Bioimaging techniques trace foliar phosphate uptake pathways and visualize whole-leaf photosynthetic restoration processes in phosphorus-deficient barley.
Abstract
Global demand for phosphorus (P) requires new agronomic practices to address sustainability challenges while increasing food production. Foliar P fertilization could increase P use efficiency; however, leaf entry pathways for inorganic phosphate ion (Pi) uptake remain unknown, and it is unclear whether foliar P applications can meet plant nutrient demands. We developed two techniques to trace foliar P uptake in P-deficient spring barley (Hordeum vulgare) and to monitor the effectiveness of the treatment on restoring P functionality. First, a whole-leaf P status assay was developed using an IMAGING PAM system; nonphotochemical quenching was a proxy for P status, as P-deficient barley developed nonphotochemical quenching at a faster rate than P-sufficient barley. The assay showed restoration of P functionality in P-deficient plants 24 h after foliar P application. Treated leaves reverted to P deficiency after 7 d, while newly emerging leaves exhibited partial restoration compared with untreated P-deficient plants, indicating Pi remobilization. Second, vanadate was tested as a possible foliar Pi tracer using high-resolution laser ablation-inductively coupled plasma-mass spectrometry elemental mapping. The strong colocalization of vanadium and P signal intensities demonstrated that vanadate was a sensitive and useful Pi tracer. Vanadate and Pi uptake predominantly occurred via fiber cells located above leaf veins, with pathways to the vascular tissue possibly facilitated by the bundle sheath extension. Minor indications of stomatal and cuticular Pi uptake were also observed. These techniques provided an approach to understand how Pi crosses the leaf surface and assimilates to meet plant nutrient demands.
By 2050, food production may need to increase by 70% to feed a projected 9.7 billion people (Hunter et al., 2017). Therefore, there is a need for increased fertilizer use efficiency, especially phosphorus (P), which is a nonrenewable mineral resource. P is a structural element for essential biomolecules involved in energy metabolism (ATP and NADPH), nucleic acids (DNA and RNA), and phospholipids of cell membranes (Vance et al., 2003). It has been estimated that 30% of the world’s agricultural soils are P deficient and need fertilizer addition to ensure yield and quality (MacDonald et al., 2011). However, phosphate rock, the main source of P fertilizers, is a finite natural resource, and the known rock phosphate reserves are estimated to last as little as 50 years in the gloomiest forecasts (Gilbert, 2009; Edixhoven et al., 2013) to another 300 to 400 years in more optimistic forecasts (Syers et al., 2011). This makes P a potential strategic natural resource similar to oil, as very few countries control the vast majority of the known reserves (Gilbert, 2009; Elser and Bennett, 2011; Edixhoven et al., 2013). Annually, over 80% of mined P is used in fertilizer manufacturing (Cordell and White, 2015). Current practices are inefficient, as only 10% to 30% of the applied soil P is taken up by crops, whereas the majority rapidly becomes unavailable to plants due to soil chemical fixation or microbial immobilization (Syers et al., 2008). Currently, an immense overuse of P is found in some parts of the world, causing eutrophication of lakes and seas, while P depletion has resulted in severe yield limitations elsewhere (Obersteiner et al., 2013). An essential aspect of solving these problems is to increase P use efficiency in agriculture to reduce the negative environmental impact while ensuring a more sustainable use of P resources to increase food production. For these reasons, new methods need to be developed to improve P fertilization practices. Foliar applications could circumvent the problems faced in the soil matrix and are already frequently used for a range of micronutrients (Fernández and Brown, 2013). However, supplying macronutrients such as P exclusively by foliar fertilization is not widely used due to an inadequate uptake efficiency, the limited canopy area at the time of application, and the risk of leaf scorching. Supplemental foliar top-ups throughout the growing season remain a viable possibility (Noack et al., 2010), but generally we need to improve the basic understanding of the uptake pathways to optimize foliar fertilization.
To maximize nutrient use efficiency, foliar fertilizers should be added in response to plant demand. While detecting P deficiency is difficult due to latent physical symptoms, hand-held fluorometers have recently made nondestructive, in-field diagnosis possible (Frydenvang et al., 2015). P deficiency decreases the photosynthetic capacity, since it lowers the bioactive inorganic phosphate ion (Pi) availability as an ATP substrate. The corresponding decreased ATP synthase activity relaxes the so-called I step of the chlorophyll a fluorescence transient (polyphasic O-J-I-P; Carstensen et al., 2018). The use of hand-held fluorometers is a noninvasive, in situ technique that is a faster, more accurate, and cheaper alternative to traditional plant nutrient analysis (Carstensen et al., 2019). Under P deficiency, excess light causes faster acidification of the thylakoid lumen due to lower cytosol Pi availability and reduced ATP synthase activity (Carstensen et al., 2018). The acidification of the thylakoid lumen initiates the xanthophyll cycle and protonation of the PsbS protein, which induces conformational changes of both PSII and light-harvesting complexes (Ruban et al., 2012). This results in nonphotochemical quenching (NPQ), a protective mechanism where absorbed energy is redirected from photochemical processes to heat. NPQ development is slower in P-sufficient plants due to their higher cytosol Pi concentrations and ATP synthase activity, which delays the acidification of the lumen (Carstensen et al., 2018).
To improve plant nutrition, foliar fertilizers must penetrate the leaf surface, which is composed of a range of structures and barriers that affect the passage of ions and molecules. To avoid water loss, leaf surfaces are covered by the cuticle, a hydrophobic surface layer (Fernández et al., 2016). The cuticle is coated by epicuticular waxes and is composed of various lipids including cutin, cutan, waxes, and phenolics, subjacent to more typical cell wall regions containing polysaccharides (Fernández et al., 2016). Epidermal cell wall compositions can influence permeability; cell walls containing higher proportions of pectins have shown higher porosity and more rapid water absorption, while cell walls with more cellulose showed ultimately greater but slower water uptake over longer periods of time (Boanares et al., 2018). Additionally, stomatal openings would allow larger molecules or particles to cross the leaf surface; ∼20 nm pore radii were found in stomatous areas, compared with 2 to 2.4 nm pore radii in astomatous parts of the same leaf (Eichert and Goldbach, 2008). Nanoparticles up to 35 nm were observed to penetrate stomata in watermelon (Citrullus lanatus), although 94 nm particles reportedly obstructed the stomata (Wang et al., 2013). However, the permeability of the stomatal pathway appears to be highly variable, with one study reporting less than 10% penetration of all stomatal openings (Eichert et al., 2008). The hydrophilic pathway model proposed by Schönherr (2006) suggests that there is also a nonstomatal pathway. This pathway consists of aqueous polar pores, supposedly resulting from water adsorbed to less hydrophobic constituents of the cuticle, and is estimated to be 0.6 to 4.8 nm in diameter (Eichert and Goldbach, 2008). There appears to be disagreement regarding true pore sizes; it is possible that pores are dynamic due to swelling properties, as up to 3-fold increases in pore size were reported due to increasing pH and relative humidity (Schönherr, 2006). These pores favorably occur at the base of trichomes and at anticlinal cell walls, suggesting that these structures could be important sites for ion movement across the leaf surface (Schönherr, 2006; Li et al., 2018a).
Identifying the dominant pathways for foliar P uptake is pivotal for improving the ability of foliar fertilizers to cross the cuticle barrier and assimilate within the photosynthetically active tissue. Fluorescent dyes such as uranine and berberine have been used to image the putative foliar uptake pathways and showed the presence of dyes around the anticlinal walls of stomatal complexes, suggesting that uptake could be occurring due to the higher permeability of guard cell cuticles compared with astomatous cuticles (Eichert and Goldbach, 2008; Fernández and Eichert, 2009). However, uncharged dyes are not ideal proxies for Pi, since the charge and basic chemistry of Pi may be of major importance as it readily forms insoluble salts with many cations typically found in plant tissues and it might interact with the ionic environment of the cell wall matrix. Tracing foliar P pathways is challenging, both in terms of detection limits and appropriate image resolution. Moreover, P concentrations in barley (Hordeum vulgare) leaves are relatively high even in P-deficient plants, making it difficult to discriminate foliar-applied P from the P already present in the native tissue. As 31P is the only stable isotope of P, radioisotopes such as 32P and 33P have been the main tracers used to investigate foliar P uptake and translocation in various species (Koontz and Biddulph, 1957; Peirce et al., 2019). However, this approach cannot be used for visualizing foliar pathways, as the resolution of autoradiography is limited to the centimeter or millimeter scale, while foliar pathways require cellular resolution capacities in the low micrometer range in order to be useful.
Laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS) is a powerful technique with the benefit of high sensitivity for light elements such as P. It uses a focused laser beam to physically ablate material into an aerosol, which is then transported by a carrier gas to an ICP-MS instrument. LA-ICP-MS has been used to study a range of biological tissues, including the effects of physical root barriers on nutrient uptake (Persson et al., 2016). It is also more accessible than other techniques, such as synchrotron radiation (Kopittke et al., 2020). Recent technical advances have led to image resolutions down to 5-μm spot sizes, which is sufficient for investigating foliar entry pathways (Chen et al., 2019).
The broad range of elements detectable by LA-ICP-MS makes it possible to trace pathways by using ions that are analogous to Pi. Plant nutrition studies have frequently used analogs to trace nutrient ion transport, such as Ca2+/Sr2+ and K+/Rb+ (Läuchli and Epstein, 1970; Storey and Leigh, 2004). A similar approach is proposed here, using vanadate (VO43−) as a phosphate (PO43−) analog. Vanadium (V) is a group 5 transition metal that primarily exists in the +4 and +5 oxidation states in living organisms (Morrell et al., 1986). Vanadium is typically present in very low concentrations in plant tissue (Morrell et al., 1986). Vanadate and Pi ions have similar radii, pKa values, and stable tetrahedral geometries. Hence, vanadates exhibit a variety of biological activities, partly because they serve as structural mimics of Pi (Cantley et al., 1977). Vanadate is commonly used for investigating transport biochemistry, since it is a potent inhibitor of ion-transporting ATPases in the plasma membrane (Bowman and Slayman, 1979). Studies in root cells showed that vanadate competitively inhibited Pi uptake and plasma membrane ATPase function under P starvation (Shen et al., 2006). Vanadate also affected stomatal opening in epidermal peels through specific inhibition of guard cell H+ ATPases (Schwartz et al., 1991). However, vanadate can be reduced to vanadyl (VO2+) once transported inside cells due to detoxification processes (Crans et al., 2010). Hence, it is likely a valuable tracer to identify entry points across the leaf surface but probably not for investigating further translocation inside plant tissues. Synchrotron X-ray absorption near-edge structure (XANES) spectroscopy can be used to resolve the actual speciation of vanadium inside the leaf tissue (Kopittke et al., 2014). Analytically, it is also advantageous to use vanadate as a phosphate tracer for LA-ICP-MS, as there are no major relevant interferences and it has a lower ionization potential than P (6.75, compared with 10.5 keV), resulting in greater sensitivity.
This study aimed to develop techniques to trace Pi entry pathways across the leaf surface and investigate the restoration of P functionality in P-deficient plants. An IMAGING PAM system was used to develop a whole-leaf P status assay using NPQ as a proxy for the P status of leaves, and vanadate was tested as a possible Pi analog by LA-ICP-MS elemental mapping of leaf cross sections. These techniques were used in concert to examine foliar P uptake pathways and to follow Pi translocation in P-deficient spring barley plants over a one-week period.
RESULTS
Assaying Whole-Leaf P Status
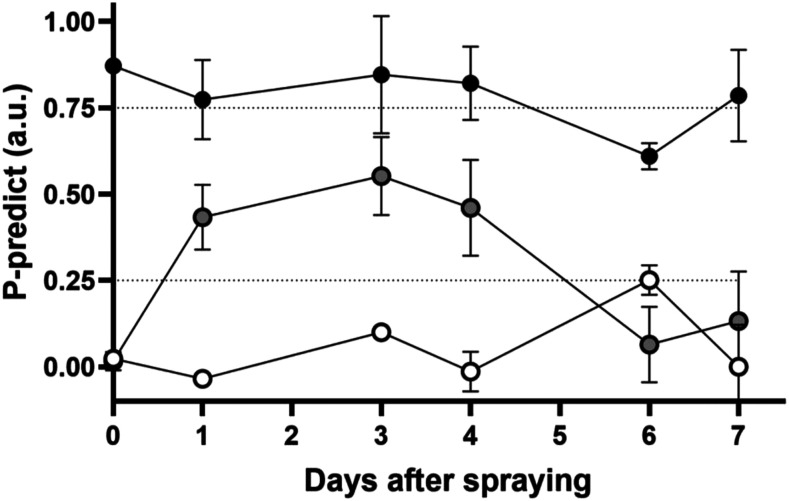
The P status of plants was analyzed using chlorophyll a fluorescence analysis (Frydenvang et al., 2015) and the algorithms presented by Carstensen et al. (2018). Control plants fully supplied with P in the hydroponic medium (P sufficient) were largely classified as fully sufficient throughout the experimental period, while P-deficient control plants remained deficient (Fig. 1). Initial measurements on day 0 were taken on the youngest fully expanded leaf (YFEL) immediately prior to foliar applications. The P status of P-deficient plants treated by foliar P fertilization was moderately restored after 1 d, and P functionality was maintained for 4 d before declining back to P-deficient status by days 6 and 7 (Fig. 1). Day 6 showed a slight anomaly for both controls, where there was a slight increase in P status in P-deficient plants and a decrease in P status for P-sufficient YFELs, although both values remained within 10% of the P status range cutoffs.
Figure 1.
P-Predict status of P-sufficient (black circles), P-deficient (white circles), and foliar P (gray circles) plants over time. Fluorescence was measured in arbitrary units (a.u.). Plant P status is classified as sufficient at P-Predict values above 0.75 a.u. and deficient at values below 0.25 a.u. (for further information, see “Materials and Methods”). Error bars indicate sd (n = 36).
The NPQ assay, based on light-response curves, showed that faster NPQ development occurred in P-deficient compared with P-sufficient YFELs (Supplemental Fig. S1). The NPQ development rate was highest at the beginning of light induction, with differences between treatments most pronounced at 15 s after actinic light was turned on (Student’s t test, P < 0.001). The NPQ value at 15 s was used as the diagnostic for P status, as P-deficient YFELs had a significantly higher average NPQ (1.59 ± 0.14) compared with P-sufficient YFELs (0.59 ± 0.12; Supplemental Fig. S1). The color scale on the IMAGING PAM corresponded to the full range of NPQ values within the light curve; lower values (P sufficient) corresponded to orange-yellow colors and higher values (P deficient) corresponded to green-blue colors (Supplemental Fig. S1).
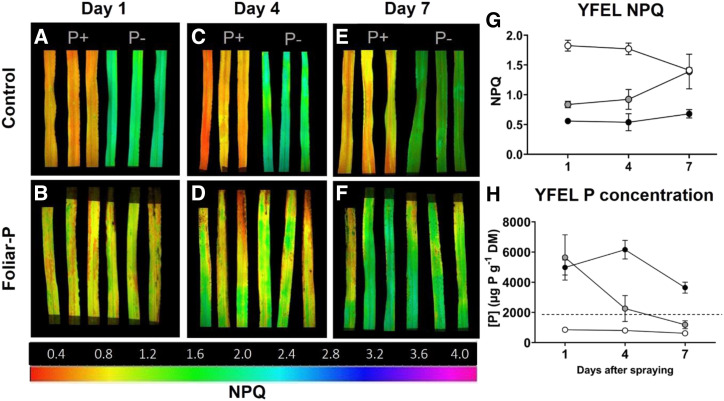
For the foliar P experiment, NPQ assay results were similar to the P-Predict results (Figs. 1 and 2). Control P-sufficient YFELs had P-sufficient status throughout the experiment (yellow-orange in color), while P-deficient YFELs also remained P deficient in status (green; Fig. 2, A, C, and E). Foliar P YFELs showed restoration of P deficiency on day 1 (0.84 ± 0.05, orange-yellow; Fig. 2B), with P status decreasing on day 4 (0.92 ± 0.17, orange-yellow/green; Fig. 2D) before returning to deficient status by day 7 (1.39 ± 0.3, slightly yellow/green; Fig. 2F). Spatial P heterogeneity was observed in foliar P YFELs on day 4, where parts of the leaf were fully restored while others were deficient (Fig. 2D). The averaged NPQ values clearly showed the return of P deficiency in foliar P-treated YFELs over time (Fig. 2G).
