Abstract
Very late stent thrombosis (VLST) is a rare but serious complication following percutaneous coronary intervention (PCI). S100A8/A9 plays an important role in thrombosis through modulating the inflammatory response. This observational study aimed to reveal the association between S100A8/A9 and VLST. Continuous blood samples were collected from patients at both the time of index PCI for acute myocardial infarction (AMI) and the time of PCI for VLST (VLST group) or follow-up coronary angiography (AMI group). In all, 56 patients were selected in each group from a cohort of 8476 patients and other 112 individuals who underwent health checkups (normal control [NC] group) were selected as controls. Serum levels of S100A8/A9 and high sensitivity C-reactive protein (hs-CRP) were tested and compared. The mean level of S100A8/A9 was 3754.4 ± 1688.9 ng/mL during index PCI and increased to 5517.8 ± 2650.9 ng/mL at the time of VLST; in the AMI group, S100A8/A9 level was 2434.9 ± 1243.4 ng/mL during index PCI and decreased to 1568.2 ± 772.1 ng/mL during follow-up, similar to that detected in the NC group (1618.2 ± 641.4 ng/mL). Of note, S100A8/A9 levels showed significant increases during VLST when compared to its own levels during index PCI, which was different from the changes of hs-CRP. Higher serum levels of S100A8/A9 are associated with the development of VLST.
Keywords: very late stent thrombosis, acute myocardial infarction, percutaneous coronary intervention, S100A8/A9
Introduction
Urgent percutaneous coronary intervention (PCI) has become the mainstay treatment for acute myocardial infarction (AMI), which is one of the most serious diseases that threaten human health globally.1 Very late stent thrombosis (VLST, stent thrombosis occurring more than 1 year after stent implantation, usually out of the time window recommended by the guidelines for antiplatelet therapy and often overlooked) is an uncommon yet catastrophic event after PCI, usually manifesting as recurrent AMI with poor prognosis or sudden death.2,3 Despite being recognized for over a decade, the detailed mechanisms underlying VLST remain poorly understood, and the insights that can translate into prediction, precaution, and management of VLST are lacking.
Inflammation is an established risk factor for arterial thrombotic diseases.4 In a large-scale study of histological thrombus analysis on patients presenting with stent thrombosis, the recruitment of leukocytes, particularly of neutrophils and eosinophils, was found to be the hallmark of stent thrombosis when compared with a control group with spontaneous AMI treated by thrombus aspiration.5 Correspondently, recent optical coherence tomography data from patients with VLST found increased macrophage infiltration in neoatherosclerosis within the stent,6 which indicates the role of inflammation in the development of VLST. S100A8/A9, also known as myeloid-related protein 8/14, has been found to play a decisive role in modulating the inflammatory response by stimulating leukocyte recruitment and inducing cytokine secretion.7 Recently, emerging evidence revealed that S100A8/A9 levels are associated with the severity of atherosclerosis and vulnerable plaque phenotypes,8,9 abnormal platelet aggregation, and arterial thrombosis.9–12 Therefore, the goal of this observational study was to characterize the association between serum levels of S100A8/A9 and the development of VLST.
Methods
Study Population
VLST Registry Study (ClinicalTrials.gov: NCT03491891) prospectively enrolled 8476 patients after PCI for acute coronary syndrome from Jilin University First Hospital and First Affiliated Hospital of Shantou University Medical College to investigate multiple aspects of VLST, including the potential mechanisms of pathogenesis. This study was approved by the institutional review boards of the First Hospital of Jilin University (no. 2013256) and the study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All patients provided written informed consent before inclusion.
Very late stent thrombosis was defined according to the 2007 definition of Academic Research Consortium stent thrombosis criteria: stent thrombosis that occurred more than 1 year after coronary stent implantation and clearly confirmed by coronary angiography. All documented cases of VLST were determined by coronary angiograms, and all procedures were independently reviewed by 2 experienced interventional cardiologists. In case of divergent results, a consensus between the 2 reviewers was sought or a third reviewer stepped in to resolve the issue.
