Abstract
P2Y12 receptor blocking drugs given at reperfusion offer protection against myocardial infarction in animal models of transient coronary occlusion. Two recent reports concluded that ticagrelor was more cardioprotective than clopidogrel, and attributed this to ticagrelor’s unique ability to raise tissue adenosine by blocking the equilibrative nucleoside transporter 1. Indeed, an adenosine receptor blocker attenuated ticagrelor’s protection. The related P2Y12 inhibitor cangrelor, which does not block the transporter, protects hearts only when platelets are in the perfusate, while adenosine is known to protect equally in situ blood-perfused and crystalloid-perfused isolated hearts. We, therefore, tested whether ticagrelor liberates a sufficient amount of adenosine to protect a Krebs buffer-perfused isolated rat heart subjected to 40 min of global ischemia followed by 2h of reperfusion. In untreated hearts 77.6±4.0% of the ventricle was infarcted as measured by triphenyltetrazolium staining. Ischemically preconditioned hearts had only 32.7±3.6% infarction (p<0.001 vs untreated) indicating that our model could be protected by preconditioning which is known to involve adenosine. Strikingly, hearts treated with 10 μM ticagrelor in the buffer throughout the reperfusion period had 77.5±2.4% infarction comparable to unprotected controls (p=NS vs untreated). These data strongly suggest that ticagrelor was unable to release sufficient adenosine from the crystalloid-perfused rat heart to protect it against infarction. Our previous studies have found no difference in the anti-infarct potency among clopidogrel, cangrelor and ticagrelor in open-chest rats and rabbits, and surprisingly adenosine receptor antagonists block protection from all three drugs. We have no explanation why ticagrelor is more protective in the pig than clopidogrel, but suspect a species or perhaps a treatment schedule difference that may or may not involve adenosine.
Keywords: clopidogrel, equilibrative nucleoside transporter, myocardial infarction, ticagrelor
Today P2Y12 antagonists, which block binding of ADP to platelet receptors, are standard-of-care in patients with acute myocardial infarction (AMI) whose arteries are stented during recanalization by primary percutaneous intervention. While the initial use of these anti-aggregant drugs was based on their anticoagulant properties, emerging evidence has revealed pleiotropic effects which could be even more important than anticoagulation. These studies clearly demonstrate that P2Y12 antagonists clopidogrel, ticagrelor, and cangrelor are all potent postconditioning-mimetics. When administered just a few minutes before reperfusion in acute animal models of myocardial ischemia/reperfusion (I/R), either ticagrelor1–3 or cangrelor2,4, both direct antagonists of the P2Y12 receptor, significantly diminish myocardial infarct size. Clopidogrel, however, is a pro-drug and must be enzymatically converted to the active P2Y12 ligand before it can protect. When it is given just prior to reperfusion in animal models, it is not protective.1 However, if sufficient time is allowed for conversion to its active form as evidenced by blockade of platelet reactivity, then clopidogrel does become cardioprotective.4,5 Vilahur and colleagues pretreated pigs with clopidogrel or ticagrelor several hours before the ischemic insult so that robust platelet inhibition occurred in both groups, and notably the anti-infarct effect of ticagrelor was greater than that of clopidogrel.5 In our rabbit model we saw equal protection from cangrelor or two-day pretreatment with clopidogrel at doses that completely abolished ADP-induced platelet aggregation.4 In contrast, Nanhwan et al.6 reported that treatment for 1 week with either ticagrelor or clopidogrel caused similar attenuation (but not complete abolition) of platelet aggregation in rats, but only ticagrelor was cardioprotective. Whether clopidogrel’s failure to limit infarct size was due to a species difference or tachyphylaxis from prolonged exposure or perhaps inadequate attenuation of aggregation is unknown.
