Cyclic nucleotide-gated ion channels in rice promote tolerance to both heat and chilling and affect the calcium response to temperature stresses.
Abstract
Calcium signaling has been postulated to be critical for both heat and chilling tolerance in plants, but its molecular mechanisms are not fully understood. Here, we investigated the function of two closely related cyclic nucleotide-gated ion channel (CNGC) proteins, OsCNGC14 and OsCNGC16, in temperature-stress tolerance in rice (Oryza sativa) by examining their loss-of-function mutants generated by genome editing. Under both heat and chilling stress, both the cngc14 and cngc16 mutants displayed reduced survival rates, higher accumulation levels of hydrogen peroxide, and increased cell death. In the cngc16 mutant, the extent to which some genes were induced and repressed in response to heat stress was altered and some Heat Shock factor (HSF) and Heat Shock Protein (HSP) genes were slightly more induced compared to the wild type. Furthermore, the loss of either OsCNGC14 or OsCNGC16 reduced or abolished cytosolic calcium signals induced by either heat or chilling stress. Therefore, OsCNGC14 and OsCNGC16 are required for heat and chilling tolerance and are modulators of calcium signals in response to temperature stress. In addition, loss of their homologs AtCNGC2 and AtCNGC4 in Arabidopsis (Arabidopsis thaliana) also led to compromised tolerance of low temperature. Thus, this study indicates a critical role of CNGC genes in both chilling and heat tolerance in plants, suggesting a potential overlap in calcium signaling in response to high- and low-temperature stress.
Plant growth and development is highly regulated by environmental factors including temperature. Extreme temperatures cause an array of biochemical, physiological, and morphological changes in plants and often negatively impact plant productivity (Hatfield and Prueger, 2015; Liu et al., 2016; Leeggangers et al., 2017). Developing plant varieties that are tolerant to high and low temperatures is a challenge for researchers in plant biology and agriculture (Atkinson and Porter, 1996). Both high and low temperatures induce complex responses in plants, some of which may provide protection against stresses and damage (Wada et al., 1990; Murakami et al., 2000). Primary temperature sensing is thought to occur on membranes potentially through membrane fluidity and membrane-localized channels or receptors (Murata and Los, 1997). Sensing is followed by the generation of secondary messengers, including Ca2+ and reactive oxygen species (ROS; Yan et al., 2006). Both heat and cold induce transient Ca2+ influx into the cell cytoplasm (Finka et al., 2012; Ma et al., 2015), and repeated cold treatment can induce repetitive Ca2+ transients (Krebs et al., 2012). ROS, at moderate amounts, may induce ROS scavengers and other protective mechanisms for stress tolerance (Prasad et al., 1994). Excess ROS cause oxidative damage to cells, leading to growth defects or initiation of programmed cell death (Mittler, 2002). The secondary Ca2+ signals and ROS can initiate another cascade of signaling events, which may have different signaling and physiological consequences depending on the primary stimuli (Zhang et al., 2019). In heat signaling, a transient increase of Ca2+ is thought to promote expression of the calmodulin (CaM) genes (Liu et al., 2003), and CaM can regulate the activities of CaM-binding protein kinase or heat-activated mitogen-activated protein kinase (Nguyen and Shiozaki, 1999; Liu et al., 2008). The activated kinases phosphorylate heat shock factors (HSFs) and promote the expression of Heat Shock Proteins (HSPs), leading to the heat shock response (HSR) and the onset of thermotolerance (Saidi et al., 2011). HSPs are chaperone proteins important for controlling damage from denatured and aggregated proteins caused by heat (Ohama et al., 2017). HSPs can be induced by both the HSR and an active unfolded protein response in the endoplasmic reticulum (ER); the unfolded protein response can stabilize, renature, and degrade unfolded proteins to enhance survival under heat (Fragkostefanakis et al., 2016; Rieu et al., 2017). In cold signaling, it is thought that cold sensors located on membranes trigger Ca2+ influx, leading to activation of kinases that ultimately modulate the activity and abundance of Inducer of C-repeat Binding Factor (CBF) Expression1 (ICE1). In Arabidopsis (Arabdopsis thaliana), AtICE1 regulates the expression of CBF genes as well as other cold-responsive transcription factors, resulting in increased freezing tolerance (Stockinger et al., 1997; Liu et al., 1998; Zhang et al., 2019). In rice (Oryza sativa), OsICE1 is phosphorylated by mitogen-activated protein kinase3, resulting in a higher protein stability, which leads to the accumulation of trehalose and improved chilling tolerance (Zhang et al., 2017).
Cyclic nucleotide-gated ion channels (CNGCs) are nonselective, ligand-gated cation channels present across eukaryotes (Liu et al., 2018). In plants, CNGCs are found to be involved in diverse processes and their functions are thought to result from their involvement in calcium influx. Arabidopsis has 20 CNGCs, which have been implicated in regulating plant growth and development, biotic/abiotic stress responses, and ion homeostasis (DeFalco et al., 2016). In growth and development, AtCNGC1, AtCNGC2, and AtCNGC10 are shown to modulate multiple processes (Chan et al., 2003; Ma et al., 2006; Borsics et al., 2007); AtCNGC5, AtCNGC6, AtCNGC9, and AtCNGC14 are required for growth of root hairs (Brost et al., 2019; Tan et al., 2019); AtCNGC7, AtCNGC8, and AtCNGC18 are essential for pollen germination and male fertility (Pan et al., 2019); and AtCNGC17 participates in protoplast expansion (Ladwig et al., 2015). In biotic stress responses, AtCNGC2, AtCNGC4, AtCNGC11, and AtCNGC12 play a role in pathogen response, whereas AtCNGC19 regulates defense against Spodoptera spp. herbivory (Yoshioka et al., 2006; Chin et al., 2013; Meena et al., 2019). In abiotic stress responses, AtCNGC3, AtCNGC10, AtCNGC19, and AtCNGC20 are involved in adaptation to salt stress (Gobert et al., 2006; Guo et al., 2008; Kugler et al., 2009), whereas AtCNGC2, AtCNGC4, AtCNGC6, and AtCNGC16 in Arabidopsis have been implicated in heat-stress adaptation (Finka et al., 2012; Gao et al., 2012; Tunc-Ozdemir et al., 2013). Disruption of AtCNGC2 and AtCNGC4 in Arabidopsis and its putative ortholog PpCNGCb in moss (Physcomitrella patens) resulted in enhanced acquired thermotolerance in seedlings, and the loss of function of PpCNGCb in moss was accompanied by a higher HSR and higher Ca2+ influx than in the wild type (Finka et al., 2012). Interestingly, deficiency in AtCNGC2 resulted in more heat susceptibility at the reproductive stage in contrast to the enhanced heat tolerance at seedling stage (Katano et al., 2018), suggesting a developmental stage-dependent function of CNGCs in stress tolerance. AtCNGC6 was shown to be activated by cytosolic cAMP and to promote expression of HSP genes and acquired thermotolerance (Gao et al., 2012). AtCNGC16 plays an essential function in pollen fertility under heat stress (Tunc-Ozdemir et al., 2013). The diverse function of CNGCs is thought to result from their functions in modulating calcium influx. AtCNGC2 is shown to affect Ca2+ influx into leaf cells to prevent Ca2+-induced hydrogen peroxide (H2O2) accumulation, cell death, and leaf senescence (Wang et al., 2017). In rice, 16 CNGC members have been identified (Nawaz et al., 2014), but only two are functionally characterized. The OsCNGC13 gene is a maternal sporophytic factor that functions in regulating rice seed setting and the OsCNGC9 gene was recently shown to mediate pathogen-associated molecular pattern-induced Ca2+ influx in plant immunity (Xu et al., 2017; Wang et al., 2019).
In this study, we found that the rice OsCNGC14 and OsCNGC16 genes play critical roles in heat tolerance as well as low-temperature tolerance. In addition, they affect Ca2+ influx to the cytosol in response to heat or cold treatments. Our study thus provides evidence for the role of CNGC proteins in calcium regulation and uncovers a further function of CNGC proteins in chilling and freezing tolerance in addition to their known roles in heat tolerance in plants.
RESULTS
Generation of the cngc14 and cngc16 Mutants
Given that AtCNGC2, AtCNGC4, and PpCNGCb play key roles in thermotolerance in Arabidopsis and moss (Finka et al., 2012), we investigated the roles of their rice homologs in temperature-stress tolerance. The complementary DNA (cDNA) sequences of these three genes were used to Blast search the rice genome sequences, and their most similar genes in rice were identified as OsCNGC14, OsCNGC15, and OsCNGC16 (Supplemental Fig. S1). Because we were not able to generate mutants in OsCNGC15 as in OsCNGC14 and OsCNGC16, we focused our study on the latter two genes. We first determined the subcellular localization of these two rice CNGC proteins by fusing them with GFP. Transient expression of these fusion proteins in Nicotiana benthamiana revealed that OsCNGC14 and OsCNGC16 are both localized to the plasma membrane (Supplemental Fig. S2). A search of the Rice Expression Profile Database showed that the OsCNGC14 and OsCNGC16 genes are expressed in most organs, including leaves, stems, inflorescence, and reproductive organs, and OsCNGC14 has a much higher expression level than OsCNGC16 in general (Supplemental Fig. S3, A and B). We assessed the expression of these genes in response to heat and cold treatments by reverse transcription quantitative PCR (RT-qPCR). The OsCNGC14 gene was induced by 42°C treatment to a maximum of 5.6-fold at 3 h, dropped at 6 h, and came down to almost the basal level at 9 h of heat treatment. The OsCNGC16 gene was induced to a maximum of 3.4-fold at 3 h and decreased to almost the basal level at 6 h of heat treatment (Supplemental Fig. S4A). In response to the 6°C chilling treatment, the expression of OsCNGC14 was increased by 1.6-fold at 1 h and dropped to the basal level at 3 h, whereas expression of the OsCNGC16 gene reached a maximum of 3.1-fold at 6 h compared to the basal level and dropped to basal level at 9 h (Supplemental Fig. S4B).
To assess the function of OsCNGC14 and OsCNGC16 genes in rice, we generated their mutants in the cv Nipponbare background via CRISPR/Cas9 technology (Xing et al., 2014). Homozygous mutants were identified among the T0 population by sequencing the region around the CRISPR target sites. Three OsCNGC14 mutants (cngc14-1, cngc14-2, and cngc14-3) and three OsCNGC16 mutants (cngc16-1, cngc16-2, and cngc16-3) were obtained. The cngc14-1 mutant contained an 8-bp deletion (193–200 bp) and a 1-bp deletion at 224 bp relative to the translation start site, leading to a predicted 3-amino acid deletion (amino acids 65–67) in its predicted coding protein (Fig. 1, A and B). The cngc14-2 mutant contained a 25-bp deletion (176–200 bp) and a 1-bp insertion at 225 bp, leading to a predicted 8-amino acid deletion (amino acids 59–66; Fig. 1, A and B). The cngc14-3 mutant contained a 28-bp deletion (197–224 bp), which is predicted to produce an altered protein sequence starting at amino acid 66 and a stop codon at amino acid 83 (Fig. 1, A and B). The cngc16-1 mutant contained 1-bp insertions at 585 and 718 bp, which are predicted to produce an altered protein sequence starting at amino acid 195 and a stop codon at amino acid 262 (Fig. 1, C and D). The cngc16-2 mutant contained a 3-bp deletion (586–588 bp) and a 1-bp insertion at 718 bp, causing a predicted 1-amino acid deletion at position 196 and a reading frame shift after position 240 (Fig. 1, C and D). The cngc16-3 contained a 7-bp deletion (585–591 bp), a 1-bp insertion at 718 bp, and a 1-bp substitution at 719 bp, which is predicted to produce an altered protein sequence starting at amino acid 196 and a stop codon at amino acid 204 (Fig. 1, C and D).