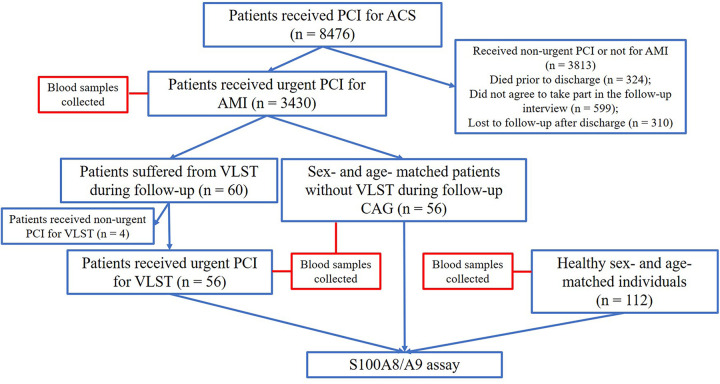
All patients enrolled in this study from January 2014 to June 2015 received second-generation drug-eluting stents and were scheduled for more than 3 years of follow-up (including scheduled telephone follow-ups and regular admission reviews) after stent implantation. Among them, 3430 patients underwent index urgent PCI for AMI. The VLST group had 56 patients enrolled who underwent urgent PCI for VLST that manifested as AMI after index PCI (also performed for AMI). The AMI group had 56 age- and sex-matched patients without VLST during 3-year follow-up after index PCI for AMI; the negative control (NC) group had selected as controls 112 healthy sex- and age-matched individuals who visited the hospital for health checkups. The study flowchart is presented in Figure 1. Blood samples were drawn from each patient into lithium-heparin tubes at the time of index PCI and at either the time of emergent PCI for VLST (VLST group) or coronary angiography during follow-up (AMI group) or during health checkups (NC group). Serum was obtained by centrifugation for 10 minutes at 1000 × g using a refrigerated centrifuge. Serum samples were then transferred to clean tubes and stored at –80 °C for further use. Serum S100A8/A9 levels were measured using ELISA (S100A8/A9 detection kit, Ray biotech Inc, Peachtree Corners, Georgia).
Figure 1.
Flowchart of enrolled patients. ACS indicates acute coronary syndrome; AMI, acute myocardial infarction; CAG, coronary angiography; PCI, percutaneous coronary intervention; VLST, very late stent thrombosis.
Statistical Analysis
Data were expressed as numbers (%) for categorical variables. Continuous variables were expressed as means ± standard deviation. Categorical data were compared using the χ2 test or Fisher exact test, whereas quantitative data were compared using the Mann-Whitney U test. All tests were 2 sided at the .05 significance level. Independent predictors for the presence of VLST were analyzed using Cox regression analysis. Possible confounding factors (P < .25) were tested using univariate regression analysis, and confounders with a P value <.25 were included in multivariate regression analysis. Statistical analysis was performed with Statistical Package for Social Sciences, version 23 (SPSS Inc).
Results
Patient Characteristics
The average age of enrolled patients was 59 years and 84% of them were men. Table 1 provides baseline characteristics of included patients at the time of index PCI for AMI. More patients in the VLST group had histories of diabetes mellitus and the number of diseased vessels and distal lesions was higher in the VLST group during index PCI; patients in the VLST group had lower left ventricle ejection fraction and larger left ventricle ending-diastolic diameter. Table 2 provides baseline characteristics of patients at the time of VLST or coronary angiography during normal follow-up. The mean time intervals between index PCI and VLST were 976.4 ± 552.6 days. Of note, fewer patients were receiving dual-antiplatelet therapy and statins treatment during follow-up in VLST group; however, the differences were not significant (57.1% vs 50%, P = .449; 60.7% vs 55.4%, P = .566).
Table 1.
