Abstract
The present study sought to estimate the applicability of apurinic/apyrimidinic endodeoxyribonuclease 1 (APE1), vascular endothelial growth factor A (VEGFA) expression and CD163+ tumor-associated macrophage (TAM) ratio as prognostic factors in bladder cancer (BCa). A total of 127 patients with bladder urothelial cancer who underwent radical cystectomy at Daping Hospital were recruited between January 2013 and January 2017, including 45 cases of non-muscle invasive BCa (NMIBC) and 82 of MIBC. Immunohistochemical detection of APE1, VEGFA and CD163, as well as multiple immunofluorescence staining for APE1, VEGFA, CD163 and CD34, were performed on tissue samples. For APE1 and VEGFA, the staining was graded based on intensity (0–3), while CD163 was graded (0–3) based on the percentage of positively stained cells. The prognostic value of APE1, VEGF and CD163 was assessed using Kaplan-Meier and Cox regression analysis. The results suggested that in BCa, high APE1 expression was associated with high VEGFA expression and more infiltration of CD163+ TAM. Furthermore, high expression of APE1 was associated with lymphovascular invasion of BCa, as well as reduced survival time. This indicates that APE1 may be associated with CD163+ TAM infiltration in BCa, with VEGFA as a possible influencing factor.
Keywords: bladder cancer, CD163, tumor-associated macrophage, apurinic/apyrimidinic endodeoxyribonuclease 1, vascular endothelial growth factor A
Introduction
Bladder cancer (BCa) is one of the most common types of urological cancer, resulting in ~150,000 deaths worldwide every year (1). Approximately 75% of new BCa cases present as non-muscle-invasive BCa (NMIBC) and up to 50% of them will recur with 20% of these recurring cases progressing (2). Treatment options for BCa vary based on the stage at diagnosis. For MIBC and recurrent NMIBC, radical cystectomy (RC) is usually the mainstay of treatment, where the goal is to provide optimal cancer control by removal of whole bladder tissue (3). Despite this, ~4-10% of patients are still susceptible to secondary urothelial tumors following RC and frequently have an adverse prognosis due to late diagnosis (4). Although numerous serum and urine biomarkers have been suggested, their precision and specificity still require further evidence (5). Therefore, identification of a novel molecular mechanism or biomarker that may provide a novel treatment option, retard the progression of Bca, improve the prediction of the prognosis of BCa may provide a significant benefit in terms of benefiting clinical treatment greatly.
Apurinic/apyrimidinic endodeoxyribonuclease 1 (APE1) protein, also known as redox factor-1 (Ref-1), was originally identified as a multifunctional protein involved in DNA base excision repair (BER) and redox signaling. Previous studies have indicated that APE1 expression is not only significantly higher in multiple cancer types, including liver cancer, ovary cancer and osteosarcoma, but also highly associated with prognosis (6–8). Although APE1 was suggested as a urinary biomarker (5,9), its prognostic value in cancer tissue has not been verified with sufficient cases.
Previously, Sun and Nelson (10) suggested that the DNA repair gene APE1 is able to promote the production of a spectrum of cytokines, growth factors and proteases, including vascular endothelial growth factor (VEGF), by the DNA damage secretory program. In the tumor microenvironment (TME), VEGF is secreted by tumor cells and diffuse through the tissue contributing to angiogenesis (11). Furthermore, VEGF has a role as an immunosuppressive gene, inducing a ‘tumor endothelial barrier’ to promote T-cell arrest, or the loss of T cell motility (12). In BCa, VEGF has been indicated to be associated with stage and recurrence. Wheeler et al (13) suggested that VEGF is able to recruit macrophages and polarize them to the M2 phenotype in the decidua. This may provide a novel mechanism to how VEGF is able to worsen the clinical outcomes of BCa through tumor-associated macrophage (TAM) manipulation. The study of TAMs impacting the progression and metastasis of cancer has been fruitful. Alternatively activated M2 macrophages in TME are responsible for angiogenesis and tissue remodeling and feature an immunosuppressive phenotype, which may have an important role in tumor progression (14). In BCa, M2 type macrophages are usually associated with poor response to BCG therapy and may significantly limit its efficacy (15). Of note, in MIBC, TAMs mostly infiltrate into the tumor areas as opposed to stroma-tumor margins in NMIBC, which is associated with an unfavorable clinical outcome (16).
Therefore, the present study sought to evaluate the association among the expression of APE1 and vascular endothelial growth factor A (VEGFA) and the infiltration of M2 macrophages, as detected via the M2 marker CD163, as well as their prognostic significance in BCa. To the best of our knowledge, the present study was the first to implicate APE1 as a key factor in the DNA damage response with polarization of TAM, laying a foundation for elucidating the mechanism of the recurrence of BCa and exploring the efficacy of immunotherapy for this neoplasm.