Figure 1.
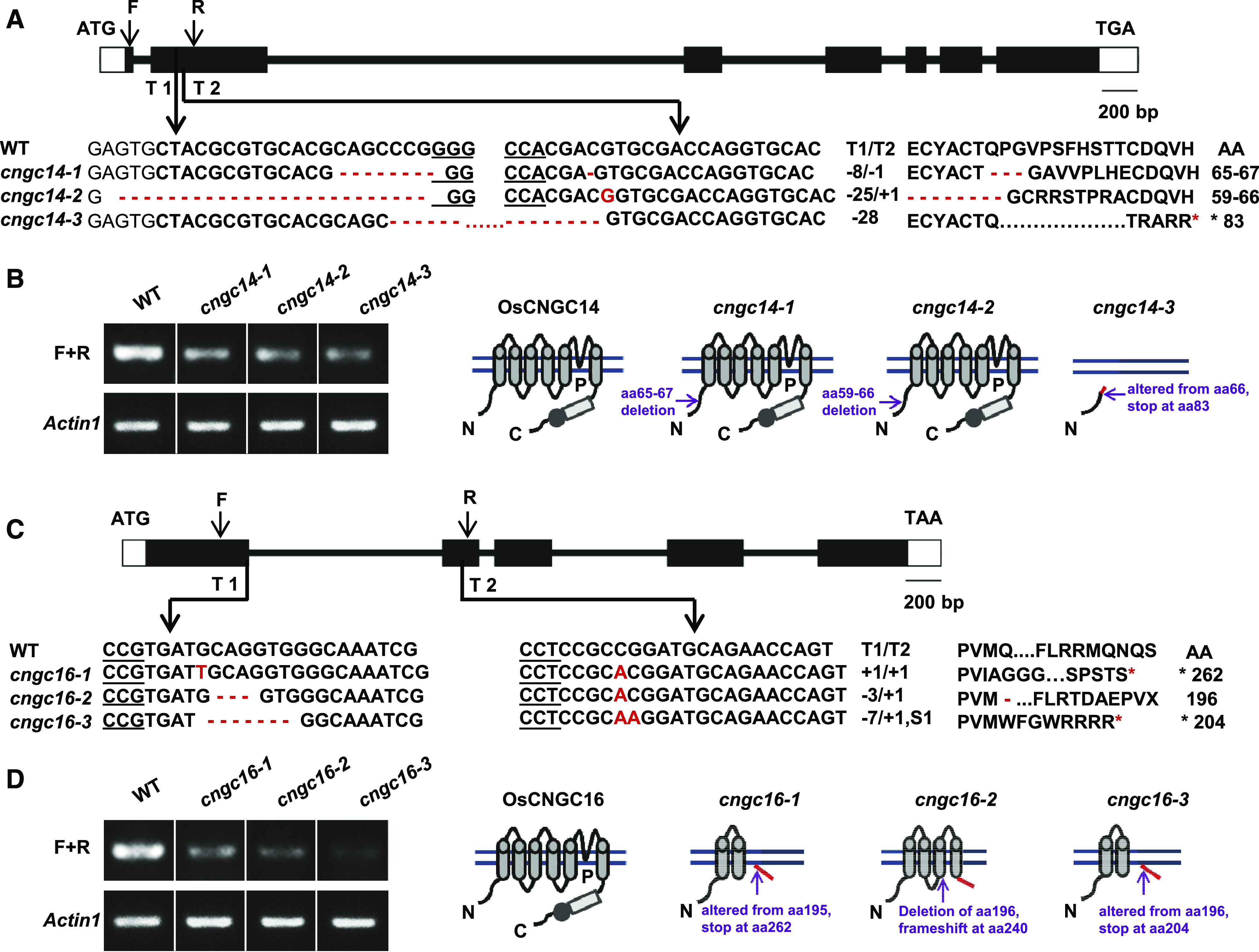
Mutants of OsCNGC14 and OsCNGC16 generated by CRISPR/Cas9 technology. Diagram of the genomic sequences (A and C) and predicted protein structures (B and D) of the wild type (WT) and mutants of OsCNGC14 (A and B) and OsCNGC16 (C and D). A and C, Diagrams of the genomic DNA, with exons, introns, and untranslated regions indicated by black boxes, lines between boxes, and white boxes, respectively. Below the diagrams are sequence alignments between the mutants and wild type, with genomic sequences on the left and protein sequence on the right. Minus (−) and plus (+) signs and the letter S indicate the number of nucleotides deleted, inserted, and substituted, respectively, within the CRISPR/Cas9 target sequences T1 and T2. The asterisk indicates a stop codon generated. F, Forward primers; R, reverse primers. B and D, RNA expression and predicted protein structures of OsCNGC14 (B) and OsCNGC16 (D) in the wild type and the cngc mutants. On the left are products of semiquantitative RT-PCR of OsCNGC14 and OsCNGC16 in their respective mutants and the wild type. Actin1 was used as a control gene. On the right are diagrams of predicted protein structure in the wild type and different mutant alleles. For each structure, transmembrane helices are indicated by cylinders, the cyclic nucleotide binding domain by a rectangle, and the calmodulin-binding domain by a solid circle. Locations of amino acid alterations are indicated by arrows. Red lines indicate altered sequences from frameshift.
We assayed the mRNA expression of OsCNGC14 and OsCNGC16 in their respective mutants by semiquantitative RT-PCR using primers spanning the CRISPR target sites. The transcripts of OsCNGC14 were found to be greatly reduced in all three cngc14 mutant alleles compared to the wild type, with cngc14-3 having the lowest expression (Fig. 1B; Supplemental Fig. S5A). The transcripts of OsCNGC16 were also greatly reduced in all three cngc16 mutant alleles compared to the wild type with cngc16-3 having the lowest expression (Fig. 1D; Supplemental Fig. S5B). The drastically reduced expression of OsCNGC14 or OsCNGC16 associated with some alleles is likely due to nonsense-mediated mRNA degradation. Taken together, cngc14-1 and cngc14-2 are likely reduction-of-function mutants, because lower levels of mRNA are produced and small deletions are present in the predicted proteins. The cngc14-3 allele and all three cngc16 alleles are most likely loss-of-function mutants, because the amounts of mRNA present are lower than in the wild type and the predicted proteins have large truncations and therefore are likely nonfunctional (Fig. 1, B and D).
OsCNGC14 and OsCNGC16 Promote Thermotolerance in Rice at Seedling Stage
To determine whether OsCNGC14 and OsCNGC16 play roles in heat tolerance, the rice cngc14 and cngc16 mutants were subjected to a heat treatment. None of these cngc14 and cngc16 mutant plants had any overt abnormalities or defects when grown under normal conditions. Two-week-old seedlings were treated at 48°C for 48 h, and the loss of green color and survival rates were analyzed after a 2-week recovery growth at 26°C. The cngc14 and cngc16 mutants had more withered and yellower leaves compared to the wild-type cv Nipponbare plants (Fig. 2A). The mutants also had lower survival rates than the wild type. The cngc14-1, cngc14-2, cngc14-3, cngc16-1, cngc16-2, and cngc16-3 had survival rates of 66%, 51%, 24%, 23%, 23%, and 30%, respectively, each of which was significantly lower than the rate of 70% for the wild type except that of cngc14-1 (Fig. 2B). The difference in survival rates among cngc14-1, cngc14-2, and cngc14-3 is consistent with the prediction that cngc14-3 is a loss-of-function allele and the other two are reduction-of-function alleles. We further tested seedling tolerance by longer exposure (72 h) to a moderate heat of 43°C. After the heat treatment, the cngc14 and cngc16 mutant plants had severely wrinkled and rough leaves and the cngc16 mutant developed necrotic spots on leaves, whereas the wild type was only slightly wrinkled at the leaf tip (Supplemental Fig. S6A). In addition, ion leakage in leaves, an indicator of membrane damage, was dramatically increased in the cngc14-2 mutant and three cngc16 mutants compared with wild-type plants after heat treatment but not in plants without heat treatment (Fig. 2C). Furthermore, whereas before heat treatment the mutants had low accumulation of H2O2, similar to that of the wild type, a higher accumulation relative to the wild type was observed after heat treatment, as assayed by staining with 3,3′-diaminobenzidine (DAB; Fig. 2D). Heat also induced moderate cell death in the wild type, as indicated by trypan blue staining, and the cngc14 and cngc16 mutants exhibited more cell death compared with the wild-type plants after heat treatment but not without heat treatment (Fig. 2E). These results indicate that OsCNGC14 and OsCNGC16 positively contribute to heat tolerance in rice and that loss or reduction of their function leads to higher heat susceptibility associated with higher accumulation of H2O2 and cell damage.
Figure 2.

Mutants of OsCNGC14 and OsCNGC16 are heat susceptible at seedling stage. A, Morphological phenotypes of the wild type (WT), cngc14, and cngc16 seedlings before and after heat treatment. Seedlings were hydroponically grown at 26°C for 2 weeks and then treated at 48°C for 48 h followed by a recovery at 26°C for 2 weeks. Plants were photographed after the recovery. Scale bars = 2 cm. B, Survival rates for the cngc14 and cngc16 mutants shown in A. Shown are the means ± sd for three biological replicates (n = 48 for each replicate). At least three independent experiments were carried out with similar results. C, Cellular ion leakage with or without 48°C treatment for 48 h in the third leaves of 2-week-old seedlings grown hydroponically at 26°C. Shown are means ± sd from four biological replicates, with 12 plants for each line. Asterisks indicate significant difference by Students t test (*P < 0.05 and **P < 0.01). D and E, H2O2 accumulation detected by DAB staining (D) and cell death detected by trypan blue staining (E) in the third leaves of 3-week-old seedlings grown in the soil before and after treatment at 48°C for 72 and 76 h, respectively (n = 15). Scale bars = 1 mm.
