosomal recessive ichthyosis and epidermolytic ichthyosis had a dramatic improvement in their skin disease when treated with 60,000 IUs of vitamin D once a day for 10 days adds strength to the argument that vitamin D itself may have its own important role in the maintenance of good health [199]. There are still open questions that need to be further investigated in order to take full advantage of the effect of vitamin D on the immune system for clinical practice. The bottom line is that there is no downside to increasing our intake of vitamin D to maintain serum 25(OH)D at at least 30 ng/mL (75 nmol/L), and preferably at 40–60 ng/mL (100–150 nmol/L) to achieve optimal overall health benefits of vitamin D.
Author Contributions
Conception or design of the article: N.C., M.F.H.; drafting the article: N.C.; critical revision of the article: N.C., M.F.H.; Illustration: N.C., M.F.H.; Final approval of the version to be published: N.C., M.F.H. All authors have read and agreed to the published version of the manuscript.
Funding
N.C. received the institutional research training grant from the Ruth L. Kirchstein National Research Service Award program from the National Institutes of Health.
Conflicts of Interest
N.C. has no conflicts of interest to disclose. M.F.H. was a consultant for Quest diagnostics and is a consultant for Ontometrics Inc. and is on the speaker’s Bureau for Abbott Inc.
References
- 1.Prietl B., Treiber G., Pieber T.R., Amrein K. Vitamin D and immune function. Nutrients. 2013;5:2502–2521. doi: 10.3390/nu5072502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holick M.F. Vitamin D deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Battault S., Whiting S.J., Peltier S.L., Sadrin S., Gerber G., Maixent J.M. Vitamin D metabolism, functions and needs: From science to health claims. Eur. J. Nutr. 2013;52:429–441. doi: 10.1007/s00394-012-0430-5. [DOI] [PubMed] [Google Scholar]
- 4.Adams J.S., Rafison B., Witzel S., Reyes R.E., Shieh A., Chun R., Zavala K., Hewison M., Liu P.T. Regulation of the extrarenal CYP27B1-hydroxylase. J. Steroid Biochem. Mol. Biol. 2014;144:22–27. doi: 10.1016/j.jsbmb.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aranow C. Vitamin D and the immune system. J. Investig. Med. 2011;59:881–886. doi: 10.2310/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charoenngam N., Shirvani A., Holick M.F. Vitamin D for skeletal and non-skeletal health: What we should know. J. Clin. Orthop. Trauma. 2019;10:1082–1093. doi: 10.1016/j.jcot.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haussler M.R., Haussler C.A., Jurutka P.W., Thompson P.D., Hsieh J.C., Remus L.S., Selznick S.H., Whitfield G.K. The vitamin D hormone and its nuclear receptor: Molecular actions and disease states. J. Endocrinol. 1997;154:S57–S73. [PubMed] [Google Scholar]
- 8.Bergwitz C., Juppner H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu. Rev. Med. 2010;61:91–104. doi: 10.1146/annurev.med.051308.111339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charoenngam N., Rujirachun P., Holick M.F., Ungprasert P. Oral vitamin D3 supplementation increases serum fibroblast growth factor 23 concentration in vitamin D-deficient patients: A systematic review and meta-analysis. Osteoporos. Int. 2019;30:2183–2193. doi: 10.1007/s00198-019-05102-7. [DOI] [PubMed] [Google Scholar]
- 10.Charoenngam N., Shirvani A., Holick M.F. The ongoing D-lemma of vitamin D supplementation for nonskeletal health and bone health. Curr. Opin. Endocrinol. Diabetes Obes. 2019;26:301–305. doi: 10.1097/MED.0000000000000508. [DOI] [PubMed] [Google Scholar]
- 11.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P., Murad M.H., Weaver C.M., Endocrine S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 12.Luxwolda M.F., Kuipers R.S., Kema I.P., van der Veer E., Dijck-Brouwer D.A.J., Muskiet F.A.J. Vitamin D status indicators in indigenous populations in East Africa. Eur. J. Nutr. 2013;52:1115–1125. doi: 10.1007/s00394-012-0421-6. [DOI] [PubMed] [Google Scholar]
- 13.Wacker M., Holick M.F. Sunlight and Vitamin D: A global perspective for health. Dermatoendocrinology. 2013;5:51–108. doi: 10.4161/derm.24494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holick M.F. The Death D-fying Vitamin. Mayo Clin. Proc. 2018;93:679–681. doi: 10.1016/j.mayocp.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Dudenkov D.V., Mara K.C., Petterson T.M., Maxson J.A., Thacher T.D. Serum 25-Hydroxyvitamin D Values and Risk of All-Cause and Cause-Specific Mortality: A Population-Based Cohort Study. Mayo Clin. Proc. 2018;93:721–730. doi: 10.1016/j.mayocp.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herr C., Greulich T., Koczulla R.A., Meyer S., Zakharkina T., Branscheidt M., Eschmann R., Bals R. The role of vitamin D in pulmonary disease: COPD, asthma, infection, and cancer. Respir. Res. 2011;12:31. doi: 10.1186/1465-9921-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams J.S., Ren S., Liu P.T., Chun R.F., Lagishetty V., Gombart A.F., Borregaard N., Modlin R.L., Hewison M. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. J. Immunol. 2009;182:4289–4295. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu P.T., Stenger S., Li H., Wenzel L., Tan B.H., Krutzik S.R., Ochoa M.T., Schauber J., Wu K., Meinken C., et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 19.Shahmiri M., Enciso M., Adda C.G., Smith B.J., Perugini M.A., Mechler A. Membrane Core-Specific Antimicrobial Action of Cathelicidin LL-37 Peptide Switches Between Pore and Nanofibre Formation. Sci. Rep. 2016;6:38184. doi: 10.1038/srep38184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barlow P.G., Svoboda P., Mackellar A., Nash A.A., York I.A., Pohl J., Davidson D.J., Donis R.O. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS ONE. 2011;6:e25333. doi: 10.1371/journal.pone.0025333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tripathi S., Tecle T., Verma A., Crouch E., White M., Hartshorn K.L. The human cathelicidin LL-37 inhibits influenza A viruses through a mechanism distinct from that of surfactant protein D or defensins. J. Gen. Virol. 2013;94:40–49. doi: 10.1099/vir.0.045013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sousa F.H., Casanova V., Findlay F., Stevens C., Svoboda P., Pohl J., Proudfoot L., Barlow P.G. Cathelicidins display conserved direct antiviral activity towards rhinovirus. Peptides. 2017;95:76–83. doi: 10.1016/j.peptides.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma O.P. Hypercalcemia in granulomatous disorders: A clinical review. Curr. Opin. Pulm. Med. 2000;6:442–447. doi: 10.1097/00063198-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Hewison M., Kantorovich V., Liker H.R., Van Herle A.J., Cohan P., Zehnder D., Adams J.S. Vitamin D-mediated hypercalcemia in lymphoma: Evidence for hormone production by tumor-adjacent macrophages. J. Bone Min. Res. 2003;18:579–582. doi: 10.1359/jbmr.2003.18.3.579. [DOI] [PubMed] [Google Scholar]