Materials and methods
Patients and data collection
A total of 167 patients with BCa subjected to radical cystectomy at Daping Hospital between January 2013 and January 2017 were recruited without any restriction by age or sex. Data collection was closed by May 2019. Those patients who did not have a reported event of death at the time of closure were included for calculation of the median follow-up time. Patient follow-up was usually performed every 3 months in the first year post-surgery and then every 6 months. The cause of death was determined by chart review from patients' medical records or from the death certificate. The follow-up time was 4–78 months and the median follow-up time was 48 months. Cases with non-urothelial carcinoma types and patients who died of causes not associated with cancer were excluded, and cases without precise follow-up were also excluded, resulting in 127 remaining cases. All subjects were from a Han Chinese population and were subjected to radical cystectomy at Daping Hospital (Chongqing, China) with the diagnosis made based on pathology. The general characteristics of the patients are summarized in Table SI. The median age was 64.25 years (range, 39–88 years). There were 45 cases of NMIBC and 82 cases of MIBC. In total, 10 cases were low-grade carcinoma and 117 were high-grade carcinoma, and 36 of the latter were indicated to have lymphovascular invasion (LVI). Regarding the smoking history, a person who had at least one pack of cigarettes/day for >1 year in his/her lifetime was regarded as having a positive smoking history; otherwise, they were considered as a non-smoker (17). The study was approved by the Ethics Committee of Daping Hospital (Chongqing, China).
Pathological evaluation and immunohistochemistry (IHC)
Tumor tissues were fixed overnight in 4% paraformaldehyde, dehydrated, embedded in paraffin and sectioned into 8–10 mm (RM2235; Leica Microsystems). Experienced pathologists blinded to the clinical outcomes reviewed these pathological specimens. The tumors were staged according to the 8th AJCC TNM staging classification (18). Grading was performed following the 1998 WHO/ISUP consensus classification (19). The presence of LVI was assessed in all specimens. IHC staining for APE1, VEGFA and CD163 was conducted utilizing the following antibodies: APE1 (1:2,000 dilution; mouse monoclonal; cat. no. ab194; Abcam), VEGFA (1:150 dilution; mouse monoclonal; cat. no. ab52917; Abcam) and CD163 (1:150 dilution; mouse monoclonal; cat. no. TA506388; Origene). Goat antibodies conjugated to horseradish peroxidase (HRP) (working solution, Kit-5030; MXB) was used as a secondary antibody, 3,3-diaminobenzidine was used as a chromogenic substrate and the sections were counterstained with hematoxylin. The results were analyzed by an experienced pathologist (HX) blinded to the clinical outcomes. APE1 and VEGFA expression were graded based on the staining intensity of the nuclei and cytoplasm, respectively, in the tumor area. Necrotic areas were ignored. Grade 0 had the weakest staining and grade 3 had the strongest staining. In the evaluation of APE1 expression, grade 0–2 was considered to indicate low expression, while grade 3 was considered to indicate high expression. For VEGFA expression, grade 0–1 was considered low expression, while grade 2–3 was considered as high expression. For CD163, the percentage of stained macrophages in the tumor stroma compared to the total number of nucleated stromal cells was scored using a scale from 0 to 100% and graded as follows: 0, 0–5%; 1, 6–30%; 2, 31–50%; and 3, >50%. For evaluation purposes, grade 0–1 is considered as low TAM ratio, inversely grade 2–3 is considered as high ratio. The cutoff is set by mainly taking the median grade value in consideration.
Multiple immunofluorescence staining
Multiple immunofluorescence staining was performed on paraffin-embedded tissues. Sections of 4 µm thickness were cut from selected BCa tissues. The slides were deparaffinized in xylene, rehydrated and washed with tap water prior to boiling in Tris-EDTA buffer (pH 9, 643901; Klinipath) for epitope retrieval/microwave treatment. Endogenous peroxidase was blocked using Antibody Diluent/Block (cat. no. 72424205; Perkin Elmer) and incubated at room temperature. Protein blocking was performed using Antibody Diluent/Block at 37°C for 1 h. The slides were incubated with primary antibodies to CD163 (1:150 dilution; cat. no. ZM0428; Zsbio), APE1 (1:2,000 dilution; cat. no. ab194; Abcam) and VEGFA (1:100 dilution; cat. no. ab52917; Abcam) for 1 h at 37°C, and then incubated with antibody to CD34 (cat. no. kit-0004; MXB) at 4°C overnight. Next, incubation with Opal Polymer HRP Ms+Rb (cat. no. 2414515; Perkin Elmer) was performed at 37°C for 10 min. Tyramide signal amplification (TSA) visualization was performed with the Opal eight-color IHC kit (cat. no. NEL797B001KT; Perkin Elmer), containing fluorophores DAPI, Opal 690 (CD34), Opal 650 (VEGFA), Opal 570 (APE1) and Opal 520 (CD163), as well as a TSA Coumarin system (cat. no. NEL703001KT; Perkin Elmer).
