Abstract
Background
Many women continue to smoke during pregnancy, despite known risks, often in response to negative affect. Recent scholarship has begun to examine factors that decrease the success of behavioral treatments for smoking cessation in pregnancy, which are the preferred interventions. Alexithymia is one factor that may interfere with smoking cessation interventions. Alexithymia restricts access to emotional information and increases propensity toward maladaptive behaviors, including smoking. However, mechanisms underlying such effects are largely unknown.
Objectives
Using data from a longitudinal treatment study, the present research examined difficulties with emotion regulation as a potential mechanism linking alexithymia and smoking. Pregnant smokers (n=73; mean age = 24.78; SD = 4.50) completed measures related to alexithymia, smoking, emotion regulation, depression, anxiety, and anger at baseline and then again following eight sessions of Cognitive-Behavioral Smoking Cessation Treatment.
Results
Nearly 40% of the sample met the criteria for alexithymia. The alexithymia group reported higher depression, anxiety, and anger. They also reported more difficulties with emotion regulation. In a path analysis, baseline alexithymia had a significant positive indirect effect on number of cigarettes smoked at the end of treatment through difficulties with emotion regulation.
Conclusions/Importance
Similar to other studies, alexithymia limits the understanding of emotional information necessary for selection and implementation of adaptive coping responses. Our results extend the literature by suggesting that smoking may be an attempt to manage undifferentiated and unpleasant sensations created by alexithymia.
Keywords: Pregnant women, smoking, alexithymia, emotion regulation
Introduction
Smoking during pregnancy is associated with negative physical consequences for both mother and baby (Banderali et al., 2015; Cnattingius, 2004). Smoking during pregnancy remains highest among minority status and socioeconomically disadvantaged women (Graham, Hawkins, & Law, 2010; Griffiths, Brown, Fulton, Tombor, & Naughton, 2016; Moore, Blatt, Aimin, Van Hook, & DeFranco, 2016; Riaz, Lewis, Naughton, & Ussher, 2018), despite significant declines in rates of smoking during pregnancy for women with greater education and higher incomes (Li et al., 2018). Consequently, smoking during pregnancy remains an urgent public health priority. Smoking cessation benefits both mother and baby. The benefits of cessation for infants include higher birth weight, better infant health outcomes, and prevention of cognitive delays that affect language development and behavior problems (Cnattingius, 2004; Godleski, Shisler, Eiden, & Huestis, 2018; Hernández-Martínez et al., 2017). For mothers, cessation may result in fewer complications during pregnancy and shorter labor (Britton, James, Collier, Sprague, & Brinthaupt, 2013). Most smokers seeking to quit are likely to benefit most from a combination of behavioral counseling and nicotine replacement therapies (NRT) or smoking cessation medications (e.g., varenicline or bupropion; Ussher et al., 2012). However, regarding smoking cessation in pregnancy, there is no consensus on the use of NRT and many women report reluctance to use NRT or are noncompliant with medication (Bittoun & Femia, 2010; Coleman, Chamberlain, Cooper, & Leonardi-Bee, 2011). Consequently, behavioral interventions remain the most accepted smoking cessation strategy for pregnant smokers.
Behavioral interventions have shown promise for smoking cessation in pregnancy (Bradizza et al., 2017; Lee et al., 2015; Su & Buttenheim, 2014). Unfortunately, they are not equally effective across all socioeconomic groups (Schneider, Huy, Schütz, & Diehl, 2010). Women with higher incomes are most likely to quit successfully during pregnancy (Dias-Damé & Cesar, 2015), while lower income women are more likely to continue to smoke (Yukiko, Kunihiko, & Setsuko, 2015). To address these disparities, recent attention has focused on identifying factors that facilitate or inhibit cessation success (Brooks et al., 2018; Mantzari, Vogt, & Marteau, 2012).
Alexithymia is one such factor that may interfere with the success of behavioral interventions for smoking cessation. Alexithymia is a disruption in the emotion regulation process (Lyvers, Brown, & Thorberg, 2018) and is distinguished by deficits in identifying and describing emotions, deficits in social attachment, and a limited fantasy life (Sifneos, 1973). Smokers score higher on measures of alexithymia than do non-smokers (Carton, Bayard, Jouanne, & Lagrue, 2008). Alexithymia is associated with adverse life events (Oyefeso, Brown, Chiang, & Clancy, 2008), and negatively related to socioeconomic status (Peters & Lumley, 2007). Notably, adverse life events and low socioeconomic status are commonly associated with smoking in pregnancy and pose a barrier to successful quit attempts (Moore, et al., 2016; Griffiths et al., 2016). Alexithymia may undermine behavioral interventions for smoking cessation, which target the relations between affective states and smoking (e.g., Bradizza et al., 2017; Ussher et al., 2012). Therefore, a better understanding of the role that alexithymia plays in disrupting adaptive emotion regulation processes may help researchers and clinicians adapt existing behavioral therapies for smoking cessation to improve cessation outcomes for pregnant women.
No known studies have examined the impact of alexithymia on smoking in pregnancy and only a few studies have examined the relationship between affect and smoking longitudinally. Thus, the purpose of the current study was to: (1) assess potential differences in emotion regulation processes between pregnant smokers with and without alexithymia and (2) utilize longitudinal data to determine the indirect effect of alexithymia on end-of-treatment smoking behavior via difficulties with emotion regulation.
Methods
Data for analyses were drawn from the baseline and post-treatment assessments of a behavioral clinical trial for pregnant women (n=73) who were identified as negative affect smokers at screening (see measures section) and expressed an interest in quitting smoking (see Bradizza et al., 2017 for more details). The University at Buffalo Institutional Review Board approved study procedures and materials.
Procedure
Study participants were recruited from a publicly-funded prenatal clinic in Buffalo, New York, USA. Participants were randomly assigned to receive standard smoking cessation treatment combined with either (a) an emotion regulation intervention (ERT) or (b) a health and lifestyle (HLS) intervention. All participants received eight individually-administered, hour-long sessions. The smoking cessation component of the intervention comprised 20 minutes of each session and the ERT or HLS intervention comprised the remaining 40 minutes of each session. Conditions were designed to be equivalent with respect to time, intensity, and client expectation of positive smoking outcomes (Bradizza et al., 2017). Consequently, participants from both conditions were combined to form a single group in the current analyses. There were no baseline differences between ERT and HLS conditions on mean alexithymia scores (47.43 [sd=10.99] and 45.35 [sd=13.05], respectively).
