Karrikins trigger degradation of SMAX1 through an F-box protein–mediated mechanism that is highly similar to strigolactone signaling.
Abstract
Karrikins (KARs) are butenolides found in smoke that can influence germination and seedling development of many plants. The KAR signaling mechanism is hypothesized to be very similar to that of the plant hormone strigolactone (SL). Both pathways require the F-box protein MORE AXILLARY GROWTH2 (MAX2), and other core signaling components have shared ancestry. Putatively, KAR activates the receptor KARRIKIN INSENSITIVE2 (KAI2), triggering its association with the E3 ubiquitin ligase complex SCFMAX2 and downstream targets SUPPRESSOR OF MAX2 1 (SMAX1) and SMAX1-LIKE2 (SMXL2). Polyubiquitination and proteolysis of SMAX1 and SMXL2 then enable growth responses to KAR. However, many of the assumptions of this model have not been demonstrated. Therefore, we investigated the posttranslational regulation of SMAX1 from the model plant Arabidopsis (Arabidopsis thaliana). We find evidence that SMAX1 is degraded by KAI2-SCFMAX2 but is also subject to MAX2-independent turnover. We identify SMAX1 domains that are responsible for its nuclear localization, KAR-induced degradation, association with KAI2, and ability to interact with other SMXL proteins. KAI2 undergoes MAX2-independent degradation after KAR treatment, which we propose results from its association with SMAX1 and SMXL2. Finally, we discover an SMXL domain that mediates receptor–target interaction preferences in KAR and SL signaling, laying the foundation for understanding how these highly similar pathways evolved to fulfill different roles.
INTRODUCTION
Strigolactones (SLs) are a class of carotenoid-derived molecules that function as hormones within plants as well as signals exuded into soil that promote association of arbuscular mycorrhizal fungi with roots (Waters et al., 2017). The core mechanism of SL perception has been revealed over the past decade. As with auxin, jasmonate, and gibberellin signaling, responses to SL are achieved through hormone-activated proteolysis of a specific set of target proteins that function as signaling repressors or growth regulators (Morffy et al., 2016; Blázquez et al., 2020). SL is bound and hydrolyzed by the ɑ/β-hydrolase DWARF14 (D14)/DECREASED APICAL DOMINANCE2 (DAD2). In the course of SL hydrolysis, a butenolide ring becomes covalently attached to the His residue in the catalytic triad of D14 (Hamiaux et al., 2012; de Saint Germain et al., 2016; Yao et al., 2016). The exact step during this process that constitutes activation of D14 is currently under debate (Carlsson et al., 2018; Shabek et al., 2018; Marzec and Brewer, 2019; Seto et al., 2019).
Regardless of how D14 is activated, it is clear that SL causes D14 to form a complex with the F-box protein MORE AXILLARY GROWTH2 (MAX2)/DWARF3 (D3) and a subset of proteins within the SMAX1-LIKE (SMXL) family (Jiang et al., 2013; Zhou et al., 2013; Soundappan et al., 2015; Wang et al., 2015; Zhao et al., 2015; Liang et al., 2016; Shabek et al., 2018). Differential scanning fluorimetry (DSF) assays show that D14 undergoes a decrease in melting temperature (Tm) in the presence of a synthetic SL analog, GR24. A strong correlation has been observed between the biological activity of SL-related compounds and their ability to induce destabilization of D14, implying that the Tm shift reflects transition of the receptor to an active state (Seto et al., 2019). SL-activated D14 undergoes a dramatic conformational rearrangement that can be observed through crystallography when it associates with D3/MAX2 (Yao et al., 2016). As an F-box protein, MAX2 confers specificity to an SCF-type E3 ubiquitin ligase complex consisting of Skp1, Cullin, and RING-BOX (RBX) that marks substrate proteins for degradation by attaching polyubiquitin chains. DWARF53 (D53) in rice (Oryza sativa) and its orthologs in Arabidopsis (Arabidopsis thaliana), SMXL6, SMXL7, and SMXL8, are polyubiquitinated and degraded by the 26S proteasome after SL treatment in a D14- and D3/MAX2-dependent manner. Because D53, SMXL6, SMXL7, and SMXL8 have an ethylene responsive element binding factor–associated amphiphilic repression (EAR) motif(s) that enables interaction with the transcriptional corepressors TOPLESS (TPL)/TOPLESS-RELATED (TPR), it is thought that their turnover activates gene expression through relief of repression (Jiang et al., 2013; Zhou et al., 2013; Soundappan et al., 2015; Wang et al., 2015; Liang et al., 2016; Ma et al., 2017). The transcription factor IDEAL PLANT ARCHITECTURE1 (IPA1) in rice and its orthologs in wheat (Triticum aestivum) interact with D53 to mediate at least some SL regulation of gene expression (Liu et al., 2017; Song et al., 2017). However, not all SMXL functions require an EAR motif, and some SL responses, such as removal of the auxin efflux carrier PIN-FORMED1 (PIN1) from the plasma membrane, do not seem to be mediated through transcriptional regulation (Shinohara et al., 2013; Liang et al., 2016).
Karrikins (KARs) are a class of butenolide molecules found in smoke and biochar that are produced by pyrolysis of carbohydrates such as cellulose (Flematti et al., 2004; Kochanek et al., 2016). KAR1 (3-methyl-2H-furo[2,3-c]pyran-2-one) and KAR2 (2H-furo[2,3-c]pyran-2-one) are among the most abundant and bioactive KARs found in smoke (Flematti et al., 2007, 2009; Nelson et al., 2009; Hrdlička et al., 2019). KARs stimulate seed germination of many species and can also influence photomorphogenic development of seedlings, root growth, and root hair development (Nelson et al., 2010, 2012; Swarbreck et al., 2019; Villaécija-Aguilar et al., 2019). Remarkably, responses to KARs are likely mediated through a signaling pathway that is highly similar to that of SLs. KAR signaling in Arabidopsis requires MAX2 and KARRIKIN INSENSITIVE2/HYPOSENSITIVE TO LIGHT (KAI2/HTL), which is an ancient paralog of D14 (Nelson et al., 2011; Waters et al., 2012; Bythell-Douglas et al., 2017). As with D14, KAI2 has a conserved Ser-His-Asp catalytic triad that is important for its function in vivo as well as for responses to GR24 in DSF assays (Waters et al., 2015a, 2015b; Yao et al., 2018). Here, we must note that although GR24 is commonly referred to and used as an analog of SLs, it is almost always used as a racemic mixture (rac-GR24) that has complex properties. The stereoisomer GR245DS has the 2′R configuration of natural SLs and predominantly signals through D14. Its enantiomer, GR24ent-5DS, which has an unnatural 2′S configuration, signals largely through KAI2 but also through D14 (Scaffidi et al., 2014; Flematti et al., 2016). Consistent with their activities in plants, GR24ent-5DS, but not GR245DS, induces a Tm shift for KAI2. There is substantial biochemical evidence that KAI2 found in multiple species can bind KAR1 (Guo et al., 2013; Kagiyama et al., 2013; Toh et al., 2014; Xu et al., 2016, 2018; Lee et al., 2018; Bürger et al., 2019). However, KAI2 does not show a response to KAR1 or KAR2 in DSF assays, suggesting that KARs might not trigger KAI2 activation in vitro (Waters et al., 2015b).
Regardless of how KARs are perceived through KAI2, there is growing evidence that the primary role of KAI2 is the recognition of an unknown endogenous signal, KAI2 ligand (KL) (Conn and Nelson, 2016; Sun et al., 2016). The significance of KL as a plant growth regulator is underscored by the diverse roles of KAI2 in germination, seedling development, leaf morphology, stress tolerance, drought tolerance, root growth, root hair development, and arbuscular mycorrhizal symbiosis (Sun and Ni, 2011; Waters et al., 2012; Gutjahr et al., 2015; Soundappan et al., 2015; Li et al., 2017; Wang et al., 2018; Swarbreck et al., 2019; Villaécija-Aguilar et al., 2019; Li et al., 2020). Therefore, identification of KL and an understanding of how KAI2-dependent signaling works will be of broad relevance to plant biology.
A screen for genetic suppressors of max2 led to the identification of SUPPRESSOR OF MAX2 1 (SMAX1; Stanga et al., 2013). Loss of SMAX1 reverses max2 phenotypes associated with KAR responses, including increased seed dormancy, reduced seedling photomorphogenic growth, and upward petiole orientation. SMAX1-LIKE2 (SMXL2) also contributes to seedling growth regulation downstream of MAX2 in a partially redundant manner with SMAX1 (Stanga et al., 2016). Thus, SL- and KAR/KL-regulated growth responses appear to be mediated through two distinct clades of the SMXL family (Soundappan et al., 2015). This has led to a model in which SMXL6, SMXL7, and SMXL8 are targets of D14-SCFMAX2 and SMAX1 and SMXL2 are targets of KAI2-SCFMAX2. According to this model, max2 phenotypes result from the combined loss of KAI2- and D14-dependent signaling, which causes overaccumulation of all five SMXL proteins. Indeed, the rosettes of smax1 max2 plants are morphologically similar to those of d14, suggesting the KAI2 pathway defect has been suppressed. Likewise, smxl6 smxl7 max2 rosettes are similar to those of kai2, suggesting the D14 pathway defect has been suppressed (Soundappan et al., 2015). Promoter-swapping experiments show that D14:KAI2 does not rescue d14 and KAI2:D14 does not rescue kai2 (Waters et al., 2015b). This lack of reciprocal function reinforces the idea that KAI2 and D14 interact with different downstream partners.
In addition to the genetic evidence that SMAX1 and SMXL2 act downstream of MAX2 as KAR/KL pathway targets, the shared ancestry of the KAR/KL and SL pathway components suggests that they may have similar signaling mechanisms (Machin et al., 2020). The hypothesis that KAI2-SCFMAX2 mediates degradation of SMAX1 and SMXL2 remains largely unproven, but there are some additional lines of evidence for it. First, rac-GR24 has been reported to stimulate yeast two-hybrid (Y2H) interactions between KAI2 and MAX2 (Toh et al., 2014; Holbrook-Smith et al., 2016), although this particular association has been considered difficult to distinguish from nonspecific KAI2 interactions by other groups (Yao et al., 2018). More compellingly, a KAI2 paralog found in Striga hermonthica that recognizes SLs, ShHTL7, can be pulled down by MAX2 in the presence of rac-GR24 (Yao et al., 2017). ShHTL7 also forms a complex with Arabidopsis MAX2 and Skp1 (ASK1) in the presence of GR24 that can be observed by size-exclusion chromatography. SMAX1 is able to pull down ShHTL7, but not Arabidopsis D14, in the presence of rac-GR24. Conversely, SMXL6 pulls down D14 in the presence of rac-GR24 and has a comparably weak interaction with ShHTL7 that is not affected by rac-GR24 (Yao et al., 2017). These results support the specificity in receptor–target interactions that has been proposed for the KAR and SL pathways, with the caveat that interactions between proteins from different species were tested. Finally, Wallner et al. (2017) reported that misexpressing an SMAX1-yellow fluorescent protein (YFP) translational fusion under the control of the SMXL5 promoter produces YFP fluorescence in the root cortex that disappears within minutes after rac-GR24 treatment. This is consistent with the current KAR signaling model, but it does not address directly whether SMAX1 degradation is mediated by KAI2-SCFMAX2.
We set out to determine how SMAX1 is posttranslationally regulated after KAR treatment. This proved to be an unexpectedly difficult undertaking, putatively due to a MAX2-independent mechanism that causes rapid turnover of SMAX1 protein. In response, we established a ratiometric reporter system to monitor SMAX1 degradation in Arabidopsis and Nicotiana benthamiana. We identified domains of SMAX1 that mediate MAX2-dependent degradation, interaction with KAI2, and interactions between SMXL proteins. We also discovered that MAX2-independent degradation of KAI2 depends upon SMAX1 and SMXL2.
RESULTS
Deletion of a Conserved Arg-Gly-Lys-Thr Motif Stabilizes SMAX1
To test whether SMAX1 is targeted for degradation by KAI2-SCFMAX2 after KAR treatment, we first attempted to develop antibodies that could detect native SMAX1 in Arabidopsis. Our extensive efforts in this area were unsuccessful. Therefore, we pursued a protein-tagging approach.
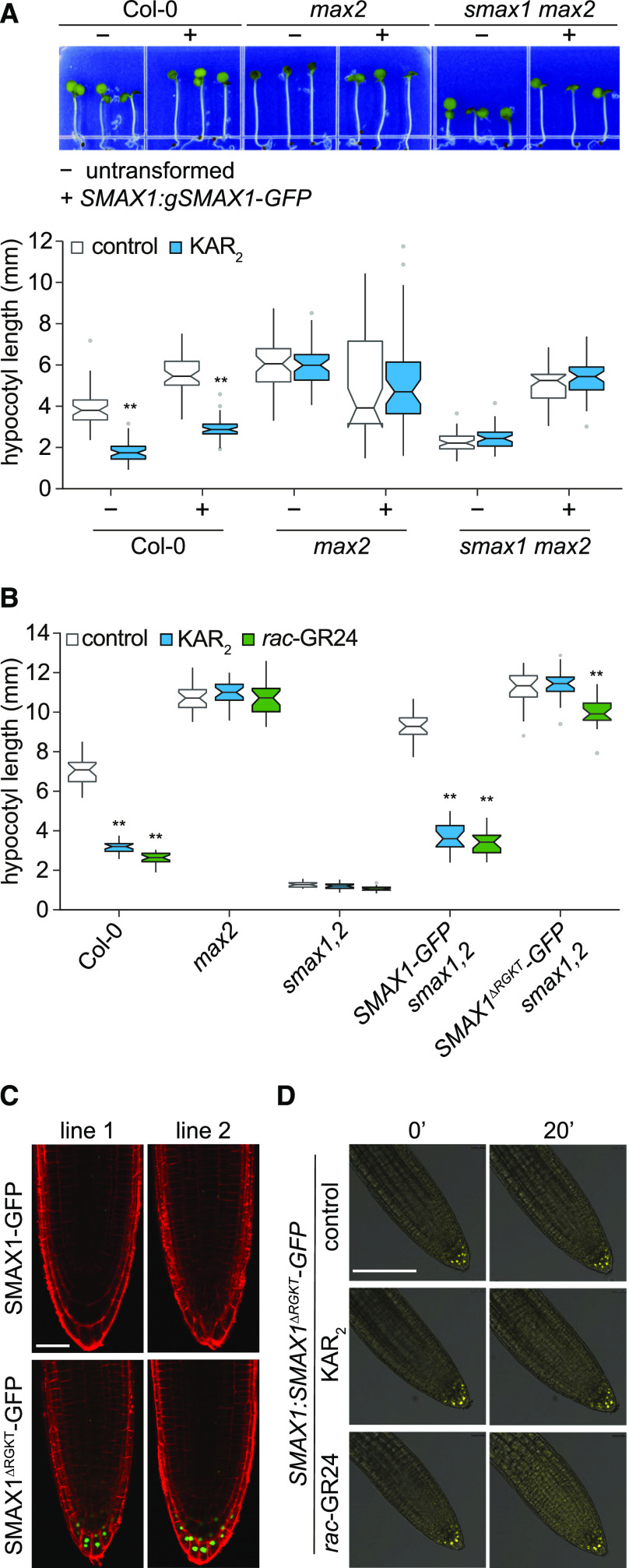
We generated a C-terminal translational fusion of eGFP to an SMAX1 genomic clone (coding sequence with introns included) under the transcriptional control of 2976 bp of sequence upstream of SMAX1. The SMAX1:gSMAX1-GFP-transgene was stably introduced by floral dip transformation into the wild type (Columbia ecotype [Col-0]), max2, and smax1 max2. The hypocotyls of gSMAX1-GFP Col-0 seedlings were similar in length to those of max2, consistent with overaccumulation of SMAX1 (Figure 1). Unlike max2, however, hypocotyl elongation of gSMAX1-GFP Col-0 seedlings was reduced by KAR2. In the max2 background, gSMAX1-GFP did not promote hypocotyl growth further, but we noted an increase in variation of the hypocotyl phenotype. The short hypocotyls of smax1 max2 seedlings were restored to max2 length by gSMAX1-GFP but remained insensitive to KAR2 (Figure 1A). Collectively, these results indicated that the gSMAX1-GFP fusion is functional in seedlings and is negatively regulated by KAR2 in a MAX2-dependent manner.
Figure 1.
SMAX1-GFP Is Functional, but Not Detectable Unless an RGKT Motif Is Removed.
(A) Hypocotyl lengths of Col-0, max2-1, and smax1-2 max2-1 seedlings that are nontransgenic (–) or homozygous transgenic (+) for SMAX1:gSMAX1-GFP. Surface-sterilized seeds were plated on solid 0.5× MS medium, stratified 3 d at 4°C, given a germination initiation treatment (3 h of white light, 21 h of dark at 21°C), and grown under continuous red light (30 µE) for 4 d at 21°C before measurement. Boxplot notches approximate the 95% CI for the median. n = 45. *P < 0.01, **P < 0.001, two-way analysis of variance with Bonferroni’s test, comparisons to control treatment.
