Abstract
Diquat is a nonselective herbicide that is used as a contact and preharvest desiccant to control terrestrial and aquatic vegetation. Increasing numbers of cases of diquat poisoning have recently been reported. Organs commonly affected by diquat poisoning include the kidney, liver, and lung. Neurological involvement of diquat poisoning is relatively rare. A 21-year-old man ingested 100 mL of diquat (20 g/100 mL) 5 hours before admission. Fifteen minutes after ingestion, he developed nausea and vomiting. The patient was sent to the emergency intensive care unit, and gastric lavage was performed. Continuous renal replacement therapy and continuous venovenous hemodiafiltration with hemoperfusion were performed, and methylprednisolone was administered. Five days after admission, the patient developed disturbance of consciousness and positive bilateral Babinski signs. Head computed tomography demonstrated hypodensity in the pons. At 11 days after admission, brain magnetic resonance imaging showed acute pontine demyelination. At 15 days after admission, the patient died of multiple organ dysfunction syndrome. We encountered a case of diquat poisoning with central pontine myelinolysis and acute kidney injury. This case highlights the clinical value of neuroimaging examination for early diagnosis of central pontine myelinolysis.
Keywords: Diquat poisoning, central pontine myelinolysis, acute kidney injury, neuroimaging, multiple organ dysfunction, consciousness disorder
Introduction
Diquat (1,1′-ethylene-2,2′-bipyridylium) is a nonselective rapidly acting herbicide that has been widely used as a contact and preharvest desiccant to control terrestrial and aquatic vegetation.1 Although the chemical constitution and pharmacological effects of diquat are similar to those of paraquat (1,1′-dimethyl-4,4′-bipyridylium), the clinical manifestations of diquat poisoning and paraquat intoxication are different. Paraquat usually causes pulmonary fibrosis, whereas diquat commonly targets the kidney, causing tubular necrosis; the occurrence of pulmonary fibrosis is rare in patients with diquat poisoning.1 No specific antidotes for diquat poisoning are available. Since paraquat was delisted in China in 2016, diquat has been used as a substitute, and increasing numbers of cases of diquat poisoning have been reported. The commonly affected organs include the kidney, liver, and lung. Brain involvement of diquat poisoning is relatively rare. We herein report a case of diquat poisoning that manifested as central pontine myelinolysis.
Case report
A 21-year-old man with a body weight of 85 kg ingested 100 mL of diquat (20 g/100 mL) 5 hours before admission because he had been quarrelling with his family. Fifteen minutes after ingestion, he developed nausea and vomiting. The patient was sent to the emergency intensive care unit, and gastric lavage was performed. Three months earlier, the patient had been diagnosed with depression. He had no history of allergy, smoking, hypertension, diabetes, coronary heart disease, or infectious diseases such as hepatitis or tuberculosis.
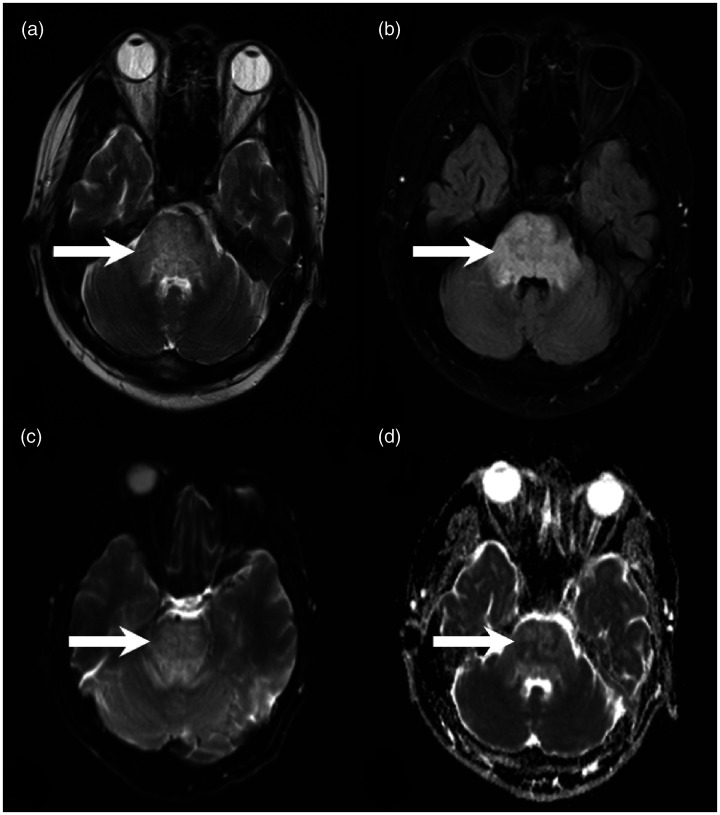