OsCNGC14 and OsCNGC16 Impact Cytosolic Calcium Increase in Response to Heat Treatment
Because heat shock induces a transient increase in cytosolic Ca2+ (Saidi et al., 2009; Zheng et al., 2012), we investigated whether OsCNGC14 and OsCNGC16 affect the production of such a calcium signal. The cytosolic calcium concentration was detected by a nuclear export signal (NES) version of the calcium sensor Yellow Cameleon 3.6 (YC3.6) based on fluorescence resonance energy transfer (FRET; Krebs et al., 2012). This NES-YC3.6 reporter was introduced into the cngc14-2 and cngc16-1 mutants by crossing, and isogenic lines of the wild type and the cngc mutants with the same reporter gene were generated (Supplemental Fig. S7, A–C). Calcium concentrations, measured by the FRET signals (cpVenus/cyan fluorescent protein), were analyzed in stomatal cells where YC3.6 has a strong expression (Supplemental Fig. S7, D–G). Under normal growth conditions, no significant difference was observed between wild-type and cngc14 or cngc16 mutants in cytosolic Ca2+ levels (Supplemental Fig. S7H). Drastic differences in Ca2+ levels were observed when heat shock was applied to leaves. In response to heat treatment, the cytosolic Ca2+ concentration rose quickly in the wild type, reached the maximum at 15 s (seconds), and declined sharply (Fig. 3, A and B; Supplemental Movie S1). This “spike and shoulder”-shaped cytosolic Ca2+ was consistent with earlier reports in Arabidopsis and rice (Behera et al., 2015). Each of the subsequent heat treatments at the frequency of once every minute induced a calcium spike with a similar shape to the first spike (at least up to the seventh treatment; Fig. 3, A and B; Supplemental Movie S1). The cngc14-2 mutant displayed a significant decrease in cytosolic calcium spike after the first heat treatment, and it showed weaker spikes with subsequent treatments compared to the wild type (Fig. 3, A and C; Supplemental Movie S2). This indicates that Ca2+ kinetics was milder in the cngc14-2 mutant compared to the wild type. The cngc16-1 mutant exhibited a much stronger defect in calcium signal compared to the cngc14-2 mutant. The heat-induced transient increase of cytosolic Ca2+ was largely abolished in the cngc16-1 mutant (Fig. 3, A and D; Supplemental Movie S3). The maximal changes in FRET signals (apparent FRET efficiency/apparent rest FRET efficiency [ΔEapp/Eapprest]) in cngc14-2 and cngc16-1 were 0.10 ± 0.09 and 0.02 ± 0.07, respectively, which were significantly lower than the signal 0.33 ± 0.15 seen in the wild type for the first peak (Fig. 3, E and F). In conclusion, OsCNGC14 and OsCNGC16 are positive modulators of heat-induced increase of cytosolic calcium. It remains to be determined whether the lesser severity of defects in the cngc14-2 mutant compared to the cngc16-1 mutant is due to the reduction-of-function nature of the cngc14-2 allele.
Figure 3.
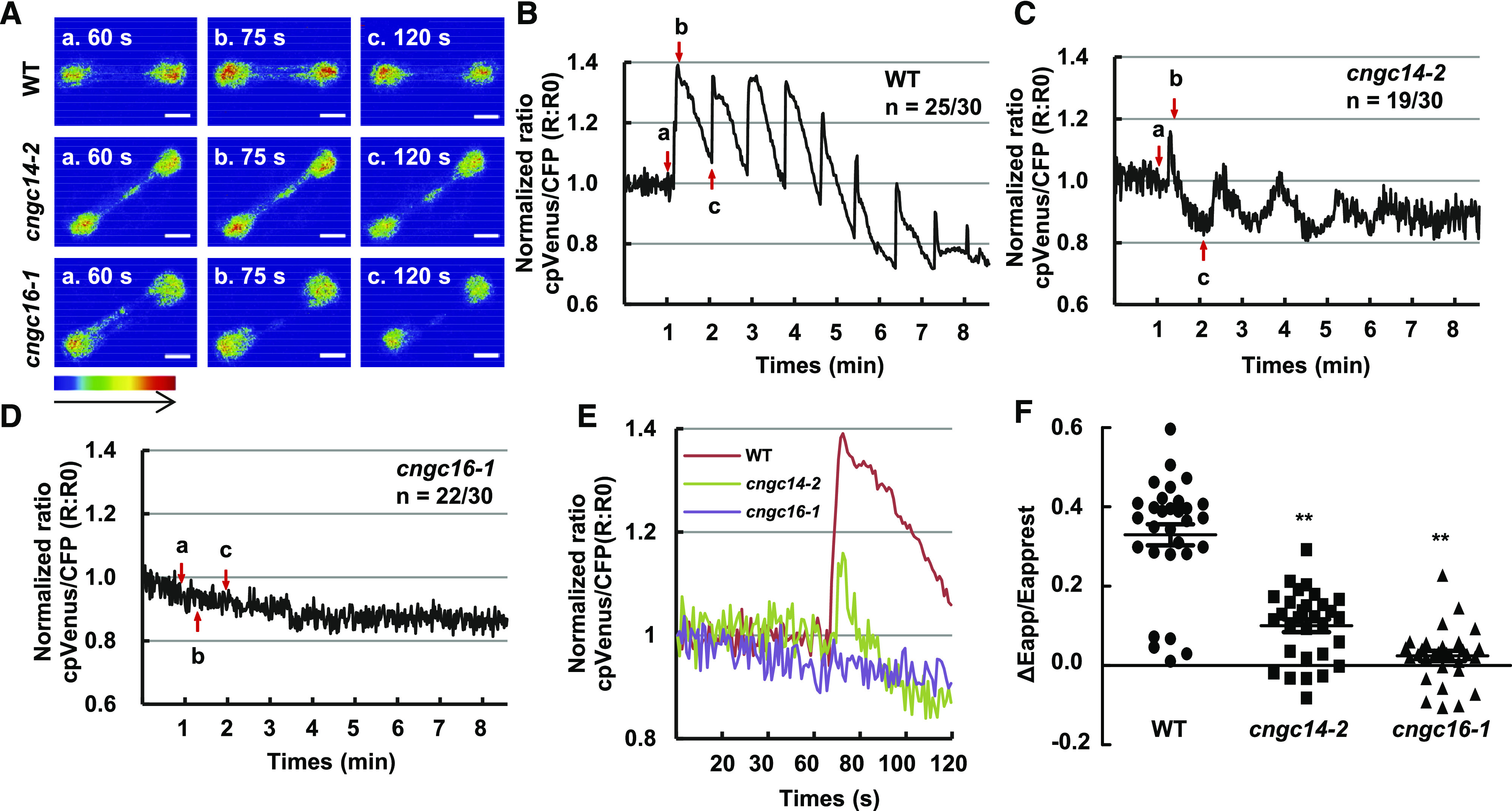
Heat-induced cytosolic Ca2+ changes in guard cells of cngc14 and cngc16 mutants. A, Emission images of cytosolic Ca2+ in the stomatal cells from leaves of wild-type (WT), cngc14-2, and cngc16-1 10-d-old seedlings expressing NES-YC3.6. Shown are representative images before heat shock treatment (60 s) and after heat shock buffer administration (75 and 120 s). The representative image of relative cytosolic Ca2+ is represented as the emission fluorescence ratio and scaled by a pseudo-color bar (bottom). Scale bars = 50 μm. B to D, Normalized cytosolic Ca2+ FRET ratio in stomatal cells. The luminescence was recorded at 1-s intervals. Shown are representative images of calcium signals in stomatal cells of the wild type, cngc14-2, and cngc16-1. Lowercase letters refer to the time points at which these ratio images were taken in A. A total of 30 stomatal cells (one leaf/plant, 1–2 stomatal cells/leaf) in the third leaves from at least 15 different seedlings were measured. The two numbers underneath each genotype indicate the number of cells with the representative calcium pattern versus the number of total cells analyzed. E, Representative traces of calcium signals from the wild type and mutants are shown together. F, Scatter plots of the heat-induced changes in cytosolic calcium (ΔEapp/Eapprest) in wild type, cngc14-2, and cngc16-1, as shown in B to D. The Δ cytosolic Ca2+ indicates the changes between the [Ca2+]peak and [Ca2+]rest. Data are from all 30 cells that were monitored for each genotype. Asterisks indicate significant difference by Student’s t test (**P < 0.01).
Transcriptome Analysis of the cngc16 Mutant
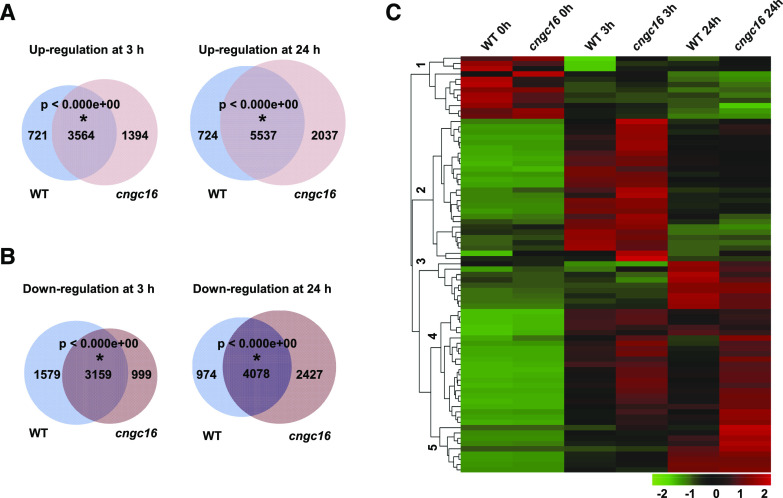
To further investigate how CNGCs regulate heat tolerance in rice, we carried out RNA sequencing (RNA-Seq) to profile transcriptomes of the wild type and the cngc16-3 mutant. Rice plants grown at 26°C for 2 weeks were heat treated at 42°C, and RNAs were extracted from leaf tissues at 0, 3, and 24 h after heat treatment. Differentially expressed genes (DEGs) among samples were defined by a fold change of |log2| ≥ 1 and a false discovery rate (FDR) ≤0.01. Overall, high correlation values were observed for three biological repeats for each genotype and time point combination (r ≥ 0.92; Supplemental Fig. S8A), indicating a good reproducibility of the samples. Principal component analysis (PCA) also revealed that the biological repeats are clustered together (Supplemental Fig. S8B). Interestingly, the 0-h samples of the wild type and the cngc16-3 mutant were closely clustered, indicating that without heat treatment, the wild type and the mutant are similar in RNA expression. PC1 divided the 0-h samples from the 3- and 24-h samples, indicating that temperature, and not genotype, has the largest impact on transcriptomes. PC2 separated the 0-, 3-, and 24-h samples and also showed that RNA expression at 3 h is more different from that at 0 and 24 h. The genotype difference was less pronounced than the heat and time differences, indicating that the wild type and the mutant are in general similar in transcriptome responses before and after heat treatment. In addition, PC1 distribution revealed that the cngc16-3 mutant at 3 and 24 h is more distant from the wild type or cngc16-3 at 0 h compared to the wild type at the corresponding 3- and 24-h time points (Supplemental Fig. S8B), suggesting that there are more changes in the cngc16-3 mutant than in the wild type in response to heat.
