Abstract
A galactan epitope is present in two woody plant cell walls and can be used for immmunological analysis of sieve element anatomical characteristics.
Dear Editor,
Carbon transport in the phloem is necessary to support growth, but questions remain about phloem physiology, such as how it changes seasonally and relates to shifts in allocation (De Schepper et al., 2013). One way to understand seasonal changes in carbon transport is through modeling. However, this requires that active sieve elements be identified, and their lumen areas measured to determine phloem conductive area (Thompson, 2006; Savage et al., 2016; Jensen, 2018). Current methods to identify active sieve elements are time consuming, requiring special sample preparation (transmission electron microscopy; Prislan et al., 2013), complex staining protocols (Cheadle et al., 1953), or assumptions about sieve element functionality based on callose accumulation (Montwé et al., 2019). Here we present another option using immunodetection of a β-1,6-galactosyl substitution of β-1,4-galactan, a branched pectin present in sieve element cell walls that is recognized by the LM26 monoclonal antibody (mAb). The LM26 mAb was previously tested with herbaceous species (Torode et al., 2018), but we report detection of the glycan epitope in two woody species and one vine by immunofluorescence (Table 1; Fig. 1). We also provide evidence that in Populus, the presence of the glycan epitope could be seasonal, that is, only detected in actively transporting sieve elements.
Table 1. Immunodetection of the LM26 mAb epitope and location of sampling.
Plus symbols indicate immunodetection of the epitope in the sieve element walls, and minus symbols indicate absence of detection. Asterisks indicate species that were grown in a greenhouse or growth chamber.
| Species | LM26 Staining | Sample |
|---|---|---|
| Acer negundo | – | Stem |
| Acer saccharum | – | Stem |
| Arabidopsis thaliana* | + | Floral stem, petiole |
| Cornus sericea | – | Stem |
| Cucurbita spp. (seedling)* | + | Hypocotyl, petiole |
| Fraxinus pennsylvanica | – | Stem |
| Pelargonium x hortorum* | – | Petiole, peduncle |
| Populus balsamifera | + | Stem, petiole |
| Populus tremuloides | + | Stem, petiole |
| Salix interior (female) | – | Stem |
Figure 1.
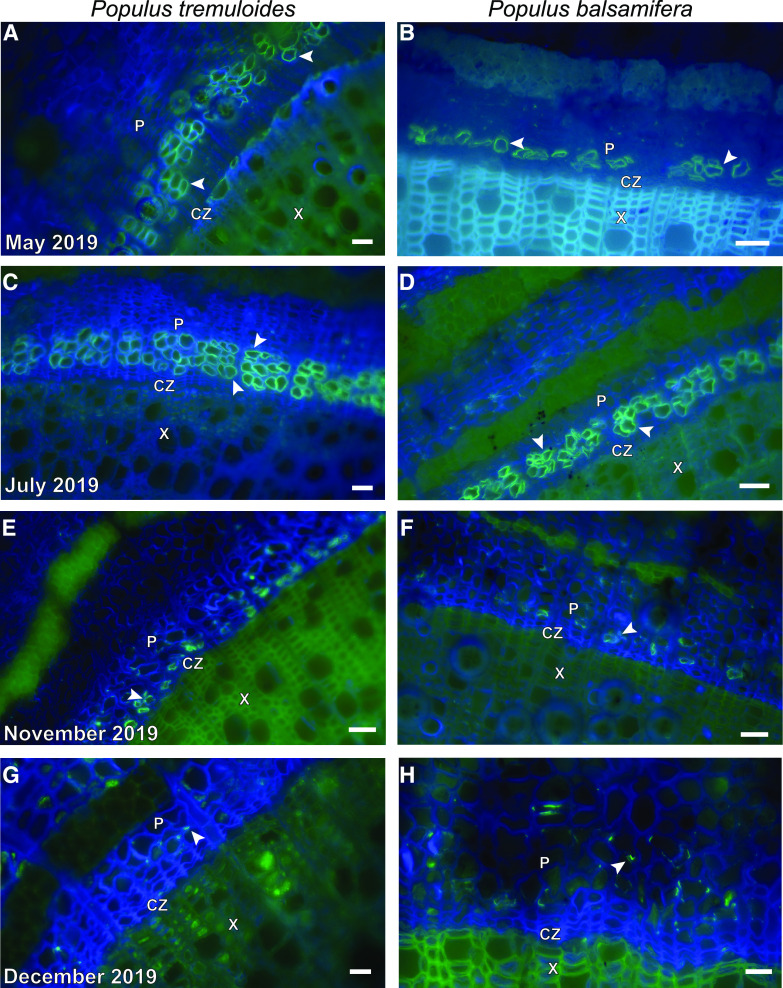
Seasonal series of Populus tremuloides (A, C, E, and G) and Populus balsamifera (B, D, F, and H) stems representative of three biological replicates (n = 3) for each time period and species. In late May, fluorescence was detected in the rows of sieve elements closest to the cambial zone (A and B). More rows of sieve elements had matured when sampled in July (C and D). In the samples from early November (E and F), fluorescence was only detected in some sieve elements within the row closest