Abstract
Programmed death-ligand 1 (PD-L1) plays an essential role in tumor cell escape from anti-tumor immunity in various types of cancer, including gastric cancer (GC). The present study investigated the intracellular and membrane-bound expression of PD-L1 in the GC cell lines MKN1, MKN74, KATO III and OCUM-1. Furthermore, soluble PD-L1 (sPD-L1) level in the supernatant of GC cells and the serum of patients with GC and healthy controls was determined by ELISA. Interferon (IFN)-γ treatment of cells resulted in increased cytoplasmic expression of PD-L1 in GC cells in a dose-dependent manner, except for MKN74 cells; however, there was no association between tumor necrosis factor-α treatment and enhanced PD-L1 expression. Concordant with these findings, results from flow cytometry analysis demonstrated that membrane-bound PD-L1 expression was also increased following GC cell treatment with IFN-γ in a dose-dependent manner. In addition, significant sPD-L1 overproduction was observed only in the culture supernatant of OCUM-1 cells. Serum level of sPD-L1 was significantly increased in patients with GC, in particular in stage IV patients, compared with healthy controls. In conclusion, the present study demonstrated that IFN-γ treatment increased the intracellular and membrane-bound PD-L1 expression in GC cells. In addition, sPD-L1 was detected not only in the supernatant of GC cells but also in the serum of patients with GC. Further investigation on the underlying mechanism of regulation of PD-L1 expression and sPD-L1 production is required.
Keywords: gastric cancer, programmed death-ligand 1, interferon γ
Introduction
Gastric cancer (GC) is the fourth most common malignancy worldwide and is the second leading cause of cancer-associated mortality (1). Patients with chronic inflammation due to Helicobacter pylori infection, heavy alcohol drinking, heavy smoking and excessive salt intake are at high risks of developing GC (2). In particular, the rate of Helicobacter pylori infection in Japanese has been reported to be high among developed countries (3). Although patients with early GC are curable by endoscopic surgery, patients with advanced stages are usually treated with systemic chemotherapy (4). Despite advances in anticancer agents, the overall 5-year survival rate of patients with GC remains low (20%) (5).
Programmed cell death protein 1 (PD-1) and its ligand programmed death-ligand 1 (PD-L1) are important immune checkpoints in the tumor (6), and it was reported that the PD-1/PD-L1 pathway functions as adaptive immune escape machinery (7,8). Therefore, blockade of the PD-1/PD-L1 pathway by immune checkpoint inhibitors (ICIs), including pembrolizumab and nivolumab, has already been clinically applied for a variety of cancers, including GC (9). PD-L1 is expressed at the surface of tumor cells, tumor-associated macrophages (TAMs) and T lymphocytes and its expression can be induced by cytokines, such as interferons (IFNs) and tumor necrosis factors (TNFs) (10,11). Recent studies reported that the soluble form of PD-L1 (sPD-L1) is detected in the blood of patients with tumors (12,13). However, the underlying mechanisms remain unknown.
The present study aimed to evaluate PD-L1 expression and sPD-L1 secretion in GC cells following treatment with IFN-γ. Furthermore, ELISA was used to examine the serum level of sPD-L1 in patients with GC to examine its utility as a candidate biomarker.
Materials and methods
Cell culture and reagents
The human GC cell lines MKN1, MKN74, KATO III, OCUM-1 were obtained from the Health Science Research Resources Bank. MKN1, MKN74, and OCUM-1 cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen; Thermo Fisher Scientific, Inc.) and KATO III cells were cultured in RPMI-1640 medium (Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal calf serum (Invitrogen; Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.) placed at 37°C in a humidified incubator containing 5% CO2. Recombinant human interferon (IFN)-γ and TNF-α were obtained from PeproTech, Inc.
Reverse transcription-quantitative (RT-q) PCR
Total RNA was extracted from all GC cells treated with 1, 10 or 100 ng/ml TNF-α or IFN-γ for 24 h using RNeasy Mini Kit (Qiagen, Inc.) according to the manufacturer's instructions. Quantitative real-time PCR was performed with an MX3000P qPCR system (Stratagene; Agilent) using the Universal Probe Library System (Roche Diagnostics GmbH) according to the manufacturer's protocol. The thermocycling conditions were: Initial denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for 30 sec, 55°C for 60 sec, and 72°C for 60 sec. The sequences of the primers were as follows: PD-L1, forward 5′-AAATGGAACCTGGCGAAAG-3′, reverse 5′-GCTCCCTGTTTGACTCCATC-3′; and GAPDH, forward 5′-CTGACTTCAACAGCGACACC-3′ and reverse 5′-TAGCCAAATTCGTTGTCATACC-3′. Calculation of relative gene expression was performed using 2−ΔΔCq method (14).