Slides were scanned using the Perkin Elmer Vectra (Vectra 3.0.5; Perkin Elmer). Multispectral images were unmixed using spectral libraries built from images of single stained tissues for each reagent using the inform Advanced Image Analysis software (inForm 2.3.0; Perkin Elmer). A selection of 5–10 representative original multispectral images was used to train the inForm software (tissue segmentation, cell segmentation, phenotyping tool and positivity score). All the settings applied to the training images were saved within an algorithm to allow the inForm software (PerkinElmer; v2.3.0) batch analysis of multiple original multispectral images of the same tissue (20).
Statistical analysis
Data analysis was performed using SPSS (version 22; IBM, Corp.) and GraphPad Prism 7 (GraphPad Software, Inc.). Cumulative survival probabilities were estimated using the Kaplan-Meier method and differences between survival rates were tested for significance using the log-rank test. Univariate and multivariate Cox regression analyses were calculated using SPSS. Hazard ratios (HRs) and 95% confidence intervals (95% CI) were used to present the associations of dependent and independent variables with the risk of mortality. The χ2 test was employed to assess differences between two groups. All tests were two-sided with a 95% CI and P<0.05 was considered to indicate statistical significance. Receiver operating characteristic (ROC) analysis was conducted for further evidence. Correlation of APE1, VEGFA and CD163 expression in muscle invasive bladder cancer tissues with multiple immunofluorescence staining was analyzed with Pearson correlation.
Results
Expression of APE1, VEGFA and CD163+ TAMs in BCa tissues
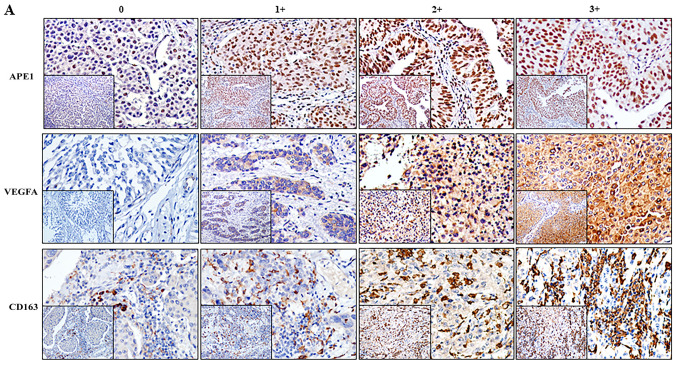
IHC was used to estimate the expression of APE1 and VEGFA, as well as the ratio of CD163+ TAMs, and the association between each of them with the clinicopathological characteristics was determined. APE1 was mainly localized in the nuclei with a certain amount residing in the cytoplasm, whereas VEGFA appeared in the cytoplasm only (Fig. 1). Among the 127 patients, 62 (48.82%) and 67 (52.76%) exhibited elevated levels of APE1 and VEGFA, respectively. The APE1 levels appeared to be associated with LVI (P=0.019) but not with the tumor stage or grade (Table SII). The levels of VEGFA were not associated with the tumor stage, grade or LVI. CD163+ TAMs were present in the tumor stroma and tumor islets. The tumor stroma included the papillary axis, lymphoid aggregates and the stroma. In certain specimens, staining for CD163 was positive in the cytoplasm and membrane of tumor cells. Of the 127 cases, 62 (48.82%) had a high grade regarding CD163+ TAMs but this was not associated with the tumor stage, grade or LVI.
Figure 1.
(A) Representative immunohistochemistry results for APE1, VEGFA and CD163 indicated that APE1 staining was mainly located in the nuclei and partly in the cytoplasm and VEGFA was present in the cytoplasm of tumor cells, while CD163 was expressed on the infiltrating macrophages (magnification, ×200). The staining was rated as 0, 1+, 2+ or 3+ based on intensity for APE1 and VEGFA and based on the percentage of positive cells for CD163. (B) Heatmap presentation of the gradings of APE1, VEGFA and CD163 among the 127 patients. VEGFA, vascular endothelial growth factor A; APE1, apurinic/apyrimidinic endodeoxyribonuclease 1.