(B) Hypocotyl lengths of Col-0, max2-1, smax1-2 smxl2-1, and smax1-2 smxl2-1 lines homozygous for SMAX1:SMAX1-GFP or SMAX1:SMAX1∆RGKT-GFP transgenes. Assay conditions as described in (A), but medium was supplemented with 1 μM KAR2, 1 μM rac-GR24, or 0.1% (v/v) acetone as a solvent control. Boxplot notches approximate the 95% CI for the median. n = 20. *P < 0.01, **P < 0.001, two-way analysis of variance with Bonferroni’s test, comparisons to control treatment.
(C) Confocal images of 4-d light-grown seedlings from two independent transgenic lines (line 1 and line 2) for SMAX1:SMAX1-GFP or SMAX1:SMAX1∆RGKT-GFP in smax1 smxl2 background. Propidium iodide staining (red) was performed to visualize cell boundaries. Bar = 25 μm.
(D) Confocal microscopy of SMAX1:SMAX1∆RGKT-GFP smax1 smxl2 seedlings treated with 10 μM KAR2, 10 μM rac-GR24, and 0.1% (v/v) acetone control. Seedlings were grown vertically on 0.5× MS medium for 4 d under white light (∼150 μE, 12 h of light and 12 h of dark) following stratification. Images are overlays of bright field (gray) and GFP channel (yellow) images. Bar = 50 μm.
We predicted that gSMAX1-GFP would be stabilized and most easily detectable in max2 or smax1 max2 backgrounds due to the loss of SCFMAX2 activity. However, we were not able to observe gSMAX1-GFP by confocal fluorescence microscopy (Supplemental Figure 1A). We considered that if SMAX1 was degraded before GFP had sufficient time to mature, we might not be able to detect SMAX1-GFP by microscopy. However, immunoblots with a polyclonal antibody against GFP (ab6556; Abcam) were also unsuccessful in detecting SMAX1-GFP in transgenic Arabidopsis lines (Supplemental Figure 1B).
Deletion of a highly conserved Arg-Gly-Lys-Thr (RGKT), or P-loop, motif in rice D53 or Arabidopsis SMXL6 and SMXL7 produces dominantly acting proteins that are resistant to GR24-induced degradation (Jiang et al., 2013; Zhou et al., 2013; Wang et al., 2015; Liang et al., 2016). To determine whether this motif also affects SMAX1 stability, we deleted it in a second SMAX1-GFP reporter construct made with the SMAX1 coding sequence (no introns) under control of the native promoter, SMAX1:SMAX1-GFP. The SMAX1-GFP and SMAX1ΔRGKT-GFP reporters were stably introduced into smax1 smxl2, which has constitutive KAR/KL responses and very short hypocotyls (Stanga et al., 2016). SMAX1-GFP rescued smax1 smxl2 seedlings, again demonstrating that a C-terminal GFP fusion to SMAX1 is functional (Figure 1B; Supplemental Figure 1C). By contrast, SMAX1ΔRGKT smax1 smxl2 seedlings were phenotypically similar to max2, with elongated hypocotyls that were insensitive to KAR2 (Figure 1B; Supplemental Figure 1C). SMAX1-GFP was not detectable by fluorescence microscopy in smax1 smxl2, consistent with the previous experiments (Figure 1C). However, SMAX1ΔRGKT-GFP was visible in the root columella and lateral root cap, particularly in the most distal cell layers (Figures 1C and 1D). The level of SMAX1ΔRGKT-GFP in root tips did not decrease within 20 min of treatment with 10 µM rac-GR24 or KAR2 (Figure 1D). Therefore, the RGKT motif influences the stability of SMAX1 as well as D53-type SMXL proteins. It is interesting that SMAX1ΔRGKT-GFP was detectable, yet we did not observe gSMAX1-GFP in a background lacking MAX2 (Supplemental Figure 1A). This raises the possibility that the ΔRGKT mutation stabilizes SMAX1 in a manner that is at least partially independent of MAX2.
Heterologous SMAX1 Is Readily Degraded in Plant Protein Extracts
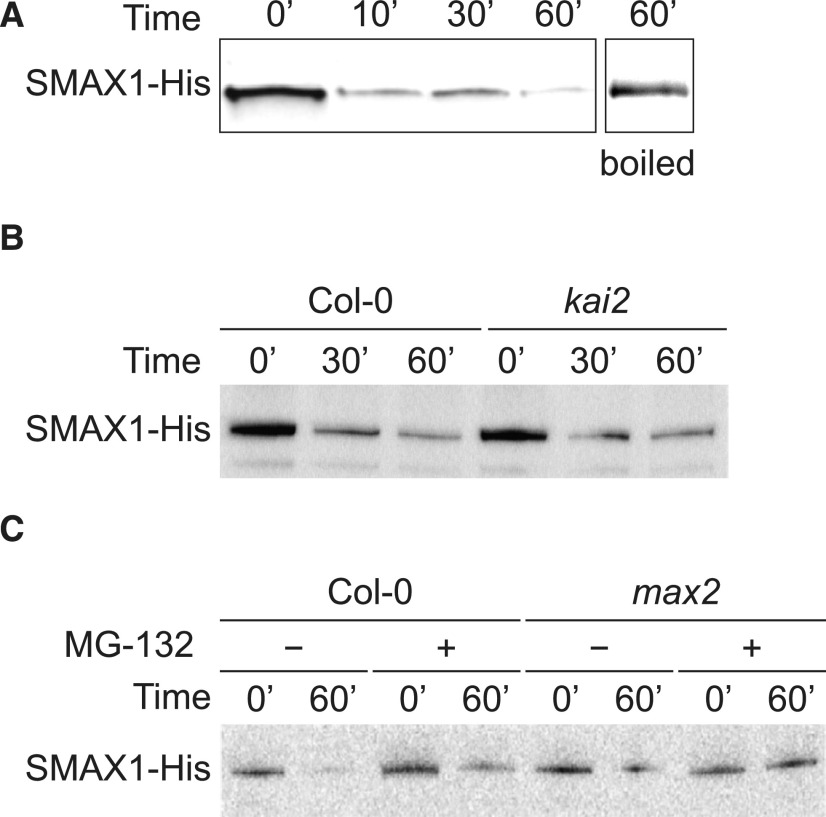
Although SMAX1ΔRGKT was detectable, it is not useful for evaluating MAX2- and KAI2-dependent degradation. Therefore, we explored an in vitro degradation assay for SMAX1 degradation. We expressed SMAX1 with a C-terminal 6×His tag in Escherichia coli and purified the fusion protein through affinity chromatography (Supplemental Figure 2). SMAX1-His was added to soluble protein extracts from 7-d-old Arabidopsis seedlings, and the mixture was assayed by immunoblot over a 60-min incubation. In preparations in which SMAX1-His made up fully 10% of the total protein mixture, we found that SMAX1-His abundance decreased within 10 min of incubation (Figure 2). SMAX1-His was stable in boiled protein extracts, implying its decline was due to proteolysis (Figure 2A). SMAX1-His degradation was comparable in Col-0 and kai2 protein extracts, demonstrating that SMAX1 turnover in this system is not KAI2 dependent (Figure 2B). SMAX1-His degradation also occurred in max2 extracts (Figure 2C). Carbobenzoxy-Leu-Leu-leucinal (MG-132) slowed SMAX1-His degradation, suggesting that the 26S proteasome may be involved in the rapid turnover of SMAX1-His in vitro (Figure 2C; Supplemental Figure 2C). We were not able to observe a consistent effect of KAR or rac-GR24 on SMAX1-His degradation in this assay. The artificial nature of this approach makes it prone to artifacts; for example, SMAX1 may be exposed to proteases it does not normally encounter in intact plant cells. However, if SMAX1 undergoes a high degree of turnover in vivo as well as in vitro, it could explain why it was not detectable in the experiments described above.
Figure 2.
Recombinant SMAX1 Is Unstable in Protein Extracts from Arabidopsis Seedlings.
(A) to (C) Immunoblots of in vitro degradation time courses of SMAX1-His in wild-type Col-0 (A), kai2 (B), and max2 (C) mutant protein extracts. Heterologously expressed and purified SMAX1-His was added to soluble protein extracts from 7-d-old Arabidopsis seedlings to compose 10% of the total protein concentration. Aliquots (50 µg) of the mixture were incubated at 24°C and boiled for 10 min at the indicated time points. Protein (33 µg) from each boiled aliquot was separated by SDS-PAGE, blotted, and probed with polyclonal anti-His antibody. The boiled plant protein extract incubated with SMAX1-His for 60 min served as the control. Where indicated in (C), 40 µM MG-132 was added to protein extracts prior to addition of SMAX1-His.
Arabidopsis SMAX1 Is Degraded after KAR and rac-GR24 Treatment in N. benthamiana
Because we had not found a viable means to detect SMAX1 in Arabidopsis, we tested whether transient expression of SMAX1 in N. benthamiana would allow us to assay its degradation. We infiltrated N. benthamiana leaves with Agrobacterium tumefaciens carrying SMAX1:gSMAX1-GFP and detected nucleus-localized GFP signal within 3 d (Supplemental Figure 3). Nuclear localization of SMAX1-GFP is consistent with our prior observation of SMAX1–TPR2 interactions in N. benthamiana nuclei by bimolecular fluorescence complementation (BiFC) and the nuclear localization of D53-type SMXLs (Zhou et al., 2013; Soundappan et al., 2015; Wang et al., 2015; Liang et al., 2016).
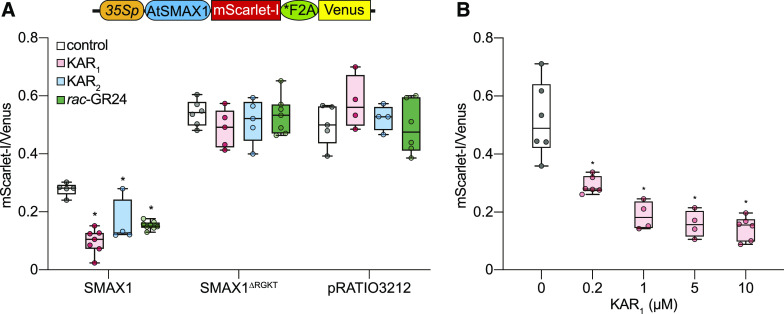
We found that gSMAX1-GFP expression tended to be too variable across infiltrated N. benthamiana leaf samples to provide robust results for degradation assays. Therefore, we adopted a ratiometric reporter system to normalize for variable rates of transformation or transgene expression (Figure 3A; Khosla et al., 2020). pRATIO3212 is a dual-fluorescent reporter construct that encodes within a single transcript a C-terminal fusion of mScarlet-I to a protein of interest (target), a modified 2A peptide (*F2A), and a Venus yellow fluorescent protein for reference (Nagai et al., 2002; Bindels et al., 2017; Khosla et al., 2020). The 2A peptide interrupts peptide-bond formation at its C-terminal end, enabling high-efficiency separation of the target and reference proteins during translation (Donnelly et al., 2001; Khosla et al., 2020).
Figure 3.
Ratiometric Detection of SMAX1 Degradation in N. benthamiana.
(A) Diagram of the pRATIO3212-SMAX1 ratiometric reporter system. The 35S promoter drives expression of a multicistronic transcript encoding an SMAX1-mScarlet-I fusion that is separately translated from the Venus fluorescent protein due to the *F2A peptide. Relative fluorescence after transient expression of pRATIO3212-SMAX1, -SMAX1∆RGKT, and empty pRATIO3212 in N. benthamiana. The empty pRATIO3212 denotes pRATIO3212 lacking the Gateway cassette. Leaf discs were treated with 0.02% (v/v) acetone or 10 μM KAR1, KAR2, and rac-GR24 for 12 h before measurement. n = 4 to 7 leaf discs.
(B) Relative fluorescence after transient expression of pRATIO3212-SMAX1 in N. benthamiana and overnight treatment with KAR1 concentrations ranging from 200 nM to 10 μM. n = 4 to 6 leaf discs. *P < 0.01, Mann–Whitney U test, comparisons to control treatment.
We transiently expressed pRATIO3212-SMAX1 in N. benthamiana. Leaf discs were removed from the infiltrated areas of the leaf and then treated with 10 µM KAR1, KAR2, or rac-GR24. We observed an >1.5-fold decrease in the mScarlet-I:Venus ratio in response to all three treatments after 12 h (P < 0.01; Figure 3A). By contrast, mScarlet-I:Venus ratios were unaffected by KAR or rac-GR24 treatments in tests of pRATIO3212-SMAX1∆RGKT and empty pRATIO3212 (Figure 3A). We examined the sensitivity of the SMAX1 ratiometric reporter to a range of KAR1 concentrations. We found that 200 nM KAR1 was sufficient to reduce SMAX1 reporter levels after 12 h, and the strongest responses were achieved with 5 and 10 µM KAR1 treatments (Figure 3B). A significant reduction in the SMAX1 reporter could be observed within 4 h of treatment with 10 µM KAR1 (P < 0.01; Supplemental Figure 4). Therefore, this ratiometric system can provide a reasonably sensitive and rapid readout of SMAX1 degradation.
We note that the exact mScarlet-I:Venus ratios produced by the ratiometric system for SMAX1 sometimes varied between experiments (see controls for Figure 3; Supplemental Figure 4). These ratios can be influenced by the age/vigor of the transformed plants and the gain settings used during signal quantitation. Nonetheless, in our characterization of SMAX1 and its domains (described below), we typically observed consistent trends in the relative abundance of different targets and their responses to chemical treatments.
The D2 Domain of SMAX1 Mediates Nuclear Localization and KAR-Induced Degradation
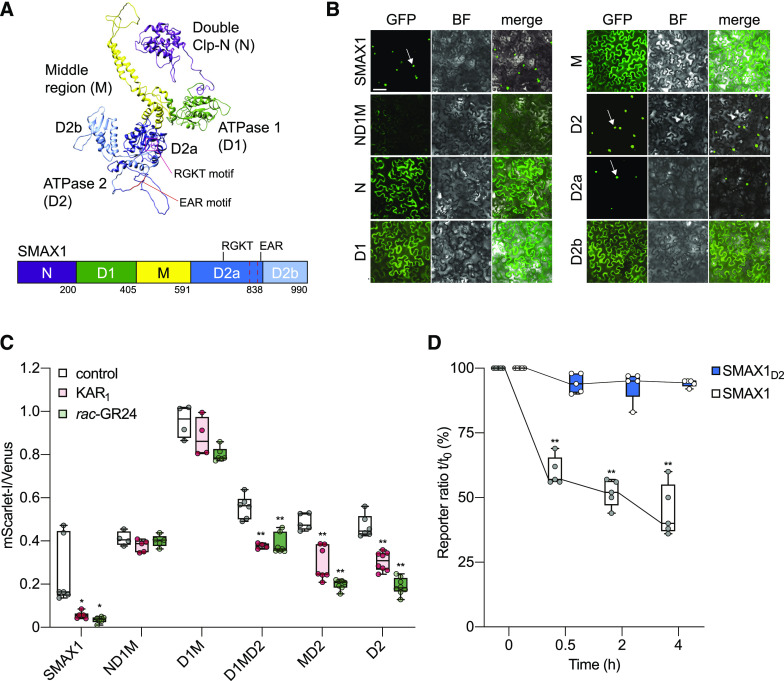
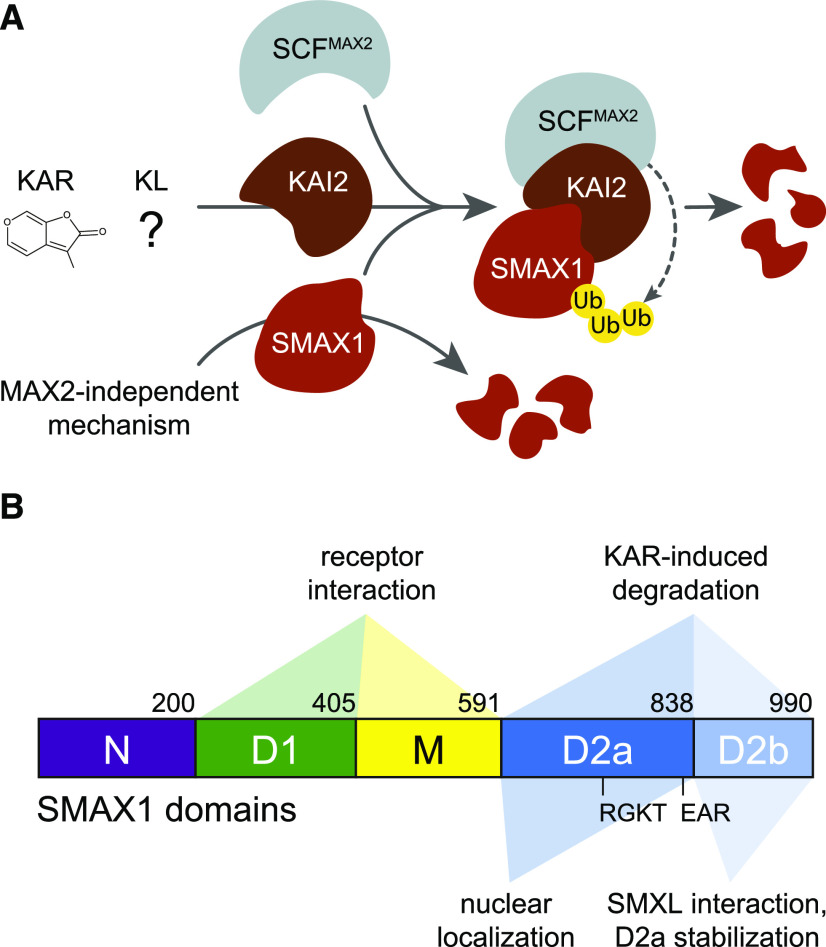
We next set out to identify the SMAX1 degron. We first used Phyre2 to predict the tertiary structure of SMAX1 (Kelley et al., 2015). Although this approach was not expected to be highly accurate, we reasoned that it might enable selection of domains that are likely to form discrete folded structures (Figure 4A). The crystal structure for bacterial ClpB/Hsp104 chaperone protein (chain B; Protein Data Bank entry: c1qvrB), was used by Phyre2 to model the tertiary structure of SMAX1. Despite the low amino acid identity between SMAX1 and Hsp104, Protein Homology/analogY Recognition Engine (Phyre) analysis returned a model with a >90% confidence score, which typically indicates high-accuracy modeling of the core of the protein. We combined this structural prediction with an examination of sequence conservation across Arabidopsis and rice SMAX1 and D53 orthologs to define five major domains of interest (Supplemental Figure 5). These correspond well with the N-terminal Double Clp-N motif (N), putative ATPase domain 1 (D1), middle region (M), and C-terminal putative ATPase domain 2 (D2) domain assignments of Shabek et al. (2018) and Zhou et al. (2013) to D53, although the exact boundaries vary slightly. We have maintained the same domain nomenclature, except for splitting D2 into subdomains D2a and D2b based upon the Phyre2 model (Figure 4A). D2a contains the RGKT motif and a conserved EAR motif that has been implicated in TOPLESS (TPL/TPR) interactions (Jiang et al., 2013; Soundappan et al., 2015; Wang et al., 2015; Liang et al., 2016; Ma et al., 2017). A comprehensive analysis of SMXL evolution in land plants supports the idea that D2 should not be treated as a single domain (Walker et al., 2019; Machin et al., 2020).