In addition to its action as a P2Y12 antagonist ticagrelor blocks the equilibrative nucleoside transporter (ENT) 1 which raises the tissue adenosine level.7 Two groups have recently suggested that this non-canonical action of ticagrelor might explain the observation that ticagrelor is a more potent cardioprotectant than clopidogrel which has no effect on ENT 1.1,5,8 Proof of this suggestion largely rests on the ability of a non-selective adenosine receptor antagonist to block ticagrelor’s infarct-sparing effect, but an adenosine receptor antagonist also blocked the protection from prolonged clopidogrel treatment.4
The actual mechanism(s) whereby P2Y12 inhibition triggers conditioning’s protective signaling remains unknown, but it appears to involve platelets. Cangrelor will not protect either a blood-free, isolated heart4 or the heart of a thrombocytopenic rat.9However, increasing adenosine concentration can trigger protection against infarction in the blood-free isolated heart.10 To objectively evaluate the potential role of ticagrelor-mediated inhibition of ENT 1 in cardioprotection, ticagrelor’s effectiveness as a cardioprotectant was examined in a buffer-perfused isolated rat heart in which the drug’s putative effect on tissue adenosine could be separated from its action as a P2Y12 receptor antagonist because platelets are absent from the system.
METHODS
All protocols were approved by the Institutional Animal Care and Use Committee of the University of South Alabama College of Medicine and conformed to published guidelines.11
Surgical preparation
Male Sprague-Dawley retired breeder rats weighing 400–600 g were anesthetized with intraperitoneal pentobarbital (100 mg/kg) and then ventilated with a positive pressure respirator and 100% oxygen. Through a left thoracotomy in the fourth intercostal space the heart was quickly removed by cutting the great vessels and mounted on a Langendorff apparatus. The aorta was retroperfused under constant pressure with oxygenated, cell-free Krebs buffer. A fluid-filled balloon was placed in the left ventricular lumen and ventricular pressure recorded. Global ischemia was produced by arresting aortic perfusion for 40 min. The heart was kept at constant temperature by being immersed in a water- jacketed chamber filled with buffer maintained at 38 °C. The heart was subsequently reperfused for 2 h.
Immediately after cardiac extirpation, blood filling the thoracic cavity was collected in a heparinized syringe for platelet aggregometry as detailed below.
Measurement of infarct size
In these hearts subjected to global ischemia, the entire ventricular mass is the risk zone. The heart was removed from the Langendorff apparatus, briefly frozen, and then sliced from apex to base into 1-mm-thick slices which were incubated in warm triphenyltetrazolium chloride (TTC) solution to visualize the infarct (TTC-negative tissue). The slices were digitally photographed and images prepared for analysis. Borders of the ventricular slices and infarct regions were determined and areas calculated by Image-J software. The observer making these measurements was blinded to the treatment applied to each heart. Infarct size was expressed as a percent of the risk zone which in this case was the entire left ventricle.
Platelet aggregometry
Platelet aggregation was determined by measuring impedance with a whole blood aggregometer (Chrono-logCorp., Haverton, PA). One-half ml of saline or buffer and 0.5 ml of heparinized blood were combined in a plastic cuvette and continuously stirred at 37 °C. Aggregation was initiated by addition of 10 μl of 1 mM ADP to produce a final concentration of 10 μM. Area under each aggregation curve was measured and areas were averaged. Aggregation curves were measured in only the groups treated with ticagrelor. For these experiments platelet aggregation was determined with blood collected when the heart was removed, and the blood in the cuvette was diluted with either saline (control) or ticagrelor-containing buffer removed from the perfusion reservoir.
Protocols
Four groups of rats were studied. In the control group (n=5) the perfusing buffer contained no additives. In the next two groups ticagrelor was added to the buffer so that the final concentration was either 3 (n=5) or 10 (n=5) μM. The hearts were perfused with standard buffer before cessation of aortic retroperfusion and initiation of global ischemia. After 40 min of ischemia aortic retroperfusion was resumed for 2 h with ticagrelor-supplemented buffer. In the fourth group (n=5) hearts were ischemically preconditioned by arresting aortic perfusion for 5 min and then resuming flow for 5 min before a second cycle of ischemia/reperfusion. After 3 cycles aortic retroperfusion was arrested for 40 min and then resumed for 2 h as above.