The smoking cessation treatment was adapted from The Tobacco Dependence Handbook: Guide to Best Practices (Abrams et al., 2003). The topics covered in each session were: (1) Reasons for Quitting, Smoking Triggers, Preparation for Session 2 Quit Day; (2) Benefits of Quitting Smoking, Urge Management, Quit Day Experiences; (3) Coping Strategies for Avoiding Smoking, Coping with Slips; (4) Identifying High-risk Situations; (5) Obtaining Social Support for Quitting; (6) Managing High Risk Situations; (7) Thoughts that Lead to Smoking, Managing High-risk Situations; and (8) Smoke-free Action Plan or Tips for Future Progress Towards Cessation Goal.
The topics covered in the ERT (Bradizza et al., 2017) condition were: (1) Program Rationale, Introduction to Emotions, and Emotions and Smoking; (2) Dedicated Mindfulness Practice and Mindfulness in Daily Activities; (3) Preparing for Guided Imagery/Exposure to Negative Affect Smoking Situations, Mindfulness; (4) Emotions and Urges, Physiologically Focused Guided Imagery/Exposure to Negative Affect Smoking Situations; (5–7) Mindfulness Review, Guided Imagery/Exposure to Negative Affect Smoking Situations; and (8) Review of Progress. Participants were also asked to complete daily homework, consisting of daily tracking of smoking, worksheets that reviewed session content, and 10 minutes of mindful breathing.
The topics covered in the HLS intervention (see Bradizza et al., 2017) were: (1) Benefits of a Healthy Lifestyle; (2) Personal Values and Priorities; (3–4) Nutrition; (5) Avoiding Carbon Monoxide Poisoning; (6) Reducing HIV Risk; (7) Balancing Life Roles; and (8) Review of Health and Lifestyle Changes. Homework in this condition involved daily tracking of smoking. monitoring changes in diet and exercise, and worksheets that reviewed the session content. Homework in this condition was designed to be equivalent in time and effort to homework in the ERT condition.
Measures
Demographic variables collected at baseline included age, education, marital status, employment status, income, and receipt of public assistance. Smoking variables collected included age first started smoking, average number of cigarettes per day, and number of prior quit attempts.
Fagerstrom Test of Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) is a six-item scale assessing quantity of cigarette consumption, compulsion to smoke, and dependence. Summing responses yields an ordinal measure of nicotine dependence: very low (0–2), low (3–4), medium (5), high (6–7), or very high (8–10). Cronbach’s α for the FTND in the present study was .58. Though low, it is common for this widely-used measure and may reflect a forced-choice answer format (Fillo, Alfano, Paulus, et al., 2016; Korte, Capron, Zvolensky, & Schmidt, 2013). Despite the low alpha, the FTND is commonly used in smoking cessation studies and, given its relationship to biological indicators of smoking (Heatherton et al., 1991), is regarded as an indicator of nicotine dependence.
Brief Smoking Consequences Questionnaire-Adult (BSCQ-A; Rash & Copeland, 2008) is a 25-item scale assessing adult smoking expectancies. Although the scale contains 10 subscales, only the 3-item negative affect reduction subscale was used to identify negative affect smokers during screening. Consistent with previous research (Copeland, Brandon, & Quinn, 1995), those who scored 5.60 or higher on the subscale were considered negative affect smokers. Negative affect reduction refers to the belief that smoking helps manage negative affect (Brandon & Baker, 1991). Cronbach’s α for the negative affect subscale was .90.
Toronto Alexithymia Scale (TAS-20; Bagby, Parker, & Taylor, 1994) is a 20-item scale that measures alexithymia. Items are rated on a scale from 1 (strongly disagree) to 5 (strongly agree) and summed to form a total score. Scores of 61 or greater indicate alexithymia, 51 to 60 indicate possible alexithymia, and 50 or lower indicate absence of alexithymia (Bagby, Parker, & Taylor, 1994). The TAS-20 total score has been shown to be a reliable and valid measure of alexithymia (Bagby, Parker, & Taylor, 1994). Cronbach’s α for all items in the scale was .72.
Emotion Regulation Questionnaire (ERQ; Gross & John, 2003) is a 10-item measure that contains two subscales: (1) Suppression (α=.73; four items) is the extent to which emotional responses are actively inhibited and is considered to be a maladaptive emotion regulation strategy, and (2) Reappraisal (α=.77; six items) is the extent to which situations are recast to change their emotional impact and is considered to be an adaptive emotion regulation strategy that has been associated with better emotional functioning (Aldao, 2012). Items are rated on a scale from 1 (strongly disagree) to 5 (strongly agree). For both subscales, higher scores indicate greater use of the particular emotion regulation strategy.
Difficulties with Emotion Regulation Scale (DERS; Gratz & Roemer, 2004) is a 36-item measure (α=.86) assessing six domains of emotion regulation difficulties: (1) Nonacceptance of emotional responses (α=.87; six items) is the tendency to experience negative secondary emotions as a result of primary negative emotions, (2) Difficulties with goal-directed behavior (α=.80; five items) refers to struggling with staying focused and accomplishing tasks when experiencing negative emotions, (3) Difficulty controlling impulses (α=.86; six items) is the tendency to remain in control when experiencing negative emotions, (4) Awareness of emotional responses (α=.73; six items) is a tendency to avoid acknowledging and attending to emotions, (5) Strategies to control emotions (α=.88; eight items) is the belief that one has little control over negative emotions, and (6) Clarity of emotional responses (α=.71; five items) is the extent to which individuals struggle to discern emotions they are experiencing. Items are rated on a scale from 1 (almost never) to 5 (almost always). Higher subscale scores or total score indicate greater difficulty with emotion regulation.
Depression, a form of negative affect, was assessed with the 21-item Beck Depression Inventory-II (BDI-II; α=.85; Beck, Steer, & Brown, 1996). Individual items are summed and higher scores indicate more severe symptoms. The BDI-II is considered a reliable and valid measure of depression (Subica et al., 2014).
Anxiety, a second form of negative affect, was assessed with the 21-item item Beck Anxiety Inventory (BAI; α=.92; Beck et al., 1988). Individual items are summed and higher scores indicate more severe levels of anxiety. The BAI is considered a reliable and valid measure of anxiety (Beck et al., 1988).