Figure 4.
Identification of SMXL Domains Mediating Nuclear Localization and KAR1-Induced Degradation.
(A) Model of the three-dimensional structure of the SMAX1 protein predicted by Phyre2. Major SMAX1 domains are indicated: (N (purple), D1 (green), M (yellow), and D2 (blue). D2 is divided into D2a (dark blue) and D2b (light blue) subdomains. RGKT motif and EAR motif are highlighted in red. The red dashed box indicates a putative NLS sequence at amino acids 797 to 824.
(B) Subcellular localization of SMAX1 domains fused to GFP after transient expression in N. benthamiana epidermal cells. Expression was driven by the 35S promoter. Bar = 50 μm. BF, bright field.
(C) Relative fluorescence of NLS-SMAX1 domains in the pRATIO1212 system after transient expression in N. benthamiana and overnight treatment with 10 μM KAR1, 10 μM rac-GR24, or 0.02% (v/v) acetone control. n = 5 to 8 leaf discs. *P < 0.01, **P < 0.001, Mann–Whitney U test, comparisons to control treatment.
(D) Time course of SMAX1 and SMAX1D2 stability in N. benthamiana after addition of cycloheximide (150 µg/mL). SMAX1 and SMAX1D2 abundance was monitored through the pRATIO1212 system. The reporter:reference ratio at each time point was scaled to the ratio measured at time 0 to show relative abundance over time. n = 5 leaf discs. **P < 0.001, paired t test, comparison to time 0.
We generated a series of C-terminal GFP fusions to SMAX1 domains to examine their subcellular localization patterns. Like full-length SMAX1, SMAX1D2 was localized to the nucleus (Figure 4B; Supplemental Figure 3). By contrast, SMAX1N, SMAX1M, SMAX1D1, and SMAX1ND1M were found in the cytoplasm and some nuclei. This localization pattern is typical of free GFP. Therefore, we concluded that D2 is sufficient and necessary for nuclear localization of SMAX1. Further examination showed that D2a is responsible for nuclear localization (Figure 4B). This was consistent with the prediction of a nuclear localization signal (NLS) at amino acids 797 to 824 of SMAX1 by the cNLS Mapper program (Kosugi et al., 2009). By contrast, an NLS was found in the SMXL7N domain (Liang et al., 2016).
We then investigated the ability of different SMAX1 domains to be degraded after KAR1 treatment in N. benthamiana. Nuclear localization is important for the function and SL-induced degradation of SMXL7 (Liang et al., 2016). Therefore, we chose to compare the degradation of SMAX1 domains within the same nuclear context. We used pRATIO1212, which is identical to pRATIO3212 but fuses an NLS from simian virus 40 (SV40) to the N terminus of the protein of interest. This NLS did not have an appreciable effect on the degradation of full-length SMAX1 (Supplemental Figures 6A to 6D).
Almost all SMAX1 truncations consistently produced mScarlet-I:Venus ratios that exceeded that of full-length SMAX1. The notable exceptions were truncations containing the D2a domain without D2b, which typically had much lower reporter ratios than full-length SMAX1 (Supplemental Figure 6E). We found that all SMAX1 truncations that contained D2 showed a significant reduction in the mScarlet-I:Venus ratio after KAR1 and rac-GR24 treatment, whereas all truncations that lacked D2 were unaffected by the treatments (Figure 4C; Supplemental Figure 6F). Therefore, D2 is necessary and sufficient for KAR1- and rac-GR24–induced degradation of Arabidopsis SMAX1 in N. benthamiana. This is consistent with prior work that implicated the D2 domain of D53 as the target of SL-induced degradation (Shabek et al., 2018).
The increased mScarlet-I:Venus ratios observed for many SMAX1 truncations could arise from these proteins having higher stability than full-length SMAX1 or being more rapidly produced. To test this possibility, we examined the half-life of SMAX1D2 and SMAX1 after treatment of N. benthamiana leaf discs with cycloheximide. The SMAX1D2 target declined by only 5% (P = 0.061, paired t test) after a 4-h treatment with cycloheximide (Figure 4D). By contrast, full-length SMAX1 was reduced by 60%. Because of its higher stability, we reasoned that SMAX1D2 might be more useful than full-length SMAX1 as a reporter of MAX2-dependent degradation in Arabidopsis.
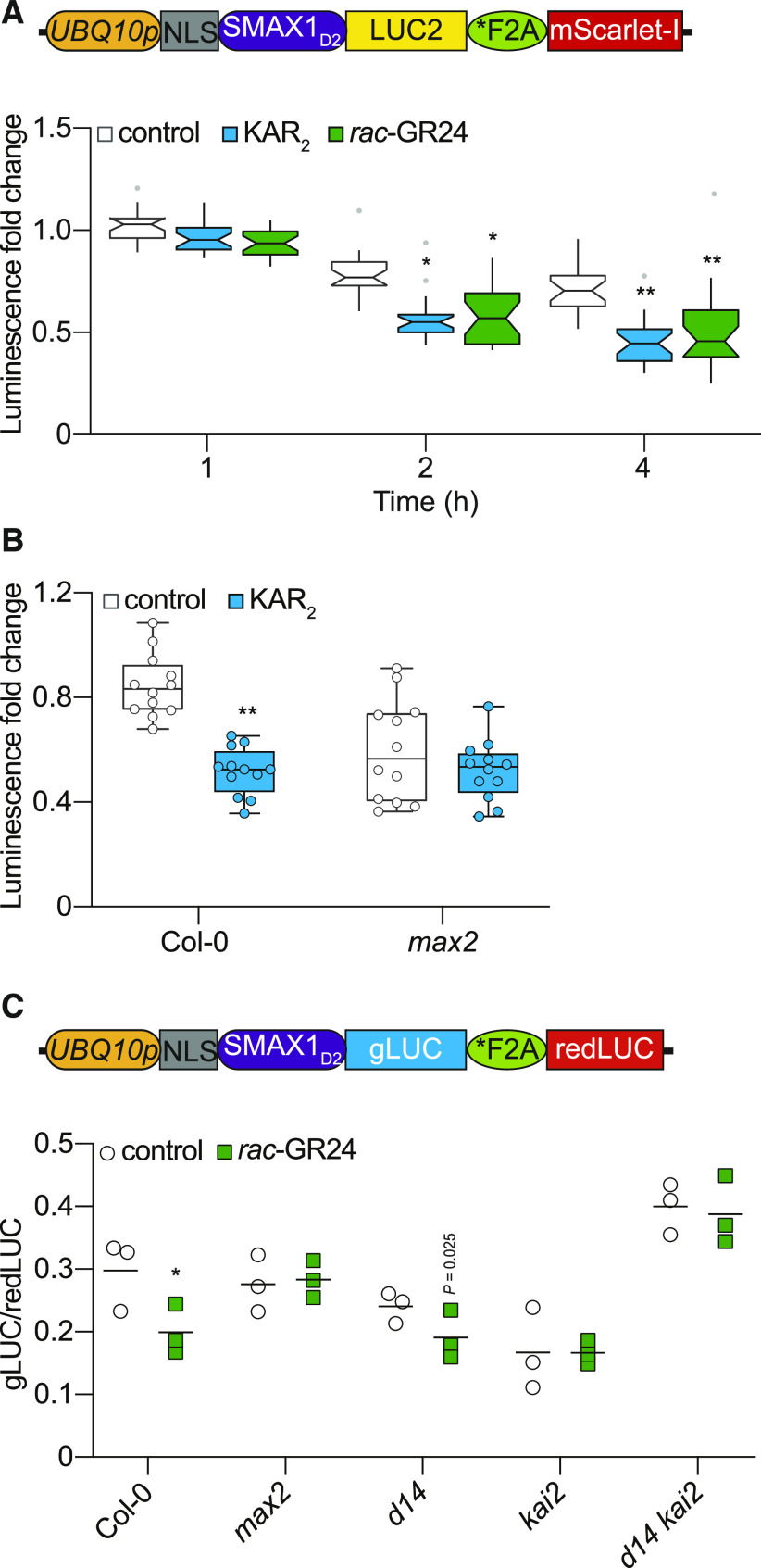
SMAX1D2 Degradation in Arabidopsis Is MAX2 and KAI2 Dependent
To test SMAX1D2 degradation in Arabidopsis, we used the ratiometric construct pRATIO2251, which uses an improved version of firefly luciferase (LUC2) as a target reporter and mScarlet-I as a reference protein (Khosla et al., 2020). We separately transformed Col-0 and max2 with pRATIO2251-SMAX1D2. Seven-day-old homozygous transgenic seedlings were treated with 10 µM KAR2 or rac-GR24, and bioluminescence was measured over the course of 4 h (Figure 5A). In the Col-0 background, we observed a significant decline in luminescence in response to both treatments within 2 h, which stabilized at an approximately twofold reduction in intensity. In the max2 background, we found no difference in luminescence between KAR2 and control-treated seedlings after 4 h. Therefore, the KAR2-induced degradation of SMAX1D2-LUC2 is MAX2 dependent (Figure 5B). Interestingly, we repeatedly noticed a stronger decline in SMAX1D2-LUC2 signal after the control treatment in max2 than Col-0, for which we do not yet have an explanation. Because mScarlet-I was not detectable above background fluorescence, it was not useful as a reference. Thus, we could not make a meaningful comparison of the baseline levels of SMAX1D2 reporter in max2 and Col-0 lines.
Figure 5.
MAX2- and KAI2-Dependent Degradation of SMAX1D2 in Arabidopsis.
(A) Diagram of the pRATIO2251-SMAX1D2 for ratiometric detection of SMAX1D2 in Arabidopsis transgenic lines. The UBQ10 promoter drives expression of a multicistronic transcript encoding an NLS-SMAX1D2-LUC2 fusion that is separately translated from the mScarlet-I fluorescent protein due to the *F2A peptide. Bioluminescence of SMAX1D2-LUC2 in homozygous Arabidopsis transgenic lines treated with 10 μM KAR2, 10 μM rac-GR24, or 0.02% (v/v) acetone control. Fold changes in bioluminescence relative to time 0 (immediately after treatment) for each seedling are shown. Boxplot notches approximate the 95% CI for the median. n = 24. *P < 0.01, **P < 0.001, two-way analysis of variance with Bonferroni’s test, comparisons to control treatment.
(B) Bioluminescence of SMAX1D2-LUC2 in the Col-0 and max2 backgrounds. Seedlings were treated with 10 µM KAR2 or acetone control for 4 h. Fold changes in bioluminescence relative to time 0 are shown. n = 12. **P < 0.001, Mann–Whitney U test, comparisons to control treatment.
(C) Diagram of the pMD19-SMAX1D2 for ratiometric detection of SMAX1D2 in Arabidopsis protoplasts. In pMD19-SMAX1D2, which is a nonbinary plasmid, the UBQ10 promoter controls expression of a multicistronic transcript encoding an NLS-SMAX1D2-gLUC fusion that is separately translated from a red-shifted firefly LUC protein due to a *F2A peptide. SMAX1D2 abundance in protoplasts isolated from Col-0, max2, d14, kai2, and d14 kai2, transformed with a dual-LUC ratiometric reporter (pMD19-SMAX1D2), recovered for 24 h, and treated with 10 μM rac-GR24 or acetone control for 4 h. SMAX1D2 was fused to gLUC and redLUC bioluminescence was used as a reference. gLUC:redLUC ratios of three independent protoplast preparations and transformations are shown; bar indicates mean. Results of a representative experiment are shown. *P < 0.01, unpaired t test, comparisons to control treatment.
To extend our analysis of SMAX1 degradation in different Arabidopsis mutant backgrounds, we developed transient expression assays in mesophyll protoplasts. We adopted a dual bioluminescent ratiometric reporter system that maximizes the ratio of signal-to-background noise, putatively enabling better detection of low-abundance proteins. The system is derived from pRATIO1267, which uses Gaussia luciferase (gLUC) as a target reporter and a red-shifted firefly luciferase (redLUC) as a reference. To reduce the size of the construct to be used in protoplast transformations, however, we used a simple cloning vector, pMD19, as the backbone instead of a binary vector (Supplemental Figure 7A). We tested full-length SMAX1 and several SMAX1 truncations. We found that gLUC fusions to SMAX1, SMAX1MD2, and SMAX1D1MD2 produced luminescence that was at or near background levels, despite having high levels of luminescence from the redLUC reference protein. By comparison, SMAX1D2-gLUC was readily detectable (Supplemental Figure 7B). The ability to detect SMAX1-gLUC was not improved in max2, kai2, or kai2 max2 protoplasts (Supplemental Figure 7C). These observations reinforce the ideas that SMAX1 is subject to MAX2- and KAI2-independent turnover and that SMAX1D2 overcomes this instability.
We tested whether KAI2- and/or D14-dependent signaling cause SMAX1D2-gLUC degradation. Protoplasts derived from Col-0, max2, d14, kai2, and kai2 d14 were treated with 10 µM rac-GR24 for 4 h and then lysed and assayed for gLUC and redLUC luminescence. We used rac-GR24 as a treatment because it can activate both KAI2 and D14 and it induces KAI2 thermal shift responses in vitro, unlike KARs (Scaffidi et al., 2014; Waters et al., 2015a). We found that Col-0 and d14 protoplasts had a reduced gLUC:redLUC ratio after rac-GR24 treatment, consistent with degradation of SMAX1D2 (Figure 5C). By contrast, max2, kai2, and kai2 d14 protoplasts did not show a response to rac-GR24. These results support MAX2- and KAI2-dependent degradation of SMAX1D2.
KAI2 Interaction with SMAX1 Occurs through the D1M Domain
A key prediction of the KAR/KL signaling model is that KAI2 associates with SCFMAX2 and SMAX1/SMXL2 after its activation, analogous to the mechanism of D14 action on D53-type SMXLs. As discussed above, there is some evidence for KAI2–MAX2 interactions from Y2H and pull-down experiments. We further argue that it is likely that KAI2 can interact with MAX2 based upon the strong amino acid conservation found among both KAI2 and D14 proteins in angiosperms at the D14 interface with D3/MAX2 (Bythell-Douglas et al., 2017).