Statistics
Infarct sizes were analyzed by one-way ANOVA. Post-hoc testing was done with Student-Newman-Keuls test. Platelet aggregation curves were analyzed by determining area beneath the curves truncated at 5 min. Paired t-test was used to compare average areas for control and ticagrelor treatment. A p value of <0.05 was considered to be significant.
RESULTS
Baseline heart rate and left ventricular developed pressure were not different in the 4 groups (data not shown). During global ischemia the hearts stopped beating but cardiac contractions resumed upon reperfusion, and some developed pressure returned in all hearts.Platelet aggregation in whole blood was almost totally blocked by ticagrelor as a verification of its efficacy. Representative platelet aggregation curves are shown in Fig. 1.
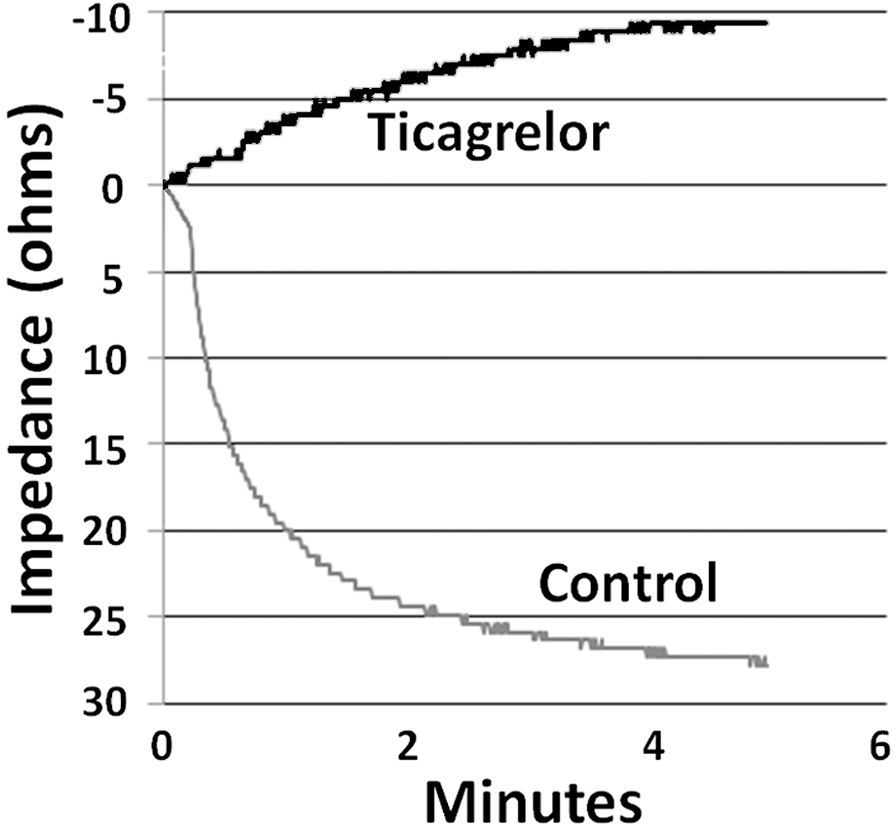
Figure 1.
Platelet aggregation curves in a representative experiment. For the control curve blood was mixed with Krebs buffer in the cuvette to which ADP was added to initiate aggregation. For the ticagrelor curve blood was mixed with buffer containing 10 μM ticagrelor. The control aggregation curve shows the result of progressive aggregation over a 5-min interval. The ticagrelor curve shows that aggregation was completely blocked and demonstrates only drift.