Comparisons of DEGs between the wild type and the cngc16-3 mutant, as well as between heat treatment and non-heat treatment, also indicated that the transcriptome responses to heat were similar in the wild type and the mutant. At 0, 3, and 24 h, a total of 150, 404, and 510 genes, respectively, had increased expression, whereas a total of 84, 44, and 576 genes had decreased expression, in the cngc16-3 mutant compared to the wild type (Supplemental Table S1). No significant gene ontology (GO) enrichment was observed for these DEGs between the cngc16-3 mutant and the wild type at 0, 3, or 24 h of heat treatment. When comparing heat treatment (3 or 24 h) to non-heat treatment (0 h), a larger number of DEGs (in thousands) was found for both the wild type and the mutant (Fig. 4, A and B; Supplemental Tables S2 and S3). A Venn diagram showed significant overlap between the wild type and the cngc16-3 mutant in both up- and down-regulated DEGs at 3 and 24 h (Fig. 4, A and B). This suggests that the mutant is not drastically different from the wild type before or after heat treatment. GO-term analysis also revealed that the wild type and the mutant had almost identically enriched GO terms in DEGs at 3 h (or 24 h) versus 0 h (Supplemental Fig. S9, A–D). Heat-induced DEGs in the wild type and the cngc16-3 mutant were both enriched in GO terms including “response to salt stress,” “response to reactive oxygen species,” “cellular response to heat,” “response to cold,” and “chaperone-mediated protein folding” (Supplemental Fig. S9, A and B). The heat-repressed DEGs in the wild type and the cngc16-3 mutant were enriched in GO terms related to growth and development, such as “plant-type cell wall biogenesis,” “sterol biosynthetic process,” “metal ion transport,” and “cellular amino acid biosynthetic process” (Supplemental Fig. S9, C and D).
Figure 4.
RNA-Seq analysis of the cngc16 mutant. A and B, Venn diagrams of heat-induced (A) and heat-repressed (B) genes in the wild type (WT) and cngc16-3. Two-week-old seedlings were treated at 42°C for 0, 3, and 24 h for RNA-Seq analysis. Expression at 3 or 24 h was compared to that at 0 h, and DEGs were defined by log2 fold change ≥1 or ≤−1, and FDR < 0.01. The DEGs were used to draw the Venn diagram via tools accessed at http://bioinfogp.cnb.csic.es/tools/venny/. Statistical significance (P < 0.01) of the overlap is indicated by an asterisk, calculated using the online program http://nemates.org/MA/progs/overlap_stats.html. C, Heatmap representation of expression levels of DEGs of HSF and HSP genes at 0, 3, and 24 h after 42°C heat treatment in wild type and the rice cngc16-3 mutant.
We further examined DEGs between the wild type and cngc16-3 mutant at 3 or 24 h to determine whether they represented any unique pathways. GO-term analysis did not reveal any enriched terms; however, these DEGs have significant overlaps with DEGs of heat versus nonheat in the wild type. Among the 404 upregulated DEGs in the cngc16-3 mutant compared to the wild type at 3 h, 105 were heat induced and 94 were heat repressed in the wild type (Supplemental Fig. S10A). This suggests that heat responses (induction or repression) are larger for some and smaller for other genes in the cngc16-3 mutant compared to the wild type (Supplemental Fig. S10A). This was similarly observed for DEGs between the wild type and the mutant at 24 h, that is, they have significant overlaps with heat-induced or heat-repressed genes in the wild type, and they are more responsive or less responsive to heat in the mutant compared to the wild type (Supplemental Fig. S10, C and D). Taken together, there was no qualitative difference between the wild type and the cngc16-3 mutant in terms of heat response at the transcriptome level, but the extent of responses for some genes differs between the mutant and the wild type.
Because the Arabidopsis cngc2 mutant had increased expression of HSP genes compared to the wild type in response to heat (Finka et al., 2012), we examined the expression of HSF and HSP genes in the cngc16 mutant. Among the 100 HSF and HSP genes in rice, 79 were identified as DEGs (log2 fold change ≥1 or ≤−1 and FDR < 0.01) using the normalized expression data in this study, which is almost exclusively due to their induction by heat (Supplemental Table S4). Among all of these HSF and HSP DEGs, only two displayed a significant expression difference between the mutant and the wild type at 24 h, and none were DEGs at 0 or 3 h (Supplemental Table S4). This indicates that expression levels of HSF and HSP genes in general are similar between the mutant and the wild type (<2-fold difference) with or without heat treatment. Cluster analyses of these 79 DEGs of HSF and HSP genes by their expression revealed five coexpression groups (Fig. 4C). Heatmap analysis showed that genes in groups 1 and 3 had lower relative expression levels in the cngc16-3 mutant compared to the wild type at 0 and 24 h, respectively (Fig. 4C; Supplemental Table S4). By contrast, genes in groups 2, 4, and 5 had higher induction by heat in the cngc16-3 mutant compared to the wild type at 3 and/or 24 h (Fig. 4C; Supplemental Table S4). These results indicate that the rice cngc16-3 mutant has slightly increased induction of primarily HSF and HSP genes after heat treatment at 3 or 24 h. cngc16-3 does not have an attenuated induction of HSFs and HSPs during heat shock, which is in contrast to the enhanced induction observed in the Arabidopsis cngc6 mutant but similar to induction observed in the cngc16 mutants (Gao et al., 2012; Tunc-Ozdemir et al., 2013).
OsCNGC14 and OsCNGC16 Enhance Chilling Tolerance in Rice at Seedling Stage
CNGCs have not been associated with cold responses in plants. The drastic calcium signal defect of the rice cngc14 and cngc16 mutants in response to heat promoted us to analyze their function in cold tolerance, where calcium signal is also thought to be a secondary messenger. The cngc14 and cngc16 mutants, as well as the wild type, were subjected to a chilling (cold but above freezing) treatment of 6°C for 72 h performed on 2-week-old seedlings followed by a 2-week recovery growth period at 26°C. Each of the mutants analyzed, namely cngc14-1, cngc14-2, cngc14-3, cngc16-1, cngc16-2, and cngc16-3, exhibited reduced growth and yielded yellower leaves after chilling treatment compared to the wild-type plants (Fig. 5A; Supplemental Fig. S6B). In addition, all mutants had lower survival rates from chilling treatment compared to the wild type (Fig. 5B). cngc14-1, cngc14-2, cngc14-3, cngc16-1, cngc16-2, and cngc16-3 exhibited survival rates of 43%, 36%, 22%, 28%, 17%, and 27%, respectively, significantly lower than the rate of 65% for the wild type (Fig. 5B).
Figure 5.
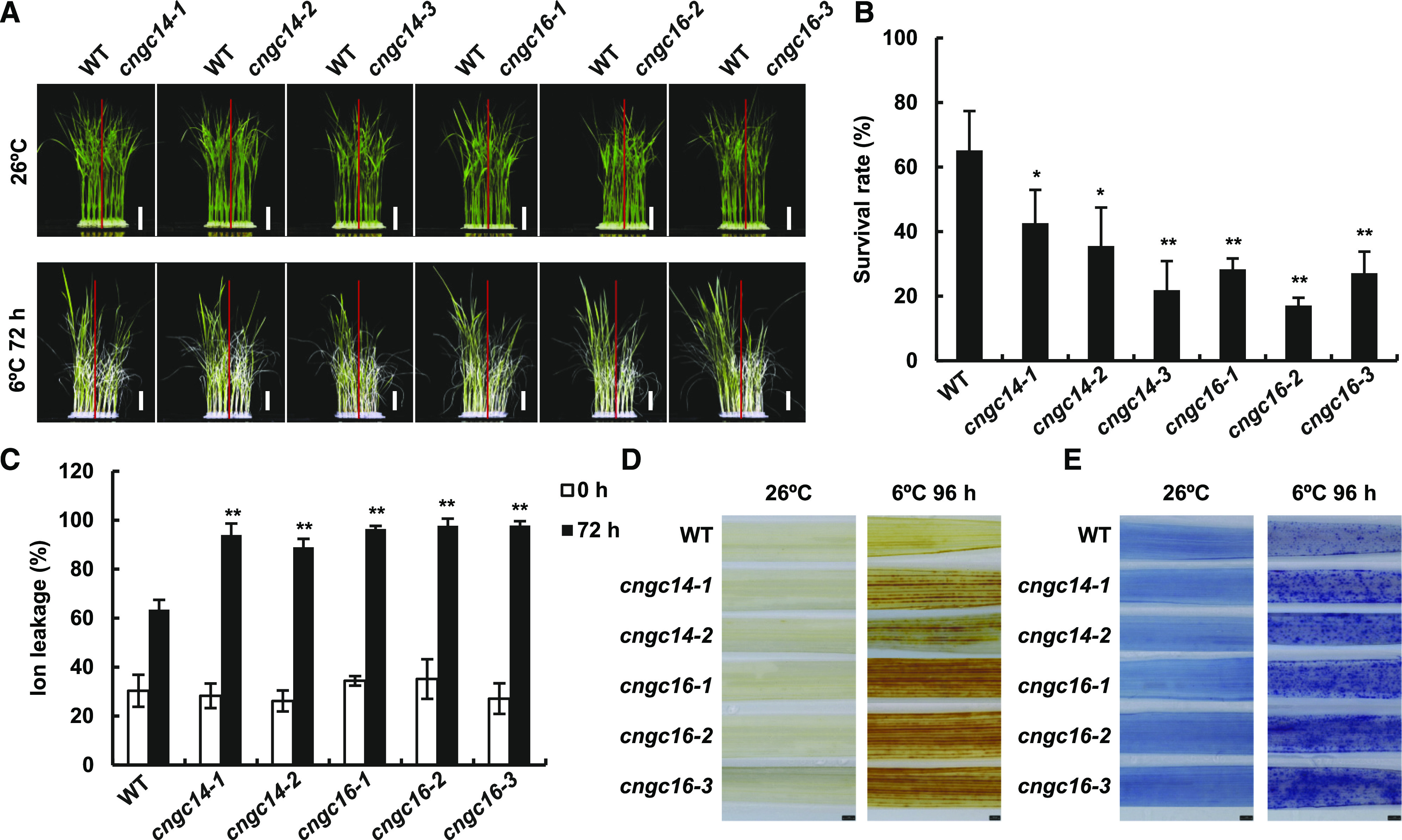
Mutants of OsCNGC14 and OsCNGC16 are chilling susceptible at seedling stage. A, Morphological phenotypes of wild-type (WT), cngc14, and cngc16 seedlings before and after chilling treatment. Seedlings were hydroponically grown at 26°C for 2 weeks and then treated at 6°C for 72 h followed by recovery at 26°C for 2 weeks. Plants were photographed after the recovery. Scale bars = 2 cm. B, Survival rates of the cngc14 and cngc16 mutants after chilling treatment as in A. The data are the means ± sd of three biological replicates (n = 48 for each replicate). Three independent experiments were carried out with similar results. C, Cellular ion leakage with or without 6°C treatment for 72 h in the third leaves of 2-week-old seedlings grown hydroponically at 26°C. Data represent means ± sd from four biological replicates with 12 plants for each line. Asterisks indicate significant difference by Student’s t test (*P < 0.05 and **P < 0.01). D and E, H2O2 accumulation detected by DAB staining (D) and cell death detected by trypan blue staining (E) in the third leaves of 3-week-old seedlings grown in soil before and after a treatment of 6°C for 96 h (n = 15). Scale bars = 1 mm.