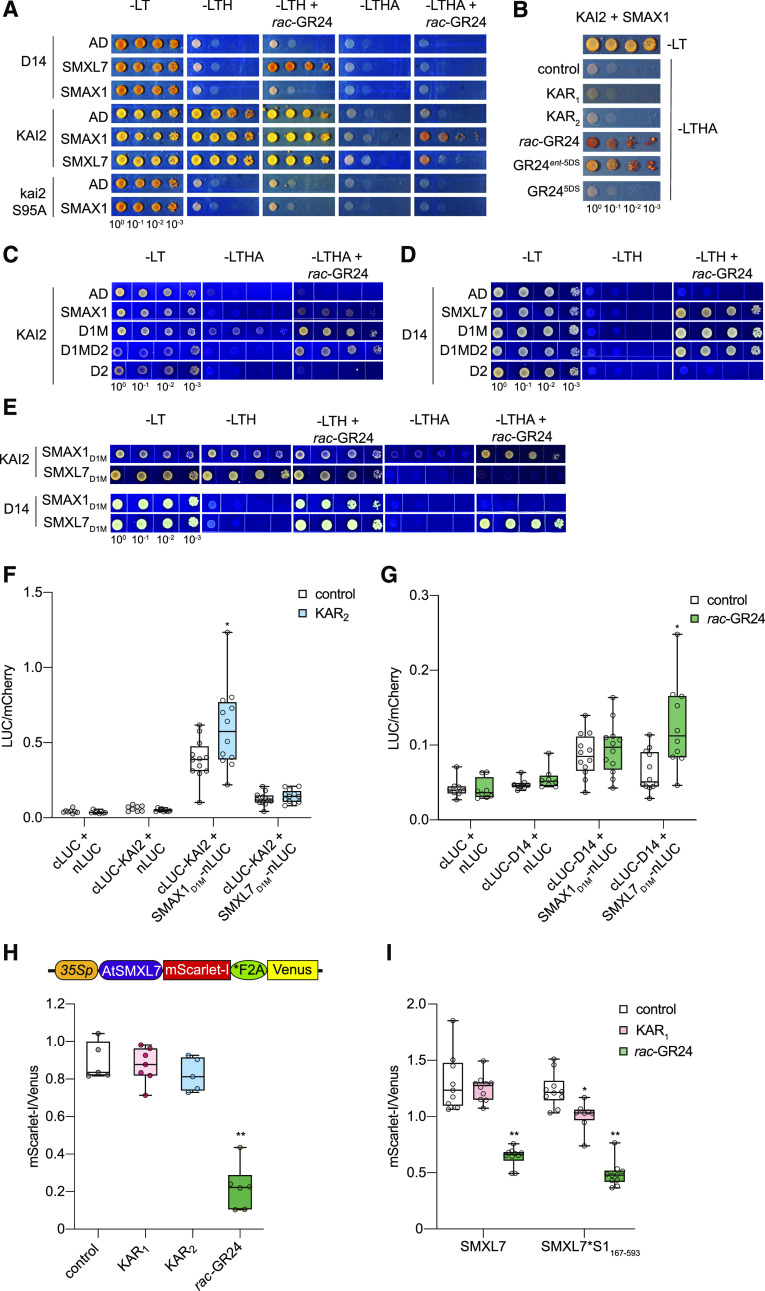
We focused our investigation on determining whether KAI2 interacts with SMAX1 and identifying the molecular basis for that interaction. First, we used Y2H to evaluate rac-GR24–induced interactions of KAI2 and D14 with SMAX1 and SMXL7. Y2H has been used previously to demonstrate SL- or SL analog–induced interactions between D14 and D53, SMXL6, or SMXL7 (Zhou et al., 2013; Umehara et al., 2015; Wang et al., 2015; Seto et al., 2019). We found that D14-SMXL7 showed a clear interaction in response to rac-GR24 on low stringency His dropout (–LTH) medium (Figure 6A). Under more stringent selection (-His-Ade media; –LTHA), D14-SMXL7 did not show growth. By contrast, KAI2 had promiscuous interactions on –LTH medium with SMAX1 and SMXL7, as well as the Gal4 DNA activation domain (GAL4-AD) negative control, regardless of whether rac-GR24 was present. However, a more specific interaction between KAI2 and SMAX1 emerged in the presence of rac-GR24 with –LTHA selection. To a weaker degree, KAI2 appeared to also interact with SMXL7. Y2H interactions with KAI2 were abolished by mutation of its catalytic Ser-95 to Ala (Figure 6A). This was consistent with the importance of the catalytic site for KAI2 signaling and DSF responses to GR24ent-5DS (Waters et al., 2015a, 2015b).
Figure 6.
KAI2 and D14 Interact with SMXL Proteins through the D1M Domains.
(A) to (E) Y2H assays for KAI2 and D14 interactions with SMAX1 and SMXL7. KAI2 and D14 were fused to GAL4-BD. SMAX1, SMXL7, and their domains were fused to GAL4-AD. Yeast cells were co-transformed with KAI2/D14 and SMAX1 and SMXL7 (A), SMAX1 only (B), SMAX1 and its domains (C), SMXL7 and its domains (D), and both SMAX1 and SMXL7 domains (E). Serial 10-fold dilutions of yeast cultures were spotted onto selective growth medium that was supplemented with 10 μM GR24, 10 μM KARs, or 0.02% acetone control. Images show growth after 3 d at 30°C. -A, -Ade; -H, -His; -L, -Leu; -T, -Trp.
(F) and (G) Split-LUC complementation assay for interactions between D1M domains of SMAX1 and SMXL7 with KAI2 (F) and D14 (G). N. benthamiana leaves were transiently cotransformed with A. tumefaciens strains carrying cLUC, nLUC, or indicated fusions as well as a strain carrying an mCherry transgene as a transformation control. Luminescence was measured 15 min after treatment with 10 μM KAR2, 10 μM rac-GR24, or 0.02% (v/v) acetone control and normalized against mCherry fluorescence. n = 10 to 12 leaf discs. *P < 0.01, Mann–Whitney U test, comparisons to control treatment.
(H) Diagram of the pRATIO3212-SMXL7 ratiometric reporter system. The 35S promoter drives expression of a multicistronic transcript encoding an SMXL7-mScarlet-I fusion that is separately translated from the Venus fluorescent protein due to the *F2A peptide. Relative fluorescence from the SMXL7-mScarlet-I reporter and the Venus reference after transient expression of the SMXL7 ratiometric system in N. benthamiana is shown. Leaf discs were treated with 0.02% (v/v) acetone or 10 μM KAR1, KAR2, and rac-GR24 for 12 h before measurement. n = 5 to 6 leaf discs.
(I) Relative fluorescence after transient expression of pRATIO1212-SMXL7 and -SMXL7*S1167-593 in N. benthamiana and overnight treatment with 10 μM KAR1, 10 μM rac-GR24, or 0.01% (v/v) acetone control. SMXL7*S1167-593 is a chimera in which amino acids 183 to 618 of SMXL7 have been exchanged for amino acids 167 to 593 from SMAX1. n = 8 to 10 leaf discs. *P < 0.01, **P < 0.001, Mann–Whitney U test, comparisons to control treatment.
We further examined the ability of different KAR and GR24 treatments to activate a KAI2-SMAX1 Y2H interaction on –LTHA medium. Interestingly, we found that only rac-GR24 and the GR24ent-5DS stereoisomer stimulated KAI2–SMAX1 interaction, whereas KAR1, KAR2, and GR245DS had no effect (Figure 6B). The responses to these compounds match their ability to stimulate Tm shifts in DSF assays of KAI2 (Waters et al., 2015b). It is possible that KAI2–SMAX1 interactions and thermal destabilization are more reliable indicators of KAI2 activation than assays that can only measure KAR1 binding to KAI2. If so, it may be that KARs require metabolism by plants into an active signal before they can be recognized by KAI2, unlike GR24ent-5DS.
Next, we investigated which SMXL domain(s) mediates interactions with KAI2 and D14. SMAX1D1MD2 and SMXL7D1MD2 maintained interactions with KAI2 and D14, respectively, demonstrating that the N domain was not necessary (Figures 6C and 6D). The D2 domains of SMAX1 and SMXL7 were not sufficient for interaction with KAI2 and D14, respectively. This narrowed the likely interaction domain to D1M, which turned out to be both necessary and sufficient for KAI2 and D14 to interact with their SMXL partners (Figures 6C and 6D). We considered that this could be an artifact of Y2H, for example, if D1M is sticky and prone to promiscuous interactions. Therefore, we tested whether KAI2 and D14 could discriminate between D1M domains from SMAX1 and SMXL7. Indeed, on –LTHA medium KAI2 showed a preference for SMAX1D1M, while D14 preferred SMXL7D1M (Figure 6E). This suggests that D1M is at least partly responsible for specific receptor–SMXL partnerships.
We sought to validate SMXLD1M–receptor interactions through split-LUC assays. N- and C-terminal portions of firefly LUC were fused to the N termini of D1M domains and KAI2 or D14. After transient coexpression in N. benthamiana, we observed that KAI2 had a stronger interaction with SMAX1D1M than SMXL7D1M (Figure 6F). The KAI2–SMAX1D1M interaction was further enhanced by KAR2 treatment (Figure 6F). D14 showed relatively weak interactions with both SMAX1D1M and SMXL7D1M, but only the latter was enhanced by rac-GR24 (Figure 6G).
We reasoned that if the D1M domain is sufficient for determining specificity in SMXL interactions with KAI2 or D14, swapping D1M domains between SMXLs should switch the proteolytic regulation of the chimeric protein. We tested SMXL7 degradation in N. benthamiana using the pRATIO3212 system and observed a clear decrease in the mScarlet-I:Venus ratio after treatment with rac-GR24, but not KAR1 or KAR2 (Figure 6H). This led us to generate a chimeric version of SMXL7 that contains SMAX1167-593, a version of SMAX1D1M that is N terminally extended by 33 amino acids. We used the pRATIO1212 system to evaluate degradation of the wild-type SMXL7 and SMXL7*S1167-593 in N. benthamiana. We found that SMXL7*S1167-593 was degraded after KAR1 treatment, but the wild-type SMXL7 was not (Figure 6I). This suggests that SMAX1D1M can confer KAR1-induced degradation to an SMXL protein, putatively through associations with one or more of the KAI2 proteins encoded in N. benthamiana. Interestingly, rac-GR24 was more effective than KAR1 at stimulating degradation of the chimeric protein. However, it is unclear whether the rac-GR24 responses in N. benthamiana are due to D14 or KAI2 activity, or both.
SMAX1 Can Form Higher Order Complexes through a C-Terminal Domain
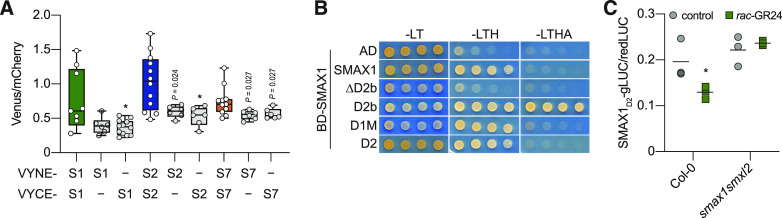
The SMXL family is most closely related to HEAT SHOCK PROTEIN101 (HSP101), a ClpB class of AAA+ chaperones that oligomerize into hexameric complexes (Gallie et al., 2002). This led us to investigate whether SMAX1 can associate with itself or other SMXL proteins. We first used BiFC in N. benthamiana to test whether SMAX1, SMXL2, and SMXL7 can interact with themselves. Transformation-normalized BiFC signals suggested that self-association can occur for all three proteins (Figure 7A). Furthermore, the BiFC assays suggested that heteromeric complexes may be possible among the SMXL family (Supplemental Figure 8A).
Figure 7.
Interactions between SMXL Proteins Are Mediated by the C-Terminal Region.
(A) BiFC tests for interactions between SMAX1 (S1), SMXL2 (S2), SMXL7 (S7), and empty BiFC vectors [pDEST-VYNE(R) and pDEST-VYCE(R)]. N. benthamiana was transiently cotransformed with Agrobacterium strains carrying mCherry and constructs expressing N- or C-terminal portions of Venus fluorescent protein fused to the N termini of SMXLs. Fluorescence intensity was quantified using a plate reader, and BiFC signals were normalized to signal from the mCherry transformation control (Venus:mCherry ratio). n = 5 to 7 leaf discs. *P < 0.01, Mann–Whitney U test, comparisons to the S1 + S1, S2 + S2, and S7 + S7 interaction tests.
(B) Y2H assays for SMAX1 interactions with itself or SMAX1 domains. Assays were performed as described in Figure 6.
(C) SMAX1D2 abundance in protoplasts isolated from Col-0 and smax1 smxl2, transformed with a dual-LUC ratiometric reporter (pMD19-SMAX1D2), recovered for 24 h, and treated with 10 μM rac-GR24 or acetone control for 4 h. SMAX1D2 was fused to gLUC, and redLUC bioluminescence was used as a reference. gLUC:redLUC ratios of three independent protoplast preparations and transformations are shown; bar indicates mean. *P < 0.01, unpaired t test, comparisons to control treatment.
BiFC can be prone to false-positive artifacts, however, because transient interactions become permanent upon reassembly of the fluorescent protein (Kudla and Bock, 2016). Appropriate negative controls, which most closely mimic the functional proteins, are critical. Therefore, we used Y2H to determine which domain(s) might be involved in SMAX1–SMAX1 interaction. We observed self-interaction for SMAX1 on –LTH selective medium (Figure 7B). SMAX1 also interacted with D1M and D2 under this selection and had a particularly strong interaction with D2b as shown on more stringent –LTHA medium. Deletion of D2b, by contrast, abolished the SMAX1–SMAX1 interaction (Figure 7B). This indicated that SMAX1D2b is necessary and sufficient for interactions with SMAX1, at least in Y2H. With this knowledge, we returned to BiFC tests and examined the function of the C-terminal region in SMAX1-SMXL complex formation. We tested C-terminal truncations of SMAX1 and SMXL7 (deletion of D2b from SMAX1 and a 217–amino acid deletion from SMXL7) and found that their interactions with full-length SMAX1 or SMXL7 were reduced (Supplemental Figure 8B). The C-terminal regions alone showed strong interactions with full-length SMXL proteins (Supplemental Figure 8B).
We do not rule out that other domains may participate in SMXL–SMXL interactions because all SMAX1 variants with D2a that lacked D2b produced low reporter signals, suggesting they were less stable (Supplemental Figure 6E). This makes it hard to judge whether a weak/negative interaction result with a ΔD2b truncated protein in plants is because D2b is necessary or because there is less protein available for the interaction. Nonetheless, D2b seems to be sufficient to mediate interactions with full-length SMXL. If so, we reasoned that it could explain how SMAX1D2 can be degraded in response to KAR treatment and yet does not appear to interact with KAI2.
We hypothesized that SMAX1D2 may be indirectly targeted for degradation through interaction with full-length SMAX1 or SMXL2 proteins, which can associate with KAI2 through their D1M domain. If this is the case, SMAX1D2 should be unaffected by rac-GR24 treatment in the absence of SMAX1 and SMXL2. We tested this through transient expression of SMAX1D2 in the dual luminescent ratiometric reporter system in the wild-type and smax1 smxl2 protoplasts, followed by treatment with 10 µM rac-GR24 for 4 h. Consistent with the hypothesis, SMAX1D2 signal decreased after rac-GR24 treatment in Col-0 protoplasts but was unresponsive to rac-GR24 in smax1 smxl2 protoplasts (Figure 7C). This finding indicated that SMAX1D2 degradation requires SMAX1/SMXL2, supporting the idea of multimeric complex formation among SMXL proteins. It also implies that the extent of the decline in SMAX1D2 reporter signal after KAI2 activation will be limited by SMAX1/SMXL2 availability and the relative rate of SMAX1D2 synthesis.
MAX2-Independent Degradation of KAI2 Occurs through Association with SMAX1/SMXL2
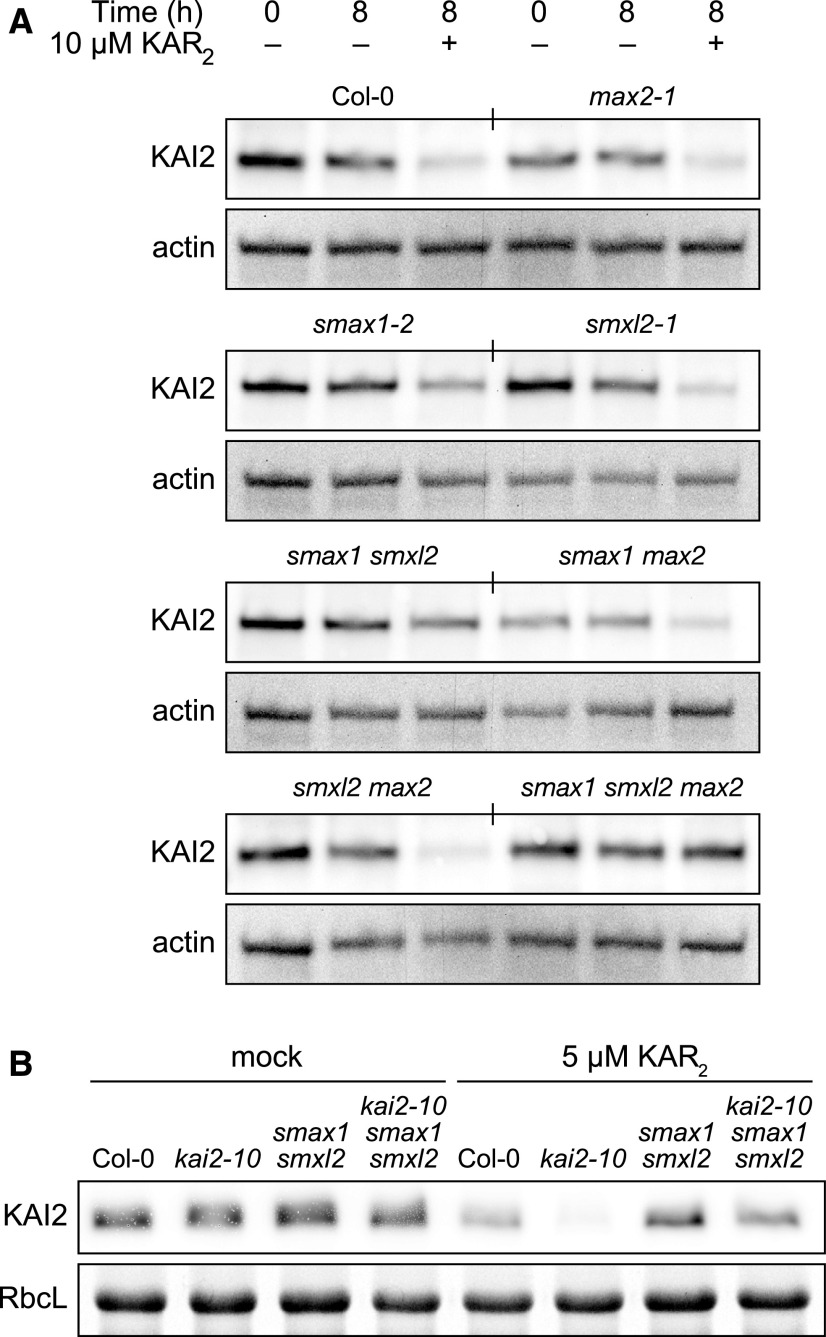
It was previously shown that KAI2 is degraded after KAR and rac-GR24 treatment of Arabidopsis seedlings in a MAX2-independent manner (Waters et al., 2015a). The results from several of the experiments described above suggested that SMAX1 is subject to MAX2-independent turnover as well as MAX2-dependent proteolysis. This led us to hypothesize that interaction with SMAX1 could be the basis of MAX2-independent degradation of KAI2 after its activation. We tested this by examining KAI2 abundance after 8 h KAR2 treatments in max2, smax1, smxl2, and higher order mutant combinations (Figure 8A). We found that KAI2 was resistant to KAR2-induced degradation in smax1 smxl2 and smax1 smxl2 max2 seedlings. We tested this further by monitoring degradation of kai2-10, a protein with a D184N substitution that causes a hypersensitive degradation response to KAR2 (Yao et al., 2018). Consistent with the wild-type KAI2, kai2-10 was strongly reduced by 5 µM KAR2 treatment within 8 h but was stabilized by smax1 smxl2 (Figure 8B).