APE1 and VEGFA expression is correlated with the CD163+ TAM ratio in BCa
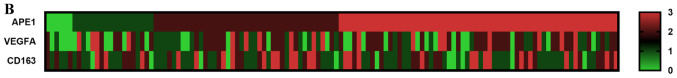
The correlation between the expression of APE1 and VEGFA and the proportion of CD163+ M2 macrophages was then assessed. The results suggested that high expression of APE1 in tumor cells was positively correlated with the grade regarding CD163+ TAMs (r=0.196, P=0.027), as well as with VEGFA expression (r=0.208, P=0.018). Furthermore, VEGFA expression was strongly correlated with the grade regarding CD163+ TAMs (r=0.408, P<0.001; Table SIII). To further assess the expression of APE1 and VEGFA in BCa tissues and their correlation with the CD163+ TAM ratio, multiple immunofluorescence staining for APE1, VEGFA, CD163 and CD34 was performed. The results suggested that APE1 and VEGFA were co-expressed in tumor cells, and CD163+ TAMs were mostly distributed around the microvasculature of tumor stroma or between tumor cells (Fig. 2). Further analysis using inForm software suggested that APE1 expression is positively correlated with VEGFA and CD163 expression (Table SIV).
Figure 2.
Multiple immunofluorescence staining for APE1, VEGFA, CD163 and CD34 in bladder cancers. (A) Representative sample with high expression of APE1 and VEGFA in which the intratumor CD163-labeled M2 macrophages are easily distinguishable. (B) Example of a sample with high expression of APE1 and VEGFA in which the CD163-labeled M2 macrophages are easily distinguishable in the tumor stroma. (C) Representative sample with low expression of APE1 and VEGFA, in which the CD163-labeled M2 macrophages are less distinguishable. APE1-positive staining in tumor epithelial cells displayed as orange and VEGFA-positive staining in tumor epithelial cells was cyan, while CD163-positive infiltrating M2 macrophages were green and CD34-positive vascular endothelial cells displayed as magenta (magnification, ×200). VEGFA, vascular endothelial growth factor A; APE1, apurinic/apyrimidinic endodeoxyribonuclease 1.
APE1 and CD163 are independent prognostic factors for OS in BCa
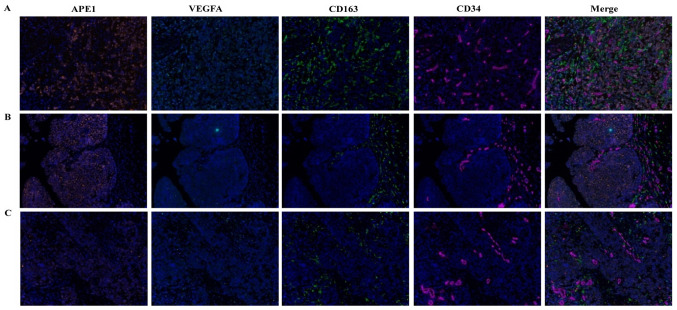
KM analysis revealed that high APE1 expression and a high CD163+ TAM ratio were associated with a shorter OS (Fig. 3). A univariate Cox regression analysis for the expression of APE1, VEGFA, CD163+ TAMs and clinical characteristics was performed, indicating that the T-stage (HR=8.279, 95% CI: 2.948–23.255, P<0.001), N-stage (HR=4.265, 95% CI: 2.262–8.045, P<0.001), LVI (HR=2.974, 95% CI: 1.642–5.387, P<0.001), APE1 expression (HR=2.797, 95% CI: 1.486–5.267, P=0.001) and CD163+ TAMs (HR=2.425, 95% CI: 1.300–4.521, P=0.005) were associated with prognosis. In addition, a multivariate analysis for OS was performed by combining the marker panel and clinical variables, suggesting that the T-stage (HR=8.279, 95% CI: 2.948–23.255, P<0.001), N-stage (HR=4.265, 95% CI: 2.262–8.045, P<0.001), APE1 expression (HR=2.797, 95% CI: 1.486–5.267, P=0.001) and CD163+ TAMs (HR=2.425, 95% CI: 1.300–4.521, P=0.005) were independent prognostic factors (Table I). Area under the curve are shown in Table SV.
Figure 3.
Factors influencing the survival of patients with bladder cancer. (A-C) Kaplan-Meier survival curves; (A) High expression of APE1 was associated with shorter OS; (B) VEGFA expression was not significantly associated with OS; (C) a high ratio of CD163+ tumor-associated macrophages was associated with shorter OS. (D) ROC curves of APE1, VEGFA and CD163 were assessed with T stage and N stage for best prediction efficiency. VEGFA, vascular endothelial growth factor A; APE1, apurinic/apyrimidinic endodeoxyribonuclease 1; OS, overall survival; ROC, receiver operating characteristic; m, months.
Table I.