- 25.Papapoulos S.E., Clemens T.L., Fraher L.J., Lewin I.G., Sandler L.M., O’Riordan J.L. 1, 25-dihydroxycholecalciferol in the pathogenesis of the hypercalcaemia of sarcoidosis. Lancet. 1979;1:627–630. doi: 10.1016/S0140-6736(79)91076-6. [DOI] [PubMed] [Google Scholar]
- 26.Adorini L., Penna G. Induction of tolerogenic dendritic cells by vitamin D receptor agonists. Handb. Exp. Pharmacol. 2009:251–273. doi: 10.1007/978-3-540-71029-5_12. [DOI] [PubMed] [Google Scholar]
- 27.Steinman R.M., Hawiger D., Nussenzweig M.C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 28.Széles L., Keresztes G., Töröcsik D., Balajthy Z., Krenács L., Póliska S., Steinmeyer A., Zuegel U., Pruenster M., Rot A., et al. 1,25-Dihydroxyvitamin D3 Is an Autonomous Regulator of the Transcriptional Changes Leading to a Tolerogenic Dendritic Cell Phenotype. J. Immunol. 2009;182:2074. doi: 10.4049/jimmunol.0803345. [DOI] [PubMed] [Google Scholar]
- 29.Piemonti L., Monti P., Sironi M., Fraticelli P., Leone B.E., Dal Cin E., Allavena P., Di Carlo V. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J. Immunol. 2000;164:4443–4451. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- 30.Urry Z., Xystrakis E., Richards D.F., McDonald J., Sattar Z., Cousins D.J., Corrigan C.J., Hickman E., Brown Z., Hawrylowicz C.M. Ligation of TLR9 induced on human IL-10-secreting Tregs by 1alpha,25-dihydroxyvitamin D3 abrogates regulatory function. J. Clin. Investig. 2009;119:387–398. doi: 10.1172/JCI32354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeffery L.E., Burke F., Mura M., Zheng Y., Qureshi O.S., Hewison M., Walker L.S., Lammas D.A., Raza K., Sansom D.M. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J. Immunol. 2009;183:5458–5467. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dickie L.J., Church L.D., Coulthard L.R., Mathews R.J., Emery P., McDermott M.F. Vitamin D3 down-regulates intracellular Toll-like receptor 9 expression and Toll-like receptor 9-induced IL-6 production in human monocytes. Rheumatology. 2010;49:1466–1471. doi: 10.1093/rheumatology/keq124. [DOI] [PubMed] [Google Scholar]
- 33.Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 34.Weeres M.A., Robien K., Ahn Y.-O., Neulen M.-L., Bergerson R., Miller J.S., Verneris M.R. The effects of 1,25-dihydroxyvitamin D3 on in vitro human NK cell development from hematopoietic stem cells. J. Immunol. 2014;193:3456–3462. doi: 10.4049/jimmunol.1400698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ota K., Dambaeva S., Kim M.W., Han A.R., Fukui A., Gilman-Sachs A., Beaman K., Kwak-Kim J. 1,25-Dihydroxy-vitamin D3 regulates NK-cell cytotoxicity, cytokine secretion, and degranulation in women with recurrent pregnancy losses. Eur. J. Immunol. 2015;45:3188–3199. doi: 10.1002/eji.201545541. [DOI] [PubMed] [Google Scholar]
- 36.Cantorna M.T., Zhao J., Yang L. Vitamin D, invariant natural killer T-cells and experimental autoimmune disease. Proc. Nutr. Soc. 2012;71:62–66. doi: 10.1017/S0029665111003193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gibson C.C., Davis C.T., Zhu W., Bowman-Kirigin J.A., Walker A.E., Tai Z., Thomas K.R., Donato A.J., Lesniewski L.A., Li D.Y. Dietary Vitamin D and Its Metabolites Non-Genomically Stabilize the Endothelium. PLoS ONE. 2015;10:e0140370. doi: 10.1371/journal.pone.0140370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andrukhova O., Slavic S., Zeitz U., Riesen S.C., Heppelmann M.S., Ambrisko T.D., Markovic M., Kuebler W.M., Erben R.G. Vitamin D is a regulator of endothelial nitric oxide synthase and arterial stiffness in mice. Mol. Endocrinol. 2014;28:53–64. doi: 10.1210/me.2013-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma R., Deng X.L., Du G.L., Li C., Xiao S., Aibibai Y., Zhu J. Active vitamin D3, 1,25-(OH)2D3, protects against macrovasculopathy in a rat model of type 2 diabetes mellitus. Genet. Mol. Res. 2016:15. doi: 10.4238/gmr.15028113. [DOI] [PubMed] [Google Scholar]
- 40.Molinari C., Uberti F., Grossini E., Vacca G., Carda S., Invernizzi M., Cisari C. 1α,25-Dihydroxycholecalciferol Induces Nitric Oxide Production in Cultured Endothelial Cells. Cell. Physiol. Biochem. 2011;27:661–668. doi: 10.1159/000330075. [DOI] [PubMed] [Google Scholar]
- 41.Kim D.-H., Meza C.A., Clarke H., Kim J.-S., Hickner R.C. Vitamin D and Endothelial Function. Nutrients. 2020;12:575. doi: 10.3390/nu12020575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vila Cuenca M., Ferrantelli E., Meinster E., Pouw S.M., Kovačević I., de Menezes R.X., Niessen H.W., Beelen R.H.J., Hordijk P.L., Vervloet M.G. Vitamin D Attenuates Endothelial Dysfunction in Uremic Rats and Maintains Human Endothelial Stability. J. Am. Heart Assoc. 2018;7:e008776. doi: 10.1161/JAHA.118.008776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He Y., Wu W., Wu S., Zheng H.-M., Li P., Sheng H.-F., Chen M.-X., Chen Z.-H., Ji G.-Y., Zheng Z.-D.-X., et al. Linking gut microbiota, metabolic syndrome and economic status based on a population-level analysis. Microbiome. 2018;6:172. doi: 10.1186/s40168-018-0557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee C., Lau E., Chusilp S., Filler R., Li B., Zhu H., Yamoto M., Pierro A. Protective effects of vitamin D against injury in intestinal epithelium. Pediatr. Surg. Int. 2019;35:1395–1401. doi: 10.1007/s00383-019-04586-y. [DOI] [PubMed] [Google Scholar]
- 45.Wang T.-T., Dabbas B., Laperriere D., Bitton A.J., Soualhine H., Tavera-Mendoza L.E., Dionne S., Servant M.J., Bitton A., Seidman E.G., et al. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. J. Biol. Chem. 2010;285:2227–2231. doi: 10.1074/jbc.C109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y.-G., Wu S., Sun J. Vitamin D, Vitamin D Receptor, and Tissue Barriers. Tissue Barriers. 2013;1:e23118. doi: 10.4161/tisb.23118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Su D., Nie Y., Zhu A., Chen Z., Wu P., Zhang L., Luo M., Sun Q., Cai L., Lai Y., et al. Vitamin D Signaling through Induction of Paneth Cell Defensins Maintains Gut Microbiota and Improves Metabolic Disorders and Hepatic Steatosis in Animal Models. Front. Physiol. 2016;7:498. doi: 10.3389/fphys.2016.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fakhoury H.M.A., Kvietys P.R., AlKattan W., Anouti F.A., Elahi M.A., Karras S.N., Grant W.B. Vitamin D and intestinal homeostasis: Barrier, microbiota, and immune modulation. J. Steroid Biochem. Mol. Biol. 2020;200:105663. doi: 10.1016/j.jsbmb.2020.105663. [DOI] [PubMed] [Google Scholar]