Multidimensional Anger Inventory (MAI; Siegel, 1986) consists of 38-items in which participants rate agreement with a series of statements on a scale from 1 (undescriptive of me) to 5 (completely descriptive of me). MAI consists of four subscales: (1) Anger eliciting situations (α=.71; nine items) refers to common experiences that elicit anger (for example, “I get angry when someone lets me down”), (2) Hostile outlook (α=.61; six items) is the tendency for anger to emerge as the first emotional response, (3) Anger out (α=.70; five items) is the tendency to express anger outwardly, and (4) Anger in (α=.73; six items) is the tendency to deal with anger internally. Items are rated on a scale from 1 (completely undescriptive of me) to 5 (completely descriptive of me). The MAI has been determined to be a reliable and valid measure of anger (Siegel, 1986).
Timeline Follow Back (Sobell & Sobell, 1995) was used to assess the mean number of cigarettes smoked in the previous seven days at baseline. TLFB uses anchor points and events of personal interest to assist participants in recalling the number of cigarettes smoked on each day. It has been shown to be a reliable measure of cigarettes smoked when administered by trained interviewers (Brown et al., 1998).
Analysis
The first aim of the present research was to assess potential differences in emotion regulation processes and affect based on baseline alexithymia status. Accordingly, independent samples t-tests were conducted using SPSS version 25 (IBM Corp., 2017) to compare group scores on the ERQ, DERS, BDI, BAI, and MAI. We used a Holm-Bonferroni correction (Holm, 1979) to control for multiple comparisons. Holm’s correction rank orders the comparisons from most to least significant and estimates the Bonferroni corrected p-value based on the location of the comparison in the rank order. Comparisons stop once the null hypothesis fails to be rejected (Holm, 1979) and all further comparisons are deemed non-significant. Hedges’ g (Hedges, 1981) was used to estimate effect size, as it is more conservative than Cohen’s d (Ferguson, 2009).
For the second aim, we conducted a path analysis using Mplus version 8 (Muthén & Muthén, 1998–2017) to test the indirect effect of baseline alexithymia on end-of-treatment smoking via difficulties with emotion regulation (DERS total score) measured at baseline and end-of-treatment (see Figure 1). This analysis also controlled for effect of nicotine dependence (FTND score). A bootstrap with 5000 iterations was used to obtain 95% confidence intervals for the indirect effect (Hayes, 2013).
Figure 1.
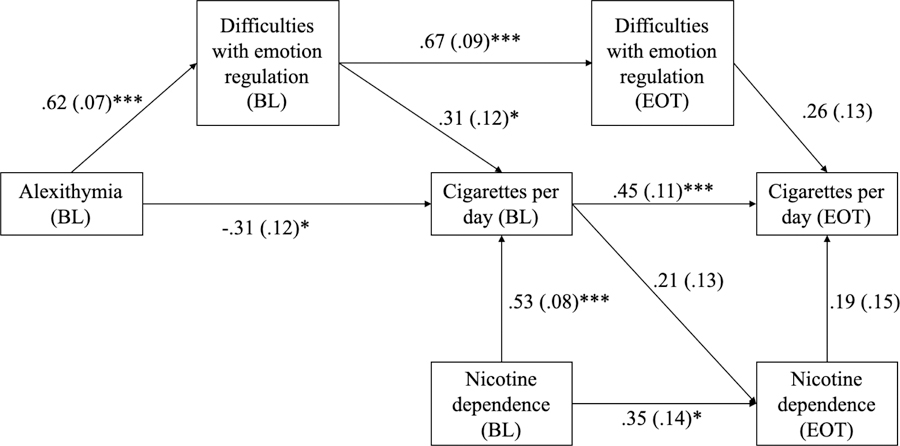
Initial path model with standardized beta weights, standard errors, and significance values depicting relationship of alexithymia, emotion regulation difficulties, and smoking.
Note. BL stands for baseline measurement; EOT stands for end of treatment measurement.
Results
Similar to prior studies examining alexithymia, we combined the possible alexithymia and alexithymia groups (Gilanifar & Delavar, 2016; Parruti et al., 2013). Thus, of the total sample (N = 73), 17 (23.3%) participants with TAS scores greater than 61 indicating alexithymia were combined with 12 (16.5%) participants with TAS scores between 52 and 61 indicating possible alexithymia for a total of 29 (39.8%) participants in the alexithymia group. Additionally, 44 (60.2%) participants with TAS scores less than 51 formed the no alexithymia group. Comparisons were conducted between the alexithymia group (n = 29) and no alexithymia (n = 44) groups. Table 1 contains demographic and smoking information for the full sample and by alexithymia status. There were no significant differences between groups on age, age began smoking, FTND score, and mean number of cigarettes smoked per day in the previous seven days. However, the alexithymia group had significantly more years of education as compared with the no alexithymia group [t(71)=2.91, p=.005]. Alexithymia did not change significantly from baseline to end of treatment for the entire sample (46.50 [sd=11.83] and 44.65 [13.88], respectively) or for either the ERT (47.43 [sd=10.99] and 45.29 [sd=13.80], respectively) or HLS control (45.35 [sd=13.05] and 43.88 [sd=14.36], respectively) conditions.
Table 1.