Baseline Characteristics of Patients at the Time of Index PCI for Acute Myocardial Infarction.
| Variables | VLST group, n = 56 | AMI group, n = 56 | P value |
|---|---|---|---|
| Age (years) | 59.57 ± 10.65 | 59.07 ± 10.45 | .77 |
| Sex (male) | 47 (83.93) | 47 (83.93) | 1 |
| BMI (kg/m2) | 23.55 ± 3.14 | 23.39 ± 3.60 | .59 |
| Systolic blood pressure (mm Hg) | 131.30 ± 20.39 | 133.96 ± 20.59 | .322 |
| Diastolic blood pressure (mm Hg) | 76.80 ± 9.92 | 77.02 ± 10.46 | .967 |
| Heart rate (bpm) | 75.52 ± 13.00 | 77.77 ± 14.27 | .312 |
| Diabetes mellitus | 19 (33.93) | 13 (23.21) | .209 |
| Hypertension | 31 (55.36) | 29 (51.79) | .705 |
| Dyslipidemia | 13 (23.21) | 14 (25.00) | .825 |
| Active smoker | 23 (41.07) | 26 (46.43) | .568 |
| History of drinking | 12 (21.43) | 11 (19.64) | .815 |
| History of heart failure | 7 (12.50) | 5 (8.93) | .541 |
| Prior myocardial infarction | 10 (17.86) | 6 (10.71) | .280 |
| Number of diseased vessels | 2.20 ± 0.88 | 2.02 ± 0.82 | .219 |
| Culprit vessel during index PCI | |||
| LM | 4 (7.14) | 6 (10.71) | .582 |
| LAD | 22 (39.29) | 20 (35.71) | .696 |
| LCX | 9 (16.07) | 16 (28.57) | .262 |
| RCA | 25 (44.64) | 31 (55.36) | .335 |
| Ostial lesion | 3 (5.36) | 4 (7.14) | .696 |
| Proximal lesion | 37 (66.07) | 44 (78.57) | .139 |
| Bifurcation lesion | 12 (21.43) | 10 (17.86) | .634 |
| Visual thrombus | 2 (3.57) | 5 (8.93) | .252 |
| Thrombus aspiration | 7 (12.50) | 12 (21.43) | .258 |
| Stent type used during index PCI | |||
| SES | 39 (69.64) | 42 (75.00) | .526 |
| ZES | 11 (19.64) | 9 (16.07) | .622 |
| EES | 6 (10.71) | 5 (8.93) | .751 |
| Stent overlap | 16 (28.57) | 13 (23.21) | .518 |
| No postdilation | 28 (50.00) | 33 (58.93) | .343 |
| Minimum stent diameter (mm) | 2.92 ± 0.39 | 2.98 ± 0.38 | .285 |
| Maximum stent diameter (mm) | 3.01 ± 0.37 | 3.04 ± 0.38 | .737 |
| Total stent length (mm) | 38.88 ± 22.07 | 34.84 ± 17.92 | .391 |
| Stent release pressure (atm) | 14.21 ± 3.42 | 14.04 ± 2.63 | .962 |
| Number of stents per vessel | 1.36 ± 0.62 | 1.23 ± 0.47 | .324 |
| LVEF | 53.11 ± 7.34 | 55.36 ± 4.70 | .173 |
| LVEDD | 50.88 ± 5.78 | 49.02 ± 4.51 | .086 |
| S100A8/A9 level (ng/mL) | 3754.40 ± 1688.86 | 2434.86 ± 1243.41 | <.001 |
| hs-CRP level (mg/dL) | 0.55 ± 0.20 | 0.54 ± 038 | .11 |
| Peak troponin I level (ng/mL) | 49.83 ± 7.65 | 46.53 ± 9.00 | .887 |
| eGFR (mL/min/1.73 m2) | 103.40±21.21 | 105.37 ± 23.46 | .898 |
| WBC count (×109/L) | 9.43 ± 3.79 | 8.56 ± 3.30 | .256 |
| HGB level (g/L) | 142.52 ± 15.78 | 139.02 ± 17.55 | .382 |
| Platelet count (×109/L) | 213.57 ± 52.42 | 212.95 ± 60.98 | .662 |
| TCL level (mmol/L) | 4.63 ± 1.35 | 4.52 ± 0.97 | .90 |
| LDL level (mmol/L) | 2.93 ± 1.08 | 2.90 ± 0.79 | .977 |
| HDL level (mmol/L) | 1.17 ± 0.30 | 1.12 ± 0.31 | .271 |