Figure 8.
KAR2-Induced Degradation of KAI2 Requires SMAX1 and SMXL2.
(A) Immunoblots of soluble protein isolated from seedlings of the indicated genotypes treated with 10 µM KAR2 or mock treated with 0.1% (v/v) acetone for 8 h. Total protein (60 µg) was loaded in each lane. Levels of ACTIN serve as a loading control.
(B) As per (A), except seedlings were treated with 5 µM KAR2 and ribulose-1,5-bis-phosphate carboxylase/oxygenase large subunit (RbcL) was used as a loading control.
DISCUSSION
This study supports the current model of KAR/KL signaling, a model that has been based mostly upon genetic analysis and the shared ancestry of the core KAR/KL and SL signaling components. We found evidence that SMAX1 degradation occurs after KAR and rac-GR24 treatment, and this response requires MAX2 and KAI2 (Figures 3, 4, and 5). We observed that KAI2 interactions with SMAX1 are enhanced in the presence of rac-GR24 and in some assays by KAR (Figure 6). Although it was not explored here, there is also reasonable evidence that KAI2 interacts with MAX2, including highly conserved residues between KAI2 and D14 at the MAX2 interaction surface and pull-downs showing association of KAI2d from parasitic plants with MAX2 after rac-GR24 treatment (Bythell-Douglas et al., 2017; Yao et al., 2017). Altogether, these data are consistent with a signaling mechanism in which KAI2 associates with SCFMAX2 and SMAX1/SMXL2 after it perceives an appropriate ligand, leading to polyubiquitination of SMAX1/SMXL2 and their degradation by the 26S proteasome (Figure 9A). In the course of our investigation, however, unexpected features of the KAR/KL signaling model also emerged.
Figure 9.
Models for SMAX1 Regulation and the Functions of Its Major Domains.
(A) Activation of KAI2 by KAR or KL promotes its association with SCFMAX2 and SMAX1, resulting in proteolysis of SMAX1. Polyubiquitination of SMAX1 is presumed, but has not been demonstrated. This proposed mechanism is highly similar to the SL signaling mechanism. MAX2-independent degradation of SMAX1 also contributes to its turnover, at least in Arabidopsis. MAX2-independent degradation of KAI2 after KAR treatment can be explained by association of KAI2 with unstable SMAX1 and SMXL2 (not depicted). Ub, ubiquitin.
(B) Diagram of the major domains of the SMAX1 protein and their proposed functions. The amino acid numbers at the boundaries of each domain of Arabidopsis SMAX1 are indicated.
MAX2-Independent Degradation of SMAX1
Several observations suggested that SMAX1 in Arabidopsis is subject to MAX2-independent turnover in addition to MAX2-dependent proteolysis (Figure 9A). First, we were unable to detect SMAX1 fusion proteins in plants by microscopy or by immunoblot analysis, even in max2 backgrounds, although the amount of tagged SMAX1 protein produced in transgenic lines was sufficient to rescue smax1 and smax1 smxl2 defects. Only mutation of the RGKT motif that is conserved in SMAX1, SMXL2, and D53-type SMXLs was able to make SMAX1 detectable in vivo (Figures 1C and 1D). Second, heterologous, purified SMAX1-His was rapidly degraded in protein extracts from seedlings. This degradation was slowed by the 26S proteasome inhibitor MG-132, but not by the absence of KAI2 or MAX2 (Figure 2; Supplemental Figure 2C). Third, full-length SMAX1 had a much shorter half-life than its C-terminal D2 domain, although SMAX1D2 retained the ability to be targeted for degradation by MAX2 (Figures 4C and 4D; Figure 5). Likewise, an SMAX1D2 reporter was readily detectable during transient expression in Arabidopsis protoplasts, while a full-length SMAX1 reporter was not (Supplemental Figure 7). It could be argued that our inability to detect SMAX1 in vivo and the rapid degradation of SMAX1 in vitro were due to technical problems or to assay conditions that are not physiologically relevant. For example, during protein extraction SMAX1 may be exposed to proteases that it does not normally encounter in the cell. However, we also demonstrated that MAX2-independent degradation of KAI2 after KAR2 treatment in seedlings is SMAX1 and SMXL2 dependent (Figure 8). This result simultaneously strengthens the conclusions that SMAX1 has high instability and that KAI2 interacts with SMAX1 after activation.
It is unclear whether the MAX2-independent instability of SMAX1 is due to nonspecific degradation or regulated proteolysis. Purified SMAX1 was stable in boiled protein extracts from seedlings, suggesting that its turnover is due to protease activity and not SMAX1 itself (Figure 2A). MAX2-independent instability of SMAX1 may be conferred by more than one domain. Reporters of SMAX1 truncations lacking either the N or D2 domain accumulated to higher levels than SMAX1, and those lacking both domains had even higher target:reference ratios (Supplemental Figure 6E). We searched for PEST motifs in SMAX1 that might explain its instability. The PEST prediction program epestfind revealed 16 candidate motifs (Rechsteiner et al., 1987). All had poor scores, so PEST-mediated degradation seems unlikely. However, the 26S proteasome inhibitor MG-132 slowed SMAX1 degradation more effectively than max2 or kai2 mutations. This suggests that a proteasome-mediated mechanism contributes to MAX2-independent turnover of SMAX1. Genetic components of such a mechanism might be identifiable through a screen for mutations that make a full-length SMAX1 reporter detectable in the max2 background.
An appealing idea—for which we currently have no evidence—is that MAX2-independent regulation of SMAX1 abundance could provide a way to potentiate the effects of MAX2-dependent signaling. That is, MAX2-independent degradation might lower SMAX1 levels to a sensitized threshold at which further regulation by MAX2-dependent mechanisms becomes physiologically relevant. Precedent is found among the Auxin/indole-3-acetic acid proteins (Aux/IAA) that are targeted for degradation by auxin signaling. These are short-lived proteins with half-lives only several minutes long, enabling rapid responses to auxin to occur (Abel et al., 1994).
How the RGKT Motif Might Influence SMAX1/SMXL Stability
We observed that deletion of the conserved RGKT motif, or P-loop, stabilizes SMAX1 in vivo (Figures 1C and 1D), as it does for D53, SMXL6, and SMXL7 (Jiang et al., 2013; Zhou et al., 2013; Wang et al., 2015; Liang et al., 2016). So far, it has been assumed that this mutation prevents MAX2-dependent degradation of D53-type SMXLs, but it is not clear how.
Interestingly, loss of the RGKT motif reduces the ability of the D53D2 domain to pull down D14 in the presence of rac-GR24 and the C-terminal helix (CTH) of D3/MAX2 (Shabek et al., 2018). This suggests that the RGKT motif facilitates interaction of D53D2 with D14. This interaction could occur directly. Alternatively, it may be that the RGKT motif mediates interactions between D53D2 and D3-CTH. We propose that D3-CTH may bind directly to both D14 and D53D2, functioning as a bridge that stabilizes their interaction. Supporting this hypothesis, association of D53D2 and D14 in size-exclusion chromatography assays requires ASK1-D3 (Shabek et al., 2018). Also, the D14-D3-ASK1 crystal structure shows D3-CTH positioned proximally to Arg-183 of D14 (Arg-233 in rice D14), a surface residue that is important for D14–SMXL7 interactions in Y2H assays (Supplemental Figure 9; Yao et al., 2016; Seto et al., 2019). Potentially, this exposed region could be an SMXL interaction surface.
Challenging the idea that the RGKT motif mediates MAX2-dependent degradation, we were able to detect SMAX1∆RGKT-GFP when MAX2 was present, but were not able to detect SMAX1-GFP in a max2 background. This indicates that the effect of the RGKT mutation on SMAX1 is not equivalent to the loss of MAX2-dependent degradation. Therefore, at least for SMAX1, it is possible that the RGKT deletion blocks MAX2-independent degradation, perhaps in addition to MAX2-dependent degradation (Figure 1D). Until it is determined whether MAX2-independent turnover is a peculiar feature of Arabidopsis SMAX1 or whether it is also found to some extent among other SMXL proteins, we propose that the regulatory effect of the RGKT motif should be considered unresolved.
Detection of SMAX1 in Root Tips
In contrast to our experiences, Wallner et al. (2017) were able to observe SMXL5:SMAX1-YFP in the cortex of smxl4,5 root tips. We cannot explain yet why we were unable to detect SMAX1 that was similarly fused to a fluorescent protein, but we can raise several hypotheses. It may be that KAI2-SCFMAX2 signaling is less active in the cortex, for example, if the availability of MAX2, KAI2, or KL is lower in the cortex than surrounding tissues. Only the last of these options seems feasible; SMAX1-YFP signal disappeared within 12 min of treatment with 10 µM rac-GR24, indicating that the cells in the cortex are capable of degradation responses that are presumably mediated by MAX2 (Wallner et al., 2017). Alternatively, it may be that SMAX1 expression driven by the SMXL5 promoter is high enough to outpace the rate of SMAX1 turnover. Perhaps MAX2-independent turnover of SMAX1 is reduced in cortical tissues. A difference between our growth conditions and those of Wallner et al. (2017) that influences SMAX1 stability is another possibility. Finally, it might be that the smxl4,5 background itself somehow stabilizes SMAX1.
We were able to observe SMAX1:SMAX1ΔRGKT-GFP in the columella and lateral root cap of seedlings (Figures 1C and 1D), consistent with an SMAX1:GUS transcriptional reporter (Soundappan et al., 2015). We do not know whether the SMAX1 protein found in root tips regulates the growth of distant tissues such as the hypocotyl. If SMAX1 can act at a distance, perhaps it does so by regulating auxin transport. This idea is suggested by the positive influence of SMXL6, SMXL7, and SMXL8 on auxin transport in the stem and PIN1 accumulation at the basal plasma membrane of xylem parenchyma cells (Soundappan et al., 2015).
Intermolecular Complex Formation among SMXL Proteins
We found evidence that SMAX1 can form complexes with itself or other SMXLs through a C-terminal domain (Figure 7; Supplemental Figure 8). This raises the question of whether SMAX1 is more susceptible to degradation as a monomer or in a multimeric complex. In our analysis of SMAX1 domains in N. benthamiana, we noted that any truncations that contained the D2a domain and not D2b produced a low mScarlet-I:Venus ratio. SMAX1 variants that lacked D2a had higher levels of mScarlet-I/Venus signal than full-length SMAX1 (Figure 4; Supplemental Figure 6E). This finding suggests that D2a has a destabilizing effect that is held in check by D2b. Because D2b appears to be important for SMAX1 complex formation, it might be that D2a-containing proteins are more prone to degradation as monomers. This will be an interesting hypothesis to resolve in the future.
Other questions that arise are whether there is partnering specificity during SMXL–SMXL complex formation? And if heteromeric SMXL complexes form, what effect does it have on their function and MAX2-dependent proteolysis? Further testing will be required to determine whether SMXLs from one clade associate with members of the other two clades in vivo. It may be that they only have the potential to do so, but do not because of spatiotemporal differences in their expression patterns.
Signaling Fidelity in KAR/KL and SL Pathways
The current models of KAR/KL and SL signaling stipulate that SMAX1 and SMXL2 are targets of KAI2-SCFMAX2, whereas SMXL6, SMXL7, and SMXL8 are targets of D14-SCFMAX2. Support for the separation of these MAX2-dependent signaling pathways comes from observations that KAR and SL influence plant growth differently. For example, KAR does not rescue the increased shoot-branching phenotype of SL-deficient mutants (Nelson et al., 2011). In addition, smax1 and smxl2 suppress max2 defects associated with KAR/KL signaling through KAI2, whereas the triple mutation smxl6,7,8 suppresses max2 phenotypes associated with SL signaling through D14 (Stanga et al., 2013, 2016; Soundappan et al., 2015; Villaécija-Aguilar et al., 2019). This is illustrated under a short-day photoperiod by the rosette phenotypes of smax1 max2 mutants, which closely resemble d14, and smxl6 smxl7 max2 mutants, which look like kai2 (Soundappan et al., 2015). Finally, promoter-swapping experiments have shown that D14:KAI2 does not enable KAR-responsive rescue of d14 shoot branching nor does KAI2:D14 rescue germination or seedling phenotypes of kai2 (Waters et al., 2015b). This lack of reciprocal activity suggests that KAI2 and D14 are not interchangeable, and their distinct developmental roles are not due merely to different expression patterns.
Nonetheless, the possibility of crosstalk in some contexts has been suggested by the observation that rac-GR24 inhibits hypocotyl elongation of kai2, but not kai2 d14. This implies that D14 can act upon SMAX1 and SMXL2, which are the only MAX2-regulated SMXLs that regulate hypocotyl growth. Because the d14 mutant has wild-type hypocotyl growth, unlike kai2, this crosstalk seems to be a result of exogenous rac-GR24 treatment that does not reflect the normal activity of D14 or endogenous SLs (Waters et al., 2012). Recent work by Swarbreck et al. (2019) also raises the possibility of KAI2–SMXL6/7/8 interactions. They discovered that the skewing angle of roots is regulated specifically by KAI2 and MAX2, but not D14. They also observed suppression of the increased-skewing phenotype of max2 by both smax1 smxl2 and smxl6,7,8, the latter of which seems to contradict the current models (Swarbreck et al., 2019). However, the effect of smxl6,7,8 on root skewing appears to vary substantially in different laboratories and may be sensitive to subtle environmental differences (Villaécija-Aguilar et al., 2019). Meanwhile, many other KAI2-dependent effects on root growth traits such as root hair length, root hair density, root straightness, and root diameter are regulated only by SMAX1 and/or SMXL2, consistent with the model (Villaécija-Aguilar et al., 2019).
Therefore, the majority of evidence to date favors the separation of KAR/KL and SL signaling pathways. We propose that under some experimental conditions (e.g., addition of exogenous agonists) nonpreferred interactions may be pushed to occur, creating crosstalk that does not have physiological relevance.
Specificity in Interactions between KAI2, D14, and Their SMXL Targets
We identified a role for the D1M domains in determining SMAX1 and SMXL7 specificity for KAI2 or D14 (Figures 6 and 9B). This aligns somewhat with the Y2H analysis of D14 and D53 by Zhou et al. (2013), which we find difficult to interpret. They showed that D53ND1, D53ND1M, D53ND1D2, and D53D1 alone could interact with D14, while other D53 domain combinations were ineffective. While this could suggest that D53D1 is necessary and sufficient for D14 interaction, D53D1MD2, D53D1M, and D53D1D2 did not interact with D14. Our results also contrast with a recent analysis of D14 and D53 interactions by Shabek et al. (2018). In an in vitro pull-down assay with recombinant proteins, they found that D53D2, but not D53N or D53D1, can associate with D14 in the presence of ASK1-D3/MAX2 and 100 µM rac-GR24. Several additional assays, including size-exclusion chromatography and Amplified Luminescent Proximity Homogeneous Assay Screen (PerkinElmer), validated complex formation between D53D2, D14, and ASK1-D3 or D3-CTH in the presence of rac-GR24 (Shabek et al., 2018).
There are a few possible explanations for our different conclusions regarding the roles of D1M and D2 domains in D14–SMXL interactions with KAI2 and D14. First, it is possible that there are differences between SMAX1 and D53 in the relative contributions of their D1M and D2 domains to receptor interactions. Second, Shabek et al. (2018) did not test interactions of D14 with D53D1M, which may have higher affinity than D53D1 alone. Third, we did not include D3/MAX2 or D3-CTH in our interaction tests. Most of the association tests reported by Shabek et al. (2018) do not reveal which components of the complex interact directly. While we do not rule out the possibility that D2 mediates direct interaction with the receptors, we found no evidence for it in our assays. Moreover, by swapping a D1M domain from SMAX1 into SMXL7, we produced a chimeric protein that could be degraded after KAR treatment (Figure 6I). This demonstrates that D1M can influence the choice of partner receptor.