Means and standard deviations of demographics and other variables in current study
| Full sample | Alexithymia group | No alexithymia group | |
|---|---|---|---|
| N=73 | n=29 | n=44 | |
| Age (y), M (SD) | 24.78 (4.50) | 24.59 (4.63) | 24.91 (4.46) |
| Number of years of education (y), M (SD) | 11.93 (1.90) | 11.17 (1.67)** | 12.43 (1.90)** |
| Race/ethnicity, n (%) | |||
| African American | 32 (43.84) | 14 (48.28) | 18 (40.91) |
| Caucasian | 22 (30.14) | 6 (20.69) | 16 (36.36) |
| Native American | 4 (5.48) | 2 (6.90) | 2 (4.55) |
| Other | 4 (5.48) | 0 (0.00) | 4 (9.09) |
| Marital status, n (%) | |||
| Single, never married | 36 (49.3) | 16 (55.2) | 20 (45.5) |
| Divorced/separated | 6 (8.2) | 3 (10.3) | 3 (6.8) |
| Married/Cohabitating | 22 (30.1) | 7 (24.1) | 15 (34.1) |
| In a relationship, not living together | 9 (12.3) | 3 (10.3) | 6 (13.6) |
| Employment status, n (%) | |||
| Not employed, looking for work | 34 (46.6) | 16 (55.2) | 18 (40.9) |
| Not employed, not looking for work/disability | 12 (16.4) | 7 (24.1) | 5 (11.4) |
| Employed, part-time | 18 (24.7) | 5 (17.2) | 13 (29.5) |
| Employed Full-time/Student | 9 (12.3) | 1 (3.4) | 8 (18.2) |
| Total income last year, n (%) | |||
| 0 to less than $10,000 | 50 (68.5) | 25 (86.2) | 25 (56.8) |
| $10,000 to $20,000 | 15 (20.5) | 1 (3.4) | 14 (31.8) |
| $20,000 or more | 8 (11.0) | 3 (10.3) | 5 (11.4) |
| Receives public assistance, n (%) | 41 (56.2%) | 21 (72.41) | 20 (45.45) |
| Smoking history | |||
| Age began smoking, M (SD) | 14.85 (2.77) | 15.03 (2.61) | 14.73 (2.89) |
| Fagerstrom Test of Nicotine Dependence, M (SD) | 2.15 (1.14) | 3.28 (2.17) | 3.41 (2.49) |
| Cigarettes per day, M (SD) | 7.48 (9.41) | 5.89 (5.18) | 8.52 (11.32) |
| Ever tried to quit smoking, n (%) | 63 (86.3) | 25 (86.21) | 38 (86.38) |
Groups are significantly different at p< .01
Table 2 reports group comparisons tested in the first aim of the study. This includes means, standard deviations, and the results of comparisons of the emotion and emotion regulation variables from the DERS, MAI, ERQ, BAI, and BDI by alexithymia status. Following application of the Holm-Bonferroni correction, the alexithymia group reported significantly higher scores on the DERS total score and also on the Goal, Strategies, Awareness, and Clarity subscales of the DERS. The alexithymia group reported higher Suppression subscale scores of the ERQ. On MAI, the alexithymia group reported significantly higher Anger-arousal, Anger-in and Anger-out subscale and total scores. Lastly, the alexithymia group reported higher scores on the BDI-II.
Table 2.
Results of Holm-corrected t-tests comparing emotion regulation processes by alexithymia status
| Alexithymia | No alexithymia | ||||
|---|---|---|---|---|---|
| Variable | M (sd) | M (sd) | t(71) | Holm corrected p-value | Hedges’ g |
| Difficulties with Emotion Regulation Questionnaire | |||||
| Non-acceptance | 14.82 (6.09) | 11.86 (5.33) | −1.95 | -- | 0.54 |
| Goal | 17.06 (4.78) | 13.70 (4.35) | −2.73 | .007** | 0.76 |
| Impulse | 14.82 (5.09) | 12.05 (5.24) | −1.92 | -- | 0.53 |
| Strategies | 21.80 (7.57) | 16.83 (5.25) | −2.531 | .005** | 0.85 |
| Aware | 17.41 (4.14) | 13.35 (4.31) | −3.43 | .003** | 0.95 |
| Clarity | 13.06 (2.93) | 9.66 (3.41) | −3.71 | .003** | 1.03 |
| Total | 99.53 (21.91) | 75.98 (20.01) | −4.16 | .03* | 1.15 |
| Emotion Regulation Questionnaire | |||||
| Reappraisal | 27.65 (7.52) | 29.57 (6.74) | 1 | -- | 0.28 |
| Suppression | 16.18 (5.07) | 12.39 (5.82) | −2.42 | .006** | 0.67 |
| Multidimensional Anger Inventory | |||||
| Anger-arousal | 27.29 (5.25) | 20.79 (7.53) | −3.32 | .003** | 0.92 |
| Anger-eliciting | 24.89 (5.28) | 24.27 (6.19) | −0.19 | -- | 0.05 |
| Hostile Outlook | 13.18 (3.47) | 12.95 (4.16) | −0.21 | -- | 0.06 |
| Anger-in | 17.12 (3.98) | 13.34 (4.88) | −2.91 | .004** | 0.81 |
| Anger-out | 6.06 (1.82) | 7.86 (2.01) | 3.28 | .004** | 0.91 |
| Total | 131.06 (14.76) | 115.34 (23.01) | −2.65 | .006** | 0.73 |
| Beck Anxiety Inventory | 22.65 (13.50) | 17.84 (13.16) | −1.31 | -- | 0.36 |
| Beck Depression Inventory | 26.12 (9.19) | 19.88 (9.69) | −2.35 | .007** | 0.65 |
p < .05
p < .01
Note. Effect sizes for Hedge’s g can be interpreted as .30 = small; .50 = medium; .80 = large.
Results of the path analysis
Figure 1 depicts the path model tested in the second aim of this study. The path model had adequate fit to the data (CFI = 1.00; TLI = 1.01; χ2 = n.s; Geiser 2013), but two paths were not significant: end of treatment nicotine dependence to end of treatment smoking, and end of treatment difficulties with emotion regulation to end of treatment smoking. End of treatment nicotine dependence did not have a significant effect on any variables.
The model was re-tested after trimming these non-significant pathways. Model fit remained in excess of established norms (CFI = 1.00; TLI = 1.07; χ2 = n.s; Gesier, 2013). All paths in the model were significant. Figure 2 depicts the final path model and displays standardized beta weights, standard errors, and significance tests for each pathway.
Figure 2.
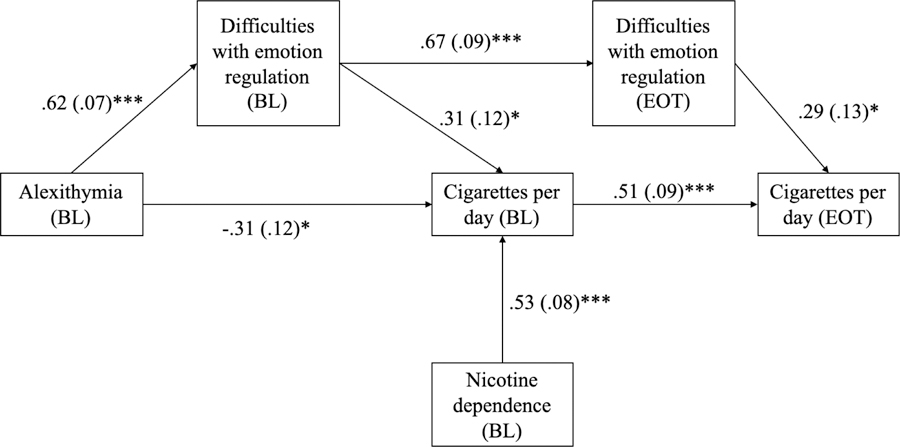
Trimmed path model with standardized beta weights, standard errors, and significance values depicting relationship of alexithymia, emotion regulation difficulties, and smoking.