Univariate and multivariate COX analysis of the prognosis of IHC marker for OS.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Agea | 1.492 | 0.829–2.687 | 0.182 | |||
| Sexb | 1.047 | 0.413–2.654 | 0.923 | |||
| Smokingc | 0.913 | 0.479–1.741 | 0.783 | |||
| LVId | 2.974 | 1.642–5.387 | <0.001 | |||
| T-stagee | 8.279 | 2.948–23.255 | <0.001 | 12.857 | 3.883–42.575 | <0.001 |
| N-stagef | 4.265 | 2.262–8.045 | <0.001 | |||
| Gradeg | 2.201 | 0.530–9.135 | 0.277 | |||
| APE1h | 2.797 | 1.486–5.267 | 0.001 | 3.644 | 1.863–7.129 | <0.001 |
| VEGFAi | 0.860 | 0.479–1.544 | 0.613 | 0.410 | 0.217–0.773 | 0.006 |
| CD163j | 2.425 | 1.300–4.521 | 0.005 | 2.883 | 1.480–5.617 | 0.002 |
Age, ≤65 vs. >65 years
sex, male vs. female
smoking: positve smoking history vs. negative smoking history
LVI, lymphovasular invasion vs. no lymphovascular invasion
T-stage, Tis and T1 vs. T2, T3 and T4
N-stage, N0 vs. N1 and N2
grade, high grade vs. low grade
APE1, high APE1 expression vs. low APE1 expression
VEGFA, high VEGFA expression vs. low VEGFA expression
CD163, high CD163 ratio vs. low CD163 ratio. Analysis were performed utilizing logistic regression using the Cox proportional hazards model on SPSS 17.0. Area under the curve is shown in Table SV. HR, hazard ratio; VEGFA, vascular endothelial growth factor A; APE1, apurinic/apyrimidinic endodeoxyribonuclease 1; LVI, lymphovascular invasion.
Discussion
Multiple nomograms and models have been constructed in the past to predict the outcomes of Bca (21). Accurate risk stratification may provide a marked benefit for patients with NMIBC that qualify for early RC, as well as for patients with MIBC, who gain from perioperative chemotherapy. The identification of new characteristics will substantially increase the fidelity of such models.
The presence of LVI, defined as the presence of tumor cells in the lymphatic vessel and in vascular walls, has been reported to be of prognostic value in patients with Bca. Evidence suggests that LVI is a characteristic of biologically and clinically aggressive BCa and may be of prognostic value. In the present study, 34 out of 127 patients were identified to have LVI, 32 of which had MIBC and 2 had NMIBC (Table SII). In addition, the presence of LVI is associated with an unfavorable prognosis. This is consistent with other studies (22).
Aromatic amines are well-known risk factors for BCa (23). Interaction between environmental exposure and genetic susceptibility may markedly increase DNA damage and thus trigger urothelial precancerous events; therefore, prompt elimination of such damage counteracts malignant transformation. The BER pathway has a critical role in DNA repair mechanisms by removing nucleobase modifications by oxidation, alkylation and deamination, and also repairs backbone single-strand DNA breaks (24). The enzyme APE1 has a mainframe role in BER by correcting apurinic/apyrimidinic sites, which are pre-mutagenic lesions able to stall DNA replication forks. Studies have indicated above-basal expression levels of APE1 and other DNA repair pathway proteins in high-grade BCa (25). Choi et al (5) proposed that APE1 may be used as a non-invasive urinary biomarker for BCa; however, its sensitivity and specificity are not satisfying for immediate clinical results. Recently, Fishel et al (26) reported that APE1 was overexpressed in human BCa tissue and that APE1 redox signaling-specific inhibitor APX3330 was able to attenuate the proliferation of BCa cells and increase their apoptosis. However, the potential capability of APE1 expression levels to differentiate between NMIBC and MIBC has not been previously reported. The present study suggested an even distribution of APE1 expression in both NMIBC and MIBC, with no significant difference between the two (P=0.711). Furthermore, no significant difference in APE1 expression was determined between high-grade and low-grade BCa (P=0.744). However, high APE1 expression was associated with the presence of LVI and unfavorable prognosis in patients with BCa. APE1 expression was also positively correlated with VEGFA and CD163+ TAM ratio in BCa tissue.
Previous studies have indicated that high expression of APE1 in lung cancer and osteosarcoma is associated with upregulated VEGF through hypoxia-inducible factor-1α activation and retardation of APE1 results in a significant drop in VEGF expression (27). Of note, Pignot et al (28) analyzed the angiogenic pathways in human transitional cell carcinoma of the bladder using a large-scale real-time reverse transcription-PCR approach, indicating that VEGFA, MET, C-X-C motif chemokine receptor 4 and interleukin-8 were significantly overexpressed in tumor samples, but only VEGFA overexpression was an independent prognostic factor for overall and disease-free survival. This did not align with the present analysis of VEGFA expression at the protein level, as no significant prognostic value for VEGFA was determined in BCa; perhaps a further study with more cases will better indicate the correlation between transcriptional levels and protein expression of VEGFA in BCa.