Immunocytochemistry
All GC cells were fixed with 2% paraformaldehyde for 10 min at room temperature and blocked with 10% normal goat serum (Invitrogen; Thermo Fisher Scientific, Inc.) for 30 min at room temperature. Cells were subsequently stained with anti-PD-L1 antibody (1:100; cat. no. 13684; Cell Signaling Technology, Inc.) at 4°C overnight, followed by incubation with Alexa 555-conjugated immunoglobulin G secondary antibody (1:400; cat. no. A27039; Molecular Probes; Thermo Fisher Scientific, Inc.) for 60 min at room temperature. Subsequently, the cover slip was mounted on each well with the help of a mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Inc.). The stained cells were then observed by fluorescence microscopy (IX70; Olympus Corp.) at ×200 magnification.
Flow cytometric analysis
GC cells non-treated (Mock) or treated with 1, 10 or 100 ng/ml IFN-γ for 24 h were subjected to flow cytometric analysis. Single GC cell suspensions were stained with allophycocyanin (APC)-conjugated anti-PD-L1 antibody (BioLegend, Inc.) at a final concentration of 1 µg/ml for 30 min at 4°C. Subsequently, 1 µg/ml propidium iodide (PI) was added to eliminate dead cells for 10 min at room temperature. Flow cytometry analyses were performed using FACSCanto II (BD Biosciences). After gating for PI-negative cells (viable cells), PD-L1 expression was analyzed. Data were analyzed using Flow Jo software (version 10.5.2; TreeStar Inc.).
Western blotting
GC cells non-treated (Mock) or treated with IFN-γ (100 ng/ml) for 24 h were subjected to western blotting according to a previous study (15). Quantification of total protein in samples was performed by bicinchoninic acid protein assay (Thermo Fisher Scientific, Inc.). Briefly, whole cell lysates were prepared using 0.1% NP-40 lysis buffer (20 mM sodium phosphate, Ph 7.0; 300 mM NaCl, 5 mM EDTA and 0.1% NP40) supplemented with protease and phosphatase inhibitor cocktails (Roche Diagnostics) or sodium dodecyl sulfate (SDS)-sample buffer (25 mM Tris, pH 6.8; 1% SDS, 5% glycerol, 0.05% bromophenol blue and 1% β-mercaptoethanol). These lysates (50 µg of protein/ lane) were separated by 8% SDS-PAGE gels (Bio-Rad Laboratories, Inc.) and then transferred to a poly vinylidene di-fluoride (PVDF) membrane (Bio-Rad Laboratories, Inc.). The membranes were blocked with 5% bovine serum albumin (Sigma-Aldrich, Merck KGaA) for 1 h at room temperature. Subsequently, they were blotted with primary antibodies against PD-L1 (1:1,000; cat. no. 13684; Cell Signaling Technology, Inc.) and heat shock protein 90 (HSP90; 1:1,000; cat. no. 610418; BD Biosciences) at 4°C overnight. After washing, the membranes were incubated with anti-rabbit horseradish peroxidase (HRP)-conjugated secondary antibody (1:4,000; cat. no. NA934; GE Healthcare Life Sciences) or anti-mouse HRP-conjugated secondary antibody (1:4,000; cat. no. NA931; GE Healthcare Life Sciences) for 1 h at room temperature. The membranes were washed again and developed with Immobilon Western Chemiluminescent HRP substrate (EMD Millipore) and the signals were detected using ChemiDoc XRS systems (Bio-Rad Laboratories, Inc.). Hsp90 was used as the loading control. To quantify band intensity, densitometry was performed using Image Lab 4.1 software (Bio-Rad Laboratories, Inc.).
Patients and blood samples
Blood samples (10 ml) were collected before treatment initiation from 40 patients with GC and 10 healthy controls. Patients with GC (median age, 71.5 years; age range, 47- 94 years) who were treated at Chiba University Hospital between June 2018 and December 2019 were analyzed. Tumor size, lymph node metastasis, distant metastasis and tumor stage were determined according to the Tumor-Node-Metastasis classification for GC (16). Healthy controls (median age, 72.5 years; age range, 37–86 years) who visited Chiba University Hospital from February to March in 2020 were studied. After centrifuging for 5 min at 1,600 × g, the supernatant serum was removed and stored at −80°C. After obtaining written informed consent, blood samples were analyzed for measurement of serum sPD-L1 concentration and data were acquired from the medical record of each participant. This study was approved by the Research Ethics Committees of the Graduate School of Medicine, Chiba University (approval. no. 3552 and 3671).