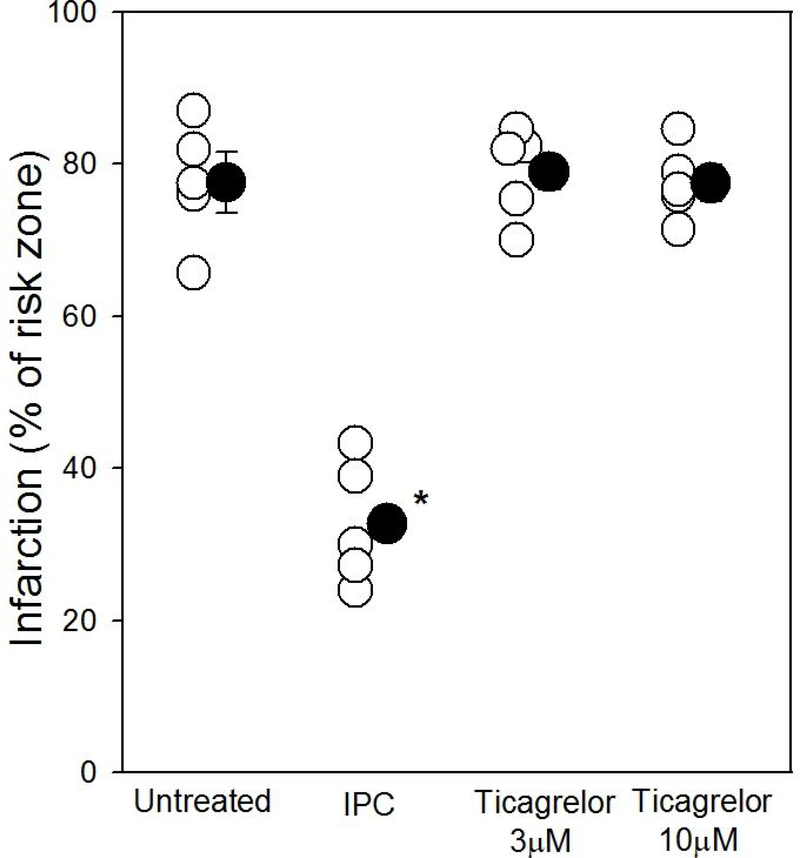
Infarct size in control, untreated hearts averaged 77.6±4.0% of the risk zone after 40 min of global ischemia and 2 h of reperfusion (Fig. 2). Neither 3 nor 10 μM ticagrelor, which demonstrated potent inhibition of platelet aggregation activity in whole blood, affected the amount of infarction (79.1±2.7% and 77.5±2.4%, respectively). Isolated hearts did appear to respond to adenosine because ischemic preconditioning as a positive control significantly decreased infarct size to 32.7±3.6% of myocardium at risk (p<0.001). Figure 3 shows 3 typical hearts sectioned into 1mm thick TTC-stained slices. Thus our experiments did not detect any protection against infarction from ticagrelor in the isolated rat heart, and hence fail to demonstrate adenosine-mediated cardioprotection from ticagrelor.
Figure 2.
Percentage of the left ventricle infarcted after 40 minutes of global ischemia and 2 h of reperfusion in Krebs-perfused isolated hearts when exposed to either ischemic preconditioning (IPC) or 1 of 2 doses of ticagrelor. Open symbols represent data from individual hearts, whereas closed symbols represent group averages ± SEM. Only ischemic preconditioning offered any protection against infarction as compared to untreated hearts or those exposed to ticagrelor. *p<0.001 vs other groups
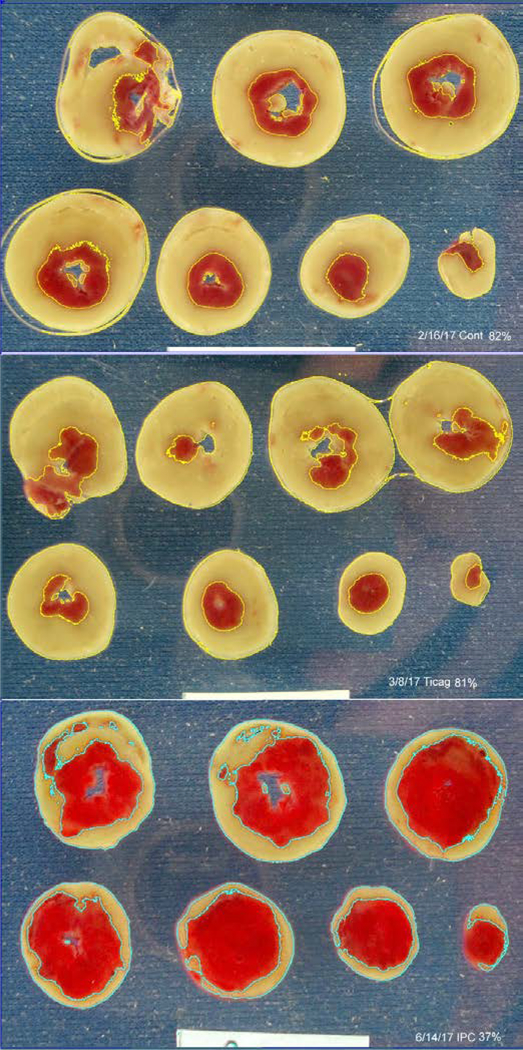
Figure 3.