VEGF and its receptors have profound effects on the early development and differentiation of both vascular endothelial and hematopoietic progenitors. Kloepper et al (29) further suggested that when subjecting glioblastoma to anti-VEGF/angiopoietin-2 blockade, TAMs along the M1 to M2 continuum are being reprogrammed toward the M1 phenotype to provide a greater survival benefit. This suggested that VEGF may have a novel role in immunosuppression by M2 polarization.
Macrophage infiltration is associated with significantly better cancer-specific survival in patients with CD163+ TAM-associated tumors. A meta-analysis of 1,400 cases of BCa including 13 studies further indicated that only CD163+ TAMs, not CD68+ TAMs, were closely associated with OS, recurrence-free survival and progression-free survival (30). Consistent with the present results, macrophage infiltration was indicated to be associated with the prognosis of BCa. The present study demonstrated through IHC and multiple immunofluorescences staining that a high CD163+ macrophage ratio is associated with an unfavorable prognosis.
Recently, Hudson et al (31) published a comparative study of 19 glioma specimens with recurrence after treatment. Through screening of 96 gene expressions, APE1 expression in tumor tissues was significantly increased after treatment. In addition, genes linked to immunosuppression, invasion and metastasis (glycoprotein nmb, C-C motif chemokine ligand 5 and killer cell lectin-like receptor C1) and an M2 phenotype (CD163) were also detected. The polarized genes were significantly upregulated. In 2018, Yan et al (32) reported that the APE1-specific redox inhibitor APX3330 directly affected the polarization of M2 macrophages in the role of neurological recovery after stroke in type I diabetic rats. However, the specific mechanism by which APE1 regulates M2 polarization has remained elusive. The present results confirmed a positive correlation between APE1, VEGFA and CD163 at the protein level, suggesting that APE1 may be involved in the regulation of macrophage polarization through VEGFA.
In the univariate linear regression analysis performed in the present study, VEGFA was indicated to have no association with OS but it was a confounding factor in the multivariate analysis. This is possibly due to several reasons: i) The number of cases was too low to accurately present the association; ii) VEGFA may not be a decisive factor on OS in BCa, but may still be a contributing factor due to its association with other meaningful factors, including APE1 and CD163 expression. Further studies on this mechanism will be required to draw a conclusion.
The present study demonstrated that high APE1 expression is associated with the presence of LVI in BCa. APE1 expression was also determined to be positively associated with VEGFA expression and increased infiltration of CD163+ TAM. LVI, high APE1 expression and a high ratio of CD163+ TAM in BCa may lead to a reduced survival time of patients. The results may add valuable insight to optimize models predicting BCa prognosis, consequently guiding perioperative treatments that benefit patients the most. The present study also indicated that there may be a mechanism by which high APE1 expression contributes to an increase of M2-type TAMs by definition as CD163+, possibly involving regulation of VEGFA expression through its redox function. However, the present results are limited in that only patients subjected to radical cystectomy were recruited, and studies including patients with broader recruitment criteria (not only restricting to patients who underwent cystectomy but, for example, also those who underwent transurethral resection) will provide a better analysis. In addition, only paraffin-embedded tissues were assessed and there was a lack of fresh tissues, which may limit the conclusions of the present study. Further prospective studies with standardized protocols should be performed to fully assess the impact of LVI, APE1 and VEGFA expression, as well as the CD163+ TAM ratio, on the outcomes for patients with BCa.
Supplementary Material
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (grant nos. 81772704 and 81972398 to JJ) and the Clinical Medical Research Personnel Training Program of Army Medical University (grant no. 2018XLC3076 to LW).
Availability of data and materials
The datasets used and analyzed during the present study are available from the corresponding author upon reasonable request.