ELISA
sPD-L1 levels in the culture supernatant of GC cells, and the serum of patients with GC and healthy controls were determined using a sandwich ELISA kit (cat. no. DB7H10; R&D Systems, Inc.) according to the manufacturer's instructions. sPD-L1 level in the supernatant of GC cells treated with IFN-γ (100 ng/ml) was determined using 100 µl culture supernatants collected 24 and 48 h after seeding 100,000 cells in 60 mm dishes. Serum sPD-L1 concentration in patients with GC and healthy controls were also measured before treatment initiation.
Statistical analysis
Data were presented as the means ± standard error of the mean. The significance of differences between two and multiple groups were analyzed using Mann-Whitney U test and two ways-ANOVA followed by post-hoc Tukey's test, respectively. P<0.05 was considered to indicate a statistically significant difference.
Results
PD-L1 expression in GC cells treated with TNF-α and IFN-γ
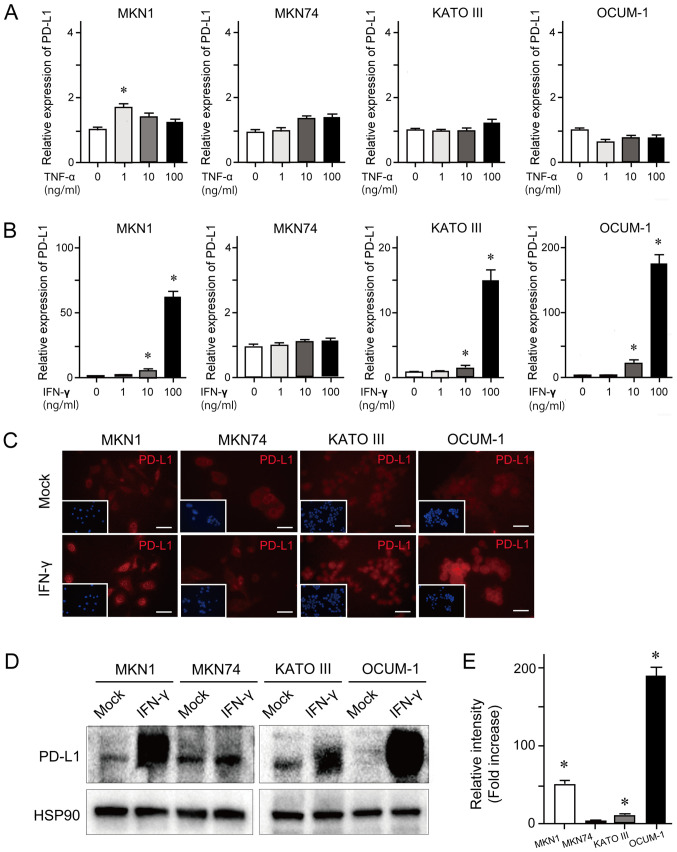
To examine cytokine-induced PD-L1 expression, RT-qPCR was performed in the GC cell lines MKN1, MKN74, KATO III and OCUM-1. These cells were treated with 1, 10 or 100 ng/ml TNF-α or IFN-γ for 24 h. The results demonstrated that TNF-α treatment at the various concentrations had no effect on PD-L1 expression level (Fig. 1A). However, IFN-γ treatment induced an increased expression level of PD-L1 in a dose-dependent manner in MKN1, KATO III and OCUM-1 cell lines (Fig. 1B). Concordant with these findings, the results from immunocytochemistry and western blotting demonstrated that IFN-γ treatment (100 ng/ml) for 24 h enhanced PD-L1 expression in MKN1, KATO III and OCUM-1 cell lines (Fig. 1C and D). The results from western blotting quantification revealed that PD-L1 expression was significantly upregulated in MKN1, KATO III and OCUM-1 cells treated with IFN-γ compared with mock-treated cells (Fig. 1E).
Figure 1.
Basal and cytokine-induced PD-L1 expression in GC cells. (A) PD-L1 mRNA expression in the presence of various concentrations of TNF-α for 24 h examined by RT-qPCR. (B) PD-L1 mRNA expression in the presence of various concentrations of IFN-γ for 24 h examined by RT-qPCR. (C) Representative images of immunocytochemistry of cells treated with IFN-γ (100 ng/ml) for 24 h. This condition demonstrated the most notable changes in PD-L1 expression among some tested conditions (1, 10 or 100 ng/ml IFN-γ for 24 h). PD-L1 (red) expression was determined at a magnification of ×200. Nuclei was stained with DAPI (blue). Scale bar, 100 µm. (D) Representative images of western blotting of cells treated with IFN-γ (100 ng/ml) for 24 h using anti-PD-L1 and anti-HSP90 (loading control) antibodies. This condition demonstrated the most notable changes in PD-L1 expression among some tested conditions (1, 10 or 100 ng/ml IFN-γ for 24 h). (E) Relative intensity of PD-L1 in IFN-γ treated cells compared to mock cells is presented. Data were expressed as the means ± standard error of the means of three independent experiments. *P<0.05. RT-qPCR, reverse transcription-quantitative PCR; PD-L1, programmed death-ligand 1; IFN-γ, interferon γ; TNF-α, tumor necrosis factor α.