Composite of typical hearts stained with triphenyltetrazolium chloride (TTC) from the study. The top panel shows an untreated heart (82% infarction), the middle a ticagrelor-treated heart (81% infarction), and the bottom an ischemically preconditioned heart (37% infarction). The tan tissue is TTC-negative and thus necrotic. The thin yellow or blue outlines mark the necrotic zones selected by Image-J’s color threshold function. The white rectangle at the bottom is a 2 cm wide standard.
DISCUSSION
Previous investigations by us2, 3,4 and others1,5,8 have demonstrated the direct cardioprotective properties of one or more of the P2Y12 antagonists clopidogrel, ticagrelor, and cangrelor. We have found that the protective effect of cangrelor is dependent on the presence of platelets. Thus in severely thrombocytopenic rats9 and in isolated hearts perfused with cell-free, crystalloid buffer,4,12 cangrelor no longer results in smaller infarcts. We now extend this observation to a second P2Y12 antagonist, ticagrelor.Although in the absence of platelets ticagrelor is no longer cardioprotective, its proposed ability to block ENT 1 should have been intact. Thus it would appear that ticagrelor’s effect on adenosine uptake by itself is not sufficient to trigger protection in the isolated rat heart.
Cangrelor and ticagrelor are equally cardioprotective in our open-chest rat model,2 but cangrelor has little effect on ENT 1.7The major metabolite of cangrelor does have a weak affinity for ENT 1, but the IC50 for inhibition of adenosine uptake in Madin-Darby canine kidney cells expressing human ENT 1 is 7-fold lower for ticagrelor than the cangrelor metabolite7 and, therefore, ENT 1 inhibition would not be expected from an anti-aggregation dose of cangrelor.Neither clopidogrel nor its active metabolite has any influence on ENT 1.7 Clopidogrel and cangrelor were also similarly protective in our rabbit heart I/R model when clopidogrel was allowed enough time to achieve platelet inhibition equal to that from cangrelor.4
We used two doses of ticagrelor in the present study. The 3 μM concentration was based on published dose response curves of ticagrelor’s ex vivo effect on ENT 1 from rat kidney.13 The 10 μM concentration was based on the treated buffer’s ability to block platelets in a blood sample. Ten μM was higher than the theoretical blocking dose for the P2Y12 receptor probably because some of the ticagrelor would have been bound by serum proteins in the blood and also because the buffer sample was diluted 100% in the aggregometer by an equal quantity of blood. Whatever the case, neither dose showed any protection.