We subsequently examined the response to chilling stress in the cngc14 and cngc16 mutants at the cellular level. Chilling induced increased ion leakage in leaf cells of the wild-type plants due to damage to cellular membranes (Fig. 5C). The cngc14 and cngc16 mutant plants all had significantly increased ion leakage in leaves compared to the wild-type plants at 72 h after chilling, whereas they did not differ from the wild type before chilling treatment (Fig. 5C). Chilling induced accumulation of ROS, including H2O2, as assayed by DAB staining (Fig. 5D). Both the cngc14 and cngc16 mutants accumulated more H2O2 than the wild-type plants after 96 h of chilling treatment, whereas both the mutants and the wild type had low amounts of H2O2 before chilling treatment (Fig. 5D). Chilling also induced cell death in the wild-type plants, as indicated by trypan blue staining (Fig. 5E). The cngc14 and cngc16 mutants exhibited more cell death compared to the wild type after 96 h of chilling treatment, whereas they did not show increased cell death before chilling (Fig. 5E). Taken together, these results indicate that OsCNGC14 and OsCNGC16 are important for chilling tolerance in rice and that the loss or reduction of their function leads to reduced chilling tolerance, which is associated with more H2O2 accumulation and cell death.
OsCNGC14 and OsCNGC16 Impact Cytosolic Calcium Increase in Response to Chilling Treatment
To investigate whether OsCNGC14 and OsCNGC16 might affect chilling-induced changes in cytosolic Ca2+, we monitored calcium levels in stomatal cells of the wild type and the cngc mutants before and after chilling treatment. Chilling triggered a large increase in cytosolic Ca2+ in the wild type in 5 s, and subsequent chilling treatments induced very mild Ca2+ spikes, with no calcium change observed at the third treatment (Fig. 6, A and B; Supplemental Movie S4). In 17 of the 24 cells observed, the cngc14-2 mutant displayed a significant decrease in cytosolic Ca2+ spike following the first cold treatment, and there was almost no calcium change in any of the 24 cells observed following the second and subsequent chilling treatments (Fig. 6A and C; Supplemental Movie S5). The cngc16-1 mutant had a stronger defect compared to cngc14-2 after the chilling treatment. No change in cytosolic Ca2+ was observed in the cngc16-1 mutant after chilling in 19 of 21 cells recorded (Fig. 6, A and D; Supplemental Movie S6). The cytosolic Ca2+ levels, measured by maximal changes in FRET signals (ΔEapp/Eapprest) of YC3.6, were 0.22 ± 0.14 in the wild type, 0.12 ± 0.09 in the cngc14-2 mutant, and 0.05 ± 0.04 in the cngc16-1 mutant (Fig. 6, E and F). These results thus indicate that OsCNGC14 and OsCNGC16 are positive modulators of chilling-induced increases in cytosolic Ca2+.
Figure 6.
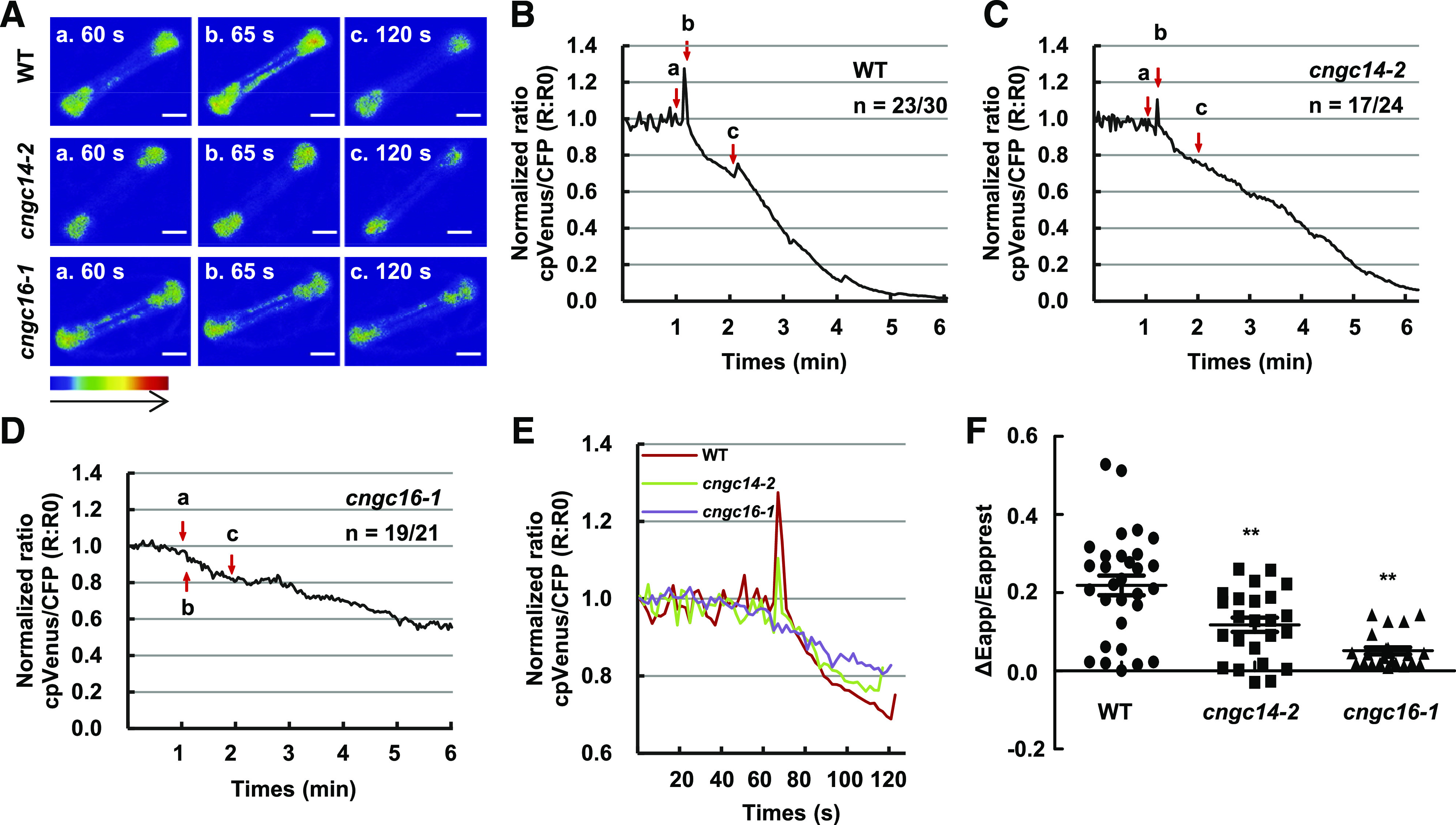
Cold-induced cytosolic Ca2+ changes in guard cells of cngc14 and cngc16 mutants. A, Emission images of cytosolic Ca2+ in the stomatal cells from leaves of wild-type (WT), cngc14-2, and cngc16-1 10-d-old seedlings expressing NES-YC3.6. Shown are representative images before chilling treatment (60 s) and after chilling treatment (65 and 120 s). The representative image of relative cytosolic Ca2+ is represented as the emission fluorescence ratio and scaled by a pseudo-color bar (bottom). Scale bars = 50 μm. B to D, Normalized cytosolic Ca2+ FRET ratio in stomatal cells. The luminescence was recorded at 2-s intervals. Shown is the representative image of calcium signals in stomatal cells of the wild type, cngc14-2, and cngc16-1. Lowercase letters refer to the time points at which these ratio images were taken in A. At least 21 stomatal cells (one leaf/plant, 1–2 stomatal cells/leaf) in the third leaves from at least 10 different seedlings were measured. The two numbers underneath each genotype indicate the number of cells with the representative calcium pattern versus the number of cells analyzed. E, Representative traces of calcium signals from the wild type and the mutants are shown together. F, Scatter plots of the cold-induced changes in cytosolic calcium (Δcytosolic Ca2+) in wild type, cngc14-2, and cngc16-1 as shown in B to D. The Δcytosolic Ca2+ indicates the changes between the [Ca2+]peak and [Ca2+]rest. Data are from all cells that were monitored (30, 24, and 21 cells for the three genotypes respectively). Asterisks indicate significant difference by Student’s t test (**P < 0.01).
Arabidopsis AtCNGC2 and AtCNGC4 Promote Chilling Growth and Freezing Tolerance
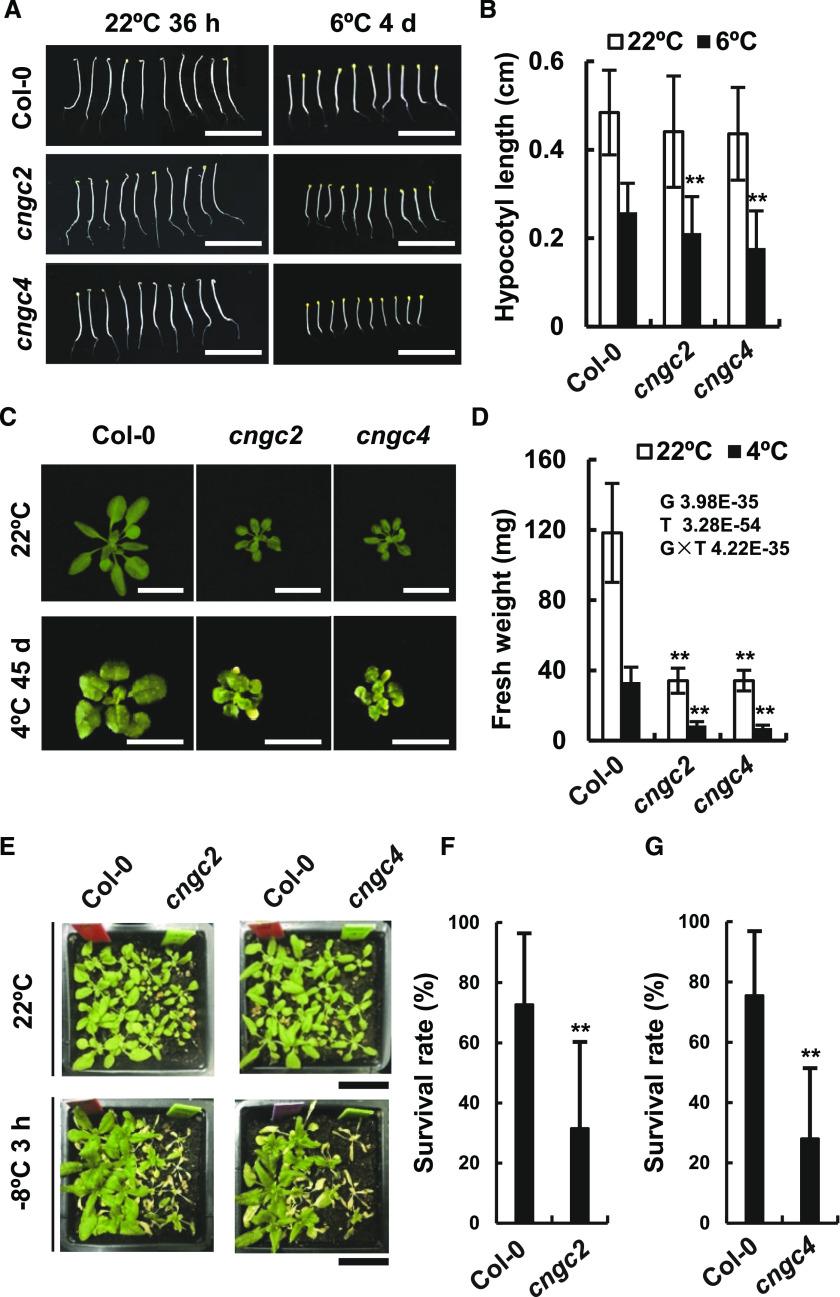
Given that the OsCNGC14 and OsCNGC16 contribute positively to both heat and chilling tolerance in rice, we asked whether Arabidopsis AtCNGC2 and AtCNGC4 affect low-temperature responses in addition to heat tolerance. The Arabidopsis cngc2 and cngc4 mutants were assayed for hypocotyl elongation and rosette growth under chilling conditions. The wild-type seedlings displayed shorter hypocotyls when grown at 6°C compared to at 22°C in darkness. The cngc2 and cngc4 mutants had shorter hypocotyls but these differences were not statistically significant compared to the wild-type plants at 22°C (Fig. 7, A and B). However, the cngc2 and cngc4 mutants displayed significantly shorter hypocotyls compared to wild-type plants at 6°C (Fig. 7, A and B). Chilling (4°C) also inhibited rosette growth in the light in the wild type. The fresh weight after 45 d of growth at 4°C was only 30% of that after 21 d of growth at 22°C (Fig. 7, C and D). Both cngc2 and cngc4 mutants exhibited more inhibited growth by chilling in the light, with smaller rosettes and reduced fresh weight compared to the wild type when grown at 22°C (Fig. 7C). Compared to the wild type, both mutants had greater reduction in fresh weight (not rosette outgrowth) at 4°C than at 22°C, as indicated by double variant analysis (G × T; Fig. 7D). In addition, both the cngc2 and cngc4 mutants exhibited yellow or chlorotic leaves when grown at 4°C, which was not observed in the wild type (Fig. 7C).