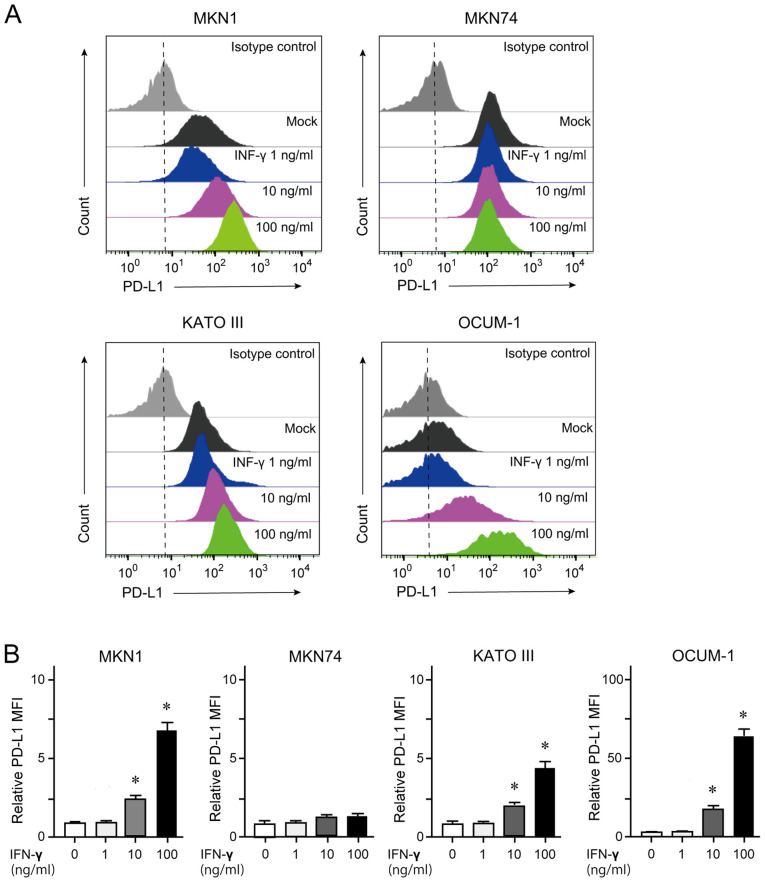
Quantification of membrane-bound PD-L1 by flow cytometry
GC cells treated with 100 ng/ml IFN-γ for 24 h were subjected to flow cytometry analyses. The results demonstrated that MKN1, KATO III and OCUM-1 cells, but not MKN74 cells, showed an increase in membrane-bound PD-L1 expression in a dose-dependent manner (Fig. 2).
Figure 2.
Flow cytometry analysis of membrane-bound PD-L1in GC cells. (A) Histogram analyses of PD-L1 expression in GC cells treated with various concentrations of IFN-γ for 24 h. (B) Mean fluorescent intensity according to data from (A) *P<0.05. PD-L1, programmed death-ligand 1; IFN-γ, interferon γ; GC, gastric cancer.
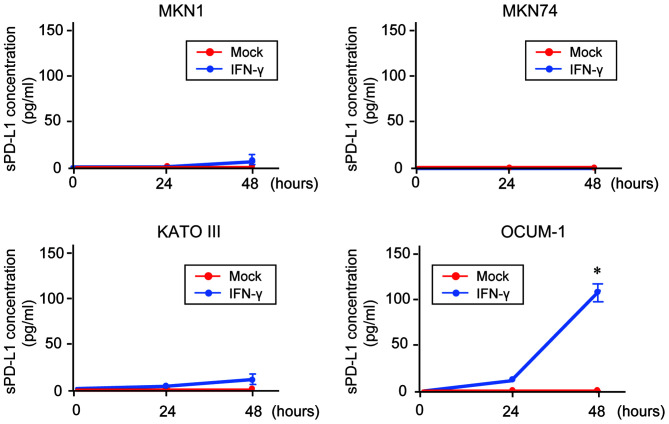
sPD-L1 detection in the culture supernatant of GC cells
To investigate the association between membrane-bound PD-L1 expression and sPD-L1 production, an ELISA was conducted for the measurement of sPD-L1 in the culture supernatant of GC cells (Fig. 3). The results demonstrated that sPD-L1 was not detected in the supernatant of untreated-GC cells. In addition, only sPD-L1 level in the supernatant of OCUM-1cells was significantly increased following IFN-γ treatment in a time-dependent manner.
Figure 3.