Figure 2.
NPQ assay of control (P-sufficient and P-deficient) and foliar P YFEL P status over time. A to F, YFELs were measured on day 1 (A and B), day 4 (C and D), and day 7 (E and F) after spraying. G, Averaged YFEL NPQ values over time for P-sufficient (black circles), P-deficient (white circles), and foliar P (gray circles) YFELs (n = 36). H, YFEL P concentrations for P-sufficient (black circles), P-deficient (white circles), and foliar P (gray circles; n = 24). The color scale indicates the range of leaf P status; green = P deficient, red = P sufficient, and orange/yellow = moderately restored P status. The dotted line indicates the 2,000 µg P g−1 dry mass (DM) threshold for P deficiency. Error bars indicate sd.
Total P concentrations supported NPQ data: P-sufficient YFEL concentrations remained high (>3,600 µg P g−1 dry mass), and P-deficient YFELs had lower concentrations (600–850 µg P g−1 dry mass) throughout the experiment (Fig. 2H). P concentrations in foliar P-treated plants were highest on day 1 (5,644 µg P g−1 dry mass), declining rapidly on day 4 (2,257 µg P g−1 dry mass), and declining further until day 7 when it reached concentrations similar to P-deficient plants (1,186 µg P g−1 dry mass; Fig. 2H). Physiological P deficiency in barley has been shown to correspond to YFEL total P concentrations below 2,000 µg P g−1 dry mass (Carstensen et al., 2019).
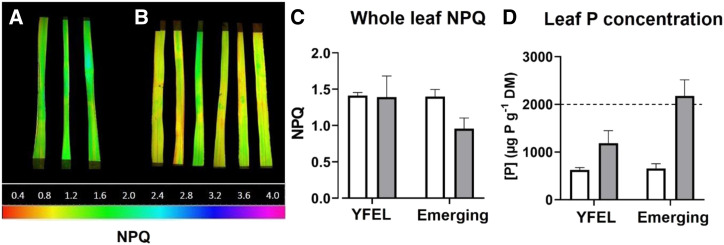
P translocation to new sink tissues appeared to occur in the foliar P-treated plants. The NPQ assay showed partial restoration of P status in the emerging leaves of foliar P-treated plants (0.96 ± 0.15, yellow), while emerging leaves of untreated plants remained deficient (1.4 ± 0.1, green; Fig. 3, A and B). This trend was supported by leaf P concentrations, which were higher in the emerging leaves of foliar P-treated plants compared with untreated plants (2,176 versus 657 µg P g−1 dry mass; Fig. 3D).
Figure 3.
NPQ assay of emerging leaves 7 d after foliar spraying. A and B, Measurements were taken from control P-deficient (A) and foliar P (B) plants. C, Averaged whole-leaf NPQ values over time for YFEL versus emerging leaves from P-deficient (white bars) and foliar P (gray bars) plants (n = 18). D, Total leaf P concentrations for treated versus emerging leaves from P-deficient (white bars) and foliar P (gray bars) plants (n = 18). The color scale indicates the range of leaf P status: green = P deficient, red = P sufficient, and orange/yellow = moderately restored P status. The dotted line indicates the 2,000 µg P g−1 dry mass (DM) threshold for P deficiency. Error bars indicate sd.
Leaf Surface Morphology
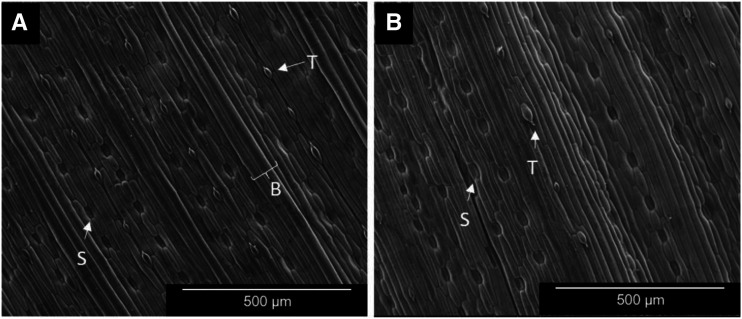
The leaf epidermis was highly structured, consisting of alternating ridges (convex) and valleys (concave) along the leaf axis. The ridges protruding from the surface corresponded to veins and varied in size depending on vein order. Valleys occurred between veins and contained stomata in two rows on either side (peristomal zone). While barley leaves are amphistomatous, stomatal densities tend to be higher on the abaxial epidermis. Trichomes occurred solely on the tops of vein ridges, where cells were noticeably narrower compared with the typical epidermal cell size on both leaf surfaces. Large bulliform cells also occurred on the adaxial epidermis between rows of stomata (Fig. 4A). The adaxial epidermis had a flatter surface plane compared with the abaxial epidermis, which had deeper valleys (Fig. 4B).
Figure 4.
Scanning electron micrographs of barley leaf surfaces in 22-d-old plants. A, Adaxial surface. B, Abaxial leaf surface. B, Bulliform cells; S, stomata; T, trichome.
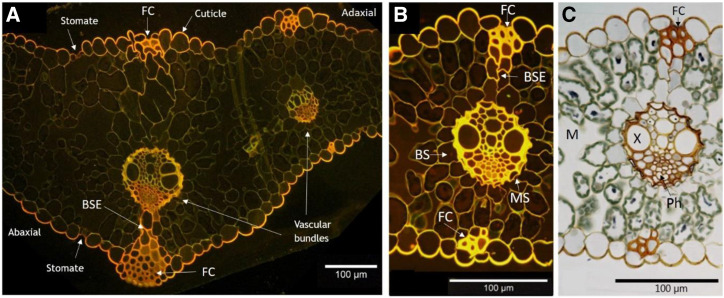
Microscopy images of leaf cross sections revealed the internal anatomy in relation to the surface morphology (Fig. 5). Histological staining using Coriphosphine O dye was previously used to identify pectins in fruit tissue (Weis et al., 1988) but has also been used to stain other cell wall components in variable colors, including lignified sclerenchyma cells (Benazir et al., 2010; Liesche et al., 2011). Coriphosphine O stained the epidermis, phloem, bundle sheath extension (BSE), and fiber cells orange, while lignified xylem cells appeared green-yellow (Fig. 5, A and B). Fiber cells had much thicker walls compared with other cells and aggregated in subepidermal caps around the vascular bundles that also extended partway down the BSE (Fig. 5).
Figure 5.
Coriphosphine O staining of barley leaf cross sections. A, Midrib. B and C, Large vein in fluorescence (B) and bright-field (C) modes. BS, Bundle sheath; FC, fiber cell; MS, mestome sheath; Ph, phloem; X, xylem.
Vanadate as a Pi Tracer
While vanadate and Pi have similar pKa values, vanadium has more complex aqueous speciation than Pi. Vanadate oxyanions can exist in a range of oligomers, including monomers, dimers, tetramers, and decamers. The presence of these species is driven by the pH, concentration, and ionic strength of the solution (Tracy et al., 1995). While VO2+, H2VO4−, HVO4−2, and VO4−3 are the major anions present in solution, equilibrium speciation modeling (Visual MINTEQ 3.1) confirms that, at the tested concentrations, various vanadium oligomers can exist within the pH range 3 to 9.5 (Supplemental Fig. S2). At physiological pH, vanadate was likely present as both H2VO4− and HVO4− species (with less than 30% present as various oligomers), while Pi most likely existed as H2PO4− (Supplemental Fig. S2). Toxic effects of vanadate were tested for by measuring leaf gas-exchange parameters; no significant differences between plants exposed to foliar applied P only and both foliar applied P and V were found (Supplemental Fig. S3).