Both Ye et al.1 and Vilahur and colleagues5 observed greater cardioprotective effects with ticagrelor than with clopidogrel and attributed the difference to the former’s ability to block adenosine uptake and increase tissue adenosine levels.Ye et al.1 administered clopidogrel and ticagrelor intraperitoneally to rats only 5 min before reperfusion, but only ticagrelor was protective. As noted above clopidogrel is a pro-drug and must be enzymatically activated by a slow, multistep process by hepatic p450 enzymes.14Therefore, it is not surprising that there was no reduction in infarct size in their clopidogrel group since little of the active metabolite would have been present during the critical first few minutes of reperfusion. We found cangrelor to be highly protective when administered to rabbits 5 min prior to reperfusion, but it offers no protection when started just 10 min after reperfusion indicating P2Y12 receptor inhibition prevents a lethal injury that occurs in the first several minutes of reperfusion.4It is important to note that the effect of clopidogrel as well as ticagrelor on platelet aggregation was not tested by Ye et al.1 until the termination of the experiment 2 h later, enough time for much of the clopidogrel to be converted to its active metabolite.
When clopidogrel was administered to pigs 4 hours before coronary occlusion, it was cardioprotective. But ticagrelor resulted in 23% smaller infarcts than clopidogrel despite the fact that platelet inhibition during coronary occlusion based on aggregometry was not different between the groups and that and bleeding time was actually higher in the clopidogrel group.5 The finding that the protection from ticagrelor could be partially blocked by the non-selective adenosine receptor blocker 8-(p-sulfophenyl)theophylline (8SPT) was offered as supportive evidence of the ENT-1 hypothesis. However, it was not tested whether 8SPT could block clopidogrel’s protection.15
Prior investigations have demonstrated that many of the signaling steps triggered by P2Y12 antagonists that lead to cardioprotection are identical to those documented for conditioning interventions. We have shown this most extensively for cangrelor,4 but also for ticagrelor3 and clopidogrel.4In the relevant signal transduction pathway there are two points at which adenosine receptors are involved.16In the trigger sequence which occurs during the preconditioning ischemia, released adenosine can bind to A1 receptors to initiate the signaling. In the mediator phase which occurs at reperfusion the A2B adenosine receptor is occupied because its affinity appears to be increased, presumably the result of phosphorylation by PKC. Both non-selective and selective A2B adenosine antagonists can block signaling and abort cardioprotection from pre- or postconditioning or the P2Y12 inhibitors cangrelor and clopidogrel.4 Obviously, blockade of ticagrelor’s protection by a non-selective adenosine receptor antagonist can no longer be used as evidence that ticagrelor’s superior protection in pigs results from ENT 1-generated adenosine.
One shortcoming of our isolated rat heart model is that it does not include circulating red blood cells. Ticagrelor has been shown to promote release of ATP from erythrocytes as well as inhibit adenosine reuptake by them.17 Since ATP in the plasma would quickly be converted to adenosine, our model may lack an important adenosine source. Dipyridamole also blocks adenosine reuptake by erythrocytes, but its effect on infarct size after myocardial ischemia/reperfusion is confusing.Dipyridamole given acutely to dogs prior to in situ regional ischemia/reperfusion had no effect on infarct size at any of 3 doses tested,18 and 3 days of oral pretreatment of rats also had no effect.19 On the other hand, acute administration of dipyridamole 5 min after onset of a 30-min coronary occlusion in rats halved infarct size.20 Dipyridamole does, however, increase the potency of ischemic preconditioning against infarction in in situ rabbit hearts, but it only did so when administered prior to the short preconditioning period of ischemia. When started after the preconditioning ischemia but before the index ischemia, dipyridamole offered no significant protection indicating that blocking adenosine reuptake only increased ischemic preconditioning’s ability to trigger the protected state.21It is possible that ticagrelor’s blockade of adenosine uptake reinforces its preconditioning-like effect when given as a pretreatment as was done by Vilahur et al.5 Because both cangrelor and ticagrelor were infused after ischemia had begun in our rat study,2 those hearts might not have benefited from the adenosine boost. In the clinical setting of primary percutaneous intervention such pretreatment with a platelet inhibitor would not be possible.
