The physical and chemical barrier provided by the cell wall is one of the first lines of defense against pathogen attack for plants. Plant cell walls provide physical protection against biotic and abiotic stresses but are also involved in a sophisticated sensing system for detecting both microbe-associated molecular patterns (MAMPs), such as flagellin (flg), and damage-associated molecular patterns (DAMPs), such as cell wall fragments, at the cell surface (Rui and Dinneny, 2020). This type of immune response is known as pattern recognition receptor-triggered immunity (PTI) and is distinct from nucleotide-binding, Leucine-rich-repeat triggered immunity (NTI), where pathogen-derived effectors are detected by receptors inside the cell. The organization of defense responses requires complex coordination between cell walls and the interior of the cell to activate downstream processes and so sensing and signaling between these two compartments is crucial. To date, few proteins that link these compartments have been identified. Understanding how signals are transduced between these compartments is of great interest to the study of pathogen resistance in plants and may reveal novel targets for crop breeding.
Plant cell wall-associated kinases (WAKs) are receptor-like kinases present in multiple plant species (Saintenac et al., 2018; Yang et al., 2019), possessing an extracellular domain and an intercellular domain that span the plasma membrane. WAK extracellular domains bind tightly to cell wall pectin (Decreux and Messiaen, 2005) and they are thought to transduce signals from the cell wall that, in part, regulate cell expansion (Wagner and Kohorn, 2001). More recently, it has been shown that WAKs have a higher affinity for small pectic oligosaccharides (such as those released during cell wall degradation) than for long-chain pectin (Kohorn et al., 2014). This has led to the suggestion that WAKs are involved in sensing cell wall integrity (Rui and Dinneny, 2020), and several strands of evidence implicate them in pathogen responses (Rosli et al., 2013; Saintenac et al., 2018; Yang et al., 2019).
In this issue of Plant Physiology, Zhang et al. (2020) report an in-depth analysis of the role of tomato (Solanum lycopersicum) WAK1 in responses to Pseudomonas syringae pv. tomato (Pst) and identify putative interaction partners. Previous work from the group implicated WAK1 in pathogen responses (Rosli et al., 2013), and in this study the authors built on that using a CRISPR/Cas9 approach to generate homozygous knockouts of WAK1 in tomato for detailed characterization.
The authors first demonstrate that WAK1 is involved in PTI responses and not NTI by using two mutant strains of Pst. When wak1 plants were challenged with a strain of Pst that is unable to activate NTI immunity, they showed enhanced disease symptoms compared to wild type, indicating that the mutant’s PTI response was deficient. By contrast, when compared to wild-type Pst, no difference was seen using Pst lacking flg, a well-known MAMP, which therefore does not activate PTI. To confirm that WAK1 is involved in flg-mediated/PTI immune responses, plants were primed using synthetic flg and then challenged using Pst lacking flg. After two d, primed wild-type plants had significantly lower bacterial counts than unprimed plants, whereas priming had no effect in wak1 mutants.
It appears that wak1 mutants are not affected in their early PTI responses such as mitogen-activated protein kinase activation or the production of reactive oxygen species, but instead are strongly impaired in later responses. Specifically, the deposition of the cell-wall component callose was greatly reduced in wak1 mutants. This suggests that WAK1 is functioning at the later stages of PTI immunity, which is consistent with previous suggestions that WAKs are involved in a cell wall integrity-sensing mechanism that monitors the status of the cell wall (Kohorn, 2015; Rui and Dinneny, 2020).
The authors also showed that flg treatment leads to an increase in WAK1 expression, and that this is dependent on the pattern recognition receptors FLS2 and FLS3. In fls2/3 knockout plants, disease symptoms occur more rapidly than in wak1 plants, suggesting that FLS genes act upstream of WAK1. To decipher the biochemical relationship among FLS2, FLS3, and WAK1, the authors transiently expressed these proteins in Nicotiana benthamiana plants and carried out coimmunoprecipitation. An interaction was found between WAK1 and both of the FLS proteins, indicating that they are within the same protein complex, but that their interaction is independent of the presence of flg.
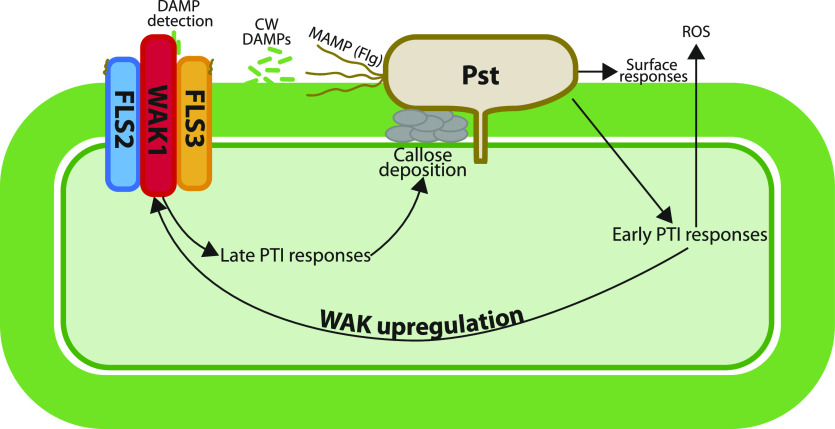
A putative model for WAK1 action is shown in Figure 1; it seems likely that expression of WAK1 is upregulated during early-stage PTI responses and acts in a complex with FLS2 and FLS3 to mediate late-stage PTI responses such as callose deposition. It is not clear yet exactly which signals WAK1 is detecting in tomato plants to stimulate callose deposition but, given our understanding of WAK1 in other species, it is likely to be perceiving cell-wall–damage products such as pectin oligosaccharides that arise in the apoplast after pathogen attack (Kohorn et al., 2014).