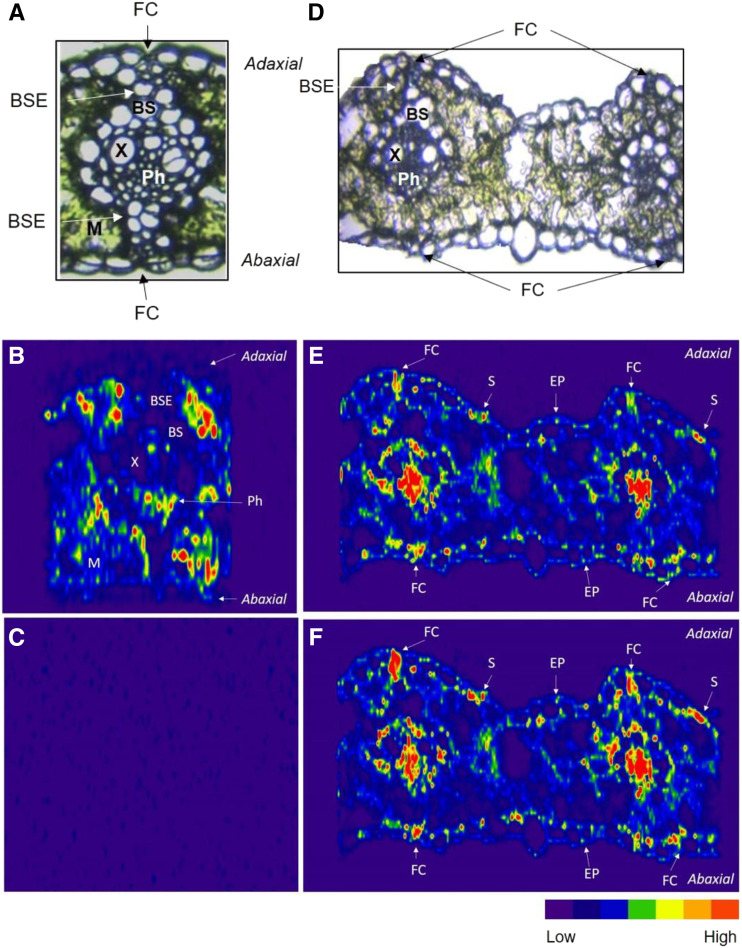
LA-ICP-MS imaging was used to analyze P-deficient and foliar P leaf cross sections taken 24 h after application (Fig. 6). Foliar P barley plants were sprayed until the dripping point with 10 mL of solution containing 0.2 mol L−1 KH2PO4 and 1 mmol L−1 Na3VO4 at pH 6 with 0.05% (v/v) Tween 20. P-deficient barley plants were sprayed with 10 mL of solution containing Milli-Q and 0.05% (v/v) Tween 20. Plants were kept under high humidity for 24 h (for details, see “Materials and Methods”). Figure 6 shows a representative sample with bright-field microscopy images of the cross sections prior to analysis and the corresponding 31P and 51V maps after LA-ICP-MS analysis. The 31P map of the P-deficient leaf cross section showed the presence of P in the mesophyll and in the vascular bundle in what appeared to be the phloem but a weak or absent signal in the epidermal cells, the bundle sheath, and the BSE (Fig. 6B; Supplemental Figs. S4 and S5). In the same image, the very low 51V signal intensities confirmed the anticipated low V background in untreated plant tissue (Fig. 6C). The foliar P-treated cross section showed higher signal intensity in the mesophyll for 31P than 51V, as expected, although the element distributions were very similar (Fig. 6, E and F). Colocalized hotspots of both 31P and 51V occurred in the epidermal cells directly above the vascular bundles, where fiber cells were typically located (Fig. 6, E and F). There were also mutual hotspots for both elements in the bundle sheath and inside the vascular bundle. Ion uptake also occurred in the stomata and at various points across the epidermis, indicating that these pathways could be of importance for foliar P uptake (Fig. 6, E and F). These images indicated that the applied ions were able to penetrate across the leaf surface within 24 h. While the 31P map showed relatively good resolution in relation to the leaf anatomy (Fig. 6E), clear replicate 31P signals were more variable and typically much more difficult to obtain than 51V signals (Supplemental Figs. S6 and S7). While this was expected, as LA-ICP-MS has lower sensitivity for lighter elements (31P), it was a further benefit associated with using 51V.
Figure 6.
LA-ICP-MS elemental maps of barley leaf cross sections. The signal intensities are represented as a heat map, where red indicates high signal intensity and purple/blue indicates low signal intensity. A, Light microscopy image of a control P-deficient cross section. B, 31P map of P-deficient (untreated) leaf. C, 51V map of P-deficient (untreated) leaf. D, Light microscopy cross section image of leaf applied foliar P. E, Foliar P 31P map. F, Foliar P 51V map. BS, Bundle sheath; EP, epidermis cell; FC, fiber cell; M, mesophyll; Ph, phloem; S, stomate; X, xylem. All data points were normalized to 13C. Similar results were observed in replicate samples (Supplemental Figs. S4–S7).
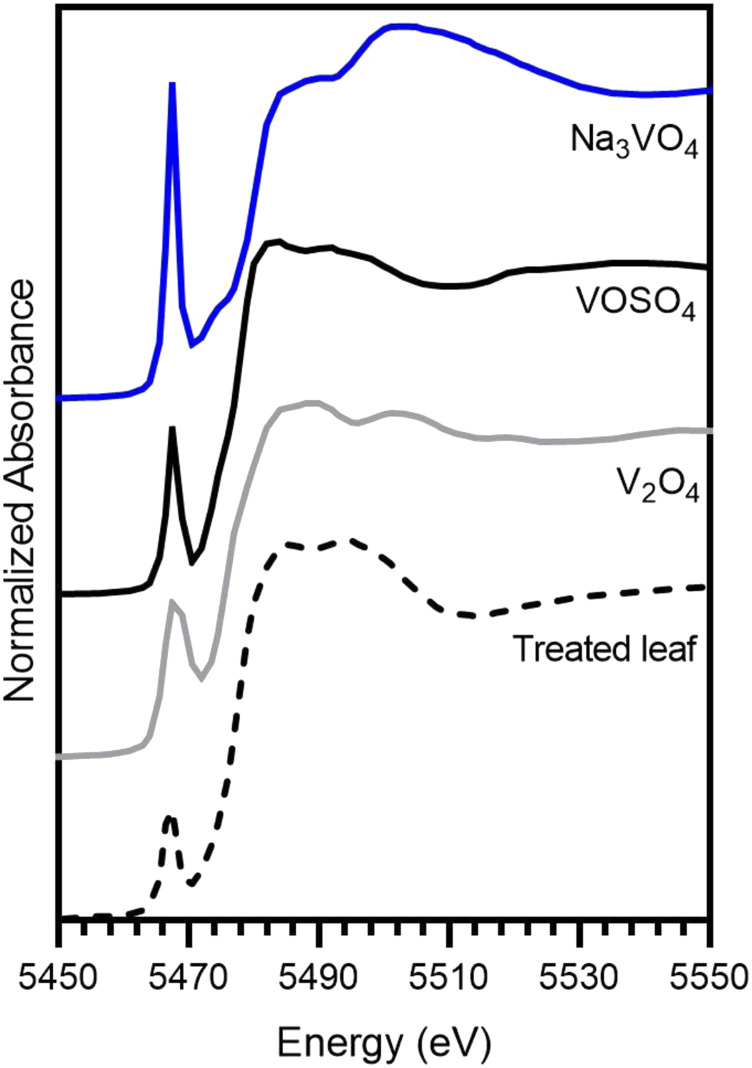
It was necessary to investigate whether the vanadate speciation changed once inside the leaf tissue, as reduction from pentavalent vanadate (VO43−) to tetravalent vanadyl (VO2+) would affect its ability to act as a P analog. A foliar P leaf cross section was analyzed using synchrotron-based µXANES spectroscopy and compared with several standards (Fig. 7). Based on the size of the pre-edge peak at 5,464 eV (Supplemental Fig. S8A), the position of the main edge region, and the shape of the post-edge peaks at the 5,475 eV (Supplemental Fig. S8B), the V present in the foliar P-treated leaf most closely resembled V2O4 and VOSO4 (Supplemental Fig. S8C). This indicated that 24 h after foliar application, the V present in the leaf tissue had been reduced to vanadyl (V→IV oxidation state).