The present study failed to provide support for the hypothesis that augmented adenosine release from ticagrelor’s effect on ENT 1 is responsible for ticagrelor’s greater potency against infarction. But it does not disprove it either. A small increase of adenosine by ENT-1 inhibition may not by itself be enough to reach the threshold for infarct size reduction directly in the blood-free isolated heart. However, it is still possible that adding adenosine to the cardioprotective process resulting from platelet P2Y12 receptor blockade in an in situ heart could have augmented the protection. We cannot explain why ticagrelor is so much more protective in the pig than clopidogrel, but since no difference in potency was found between cangrelor and ticagrelor in our recent rat study2 we suspect either a possible species difference that may or may not involve adenosine or perhaps a protocol difference as discussed above.
Regardless of whether adenosine plays a role in its protection, we agree that in the setting of reperfusion therapy for acute myocardial infarction ticagrelor is a superior drug compared to clopidogrel. The latter suffers from an onset of effect that is too slow for acute treatment as well as a common genetic variation that negates its metabolic activation in a significant portion of the patient population.22
The importance of the present study is underscored by controversies in the literature regarding the proposed mechanism of action of P2Y12 drugs solely as anti-platelet aggregants and a failure to fully appreciate their pleiotropic cardioprotective effects. Indeed, our recent work shows that drugs such as the caspase-1 inhibitor VX-765, that act via mechanisms distinct to the adenosine-dependent cardioprotective pathways, can significantly add to the cardioprotective effects of P2Y12 drugs that are delivered to patients with AMI as standard-of-care.2 Together, ours and other studies suggest that all new anti-infarct interventions should be shown to be capable of adding protection to that from a P2Y12 inhibitor as part of the consideration for their clinical testing.
Acknowledgments
Funding
This study was supported in part by grant HL-118334 (DFA and JPA) from the Heart, Lung, and Blood Institute of the National Institutes of Health.
Footnotes
Conflict of interest
All authors declare absence of conflicts of interest.
COMPLIANCE WITH ETHICAL STANDARDS
Ethical Approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed.
This article does not contain any studies with human participants performed by any of the authors.
References
- 1.Ye Y, Birnbaum GD, Perez-Polo JR, Nanhwan MK, Nylander S, Birnbaum Y. Ticagrelor protects the heart against reperfusion injury and improves remodeling after myocardial infarction. Arterioscler Thromb Vasc Biol. 2015;35:1805–1814. [DOI] [PubMed] [Google Scholar]
- 2.Audia JP, Yang XM, Crockett ES, et al. Caspase-1 Inhibition by VX-765 Administered at Reperfusion in P2Y12 Receptor Antagonist-Treated Rats Provides Long-Term Reduction in Myocardial Infarct Size and Preservation of Ventricular Function. Basic Res Cardiol. 2018;113:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X-M, Cui L, Alhammouri A, Downey JM, Cohen MV. Triple therapy greatly increases myocardial salvage during ischemia/reperfusion in the in situ rat heart. Cardiovasc Drugs Ther. 2013;27:403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X-M, Liu Y, Cui L, et al. Platelet P2Y12 blockers confer direct postconditioning-like protection in reperfused rabbit hearts. J Cardiovasc Pharmacol Ther. 2013;18:251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vilahur G, Gutiérrez M, Casani L, et al. Protective effects of ticagrelor on myocardial injury after infarction. Circulation. 2016;134:1708–1719. [DOI] [PubMed] [Google Scholar]