After admission, physical examination showed that the patient’s vital signs were stable and that his oral mucosa was eroded. He had remarkable subxiphoid tenderness without rebound pain or muscle tension. Cardiopulmonary function was normal. The laboratory examination results are shown in Table 1. Hemoperfusion was performed twice daily after admission. Anti-infection, organ protection, and anti-oxidation drugs were administered. Two days after admission, chest computed tomography showed patchy hyperdensities with poorly defined boundaries in the upper lobe of the right lung and the lower lobes of both lungs. The daily urine volume was 100 mL. Continuous renal replacement therapy and continuous venovenous hemodiafiltration with hemoperfusion were performed. The patient received methylprednisolone (80 mg twice daily in the first 3 days and 40 mg twice daily thereafter), sulbenicillin (4 g three times daily for 10 days; this treatment was then changed to moxifloxacin at 0.4 g for 5 days), ulinastatin (200,000 IU twice daily for 10 days), and acetylcysteine (8 g once daily for 7 days). Five days after admission, the patient developed disturbance of consciousness. Considering the disturbance of consciousness and significantly increased creatinine level, renal encephalopathy was suspected. Ten days after admission, the patient exhibited quadriplegia, chewing and swallowing disorders, and positive bilateral Babinski signs. Head computed tomography was performed to exclude concomitant damage in the central nervous system as the cause of the consciousness disorder, and hypodensity in the pons was found. At 11 days, brain magnetic resonance imaging showed a pontine lesion with slight hypointensity on T1-weighted imaging, hyperintensity on T2-weighted imaging, and heterogeneous intensities on diffusion-weighted imaging and apparent diffusion coefficient imaging (Figure 1). A diagnosis of acute pontine demyelination was made by collaboration among poisoning experts, neurologists, and neuroradiologists. At 15 days after admission, the patient died of multiple organ dysfunction syndrome.
Table 1.
Laboratory profile.
| BUN mmol/L |
Cr μmol/L |
Na+ mmol/L |
K+ mmol/L |
AST U/L |
ALT U/L |
PaO2 mmHg |
SaO2 % |
WBC ×109/L |
|
|---|---|---|---|---|---|---|---|---|---|
| Day 0 | 4.78 | 101.2 | 140.8 | 3.93 | 40.9 | 68.7 | 110 | 98 | 27.13 |
| Day 1 | 13.38 | 507.4 | 134.5 | 3.66 | 268.0 | 371.6 | 94 | 97 | 26.53 |
| Day 2 | 11.56 | 402.1 | 133.3 | 3.39 | 84.1 | 261.6 | 98 | 98 | 20.67 |
| Day 3 | 19.79 | 687.0 | 137.2 | 3.75 | 40.9 | 127.0 | 120 | 99 | 14.10 |
| Day 4 | 14.62 | 408.3 | 133.2 | 3.06 | 23.6 | 85.0 | 65 | 91 | 18.25 |
| Day 5 | 29.07 | 727.2 | 136.7 | 3.62 | 18.4 | 55.8 | 88 | 96 | 20.44 |
| Day 6 | 27.57 | 606.4 | 139.1 | 2.86 | 58 | 91 | 22.34 | ||
| Day 7 | 27.73 | 623.4 | 139.0 | 3.53 | 76 | 95 | 22.74 | ||
| Day 8 | 16.83 | 431.4 | 142.4 | 3.54 | 50.5 | 85.2 | 58 | 91 | 30.83 |
| Day 9 | 11.82 | 248.5 | 139.1 | 3.32 | 90 | 97 | 32.71 | ||
| Day 10 | 29.46 | 563.3 | 135.7 | 3.44 | 35.67 | ||||
| Day 11 | 42.28 | 764.0 | 136.0 | 3.42 | 32.08 | ||||
| Day 12 | 20.67 | 275.9 | 136.7 | 3.46 | 89 | 97 | 23.98 | ||
| Day 13 | 22.04 | 402.7 | 137.3 | 3.61 | 59.0 | 189.5 | 26.52 | ||
| Day 14 | 39.11 | 716.9 | 135.0 | 4.24 | 92.5 | 156.5 | 48 | 81 | 24.89 |
| Day 15 | 48.73 | 973.7 | 135.9 | 4.91 | 40.4 | 110.6 | 64 | 90 | 23.42 |
BUN, blood urea nitrogen; Cr, creatinine; Na+, sodium; K+, potassium; AST, aspartate aminotransferase; ALT, alanine aminotransferase; PaO2, partial pressure of oxygen in arterial blood; SaO2, oxygen saturation of arterial blood; WBC, white blood cells.