to the vascular cambium. In early December (G and H), fluorescence was only detected in portions of a small number of cell walls within the phloem. Representative sieve elements within the phloem tissue (P) are indicated by arrowheads in all panels. The cambial zone (CZ) and xylem tissue (X) are also labeled in each panel. Freeze-substitution (C) reduced autofluorescence in the fluorescein channel from the xylem, compared with staining of fresh tissue (A, B, D, E, F, G, and H). Micrographs were captured using a Photometrics Coolsnap Dyno charge-coupled device (CCD) camera attached to a Nikon Eclipse Ts2-2-FL inverted microscope. All scale bars = 30 µm.
We conducted immunofluorescence labeling with the LM26 mAb as the primary antibody according to Xue et al. (2013), but used fresh, rather than fixed, tissue to ensure maximal antigenicity (Supplemental Material S1). We also fixed liquid nitrogen-frozen tissue from the two Populus species by freeze-substitution in ethanol (McDonald, Webb, 2011) and freeze drying at –50°C in a benchtop freeze dryer. Both methods using fixed tissue resulted in immunodetection comparable with that obtained using fresh tissue and appeared to reduce the amount of xylem autofluorescence detected at fluorescein excitation wavelengths (Fig. 1C). In future studies, xylem autofluorescence could also be reduced by staining with Toluidine Blue after immunofluorescence labeling (O’Brien et al., 1964; Xue et al., 2013). Preliminary sampling of P. tremuloides leaves and stems suggested that if sieve element walls contained the epitope recognized by the LM26 mAb, it would be present in multiple organs within the plant body, consistent with Torode et al. (2018). Negative controls contained little fluorescence from nonspecific binding of the secondary antibody controls (no primary antibody; Supplemental Fig. S1).
We followed the course of P. tremuloides and P. balsamifera sieve elements throughout the growing season (May, June, July, October, November, and December 2019) to determine if there was seasonality associated with detection of the epitope (Fig. 1). We observed immunofluorescence in the cell walls of all mature sieve elements until November 2019, after which it was only detected in the walls of the most recently matured sieve elements nearest the cambial zone (Fig. 1, E and F). The fluorescent signal associated with the presence of the epitope was only observed in scattered sections of the sieve tube walls of both species in December 2019 (Fig. 1, G and H). The timing of this absence of fluorescence in the sieve elements after immunolabeling with the LM26 antibody coincides with the timing of loss of sieve element function in Populus (Davis and Evert, 1968; Montwé et al., 2019), suggesting that the epitope associates with walls of functional sieve elements.