Therefore, we propose that direct interactions between D14/KAI2 and their SMXL targets may occur exclusively or mostly through the D1M domain, which could explain our ability to observe KAI2–SMAX1D1M and D14–SMXL7D1M interactions in the absence of MAX2. Perhaps the D2 domain acts to stabilize the tripartite interaction between SMXLs, a D14 or KAI2 receptor, and D3/MAX2 via the D3-CTH. Discovery of the role of the SMXL D1M domains in KAI2 and D14 interactions lays a foundation for a more refined understanding of receptor–target pairing. It will also enable a better understanding of how highly similar MAX2-dependent signaling pathways evolved to become insulated from crosstalk.
A Potential New Bioassay for KL Isolation
A major unresolved goal of the SL and KAR field is identification of the putative KAI2 ligand, KL. A bioassay based on induction of the KAR-response marker DLK2 was previously developed to help solve this problem (Sun et al., 2016). In it, the 3597-bp region upstream of the DLK2 translational start site was used to drive expression of firefly LUC. DLK2p:LUC seed showed an ∼15-fold induction in luminescence after treatment with 100 nM KAR2, but was not responsive to several plant hormones or nitrates. Moreover, a promising observation was a positive DLK2p:LUC response to aqueous extracts from Arabidopsis leaves, which suggested this reporter could be used in bioassay-guided fractionation to identify KL (Sun et al., 2016). An inherent limitation of this system is that DLK2 regulation is an indirect downstream effect of KAI2 activation that might be influenced by other signaling pathways.
Degradation of the SMAX1D2 reporter developed in this work offers a potential alternative bioassay for KL that is appealing as a direct and specific readout of KAI2 activation. However, there are likely to be some limitations to using SMAX1D2 for KL detection, foremost of which is that the dynamic range for the reporter is restricted by the availability of SMAX1 and SMXL2 that can interact with KAI2 (Figure 7C). The reduction in SMAX1D2 reporter after KAI2 activation is expected to plateau as the initial pool of SMAX1 and SMXL2 is depleted and an equilibrium is established based on the relative rates of synthesis of the reporter and SMAX1/SMXL2. Including the D1M domains, which mediate KAI2 interaction, could reduce the dependency of the SMAX1D2 reporter on full-length SMAX1 and SMXL2. At least in Arabidopsis protoplasts, however, we have found that SMAX1D1MD2 has an ∼10-fold lower signal than SMAX1D2, which imposes another limit on the dynamic range of the bioassay. Regardless of their ultimate utility in bioassay-guided fractionation for KL, SMAX1 degradation reporters may prove useful in genetic screens for KL-metabolism mutants.
METHODS
Data Visualization and Statistical Tests
Graphs were produced with the ggplot2 package of R or in Prism v8.2.0 (GraphPad Software). Notched boxplots were used for analysis of large sample sizes (n ≥ 20). Notches approximate the 95% confidence interval (CI). Whiskers for notched boxplots extend to the last observation that is within or meets 1.5 times the interquartile range from the 25th and 75th percentiles; outliers are indicated by gray dots. Box-whisker plots were used for medium sample sizes (n ≥ 4). Whiskers indicate the minimum and maximum of the data range, and individual data points are overlaid. Center lines of notched boxplots and box-whisker plots indicate the median. For n < 4, individual data points and the mean value are shown. Statistical analyses were performed with Prism as indicated in figure legends (Supplemental Data Set 1). For sample sizes with n < 20, data were not assumed to be normally distributed, and the nonparametric Mann–Whitney U test was applied unless otherwise indicated.
Plant Material, Growth Conditions, and Plant Growth Regulators
The Arabidopsis (Arabidopsis thaliana) max2-1, d14-1, htl-3, d14-1 htl-3, smax1-2 max2-1, and smax1-2 smxl2-1 mutants have been previously described by Stirnberg et al. (2002), Waters et al. (2012), Stanga et al. (2013), 2016, and Toh et al. (2014). For clarity, the htl-3 allele of KAI2 is here referred to as kai2. The kai2-10 allele in the Landsberg erecta background was isolated from an ethyl methanesulfonate mutagenesis screen (Yao et al., 2018). The kai2-10 mutation destroys a Hpy188III restriction site, which assisted in the construction of kai2-10 smax1-2 smxl2-1. Note this triple mutant is a Landsberg erecta/Col-0 hybrid. KAI2 degradation experiments using this triple mutant were performed on the F4 generation. Unless otherwise stated, plants were propagated under white light (∼80 to 110 µmol m–2 s−1; MaxLite 16.5W 4000k light-emitting diode bulbs) with 16-h-light/8-h-dark cycles at ∼21 to 24°C. Soil was supplemented with Gnatrol WDG, Marathon (imidacloprid), and Osmocote 14–14–14 fertilizer. Agrobacterium tumefaciens (GV3101 pMP90)–mediated transformation of Arabidopsis was performed using the floral dip method as previously described by Clough and Bent (1998). Homozygous transgenic lines were identified and used in subsequent characterization.
Chemical Compounds
KAR1 and KAR2 were synthesized as previously reported by Goddard-Borger et al. (2007). rac-GR24 was synthesized according to Mangnus et al. (1992) and recrystallized from diethyl ether/hexanes. GR245DS and GR24ent-5DS enantiomers were purified from rac-GR24 by chiral-phase HPLC as previously described by Scaffidi et al. (2014). Stocks (10 or 50 mM) were prepared in acetone and stored at –20°C and freshly diluted in aqueous solutions before use.
SMAX1-GFP and SMAX1∆RGKT-GFP Analysis in Arabidopsis
To construct SMAX1:gSMAX1-GFP, gSMAX1 insert with SMAX1 promoter was amplified from Arabidopsis (Col-0) genomic DNA with primers SMAX1-2976-GW-F and SMAX1-GW-R, cloned into the pDONR207 entry vector (Invitrogen) by Gateway BP reaction, sequence verified, and subsequently transferred into the pGWB504 binary vector by Gateway LR reaction (Nakagawa et al., 2007). For the SMAX1:SMAX1-GFP and SMAX1:SMAX1∆RGKT-GFP constructs, a 2747-bp fragment upstream from SMAX1 start codon, a full-length SMAX1 coding sequence without stop codon, and a 720-bp GFP gene were amplified from Col-0 genomic DNA, Arabidopsis cDNA, and pGWB405 binary vector, respectively, using Primestar GXL high-fidelity DNA polymerase (Takara Bio) and primer pairs of pSMAX1-F and pSMAX1-R, SMAX1-F and SMAX1-R, and GFP-F and GFP-R, respectively. Fragments were amplified with overlapping sequences to enable assembly with each other and BamHI-SacI–digested pCAMBIA2300 using NEBuilder (New England Biolabs). Primers for SMAX1∆RGKT were designed to delete the RGKT coding region. Purified PCR products that used pSMAX1-F/SMAX1∆RGKT-R and SMAX1∆RGKT-F/GFP-R as primer pairs and SMAX1:SMAX1-GFP plasmid as template were assembled together into digested pCAMBIA2300 with NEBuilder. Oligonucleotides used to make DNA constructs are listed in Supplemental Data Set 2.
Hypocotyl growth under red light was assayed as previously described by Nelson et al. (2011). The assay medium was 0.5× Murashige and Skoog (MS), with Gamborg’s vitamins and MES, pH 5.8, solidified with 0.8% (w/v) Bacto-agar. The medium was supplemented as indicated with 1 µM KAR2, 1 µM rac-GR24, or 0.1% (v/v) acetone as a control. Seeds were surface sterilized, plated, stratified for 3 d at 4°C in darkness, treated with 3 h of white light (∼170 µmol m-2s−1) at 21°C, returned to darkness for 21 h at 21°C, and grown under continuous red light (30 µmol m–2 s−1) at 21°C for 4 d before being photographed. Hypocotyl length was measured using ImageJ software.
Arabidopsis roots were visualized with a Leica SP5 microscope at 5 d poststratification. Seedlings were mounted in liquid 0.5× MS medium, and treatments (KAR2, rac-GR24, or 0.1% [v/v] acetone) were applied in the same solution. Propidium iodide (0.1 mg/mL; ACROS) was used to visualize cell borders in roots. GFP was excited with an argon laser (488 nm), and emitted light was collected between 510 and 540 nm. Propidium iodide fluorescence was imaged using an excitation wavelength of 543 nm with emission wavelengths of 587 to 625 nm.
Protein Extraction and Immunoblotting
For gSMAX1-GFP detection, 7-d-old Arabidopsis seedlings grown on 0.5× MS medium, or cauline leaves from adult plants, were harvested and snap frozen in liquid nitrogen and ground to a fine powder in a prechilled mortar and pestle. Homogenization buffer (1 mM DTT, 1 mM EDTA, 10% [v/v] glycerol, 50 mM Tris-HCl, pH 7.6, 0.1% [v/v] Triton X-100, 150 mM NaCl, and 1 tablet of Roche Complete Protease Inhibitor per 10 mL of buffer) was added to the powder and ground further. Collected tissue in the homogenization buffer was vortexed and incubated on ice for 30 min. Samples were then spun down at >6000g at 4°C for 30 min. Supernatant was collected and used for downstream analysis. For immunoblots, protein concentrations were quantified via Bradford assay and normalized with more homogenization buffer. gSMAX1-GFP was probed with anti-GFP primary antibody (catalog no. ab6556; Abcam) at 1:3000 dilution in milk block solution, followed by horseradish peroxidase–linked goat anti-rabbit secondary antibody (catalog no. 32460; Thermo Fisher Scientific) at 1:5000 dilution in milk block. Blots were probed with Super Signal West Femto ECL Substrate (Thermo Fisher Scientific) and visualized on a Fluor Chem E detector (Protein Simple) under auto-exposure conditions.
Heterologous Expression and Purification of SMAX1
The full coding sequence of Arabidopsis SMAX1 without the stop codon was amplified by PCR using Q5 hot-start high-fidelity polymerase (New England Biolabs) and introduced into the pET26b(+) vector (Novagen), which encodes a C-terminal 6×His tag, by enzymatic restriction ligation and transformed into chemically competent Escherichia coli strain BL21 (New England Biolabs). Bacteria were cultured in 600 mL of Luria-Bertani (LB) broth with 50 µg mL−1 filtered kanamycin at 37°C and 220 rpm shaking, until OD600 reached 0.6. Recombinant SMAX1-His protein then expressed after addition of 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) for 3 h at 20°C and 220 rpm shaking. Culture was centrifuged at 3900g for 15 min at 4°C. The cell pellet was stored at –20°C overnight. The pellet was resuspended in 8 mL of lysis buffer (300 mM NaCl, 50 mM NaH2PO4, 5 mM imidazole, 1 mM phenylmethylsulfonyl fluoride [PMSF], and 0.1% [v/v] Tween 20, pH 7.6) and incubated for 30 min on ice. One tablet of cOmplete Mini EDTA-free Protease Inhibitor Cocktail (Roche Diagnostics) and 1 mL of 10 mg mL−1 lysozyme were added to the resuspended cells, followed by incubation on ice for 60 min and then at room temperature for a further 10 min at 125 rpm. After incubation on ice for 30 min, prelysate cells were sonicated on ice with 10 cycles of 10-s bursts (50% intensity), followed by 30-s cooling period between each burst. Lysed cells were centrifuged at 10,000g for 30 min at 4°C. The supernatant was then incubated with 2 mL of pre-equilibrated nickel-nitrilotriacetic acid (Ni-NTA) agarose beads (Qiagen) at 4°C under agitation. All purification steps were done at 4°C. Beads were packed into column, and the flowthrough was collected and set aside on ice. Column was washed with 50 mL of wash buffer no. 1 (300 mM NaCl, 50 mM NaH2PO4, and 5 mM imidazole, pH 7.6) and then with 50 mL of wash buffer no. 2 (300 mM NaCl, 50 mM NaH2PO4, and 20 mM imidazole, pH 7.6). Proteins were eluted using 3 mL of elution buffer (300 mM NaCl, 50 mM NaH2PO4, and 250 mM imidazole, pH 7.6). Fractions were concentrated and buffer exchanged using Amicon Ultra centrifugal filter units, molecular weight cutoff 50 kD (Millipore Sigma) and a 1× PBS solution.
SMAX1 Stability in a Reconstituted Cell-Free System
Seven-day-old Arabidopsis seedlings were flash frozen and ground in liquid nitrogen using a prechilled mortar and pestle. Ground tissue was further ground in native protein extraction buffer (10 mM KCl, 1 mM EDTA, 1 mM EGTA, 1 mM MgCl2, 400 mM Suc, and 100 mM potassium phosphate buffer, pH 7.2) and kept on ice for 30 min. Following incubation, samples were centrifuged in an FA-45-30-11 rotor (Eppendorf) at 5000 rpm at 4°C for 20 min. Supernatant was moved to new prechilled tubes, and the pellet was discarded. Extracted protein concentration was determined by Bradford assay, and all samples were diluted to the same concentration. As indicated, MG-132 (40 µM) was added to protein preparations at this time and incubated on ice for 5 min. Recombinant, purified SMAX1-His was added to samples such that the concentration of SMAX1 was one-tenth of the total concentration of the mixture. Samples were incubated at 24°C for the indicated time and then boiled at 100°C for 10 min to stop degradation. SMAX1 was probed with anti-HIS primary antibody (catalog no. MA1-21315; Thermo Fisher Scientific) at 1:5000 dilution in milk block solution, followed by horseradish peroxidase–linked horse anti-mouse IgG secondary antibody (catalog no. 7076; Cell Signaling) at 1:10,000 dilution.
Tertiary Structure Prediction
Two independent methods were used to generate a predicted structure for SMAX1. First, the primary amino acid sequence of AtSMAX1 (AT3G20600) was submitted to the protein fold recognition server Phyre (http://www.sbg.bio.ic.ac.uk/phyre/; Kelley et al., 2015). Further analysis was performed by submitting the primary amino acid sequence of SMAX1 to I-TASSER (http://zhanglab.ccmb.med.umich.edu/I-TASSER/), an Internet-based structure prediction service (Roy et al., 2010). Similar results were obtained. The predicted structure was then viewed using Chimera (Pettersen et al., 2004).
Gateway Entry Clones for KAI2, D14, SMAX1, SMXL7, and Their Domains
Full-length SMAX1 and SMXL7 coding sequences were amplified from Col-0 cDNA using primers SMAX1-GW-F/SMAX1-GW-R and SMXL7-GW-F/SMXL7-GW-R and inserted into Gateway entry vectors pDONR221 and pDONR207, respectively. For SMAX1 domains N, D1, M, D2, D2a, D2b, ND1, ND1M, ND1MD2a, D1M, D1MD2a, D1MD2, MD2a, and MD2, the corresponding cDNA sequences were PCR amplified with primers SMAX1_N-GW-F and SMAX1_N-GW-R, SMAX1_D1-GW-F and SMAX1_D1-GW-R, SMAX1_M-GW-F and SMAX1_M-GW-R, SMAX1_D2-GW-F and SMAX1_D2-GW-R, SMAX1_D2a-GW-F and SMAX1_D2a-GW-R, SMAX1_D2b-GW-F and SMAX1_D2b-GW-R, SMAX1_ND1-GW-F and SMAX1_ND1-GW-R, SMAX1_ND1M-GW-F and SMAX1_ND1M-GW-R, SMAX1_ND1MD2a-GW-F and SMAX1_ND1MD2a-GW-R, SMAX1_D1M-GW-F and SMAX1_D1M-GW-R, SMAX1_D1MD2a-GW-F and SMAX1_D1MD2a-GW-R, SMAX1_D1MD2-GW-F and SMAX1_D1MD2-GW-R, SMAX1_MD2a-GW-F and SMAX1_MD2a-GW-R, and SMAX1_MD2-GW-F and SMAX1_MD2-GW-R, respectively, and cloned into pDONR221. The coding sequences of SMXL7 domains D1M, D1MD2, and D2 were amplified with primers SMXL7_D1M-GW-F and SMXL7_D1M-GW-R, SMXL7_D1MD2-GW-F and SMXL7_D1MD2-GW-R, and SMXL7_D2-GW-F and SMXL7_D2-GW-R, respectively, and cloned into pDONR221. KAI2 and D14 were amplified from Col-0 cDNA with primer pairs of D14-GW-F/D14-GW-R and KAI2-GW-F/KAI2-GW-R that excluded the stop codon and had Gateway-compatible adapters and shuttled into pDONR207. The sequences of the PCR primers used for vector construction are given in Supplemental Data Set 2.
Transient Expression in Nicotiana benthamiana
N. benthamiana plants (3 weeks old) were used to express the various construct combinations by Agrobacterium (GV3101 pMP90)–mediated transient transformation of lower epidermal leaf cells as previously described by Sparkes et al. (2006).
Subcellular Localization of SMAX1 Domains in N. benthamiana
SMAX1 and SMAX1 domain entry clones were recombined with the binary vector pGWB451 containing C‐terminal GFP by Gateway LR reaction (Nakagawa et al., 2007). N. benthamiana leaf discs were imaged 3 d after infiltration with A. tumefaciens carrying destination clones using a Keyence BZ-X710 epifluorescence microscope. Images were taken using the GFP setting (excitation, 470/40 nm; emission, 535/50 nm). For 4′,6-diamino-2-phenylindole dihydrochloride (DAPI) staining, leaves were incubated in distilled water supplemented with 5 µg/mL DAPI for 20 min and visualized using the DAPI setting (excitation, 360/40 nm; emission, 460/50 nm).