Figure 7.
Arabidopsis mutants of AtCNGC2 and AtCNGC4 are chilling and freezing susceptible. A and B, Phenotypes (A) and quantification (B) of hypocotyl elongation of cngc2 and cngc4 in the dark at 22°C (for 36 h) and 6°C (for 4 d). Data represent means ± sd from three biological replicates, each of which had at least 15 seedlings. C and D, Phenotypes (C) and quantification (D) of rosette growth of the wild type, cngc2, and cngc4 grown on soil at 22°C for 3 weeks and at 6°C for 45 d. Data represent means ± sd from three biological replicates with 10 plants per replica. Double factor variance analysis shows interaction between genotype and temperature. E to G, Phenotypes (E) and survival rate quantifications (F and G) of the wild type and mutants after −8°C treatment for 3 h followed by a 1-week recovery. Data represent means ± sd from nine repeats (one pot per repeat) for each genotype. Asterisks indicate significant difference by Student’s t test (**P < 0.01). Scale bars = 0.5 cm (A), 1 cm (C and D), and 2 cm (E).
We further examined responses to freezing treatments in the Arabidopsis cngc2 and cngc4 mutants. When treated at −8°C for 3 h followed by a 1-week recovery period, the cngc2 mutants displayed a 32% survival rate, significantly lower than the 73% survival rate for the wild-type plants grown side-by-side in the same pot (Fig. 7, E and F). Similarly, the cngc4 mutants had a 28% survival rate, significantly lower than the 76% rate for the wild type grown in the same pot (Fig. 7, E and G). Taken together, these results indicate that the Arabidopsis cngc2 and cngc4 mutants are both impaired in chilling growth and freezing tolerance.
DISCUSSION
Here we report that the rice genes OsCNGC14 and OsCNGC16 have critical functions in heat and chilling tolerance. The loss of either OsCNGC14 or OsCNGC16 leads to a higher susceptibility to heat and chilling compared to the wild type. In addition, these two rice CNGCs are important for generating cytosolic calcium signals in response to heat and cold, which might further influence transcriptional reprogramming under these stress conditions. Heat tolerance was shown to involve the functions of four Arabidopsis CNGC genes (AtCNGC2, AtCNGC4, AtCNGC6, and AtCNGC 16) and a moss gene, PpCNGCb (Finka et al., 2012; Gao et al., 2012; Tunc-Ozdemir et al., 2013). This study demonstrates that rice CNGCs function in heat-stress tolerance. Moreover, these data provide evidence for a function of plant CNGCs in chilling/freezing tolerance. The Arabidopsis AtCNGC2 and AtCNGC4 genes, similar to rice OsCNGC14 and OsCNGC16 genes, are required for growth at chilling temperatures and for freezing tolerance. Although the interpretation of this defect could be complicated by the growth defect in the Arabidopsis cngc2 and cngc4 mutants, it corroborates a positive role of CNGCs in low-temperature responses observed in rice. Therefore, these findings suggest that the role of CNGCs encompasses a further stress tolerance: chilling and freezing tolerance.
Early studies found a negative impact on heat-stress tolerance from AtCNGC2 and AtCNGC4 in Arabidopsis as well as PpCNGCb in moss (Finka et al., 2012). Here, we find that two of their closest homologs in rice, OsCNGC14 and OsCNGC16, contribute positively to heat tolerance. The first scenario explaining this apparent contradiction is that basal tolerance is assayed in rice and acquired tolerance is assayed in Arabidopsis. A more comprehensive analysis of basal and acquired heat tolerance under different treatment conditions would be needed to compare the roles of Arabidopsis and rice CNGCs. Second, the function of CNGC in heat tolerance could be tissue- or developmental-stage dependent. Consistent with this hypothesis, the AtCNGC2 gene was found to be a negative regulator of heat tolerance at the seedling stage but a positive regulator of heat tolerance at the reproductive stage (Katano et al., 2018). Third, the loss of CNGC function might generate stress conditions that trigger acclimation when the stress level is above a threshold. It is possible that the extent of stress induced by the loss of AtCNGC2 or AtCNGC4 in Arabidopsis might be larger than that induced by the loss of OsCNGC14 or OsCNGC16 in rice, and therefore mutations of AtCNGC2 or AtCNGC4 in Arabidopsis induce stress acclimation and enhanced heat tolerance. Consistent with this hypothesis, the rice cngc16 mutant and the wild type had low numbers of DEGs and no enriched GO terms under nonstress conditions, suggesting that the mutant does not significantly up- or downregulate a pathway or process. Nevertheless, in contrast to AtCNGC2 and AtCNGC4, a distantly related gene in Arabidopsis, AtCNGC6, was shown to be a positive regulator of acquired heat tolerance (Gao et al., 2012). This indicates that different CNGCs might have different or even opposite impacts on heat tolerance in the same plant.
OsCNGC14 and OsCNGC16 likely are involved in the early signaling events of both heat and cold responses. Without OsCNGC14 or OsCNGC16, calcium spikes, whether from the first treatment or subsequent treatments of heat or chilling, were reduced in amplitude or totally abolished (Figs. 3 and 6). These results indicate that OsCNGC14 and OsCNGC16 are directly or indirectly required for Ca2+ influx to the plant cells in response to heat or cold. Similar calcium dynamics in response to heat or chilling have been observed between Arabidopsis and rice (Zheng et al., 2012; Kiegle et al., 2000; Mori et al., 2018; Ma et al., 2015). In Arabidopsis, two Ca2+-permeable mechanosensitive channels, MCA1 and MCA2, were found to mediate chilling-induced calcium influx (Mori et al., 2018). The cold-induced cytosolic Ca2+ increase was reduced, but not abolished, in the mca1 mca2 double mutant, suggesting additional modulators or channels for cold sensing. In rice, chilling-tolerance divergence1 (COLD1), a G-protein signaling regulator, can modulate the activity of the channel protein for calcium influx (Ma et al., 2015). In Arabidopsis, AtCNGC6, which mediates the acquisition of thermotolerance, was reported to be required for heat-induced Ca2+ influx using the whole-cell patch-clamp technique (Gao et al., 2012). In moss, PpCNGCb is also required for calcium influx under heat, although the calcium dynamics after heat is different from that in rice (Finka et al., 2012). The impact of OsCNGC14 and OsCNGC16 on calcium signals and tolerance to heat and chilling makes them candidates for channels responsible for the chilling response. Consistent with a function of OsCNGC14 or OsCNGC16 in early temperature-stress signaling, their loss of function or reduction of function led to more signs of stress and damage, such as ROS accumulation, and more cell death in leaves under heat or chilling (Figs. 2, D and E, and 5, D and E). The slightly higher induction of some HSF and HSP genes in the cngc16 mutant (Fig. 4C; Supplemental Table S4) also suggests the perception of more severe stress in the mutant after heat treatment.
The study here reveals that the homologous genes OsCNGC14 and OsCNGC16 have similar functions in temperature-stress tolerance, but their functions are not redundant or totally overlapping. This indicates that either they function in parallel for maximum responses or they interact to form functional complexes. Complexes of heterodimers or heterotetramers have been observed for CNGCs. In Arabidopsis, a homotetramer of the pollen tube-specific AtCNGC18 is an active Ca2+ channel, but the heterotetramer of AtCNGC18 with AtCNGC7 (or AtCNGC8) is inactive (Pan et al., 2019). In rice, OsCNGC15 is very closely related to OsCNGC14 and OsCNGC16 (Supplemental Fig. S1), but a failure to generate its knockout mutant prevented investigation of its function. It will be interesting to examine whether these proteins form complexes among themselves and how complex formation affects their activities in calcium signal generation.
In sum, this study reveals a critical role of CNGCs in low-temperature tolerance as well as in heat tolerance in plants and suggests a shared molecular mechanism in calcium signaling in response to both temperature-stress conditions. Future study should reveal whether and how CNGCs form channels for calcium influx and how they respond to temperature changes and impact temperature-stress signaling.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
The rice genotype used in this study is Oryza sativa ‘Nipponbare’. Freshly harvested seeds were treated for 2 weeks or more at 42°C to break dormancy, soaked in water at room temperature for 3 to 5 d, and then germinated for 1 d at 37°C. For hydroponic growth, seeds at a similar germination stage were transferred to a 96-well plate (with its bottom cut) sitting in water, as described previously (Huang et al., 2009). Seedlings were grown in chambers with 16 h light at 26°C. For soil growth, germinated seeds were transferred to a mix of vermiculite:special substrate:organic substrate (1:1:1) and cultured at 26°C in the greenhouse.
The Arabidopsis (Arabidopsis thaliana) mutants cngc2 (stock no. CS6523) and cngc4 (stock no. CS6523) were obtained from the Arabidopsis Biological Resource Center. For Arabidopsis growth, seeds were sown in a mixture of vermiculite:soil (1:1), cold treated at 4°C for 3 d, and germinated and grown in controlled chambers.
Plasmid Constructions and Rice Transformation
For generating CRISPR/Cas9 constructs, specific targets of guide RNA spacer sequences were designed using the CRISPR-PLANT web site (http://www.genome.arizona.edu/crispr/CRISPRsearch.html). Two guide RNA targets were cloned into the PHUE411 construct (Xing et al., 2014) and expressed in cv Nipponbare by Agrobacterium tumefaciens-mediated transformation (Nishimura et al., 2006). For subcellular localization, the coding sequences from the genomic DNA were cloned first into the entry vector pDONR221 (Magnani et al., 2006) by BP Clonase II Enzyme mix (Invitrogen) and then into the destination vector phpt-pSATN1-GW (Tzfira et al., 2005) using LR Clonase II Enzyme mix (Invitrogen).
All primer sequences are listed in Supplemental Table S5.