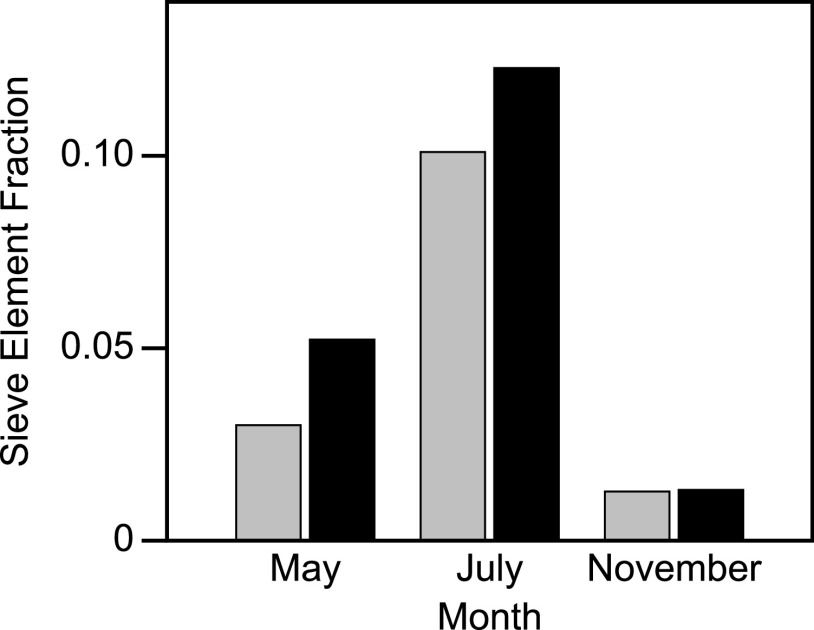
We quantified the sieve element area as a fraction of the measured phloem tissue area (sieve element fraction; Fig. 2) in micrographs from May, July, and November (Fig. 1, A–F) to quantify changes in conducting area over time. Consistent with a previous study (Davis and Evert, 1968), the sieve element area fraction peaked in the summer after the majority of sieve elements had formed. The sieve element area fraction in July was 0.10 for P. balsamifera and 0.12 for P. tremuloides (Fig. 2), which are lower than the value of 0.2 originally reported by Crafts (1931). Canny (1973) suggested that sieve tube area fractions vary between species, an idea that is supported by more recent studies (Khan et al., 1992). Unlike Crafts (1931), we measured the areas only of the sieve element lumens and did not include the cell walls. Although our data are subsampled and not replicated, our measurements suggest that the presence of the branched pectic glycan in the cell walls of the sieve tube elements might be linked to functionality. If this is the case, our results indicate an increase in transport capacity in the spring and a decrease in the fall.
Figure 2.
Sieve element lumen area as a fraction of the measured phloem area for the May, July, and November 2019 images in Figure 1. Sieve tube fractions for P. tremuloides (black bars) and P. balsamifera (gray bars) were 0.05 and 0.03, respectively, in May. Those fractions increased to 0.12 and 0.10 in July, before both decreasing to 0.013 in November as the sieve tubes began to collapse. We quantified the change in sieve element areas between May, July, and November by measuring the sieve element lumen areas within a 3 cm2 (P. balsamifera) or 4 cm2 (P. tremuloides) area of the current season phloem tissue shown, using ImageJ (Schindelin et al., 2012). Sieve element area fraction was the quotient of sieve tube lumen and the total measured phloem area. Populus tremuloides phloem had more tissue between the vascular cambium and the phloem fibers that are formed between seasons, resulting in more total phloem area in that species. We note that we only quantified sieve tubes that fluoresced, indicating the presence of the pectic galactan epitope; therefore, our observation of fewer sieve tubes in November reflects detection of the epitope and not necessarily whether the sieve tubes were actively transporting. No error bars are shown because these measurements were made without replication from the images in Figure 1.
It is unclear why the fluorescence signal associated with the glycan epitope recognized by the LM26 mAb appears to be seasonal in the sieve tube elements of Populus. The epitope has been linked to cell wall elasticity (Torode et al., 2018); however, this elasticity may change seasonally (Wright and Fisher, 1983). Previous research indicates that pectin composition can change seasonally in the vascular cambium and newly formed vascular tissue (Catesson, 1994; Viëtor et al., 1995); therefore, this glycan epitope may not be present in the walls of inactive sieve elements, or wall composition and architecture may change such that detection by the LM26 mAb is physically restricted. If loss of detection of this particular epitope reflects a change in pectin composition, when sieve elements lose function during the transition to winter, species that retain open sieve elements and/or reuse them the following year may retain this specific β-1,6-galactosyl substitution of the β-1,4-galactan year-round. Broader screening of species with knowledge of their sieve tube overwintering strategy would test this hypothesis.
Use of the LM26 mAb, combined with secondary antibodies tagged with fluorescent compounds in seasonal studies, will allow for objective quantification of sieve elements without the need to stain callose, subjective interpretation of active sieve tubes from brightfield micrographs, or the time-consuming use of electron microscopy. We are currently screening more woody and herbaceous species to determine common characteristics that underlie the presence of the glycan epitope in sieve tube walls and the factors that affect its seasonality.
Supplemental Data
The following supplemental materials are available.