Figure 1.
Putative model of WAK1 function in pattern-recognition PTI to Pst. Early responses to Pst, such as the production of reactive oxygen species (ROS), are activated after the detection of the MAMP flg by the receptors FLS2 and FLS3. This leads to the upregulation of WAK1, which forms a complex with FLS2 and FLS3. WAK detects cell-wall (CW) DAMPs and activates late-stage PTI responses including callose deposition into the cell wall. Figure redrawn from figure 9 of Zhang et al. (2020).
Zhang et al. (2020) provide an in-depth analysis of the role of WAK1 in tomato immunity to Pst. This study sheds some light on the workings of this fascinating but little understood group of receptor-like kinases that may provide a crucial signaling link between the cell wall and the cytosol. The economic impact of crop losses in tomato due to Pst infection can be substantial, and a better understanding of how resistance occurs could lead to novel breeding targets for developing pathogen-resistant cultivars.
This work raises additional questions. For example, the mechanism of WAK1 transcriptional activation during late-stage PTI responses in tomato remains unknown. It is also not yet clear why WAK1 acts later in the PTI response—is it because the protein levels are too low in these early stages, or could its activity require some signals generated in the early stage? For example, the movement of Ca2+ from the apoplast into the cytosol is an important early response in PTI (Ranf et al., 2011). This movement of Ca2+ leads to alkalization of the cell wall, which alters the activity of pectin-remodeling enzymes (Hocq et al., 2017), possibly promoting the generation of the pectic oligosaccharides that are characteristic of the DAMP signals to which WAK1 is known to bind. Piecing together the missing steps in this important defense response will be essential for informing future breeding strategies for developing pathogen-resistant crops.
References
- Decreux A, Messiaen J(2005) Wall-associated kinase WAK1 interacts with cell wall pectins in a calcium-induced conformation. Plant Cell Physiol 46: 268–278 [DOI] [PubMed] [Google Scholar]
- Hocq L, Pelloux J, Lefebvre V(2017) Connecting homogalacturonan-type pectin remodeling to acid growth. Trends Plant Sci 22: 20–29 [DOI] [PubMed] [Google Scholar]
- Kohorn BD.(2015) The state of cell wall pectin monitored by wall associated kinases: A model. Plant Signal Behav 10: e1035854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohorn BD, Kohorn SL, Saba NJ, Martinez VM(2014) Requirement for pectin methyl esterase and preference for fragmented over native pectins for wall-associated kinase-activated, EDS1/PAD4-dependent stress response in Arabidopsis. J Biol Chem 289: 18978–18986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranf S, Eschen-Lippold L, Pecher P, Lee J, Scheel D(2011) Interplay between calcium signalling and early signalling elements during defence responses to microbe- or damage-associated molecular patterns. Plant J 68: 100–113 [DOI] [PubMed] [Google Scholar]
- Rosli HG, Zheng Y, Pombo MA, Zhong S, Bombarely A, Fei Z, Collmer A, Martin GB(2013) Transcriptomics-based screen for genes induced by flagellin and repressed by pathogen effectors identifies a cell wall-associated kinase involved in plant immunity. Genome Biol 14: R139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui Y, Dinneny JR(2020) A wall with integrity: Surveillance and maintenance of the plant cell wall under stress. New Phytol 225: 1428–1439 [DOI] [PubMed] [Google Scholar]
- Saintenac C, Lee W-S, Cambon F, Rudd JJ, King RC, Marande W, Powers SJ, Bergès H, Phillips AL, Uauy C, et al. (2018) Wheat receptor-kinase-like protein Stb6 controls gene-for-gene resistance to fungal pathogen Zymoseptoria tritici. Nat Genet 50: 368–374 [DOI] [PubMed] [Google Scholar]
- Wagner TA, Kohorn BD(2001) Wall-associated kinases are expressed throughout plant development and are required for cell expansion. Plant Cell 13: 303–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Praz C, Li B, Singla J, Robert CAM, Kessel B, Scheuermann D, Lüthi L, Ouzunova M, Erb M, et al. (2019) Fungal resistance mediated by maize wall-associated kinase ZmWAK-RLK1 correlates with reduced benzoxazinoid content. New Phytol 221: 976–987 [DOI] [PubMed] [Google Scholar]
- Zhang N, Pombo MA, Rosli HG, Martin GB (2020) Tomato wall-associated kinase SlWak1 depends on Fls2/Fls3 to promote 6 apoplastic immune responses to Pseudomonas syringae. Plant Physiol 183: 1869–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]