- 49.Chakaroun R.M., Massier L., Kovacs P. Gut Microbiome, Intestinal Permeability, and Tissue Bacteria in Metabolic Disease: Perpetrators or Bystanders? Nutrients. 2020;12:1082. doi: 10.3390/nu12041082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan M.F., Wang H. Environmental Exposures and Autoimmune Diseases: Contribution of Gut Microbiome. Front. Immunol. 2020;10:3094. doi: 10.3389/fimmu.2019.03094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhalla A.K., Amento E.P., Clemens T.L., Holick M.F., Krane S.M. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: Presence in monocytes and induction in T lymphocytes following activation. J. Clin. Endocrinol. Metab. 1983;57:1308–1310. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- 52.Amento E.P., Bhalla A.K., Kurnick J.T., Kradin R.L., Clemens T.L., Holick S.A., Holick M.F., Krane S.M. 1 alpha,25-dihydroxyvitamin D3 induces maturation of the human monocyte cell line U937, and, in association with a factor from human T lymphocytes, augments production of the monokine, mononuclear cell factor. J. Clin. Investig. 1984;73:731–739. doi: 10.1172/JCI111266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hewison M. Vitamin D and the intracrinology of innate immunity. Mol. Cell. Endocrinol. 2010;321:103–111. doi: 10.1016/j.mce.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kongsbak M., von Essen M.R., Levring T.B., Schjerling P., Woetmann A., Odum N., Bonefeld C.M., Geisler C. Vitamin D-binding protein controls T cell responses to vitamin D. BMC Immunol. 2014;15:35. doi: 10.1186/s12865-014-0035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cantorna M.T., Snyder L., Lin Y.D., Yang L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients. 2015;7:3011–3021. doi: 10.3390/nu7043011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lemire J.M., Archer D.C., Beck L., Spiegelberg H.L. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: Preferential inhibition of Th1 functions. J. Nutr. 1995;125:1704S–1708S. doi: 10.1093/jn/125.suppl_6.1704S. [DOI] [PubMed] [Google Scholar]
- 57.Boonstra A., Barrat F.J., Crain C., Heath V.L., Savelkoul H.F.J., O’Garra A. 1α,25-Dihydroxyvitamin D3 Has a Direct Effect on Naive CD4+ T Cells to Enhance the Development of Th2 Cells. J. Immunol. 2001;167:4974. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 58.Tang J., Zhou R., Luger D., Zhu W., Silver P.B., Grajewski R.S., Su S.B., Chan C.C., Adorini L., Caspi R.R. Calcitriol suppresses antiretinal autoimmunity through inhibitory effects on the Th17 effector response. J. Immunol. 2009;182:4624–4632. doi: 10.4049/jimmunol.0801543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mocanu V., Oboroceanu T., Zugun-Eloae F. Current status in vitamin D and regulatory T cells--immunological implications. Rev. Med. Chir. Soc. Med. Nat. Iasi. 2013;117:965–973. [PubMed] [Google Scholar]
- 60.Gregori S., Giarratana N., Smiroldo S., Uskokovic M., Adorini L. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51:1367–1374. doi: 10.2337/diabetes.51.5.1367. [DOI] [PubMed] [Google Scholar]
- 61.Kongsbak M., Levring T.B., Geisler C., von Essen M.R. The vitamin d receptor and T cell function. Front. Immunol. 2013;4:148. doi: 10.3389/fimmu.2013.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sarkar S., Hewison M., Studzinski G.P., Li Y.C., Kalia V. Role of vitamin D in cytotoxic T lymphocyte immunity to pathogens and cancer. Crit. Rev. Clin. Lab. Sci. 2016;53:132–145. doi: 10.3109/10408363.2015.1094443. [DOI] [PubMed] [Google Scholar]
- 63.Mao X., Hu B., Zhou Z., Xing X., Wu Y., Gao J., He Y., Hu Y., Cheng Q., Gong Q. Vitamin D levels correlate with lymphocyte subsets in elderly patients with age-related diseases. Sci. Rep. 2018;8:7708. doi: 10.1038/s41598-018-26064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eckard A.R., O’Riordan M.A., Rosebush J.C., Lee S.T., Habib J.G., Ruff J.H., Labbato D., Daniels J.E., Uribe-Leitz M., Tangpricha V., et al. Vitamin D supplementation decreases immune activation and exhaustion in HIV-1-infected youth. Antivir. Ther. 2018;23:315–324. doi: 10.3851/IMP3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stallings V.A., Schall J.I., Hediger M.L., Zemel B.S., Tuluc F., Dougherty K.A., Samuel J.L., Rutstein R.M. High-dose vitamin D3 supplementation in children and young adults with HIV: A randomized, placebo-controlled trial. Pediatr. Infect. Dis. J. 2015;34:e32–e40. doi: 10.1097/INF.0000000000000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen S., Sims G.P., Chen X.X., Gu Y.Y., Chen S., Lipsky P.E. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007;179:1634–1647. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 67.Lemire J.M., Adams J.S., Sakai R., Jordan S.C. 1 alpha,25-dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. J. Clin. Investig. 1984;74:657–661. doi: 10.1172/JCI111465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Geldmeyer-Hilt K., Heine G., Hartmann B., Baumgrass R., Radbruch A., Worm M. 1,25-dihydroxyvitamin D3 impairs NF-kappaB activation in human naive B cells. Biochem. Biophys. Res. Commun. 2011;407:699–702. doi: 10.1016/j.bbrc.2011.03.078. [DOI] [PubMed] [Google Scholar]
- 69.Heine G., Niesner U., Chang H.D., Steinmeyer A., Zugel U., Zuberbier T., Radbruch A., Worm M. 1,25-dihydroxyvitamin D(3) promotes IL-10 production in human B cells. Eur. J. Immunol. 2008;38:2210–2218. doi: 10.1002/eji.200838216. [DOI] [PubMed] [Google Scholar]
- 70.Shirakawa A.K., Nagakubo D., Hieshima K., Nakayama T., Jin Z., Yoshie O. 1,25-dihydroxyvitamin D3 induces CCR10 expression in terminally differentiating human B cells. J. Immunol. 2008;180:2786–2795. doi: 10.4049/jimmunol.180.5.2786. [DOI] [PubMed] [Google Scholar]
- 71.Yamamoto E.A., Nguyen J.K., Liu J., Keller E., Campbell N., Zhang C.J., Smith H.R., Li X., Jorgensen T.N. Low Levels of Vitamin D Promote Memory B Cells in Lupus. Nutrients. 2020;12:291. doi: 10.3390/nu12020291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koeffler H.P., Hirji K., Itri L. 1,25-Dihydroxyvitamin D3: In vivo and in vitro effects on human preleukemic and leukemic cells. Cancer Treat. Rep. 1985;69:1399–1407. [PubMed] [Google Scholar]
- 73.Parisi R., Symmons D.P., Griffiths C.E., Ashcroft D.M., Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J. Investig. Dermatol. 2013;133:377–385. doi: 10.1038/jid.2012.339. [DOI] [PubMed] [Google Scholar]
- 74.Holick M.F. The photobiology of vitamin D and its consequences for humans. Ann. N. Y. Acad. Sci. 1985;453:1–13. doi: 10.1111/j.1749-6632.1985.tb11793.x. [DOI] [PubMed] [Google Scholar]