Figure 7.
Normalized V K-edge XANES for three standard vanadium compounds (Na3VO4, VOSO4, and V2O4) and the treated foliar P leaf cross section, measured 24 h after the foliar solution was applied.
DISCUSSION
In this study, we developed two techniques to trace foliar P uptake and translocation in P-deficient spring barley and to monitor the actual effect the treatment had on the restoration of P functionality. First, the NPQ method showed that foliar P was able to restore P functionality within 24 h and that the effect lasted approximately 4 d (Fig. 2). Second, strong colocalization of 51V and 31P element signal intensities in LA-ICP-MS maps (Fig. 6) validated vanadate as a suitable foliar P tracer and supported its use to visualize foliar P entry pathways for the first time in any plant species. High signal intensities were observed in fiber cells located at the nexus between the BSE and the epidermis (Fig. 6). Stomatal and cuticular penetration was also observed, although there was no accumulation within stomatal cavities, indicating their probable role as minor pathways. Future avenues for investigating foliar P fertilization effectiveness in relation to these methods are discussed below.
Imaging Whole-Leaf P Status
While rapid foliar P uptake and translocation was imaged as early as 1957 using autoradiography, the NPQ assay shows such uptake in relation to plant nutritional demand. Our assay showed that foliar P restored P deficiency within 24 h, lasting for several days before reverting to P deficiency 7 d after application (Fig. 2). Newly emerging leaves also showed evidence of P deficiency restoration through higher NPQ values, indicating that foliar P was translocated to the new sink tissues. This was confirmed by leaf P concentrations showing a corresponding increase (Fig. 3). This rapid translocation indicated that foliar P only provided a temporary nutritional relief for the deficient leaves due to the rapid remobilization of P to developing leaves. This was also supported by the P concentrations in the YFEL, which were highest 24 h after application and declined to concentrations similar to control P-deficient leaves by day 7 (Fig. 2). The chlorophyll a fluorescence analysis and the derived P-Predict results also showed restoration of the P status after 24 h, although results indicated that maximum restoration occurred after 4 d (Fig. 1). The major limitation with the P-Predict method was that it captured a relatively small sample area per leaf sample and was more vulnerable to the effects of sample clip placement along the leaf; results representing whole-leaf P status could be affected by spatial heterogeneity, as seen in NPQ images on day 4 (Fig. 2D).
The NPQ assay is an approach to tracing foliar P uptake and translocation by a direct visualization of the plant physiological responses and may be a useful tool for investigating current issues associated with foliar P fertilization. Foliar P studies have commonly applied phosphoric acid, which often causes leaf scorching due to low pH and/or high salt load (Peirce et al., 2014). Scorching could limit potential yield increases due to decreased leaf photosynthesis or localized cell death from rapid uptake. However, this leaf damage could also improve ion penetration. The NPQ assay could visualize leaf scorch and analyze the effects on leaf P status. Due to the rapid nature of P translocation observed using the NPQ assay, foliar P solutions could be used to provide temporary nutrient relief in the form of fertilizer top-ups. However, achieving improvements in grain yields or nutritional composition may require P provision over longer periods, possibly through multiple sprayings or slow-release formulations. The NPQ assay could be a powerful tool to visualize the effects of these alternative strategies.
Foliar P Entry Pathways
This method has successfully visualized P entry via foliar pathways. Indeed, P-deficient, untreated plants had very low 51V background signal intensities, which was ideal for using 51V as a Pi tracer in foliar P cross sections (Fig. 6C). 51V signal intensities in the treated cross sections were high enough to resolve leaf anatomical features based on the 5-μm laser ablation spot size. 51V had very similar distributions to 31P signal intensities, supporting the assumption that vanadate behaved as a suitable Pi tracer (Fig. 6, E and F). 31P signal intensities were highest in the mesophyll and the vascular bundle in what appeared to be the phloem in the P-deficient cross sections but were absent from the BSE and the epidermis (Fig. 6B). Element maps showed the accumulation of 51V and 31P in fiber cells, which occurred at the nexus between the BSE and the adaxial and abaxial epidermis (Fig. 6, E and F). Strong signal intensities from both elements in the vascular bundles indicated rapid movement of applied ions from the leaf surface to the vasculature within 24 h. Both elements also accumulated at points across the epidermis and stomata, indicating that these zones could be additional pathways for ion entry. Nevertheless, the LA-ICP-MS maps showed that a majority of the added 51V and 31P accumulated in fiber cells occurring between the BSE and the epidermis (Figs. 5 and 6, E and F).
The fiber cells were located along the ridge peaks, where trichomes are typically found in parallel epidermal cell lines above and below the vascular bundles on both leaf surfaces (Figs. 4 and 5). It has been theorized that this zone could be a preferential pathway for ion penetration due to the favorable occurrence of aqueous polar pores at the base of trichomes or anticlinal cell walls (Schönherr, 2006). Leaf surface scars formed after trichome shedding contributed to foliar water uptake in one study (Fernández et al., 2014). The terminology for these cells varies in the literature; they have been called hypodermal sclerenchyma or structural cells in barley leaves (Williams et al., 1989; Karley et al., 2000). Here, we use fiber cells, a type of slender, long extraxylary sclerenchyma cell with thick, lignified, and strong secondary cell walls, possessing great tensile strength. These cells commonly occur in compact perivascular bundles or caps between the epidermis and the BSE. It is likely that Coriphosphine O staining added to the lignin autofluorescence in several tissue types in our images, including sclerenchyma in the xylem (appearing green-yellow) and in fiber cells (orange) as well as cutin (orange; Fig. 5, A and B). The difference in fluorescence emission wavelengths between sclerenchyma tissue in xylem and fiber cells could be due to the different lignin compositions (Lourenço et al., 2016). These hard fibers provide support in monocotyledonous leaves and constitute between 5% and 10% of total cells in grass leaves, where they are responsible for up to 95% of longitudinal leaf stiffness (Vincent, 1983; Crang et al., 2018).
While fiber cells are usually dead at maturity, plasmodesmata are often observed in simple cell lumen pits both between individual fiber cells and their surrounding cells, which could indicate symplastic continuity while cells were still growing (Walsh et al., 2005). The composition of cell walls influences their properties, including permeability and elasticity. The presence of lignified fiber cells at the termination of the BSE is hypothesized to be related to minimizing water loss from the associated vascular bundle. However, significantly higher evaporation was measured from fiber cells compared with typical epidermal cells in an edible grass species. Here, the role of large pits was implicated as a possible explanation (Aston and Jones, 1976). A recent study examined foliar water uptake pathways in beech (Fagus spp.) using an AgNO3 precipitation tracer method, where black deposits formed when Ag+ ions complexed with anions to precipitate as Ag nanoparticles (Schreel et al., 2020). Uptake was found to be highest in trichomes and Ag nanoparticle deposits were observed in subepidermal sclerenchyma cell lumens and interconnecting pit pairs, supporting the results observed here that trichome absorption and redistribution to sclerenchyma fiber cells could be a key ionic foliar pathway. It is possible that the dense networks of pit pairs connecting fiber cells could play an important role in facilitating ion transport to the vascular tissues.