Degradation Assays in N. benthamiana
To generate ratiometric constructs for N. benthamiana degradation assays, SMXL7, SMAX1, and SMAX1 domain entry clones were moved to the pRATIO1212 and 3212 destination vectors by Gateway LR reaction. The design and construction of these ratiometric vectors have been previously described by Khosla et al. (2020). To create a chimeric version of SMXL7 (SMXL7*S1167-593), coding sequences of full-length SMXL7 without stop codon, SMXL71-182, SMAX1167-593, and SMXL7619-1003 without stop codon were amplified using the primers SMXL7-GW-F and SMXL7D1-R, OE-SMAX1D2-F and OE-SMAX1D3-R, and SMXL7D4-F and SMXL7-GW-R, respectively. The DNA fragments for SMXL71-182, SMAX1167-593, and SMXL7619-1003 were combined in a subsequent overlap extension PCR reaction to create the chimeric SMXL7*S1167-593. The full-length SMXL7 and chimeric SMXL7*S1167-593 were cloned into pDONR221 vector by Gateway BP reaction. The resulting entry clones were then recombined into pRATIO1212 binary vector by Gateway LR reaction to generate 35S:SMXL7-mScarlet-I-*F2A-Venus and 35S:SMXL7*S1167-593-mScarlet-I-*F2A-Venus, respectively.
To perform degradation assays, leaf discs were excised 3 d postinfiltration and incubated at room temperature for 12 h in the presence or absence of 10 µM KAR1, KAR2, rac-GR24, or 0.02% (v/v) acetone control. Relative fluorescence was measured in a well scan mode of density 9 × 9 using the CLARIOstar plate reader (BMG Labtech) and 96-well black polystyrene plates (Corning Costar). mScarlet-I (reporter) was monitored using the excitation filter 560 nm (10 nm bandwidth), dichroic filter 573.5 nm and emission filter 595 nm (10 nm bandwidth); Venus (reference) was measured using the excitation filter 497 nm (15 nm bandwidth), dichroic filter auto 527.2 nm and emission filter 540 nm (20 nm bandwidth). Degradation was quantified as mScarlet-I:Venus ratio after background subtraction using nontransformed leaf disc. To compare the stability of the pRATIO1212-SMAX1 and -SMAX1D2 proteins, leaf discs were incubated with 150 µg/mL cycloheximide or DMSO mock solution, and mScarlet-I and Venus were measured at the indicated time points as described above.
Protoplast Isolation and Transient Expression
To construct plasmids for degradation assays in Arabidopsis protoplasts, pRATIO1212 vector expressing SMAX1D2 was used to amplify the expression cassette using PrimeSTAR HS DNA Polymerase (Takara Bio) and primers pMD19-F and pMD19-R, that are specific for the cauliflower mosaic virus 35S promoter and the nopaline synthase terminator. Extra adenosine bases were added to the 3′ ends of the cassette using Taq (New England Biolabs) and 10 mM dATP, and it was ligated into linearized pMD19 (Takara Bio) using T4 DNA ligase (New England Biolabs). To generate UBQ10:SMAX1D2-gLUC-*F2A-redLUC, the coding sequences of gLUC, *F2A, and redLUC were amplified with primer pairs of gLUC-F and gLUC-R, *F2A-F and *F2A-R, and redLUC-F and redLUC-R, respectively, and assembled into PCR-linearized pRATIO1212-SMAX1D2 using NEBuilder.
Protoplasts were isolated from the wild-type Arabidopsis (Col-0), Atd14-1, max2-1, htl3-3, d14-1 htl3-3, and htl3-3 max2-1 mutant plants using a modified version of the tape-sandwich protocol (Wu et al., 2009). Briefly, protoplasts were isolated from 3- to 4-week-old plants grown in short-day (8 h light/16 h dark) conditions. Protoplasts (2 × 105) were incubated in 400 mM mannitol, 15 mM MgCl2, and 4 mM MES, pH 5.7; 40% (w/v) polyethylene glycol 4000 (Sigma-Aldrich); and 20 μg of SMAX1D2-gLUC-*F2A-redLUC fusion plasmid for 15 min to transform cells. Protoplasts were washed and incubated overnight in W5 (154 mM NaCl, 125 mM CaCl2, 5 mM KCl, 5 mM Glc, and 2 mM MES, pH 5.7) before induction with the substrates. To prepare protoplasts from smax1-2 smxl2-1 mutant plants, leaf strips (0.5 to 1 mm) were cut from the middle part of a leaf using a fresh sharp razor blade. The obtained leaf strips were gently transferred into the enzymatic solution (400 mM mannitol, 40 mM MES, 10 mM CaCl2, 20 mM KCl, 1% [w/v] cellulase Onozuka R10, and 0.25% [w/v] Macerozyme R10 at pH 5.6), and digestion was performed overnight in the dark at room temperature. The protoplast solution was diluted with an equal volume of W5 solution and filtered with 40-μm nylon mesh to remove cell debris or undigested leaf tissue and centrifuged at 100g for 4 min to pellet the protoplasts. Protoplast transformation, washing, and incubation steps were performed as described above.
Luminescence Assays
Arabidopsis protoplasts expressing UBQ10:SMAX1D2-gLUC-*F2A-redLUC were incubated in the presence or absence of 10 µM rac-GR24. After 4 h of incubation, luminescence analysis was performed. For this, 100 μL of protoplast suspension was transferred into a white 96-well plate (Optiplate-96; PerkinElmer) to simultaneously determine the luminescence of both LUCs. For each well, redLUC and gLUC activities were determined by adding 100 μL of ONE-Glo reagent (Promega) followed by injecting 100 μL of gLUC substrate (20 µM coelenterazine), respectively. Luminescence was determined using emission at 600 nm with a 40 nm bandwidth (emission of redLUC) and 480 nm with a 80 nm bandwidth (emission of gLUC), and SMAX1D2 degradation was quantified as gLUC:redLUC ratio after subtracting luminescence counts obtained from mock-transfected protoplasts in which no plasmid DNA was included.
To construct UBQ10:SMAX1D2-LUC2-*F2A-mScarlet-I, SMAX1D2 cDNA coding sequence was amplified with primers SMAX1D2-GW-F and SMAX1D2-GW-R and then cloned into pDONR221 and moved into pRATIO2251 (Khosla et al., 2020) by Gateway LR reaction. Transgenic plants were generated as described above. Seeds were surface sterilized with a 70% (v/v) ethanol, 0.05% (v/v) Triton X-100 solution, rinsed with 70 and 95% (v/v) ethanol, and air dried. Sterile seeds were plated on solid 0.5× MS (RPI M70800-25.0, 2.4 g/L) medium, pH 5.8, without Suc, and kept at 4°C for 3 d (stratification). Seeds were then incubated for 7 d in a growth chamber at 25°C and a 12 h light/12 h dark photoperiod. Using tweezers, 7-d-old Arabidopsis seedlings were placed in a 96-well white microtiter plate with their cotyledons facing up (one seedling per well in 180 μL of 1.5 mM luciferin solution). Measurements were taken every hour in a microplate reader for 4 h (endpoint, 10 s); the signal was allowed to stabilize for 6 to 8 h in the light before treatments were started by adding 20 μL per well of rac-GR24, KAR2, or acetone blank control, thus reaching a final volume in each well of 200 µL. rac-GR24 and KAR2 were dissolved as 50 mM stock solutions in acetone, and compound stocks were further diluted in water and added to each well at a final concentration of 10 µM. Each treatment was applied to a minimum of 12 wells (seedlings), each of which was measured individually over time. After measurements were completed, the mean luminescence signals produced by the untransformed Col-0 or max2-1 seedlings was subtracted from each luminescence reading produced by SMAX1D2 seedlings, to obtain the net reading for each sample.
Y2H Assays
To construct plasmids for Y2H assays, D14 and KAI2 entry clones were recombined into Gateway yeast expression vector pDest-GBKT7 to generate BD-D14 and BD-KAI2, respectively. To make Gal4 DNA AD constructs, the coding sequences for SMAX1, SMAX1 truncations (D1M, D1MD2, and D2), SMXL7, and SMXL7 truncations (D1M, D1MD2, and D2) were PCR amplified using primers mentioned above, cloned into pDONR221, and moved into Gateway yeast expression vector pDest-GADT7. For SMXL7, D1M, D1MD2, and D2 correspond to 172 to 599, 172 to 1002, and 600 to 1002 amino acids, respectively. The PCR primers used for Y2H assays are listed in Supplemental Data Set 2.
Direct interaction of two proteins was investigated by cotransformation of the respective plasmids in the yeast strain Y2H Gold (Clontech) by the standard lithium acetate–mediated method. The transformed yeast strains were plated on synthetic dropout nutrient medium (SD/-Leu-Trp; Clontech) at 30°C for 3 d. Interactions in yeast were tested on SD/-Leu-Trp-His and SD/-Leu-Trp-His-Ade medium (Clontech) in the presence or absence of 10 µM rac-GR24, KAR1, KAR2, GR245DS, and GR24ent-5DS.
Split-LUC Assays
To construct plasmids used in split-LUC assays, D14 and KAI2 coding sequences were amplified with the primer pairs of D14-cLuc-F and D14-cLuc-R and KAI2-cLuc-F and KAI2-cLuc-R, respectively, and cloned into KpnI and SalI site of pCAMBIA1300-cLUC (Chen et al., 2008) using NEBuilder. Similarly, cDNA sequences for SMAX1D1M and SMXL7D1M were amplified using primers of SMAX1D1M-nLuc-F and SMAX1D1M-nLuc-R and SMXL7D1M-nLuc-F and SMXL7D1M-nLuc-R, respectively, and cloned into KpnI- and SalI-digested pCAMBIA1300-nLUC using NEBuilder. To construct the 35S:mCherry plasmid, mCherry coding sequence amplified by primers mCherry-GW-F and mCherry-GW-R was inserted into pDONR221 vector and then into pGWB451 binary vector using Gateway LR reaction. Oligonucleotides used for vector construction are listed in Supplemental Data Set 2.
For split-LUC complementation assay, the various combinations of nLUC and cLUC fusion plasmids were transformed into N. benthamiana leaves. The Agrobacterium strain containing 35S:mCherry was used as a control. After 3 d, leaf discs were excised and immersed into 200 μL of luciferin solution (1.5 mM working solution prepared in distilled water) in a 96-well white plate and kept in the dark for 10 min. Fifteen minutes after addition of substrates (10 µM rac-GR24, 10 µM KAR2, or 0.02% [v/v] acetone), luminescence was measured using the emission filter 580 nm (80 nm bandwidth) for 10 s in the top optic mode and expressed as luminescence normalized to mCherry transformation control. mCherry filter settings are described below.
BiFC Assays
Binary vectors pDEST-VYNE(R)GW and pDEST-VYCE(R)GW (Gehl et al., 2009) were used to generate constructs for the BiFC assay. Entry clones with stop codon for each test candidate (SMAX1, SMXL7, SMXL2, SMAX1D2b, SMAX1∆D2b, SMXL7786-1002, and SMXL71-785) were recombined into BiFC vectors by Gateway LR reaction.
These constructs produce fusion proteins with either the N- or C-terminal half of the VENUS fused to the N terminus of the test candidate. The various combinations of n-VENUS or c-VENUS fusion plasmids and mCherry control were cotransformed into 3- to 4-week-old N. benthamiana leaves. Fluorescence images were captured using Keyence BZ-X710 fluorescence microscope at 2 d postinfiltration. Images were taken with the YFP filter setting. BiFC signal was quantified as Venus:mCherry obtained on expressing each of the construct combinations and analyzed for Venus and mCherry fluorescence intensities after background subtraction using untransformed leaf disc. The fluorescence intensity was measured in a CLARIOstar machine (BMG labtech) using the following settings: Venus fluorescence; excitation wavelength-bandwidth (497-15 nm), emission wavelength-bandwidth (540-20 nm), mCherry fluorescence; excitation wavelength-bandwidth (570-15 nm), emission wavelength-bandwidth (620-20 nm).
KAI2 Degradation Assays
Arabidopsis seeds were sown on 0.5× MS medium, pH 5.9, solidified with 0.7% (w/v) agar, stratified at 4°C in the dark for 3 d, and then transferred to a growth room with a 16 h light/8 h dark and 22°C/16°C diurnal cycle for a further 7 d. Light was provided by broad-spectrum, white light-emitting diodes (90 to 100 µmol−2 s−1). On day 7, seedlings were collected from the plate using spatula and forceps and transferred into a sterile 12-well assay plate (Corning 3513). Each well contained 3 mL of liquid 0.5× MS medium and ∼30 to 40 seedlings. The plate was shaken gently at 70 rpm for 24 h under the same growth conditions, after which the liquid medium was removed by pipette and replaced with fresh medium containing KAR2 (from 1000× stocks in acetone) or an equivalent concentration of acetone as a mock-treated control. After 8 h, seedlings were blotted dry, frozen in liquid nitrogen, and ground to a fine powder. Soluble protein was extracted, separated by SDS-PAGE, and blotted onto polyvinylidene fluoride membrane as described previously by Waters et al. (2015a). KAI2 was detected using a custom-made rabbit polyclonal antibody described previously by Waters et al. (2015a); ACTIN was detected using a commercial mouse monoclonal anti-actin (plant) antibody (catalog no. A0480, clone 10-B3; Sigma-Aldrich). The ribulose-1,5-bis-phosphate carboxylase/oxygenase large subunit was imaged directly from the gel prior to blotting by UV light fluorescence of SDS-PAGE gels containing 1% (v/v) 2,2,2-trichloroethanol.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: pRATIO1212-SMAX1D2 (MN508832), pRATIO3212-SMAX1 (MN508833), pRATIO3212-SMXL7 (MN508834), pMD19-SMAX1D2 (MN508835), pRATIO2251-SMAX1D2 (MN508836), D14 (AT3G03990), KAI2 (AT4G37470), SMAX1 (AT5G57710), SMXL2 (AT4G30350), SMXL7 (AT2G29970) , and MAX2 (AT2G42620).
Supplemental Data
Supplemental Figure 1. SMAX1 is undetectable in transgenic Arabidopsis lines. (Supports Figure 1)
Supplemental Figure 2. Purification of SMAX1-His expressed in E. coli. (Supports Figure 2)
Supplemental Figure 3. SMAX1-GFP is nucleus-localized in N. benthamiana cells. (Supports Figure 4)
Supplemental Figure 4. Time course of SMAX1 degradation in N. benthamiana. (Supports Figure 3)
Supplemental Figure 5. Major domains in SMXL proteins. (Supports Figure 4A)
Supplemental Figure 6. KAR1-induced degradation of NLS-SMAX1 and nucleus-localized SMAX1 domains. (Supports Figure 3 and Figure 4)
Supplemental Figure 7. Bioluminescence assays for SMAX1 degradation in protoplasts. (Supports Figure 5)
Supplemental Figure 8. The C-terminus is necessary and sufficient for SMXL dimerization. (Supports Figure 7)
Supplemental Figure 9. C-terminal helix of D3/MAX2 is adjacent to a D14 residue required for SMXL7 interaction. (Supports Figure 6)
Supplemental Data Set 1. Statistical analysis data.
Supplemental Data Set 2. Cloning primers used in this study.
DIVE Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Katharina Bursch (Freie Universität Berlin) for sharing a protocol for protoplast preparation and Adrian Scaffidi (University of Western Australia) for chemical synthesis and purification.Support was provided by the National Science Foundation (grants IOS-1737153 and IOS-1740560, to D.C.N.) and the Australian Research Council (grants FT150100162 and DP160102888 to M.T.W. and G.R.F.).
AUTHOR CONTRIBUTIONS
Experiments were designed, carried out, and analyzed by A.K., N.M., Q.L., L.F., S.H.C., J.Y., J.Z., M.L.C., J.S., M.T.W., and D.C.N. Critical chemical reagents were provided by G.R.F. Figure preparation was by A.K., N.M., Q.L., L.F., S.C., M.T.W., and D.C.N. Article preparation was by D.C.N. and A.K., with contributions and final approval from all authors. Project design was by D.C.N. Funding to support the project was secured by D.C.N., M.T.W., and G.R.F.