Figure 1.
Brain magnetic resonance imaging. Brain magnetic resonance imaging showed a pontine lesion, which appeared hyperintense on (a) T2-weighted imaging and (b) fluid-attenuated inversion-recovery imaging. The lesion showed heterogeneous intensities on (c) diffusion-weighted imaging and (d) apparent diffusion coefficient imaging.
Discussion
Diquat is slightly less toxic than paraquat. Animal experiments have indicated that the median lethal dose of oral diquat is 30 to 400 mg/kg,2 and the dose that causes adult mortality is 6 to 12 g.3 Hantson et al.4 performed an autopsy on a 37-year-old patient who died after oral ingestion of diquat, and the authors found that the relative concentration of diquat was highest in the kidney, followed by the lung, liver, brain, and heart.
Central nervous system complications after diquat poisoning are rare.5,6 In 2001, Saeed et al.7 reported a case of intracerebral hematoma in the right basal ganglia and external capsule. In patients presenting with coma, pontine hemorrhage or infarction was noted during life or postmortem.3,6,8,9 The autopsy of a 64-year-old man who died 18 days after diquat poisoning revealed brain stem hemorrhage and infarction.3
The administration of heparin during hemodialysis or hemoperfusion has been considered a contributor to intracranial hemorrhage, although this hypothesis cannot explain why only the pons was affected in the present case.11 Rudez et al.12 reported a case of poisoning by injection of 20 mL of diquat into the vagina; 3 months later, the patient developed quadriplegia and dysarthria. The mechanism of diquat-induced neurotoxicity remains unclear. Diquat mediates the redox cycle in cytochrome P450 reductase recombinant cells and participates in lipid peroxidation and reactive oxygen species production by NADPH. Djurdjevic et al.13 reported that oxidation of glutathione was the cause of the reduced antioxidative defense against diquat neurotoxicity. Some authors have also reported the role of nitridation stress in diquat neurotoxicity. Nitridation stress produces reactive nitrogen species, which cause neuronal damage. Pretreatment with NG-nitro-L-arginine methyl ester (L-NAME), a nonselective inhibitor of nitric oxide synthase, in a rat model of diquat poisoning reduced its neurotoxic effects.14 Singh et al.15 proposed that Bacopa monniera can protect PC12 cells through modulating cellular redox pathways that are altered in Parkinson’s disease.
In the current study, we reported a case of acute pontine demyelination and kidney injury in a young man with diquat poisoning. During the clinical course, the patient developed disturbance of consciousness, and his urea and creatinine levels gradually increased. The diagnosis of renal encephalopathy was suspected, and continuous renal replacement therapy was performed. Neuroimaging examination was performed to exclude central nervous system damage. Moreover, brain magnetic resonance imaging showed abnormal signals in the pons, and diffusion-weighted imaging and apparent diffusion coefficient imaging showed mixed signals that were inconsistent with the clinical manifestations of cerebral infarction or hemorrhage. To the best of our knowledge, this is the first reported case of acute pontine demyelination following diquat poisoning. Notably, the patient’s serum sodium ion concentration was normal, and sodium-related demyelination could be ruled out. According to existing evidence, hemorrhage, infarction, and demyelination usually affect the pons in patients with diquat poisoning. The specific mechanism remains unclear, and further studies are warranted.