The connected BSE provides a hydraulic link between the leaf epidermis and vascular bundles (Buckley et al., 2011). Fluorescent dye tracers showed water diffusion occurring along apoplastic pathways in BSEs from the xylem to epidermal cells, and it is possible that foliar ions could also travel apoplastically in the opposite direction along this route, to the veins (Canny, 1990). BSE in sunflower (Helianthus spp.) facilitated the penetration of zinc (Zn) to the epidermal cell walls and vascular tissue through nonglandular trichomes (Li et al., 2019). Greater accumulation of foliar nutrients above the veins compared with the interveinal tissue was found also for iron (Fe) and manganese (Mn) in sunflower and tomato (Solanum lycopersicum), calcium (Ca) in beech leaves, and Zn in citrus, tomato, soybean (Glycine max), and sunflower (Du et al., 2015; Li et al., 2017, 2018a; Bahamonde et al., 2018). It is possible that rapid accumulation occurred in this zone due to higher leaf wettability of the veins, which reduced the contact angle of foliar droplets (Li et al., 2018a).
High 51V and 31P signal intensities were also observed in some stomatal regions and at various points across the cuticle in epidermal cells (Fig. 6, E and F). Stomatal pathways were critical for foliar boron uptake in soybean and lychee (Litchi chinensis) leaves, where the stomatal abaxial epidermis was permeable to foliar solutions while the astomatous adaxial epidermis was not (Will et al., 2012). It is likely that high-humidity conditions influenced uptake, as increasing relative humidity promotes stomatal opening (Lange et al., 1971). Furthermore, hygroscopic particle accumulation around the stomatal pore can trigger the hydraulic action of stomata, where a thin liquid film connecting the inner and outer cuticular walls of the stomata can facilitate ion uptake (Burkhardt, 2010). However, the LA-ICP-MS signal intensities were not high in the subjacent stomatal cavity, making it unlikely that this is a dominant pathway for foliar P uptake. The movement of ions across the cuticle could also have been promoted by increased permeability due to high relative humidity, as was suggested for Zn in sunflower (Fernández and Eichert, 2009; Li et al., 2019). The permeability of the cuticle also depends on its chemical composition, which varies across plant species and can be affected by nutrient deficiencies (Schreiber and Schönherr, 2009). Zn-deficient sunflower leaves were less permeable to foliar Zn, possibly due to decreased trichome density and an altered chemical composition of the adaxial leaf surface, which contained higher proportions of cutin, waxes, and phenolics but less polysaccharides (Li et al., 2018b). While extremely P-deficient wheat (Triticum aestivum) leaves had enhanced wettability and decreased cuticle thickness, they were impermeable to radiolabeled foliar phosphoric acid, possibly due to reduced trichome and stomatal densities and associated cuticular pores (Fernández et al., 2014). Further studies should investigate the effects of P deficiency on the physical and chemical properties of barley leaf cuticles, as cuticle penetration mechanisms are still not well understood.
Finally, synchrotron-based XANES spectroscopy indicated that vanadate had been reduced to vanadyl after 24 h (Fig. 7). This was expected, as vanadate has been shown to be reduced by glutathione or catechol once transported inside vacuoles, as a mechanism to alleviate toxicity (Crans et al., 2010). However, there was strong similarity between the 51V and 31P signal intensities in the anatomical structures of the cross sections, such as the vascular bundles and the bundle sheaths (Fig. 6, E and F). Hence, it is possible that vanadate and Pi are analogous ions during apoplastic transport, with vanadate reduction occurring only after symplastic entrance. Regardless, it is recommended that vanadate is primarily used as a surface entry point tracer, since its speciation, and therefore its behavior, might not be fully analogous to Pi once inside the plant.
CONCLUSION
This study successfully traced the uptake, translocation, and effect of foliar P using two imaging approaches: an NPQ assay and LA-ICP-MS. The NPQ assay visualized whole-leaf P status and showed that P deficiency could be restored using foliar P within 24 h under high humidity, with rapid translocation of foliar P ions to new sink tissues within a 1-week period. Second, the strong colocalization of 51V and 31P signal intensities in the YFEL cross sections analyzed by LA-ICP-MS demonstrated that vanadate was a sensitive foliar P tracer. Based on this tracer, the fiber cells and associated BSE appeared to be the most important pathways for P uptake across the leaf surface, with evidence also suggesting some stomatal and cuticular penetration. The combination of these two imaging techniques has provided new insights into foliar Pi uptake and the accompanying physiological responses and will be useful for investigating foliar P fertilization under field conditions in future studies.
MATERIALS AND METHODS
Hydroponic Cultivation
Spring barley (Hordeum vulgare) ‘Golden Promise’ was germinated in Sorbix vermiculite. After 5 d, uniform seedlings were selected and transferred to light-impermeable black hydroponic cultivation units. Plants were grown in a greenhouse with a minimum 250 to 300 µmol m−2 s−1 photon flux density and a 20°C/15°C (16/8 h) day/night temperature regime. A misting system was used to maintain setpoint relative humidity at 65%.
Uniform barley seedlings were transferred to 4-L hydroponic cultivation units; each unit contained four individual plants held upright through drilled holes in the lid to allow access to the nutrient solution. The units were filled with a chelate-buffered solution prepared in 18.2 MΩ Milli-Q water (Milli-Q Plus; Millipore) containing 200 µmol L−1 KH2PO4, 200 µmol L−1 K2SO4, 300 µmol L−1 MgSO4·7H2O, 100 µmol L−1 NaCl, 300 µmol L−1 Mg(NO3)2·6H2O, 900 µmol L−1 Ca(NO3)2·4H2O, 600 µmol L−1 KNO3, 50 µmol L−1 Fe(III)-EDTA-Na, 0.8 µmol L−1 Na2MoO4·2H2O, 0.7 µmol L−1 ZnCl2, 1 µmol L−1 MnCl20.4H2O, 0.8 µmol L−1 CuSO4·5H2O, and 2 µmol L−1 H2BO3 for the P-sufficient plants. P-deficient plants were grown in 9 µmol L−1 KH2PO4 and 200 µmol L−1 KCl instead of 200 µmol L−1 KH2PO4. Nutrient solutions were renewed once per week, with pH adjusted every third day to 6 ± 0.5 with 1 m HCl or 1 m NaOH.
Foliar Application Conditions
Foliar P was applied when the plants reached 21 d after sowing. The P status of plants grown under P-sufficient and P-deficient conditions was confirmed using the Handy-Pea fluorimeter before spraying commenced. Shoots were sprayed with 10 mL (per each hydroponic unit containing four plants) of a solution containing 0.2 mol L−1 KH2PO4 and 1 mmol L−1 Na3VO4 at pH 6 using a hand-held diffusive sprayer. Untreated plants were sprayed with Milli-Q water. All solutions contained 0.05% (v/v) Tween 20 to ensure spray retention on the leaves. Each control treatment (P deficient and P sufficient) consisted of three replicate hydroponic cultivation units, representing three independent biological units, while the foliar P treatment consisted of six replicate hydroponic units. To prevent foliar P spray runoff entering the hydroponic solution, the base of the cultivation unit lids containing the plants was covered in a layer of plastic wrap and removed from the solution. The lids were turned upside down and suspended between two support beams, while the roots were covered using empty cultivation units. The shoots were carefully sprayed and remained suspended until no more dripping was observed. Small amounts of paper towel and plastic wrap were wrapped 1 cm above the base of the tillers to prevent the foliar spray running down into the hydroponic solution before plants were returned to the hydroponic cultivation unit. Humidity was artificially increased by enclosing shoots in a plastic bag where the relative humidity quickly reached greater than 90%. The bag had openings to ensure that atmospheric exchange could still occur. The humidity was kept high for 20 h after P application, with day-1 measurements performed on dry leaves 4 h after removing the bag. Measurements were taken at the same time each day (10 am) under greenhouse conditions. All measurements were taken from the YFEL from each individual plant at the time of sampling (four YFEL measurements per each hydroponic cultivation unit).