Detection of sPD-L1 in GC cells. sPD-L1 in supernatant of GC cells treated with IFN-γ (100 ng/ml) for 24 or 48 h measured by ELISA. *P<0.05. sPD-L1, soluble programmed death-ligand 1; IFN-γ, interferon γ; GC, gastric cancer.
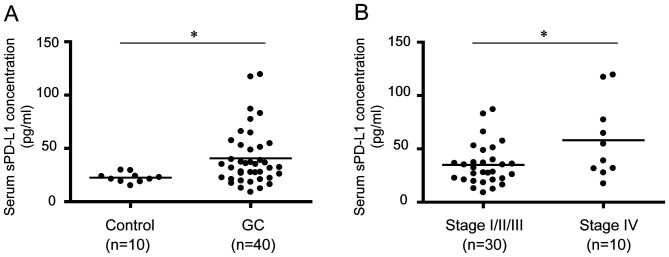
Serum sPD-L1 concentrations in GC patients
ELISA was conducted to measure the serum PD-L1 concentration in patients with GC and healthy controls (Table I). The serum PD-L1 level in patients with GC was significantly increased compared with controls (P<0.05; Fig. 4A). The median sPD-L1 levels in controls and patients with GC were 20.8 and 33.8 pg/ml, respectively. Furthermore, the serum PD-L1 level in patients with GC was evaluated according to stage progression. The results demonstrated that the serum PD-L1 level in patients with stage IV GC was significantly increased compared with that in patients with stages I, II or III GC (P<0.05; Fig. 4B).
Table I.
Clinicopathological characteristics of patients with gastric cancer.
| Characteristics | Value (n=40) |
|---|---|
| Age, years (range) | 71.5 (47–94) |
| Sex, n (male/female) | 26/14 |
| BMI, kg/m2 (range) | 21.2 (15.6–32.0) |
| Smoking, n | |
| Yes | 25 |
| No | 15 |
| Alcohol intake, n | |
| Yes | 24 |
| No | 16 |
| Helicobacter pylori, n | |
| Positive | 20 |
| Negative | 20 |
| Tumor markers, ng/ml (range) | |
| CEA | 2.85 (0.5–86.9) |
| CA19-9 | 19.1 (0–719.0) |
| Stagea, n (%) | |
| I | 10 (25) |
| II | 10 (25) |
| III | 10 (25) |
| IV | 10 (25) |
| Tumora, n (%) | |
| T1 | 8 (20) |
| T2 | 4 (10) |
| T3 | 14 (35) |
| T4 | 14 (35) |
| Lymph node metastasisa, n (%) | |
| N1 | 14 (35) |
| N2 | 5 (12.5) |
| N3 | 8 (20) |
| N4 | 13 (32.5) |
| Distant metastasisa, n (%) | |
| M0 | 30 (75.0) |
| M1 | 10 (25.0) |
| Histological finding, n (%) | |
| Diffuse | 19 (47.5) |
| Intestinal | 17 (42.5) |
| Mix | 4 (10.0) |
Tumor size, lymph node metastasis, distant metastasis and tumor stage were determined according to the Tumor-Node-Metastasis classification for gastric cancer (Union for International Cancer Control, 8th edition) (16).
Figure 4.
Measurement of sPD-L1 in the serum of patients with GC. (A) sPD-L1 in the serum of healthy controls and patients with GC. (B) sPD-L1 levels in the serum of patients with GC according to stage progression. *P<0.05. sPD-L1, soluble programmed death-ligand 1; GC, gastric cancer.
Discussion
PD-1 is a single-pass type I membrane protein that belongs to the CD28/CTLA-4 family (17). PD-1 is mainly expressed at the surface of immunocompetent cells, including T lymphocytes, B lymphocytes and natural killer cells (18). In addition, high expression level of PD-1 is associated with T-cell exhaustion (19). PD-L1 has been determined as B7 homolog 1 (B7-H1) and functions as a ligand for PD-1 (20). In normal tissues, PD-1/PD-L1 binding prevents an excessive immune response and protects tissues from damage through the induction of immune tolerance (21). However, PD-L1/PD-1-mediated tumor immune escape attenuates the immune response in cancer tissues and makes the elimination of cancer cells difficult (22). Furthermore, PD-L1 is a target for hypoxia-inducible factor-1, and PD-L1 expression is further upregulated under hypoxia (23). Overall, PD-L1 expression is closely associated with cancer development and progression.