Note. Indirect effect of alexithymia on mean cigarettes via baseline and end of treatment difficulties with emotion regulation was significant following a bootstrap with 5000 iterations (β = .12; 95% CI [.01–.24]). BL stands for baseline measurement; EOT stands for end of treatment measurement.
A bootstrap with 5000 iterations was run on the final model presented in Figure 2. Baseline alexithymia had a significant indirect effect on end of treatment smoking through baseline difficulties with emotion regulation and end of treatment difficulties with emotion regulation (β = .12; 95% CI [.01, 24]).
The full path model accounted for 34% (p < .000) of the variance in smoking at baseline and 38% (p < .000) of the variance in smoking at end of treatment. Baseline nicotine dependence alone accounted for 28% of the variance in baseline smoking. Adding baseline alexithymia and difficulties in emotion regulation accounted for an additional 7% of the variance and represented a statistically significant increase (p = .01) over variance in baseline smoking accounted for by baseline nicotine dependence alone.
Discussion
Understanding the effects of alexithymia on emotion regulation may assist researchers and clinicians in adapting and refining behavioral interventions that could ultimately lead to improved smoking cessation outcomes for women attempting to quit smoking during pregnancy. To that end, the present research sought to: (1) assess potential differences in emotion regulation processes between pregnant smokers with and without alexithymia and (2) determine the indirect effect of alexithymia on smoking behavior via difficulties with emotion regulation. After a Holm-Bonferroni correction, results indicate that pregnant smokers with alexithymia score higher than those without alexithymia on measures of difficulties with emotion regulation and negative affect. Additionally, this study is the first known study to empirically and longitudinally examine the impact of alexithymia on smoking outcomes among pregnant smokers and found that emotion regulation difficulties mediated the relationship of alexithymia and smoking. The results of this analysis clarify the relationship between alexithymia and maladaptive coping strategies and illuminate clinical intervention targets.
Alexithymia may make quitting smoking especially difficult given that it restricts access to emotional information necessary for selection and implementation of adaptive emotion regulation (Taylor, 2018). Pregnant smokers report significant negative affect including depression, relationship problems, stressors and uncertainties associated with low socioeconomic status (Moore et al., 2016; Riaz, Lewis, Naughton, & Ussher, 2018; Smedberg, Lupattelli, Mårdby, Øverland, & Nordeng, 2015; Yukiko, Kunihiko, & Setsuko, 2015). Pregnancy is also a significant stressor for smokers (Schneider, Huy, Schütz, & Diehl, 2010). Among pregnant women, particularly those with low incomes, smoking may provide a brief respite during times of stress. However, given that access to emotional information is restricted for individuals with alexithymia, negative affect is experienced as an unpleasant bodily sensation rather than as an emotion (Betka et al., 2018; Mueller & Alpers, 2006). Smoking may be an attempt to manage this undifferentiated aversive bodily sensation. For an alexithymic individual, these undifferentiated, aversive bodily sensations are experienced whenever negative affect is encountered (Pollatos & Herbert, 2018; Porcelli & Taylor, 2018). Thus, long-term reliance on smoking as a preferred method for managing these aversive bodily sensations may make smoking cessation particularly difficult.
Previous research has shown that alexithymia results in increased use of unhealthy coping behaviors, including smoking (Carton, Bayard, Jouanne, & Lagrue, 2008; Peters & Lumley, 2007), though this relationship is poorly understood. The current results extend this literature by illuminating a possible pathway by which alexithymia motivates continued smoking. In our study, baseline alexithymia had a significant and positive indirect effect on end of treatment smoking through baseline and end-of-treatment difficulties with emotion regulation. The indirect effect of alexithymia is positive, indicating that alexithymia is associated with greater emotion regulation difficulties, which are in turn, associated with greater smoking. These findings are consistent with other studies of substance use disorders and alexithymia (Morie & Ridout, 2018). This model of alexithymia is consistent with the prevailing view that it impairs the regulation of emotions, which in turn motivates smoking behavior (Bonnet, Bréjard, & Pedinielli, 2013; Lyvers, Brown, & Thorberg, 2018; Zdankiewicz-Ścigała & Ścigała, 2018).
A model that incorporates emotion regulation as a mediator of the relationship between alexithymia and smoking also has valuable clinical utility. There is a substantial body of evidence indicating that emotion regulation is modifiable through clinical intervention (Fairholme, Boisseau, Ellard, Ehrenreich, & Barlow, 2010; Fehlinger, Stumpenhorst, Stenzel, & Rief, 2013; Stasiewicz et al., 2012). Thus, current findings highlight emotion regulation as a promising clinical target in a population that has demonstrated poor smoking cessation outcomes with a range of cognitive and behavioral smoking cessation interventions (Jones, Lewis, Parrott, Wormall, & Coleman, 2016; Ussher et al., 2012).
The identification of emotion regulation as a clinical target is particularly important given the ongoing controversy regarding whether or not alexithymia is modifiable through clinical intervention (de Haan, van der Palen, Wijdeveld, Buitelaar, & De Jong, 2014; Ogrodniczuk, Kealy, Hadjipavlou, & Cameron, 2018; Silva, Vasco, & Watson, 2017). In the absence of rigorous evidence indicating that alexithymia can be modified, targeting the specific domains of emotion regulation via existing therapeutic techniques may help increase quit rates for pregnant smokers (e.g., Bradizza et al., 2017). In the present study, alexithymia and diffculties with emotion regulation accounted for a significant portion of the variance over and above nicotine dependence. The unique variance accounted for by these variables suggests that interventions aimed at ameloriating emotion regulation difficulties could result in meaningful changes in rates of smoking cessation.
Alexithymia may also account for some of the challenges noted in engaging low socioeconomic status pregnant smokers in smoking cessation treatments (Giatras et al., 2017). The results of this study suggest that pregnant women with alexithymia may present with greater levels of anger and depression and thus may be more difficult for clinicians to engage in treatment (Probst et al., 2017; Quilty, Taylor, McBride, & Bagby, 2017). Therefore, additional efforts on the part of clinicians may be required to establish a strong therapeutic alliance. Given the prevalence of alexithymia among smokers (Carton et al., 2008), and among individuals who have experienced adversity (Oyefeso et al., 2008), screening for alexithymia among pregnant smokers, and among all smokers, may help to identify those in need of specialized clinical attention.