Authors contributions
LAW contributed to the conception and design, acquisition of data, analysis and interpretation of the data and IHC of the tissue and was a major contributor in writing the manuscript. BY was a major contributor to the analysis and interpretation of the data. TT contributed to the acquisition of data. YY, JX and LL contributed to the IHC analysis of the tissues. DZ contributed to the writing and critical revision of the manuscript, as well as towards the conception of the study. HX is an experienced pathologist and contributed to reviewing and analyzing the results of IHC and multiple immunofluorescence. LW contributed to the retrieval of funding for the study and revision of the manuscript. JJ contributed to the conception and design, writing and revision of the manuscript and retrieval of funding for the study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study involved the use of patient tissues and data. Approval was acquired from the Ethics Committee of the Research Institute of Surgery and Daping Hospital, Army Medical University (Chongqing, China), serial number 2018 No.25. Written informed consent was obtained from a patient representative who is involved in this study for the usage of the lesion tissue for research purposes was acquired at the time-point of surgery.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Cambier S, Sylvester RJ, Collette L, Gontero P, Brausi MA, van Andel G, Kirkels WJ, Silva FC, Oosterlinck W, Prescott S, et al. EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage Ta-T1 urothelial bladder cancer patients treated with 1–3 years of maintenance Bacillus Calmette-Guérin. Eur Urol. 2016;69:60–69. doi: 10.1016/j.eururo.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 3.Gakis G, Efstathiou J, Lerner SP, Cookson MS, Keegan KA, Guru KA, Shipley WU, Heidenreich A, Schoenberg MP, Sagaloswky AI, et al. International Consultation on Urologic Disease-European Association of Urology Consultation on Bladder Cancer 2012 ICUD-EAU International Consultation on Bladder Cancer 2012: Radical cystectomy and bladder preservation for muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2013;63:45–57. doi: 10.1016/j.eururo.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Gakis G, Black PC, Bochner BH, Boorjian SA, Stenzl A, Thalmann GN, Kassouf W. Systematic Review on the Fate of the Remnant Urothelium after Radical Cystectomy. Eur Urol. 2017;71:545–557. doi: 10.1016/j.eururo.2016.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi S, Shin JH, Lee YR, Joo HK, Song KH, Na YG, Chang SJ, Lim JS, Jeon BH. Urinary APE1/Ref-1: A potential bladder cancer biomarker. Dis Markers. 2016;2016:7276502. doi: 10.1155/2016/7276502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Q, Wei X, Zhou ZW, Wang SN, Jin H, Chen KJ, Luo J, Westover KD, Wang JM, Wang D, et al. GADD45α sensitizes cervical cancer cells to radiotherapy via increasing cytoplasmic APE1 level. Cell Death Dis. 2018;9:524. doi: 10.1038/s41419-018-0452-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu GS, Li M, Xu CX, Wang D. APE1 stimulates EGFR-TKI resistance by activating Akt signaling through a redox-dependent mechanism in lung adenocarcinoma. Cell Death Dis. 2018;9:1111. doi: 10.1038/s41419-018-1162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang S, He L, Dai N, Guan W, Shan J, Yang X, Zhong Z, Qing Y, Jin F, Chen C, et al. Serum APE1 as a predictive marker for platinum-based chemotherapy of non-small cell lung cancer patients. Oncotarget. 2016;7:77482–77494. doi: 10.18632/oncotarget.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin JH, Choi S, Lee YR, Park MS, Na YG, Irani K, Lee SD, Park JB, Kim JM, Lim JS, et al. APE1/Ref-1 as a serological biomarker for the detection of bladder cancer. Cancer Res Treat. 2015;47:823–833. doi: 10.4143/crt.2014.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun Y, Nelson PS. Molecular pathways: Involving microenvironment damage responses in cancer therapy resistance. Clin Cancer Res. 2012;18:4019–4025. doi: 10.1158/1078-0432.CCR-11-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 12.Georganaki M, van Hooren L, Dimberg A. Vascular targeting to increase the efficiency of immune checkpoint blockade in cancer. Front Immunol. 2018;9:3081. doi: 10.3389/fimmu.2018.03081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wheeler KC, Jena MK, Pradhan BS, Nayak N, Das S, Hsu CD, Wheeler DS, Chen K, Nayak NR. VEGF may contribute to macrophage recruitment and M2 polarization in the decidua. PLoS One. 2018;13:e0191040. doi: 10.1371/journal.pone.0191040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sica A, Allavena P, Mantovani A. Cancer related inflammation: The macrophage connection. Cancer Lett. 2008;267:204–215. doi: 10.1016/j.canlet.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 15.Takayama H, Nishimura K, Tsujimura A, Nakai Y, Nakayama M, Aozasa K, Okuyama A, Nonomura N. Increased infiltration of tumor associated macrophages is associated with poor prognosis of bladder carcinoma in situ after intravesical bacillus Calmette-Guerin instillation. J Urol. 2009;181:1894–1900. doi: 10.1016/j.juro.2008.11.090. [DOI] [PubMed] [Google Scholar]