| TG level (mmol/L) | 2.37 ± 1.52 | 2.33 ± 1.40 | .807 |
| Fasting blood-glucose level (mmol/L) | 6.68 ± 1.96 | 6.54 ± 2.53 | .303 |
| Fibrinogen level (g/L) | 3.19 ± 0.89 | 3.08 ± 0.84 | .919 |
| HbA1c level (%) | 6.26 ± 1.62 | 6.13 ± 1.62 | .738 |
Abbreviations: AMI, acute myocardial infarction; BMI, body mass index; EES, everolimus-eluting stent; eGFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin; HDL, high-density lipoprotein cholesterol; HGB, hemoglobin; hs-CRP, high sensitivity C-reactive protein; LAD, left anterior descending artery; LCX, left circumflex coronary artery; LDL, low-density lipoprotein cholesterol; LM, left main coronary artery; LVEDD, left ventricle ending-diastolic diameter; LVEF, left ventricle ejection fraction; PCI, percutaneous coronary intervention; RCA, right coronary artery; SES, sirolimus-eluting stent; TCL, total cholesterol; TG, triglycerides; WBC, white blood cell; VLST, very late stent thrombosis; ZES, zotarolimus-eluting stent.
Table 2.
Baseline Characteristics of Patients at the Time of VLST or Coronary Angiography During Follow-Up After Index PCI.
| Variables | VLST group, n = 56 | AMI group, n = 56 | P value |
|---|---|---|---|
| LVEF | 51.89 ± 8.30 | 53.93 ± 4.66 | .730 |
| LVEDD | 51.43 ± 5.47 | 49.59 ± 3.97 | .073 |
| eGFR (mL/min/1.73 m2) | 100.63 ± 24.23 | 103.55 ± 23.36 | .926 |
| WBC count (×109/L) | 10.06 ± 3.49 | 7.80 ± 2.62 | .228 |
| HGB level (g/L) | 141.96 ± 20.73 | 142.32 ± 14.03 | .570 |
| Platelet count (×109/L) | 217.95 ± 67.10 | 236.13 ± 58.96 | .064 |
| TCL level (mmol/L) | 4.17 ± 1.41 | 4.18 ± 0.67 | .135 |
| LDL level (mmol/L) | 2.44 ± 1.29 | 2.59 ± 0.61 | .061 |
| HDL level (mmol/L) | 1.11 ± 0.27 | 1.09 ± 0.23 | .951 |
| TG level (mmol/L) | 2.06 ± 1.16 | 2.33 ± 1.42 | .449 |
| Fasting blood-glucose level (mmol/L) | 6.04 ± 2.61 | 6.35 ± 2.44 | .136 |
| HbA1c level (%) | 6.06 ± 1.32 | 6.10 ± 1.60 | .437 |
| Fibrinogen level (g/L) | 3.27±0.95 | 3.15 ± 0.77 | .481 |
| hs-CRP level (mg/dL) | 0.56 ± 0.21 | 0.30 ± 0.22 | <.001 |
| S100A8/A9 level (ng/mL) | 5517.79 ± 2650.86 | 1568.16 ± 772.09 | <.001 |
| Statin treatment | 31 (55.36) | 34 (60.71) | .566 |
| DAPT | 28 (50.00) | 32 (57.14) | .449 |
Abbreviations: AMI, acute myocardial infarction; CAG, coronary angiography; DAPT, dual antiplatelet therapy; eGFR, estimated glomerular filtration rate; HbA1c, glycosylated haemoglobin; hs-CRP, high sensitivity C-reactive protein; HDL, high-density lipoprotein cholesterol; HGB, haemoglobin; LDL, low-density lipoprotein cholesterol; LVEDD, left ventricle ending diastolic diameter; LVEF, left ventricle ejection fraction; PCI, percutaneous coronary intervention; TCL, total cholesterol; TG, triglycerides; VLST, very late stent thrombosis; WBC, white blood cell.