- 75.Reichrath J., Perez A., Muller S.M., Chen T.C., Kerber A., Bahmer F.A., Holick M.F. Topical calcitriol (1,25-dihydroxyvitamin D3) treatment of psoriasis: An immunohistological evaluation. Acta Dermatol. Venereol. 1997;77:268–272. doi: 10.2340/0001555577268272. [DOI] [PubMed] [Google Scholar]
- 76.Smith E.L., Walworth N.C., Holick M.F. Effect of 1 alpha,25-dihydroxyvitamin D3 on the morphologic and biochemical differentiation of cultured human epidermal keratinocytes grown in serum-free conditions. J. Investig. Dermatol. 1986;86:709–714. doi: 10.1111/1523-1747.ep12276343. [DOI] [PubMed] [Google Scholar]
- 77.Holick M.F. Active vitamin D compounds and analogues: A new therapeutic era for dermatology in the 21st century. Mayo Clin. Proc. 1993;68:925–927. doi: 10.1016/S0025-6196(12)60704-6. [DOI] [PubMed] [Google Scholar]
- 78.MacLaughlin J.A., Gange W., Taylor D., Smith E., Holick M.F. Cultured psoriatic fibroblasts from involved and uninvolved sites have a partial but not absolute resistance to the proliferation-inhibition activity of 1,25-dihydroxyvitamin D3. Proc. Natl. Acad. Sci. USA. 1985;82:5409–5412. doi: 10.1073/pnas.82.16.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith E.L., Pincus S.H., Donovan L., Holick M.F. A novel approach for the evaluation and treatment of psoriasis. Oral or topical use of 1,25-dihydroxyvitamin D3 can be a safe and effective therapy for psoriasis. J. Am. Acad. Dermatol. 1988;19:516–528. doi: 10.1016/S0190-9622(88)70207-8. [DOI] [PubMed] [Google Scholar]
- 80.Dubertret L., Wallach D., Souteyrand P., Perussel M., Kalis B., Meynadier J., Chevrant-Breton J., Beylot C., Bazex J.A., Jurgensen H.J. Efficacy and safety of calcipotriol (MC 903) ointment in psoriasis vulgaris. A randomized, double-blind, right/left comparative, vehicle-controlled study. J. Am. Acad. Dermatol. 1992;27:983–988. doi: 10.1016/0190-9622(92)70299-U. [DOI] [PubMed] [Google Scholar]
- 81.Kagami S., Rizzo H.L., Lee J.J., Koguchi Y., Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J. Investig. Dermatol. 2010;130:1373–1383. doi: 10.1038/jid.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barrea L., Savanelli M.C., Di Somma C., Napolitano M., Megna M., Colao A., Savastano S. Vitamin D and its role in psoriasis: An overview of the dermatologist and nutritionist. Rev. Endocr. Metab. Disord. 2017;18:195–205. doi: 10.1007/s11154-017-9411-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Orgaz-Molina J., Buendia-Eisman A., Arrabal-Polo M.A., Ruiz J.C., Arias-Santiago S. Deficiency of serum concentration of 25-hydroxyvitamin D in psoriatic patients: A case-control study. J. Am. Acad. Dermatol. 2012;67:931–938. doi: 10.1016/j.jaad.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 84.Disphanurat W., Viarasilpa W., Chakkavittumrong P., Pongcharoen P. The Clinical Effect of Oral Vitamin D2 Supplementation on Psoriasis: A Double-Blind, Randomized, Placebo-Controlled Study. Dermatol. Res. Pract. 2019;2019:5237642. doi: 10.1155/2019/5237642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Finamor D.C., Sinigaglia-Coimbra R., Neves L.C.M., Gutierrez M., Silva J.J., Torres L.D., Surano F., Neto D.J., Novo N.F., Juliano Y., et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatol. Endocrinol. 2013;5:222–234. doi: 10.4161/derm.24808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ingram M.A., Jones M.B., Stonehouse W., Jarrett P., Scragg R., Mugridge O., von Hurst P.R. Oral vitamin D3 supplementation for chronic plaque psoriasis: A randomized, double-blind, placebo-controlled trial. J. Dermatol. Treat. 2018;29:648–657. doi: 10.1080/09546634.2018.1444728. [DOI] [PubMed] [Google Scholar]
- 87.Perez A., Raab R., Chen T.C., Turner A., Holick M.F. Safety and efficacy of oral calcitriol (1,25-dihydroxyvitamin D3) for the treatment of psoriasis. Br. J. Dermatol. 1996;134:1070–1078. doi: 10.1111/j.1365-2133.1996.tb07945.x. [DOI] [PubMed] [Google Scholar]
- 88.Ezquerra G.M., Regana M.S., Millet P.U. Combination of acitretin and oral calcitriol for treatment of plaque-type psoriasis. Acta Dermatol. Venereol. 2007;87:449–450. doi: 10.2340/00015555-0290. [DOI] [PubMed] [Google Scholar]
- 89.Harjutsalo V., Sund R., Knip M., Groop P.H. Incidence of type 1 diabetes in Finland. JAMA. 2013;310:427–428. doi: 10.1001/jama.2013.8399. [DOI] [PubMed] [Google Scholar]
- 90.Junnila S.K. Type 1 diabetes epidemic in Finland is triggered by zinc-containing amorphous silica nanoparticles. Med. Hypotheses. 2015;84:336–340. doi: 10.1016/j.mehy.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 91.Hyöty H., Leon F., Knip M. Developing a vaccine for type 1 diabetes by targeting coxsackievirus B. Expert Rev. Vaccines. 2018;17:1071–1083. doi: 10.1080/14760584.2018.1548281. [DOI] [PubMed] [Google Scholar]
- 92.Holick M.F. Vitamin D: A millenium perspective. J. Cell. Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 93.Webb A.R., Kline L., Holick M.F. Influence of Season and Latitude on the Cutaneous Synthesis of Vitamin D3: Exposure to Winter Sunlight in Boston and Edmonton Will Not Promote Vitamin D3 Synthesis in Human Skin*. J. Clin. Endocrinol. Metab. 1988;67:373–378. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 94.Chen Y.-L., Huang Y.-C., Qiao Y.-C., Ling W., Pan Y.-H., Geng L.-J., Xiao J.-L., Zhang X.-X., Zhao H.-L. Climates on incidence of childhood type 1 diabetes mellitus in 72 countries. Sci. Rep. 2017;7:12810. doi: 10.1038/s41598-017-12954-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li M., Song L.-J., Qin X.-Y. Advances in the cellular immunological pathogenesis of type 1 diabetes. J. Cell. Mol. Med. 2014;18:749–758. doi: 10.1111/jcmm.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoon J.W., Jun H.S. Autoimmune destruction of pancreatic beta cells. Am. J. Ther. 2005;12:580–591. doi: 10.1097/01.mjt.0000178767.67857.63. [DOI] [PubMed] [Google Scholar]
- 97.Lee S., Clark S.A., Gill R.K., Christakos S. 1,25-Dihydroxyvitamin D3 and pancreatic beta-cell function: Vitamin D receptors, gene expression, and insulin secretion. Endocrinology. 1994;134:1602–1610. doi: 10.1210/endo.134.4.8137721. [DOI] [PubMed] [Google Scholar]
- 98.The EURODIAB Substudy 2 Study Group Vitamin D supplement in early childhood and risk for Type I (insulin-dependent) diabetes mellitus. Diabetologia. 1999;42:51–54. doi: 10.1007/s001250051112. [DOI] [PubMed] [Google Scholar]