- 16.Sjödahl G, Lövgren K, Lauss M, Chebil G, Patschan O, Gudjonsson S, Månsson W, Fernö M, Leandersson K, Lindgren D, et al. Infiltration of CD3+ and CD68+ cells in bladder cancer is subtype specific and affects the outcome of patients with muscle-invasive tumors. Urol Oncol. 2014;32:791–797. doi: 10.1016/j.urolonc.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Wang Q, Zhang T, Wu J, Wen J, Tao D, Wan T, Zhu W. Prognosis and risk factors of patients with upper urinary tract urothelial carcinoma and postoperative recurrence of bladder cancer in central China. BMC Urol. 2019;19:24. doi: 10.1186/s12894-019-0457-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Epstein JI, Amin MB, Reuter VR, Mostofi FK, Bladder Consensus Conference Committee The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Am J Surg Pathol. 1998;22:1435–1448. doi: 10.1097/00000478-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Amin MB, Edge SB, Greene FL, Schilsky RL, Gaspar LE, Washington M. Springer; New York, NY: 2017. AJCC Cancer Staging Manual. [DOI] [Google Scholar]
- 20.Gorris MA, Halilovic A, Rabold K, van Duffelen A, Wickramasinghe IN, Verweij D, Wortel IM, Textor JC, de Vries IJ, Figdor CG. Eight-color multiplex immunohistochemistry for simultaneous detection of multiple immune checkpoint molecules within the tumor microenvironment. J Immunol. 2018;200:347–354. doi: 10.4049/jimmunol.1701262. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Hong YK, Zhuang DW, He XJ, Lin ME. Bladder cancer survival nomogram: Development and validation of a prediction tool, using SEER and TCGA databases. Medicine (Baltimore) 2019;98:e17725. doi: 10.1097/MD.0000000000017725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim H, Kim M, Kwak C, Kim HH, Ku JH. Prognostic significance of lymphovascular invasion in radical cystectomy on patients with bladder cancer: A systematic review and meta-analysis. PLoS One. 2014;9:e89259. doi: 10.1371/journal.pone.0089259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C, Shariat S, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63:234–241. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 24.Zharkov DO. Base excision DNA repair. Cell Mol Life Sci. 2008;65:1544–1565. doi: 10.1007/s00018-008-7543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans AR, Limp-Foster M, Kelley MR. Going APE over ref-1. Mutat Res. 2000;461:83–108. doi: 10.1016/S0921-8777(00)00046-X. [DOI] [PubMed] [Google Scholar]
- 26.Fishel ML, Xia H, McGeown J, McIlwain DW, Elbanna M, Craft AA, Kaimakliotis HZ, Sandusky GE, Zhang C, Pili R, et al. Anti-tumor activity and mechanistic characterization of APE1/Ref-1 inhibitors in bladder cancer. Mol Cancer Ther. 2019;18:1947–1960. doi: 10.1158/1535-7163.MCT-18-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D, Zhong ZY, Li MX, Xiang DB, Li ZP. Vector-based Ape1 small interfering RNA enhances the sensitivity of human osteosarcoma cells to endostatin in vivo. Cancer Sci. 2007;98:1993–2001. doi: 10.1111/j.1349-7006.2007.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pignot G, Bieche I, Vacher S, Güet C, Vieillefond A, Debré B, Lidereau R, Amsellem-Ouazana D. Large-scale real-time reverse transcription-PCR approach of angiogenic pathways in human transitional cell carcinoma of the bladder: Identification of VEGFA as a major independent prognostic marker. Eur Urol. 2009;56:678–688. doi: 10.1016/j.eururo.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 29.Kloepper J, Riedemann L, Amoozgar Z, Seano G, Susek K, Yu V, Dalvie N, Amelung RL, Datta M, Song JW, et al. Ang-2/VEGF bispecific antibody reprograms macrophages and resident microglia to anti-tumor phenotype and prolongs glioblastoma survival. Proc Natl Acad Sci USA. 2016;113:4476–4481. doi: 10.1073/pnas.1525360113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Yang TH, Porta-Pardo E, Gao GF, Plaisier CL, Eddy JA, et al. The Immune Landscape of Cancer. Immunity. 2018;48:812–830.e814. doi: 10.1016/j.immuni.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hudson AL, Parker NR, Khong P, Parkinson JF, Dwight T, Ikin RJ, Zhu Y, Chen J, Wheeler HR, Howell VM. Glioblastoma Recurrence Correlates With Increased APE1 and Polarization Toward an Immuno-Suppressive Microenvironment. Front Oncol. 2018;8:314. doi: 10.3389/fonc.2018.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan T, Venkat P, Chopp M, Zacharek A, Yu P, Ning R, Qiao X, Kelley MR, Chen J. APX3330 promotes neurorestorative effects after stroke in type one diabetic rats. Aging Dis. 2018;9:453–466. doi: 10.14336/AD.2017.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the present study are available from the corresponding author upon reasonable request.