High Sensitivity C-Reactive Protein and S100A8/A9 Changes During VLST
The mean level of S100A8/A9 was 3754.4 ± 1688.9 ng/mL during index PCI and increased to 5517.8 ± 2650.9 ng/mL at the time of VLST; in the AMI group, S100A8/A9 level was 2434.9 ± 1243.4 ng/mL during index PCI and decreased to 1568.2 ± 772.1 ng/mL during follow-up, similar to that detected in the NC group (1618.2 ± 641.4 ng/mL; Figure 2). The mean level of high sensitivity C-reactive protein (hs-CRP), a commonly used biomarker of inflammation, was 0.55 ± 0.20 mg/dL during index PCI in the VLST group, similar to that in the AMI group (0.54 ± 0.38 mg/dL), and at the time of VLST, no significant changes were seen in VLST group (0.56 ± 0.21 mg/dL); however, as expected, levels reduced to 0.30 ± 0.22 mg/dL in AMI group during follow-up, similar to that in the NC group (0.33 ± 0.15 mg/dL; Figure 2). Of note, as shown in Figure 3, S100A8/A9 levels showed significant increases during VLST when compared to its own levels during index PCI, while no significant changes were seen in hs-CRP, further suggesting its association with the development of VLST.
Figure 2.
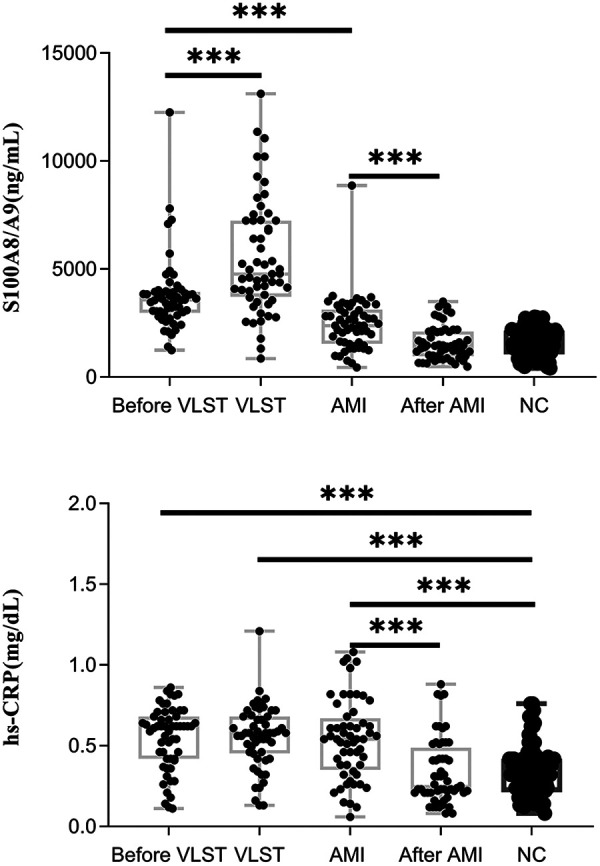
S100A8/A9 and hs-CRP levels in different cases. AMI indicates acute myocardial infarction; hs-CRP, high sensitivity C-reactive protein; NC, normal control; VLST, very late stent thrombosis. *** P < .001.
Figure 3.
S100A8/A9 and hs-CRP levels increase in different cases. AMI indicates acute myocardial infarction; hs-CRP, high sensitivity C-reactive protein; VLST, very late stent thrombosis.
To define whether serum S100A8/A9 levels during index PCI could be used to predict the development of VLST, Cox univariate regression analysis was performed; the results are presented in Supplementary Table 1. In multivariate regression analysis, serum S100A8/A9 levels during index PCI remained independently correlated with the development of VLST (Table 3); however, the hazard ratio (HR) was only 1.001, which limits its use as a biomarker for predicting the development of VLST.
Table 3.