Despite a number of study strengths, including temporal precedence of variables in the path analysis and cotinine (a metabolite of nicotine) verification of smoking status at baseline and end of treatment, this study is not without limitations. Data were drawn from women in a single mid-sized northeastern U.S. city and may not generalize to all pregnant smokers. In addition, given the small sample size, findings may not generalize to all pregnant smokers. Additionally, data may contain self-report bias given that women recruited for this study led stressful lives and it was common for them to report feeling they needed to rush through research assessments, which is consistent with other reports of studies with this population (Giatras et al., 2017). To reduce self-report bias, this study used trained interviewers and participants were assured of confidentiality. Concerns regarding self-report may also be mitigated by a broad consensus that smoking can be reliably assessed via self-report (Blank et al., 2016; Kenkel, Lillard, & Mathios, 2003) when conducted in the context of a research study (Babor, Stephens, & Marlatt, 1987).
Conclusion
The results of this study suggest that pregnant smokers with alexithymia have greater difficulties with emotion regulation, and engage in more maladaptive emotion regulation strategies, relative to those without alexithymia. They also experience high levels of negative affect, making them more challenging to engage in treatment. Lastly, results suggest that difficulties with emotion regulation play a key role in the link between alexithymia and smoking. Taken together, these findings suggest that bolstering adaptive emotion regulation strategies would be a fruitful direction for future interventions. The opportunity to contribute to public health by assisting pregnant smokers with quitting is significant and requires immediate attention from scholars.
Acknowledgements:
This research was supported in part y R01 DA 021802 from the National Institute on Drug Abuse and the Office of Research on Women’s Health. Preparation of this manuscript was partially supported by T32 AA007583 and K01 AA027547 from the National Institute on Alcohol Abuse and Alcoholism.
References
- Abrams DB, Niaura R, Brown RA, M., E. K., Goldstein MG, & Monti PM (2003). The Tobacco Dependence Handbook: Guide to Best Practices. New York: The Guilford Press. [Google Scholar]
- Aldao A (2012). Emotion regulation strategies as transdiagnostic processes: A closer look at the invariance of their form and function. Revista de Psicopatologia y Psicologia Clinica 17(3), 261–277. doi: 10.5944/rppc.vol.17.num.3.2012.11843 [DOI] [Google Scholar]
- Babor TF, Stephens RS, & Marlatt GA (1987). Verbal report methods in clinical research on alcoholism: response bias and its minimization. Journal of Studies on Alcohol, 48, 410–424. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Parker JDA, & Taylor GJ (1994). The twenty-item Toronto Alexithymia Scale: I Item selection and cross-validation of the factor structure. Journal of Psychosomatic Research, 38(1), 23–32. [DOI] [PubMed] [Google Scholar]
- Banderali G, Martelli A, Landi M, Moretti F, Betti F, Radaelli G, … Verduci E (2015). Short and long term health effects of parental tobacco smoking during pregnancy and lactation: a descriptive review. Journal of Translational Medicine, 13, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, & Steer RA (1988). An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology, 56(6), 893–897. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Betka S, Pfeifer G, Garfinkel S, Prins H, Bond R, Sequeira H, … Critchley H (2018). How Do Self-Assessment of Alexithymia and Sensitivity to Bodily Sensations Relate to Alcohol Consumption? Alcoholism, Clinical And Experimental Research, 42(1), 81–88. doi: 10.1111/acer.13542 [DOI] [PubMed] [Google Scholar]
- Bittoun R, & Femia G (2010). Smoking cessation in pregnancy. Obstetric Medicine (1753–495X), 3(3), 90–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank MD, Breland AB, Enlow PT, Duncan C, Metzger A, & Cobb CO (2016). Measurement of smoking behavior: Comparison of self-reports, returned cigarette butts, and toxicant levels. Experimental and Clinical Psychopharmacology, 24(5), 348–355. doi: 10.1037/pha0000083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet A, Bréjard V, & Pedinielli J-L (2013). Emotional dispositions and substance use: Mediating effect of alexithymia. Psychological Reports, 112(1), 289–302. doi: 10.2466/18.09.20.PR0.112.1.289-302 [DOI] [PubMed] [Google Scholar]
- Bradizza CM, Stasiewicz PR, Yue Z, Ruszczyk M, Maisto SA, Lucke JF, … Zhuo Y (2017). Smoking cessation for pregnant smokers: Development and pilot test of an emotion regulation treatment supplement to standard smoking cessation for negative affect smokers. Nicotine & Tobacco Research, 19(5), 578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon TH, & Baker TB (1991). The Smoking Consequences Questionnaire: The subjective expected utility of smoking in college students. Psychological Assessment: A Journal of Consulting and Clinical Psychology, 3(3), 484–491. doi: 10.1037/1040-3590.3.3.484 [DOI] [Google Scholar]
- Britton GR, James GD, Collier R, Sprague LM, & Brinthaupt J (2013). The effects of smoking cessation and a programme intervention on birth and other perinatal outcomes among rural pregnant smokers. Annals of Human Biology, 40(3), 256–265. [DOI] [PubMed] [Google Scholar]
- Brooks DR, Burtner JL, Borrelli B, Heeren TC, Evans T, Davine JA, … Geller AC (2018). Twelve-month outcomes of a group-randomized community health advocate-led smoking cessation intervention in public housing. Nicotine & Tobacco Research, 20(12), 1434–1441. doi: 10.1093/ntr/ntx193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, & Miller IW (1998). Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors, 12(2), 101–112. doi: 10.1037/0893-164X.12.2.101 [DOI] [Google Scholar]
- Carton S, Bayard S, Jouanne C, & Lagrue G (2008). Emotional awareness and alexithymia in smokers seeking help for cessation: A clinical analysis. Journal of Smoking Cessation, 3(2), 81–91. [Google Scholar]
- Cnattingius S (2004). The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine & Tobacco Research, 6, S125–S140. [DOI] [PubMed] [Google Scholar]
- Coleman T, Chamberlain C, Cooper S, & Leonardi-Bee J (2011). Efficacy and safety of nicotine replacement therapy for smoking cessation in pregnancy: systematic review and meta-analysis. Addiction, 106(1), 52–61. [DOI] [PubMed] [Google Scholar]