Chlorophyll a Fluorescence Transients
Chlorophyll a fluorescence transients were measured on the YFEL using a Handy-Pea Advanced Continuous Excitation Chlorophyll Fluorometer (Hansatech Instruments). Leaves were dark adapted for at least 25 min using Handy-Pea clips before being illuminated with saturating light at 3,000 µmol photons m−2 s−1 photosynthetically active radiation (PAR) for 10 s. The fluorescence was recorded with a temporal resolution of 10 µs to identify high-resolution steps in the transient. Data were extracted using PEA Plus V1.10, and the relative variable fluorescence at time t was calculated by double normalizing transients between minimum and maximum PSII fluorescence in the dark-adapted state (F0 and Fm, respectively) according to the formula V (t) = [fluorescence (t) − F0]/(Fm − F0). The chlorophyll a fluorescence transients were used to calculate the P status of all plants based on the shape of the I-step transient and the status in the P-Predict regression model as calculated in Python 3.6.3 (Python Software Foundation [available at http://www.python.org], sourced using the Anaconda Distribution [available at http://www.anaconda.com]; Frydenvang et al., 2015; Carstensen et al., 2019).
IMAGING PAM NPQ Assay
NPQ was measured on the YFEL using a Walz IMAGING PAM M-series MAXI (Heinz Walz). NPQ spatial distribution was imaged using ImagingWin v2.46i, where NPQ was calculated as (Fm – Fm′)/Fm′, where Fm is the maximum fluorescence of the dark-adapted leaf with a saturating light pulse and Fm′ is the maximum fluorescence at a saturating light pulse in a light-adapted sample. The measurements were visualized on a false-color scale, with colors corresponding to values between 0% and 100% of the NPQ value range during the light-response curve (0–4; Heinz Walz). Leaves were dark adapted for at least 25 min under green light before being excised at the base and mounted in the IMAGING PAM chamber. The yield was measured with saturating light pulses of 720-ms illumination with 4,700 µmol photons m−2 s−1 PAR. The PAM NPQ script was as follows: Fv/Fm was measured with a saturating light pulse, followed by 30 s of darkness. Actinic light was turned on at 926 µmol photons m−2 s−1 PAR with an immediate saturating light pulse, followed by additional saturating light pulses with 15- to 60-s intervals to track NPQ development in actinic light. Saturating light pulses consisted of 720 ms of illumination with 4,700 µmol photons m−2 s−1 PAR. NPQ was reported from the saturating pulse after 15 s in actinic light. The whole-leaf region was selected using a polygon freeform tool, and the average NPQ value was exported to Microsoft Excel.
Scanning Electron Microscopy
Small leaf pieces (1 cm × 1 cm) were cut using a scalpel, fixed in Karnovsky’s solution for 24 h under vacuum, and sequentially dehydrated using acetone. Samples then underwent critical point drying and were sputter coated with a mixture of palladium and gold. Both adaxial and abaxial leaf surfaces were examined using an FEI Quanta 200 scanning electron microscope.
Light Microscopy
Leaf samples (5 mm × 20 mm) were cut using a scalpel and fixed under vacuum in Karnovsky’s solution for 24 h. Samples were dehydrated in an acetone series and embedded in Spurr’s resin overnight. Sections (2.5 µm thick) were cut using a microtome and stained using Coriphosphine O for 2 min (Weis et al., 1988). Samples were observed on a Leica DM 5000B light and fluorescence microscope with a Canon EOS 90D camera. Whole-image postprocessing to minimally adjust contrast and scale bar addition were done using Fiji ImageJ 2.0 (Schindelin et al., 2012).
YFEL Gas-Exchange Measurements
YFEL transpiration, respiration, stomatal conductance, and substomatal CO2 concentration measurements were made using a CIRAS-3 instrument (Portable Photosynthesis Systems). Each YFEL was measured five times between 9 and 11 am, and three biological replicates for each treatment were measured. The internal CO2 concentration was maintained at 390 mmol mol−1, with the relative humidity and light intensity as recorded under greenhouse conditions.
ICP-OES Analysis
Barley leaves were scrubbed gently with a sponge three times in a solution of 1% (v/v) Tween 20 to ensure that the surface spray was removed. Hereafter, the samples were dried in an oven at 60°C for 48 h before digestion in a Milestone UltraWAVE before analysis for total P and trace metals using a 5100 ICP-OES device (Agilent Technologies).
Sample Preparation for LA-ICP-MS
Whole leaves were removed at the base 24 h after spraying and rinsed using 2% (v/v) HNO3, 3% (v/v) ethanol, and Milli-Q water sequentially (three times per rinse solution; Du et al., 2015). Leaf samples were cut 10 cm from the leaf tip of the YFEL. These samples were dried on a paper towel before being placed in a handmade aluminum foil mold containing Optimal Cutting Temperature compound (Tissue-Tek; Sakura Finetek). This mold was placed in a precooled 96% (v/v) ethanol bath, which was suspended in an aluminum foil boat floating in liquid nitrogen, to ensure a slow freezing process. Samples (16 µm thick) were then sectioned using a Leica CM050S cryotome and prepared according to Persson et al. (2016).
LA-ICP-MS Analysis
Samples were analyzed using a nanosecond LA unit (NWR193; New Wave Research) equipped with an argon fluoride excimer laser source operating at 193 nm using the following settings: energy, 1.4 to 2.3 J cm−2 (30%–40% of maximum energy); scan speed, 10 µm s−1; repetition rate, 30 to 40 Hz; spot size, 5 µm. Element signals were obtained using an Agilent Technologies 7900 ICP-MS instrument operated in hydrogen mode (1 mL min−1). The isotopes analyzed were 13C, 44Ca, 39K, 24Mg, 31P, 85Rb, and 51V using an integration time of 0.1 s per element. The ICP-MS settings were as follows: sample cone depth, 5 mm; carrier gas, 1 mL min−1. A rubidium (Rb) standard was used to monitor the sensitivity prior to and between analyses, as described by Persson et al. (2016). All element signals were normalized to 13C to account for differences in sample topography and signal drift during analysis. Elemental maps were generated using SigmaPlot version 14.0 (Systat Software). Samples were corrected using the gas blank by subtracting the average elemental counts during the gas blank measurements on the sample scan immediately before the sample was ablated.
Synchrotron μXANES
To investigate the chemical speciation of absorbed foliar-applied V, μXANES analysis of barley leaves was performed. Living barley plants, grown for 21 d under the same conditions as described above, were transported to the Australian Synchrotron. One 50-µL droplet of a solution consisting of 0.2 mol L−1 KH2PO4 and 1 mmol L−1 Na3VO4 (0.05% [v/v] Tween 20) was then applied to the adaxial side of the YFEL while still attached to the plant. To ensure that the droplet remained as a liquid for the duration of the V exposure, the attached leaf was placed in a covered petri dish to maintain high humidity (greater than 95%) as described previously (Li et al., 2018b). After 24 h, the leaf was carefully rinsed using 2% (v/v) HNO3, 3% (v/v) ethanol, and deionized water three times. The fresh leaf was then turned upside down (i.e. abaxial side facing up) to prevent contamination during sectioning, and a pair of razor blades 100 µm apart were used to prepare fresh cross sections. The 100-μm leaf sections were placed between two layers of 4-µm-thick Ultralene Thin Film (SPEX SamplePrep 3525), taped to a Perspex sample holder, and analyzed immediately. μXANES analysis was used to determine V speciation in leaves 24 h after foliar application (step size of 0.003 mm, 65-mm × 35-mm scan area, dwell time of 5 mm s−1). Finely ground reference standards were dusted onto Kapton tape for analysis (Sigma-Aldrich): vanadyl sulfate hydrate (VOSO4·5H2O, 99.99%), vanadium(IV) oxide (V2O4, 99.99%), and sodium orthovanadate (Na3VO4, 99.99%), as these oxidation states could potentially be present in plant tissue following foliar V uptake. Analysis was conducted at the XFM Beamline at the Australian Synchrotron. The X-ray beam was tuned with an Si(111) monochromator, where data were collected at 110 incident energies across the V K-edge at a spatial resolution of 1 to 2 μm. Incident energy step sizes decreased sequentially (1-eV step size over the pre-edge region, 0.5-eV step size over the main-edge region, and 1-eV step size throughout the post-edge region), and a maximum velocity of 0.2 mm s−1 was used. The X‐ray fluorescence emitted by the specimen was collected using a 384‐element Maia detector. XANES spectra were extracted from relevant regions using GeoPIXE, while energy normalization and background correction were performed using the Athena software package (Ravel and Newville, 2005; Kopittke et al., 2014).