Results of Multivariate Cox Regression Analysis.
| Variables | HR | 95% CI | P value |
|---|---|---|---|
| Diabetes mellitus | 1.599 | 0.606-4.217 | .343 |
| Proximal lesion | 0.626 | 0.235-1.668 | .349 |
| LVEF | 0.949 | 0.875-1.029 | .208 |
| LVEDD | 1.005 | 0.912-1.1.106 | .927 |
| S100A8/A9 level | 1.001 | 1.000-1.001 | <.001 |
Abbreviations: CI, confidence interval; HR, hazard ratio; LVEF, left ventricle ejection fraction; LVEDD, left ventricle ending-diastolic diameter.
Discussion
The present study found that higher serum levels of S100A8/A9 are associated with the development of VLST. Mechanisms underlying VLST are complicated and might include intense platelet reaction13 and hypercoagulable state,14 as well as various kinds of changes within the artery accompanying stent implantation.15,16 Hypersensitivity and inflammation reactions to drug-eluting stents have been described in VLST for both first-generation17,18 and second-generation drug-eluting stents.19 Infiltration of pro-inflammatory cells, including eosinophils, lymphocytes, palisading macrophages, and giant cells, has been observed in both the stented vessel wall and harvested thrombi.5,18 Recently, with the progression of intravascular imaging technology, infiltration of macrophages was also identified by optical coherence tomography in plaques within the stents of patients with VLST.6
In our study, we found that levels of S100A8/A9, an important regulator and indicator of inflammation, were associated with the development of VLST; this relationship manifested as a sharp increase in S100A8/A9 level during VLST compared to that during index PCI, while in patients without VLST during follow-up, its levels decreased to levels detected in NCs. The possible mechanisms might due to the role of S100A8/A9 in atherosclerosis and thrombosis through regulating inflammatory response. S100A8/A9 was found to be elevated in patients with cardiovascular disease, particularly in AMI.9,12,20 Further studies revealed that the S100A8/A9 complex may be involved in the inflammatory process of coronary atherosclerotic plaques and correlates with a vulnerable plaque phenotype; immune-double staining clearly showed that the S100A8/A9 complex was expressed in infiltrated neutrophils and some macrophages in patients with unstable angina, while the S100A8/A9-positive areas were significantly higher in patients with unstable angina than in those with stable angina.21 S100A8/A9 also plays a pivotal role in arterial embolism22–25 on account of the vicious inflammation/thrombosis cycle.26 S100A8/A9 could increase the production of interleukin-6, a pleiotropic cytokine that is implicated in inflammatory thrombocytosis, and acts on hepatocytes to enhance the production of thrombopoietin, which in turn results in reticulated thrombocytosis and atherogenesis.27 Therefore, further preclinical study is needed to define the role of S100A8/A9 in the development of VLST in patients with AMI.
In clinical practice, identification of potential high-risk patients with VLST occurring more than 1 year after index stent placement is appealing, because doing so may reduce the occurrence of VLST by means of intensive follow-up and even preventive interventions. In our observational study, the serum S100A8/A9 level during index PCI remained independently correlated with the development of VLST; however, the HR was only 1.001, which limits its use as a biomarker for predicting the development of VLST. In fact, elevated plasma levels of S100A8/A9 were previously found to be associated with increased risk of future coronary events in healthy individuals and in myocardial infarction survivors.8,28 In 237 case–control pairs selected from the Pravastatin or Atorvastatin Evaluation and Infection Therapy: Thrombolysis in Myocardial Infarction (PROVE IT-TIMI 22) statin therapy trial cohort, S100A8/A9 levels were measured 30 days after the acute event and found to be elevated in patients who had recurrent events (MI or cardiovascular death) during the subsequent 30 days. Patients with S100A8/A9 levels within the top quartile had a 2-fold higher risk of developing recurrent events compared to those in the lowest quartile, independently of the presence of diabetes mellitus, hypertension, previous cardiovascular disease, heart failure, or elevated hs-CRP levels.28 Therefore, whether the level of S100A8/A9 during index PCI can be used to predict the development of VLST remains to be investigated by future studies.
The present study has certain limitations. First, given the incidence of stent thrombosis was 0.5% to 4%, the number of included patients was small. Second, we did not have the information about the patients who experienced another MI after the index PCI that were not related to VLST.