- Copeland AL, Brandon TH, & Quinn EP (1995). The Smoking Consequences Questionnaire—Adult: Measurement of smoking outcome expectancies of experienced smokers. Psychological Assessment, 7(4), 484–494. doi: 10.1037/1040-3590.7.4.484 [DOI] [Google Scholar]
- de Haan HA, van der Palen J, Wijdeveld TGM, Buitelaar JK, & De Jong CAJ (2014). Alexithymia in patients with substance use disorders: State or trait? Psychiatry Research, 216(1), 137–145. [DOI] [PubMed] [Google Scholar]
- Dias-Damé JL, & Cesar JA (2015). Disparities in prevalence of smoking and smoking cessation during pregnancy: A population-based study. BioMed Research International, 2015, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairholme CP, Boisseau CL, Ellard KK, Ehrenreich JT, & Barlow DH (2010). Emotions, emotion regulation, and psychological treatment: A unified perspective In Kring AM & Sloan DM (Eds.), Emotion Regulation and Psychopathology: A Transdiagnotic Approach to Etiology and Treatment. New York: The Guilford Press. [Google Scholar]
- Fehlinger T, Stumpenhorst M, Stenzel N, & Rief W (2013). Emotion regulation is the essential skill for improving depressive symptoms. Journal of Affective Disorders, 144(1–2), 116–122. doi: 10.1016/j.jad.2012.06.015 [DOI] [PubMed] [Google Scholar]
- Ferguson CJ (2009). An effect size primer: A guide for clinicians and researchers. Professional Psychology: Research and Practice, 40(5), 532–538. [Google Scholar]
- Fillo J, Alfano CA, Paulus DJ, Smits JAJ, Davis ML, Rosenfield D, … Zvolensky MJ (2016). Emotion dysregulation explains relations between sleep disturbance and smoking quit-related cognition and behavior. Addictive Behaviors, 57, 6–12. doi: 10.1016/j.addbeh.2016.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser C (2013). Data Analysis with MPlus. New York: The Guilford Press. [Google Scholar]
- Giatras N, Wanninkhof E, Leontowitsch M, Lewis B, Taylor A, Cooper S, & Ussher M (2017). Lessons learned from the London Exercise and Pregnant (LEAP) Smokers randomised controlled trial process evaluation: implications for the design of physical activity for smoking cessation interventions during pregnancy. BMC Public Health, 17(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilanifar M, & Delavar MA (2016). Alexithymia in pregnant women: Its relationship with depression. ASEAN Journal of Psychiatry, 17(1), 35–41. [Google Scholar]
- Godleski SA, Shisler S, Eiden RD, & Huestis MA (2018). Co-use of tobacco and marijuana during pregnancy: Pathways to externalizing behavior problems in early childhood. Neurotoxicology and Teratology, 69, 39–48. doi: 10.1016/j.ntt.2018.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham H, Hawkins SS, & Law C (2010). Lifecourse influences on women’s smoking before, during and after pregnancy. Social Science & Medicine, 70(4), 582–587. [DOI] [PubMed] [Google Scholar]
- Gratz KL, & Roemer L (2004). Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the Difficulties in Emotion Regulation Scale. Journal of Psychopathology & Behavioral Assessment, 26(1), 41–54. [Google Scholar]
- Griffiths SE, Brown KE, Fulton EA, Tombor I, & Naughton F (2016). Are digital interventions for smoking cessation in pregnancy effective? A systematic review protocol. Systematic Reviews, 5, 1–8. doi: 10.1186/s13643-016-0390-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, & John OP (2003). Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality & Social Psychology, 85(2), 348–362. [DOI] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to Mediation, Moderation, and Conditional Process Analysis. New York: The Guilford Press. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, & Fagerstrom K-O (1991). The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction, 86(9), 1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- Hedges LV (1981). Distribution theory for Glass’s estimator of effect size and related estimators. Journal of Educational Statistics, 6(2), 107–128. [Google Scholar]
- Hernández-Martínez C, Voltas Moreso N, Ribot Serra B, Arija Val V, Escribano Macías J, & Canals Sans J (2017). Effects of Prenatal Nicotine Exposure on Infant Language Development: A Cohort Follow Up Study. Maternal & Child Health Journal, 21(4), 734–744. doi: 10.1007/s10995-016-2158-y [DOI] [PubMed] [Google Scholar]
- Holm S (1979). A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. Theory and Applications, 6(2), 65. [Google Scholar]
- IBM Corporation. (2017). IBM SPSS Statistics for Macintosh (Version 25) [Computer software]. Armonk, NY: IBM Corporation. [Google Scholar]
- Jones M, Lewis S, Parrott S, Wormall S, & Coleman T (2016). Re-starting smoking in the postpartum period after receiving a smoking cessation intervention: a systematic review. Addiction, 111(6), 981–990. doi: 10.1111/add.13309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel D, Lillard DR, & Mathios A (2003). Smoke or fog? The usefulness of retrospectively reported information about smoking. Addiction, 98(9), 1307–1313. [DOI] [PubMed] [Google Scholar]
- Korte KJ, Capron DW, Zvolensky M, & Schmidt NB (2013). The Fagerström Test for Nicotine Dependence: Do revisions in the item scoring enhance the psychometric properties? Addictive Behaviors, 38(3), 1757–1763. doi: 10.1016/j.addbeh.2012.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Miller S, Wen K-Y, Hui S. k., Roussi P, & Hernandez E (2015). Cognitive-behavioral intervention to promote smoking cessation for pregnant and postpartum inner city women. Journal of Behavioral Medicine, 38(6), 932–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Hansen AR, McGalliard Z, Gover L, Yan F, & Zhang J (2018). Trends in smoking and smoking cessation during pregnancy from 1985 to 2014: Racial and ethnic disparity observed from multiple national surveys. Maternal & Child Health Journal, 22(5), 685–693. [DOI] [PubMed] [Google Scholar]
- Lyvers M, Brown T, & Thorberg FA (2018). Is it the taste or the buzz? Alexithymia, caffeine, and emotional eating. Substance Use & Misuse. [DOI] [PubMed]