- 99.Hypponen E., Laara E., Reunanen A., Jarvelin M.R., Virtanen S.M. Intake of vitamin D and risk of type 1 diabetes: A birth-cohort study. Lancet. 2001;358:1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 100.Ataie-Jafari A., Loke S.C., Rahmat A.B., Larijani B., Abbasi F., Leow M.K., Yassin Z. A randomized placebo-controlled trial of alphacalcidol on the preservation of beta cell function in children with recent onset type 1 diabetes. Clin. Nutr. 2013;32:911–917. doi: 10.1016/j.clnu.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 101.Gabbay M.A.L., Sato M.N., Finazzo C., Duarte A.J.S., Dib S.A. Effect of Cholecalciferol as Adjunctive Therapy With Insulin on Protective Immunologic Profile and Decline of Residual β-Cell Function in New-Onset Type 1 Diabetes Mellitus. Arch. Pediatr. Adolesc. Med. 2012;166:601–607. doi: 10.1001/archpediatrics.2012.164. [DOI] [PubMed] [Google Scholar]
- 102.Treiber G., Prietl B., Frohlich-Reiterer E., Lechner E., Ribitsch A., Fritsch M., Rami-Merhar B., Steigleder-Schweiger C., Graninger W., Borkenstein M., et al. Cholecalciferol supplementation improves suppressive capacity of regulatory T-cells in young patients with new-onset type 1 diabetes mellitus—A randomized clinical trial. Clin. Immunol. 2015;161:217–224. doi: 10.1016/j.clim.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 103.Gregoriou E., Mamais I., Tzanetakou I., Lavranos G., Chrysostomou S. The Effects of Vitamin D Supplementation in Newly Diagnosed Type 1 Diabetes Patients: Systematic Review of Randomized Controlled Trials. Rev. Diabetes Stud. 2017;14:260–268. doi: 10.1900/RDS.2017.14.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dong J.-Y., Zhang W.-G., Chen J.J., Zhang Z.-L., Han S.-F., Qin L.-Q. Vitamin D intake and risk of type 1 diabetes: A meta-analysis of observational studies. Nutrients. 2013;5:3551–3562. doi: 10.3390/nu5093551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Simpson S., Blizzard L., Otahal P., Van der Mei I., Taylor B. Latitude is significantly associated with the prevalence of multiple sclerosis: A meta-analysis. J. Neurol. Neurosurg. Psychiatry. 2011;82:1132. doi: 10.1136/jnnp.2011.240432. [DOI] [PubMed] [Google Scholar]
- 106.VanAmerongen B.M., Dijkstra C.D., Lips P., Polman C.H. Multiple sclerosis and vitamin D: An update. Eur. J. Clin. Nutr. 2004;58:1095–1109. doi: 10.1038/sj.ejcn.1601952. [DOI] [PubMed] [Google Scholar]
- 107.Munger K.L., Levin L.I., Hollis B.W., Howard N.S., Ascherio A. Serum 25-Hydroxyvitamin D Levels and Risk of Multiple Sclerosis. JAMA. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 108.Munger K.L., Zhang S.M., O’Reilly E., Hernan M.A., Olek M.J., Willett W.C., Ascherio A. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62:60–65. doi: 10.1212/01.WNL.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 109.Haines J.D., Inglese M., Casaccia P. Axonal damage in multiple sclerosis. Mt. Sinai J. Med. 2011;78:231–243. doi: 10.1002/msj.20246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ghasemi N., Razavi S., Nikzad E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J. 2017;19:1–10. doi: 10.22074/cellj.2016.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mosca L., Mantero V., Penco S., La Mantia L., De Benedetti S., Marazzi M.R., Spreafico C., Erminio C., Grassi L., Lando G., et al. HLA-DRB1*15 association with multiple sclerosis is confirmed in a multigenerational Italian family. Funct. Neurol. 2017;32:83–88. doi: 10.11138/FNeur/2017.32.2.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cocco E., Meloni A., Murru M.R., Corongiu D., Tranquilli S., Fadda E., Murru R., Schirru L., Secci M.A., Costa G., et al. Vitamin D responsive elements within the HLA-DRB1 promoter region in Sardinian multiple sclerosis associated alleles. PLoS ONE. 2012;7:e41678. doi: 10.1371/journal.pone.0041678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ramagopalan S.V., Maugeri N.J., Handunnetthi L., Lincoln M.R., Orton S.-M., Dyment D.A., Deluca G.C., Herrera B.M., Chao M.J., Sadovnick A.D., et al. Expression of the multiple sclerosis-associated MHC class II Allele HLA-DRB1*1501 is regulated by vitamin D. PLoS Genet. 2009;5:e1000369. doi: 10.1371/journal.pgen.1000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McLaughlin L., Clarke L., Khalilidehkordi E., Butzkueven H., Taylor B., Broadley S.A. Vitamin D for the treatment of multiple sclerosis: A meta-analysis. J. Neurol. 2018;265:2893–2905. doi: 10.1007/s00415-018-9074-6. [DOI] [PubMed] [Google Scholar]
- 115.Lo C.W., Paris P.W., Clemens T.L., Nolan J., Holick M.F. Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. Am. J. Clin. Nutr. 1985;42:644–649. doi: 10.1093/ajcn/42.4.644. [DOI] [PubMed] [Google Scholar]
- 116.Farraye F.A., Nimitphong H., Stucchi A., Dendrinos K., Boulanger A.B., Vijjeswarapu A., Tanennbaum A., Biancuzzo R., Chen T.C., Holick M.F. Use of a novel vitamin D bioavailability test demonstrates that vitamin D absorption is decreased in patients with quiescent crohn’s disease1,2,3. Inflamm. Bowel Dis. 2011;17:2116–2121. doi: 10.1002/ibd.21595. [DOI] [PubMed] [Google Scholar]
- 117.Ludvigsson J.F., Mahl M., Sachs M.C., Bjork J., Michaelsson K., Ekbom A., Askling J., Backman A.S., Olen O. Fracture Risk in Patients With Inflammatory Bowel Disease: A Nationwide Population-Based Cohort Study From 1964 to 2014. Am. J. Gastroenterol. 2019;114:291–304. doi: 10.14309/ajg.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 118.Van staa T.-P., Cooper C., Samuels Brusse L., Leufkens H., Javaid M.K., Arden N.K. Inflammatory bowel disease and the risk of fracture. Gastroenterology. 2003;125:1591–1597. doi: 10.1053/j.gastro.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 119.Schultz M., Butt A.G. Is the north to south gradient in inflammatory bowel disease a global phenomenon? Expert Rev. Gastroenterol. Hepatol. 2012;6:445–447. doi: 10.1586/egh.12.31. [DOI] [PubMed] [Google Scholar]
- 120.Ananthakrishnan A.N., Khalili H., Higuchi L.M., Bao Y., Korzenik J.R., Giovannucci E.L., Richter J.M., Fuchs C.S., Chan A.T. Higher predicted vitamin D status is associated with reduced risk of Crohn’s disease. Gastroenterology. 2012;142:482–489. doi: 10.1053/j.gastro.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yue B., Luo X., Yu Z., Mani S., Wang Z., Dou W. Inflammatory Bowel Disease: A Potential Result from the Collusion between Gut Microbiota and Mucosal Immune System. Microorganisms. 2019;7:440. doi: 10.3390/microorganisms7100440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Matricon J., Barnich N., Ardid D. Immunopathogenesis of inflammatory bowel disease. Self Nonself. 2010;1:299–309. doi: 10.4161/self.1.4.13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nemeth Z.H., Bogdanovski D.A., Barratt-Stopper P., Paglinco S.R., Antonioli L., Rolandelli R.H. Crohn’s Disease and Ulcerative Colitis Show Unique Cytokine Profiles. Cureus. 2017;9:e1177. doi: 10.7759/cureus.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gálvez J. Role of Th17 Cells in the Pathogenesis of Human IBD. ISRN Inflamm. 2014;2014:928461. doi: 10.1155/2014/928461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gubatan J., Moss A.C. Vitamin D in inflammatory bowel disease: More than just a supplement. Curr. Opin. Gastroenterol. 2018;34:217–225. doi: 10.1097/MOG.0000000000000449. [DOI] [PubMed] [Google Scholar]