Conclusions
In summary, higher serum levels of S100A8/A9 are associated with the development of VLST, and the underlying mechanism need to be defined by future preclinical studies.
Supplemental Material
Supplemental Material, Supplementary_material for The Association Between S100A8/A9 and the Development of Very Late Stent Thrombosis in Patients With Acute Myocardial Infarction by Xiang Wang, Meng Guan, Xiuhang Zhang, Taiyuan Ma, Muli Wu, Yulin Li, Xinxin Chen and Yang Zheng in Clinical and Applied Thrombosis/Hemostasis
Footnotes
Authors’ Note: This study was approved by the institutional review boards of the First Hospital of Jilin University (no. 2013256). In addition, informed consent was obtained from all the participants.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research was supported by the National Key R&D Program of China (grant number 2016YFC0900903).
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Go AS, Mozaffarian D, Roger VL, et al. Heart Disease and Stroke Statistics—2014 update: a report from the American Heart Association. Circulation. 2013;129(3):e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang X, Chen X, Sun W, et al. Very late stent thrombosis in drug-eluting stents new observations and clinical implications. Cardiol Rev. 2019;27(6):279–285. [DOI] [PubMed] [Google Scholar]
- 3. Wang X, Chen X, Tian T, et al. A scoring system to predict the occurrence of very late stent thrombosis following percutaneous coronary intervention for acute coronary syndrome. Sci Rep. 2020;10:6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rivers JP, Mitchell V, Peters-Lawrence M. Vascular inflammation as a subclinical marker of atherosclerosis demonstrates high cardiometabolic risk in a resource-limited, community-based population. Circulation. 2017;135:AP381. [Google Scholar]
- 5. Riegger J, Byrne RA, Joner M, et al. Histopathological evaluation of thrombus in patients presenting with stent thrombosis. A multicenter European study: a report of the prevention of late stent thrombosis by an Interdisciplinary Global European Effort Consortium. Eur Heart J. 2016;379(19):1538–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Joner M, Koppara T, Byrne RA, et al. Neoatherosclerosis in patients with coronary stent thrombosis: findings from optical coherence tomography imaging (a report of the PRESTIGE consortium). JACC Cardiovasc Interv. 2018;11(14):1340–1350. [DOI] [PubMed] [Google Scholar]
- 7. Wang S, Song R, Wang Z, et al. S100A8/A9 in inflammation. Front Immunol. 2018;9:1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schiopu A, Cotoi OS. S100A8 and S100A9: DAMPs at the crossroads between innate immunity, traditional risk factors, and cardiovascular disease. Mediators Inflamm. 2013;2013:828354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen X, Tao T, Wang H, et al. Arterial thrombosis is accompanied by elevated mitogen-activated protein kinase (MAPK) and cyclooxygenase-2 (COX-2) expression via toll-like receptor 4 (TLR-4) activation by S100A8/A9. Med Sci Monit. 2018;24:7673–7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Y, Fang C, Gao H, et al. Platelet-derived S100 family member myeloid-related protein-14 regulates thrombosis. J Clin Invest. 2014;124(5):2160–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berezin AE. The cardiovascular risk prognostication in diabetes mellitus: the role of myeloid-related protein complex calprotectin. Int J Pathol Clin Res. 2016;2(1):4. [Google Scholar]
- 12. Buyukterzi Z CU, Alpaydin S, Guzelant A, et al. Enhanced S100A9 and S100A12 expression in acute coronary syndrome. Biomark Med. 2017;11(3):8. [DOI] [PubMed] [Google Scholar]
- 13. Godschalk TC, Byrne RA, Adriaenssens T, et al. Observational study of platelet reactivity in patients presenting with ST-segment elevation myocardial infarction due to coronary stent thrombosis undergoing primary percutaneous coronary intervention: results from the European Prevention of Stent Thrombosis by an Interdisciplinary Global European Effort Registry. JACC Cardiovasc Interv. 2017;10(24):2548–2556. [DOI] [PubMed] [Google Scholar]