Both aberrant expression of PD-L1 and uncontrolled PD-L1/PD-1 signaling are observed with variable frequency in various types of cancer, such as lung cancer and GC (24). A previous study demonstrated by immunohistochemistry that PD-L1 expression is detected in ~40% of GC tissues analyzed and is correlated with both aggressiveness and unfavorable prognosis (25). PD-L1 expression is regulated by inflammatory signaling, oncogenic signaling and genetic and epigenetic alterations (26,27). In addition, the co-existence of PD-L1-positive cancer cells and tumor infiltrating lymphocytes (TILs) has been reported to be associated with a poor prognosis in patients with GC (28).
Inflammatory cytokines, including IFNs, TNFs and interleukins, are mainly released form TILs and upregulate PD-L1 expression in various types of cancer cells, such as lung cancer, breast cancer, and GC (29). Similarly, the present study demonstrated that IFN-γ treatment induced a significant increase in PD-L1 expression in the GC cell lines MKN1, KATO III and OCUM-1. Furthermore, TNF-α treatment modestly increased PD-L1 expression in these cell lines. Although IFN-γ induces PD-L1 expression by stimulating the janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling pathway, scarce expression of STAT1 have been reported in some GC cells (30). This might contribute to the lack of response to IFN treatment in MKN74 cells. Subsequently, we examined whether an increase in membranous PD-L1 expression was accompanied by an upregulation of PD-L1 induced by IFN-γ treatment. The results from flow cytometry demonstrated that IFN-γ treatment induced an increase in membrane-bound PD-L1 expression in a dose-dependent manner. It is well known that membranous PD-L1 expression is closely associated with PD-1/PD-L1-mediated tumor immune escape (31). In addition, intracellular PD-L1, but not membranous PD-L1, serves a crucial role in the proliferation and migration of melanoma and ovarian cancer cells (30,32).
A recent study demonstrated that sPD-L1 is detected not only in human serum but also in culture supernatants of PD-L1-expressing cell lines (33). The present study aimed therefore to detect sPD-L1 in the supernatant of GC cell lines using ELISA. Although both intracellular and membrane-bound PD-L1 was upregulated in three cell lines treated with IFN-γ, sPD-L1 overproduction was only observed in IFN-γ-treated OCUM-1 cells. Although the accurate source of sPD-L1 remains unclear, sPD-L1 might be released or shed from PD-L1-positive tumor cells. Considering that some of disintegrin and metalloproteinase (ADAM) proteases, transmembrane protein shedding enzymes, are overexpressed in undifferentiated GC tissues (34), enhanced ADAM activity might contribute to sPD-L1 production in OCUM-1 cells. It is also possible that matrix metalloproteinases might partly be associated with sPD-L1 release (35). Alternatively, sPD-L1 has been reported to originate from its splicing variants lacking the transmembrane domain (36). Further investigation is required to elucidate the underlying mechanisms of sPD-L1 production in GC cells.
sPD-L1 is used as a prognostic biomarker in various types of cancer (37). It has been reported that sPD-L1 functions as a lure and attenuates the effect of ICI in lung cancer (38). Furthermore, the exposure of CD4+ and CD8+ lymphocytes to sPD-L1 induces their apoptosis (39). Previous studies reported that a high sPD-L1 level is closely associated with an unfavorable prognosis in many types of cancer, including GC (28,40–43). However, Zheng et al (44) reported opposite results, where patients with GC and high sPD-L1 levels have a better prognosis than those with low sPD-L1 levels. Concordant with these findings, the present study demonstrated that sPD-L1 level in the serum of patients with stage IV GC was significantly higher than in those with stages I–III GC. Further investigation using a larger number of patients is required to determine the role of sPD-L1 in GC.
In conclusion, the present study demonstrated that IFN-γ treatment simultaneously enhanced the intracellular and membranous PD-L1 expression in some GC cells. In addition, a significantly high concentration of sPD-L1 was also detected in the serum of patients with GC. Further investigation on the underlying mechanism of the regulation of PD-L1 expression and sPD-L1 production is required and would serve the development of novel therapeutic approaches in GC.
Acknowledgements
Not applicable.
Funding
This work was partly funded by the Japan Society for the Promotion of Science (grant no. 16K09340) and the Program for Basic and Clinical Research on Hepatitis from Japan Agency for Medical Research and Development (AMED; grant no. JP20fk0210054).
Availability of data and materials
All results and data obtained from the present study are available from the corresponding author on reasonable request.
Authors' contributions
YI and TC designed this study. TK, HK, KK, JA, RK, YK and MN performed experiments and analyzed data. TS, RN, ES, SN, RM, AT, TM, TN and JK checked all data and analyzed data. YI and HM collected samples and data. AK and NK contributed to data interpretation and writing of the manuscript. The manuscript was written by YI and TC. All authors read and approved the final the manuscript.