- 126.Fletcher J., Cooper S.C., Ghosh S., Hewison M. The Role of Vitamin D in Inflammatory Bowel Disease: Mechanism to Management. Nutrients. 2019;11:1019. doi: 10.3390/nu11051019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li J., Chen N., Wang D., Zhang J., Gong X. Efficacy of vitamin D in treatment of inflammatory bowel disease: A meta-analysis. Medicine. 2018;97:e12662. doi: 10.1097/MD.0000000000012662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schäffler H., Herlemann D.P.R., Klinitzke P., Berlin P., Kreikemeyer B., Jaster R., Lamprecht G. Vitamin D administration leads to a shift of the intestinal bacterial composition in Crohn’s disease patients, but not in healthy controls. J. Dig. Dis. 2018;19:225–234. doi: 10.1111/1751-2980.12591. [DOI] [PubMed] [Google Scholar]
- 129.Charoenngam N., Shirvani A., Kalajian T.A., Song A., Holick M.F. The Effect of Various Doses of Oral Vitamin D3 Supplementation on Gut Microbiota in Healthy Adults: A Randomized, Double-blinded, Dose-response Study. Anticancer Res. 2020;40:551–556. doi: 10.21873/anticanres.13984. [DOI] [PubMed] [Google Scholar]
- 130.Merlino L.A., Curtis J., Mikuls T.R., Cerhan J.R., Criswell L.A., Saag K.G. Vitamin D intake is inversely associated with rheumatoid arthritis: Results from the Iowa Women’s Health Study. Arthritis Rheum. 2004;50:72–77. doi: 10.1002/art.11434. [DOI] [PubMed] [Google Scholar]
- 131.Lee Y.H., Bae S.C. Vitamin D level in rheumatoid arthritis and its correlation with the disease activity: A meta-analysis. Clin. Exp. Rheumatol. 2016;34:827–833. [PubMed] [Google Scholar]
- 132.Kostoglou-Athanassiou I., Athanassiou P., Lyraki A., Raftakis I., Antoniadis C. Vitamin D and rheumatoid arthritis. Ther. Adv. Endocrinol. Metab. 2012;3:181–187. doi: 10.1177/2042018812471070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Meena N., Singh Chawla S.P., Garg R., Batta A., Kaur S. Assessment of Vitamin D in Rheumatoid Arthritis and Its Correlation with Disease Activity. J. Nat. Sci. Biol. Med. 2018;9:54–58. doi: 10.4103/jnsbm.JNSBM_128_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Aslam M.M., John P., Bhatti A., Jahangir S., Kamboh M.I. Vitamin D as a Principal Factor in Mediating Rheumatoid Arthritis-Derived Immune Response. Biomed. Res. Int. 2019;2019:3494937. doi: 10.1155/2019/3494937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guo Q., Wang Y., Xu D., Nossent J., Pavlos N.J., Xu J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6:15. doi: 10.1038/s41413-018-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kosmaczewska A., Swierkot J., Ciszak L., Wiland P. The role of Th1, Th17, and Treg cells in the pathogenesis of rheumatoid arthritis including anti-inflammatory action of Th1 cytokines. Postepy Hig. Med. Dosw. 2011;65:397–403. doi: 10.5604/17322693.948971. [DOI] [PubMed] [Google Scholar]
- 137.Li S., Yin H., Zhang K., Wang T., Yang Y., Liu X., Chang X., Zhang M., Yan X., Ren Y., et al. Effector T helper cell populations are elevated in the bone marrow of rheumatoid arthritis patients and correlate with disease severity. Sci. Rep. 2017;7:4776. doi: 10.1038/s41598-017-05014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gopinath K., Danda D. Supplementation of 1,25 dihydroxy vitamin D3 in patients with treatment naive early rheumatoid arthritis: A randomised controlled trial. Int. J. Rheum. Dis. 2011;14:332–339. doi: 10.1111/j.1756-185X.2011.01684.x. [DOI] [PubMed] [Google Scholar]
- 139.Li C., Yin S., Yin H., Cao L., Zhang T., Wang Y. Efficacy and Safety of 22-Oxa-Calcitriol in Patients with Rheumatoid Arthritis: A Phase II Trial. Med. Sci. Monit. 2018;24:9127–9135. doi: 10.12659/MSM.911628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hansen K.E., Bartels C.M., Gangnon R.E., Jones A.N., Gogineni J. An evaluation of high-dose vitamin D for rheumatoid arthritis. J. Clin. Rheumatol. 2014;20:112–114. doi: 10.1097/RHU.0000000000000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dehghan A., Rahimpour S., Soleymani-Salehabadi H., Owlia M.B. Role of vitamin D in flare ups of rheumatoid arthritis. Z. Rheumatol. 2014;73:461–464. doi: 10.1007/s00393-013-1297-4. [DOI] [PubMed] [Google Scholar]
- 142.Salesi M., Farajzadegan Z. Efficacy of vitamin D in patients with active rheumatoid arthritis receiving methotrexate therapy. Rheumatol. Int. 2012;32:2129–2133. doi: 10.1007/s00296-011-1944-5. [DOI] [PubMed] [Google Scholar]
- 143.Yang J., Liu L., Zhang Q., Li M., Wang J. Effect of vitamin D on the recurrence rate of rheumatoid arthritis. Exp. Ther. Med. 2015;10:1812–1816. doi: 10.3892/etm.2015.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Floyd K., Glaziou P., Zumla A., Raviglione M. The global tuberculosis epidemic and progress in care, prevention, and research: An overview in year 3 of the End TB era. Lancet Respir. Med. 2018;6:299–314. doi: 10.1016/S2213-2600(18)30057-2. [DOI] [PubMed] [Google Scholar]
- 145.Sasindran S.J., Torrelles J.B. Mycobacterium Tuberculosis Infection and Inflammation: What is Beneficial for the Host and for the Bacterium? Front. Microbiol. 2011;2:2. doi: 10.3389/fmicb.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Aibana O., Huang C.C., Aboud S., Arnedo-Pena A., Becerra M.C., Bellido-Blasco J.B., Bhosale R., Calderon R., Chiang S., Contreras C., et al. Vitamin D status and risk of incident tuberculosis disease: A nested case-control study, systematic review, and individual-participant data meta-analysis. PLoS Med. 2019;16:e1002907. doi: 10.1371/journal.pmed.1002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nnoaham K.E., Clarke A. Low serum vitamin D levels and tuberculosis: A systematic review and meta-analysis. Int. J. Epidemiol. 2008;37:113–119. doi: 10.1093/ije/dym247. [DOI] [PubMed] [Google Scholar]
- 148.Holick M.F. Vitamin D: A d-lightful solution for health. J. Investig. Med. 2011;59:872–880. doi: 10.2310/JIM.0b013e318214ea2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Naik A.L., Rajan M.G., Manjrekar P.A., Shenoy M.T., Shreelata S., Srikantiah R.M., Hegde A. Effect of DOTS Treatment on Vitamin D Levels in Pulmonary Tuberculosis. J. Clin. Diagn. Res. 2017;11:BC18–BC22. doi: 10.7860/JCDR/2017/24501.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nursyam E.W., Amin Z., Rumende C.M. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med. Indones. 2006;38:3–5. [PubMed] [Google Scholar]