- 14. Loeffen R, Godschalk TC, van Oerle R, et al. The hypercoagulable profile of patients with stent thrombosis. Heart. 2015;101(14):1126–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Otsuka F, Byrne RA, Yahagi K, et al. Neoatherosclerosis: overview of histopathologic findings and implications for intravascular imaging assessment. Eur Heart J. 2015;36(32):2147–2159. [DOI] [PubMed] [Google Scholar]
- 16. Taniwaki M, Radu MD, Zaugg S, et al. Mechanisms of very late drug-eluting stent thrombosis assessed by optical coherence tomography. Circulation. 2016;133(7):650–660. [DOI] [PubMed] [Google Scholar]
- 17. Virmani R, Guagliumi G, Farb A, et al. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation. 2004;109(6):701–705. [DOI] [PubMed] [Google Scholar]
- 18. Cook S, Ladich E, Nakazawa G, et al. Correlation of intravascular ultrasound findings with histopathological analysis of thrombus aspirates in patients with very late drug-eluting stent thrombosis. Circulation. 2009;120(5):391–399. [DOI] [PubMed] [Google Scholar]
- 19. Otsuka F, Kazuyuki Y, Ladich E, et al. Hypersensitivity reaction in the US Food and Drug Administration-approved second-generation drug-eluting stents: histopathological assessment with ex vivo optical coherence tomography. Circulation. 2015;131(3):3. [DOI] [PubMed] [Google Scholar]
- 20. Lood C, Tyden H, Gullstrand B, et al. Platelet-derived S100A8/A9 and cardiovascular disease in systemic lupus erythematosus. Arthritis Rheumatol. 2016;68(8):1970–1980. [DOI] [PubMed] [Google Scholar]
- 21. Miyamoto S, Ueda M, Ikemoto M, et al. Increased serum levels and expression of S100A8/A9 complex in infiltrated neutrophils in atherosclerotic plaque of unstable angina. Heart. 2008;94(8):1002–1007. [DOI] [PubMed] [Google Scholar]
- 22. Croce K, Gao H, Wang Y, et al. Myeloid-related protein-8/14 is critical for the biological response to vascular injury. Circulation. 2009;120(5):427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Crawford JR, Trial J, Nambi V, et al. Plasma levels of endothelial microparticles bearing monomeric C-reactive protein are increased in peripheral artery disease. J Cardiovasc Transl Res. 2016;9(3):184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li X, Ballantyne LL, Che X, et al. Endogenously generated omega-3 fatty acids attenuate vascular inflammation and neointimal hyperplasia by interaction with free fatty acid receptor 4 in mice. J Am Heart Assoc. 2015;4(4):e001856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mondal NK, Roychoudhury S, Mukherjee S, et al. Increased risk of cardiovascular disease in premenopausal female ragpickers of Eastern India: involvement of inflammation, oxidative stress, and platelet hyperactivity. Mol Cell Biochem. 2016;419(1-2):193–203. [DOI] [PubMed] [Google Scholar]
- 26. Arthur J C. Blood coagulation as an intrinsic pathway for proinflammation: a mini review. Inflamm Allergy Drug Targets. 2010;9(1):12. [DOI] [PubMed] [Google Scholar]
- 27. Kraakman MJ, Lee MK, Al-Sharea A, et al. Neutrophil-derived S100 calcium-binding proteins A8/A9 promote reticulated thrombocytosis and atherogenesis in diabetes. J Clin Invest. 2017;127(6):2133–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morrow DA, Wang Y, Croce K, et al. Myeloid-related protein 8/14 and the risk of cardiovascular death or myocardial infarction after an acute coronary syndrome in the Pravastatin or Atorvastatin Evaluation and Infection Therapy: Thrombolysis in Myocardial Infarction (PROVE IT-TIMI 22) trial. Am Heart J. 2008;155(1):49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Supplementary_material for The Association Between S100A8/A9 and the Development of Very Late Stent Thrombosis in Patients With Acute Myocardial Infarction by Xiang Wang, Meng Guan, Xiuhang Zhang, Taiyuan Ma, Muli Wu, Yulin Li, Xinxin Chen and Yang Zheng in Clinical and Applied Thrombosis/Hemostasis