References
- Abel S., Oeller P.W., Theologis A.(1994). Early auxin-induced genes encode short-lived nuclear proteins. Proc. Natl. Acad. Sci. USA 91: 326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindels D.S., Haarbosch L., van Weeren L., Postma M., Wiese K.E., Mastop M., Aumonier S., Gotthard G., Royant A., Hink M.A., Gadella T.W.J. Jr.(2017). mScarlet: A bright monomeric red fluorescent protein for cellular imaging. Nat. Methods 14: 53–56. [DOI] [PubMed] [Google Scholar]
- Blázquez M.A., Nelson D.C., Weijers D.(2020). Evolution of plant hormone response pathways. Annu. Rev. Plant Biol. 71: 327–353. [DOI] [PubMed] [Google Scholar]
- Bürger M., Mashiguchi K., Lee H.J., Nakano M., Takemoto K., Seto Y., Yamaguchi S., Chory J.(2019). Structural basis of karrikin and non-natural strigolactone perception in Physcomitrella patens. Cell Rep. 26: 855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bythell-Douglas R., Rothfels C.J., Stevenson D.W.D., Graham S.W., Wong G.K.-S., Nelson D.C., Bennett T.(2017). Evolution of strigolactone receptors by gradual neo-functionalization of KAI2 paralogues. BMC Biol. 15: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson G.H., Hasse D., Cardinale F., Prandi C., Andersson I.(2018). The elusive ligand complexes of the DWARF14 strigolactone receptor. J. Exp. Bot. 69: 2345–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Zou Y., Shang Y., Lin H., Wang Y., Cai R., Tang X., Zhou J.-M.(2008). Firefly luciferase complementation imaging assay for protein-protein interactions in plants. Plant Physiol. 146: 368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F.(1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743. [DOI] [PubMed] [Google Scholar]
- Conn C.E., Nelson D.C.(2016). Evidence that KARRIKIN-INSENSITIVE2 (KAI2) receptors may perceive an unknown signal that is not karrikin or strigolactone. Front. Plant Sci. 6: 1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Saint Germain A., et al. (2016). An histidine covalent receptor and butenolide complex mediates strigolactone perception. Nat. Chem. Biol. 12: 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly M.L.L., Hughes L.E., Luke G., Mendoza H., Ten Dam E., Gani D., Ryan M.D.(2001). The ‘cleavage’ activities of foot-and-mouth disease virus 2A site-directed mutants and naturally occurring ‘2A-like’ sequences. J. Gen. Virol. 82: 1027–1041. [DOI] [PubMed] [Google Scholar]
- Flematti G.R., Ghisalberti E.L., Dixon K.W., Trengove R.D.(2004). A compound from smoke that promotes seed germination. Science 305: 977. [DOI] [PubMed] [Google Scholar]
- Flematti G.R., Ghisalberti E.L., Dixon K.W., Trengove R.D.(2009). Identification of alkyl substituted 2H-furo[2,3-c]pyran-2-ones as germination stimulants present in smoke. J. Agric. Food Chem. 57: 9475–9480. [DOI] [PubMed] [Google Scholar]
- Flematti G.R., Goddard-Borger E.D., Merritt D.J., Ghisalberti E.L., Dixon K.W., Trengove R.D.(2007). Preparation of 2H-furo[2,3-c]pyran-2-one derivatives and evaluation of their germination-promoting activity. J. Agric. Food Chem. 55: 2189–2194. [DOI] [PubMed] [Google Scholar]
- Flematti G.R., Scaffidi A., Waters M.T., Smith S.M.(2016). Stereospecificity in strigolactone biosynthesis and perception. Planta 243: 1361–1373. [DOI] [PubMed] [Google Scholar]
- Gallie D.R., Fortner D., Peng J., Puthoff D.(2002). ATP-dependent hexameric assembly of the heat shock protein Hsp101 involves multiple interaction domains and a functional C-proximal nucleotide-binding domain. J. Biol. Chem. 277: 39617–39626. [DOI] [PubMed] [Google Scholar]
- Gehl C., Waadt R., Kudla J., Mendel R.-R., Hänsch R.(2009). New GATEWAY vectors for high throughput analyses of protein-protein interactions by bimolecular fluorescence complementation. Mol. Plant 2: 1051–1058. [DOI] [PubMed] [Google Scholar]
- Goddard-Borger E.D., Ghisalberti E.L., Stick R.V.(2007). Synthesis of the germination stimulant 3-methyl-2H-furo[2,3-c]pyran-2-one and analogous compounds from carbohydrates. ChemInform 2007: 3925–3934. [Google Scholar]
- Guo Y., Zheng Z., La Clair J.J., Chory J., Noel J.P.(2013). Smoke-derived karrikin perception by the α/β-hydrolase KAI2 from Arabidopsis. Proc. Natl. Acad. Sci. USA 110: 8284–8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutjahr C., et al. (2015). Rice perception of symbiotic arbuscular mycorrhizal fungi requires the karrikin receptor complex. Science 350: 1521–1524. [DOI] [PubMed] [Google Scholar]
- Hamiaux C., Drummond R.S.M., Janssen B.J., Ledger S.E., Cooney J.M., Newcomb R.D., Snowden K.C.(2012). DAD2 is an α/β hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Curr. Biol. 22: 2032–2036. [DOI] [PubMed] [Google Scholar]
- Holbrook-Smith D., Toh S., Tsuchiya Y., McCourt P.(2016). Small-molecule antagonists of germination of the parasitic plant Striga hermonthica. Nat. Chem. Biol. 12: 724–729. [DOI] [PubMed] [Google Scholar]
- Hrdlička J., Gucký T., Novák O., Kulkarni M., Gupta S., van Staden J., Doležal K.(2019). Quantification of karrikins in smoke water using ultra-high performance liquid chromatography-tandem mass spectrometry. Plant Methods 15: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., et al. (2013). DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature 504: 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagiyama M., Hirano Y., Mori T., Kim S.-Y., Kyozuka J., Seto Y., Yamaguchi S., Hakoshima T.(2013). Structures of D14 and D14L in the strigolactone and karrikin signaling pathways. Genes Cells 18: 147–160. [DOI] [PubMed] [Google Scholar]
- Kelley L.A., Mezulis S., Yates C.M., Wass M.N., Sternberg M.J.E.(2015). The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 10: 845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla A., Rodriguez-Furlan C., Kapoor S., Van Norman J.M., Nelson D.C.(2020). A series of dual-reporter vectors for ratiometric analysis of protein abundance in plants. bioRxiv 2020.02.10.939363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek J., Long R.L., Lisle A.T., Flematti G.R.(2016). Karrikins identified in biochars indicate post-fire chemical cues can influence community diversity and plant development. PLoS One 11: e0161234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi S., Hasebe M., Tomita M., Yanagawa H.(2009). Systematic identification of cell cycle-dependent yeast nucleocytoplasmic shuttling proteins by prediction of composite motifs. Proc. Natl. Acad. Sci. USA 106: 10171–10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla J., Bock R.(2016). Lighting the way to protein-protein interactions: Recommendations on best practices for bimolecular fluorescence complementation analyses. Plant Cell 28: 1002–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I., et al. (2018). A missense allele of KARRIKIN-INSENSITIVE2 impairs ligand-binding and downstream signaling in Arabidopsis thaliana. J. Exp. Bot. 69: 3609–3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Ward S., Li P., Bennett T., Leyser O.(2016). SMAX1-LIKE7 signals from the nucleus to regulate shoot development in Arabidopsis via partially EAR motif-independent mechanisms. Plant Cell 28: 1581–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Cheng X., Liu P., Sun J.(2017). miR156-targeted SBP-Box transcription factors interact with DWARF53 to regulate TEOSINTE BRANCHED1 and BARREN STALK1 expression in bread wheat. Plant Physiol. 174: 1931–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., et al. (2017). The karrikin receptor KAI2 promotes drought resistance in Arabidopsis thaliana. PLoS Genet. 13: e1007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., et al. (2020). Comparative functional analyses of DWARF14 and KARRIKIN INSENSITIVE2 in drought adaptation of Arabidopsis thaliana. Plant J. In press. [DOI] [PubMed] [Google Scholar]
- Ma H., et al. (2017). A D53 repression motif induces oligomerization of TOPLESS corepressors and promotes assembly of a corepressor-nucleosome complex. Sci. Adv. 3: e1601217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machin DARREN C, Hamon-Josse MAXIME, Bennett TOM(2020). Fellowship of the rings: a saga of strigolactones and other small signals. New Phytol. 225: 621–636. [DOI] [PubMed] [Google Scholar]
- Mangnus E.M., Dommerholt F.J., De Jong R.L.P., Zwanenburg B.(1992). Improved synthesis of strigol analog GR24 and evaluation of the biological activity of its diastereomers. J. Agric. Food Chem. 40: 1230–1235. [Google Scholar]
- Marzec M., Brewer P.(2019). Binding or hydrolysis? How does the strigolactone receptor work? Trends Plant Sci. 24: 571–574. [DOI] [PubMed] [Google Scholar]
- Morffy N., Faure L., Nelson D.C.(2016). Smoke and hormone mirrors: Action and evolution of karrikin and strigolactone signaling. Trends Genet. 32: 176–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T., Ibata K., Park E.S., Kubota M., Mikoshiba K., Miyawaki A.(2002). A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 20: 87–90. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., et al. (2007). Improved Gateway binary vectors: High-performance vectors for creation of fusion constructs in transgenic analysis of plants. Biosci. Biotechnol. Biochem. 71: 2095–2100. [DOI] [PubMed] [Google Scholar]
- Nelson D.C., Flematti G.R., Ghisalberti E.L., Dixon K.W., Smith S.M.(2012). Regulation of seed germination and seedling growth by chemical signals from burning vegetation. Annu. Rev. Plant Biol. 63: 107–130. [DOI] [PubMed] [Google Scholar]
- Nelson D.C., Flematti G.R., Riseborough J.-A., Ghisalberti E.L., Dixon K.W., Smith S.M.(2010). Karrikins enhance light responses during germination and seedling development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 107: 7095–7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D.C., Riseborough J.-A., Flematti G.R., Stevens J., Ghisalberti E.L., Dixon K.W., Smith S.M.(2009). Karrikins discovered in smoke trigger Arabidopsis seed germination by a mechanism requiring gibberellic acid synthesis and light. Plant Physiol. 149: 863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D.C., Scaffidi A., Dun E.A., Waters M.T., Flematti G.R., Dixon K.W., Beveridge C.A., Ghisalberti E.L., Smith S.M.(2011). F-box protein MAX2 has dual roles in karrikin and strigolactone signaling in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 108: 8897–8902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E.(2004). UCSF Chimera--A visualization system for exploratory research and analysis. J. Comput. Chem. 25: 1605–1612. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M., Rogers S., Rote K.(1987). Protein-structure and intracellular stability. Trends Biochem. Sci. 12: 390–394. [Google Scholar]
- Roy A., Kucukural A., Zhang Y.(2010). I-TASSER: A unified platform for automated protein structure and function prediction. Nat. Protoc. 5: 725–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi A., Waters M.T., Sun Y.K., Skelton B.W., Dixon K.W., Ghisalberti E.L., Flematti G.R., Smith S.M.(2014). Strigolactone hormones and their stereoisomers signal through two related receptor proteins to induce different physiological responses in Arabidopsis. Plant Physiol. 165: 1221–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto Y., et al. (2019). Strigolactone perception and deactivation by a hydrolase receptor DWARF14. Nat. Commun. 10: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabek N., Ticchiarelli F., Mao H., Hinds T.R., Leyser O., Zheng N.(2018). Structural plasticity of D3-D14 ubiquitin ligase in strigolactone signalling. Nature 563: 652–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara N., Taylor C., Leyser O.(2013). Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane. PLoS Biol. 11: e1001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., et al. (2017). IPA1 functions as a downstream transcription factor repressed by D53 in strigolactone signaling in rice. Cell Res. 27: 1128–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soundappan I., Bennett T., Morffy N., Liang Y., Stanga J.P., Abbas A., Leyser O., Nelson D.C.(2015). SMAX1-LIKE/D53 family members enable distinct MAX2-dependent responses to strigolactones and karrikins in Arabidopsis. Plant Cell 27: 3143–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkes I.A., Runions J., Kearns A., Hawes C.(2006). Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 1: 2019–2025. [DOI] [PubMed] [Google Scholar]
- Stanga J.P., Morffy N., Nelson D.C.(2016). Functional redundancy in the control of seedling growth by the karrikin signaling pathway. Planta 243: 1397–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanga J.P., Smith S.M., Briggs W.R., Nelson D.C.(2013). SUPPRESSOR OF MORE AXILLARY GROWTH2 1 controls seed germination and seedling development in Arabidopsis. Plant Physiol. 163: 318–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirnberg P., van De Sande K., Leyser H.M.O.(2002). MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development 129: 1131–1141. [DOI] [PubMed] [Google Scholar]
- Sun X.-D., Ni M.(2011). HYPOSENSITIVE TO LIGHT, an alpha/beta fold protein, acts downstream of ELONGATED HYPOCOTYL 5 to regulate seedling de-etiolation. Mol. Plant 4: 116–126. [DOI] [PubMed] [Google Scholar]
- Sun Y.K., Flematti G.R., Smith S.M., Waters M.T.(2016). Reporter gene-facilitated detection of compounds in Arabidopsis leaf extracts that activate the karrikin signaling pathway. Front. Plant Sci 7: 1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarbreck S.M., Guerringue Y., Matthus E., Jamieson F.J.C., Davies J.M.(2019). Impairment in karrikin but not strigolactone sensing enhances root skewing in Arabidopsis thaliana. Plant J. 98: 607–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh S., Holbrook-Smith D., Stokes M.E., Tsuchiya Y., McCourt P.(2014). Detection of parasitic plant suicide germination compounds using a high-throughput Arabidopsis HTL/KAI2 strigolactone perception system. Chem. Biol. 21: 988–998. [DOI] [PubMed] [Google Scholar]
- Umehara M., Cao M., Akiyama K., Akatsu T., Seto Y., Hanada A., Li W., Takeda-Kamiya N., Morimoto Y., Yamaguchi S.(2015). Structural requirements of strigolactones for shoot branching inhibition in rice and Arabidopsis. Plant Cell Physiol. 56: 1059–1072. [DOI] [PubMed] [Google Scholar]
- Villaécija-Aguilar J.A., Hamon-Josse M., Carbonnel S., Kretschmar A., Schmidt C., Dawid C., Bennett T., Gutjahr C.(2019). SMAX1/SMXL2 regulate root and root hair development downstream of KAI2-mediated signalling in Arabidopsis. PLoS Genet. 15: e1008327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CATRIONA H, Siu-Ting KAREN, Taylor ALYSHA, O’Connell MARY J, Bennett TOM(2019). Strigolactone synthesis is ancestral in land plants, but canonical strigolactone signalling is a flowering plant innovation. BMC Biol. 17: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner E.-S., et al. (2017). Strigolactone- and karrikin-independent SMXL proteins are central regulators of phloem formation. Curr. Biol. 27: 1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Wang B., Jiang L., Liu X., Li X., Lu Z., Meng X., Wang Y., Smith S.M., Li J.(2015). Strigolactone signaling in Arabidopsis regulates shoot development by targeting D53-like SMXL repressor proteins for ubiquitination and degradation. Plant Cell 27: 3128–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Waters M.T., Smith S.M.(2018). Karrikin-KAI2 signalling provides Arabidopsis seeds with tolerance to abiotic stress and inhibits germination under conditions unfavourable to seedling establishment. New Phytol. 219: 605–618. [DOI] [PubMed] [Google Scholar]
- Waters M.T., Gutjahr C., Bennett T., Nelson D.C.(2017). Strigolactone signaling and evolution. Annu. Rev. Plant Biol. 68: 291–322. [DOI] [PubMed] [Google Scholar]
- Waters M.T., Nelson D.C., Scaffidi A., Flematti G.R., Sun Y.K., Dixon K.W., Smith S.M.(2012). Specialisation within the DWARF14 protein family confers distinct responses to karrikins and strigolactones in Arabidopsis. Development 139: 1285–1295. [DOI] [PubMed] [Google Scholar]
- Waters M.T., Scaffidi A., Flematti G., Smith S.M.(2015a). Substrate-induced degradation of the α/β-fold hydrolase KARRIKIN INSENSITIVE2 requires a functional catalytic triad but is independent of MAX2. Mol. Plant 8: 814–817. [DOI] [PubMed] [Google Scholar]
- Waters M.T., Scaffidi A., Moulin S.L.Y., Sun Y.K., Flematti G.R., Smith S.M.(2015b). A Selaginella moellendorffii ortholog of KARRIKIN INSENSITIVE2 functions in Arabidopsis development but cannot mediate responses to karrikins or strigolactones. Plant Cell 27: 1925–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F.-H., Shen S.-C., Lee L.-Y., Lee S.-H., Chan M.-T., Lin C.-S.(2009). Tape-Arabidopsis sandwich - A simpler Arabidopsis protoplast isolation method. Plant Methods 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Miyakawa T., Nakamura H., Nakamura A., Imamura Y., Asami T., Tanokura M.(2016). Structural basis of unique ligand specificity of KAI2-like protein from parasitic weed Striga hermonthica. Sci. Rep. 6: 31386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Miyakawa T., Nosaki S., Nakamura A., Lyu Y., Nakamura H., Ohto U., Ishida H., Shimizu T., Asami T., Tanokura M.(2018). Structural analysis of HTL and D14 proteins reveals the basis for ligand selectivity in Striga. Nat. Commun. 9: 3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J., Mashiguchi K., Scaffidi A., Akatsu T., Melville K.T., Morita R., Morimoto Y., Smith S.M., Seto Y., Flematti G.R., Yamaguchi S., Waters M.T.(2018). An allelic series at the KARRIKIN INSENSITIVE 2 locus of Arabidopsis thaliana decouples ligand hydrolysis and receptor degradation from downstream signalling. Plant J. 96: 75–89. [DOI] [PubMed] [Google Scholar]
- Yao R., et al. (2016). DWARF14 is a non-canonical hormone receptor for strigolactone. Nature 536: 469–473. [DOI] [PubMed] [Google Scholar]
- Yao R., Wang F., Ming Z., Du X., Chen L., Wang Y., Zhang W., Deng H., Xie D.(2017). ShHTL7 is a non-canonical receptor for strigolactones in root parasitic weeds. Cell Res. 27: 838–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L.-H., et al. (2015). Destabilization of strigolactone receptor DWARF14 by binding of ligand and E3-ligase signaling effector DWARF3. Cell Res. 25: 1219–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., et al. (2013). D14-SCF(D3)-dependent degradation of D53 regulates strigolactone signalling. Nature 504: 406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]