Ethics approval and consent to participate
This study was approved by the Research Ethics Committees of the Graduate School of Medicine, Chiba University (approval no. 3552 and 3671). Informed consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Khasag O, Boldbaatar G, Tegshee T, Duger D, Dashdorj A, Uchida T, Matsuhisa T, Yamaoka Y. The prevalence of helicobacter pylori infection and other risk factors among mongolian dyspeptic patients who have a high incidence and mortality rate of gastric cancer. Gut Pathog. 2018;10:14. doi: 10.1186/s13099-018-0240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiota S, Murakawi K, Suzuki R, Fujioka T, Yamaoka Y. Helicobacter pylori infection in Japan. Expert Rev Gastroenterol Hepatol. 2013;7:35–40. doi: 10.1586/egh.12.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selim JH, Shaheen S, Sheu WC, Hsueh CT. Targeted and novel therapy in advanced gastric cancer. Exp Hematol Oncol. 2019;8:25. doi: 10.1186/s40164-019-0149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700–713. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 7.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: Integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 8.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 10.Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol. 2007;19:309–314. doi: 10.1016/j.coi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Guan J, Lim KS, Mekhail T, Chang CC. Programmed death ligand-1 (PD-L1) expression in the programmed death receptor-1 (PD-1)/PD-L1 blockade: A key player against various cancers. Arch Pathol Lab Med. 2017;141:851–861. doi: 10.5858/arpa.2016-0361-RA. [DOI] [PubMed] [Google Scholar]
- 12.Nagato T, Ohkuri T, Ohara K, Hirata Y, Kishibe K, Komabayashi Y, Ueda S, Takahara M, Kumai T, Ishibashi K, et al. Programmed death-ligand 1 and its soluble form are highly expressed in nasal natural killer/T-cell lymphoma: A potential rationale for immunotherapy. Cancer Immunol Immunother. 2017;66:877–890. doi: 10.1007/s00262-017-1987-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang B, Huang T, Wei H, Shen L, Zhu D, He W, Chen Q, Zhang H, Li Y, Huang R, et al. The correlation and prognostic value of serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death-ligand 1 (sPD-L1) in patients with hepatocellular carcinoma. Cancer Immunol Immunother. 2019;68:353–363. doi: 10.1007/s00262-018-2271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Deng R, Zhang P, Liu W, Zeng X, Ma X, Shi L, Wang T, Yin Y, Chang W, Zhang P, et al. HDAC is indispensable for IFN-γ-induced B7-H1 expression in gastric cancer. Clin Epigenetics. 2018;10:153. doi: 10.1186/s13148-018-0589-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Briely JD, Gospodarowicz MK, Wittekind CH. Union for International Cancer Control, corp-editor. TNM Classification of Malignant Tumors. 8th. Wiley-Blackwell; Hoboken, NJ: 2017. [Google Scholar]
- 17.Intlekofer AM, Thompson CB. At the bench: Preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J Leukoc Biol. 2013;94:25–39. doi: 10.1189/jlb.1212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma J, Zheng B, Goswami S, Meng L, Zhang D, Cao C, Li T, Zhu F, Ma L, Zhang Z, et al. PD1Hi CD8+ T cells correlate with exhausted signature and poor clinical outcome in hepatocellular carcinoma. J Immunother Cancer. 2019;7:331. doi: 10.1186/s40425-019-0814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishida M, Iwai Y, Tanaka Y, Okazaki T, Freeman GJ, Minato N, Honjo T. Differential expression of PD-L1 and PD-L2, ligands for an inhibitory receptor PD-1, in the cells of lymphohematopoietic tissues. Immunol Lett. 2002;84:57–62. doi: 10.1016/S0165-2478(02)00142-6. [DOI] [PubMed] [Google Scholar]
- 21.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on t-cell infiltration and PD-L1. Cancer Res. 2015;75:2139–2145. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp Mol Med. 2018;50:1–11. doi: 10.1038/s12276-018-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006;108:19–24. doi: 10.1016/j.acthis.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48:434–452. doi: 10.1016/j.immuni.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cha JH, Chan LC, Li CW, Hsu JL, Hung MC. Mechanisms controlling PD-L1 expression in cancer. Mol Cell. 2019;76:359–370. doi: 10.1016/j.molcel.2019.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shigemori T, Toiyama Y, Okugawa Y, Yamamoto A, Yin C, Narumi A, Ichikawa T, Ide S, Shimura T, Fujikawa H, et al. Soluble PD-L1 expression in circulation as a predictive marker for recurrence and prognosis in gastric cancer: Direct comparison of the clinical burden between tissue and serum PD-L1 expression. Ann Surg Oncol. 2019;26:876–883. doi: 10.1245/s10434-018-07112-x. [DOI] [PubMed] [Google Scholar]