Supplemental Material S1. Immunostaining methods.
Supplemental Figure S1. Representative negative control image.
Acknowledgments
We acknowledge J. Paul Knox for kindly providing a sample of the LM26 antibody during preliminary phases of antibody testing. We thank Juan M. Losada and two anonymous reviewers for insightful comments on the manuscript. The Swenson College of Science and Engineering Greenhouse at the University of Minnesota Duluth provided the greenhouse-grown plants that were screened for the gylcan epitope.
Footnotes
This work was supported by the NSF | BIO | Division of Integrative Organismal Systems (IOS; grant no. NSF IOS 1656318 to J.A.S.).
Articles can be viewed without a subscription.
References
- Canny MJ.(1973) Phloem Translocation. Cambridge University Press, London [Google Scholar]
- Catesson A-M.(1994) Cambial ultrastructure and biochemistry: Changes in relation to vascular tissue differentiation and the seasonal cycle. Int J Plant Sci 155: 251–261 [Google Scholar]
- Cheadle VI, Gifford EM Jr., Esau K(1953) A staining combination for phloem and contiguous tissues. Stain Technol 28: 49–53 [DOI] [PubMed] [Google Scholar]
- Crafts AS.(1931) Movement of organic materials in plants. Plant Physiol 6: 1–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JD, Evert RF(1968) Seasonal development of the secondary phloem in populus tremuloides. Bot Gaz 129: 1–8 [Google Scholar]
- De Schepper V, De Swaef T, Bauweraerts I, Steppe K(2013) Phloem transport: A review of mechanisms and controls. J Exp Bot 64: 4839–4850 [DOI] [PubMed] [Google Scholar]
- Jensen KH.(2018) Phloem physics: Mechanisms, constraints, and perspectives. Curr Opin Plant Biol 43: 96–100 [DOI] [PubMed] [Google Scholar]
- Khan MA, Kalimullah, Ahmad Z(1992) Area occupied by sieve elements in the secondary phloem of some leguminous forest trees of Madhya Pradesh, India. Flora 186: 311–315 [Google Scholar]
- McDonald KL, Webb RI(2011) Freeze substitution in 3 hours or less. J Microsc 243: 227–233 [DOI] [PubMed] [Google Scholar]
- Montwé D, Hacke U, Schreiber SG, Stanfield RC(2019) Seasonal vascular tissue formation in four boreal tree species with a focus on callose deposition in the phloem. Front For Glob Change 2: 58 [Google Scholar]
- O’Brien TP, Feder N, McCully ME(1964) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59: 368–373 [Google Scholar]
- Prislan P, Čufar K, Koch G, Schmitt U, Gričar J(2013) Review of cellular and subcellular changes in the cambium. IAWA J 34: 391–407 [Google Scholar]
- Savage JA, Clearwater MJ, Haines DF, Klein T, Mencuccini M, Sevanto S, Turgeon R, Zhang C(2016) Allocation, stress tolerance and carbon transport in plants: How does phloem physiology affect plant ecology? Plant Cell Environ 39: 709–725 [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012) Fiji: An open-source platform for biological-image analysis. Nat Methods 9: 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MV.(2006) Phloem: The long and the short of it. Trends Plant Sci 11: 26–32 [DOI] [PubMed] [Google Scholar]
- Torode TA, O’Neill R, Marcus SE, Cornuault V, Pose S, Lauder RP, Kračun SK, Rydahl MG, Andersen MCF, Willats WGT, et al. (2018) Branched pectic galactan in phloem-sieve-element cell walls: Implications for cell mechanics. Plant Physiol 176: 1547–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viëtor RJ, Renard CMGC, Goldberg R, Catesson A-M(1995) Cell-wall polysaccharides in growing poplar bark tissue. Int J Biol Macromol 17: 341–344 [DOI] [PubMed] [Google Scholar]
- Wright JP, Fisher DB(1983) Estimation of the volumetric elastic modulus and membrane hydraulic conductivity of willow sieve tubes. Plant Physiol 73: 1042–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Bosch M, Knox JP(2013) Heterogeneity and glycan masking of cell wall microstructures in the stems of Miscanthus x giganteus, and its parents M. sinensis and M. sacchariflorus. PLoS One 8: e82114. [DOI] [PMC free article] [PubMed] [Google Scholar]