- 6.Nanhwan MK, Ling S, Kodakandla M, Nylander S, Ye Y, Birnbaum Y. Chronic treatment with ticagrelor limits myocardial infarct size: an adenosine and cyclooxygenase-2-dependent effect. Arterioscler Thromb Vasc Biol. 2014;34:2078–2085. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong D, Summers C, Ewart L, Nylander S, Sidaway JE, van Giezen JJJ. Characterization of the adenosine pharmacology of ticagrelor reveals therapeutically relevant inhibition of equilibrative nucleoside transporter 1. J Cardiovasc Pharmacol Ther. 2014;19:209–219. [DOI] [PubMed] [Google Scholar]
- 8.Birnbaum Y, Birnbaum GD, Birnbaum I, Nylander S, Ye Y. Ticagrelor and rosuvastatin have additive cardioprotective effects via adenosine. Cardiovasc Drugs Ther. 2016;30:539–550. [DOI] [PubMed] [Google Scholar]
- 9.Cohen MV, Yang X-M, White J, Yellon DM, Bell RM, Downey JM. Cangrelor-mediated cardioprotection requires platelets and sphingosine phosphorylation. Cardiovasc Drugs Ther. 2016;30:229–232. [DOI] [PubMed] [Google Scholar]
- 10.Woolfson RG, Patel VC, Yellon DM. Pre-conditioning with adenosine leads to concentration-dependent infarct size reduction in the isolated rabbit heart. Cardiovasc Res. 1996;31:148–151. [PubMed] [Google Scholar]
- 11.Council NR. Guide for the Care and Use of Laboratory Animals: Eighth Edition. et al. ed. ed. Washington, D.C.: National Academy Press; 2011. [Google Scholar]
- 12.Bell RM, Sivaraman V, Kunuthur SP, Cohen MV, Downey JM, Yellon DM. Cardioprotective properties of the platelet P2Y12 receptor inhibitor, cangrelor: protective in diabetics and reliant upon the presence of blood. Cardiovasc Drugs Ther. 2015;29:415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Giezen JJJ, Sidaway J, Glaves P, Kirk I, Björkman J-A. Ticagrelor inhibits adenosine uptake in vitro and enhances adenosine-mediated hyperemia responses in a canine model. J Cardiovasc Pharmacol Ther. 2012;17:164–172. [DOI] [PubMed] [Google Scholar]
- 14.Sangkuhl K, Klein TE, Altman RB. Clopidogrel pathway. Pharmacogenet Genomics. 2010;20:463–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Downey JM, Cohen MV. Letter by Downey and Cohen regarding article, “Protective effects of ticagrelor on myocardial injury after infarction”. Circulation. 2017;135:e1000–e1001. [DOI] [PubMed] [Google Scholar]
- 16.Cohen MV, Downey JM. Signalling pathways and mechanisms of protection in pre- and postconditioning: historical perspective and lessons for the future. Br J Pharmacol. 2015;172:1913–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohman J, Kudira R, Albinsson S, Olde B, Erlinge D. Ticagrelor induces adenosine triphosphate release from human red blood cells. Biochem Biophys Res Commun. 2012;418:754–758. [DOI] [PubMed] [Google Scholar]
- 18.Matsuoka H, Henrichs KJ, Schaper W. Influence of dipyridamole on infarct size and on cardiac nucleoside content following coronary occlusion in the dog. Basic Res Cardiol. 1985;80:682–692. [DOI] [PubMed] [Google Scholar]
- 19.Ye Y, Lin Y, Perez-Polo R, et al. Enhanced cardioprotection against ischemia-reperfusion injury with a dipyridamole and low-dose atorvastatin combination. Am J Physiol. 2007;293:H813–H818. [DOI] [PubMed] [Google Scholar]
- 20.Ye Y, Long B, Qian J, Perez-Polo JR, Birnbaum Y. Dipyridamole with low-dose aspirin augments the infarct size-limiting effects of simvastatin. Cardiovasc Drugs Ther. 2010;24:391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki K, Miura T, Miki T, Tsuchida A, Shimamoto K. Infarct-size limitation by preconditioning is enhanced by dipyridamole administered before but not after preconditioning: evidence for the role of interstitial adenosine level during preconditioning as a primary determinant of cardioprotection. J Cardiovasc Pharmacol. 1998;31:1–9. [DOI] [PubMed] [Google Scholar]
- 22.Frere C, Cuisset T, Morange P-E, et al. Effect of cytochrome p450 polymorphisms on platelet reactivity after treatment with clopidogrel in acute coronary syndrome. Am J Cardiol. 2008;101:1088–1093. [DOI] [PubMed] [Google Scholar]