Protein Subcellular Location
Nicotiana benthamiana leaves expressing GFP proteins were analyzed with a ZEISS LSM 780 confocal microscope (ZEISS Microsystems). The excitation lines for imaging GFP and mCherry fusions were 488 and 580 nm, respectively. Images were analyzed using ZEISS Microsystems software.
Heat and Cold Tolerance Assays
Heat and cold tolerance assays were performed using 2-week-old rice plants. Genotypes to be compared were planted side by side each occupying half a plate in the same 96-well plate and treated with 48°C for 48 h (heat) or 6°C for 72 h (chilling). Three plates were treated at the same time and at least three independent treatments were conducted.
Hypocotyl elongation assays were done on seeds sown on one-half strength Murashige and Skoog plates, stratified at 4°C for 3 d, and allowed to germinate at 22°C for 24 h. Seeds at a similar germination stage were then grown at 22°C for 36 h or at 6°C for 4 d in the dark. For measurement of rosette growth under chilling, seedlings were grown first at 22°C for 1 week followed by 45 d at 4°C. Freezing tolerance assays were done on 2-week-old seedlings grown at 22°C in chambers. Plants were moved to a −8°C chamber for 3 h before moving back to the 22°C chamber for 1 week of recovery. Plants of two different genotypes were planted side by side in the same pot for comparison. Nine replicate pots were freezing-stress treated at the same time.
DAB and Trypan Blue Staining
Accumulation of H2O2 and cell death were detected by DAB and trypan blue staining, respectively, as described previously (Shen et al., 2015; Thordal-Christensen et al.,1997).
Ion Leakage Analysis
Ion leakage was measured as previously described, with some modifications (Charng et al., 2007). For each sample, three leaves were combined and cut into 0.5-cm pieces and incubated in 0.4 m mannitol at room temperature with gentle shaking for 3 h before the initial conductivity of the solution was measured with a conductivity meter (DDS-307A, Ningbo Hinotek Technology). Total conductivity of the solution was measured after incubation at 85°C for 20 min. The ion leakage rate was expressed as the percentage of initial conductivity divided by total conductivity.
RNA Extraction, RT-PCR, and RT-qPCR
Total RNAs were isolated from seedling tissues using TRIpure Reagent (Bioteke) according to the manufacturer’s protocols. One microgram total RNA was reverse transcribed into cDNA using HiScript II RT SuperMix (Vazyme). The cDNAs were then used as templates for qPCR with gene-specific primers. Relative quantitative expression was normalized to the reference gene OsActin1 (LOC_Os03g61970) and calculated as described previously (Gao et al., 2012). The RT-qPCR was performed using AceQ qPCR SYBR Green Master Mix (Vazyme) and the Bio-Rad CFX96 real-time PCR detection system (Bio-Rad Laboratories). Primer sequences are listed in Supplemental Table S5.
RNA-Seq and Data Analysis
Two-week-old rice seedlings grown in soil at 26°C were heat treated (42°C), and leaf tissues were collected at 0, 3, and 24 h after treatment. Total RNAs were extracted using TRIpure Reagent (Bioteke) according to the manufacturer’s protocol. The transcriptomes were sequenced using Illumina HiSeq X Ten (PG150, Illumina). The adaptor sequences and low-quality sequences were removed using Hisat2 (Kim et al., 2015). Approximately 4.7 GB of clean reads were generated from each sample. The clean reads were mapped to the rice reference genome (IRGSP-1.0). Gene expression normalization among samples was performed using DESeq2 (Love et al., 2014).
PCA analysis was carried out via the web tool https://www.omicshare.com/tools/Home/Soft/pca. The spearman correlation was calculated by the Python Pandas package. Venn diagram analysis was done via the web tool http://bioinfogp.cnb.csic.es/tools/venny/, and the statistical test for overlapping significance was based on the online tool http://nemates.org/MA/progs/overlap_stats.html.
Ca2+ Imaging
FRET-based Ca2+ imaging was performed as described previously (Li et al., 2017). For heat and cold treatment, images were acquired every 1 and 2 s, respectively.
Statistical Analysis
Data were processed in Excel version 2010 (Microsoft Corporation). Comparisons between group means were performed with Student’s t test.
Accession Numbers
Sequence data were obtained from the Rice Annotation Project Database (http://rice.plantbiology.msu.edu/index.shtml) according to the following accession numbers: LOC_Os03g55100 (OsCNGC14) and LOC_Os05g42250 (OsCNGC16). The GenBank accession numbers are as follows: NC_029258.1 (OsCNGC14) and NC_029260.1 (OsCNGC16).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Phylogenetic tree of CNGC proteins in Arabidopsis, moss, and rice.
Supplemental Figure S2. Subcellular localization of OsCNGC14 and OsCNGC16.
Supplemental Figure S3. Global expression profiles of OsCNGC14 and OsCNGC16 at various stages of development.
Supplemental Figure S4. Expression pattern of OsCNGC14 and OsCNGC16 genes in response to heat- and cold-stress conditions.
Supplemental Figure S5. RNA expression of OsCNGC14 and OsCNGC16 in the wild type and the cngc mutants.
Supplemental Figure S6. Mutants of OsCNGC14 and OsCNGC16 are heat and cold susceptible at seedling stage.
Supplemental Figure S7. Signals of NES-YC3.6 in the wild type and the cngc14 and cngc16 mutants.
Supplemental Figure S8. Overall analyses of transcriptomes of the wild type and the cngc16-3 mutant.
Supplemental Figure S9. GO enrichment analysis of DEGs between the cngc16-3 mutant and the wild type at 3 h and 24 h.
Supplemental Figure S10. Venn diagram showing overlaps of high temperature-responsive genes and DEGs of cngc16-3 mutant.
Supplemental Table S1. DEGs between cngc16-3 mutant and the wild type at 0, 3, and 24 h.
Supplemental Table S2. Heat-induced and heat-repressed genes in wild-type plants at 3 and 24 h.
Supplemental Table S3. Heat-induced and heat-repressed genes in the cngc16-3 mutant at 3 and 24 h.
Supplemental Table S4. Expression levels of HSFs and HSPs in the wild type and the cngc16-3 mutant at 0, 3, and 24 h.
Supplemental Table S5. List of primers and their sequences used in this study.
Supplemental Movie S1. Cytosolic Ca2+ transient induced in wild-type plants by heat treatment.
Supplemental Movie S2. Cytosolic Ca2+ transient induced in cngc14-2 plants by heat treatment.
Supplemental Movie S3. Cytosolic Ca2+ transient induced in cngc16-1 plants by heat treatment.
Supplemental Movie S4. Cytosolic Ca2+ transient induced in wild-type plants by cold treatment.
Supplemental Movie S5. Cytosolic Ca2+ transient induced in cngc14-2 plants by cold treatment.
Supplemental Movie S6. Cytosolic Ca2+ transient induced in cngc16-1 plants by cold treatment.
Acknowledgments
We thank Dr. Jörg Kudla for the NES-YC3.6 transgenic materials, Qianru Jia and other members of Dr. Wenhua Zhang’s group for assisting calcium imaging, and Kejian Shi for assisting in mutant material generation.
Footnotes
This work was supported by the National Science Foundation of China (grant nos. 31700223 to S.L. and 31670269 to J.H.), Fundamental Research Funds for the Central Universities (grant nos. KJQN201807 to S.L., and KYZ201813 to J.H.), open funds of the State Key Laboratory of Plant Physiology and Biochemistry (grant no. SKLPPBKF1501 to B.Z.), the Jiangsu Collaborative Innovation Center for Modern Crop Production, and the Cyrus Tang Innovation Center for Seed Industry.
Articles can be viewed without a subscription.
References
- Atkinson D, Porter JR(1996) Temperature, plant development and crop yields. Trends Plant Sci 1: 119–124 [Google Scholar]
- Behera S, Wang N, Zhang C, Schmitz-Thom I, Strohkamp S, Schültke S, Hashimoto K, Xiong L, Kudla J(2015) Analyses of Ca2+ dynamics using a ubiquitin-10 promoter-driven Yellow Cameleon 3.6 indicator reveal reliable transgene expression and differences in cytoplasmic Ca2+ responses in Arabidopsis and rice (Oryza sativa) roots. New Phytol 206: 751–760 [DOI] [PubMed] [Google Scholar]
- Borsics T, Webb D, Andeme-Ondzighi C, Staehelin LA, Christopher DA(2007) The cyclic nucleotide-gated calmodulin-binding channel AtCNGC10 localizes to the plasma membrane and influences numerous growth responses and starch accumulation in Arabidopsis thaliana. Planta 225: 563–573 [DOI] [PubMed] [Google Scholar]
- Brost C, Studtrucker T, Reimann R, Denninger P, Czekalla J, Krebs M, Fabry B, Schumacher K, Grossmann G, Dietrich P(2019) Multiple cyclic nucleotide-gated channels coordinate calcium oscillations and polar growth of root hairs. Plant J 99: 910–923 [DOI] [PubMed] [Google Scholar]
- Chan CWM, Schorrak LM, Smith RKW Jr., Bent AF, Sussman MR(2003) A cyclic nucleotide-gated ion channel, CNGC2, is crucial for plant development and adaptation to calcium stress. Plant Physiol 132: 728–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charng YY, Liu HC, Liu NY, Chi WT, Wang CN, Chang SH, Wang TT(2007) A heat-inducible transcription factor, HsfA2, is required for extension of acquired thermotolerance in Arabidopsis. Plant Physiol 143: 251–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin K, DeFalco TA, Moeder W, Yoshioka K(2013) The Arabidopsis cyclic nucleotide-gated ion channels AtCNGC2 and AtCNGC4 work in the same signaling pathway to regulate pathogen defense and floral transition. Plant Physiol 163: 611–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFalco TA, Moeder W, Yoshioka K(2016) Opening the gates: Insights into cyclic nucleotide-gated channel-mediated signaling. Trends Plant Sci 21: 903–906 [DOI] [PubMed] [Google Scholar]
- Finka A, Cuendet AFH, Maathuis FJM, Saidi Y, Goloubinoff P(2012) Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired thermotolerance. Plant Cell 24: 3333–3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragkostefanakis S, Mesihovic A, Hu Y, Schleiff E(2016) Unfolded protein response in pollen development and heat stress tolerance. Plant Reprod 29: 81–91 [DOI] [PubMed] [Google Scholar]
- Gao F, Han X, Wu J, Zheng S, Shang Z, Sun D, Zhou R, Li B(2012) A heat-activated calcium-permeable channel—Arabidopsis cyclic nucleotide-gated ion channel 6—is involved in heat shock responses. Plant J 70: 1056–1069 [DOI] [PubMed] [Google Scholar]
- Gobert A, Park G, Amtmann A, Sanders D, Maathuis FJM(2006) Arabidopsis thaliana cyclic nucleotide gated channel 3 forms a non-selective ion transporter involved in germination and cation transport. J Exp Bot 57: 791–800 [DOI] [PubMed] [Google Scholar]