- Mantzari E, Vogt F, & Marteau TM (2012). The effectiveness of financial incentives for smoking cessation during pregnancy: is it from being paid or from the extra aid? BMC Pregnancy And Childbirth, 12, 24–24. doi: 10.1186/1471-2393-12-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E, Blatt K, Aimin C, Van Hook J, & DeFranco EA (2016). Factors associated with smoking cessation in pregnancy. American Journal of Perinatology, 33(6), 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morie KP, & Ridout N (2018). Alexithymia and maladaptive regulatory behaviors in substance use disorders and eating disorders In Luminet O, Bagby RM, & Taylor GJ (Eds.), Alexithymia: Advances in Research, Theory, and Clinical Practice. Cambridge, United Kingdom: Cambridge University Press [Google Scholar]
- Mueller J, & Alpers GW (2006). Two facets of being bothered by bodily sensations: Anxiety sensitivity and alexithymia in psychosomatic patients. Comprehensive Psychiatry, 47(6), 489–495. doi: 10.1016/j.comppsych.2006.03.001 [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998–2017). Mplus User’s Guide (8th Edition). Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Ogrodniczuk JS, Kealy D, Hadjipavlou GA, & Cameron K (2018). Therapeutic Issues In Luminet O, Bagby RM, & Taylor GJ (Eds.), Alexithymia: Advances in Research, Theory, and Clinical Practice. Cambridge, United Kingdom: Cambridge University Press. [Google Scholar]
- Oyefeso A, Brown S, Chiang Y, & Clancy C (2008). Self-injurious behaviour, traumatic life events and alexithymia among treatment-seeking opiate addicts: Prevalence, pattern and correlates. Drug & Alcohol Dependence, 98(3), 227–234. [DOI] [PubMed] [Google Scholar]
- Parruti G, Vadini F, Sozio F, Mazzott E, Ursini T, Polill E, … Manzoli L (2013). Psychological factors, including alexithymia, in the prediction of cardiovascular risk in HIV infected patients: Results of a cohort study. PLoS ONE, 8(1), 1–12. doi: 10.1371/journal.pone.0054555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters RM, & Lumley MA (2007). Relationship of alexithymia to cardiovascular disease risk factors among African Americans. Comprehensive Psychiatry, 48(1), 34–41. [DOI] [PubMed] [Google Scholar]
- Probst T, Sattel H, Gündel H, Henningsen P, Kruse J, Schneider G, & Lahmann C (2017). Moderating effects of alexithymia on associations between the therapeutic alliance and the outcome of brief psychodynamic-interpersonal psychotherapy for multisomatoform disorder. Frontiers in Psychiatry, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O & Herbert BM (2018). Alexithymia and Body Awareness In Luminet O, Bagby RM, & Taylor GJ (Eds.), Alexithymia: Advances in Research, Theory, and Clinical Practice. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Porcelli P, & Taylor GJ (2018). Alexithymia and Physical Illness: A Psychosomatic Approach In Luminet O, Bagby RM, & Taylor GJ (Eds.), Alexithymia: Advances in Research, Theory, and Clinical Practice. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Quilty LC, Taylor GJ, McBride C, & Bagby RM (2017). Relationships among alexithymia, therapeutic alliance, and psychotherapy outcome in major depressive disorder. Psychiatry Research, 254, 75–79. [DOI] [PubMed] [Google Scholar]
- Rash CJ, & Copeland AL (2008). The Brief Smoking Consequences Questionnaire-Adult (BSCQ-A): Development of a short form of the SCQ-A. Nicotine & Tobacco Research, 10(11), 1633–1643. [DOI] [PubMed] [Google Scholar]
- Riaz M, Lewis S, Naughton F, & Ussher M (2018). Predictors of smoking cessation during pregnancy: a systematic review and meta‐analysis. Addiction, 113(4), 610–622. [DOI] [PubMed] [Google Scholar]
- Schneider S, Huy C, Schütz J, & Diehl K (2010). Smoking cessation during pregnancy: A systematic literature review. Drug & Alcohol Review, 29(1), 81–90. [DOI] [PubMed] [Google Scholar]
- Siegel JM (1986). The Multidimensional Anger Inventory. Journal of Personality and Social Psychology, 51(1), 191–200. doi: 10.1037/0022-3514.51.1.191 [DOI] [PubMed] [Google Scholar]
- Sifneos PE (1973). The prevalence of ‘Alexithymic’ characteristics in psychosomatic patients. Psychotherapy and psychosomatics, 22(2), 255–262. [DOI] [PubMed] [Google Scholar]
- Silva AN, Vasco AB, & Watson JC (2017). Alexithymia and emotional processing: A mediation model. Journal of Clinical Psychology, 73(9), 1196–1205. [DOI] [PubMed] [Google Scholar]
- Smedberg J, Lupattelli A, Mårdby A-C, Øverland S, & Nordeng H (2015). The relationship between maternal depression and smoking cessation during pregnancy-a cross-sectional study of pregnant women from 15 European countries. Archives of Women’s Mental Health, 18(1), 73–84. doi: 10.1007/s00737-014-0470-3 [DOI] [PubMed] [Google Scholar]
- Sobell LC, & Sobell MB (1995). Alcohol Timeline Followback Users’ Manual. Toronto, Canada: Addiction Research Foundation. [Google Scholar]
- Stasiewicz PR, Bradizza CM, Gudleski GD, Coffey SF, Schlauch RC, Bailey ST, … Gulliver SB (2012). The relationship of alexithymia to emotional dysregulation within an alcohol dependent treatment sample. Addictive Behaviors, 37(4), 469–476. doi: 10.1016/j.addbeh.2011.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su A, & Buttenheim A (2014). Maintenance of smoking cessation in the postpartum period: Which interventions work best in the long-term? Maternal & Child Health Journal, 18(3), 714–728. [DOI] [PubMed] [Google Scholar]
- Subica AM, Fowler JC, Elhai JD, Frueh BC, Sharp C, Kelly EL, & Allen JG (2014). Factor structure and diagnostic validity of the Beck Depression Inventory-II with adult clinical inpatients: Comparison to a gold-standard diagnostic interview. Psychological Assessment, 26(4), 1106–1115. doi: 10.1037/a0036998 [DOI] [PubMed] [Google Scholar]
- Taylor GJ (2018). History of Alexithymia: The Contributions of Psychoanalysis In Bagby OLRM & Taylor GJ (Eds.), Alexithymia: Advances in Research, Theory, and Clinical Practice. Cambridge, United Kingdom: Cambridge University Press. [Google Scholar]
- Ussher M, Aveyard P, Manyonda I, Lewis S, West R, Lewis B, … Coleman T (2012). Physical activity as an aid to smoking cessation during pregnancy (LEAP) trial: study protocol for a randomized controlled trial. Trials, 13(1), 186–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukiko M, Kunihiko H, & Setsuko I (2015). Smoking cessation in pregnancy: psychosocial interventions and patient-focused perspectives. International Journal of Women’s Health, 7, 415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdankiewicz-Ścigała E, & Ścigała DK (2018). Relationship between attachment style in adulthood, alexithymia, and dissociation in alcohol use disorder inpatients: Mediational model. Frontiers In Psychology, 9, 2039–2039. doi: 10.3389/fpsyg.2018.02039 [DOI] [PMC free article] [PubMed] [Google Scholar]