- 151.Mily A., Rekha R.S., Kamal S.M., Arifuzzaman A.S., Rahim Z., Khan L., Haq M.A., Zaman K., Bergman P., Brighenti S., et al. Significant Effects of Oral Phenylbutyrate and Vitamin D3 Adjunctive Therapy in Pulmonary Tuberculosis: A Randomized Controlled Trial. PLoS ONE. 2015;10:e0138340. doi: 10.1371/journal.pone.0138340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Martineau A.R., Timms P.M., Bothamley G.H., Hanifa Y., Islam K., Claxton A.P., Packe G.E., Moore-Gillon J.C., Darmalingam M., Davidson R.N., et al. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: A double-blind randomised controlled trial. Lancet. 2011;377:242–250. doi: 10.1016/S0140-6736(10)61889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Salahuddin N., Ali F., Hasan Z., Rao N., Aqeel M., Mahmood F. Vitamin D accelerates clinical recovery from tuberculosis: Results of the SUCCINCT Study [Supplementary Cholecalciferol in recovery from tuberculosis]. A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis’. BMC Infect. Dis. 2013;13:22. doi: 10.1186/1471-2334-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Daley P., Jagannathan V., John K.R., Sarojini J., Latha A., Vieth R., Suzana S., Jeyaseelan L., Christopher D.J., Smieja M., et al. Adjunctive vitamin D for treatment of active tuberculosis in India: A randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2015;15:528–534. doi: 10.1016/S1473-3099(15)70053-8. [DOI] [PubMed] [Google Scholar]
- 155.Tukvadze N., Sanikidze E., Kipiani M., Hebbar G., Easley K.A., Shenvi N., Kempker R.R., Frediani J.K., Mirtskhulava V., Alvarez J.A., et al. High-dose vitamin D3 in adults with pulmonary tuberculosis: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2015;102:1059–1069. doi: 10.3945/ajcn.115.113886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ganmaa D., Munkhzul B., Fawzi W., Spiegelman D., Willett W.C., Bayasgalan P., Baasansuren E., Buyankhishig B., Oyun-Erdene S., Jolliffe D.A., et al. High-Dose Vitamin D(3) during Tuberculosis Treatment in Mongolia. A Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2017;196:628–637. doi: 10.1164/rccm.201705-0936OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wejse C., Gomes V.F., Rabna P., Gustafson P., Aaby P., Lisse I.M., Andersen P.L., Glerup H., Sodemann M. Vitamin D as Supplementary Treatment for Tuberculosis. Am. J. Respir. Crit. Care Med. 2009;179:843–850. doi: 10.1164/rccm.200804-567OC. [DOI] [PubMed] [Google Scholar]
- 158.Rello J., Valenzuela-Sánchez F., Ruiz-Rodriguez M., Moyano S. Sepsis: A Review of Advances in Management. Adv. Ther. 2017;34:2393–2411. doi: 10.1007/s12325-017-0622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.de Haan K., Groeneveld A.B.J., de Geus H.R.H., Egal M., Struijs A. Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: Systematic review and meta-analysis. Crit. Care. 2014;18:660. doi: 10.1186/s13054-014-0660-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vipul P., Shuchi C., Avinash A., Manish G., Sukriti K., Ved P. Correlation of Serum Vitamin D Level with Mortality in Patients with Sepsis. Indian J. Crit. Care Med. 2017;21:199–204. doi: 10.4103/ijccm.IJCCM_192_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kempker J.A., Han J.E., Tangpricha V., Ziegler T.R., Martin G.S. Vitamin D and sepsis: An emerging relationship. Dermatol. Endocrinol. 2012;4:101–108. doi: 10.4161/derm.19859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rübsamen D., Kunze M.M., Buderus V., Brauß T.F., Bajer M.M., Brüne B., Schmid T. Inflammatory conditions induce IRES-dependent translation of cyp24a1. PLoS ONE. 2014;9:e85314. doi: 10.1371/journal.pone.0085314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dahl B., Schiodt F.V., Ott P., Wians F., Lee W.M., Balko J., O’Keefe G.E. Plasma concentration of Gc-globulin is associated with organ dysfunction and sepsis after injury. Crit. Care Med. 2003;31:152–156. doi: 10.1097/00003246-200301000-00024. [DOI] [PubMed] [Google Scholar]
- 164.Quraishi S.A., De Pascale G., Needleman J.S., Nakazawa H., Kaneki M., Bajwa E.K., Camargo C.A., Jr., Bhan I. Effect of Cholecalciferol Supplementation on Vitamin D Status and Cathelicidin Levels in Sepsis: A Randomized, Placebo-Controlled Trial. Crit. Care Med. 2015;43:1928–1937. doi: 10.1097/CCM.0000000000001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kew R.R. The Vitamin D Binding Protein and Inflammatory Injury: A Mediator or Sentinel of Tissue Damage? Front. Endocrinol. (Lausanne) 2019;10:470. doi: 10.3389/fendo.2019.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Leaf D.E., Raed A., Donnino M.W., Ginde A.A., Waikar S.S. Randomized controlled trial of calcitriol in severe sepsis. Am. J. Respir. Crit. Care Med. 2014;190:533–541. doi: 10.1164/rccm.201405-0988OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Amrein K., Schnedl C., Holl A., Riedl R., Christopher K.B., Pachler C., Urbanic Purkart T., Waltensdorfer A., Munch A., Warnkross H., et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: The VITdAL-ICU randomized clinical trial. JAMA. 2014;312:1520–1530. doi: 10.1001/jama.2014.13204. [DOI] [PubMed] [Google Scholar]
- 168.Martucci G., McNally D., Parekh D., Zajic P., Tuzzolino F., Arcadipane A., Christopher K.B., Dobnig H., Amrein K. Trying to identify who may benefit most from future vitamin D intervention trials: A post hoc analysis from the VITDAL-ICU study excluding the early deaths. Crit. Care. 2019;23:200. doi: 10.1186/s13054-019-2472-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Han J.E., Jones J.L., Tangpricha V., Brown M.A., Brown L.A.S., Hao L., Hebbar G., Lee M.J., Liu S., Ziegler T.R., et al. High Dose Vitamin D Administration in Ventilated Intensive Care Unit Patients: A Pilot Double Blind Randomized Controlled Trial. J. Clin. Transl. Endocrinol. 2016;4:59–65. doi: 10.1016/j.jcte.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.National Heart L., Blood Institute P.C.T.N., Ginde A.A., Brower R.G., Caterino J.M., Finck L., Banner-Goodspeed V.M., Grissom C.K., Hayden D., Hough C.L., et al. Early High-Dose Vitamin D3 for Critically Ill, Vitamin D-Deficient Patients. N. Engl. J. Med. 2019;381:2529–2540. doi: 10.1056/NEJMoa1911124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hope-Simpson R.E. The role of season in the epidemiology of influenza. J. Hyg. (Lond.) 1981;86:35–47. doi: 10.1017/S0022172400068728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cannell J.J., Vieth R., Umhau J.C., Holick M.F., Grant W.B., Madronich S., Garland C.F., Giovannucci E. Epidemic influenza and vitamin D. Epidemiol. Infect. 2006;134:1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gunville C.F., Mourani P.M., Ginde A.A. The role of vitamin D in prevention and treatment of infection. Inflamm. Allergy Drug Targets. 2013;12:239–245. doi: 10.2174/18715281113129990046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ingham T.R., Jones B., Camargo C.A., Kirman J., Dowell A.C., Crane J., Stanley T.V., Grimwood K., The Whiti Te Ra Study G. Association of vitamin D deficiency with severity of acute respiratory infection: A case-control study in New Zealand children. Eur. Respir. J. 2014;44:439. [Google Scholar]