- 29.Jiang X, Wang J, Deng X, Xiong F, Ge J, Xiang B, Wu X, Ma J, Zhou M, Li X, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. 2019;18:10. doi: 10.1186/s12943-018-0928-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mimura K, Teh JL, Okayama H, Shiraishi K, Kua LF, Koh V, Smoot DT, Ashktorab H, Oike T, Suzuki Y, et al. PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci. 2018;109:43–53. doi: 10.1111/cas.13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Feng Y, Lu L, Wang H, Dai L, Li Y, Zhang P. Interferon-gamma-induced PD-L1 surface expression on human oral squamous carcinoma via PKD2 signal pathway. Immunobiology. 2012;217:385–393. doi: 10.1016/j.imbio.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 32.Clark CA, Gupta HB, Curiel TJ. Tumor cell-intrinsic CD274/PD-L1: A novel metabolic balancing act with clinical potential. Autophagy. 2017;13:987–988. doi: 10.1080/15548627.2017.1280223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qu QX, Xie F, Huang Q, Zhang XG. Membranous and cytoplasmic expression of PD-L1 in ovarian cancer cells. Cell Physiol Biochem. 2017;43:1893–1906. doi: 10.1159/000484109. [DOI] [PubMed] [Google Scholar]
- 34.Aydin D, Bilici A, Yavuzer D, Kefeli U, Tan A, Ercelep O, Mert A, Yuksel S, Ozcelik M, Isik D, et al. Prognostic significance of ADAM17 expression in patients with gastric cancer who underwent curative gastrectomy. Clin Transl Oncol. 2015;17:604–611. doi: 10.1007/s12094-015-1283-1. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Wang Q, Shi B, Xu P, Hu Z, Bai L, Zhang X. Development of a sandwich ELISA for evaluating soluble PD-L1 (CD274) in human sera of different ages as well as supernatants of PD-L1+ cell lines. Cytokine. 2011;56:231–238. doi: 10.1016/j.cyto.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Zhou J, Mahoney KM, Giobbie-Hurder A, Zhao F, Lee S, Liao X, Rodig S, Li J, Wu X, Butterfield LH, et al. Soluble PD-L1 as a biomarker in malignant melanoma treated with checkpoint blockade. Cancer Immunol Res. 2017;5:480–492. doi: 10.1158/2326-6066.CIR-16-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu X, Lang J. Soluble PD-1 and PD-L1: Predictive and prognostic significance in cancer. Oncotarget. 2017;8:97671–97682. doi: 10.18632/oncotarget.18311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gong B, Kiyotani K, Sakata S, Nagano S, Kumehara S, Baba S, Besse B, Yanagitani N, Friboulet L, Nishio M, et al. Secreted PD-L1 variants mediate resistance to PD-L1 blockade therapy in non-small cell lung cancer. J Exp Med. 2019;216:982–1000. doi: 10.1084/jem.20180870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frigola X, Inman BA, Krco CJ, Liu X, Harrington SM, Bulur PA, Dietz AB, Dong H, Kwon ED. Soluble B7-H1: Differences in production between dendritic cells and T cells. Immunol Lett. 2012;142:78–82. doi: 10.1016/j.imlet.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frigola X, Inman BA, Lohse CM, Krco CJ, Cheville JC, Thompson RH, Leibovich B, Blute ML, Dong H, Kwon ED. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res. 2011;17:1915–1923. doi: 10.1158/1078-0432.CCR-10-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossille D, Azzaoui I, Feldman AL, Maurer MJ, Labouré G, Parrens M, Pangault C, Habermann TM, Ansell SM, Link BK, et al. Soluble programmed death-ligand 1 as a prognostic biomarker for overall survival in patients with diffuse large B-cell lymphoma: A replication study and combined analysis of 508 patients. Leukemia. 2017;31:988–991. doi: 10.1038/leu.2016.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finkelmeier F, Canli Ö, Tal A, Pleli T, Trojan J, Schmidt M, Kronenberger B, Zeuzem S, Piiper A, Greten FR, Waidmann O. High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis. Eur J Cancer. 2016;59:152–159. doi: 10.1016/j.ejca.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Ito M, Oshima Y, Yajima S, Suzuki T, Nanami T, Shiratori F, Funahashi K, Nemoto T, Shimada H. Is high serum programmed death ligand 1 level a risk factor for poor survival in patients with gastric cancer? Ann Gastroenterol Surg. 2018;2:313–318. doi: 10.1002/ags3.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng Z, Bu Z, Liu X, Zhang L, Li Z, Wu A, Wu X, Cheng X, Xing X, Du H, et al. Level of circulating PD-L1 expression in patients with advanced gastric cancer and its clinical implications. Chin J Cancer Res. 2014;26:104–111. doi: 10.3978/j.issn.1000-9604.2014.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All results and data obtained from the present study are available from the corresponding author on reasonable request.