- Guo KM, Babourina O, Christopher DA, Borsics T, Rengel Z(2008) The cyclic nucleotide-gated channel, AtCNGC10, influences salt tolerance in Arabidopsis. Physiol Plant 134: 499–507 [DOI] [PubMed] [Google Scholar]
- Hatfield JL, Prueger JH(2015) Temperature extremes: Effect on plant growth and development. Weather Clim Extrem 10: 4–10 [Google Scholar]
- Huang XY, Chao DY, Gao JP, Zhu MZ, Shi M, Lin HX(2009) A previously unknown zinc finger protein, DST, regulates drought and salt tolerance in rice via stomatal aperture control. Genes Dev 23: 1805–1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katano K, Kataoka R, Fujii M, Suzuki N(2018) Differences between seedlings and flowers in anti-ROS based heat responses of Arabidopsis plants deficient in cyclic nucleotide gated channel 2. Plant Physiol Biochem 123: 288–296 [DOI] [PubMed] [Google Scholar]
- Kiegle E, Moore CA, Haseloff J, Tester MA, Knight MR(2000) Cell-type-specific calcium responses to drought, salt and cold in the Arabidopsis root. Plant J 23: 267–278 [DOI] [PubMed] [Google Scholar]
- Kim D, Langmead B, Salzberg SL(2015) HISAT: A fast spliced aligner with low memory requirements. Nat Methods 12: 357–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs M, Held K, Binder A, Hashimoto K, Den Herder G, Parniske M, Kudla J, Schumacher K(2012) FRET-based genetically encoded sensors allow high-resolution live cell imaging of Ca2+ dynamics. Plant J 69: 181–192 [DOI] [PubMed] [Google Scholar]
- Kugler A, Köhler B, Palme K, Wolff P, Dietrich P(2009) Salt-dependent regulation of a CNG channel subfamily in Arabidopsis. BMC Plant Biol 9: 140–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladwig F, Dahlke RI, Stührwohldt N, Hartmann J, Harter K, Sauter M(2015) Phytosulfokine regulates growth in Arabidopsis through a response module at the plasma membrane that includes CYCLIC NUCLEOTIDE-GATED CHANNEL17, H+-ATPase, and BAK1. Plant Cell 27: 1718–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeggangers HACF, Nijveen H, Bigas JN, Hilhorst HWM, Immink RGH(2017) Molecular regulation of temperature-dependent floral induction in Tulipa gesneriana. Plant Physiol 173: 1904–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wang F, Yan P, Jing W, Zhang C, Kudla J, Zhang W(2017) A phosphoinositide-specific phospholipase C pathway elicits stress-induced Ca2+ signals and confers salt tolerance to rice. New Phytol 214: 1172–1187 [DOI] [PubMed] [Google Scholar]
- Liu HT, Gao F, Li GL, Han JL, Liu DL, Sun DY, Zhou RG(2008) The calmodulin-binding protein kinase 3 is part of heat-shock signal transduction in Arabidopsis thaliana. Plant J 55: 760–773 [DOI] [PubMed] [Google Scholar]
- Liu HT, Li B, Shang ZL, Li XZ, Mu RL, Sun DY, Zhou RG(2003) Calmodulin is involved in heat shock signal transduction in wheat. Plant Physiol 132: 1186–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Niu Y, Zhang J, Zhou Y, Ma Z, Huang X(2018) Ca2+ channels and Ca2+ signals involved in abiotic stress responses in plant cells: Recent advances. Plant Cell Tissue Organ Cult 132: 413–424 [Google Scholar]
- Liu J, Zhang C, Wei C, Liu X, Wang M, Yu F, Xie Q, Tu J(2016) The RING finger ubiquitin E3 ligase OsHTAS enhances heat tolerance by promoting H2O2-induced stomatal closure in rice. Plant Physiol 170: 429–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K(1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10: 1391–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S(2014) Moderated estimation of fold change and dispersion for RNA-seq with DESeq2. Genome Biol 15: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Ali R, Berkowitz GA(2006) Characterization of plant phenotypes associated with loss-of-function of AtCNGC1, a plant cyclic nucleotide gated cation channel. Plant Physiol Biochem 44: 494–505 [DOI] [PubMed] [Google Scholar]
- Ma Y, Dai X, Xu Y, Luo W, Zheng X, Zeng D, Pan Y, Lin X, Liu H, Zhang D, et al. (2015) COLD1 confers chilling tolerance in rice. Cell 160: 1209–1221 [DOI] [PubMed] [Google Scholar]
- Magnani E, Bartling L, Hake S(2006) From Gateway to MultiSite Gateway in one recombination event. BMC Mol Biol 7: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meena MK, Prajapati R, Krishna D, Divakaran K, Pandey Y, Reichelt M, Mathew MK, Boland W, Mithöfer A, Vadassery J(2019) The Ca2+ channel CNGC19 regulates Arabidopsis defense against Spodoptera herbivory. Plant Cell 31: 1539–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R.(2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7: 405–410 [DOI] [PubMed] [Google Scholar]
- Mori K, Renhu N, Naito M, Nakamura A, Shiba H, Yamamoto T, Suzaki T, Iida H, Miura K(2018) Ca2+-permeable mechanosensitive channels MCA1 and MCA2 mediate cold-induced cytosolic Ca2+ increase and cold tolerance in Arabidopsis. Sci Rep 8: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y, Tsuyama M, Kobayashi Y, Kodama H, Iba K(2000) Trienoic fatty acids and plant tolerance of high temperature. Science 287: 476–479 [DOI] [PubMed] [Google Scholar]
- Murata N, Los DA(1997) Membrane fluidity and temperature perception. Plant Physiol 115: 875–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz Z, Kakar KU, Saand MA, Shu Q-Y(2014) Cyclic nucleotide-gated ion channel gene family in rice, identification, characterization and experimental analysis of expression response to plant hormones, biotic and abiotic stresses. BMC Genomics 15: 853–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AN, Shiozaki K(1999) Heat-shock-induced activation of stress MAP kinase is regulated by threonine- and tyrosine-specific phosphatases. Genes Dev 13: 1653–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura A, Aichi I, Matsuoka M(2006) A protocol for Agrobacterium-mediated transformation in rice. Nat Protoc 1: 2796–2802 [DOI] [PubMed] [Google Scholar]
- Ohama N, Sato H, Shinozaki K, Yamaguchi-Shinozaki K(2017) Transcriptional regulatory network of plant heat stress response. Trends Plant Sci 22: 53–65 [DOI] [PubMed] [Google Scholar]
- Pan Y, Chai X, Gao Q, Zhou L, Zhang S, Li L, Luan S(2019) Dynamic interactions of plant CNGC subunits and calmodulins drive oscillatory Ca2+ channel activities. Dev Cell 48: 710–725.e5 [DOI] [PubMed] [Google Scholar]
- Prasad TK, Anderson MD, Martin BA, Stewart CR(1994) Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell 6: 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieu I, Twell D, Firon N(2017) Pollen development at high temperature: From acclimation to collapse. Plant Physiol 173: 1967–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidi Y, Finka A, Goloubinoff P(2011) Heat perception and signalling in plants: A tortuous path to thermotolerance. New Phytol 190: 556–565 [DOI] [PubMed] [Google Scholar]
- Saidi Y, Finka A, Muriset M, Bromberg Z, Weiss YG, Maathuis FJM, Goloubinoff P(2009) The heat shock response in moss plants is regulated by specific calcium-permeable channels in the plasma membrane. Plant Cell 21: 2829–2843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Zhong X, Zhao F, Wang Y, Yan B, Li Q, Chen G, Mao B, Wang J, Li Y, et al. (2015) Overexpression of receptor-like kinase ERECTA improves thermotolerance in rice and tomato. Nat Biotechnol 33: 996–1003 [DOI] [PubMed] [Google Scholar]
- Stockinger EJ, Gilmour SJ, Thomashow MF(1997) Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci USA 94: 1035–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y-Q, Yang Y, Zhang A, Fei C-F, Gu L-L, Sun S-J, Xu W, Wang L, Liu H, Wang Y-F(2019) Three CNGC family members, CNGC5, CNGC6, and CNGC9, are required for constitutive growth of Arabidopsis root hairs as Ca2+-permeable channels. Plant Commun 1: 100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei YD, Collinge DB(1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11: 1187–1194 [Google Scholar]
- Tunc-Ozdemir M, Tang C, Ishka MR, Brown E, Groves NR, Myers CT, Rato C, Poulsen LR, McDowell S, Miller G, et al. (2013) A cyclic nucleotide-gated channel (CNGC16) in pollen is critical for stress tolerance in pollen reproductive development. Plant Physiol 161: 1010–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzfira T, Tian GW, Lacroix B, Vyas S, Li J, Leitner-Dagan Y, Krichevsky A, Taylor T, Vainstein A, Citovsky V(2005) pSAT vectors: A modular series of plasmids for autofluorescent protein tagging and expression of multiple genes in plants. Plant Mol Biol 57: 503–516 [DOI] [PubMed] [Google Scholar]
- Wada H, Gombos Z, Murata N(1990) Enhancement of chilling tolerance of a cyanobacterium by genetic manipulation of fatty acid desaturation. Nature 347: 200–203 [DOI] [PubMed] [Google Scholar]
- Wang J, Liu X, Zhang A, Ren Y, Wu F, Wang G, Xu Y, Lei C, Zhu S, Pan T, et al. (2019) A cyclic nucleotide-gated channel mediates cytoplasmic calcium elevation and disease resistance in rice. Cell Res 29: 820–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kang Y, Ma C, Miao R, Wu C, Long Y, Ge T, Wu Z, Hou X, Zhang J, et al. (2017) CNGC2 is a Ca2+ influx channel that prevents accumulation of apoplastic Ca2+ in the leaf. Plant Physiol 173: 1342–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing HL, Dong L, Wang ZP, Zhang HY, Han CY, Liu B, Wang XC, Chen QJ(2014) A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol 14: 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Yang J, Wang Y, Wang J, Yu Y, Long Y, Wang Y, Zhang H, Ren Y, Chen J, et al. (2017) OsCNGC13 promotes seed-setting rate by facilitating pollen tube growth in stylar tissues. PLoS Genet 13: e1006906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Wei CL, Zhang WR, Cheng HP, Liu J(2006) Cross-talk between calcium and reactive oxygen species signaling. Acta Pharmacol Sin 27: 821–826 [DOI] [PubMed] [Google Scholar]
- Yoshioka K, Moeder W, Kang HG, Kachroo P, Masmoudi K, Berkowitz G, Klessig DF(2006) The chimeric Arabidopsis CYCLIC NUCLEOTIDE-GATED ION CHANNEL11/12 activates multiple pathogen resistance responses. Plant Cell 18: 747–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li XM, Lin HX, Chong K(2019) Crop improvement through temperature resilience. Annu Rev Plant Biol 70: 753–780 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Li J, Li F, Liu H, Yang W, Chong K, Xu Y(2017) OsMAPK3 phosphorylates OsbHLH002/OsICE1 and inhibits its ubiquitination to activate OsTPP1 and enhances rice chilling tolerance. Dev Cell 43: 731–743.e5 [DOI] [PubMed] [Google Scholar]
- Zheng SZ, Liu YL, Li B, Shang ZL, Zhou RG, Sun DY(2012) Phosphoinositide-specific phospholipase C9 is involved in the thermotolerance of Arabidopsis. Plant J 69: 689–700 [DOI] [PubMed] [Google Scholar]