- 175.Sabetta J.R., DePetrillo P., Cipriani R.J., Smardin J., Burns L.A., Landry M.L. Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults. PLoS ONE. 2010;5:e11088. doi: 10.1371/journal.pone.0011088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kuchar E., Miśkiewicz K., Nitsch-Osuch A., Szenborn L. Pathophysiology of Clinical Symptoms in Acute Viral Respiratory Tract Infections. Adv. Exp. Med. Biol. 2015;857:25–38. doi: 10.1007/5584_2015_110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Matthay M.A., Zemans R.L. The acute respiratory distress syndrome: Pathogenesis and treatment. Annu. Rev. Pathol. 2011;6:147–163. doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Beard J.A., Bearden A., Striker R. Vitamin D and the anti-viral state. J. Clin. Virol. 2011;50:194–200. doi: 10.1016/j.jcv.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Martineau A.R., Jolliffe D.A., Hooper R.L., Greenberg L., Aloia J.F., Bergman P., Dubnov-Raz G., Esposito S., Ganmaa D., Ginde A.A., et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fouad M.N., Ruffin J., Vickers S.M. COVID-19 is Out of Proportion in African Americans. This Will Come as No Surprise. Am. J. Med. 2020:30411–30413. doi: 10.1016/j.amjmed.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dietz W., Santos-Burgoa C. Obesity and its Implications for COVID-19 Mortality. Obesity. 2020;28:1005. doi: 10.1002/oby.22818. [DOI] [PubMed] [Google Scholar]
- 182.Cui C., Xu P., Li G., Qiao Y., Han W., Geng C., Liao D., Yang M., Chen D., Jiang P. Vitamin D receptor activation regulates microglia polarization and oxidative stress in spontaneously hypertensive rats and angiotensin II-exposed microglial cells: Role of renin-angiotensin system. Redox Biol. 2019;26:101295. doi: 10.1016/j.redox.2019.101295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., Bhattoa H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients. 2020;12:988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mendy A., Apewokin S., Wells A.A., Morrow A.L. Factors Associated with Hospitalization and Disease Severity in a Racially and Ethnically Diverse Population of COVID-19 Patients. medRxiv. 2020 doi: 10.1101/2020.06.25.20137323. [DOI] [Google Scholar]
- 185.Hossein-nezhad A., Holick M.F. Vitamin D for Health: A Global Perspective. Mayo Clin. Proc. 2013;88:720–755. doi: 10.1016/j.mayocp.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shirvani A., Kalajian T.A., Song A., Holick M.F. Disassociation of Vitamin D’s Calcemic Activity and Non-calcemic Genomic Activity and Individual Responsiveness: A Randomized Controlled Double-Blind Clinical Trial. Sci. Rep. 2019;9:17685. doi: 10.1038/s41598-019-53864-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Carlberg C., Seuter S., de Mello V.D.F., Schwab U., Voutilainen S., Pulkki K., Nurmi T., Virtanen J., Tuomainen T.-P., Uusitupa M. Primary vitamin D target genes allow a categorization of possible benefits of vitamin D₃ supplementation. PLoS ONE. 2013;8:e71042. doi: 10.1371/journal.pone.0071042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Carlberg C., Haq A. The concept of the personal vitamin D response index. J. Steroid Biochem. Mol. Biol. 2018;175:12–17. doi: 10.1016/j.jsbmb.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 189.Shirvani A., Kalajian T.A., Song A., Allen R., Charoenngam N., Lewanczuk R., Holick M.F. Variable Genomic and Metabolomic Responses to Varying Doses of Vitamin D Supplementation. Anticancer Res. 2020;40:535–543. doi: 10.21873/anticanres.13982. [DOI] [PubMed] [Google Scholar]
- 190.Manson J.E., Cook N.R., Lee I.M., Christen W., Bassuk S.S., Mora S., Gibson H., Gordon D., Copeland T., D’Agostino D., et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2018;380:33–44. doi: 10.1056/NEJMoa1809944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pittas A.G., Dawson-Hughes B., Sheehan P., Ware J.H., Knowler W.C., Aroda V.R., Brodsky I., Ceglia L., Chadha C., Chatterjee R., et al. Vitamin D Supplementation and Prevention of Type 2 Diabetes. N. Engl. J. Med. 2019;381:520–530. doi: 10.1056/NEJMoa1900906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lu S., Guo S., Hu F., Guo Y., Yan L., Ma W., Wang Y., Wei Y., Zhang Z., Wang Z. The Associations Between the Polymorphisms of Vitamin D Receptor and Coronary Artery Disease: A Systematic Review and Meta-Analysis. Medicine. 2016;95:e3467. doi: 10.1097/MD.0000000000003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Xu Y., He B., Pan Y., Deng Q., Sun H., Li R., Gao T., Song G., Wang S. Systematic review and meta-analysis on vitamin D receptor polymorphisms and cancer risk. Tumor Biol. 2014;35:4153–4169. doi: 10.1007/s13277-013-1544-y. [DOI] [PubMed] [Google Scholar]
- 194.Monticielo O.A., Teixeira T.d.M., Chies J.A.B., Brenol J.C.T., Xavier R.M. Vitamin D and polymorphisms of VDR gene in patients with systemic lupus erythematosus. Clin. Rheumatol. 2012;31:1411–1421. doi: 10.1007/s10067-012-2021-5. [DOI] [PubMed] [Google Scholar]
- 195.Wang L., Wang Z.T., Hu J.J., Fan R., Zhou J., Zhong J. Polymorphisms of the vitamin D receptor gene and the risk of inflammatory bowel disease: A meta-analysis. Genet. Mol. Res. 2014;13:2598–2610. doi: 10.4238/2014.April.8.2. [DOI] [PubMed] [Google Scholar]
- 196.Gao X.-R., Yu Y.-G. Meta-Analysis of the Association between Vitamin D Receptor Polymorphisms and the Risk of Autoimmune Thyroid Disease. Int. J. Endocrinol. 2018;2018:2846943. doi: 10.1155/2018/2846943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bikle D.D., Schwartz J. Vitamin D Binding Protein, Total and Free Vitamin D Levels in Different Physiological and Pathophysiological Conditions. Front. Endocrinol. (Lausanne) 2019;10:317. doi: 10.3389/fendo.2019.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hossein-nezhad A., Spira A., Holick M.F. Influence of Vitamin D Status and Vitamin D3 Supplementation on Genome Wide Expression of White Blood Cells: A Randomized Double-Blind Clinical Trial. PLoS ONE. 2013;8:e58725. doi: 10.1371/journal.pone.0058725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sethuraman G., Marwaha R.K., Challa A., Yenamandra V.K., Ramakrishnan L., Thulkar S., Sharma V.K. Vitamin D: A New Promising Therapy for Congenital Ichthyosis. Pediatrics. 2016;137 doi: 10.1542/peds.2015-1313. [DOI] [PubMed] [Google Scholar]