Conclusion
We have herein reported a case of diquat poisoning with acute pontine demyelination and acute kidney injury. Clinicians should be aware of the potential for pontine damage in patients with diquat poisoning and the clinical value of neuroimaging examination for early diagnosis of acute pontine demyelination. Acute kidney injury is the most common complication in patients with diquat poisoning. If a patient presents with concomitant consciousness disorder and a significantly increased creatinine level, the differential diagnoses include renal encephalopathy and central nervous system injury. In such cases, neuroimaging examination should be promptly performed to avoid misdiagnosis and underdiagnosis.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethics
This study was approved by the Institutional Review Board and Ethics Committee of the First Hospital of Jilin University. Informed consent was obtained from the patient’s parents.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Lichao Sun https://orcid.org/0000-0001-7471-3503
References
- 1.Magalhães N, Carvalho F, Dinis-Oliveira RJ. Human and experimental toxicology of diquat poisoning: toxicokinetics, mechanisms of toxicity, clinical features, and treatment. Hum Exp Toxicol 2018; 37: 1131–1160. 10.1177/0960327118765330. [DOI] [PubMed] [Google Scholar]
- 2.Tanen DA, Curry SC, Laney RF. Renal failure and corrosive airway and gastrointestinal injury after ingestion of diluted diquat solution. Ann Emerg Med 1999; 34: 542–545. 10.1016/s0196-0644(99)80059-6. [DOI] [PubMed] [Google Scholar]
- 3.Vanholder R, Colardyn F, De Reuck J, et al. Diquat intoxication: report of two cases and review of the literature. Am J Med 1981; 70: 1267–1271. 10.1016/0002-9343(81)90836-6. [DOI] [PubMed] [Google Scholar]
- 4.Hantson P, Wallemacq P, Mahieu P. A case of fatal diquat poisoning: toxicokinetic data and autopsy findings. J Toxicol Clin Toxicol 2000; 38: 149–152. 10.1081/clt-100100930. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt DM, Neale J, Olson KR. Clinical course of a fatal ingestion of diquat. J Toxicol Clin Toxicol 1999; 37: 881–884. DOI: 10.1081/clt-100102471. [DOI] [PubMed] [Google Scholar]
- 6.Powell D, Pond SM, Allen TB, et al. Hemoperfusion in a child who ingested diquat and died from pontine infarction and hemorrhage. J Toxicol Clin Toxicol 1983; 20: 405–420. DOI: 10.3109/15563658308990609. [DOI] [PubMed] [Google Scholar]
- 7.Saeed SA, Wilks MF, Coupe M. Acute diquat poisoning with intracerebral bleeding. Postgrad Med J 2001; 77: 329–332. 10.1081/clt-100100930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schönborn H, Schuster HP, Kössling FK. Klinik und Morphologie der akuten peroralen Diquatintoxikation (Reglone). Arch Toxikol 1971; 27: 204–216. [PubMed] [Google Scholar]
- 9.Van Den Heede M, Heyndrickx A, Timperman J. Thin layer chromatography as a routine appropriate technique for the determination of bipyridylium herbicides in post mortem human tissues. Med Sci Law 1982; 22: 57–62. 10.1177/002580248202200111. [DOI] [PubMed] [Google Scholar]
- 10.Jović-Stosić J, Babić G, Todorović V. Fatal diquat intoxication. Vojnosanit Pregl 2009; 66: 477–481. 10.2298/vsp0906477j. [DOI] [PubMed] [Google Scholar]
- 11.Jones GM, Vale JA. Mechanisms of toxicity, clinical features, and management of diquat poisoning: a review. J Toxicol Clin Toxicol 2000; 38: 123–128. 10.1081/clt-100100926. [DOI] [PubMed] [Google Scholar]
- 12.Rudez J, Sepcić K, Sepcić J. Vaginal applied diquat intoxication. J Toxicol Clin Toxicol 1999; 37: 877–879. 10.1081/clt-100102470. [DOI] [PubMed] [Google Scholar]
- 13.Djurdjevic D, Djukic M, Ninkovic M, et al. Glutathione cycle in diquat neurotoxicity: assessed by intrastriatal pre-treatment with glutathione reductase. Acta Vet 2013; 63: 159–175. 10.2298/AVB1303159D. [DOI] [Google Scholar]
- 14.Djukic M, Jovanovic MD, Ninkovic M, et al. Intrastriatal pre-treatment with l-NAME protects rats from diquat neurotoxcity. Ann Agric Environ Med 2012; 19: 666–672. [PubMed] [Google Scholar]
- 15.Singh M, Murthy V, Ramassamy C. Neuroprotective mechanisms of the standardized extract of Bacopa monniera in a paraquat/diquat-mediated acute toxicity. Neurochem Int 2013; 62: 530–539. 10.2298/AVB1303159D. [DOI] [PubMed] [Google Scholar]