Statistical Analyses
Statistical differences between P-deficient and P-sufficient YFEL for the NPQ light-response curves were calculated using an unpaired two-sample Student’s t test (GraphPad Prism version 8.3.1 for Windows). Samples were considered significantly different at P < 0.05.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. NPQ response in the YFEL of 22-d-old P-sufficient and P-deficient plants 15 s after actinic light was turned on.
Supplemental Figure S2. Aqueous speciation modeling of P, vanadium monomeric species, and vanadium polymeric species.
Supplemental Figure S3. CIRAS gas measurements for control foliar solutions.
Supplemental Figure S4. Replicate LA-ICP-MS elemental maps of control P-deficient leaf, bright-field microscopy image, 31P elemental scan, and 51V elemental scan.
Supplemental Figure S5. Replicate LA-ICP-MS elemental maps of control P-deficient leaf, bright-field microscopy image, 31P elemental scan, and 51V elemental scan.
Supplemental Figure S6. Replicate LA-ICP-MS elemental maps of foliar P leaf, 31P elemental scan, and 51V elemental scan.
Supplemental Figure S7. Replicate LA-ICP-MS elemental maps of foliar P leaf, bright-field microscopy image, 31P elemental scan, and 51V elemental scan.
Supplemental Figure S8. Normalized V K-edge XANES for three standard vanadium compounds and the treated foliar P leaf cross section, measured 24 h after the foliar solution was applied.
Footnotes
This work was supported by the Innovation Fund Denmark (grant no. 7045–00010A to the Smart-P project) and by the Australian Synchrotron (grant no. AS191/XFM/14515).
Articles can be viewed without a subscription.
References
- Aston MJ, Jones MM(1976) A study of the transpiration surfaces of Avena sterilis L. var. Algerian leaves using monosilicic acid as a tracer for water movement. Planta 130: 121–129 [DOI] [PubMed] [Google Scholar]
- Bahamonde HA, Gil L, Fernández V(2018) Surface properties and permeability to calcium chloride of Fagus sylvatica and Quercus petraea leaves of different canopy heights. Front Plant Sci 9: 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benazir J, Manimekalai V, Ravichandran P, Suganthi R, Dinesh DC(2010) Properties of fibres/culm strands from mat sedge—Cyperus pangorei Rottb. BioResources 5: 951–967 [Google Scholar]
- Boanares D, Ferreira BG, Kozovits AR, Sousa HC, Isaias RMS, França MGC(2018) Pectin and cellulose cell wall composition enables different strategies to leaf water uptake in plants from tropical fog mountain. Plant Physiol Biochem 122: 57–64 [DOI] [PubMed] [Google Scholar]
- Bowman BJ, Slayman CW(1979) The effects of vanadate on the plasma membrane ATPase of Neurospora crassa. J Biol Chem 254: 2928–2934 [PubMed] [Google Scholar]
- Buckley TN, Sack L, Gilbert ME(2011) The role of bundle sheath extensions and life form in stomatal responses to leaf water status. Plant Physiol 156: 962–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt J.(2010) Hygroscopic particles on leaves: Nutrients or desiccants? Ecol Monogr 80: 369–399 [Google Scholar]
- Canny MJ.(1990) Tansley Review No. 22. What becomes of the transpiration stream? New Phytol 114: 341–368 [DOI] [PubMed] [Google Scholar]
- Cantley LC Jr., Josephson L, Warner R, Yanagisawa M, Lechene C, Guidotti G(1977) Vanadate is a potent (Na,K)-ATPase inhibitor found in ATP derived from muscle. J Biol Chem 252: 7421–7423 [PubMed] [Google Scholar]
- Carstensen A, Herdean A, Schmidt SB, Sharma A, Spetea C, Pribil M, Husted S(2018) The impacts of phosphorus deficiency on the photosynthetic electron transport chain. Plant Physiol 177: 271–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen A, Szameitat AE, Frydenvang J, Husted S(2019) Chlorophyll a fluorescence analysis can detect phosphorus deficiency under field conditions and is an effective tool to prevent grain yield reductions in spring barley (Hordeum vulgare L.). Plant Soil 434: 79–91 [Google Scholar]
- Chen A, Husted S, Salt DE, Schjoerring JK, Persson DP(2019) The intensity of manganese deficiency strongly affects root endodermal suberization and ion homeostasis. Plant Physiol 181: 729–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell D, White S(2015) Tracking phosphorus security: Indicators of phosphorus vulnerability in the global food system. Food Secur 7: 337–350 [Google Scholar]
- Crang R, Lyons-Sobaski S, Wise R(2018) Parenchyma, collenchyma, and sclerenchyma In Plant Anatomy. Springer, Cham, Switzerland, pp 181–213 [Google Scholar]
- Crans DC, Zhang B, Gaidamauskas E, Keramidas AD, Willsky GR, Roberts CR(2010) Is vanadate reduced by thiols under biological conditions? Changing the redox potential of V(V)/V(IV) by complexation in aqueous solution. Inorg Chem 49: 4245–4256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Kopittke PM, Noller BN, James SA, Harris HH, Xu ZP, Li P, Mulligan DR, Huang L(2015) In situ analysis of foliar zinc absorption and short-distance movement in fresh and hydrated leaves of tomato and citrus using synchrotron-based x-ray fluorescence microscopy. Ann Bot 115: 41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edixhoven JD, Gupta J, Savenije HHG(2013) Recent revisions of phosphate rock reserves and resources: Reassuring or misleading? An in-depth literature review of global estimates of phosphate rock reserves and resources. Earth Syst Dyn Discuss 4: 1005–1034 [Google Scholar]
- Eichert T, Goldbach HE(2008) Equivalent pore radii of hydrophilic foliar uptake routes in stomatous and astomatous leaf surfaces: Further evidence for a stomatal pathway. Physiol Plant 132: 491–502 [DOI] [PubMed] [Google Scholar]
- Eichert T, Kurtz A, Steiner U, Goldbach HE(2008) Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiol Plant 134: 151–160 [DOI] [PubMed] [Google Scholar]
- Elser J, Bennett E(2011) Phosphorus cycle: A broken biogeochemical cycle. Nature 478: 29–31 [DOI] [PubMed] [Google Scholar]
- Fernández V, Brown PH(2013) From plant surface to plant metabolism: The uncertain fate of foliar-applied nutrients. Front Plant Sci 4: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández V, Eichert T(2009) Uptake of hydrophilic solutes through plant leaves: Current state of knowledge and perspectives of foliar fertilization. CRC Crit Rev Plant Sci 28: 36–68 [Google Scholar]
- Fernández V, Guzmán P, Peirce CAE, McBeath TM, Khayet M, Mclaughlin MJ(2014) Effect of wheat phosphorus status on leaf surface properties and permeability to foliar-applied phosphorus. Plant Soil 384: 7–20 [Google Scholar]
- Fernández V, Guzmán-Delgado P, Graça J, Santos S, Gil L(2016) Cuticle structure in relation to chemical composition: Re-assessing the prevailing model. Front Plant Sci 7: 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydenvang J, van Maarschalkerweerd M, Carstensen A, Mundus S, Schmidt SB, Pedas PR, Laursen KH, Schjoerring JK, Husted S(2015) Sensitive detection of phosphorus deficiency in plants using chlorophyll a fluorescence. Plant Physiol 169: 353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert N.(2009) Environment: The disappearing nutrient. Nature 461: 716–718 [DOI] [PubMed] [Google Scholar]
- Hunter MC, Smith RG, Schipanski ME, Atwood LW, Mortensen DA(2017) Agriculture in 2050: Recalibrating targets for sustainable intensification. Bioscience 67: 386–391 [Google Scholar]
- Karley AJ, Leigh RA, Sanders D(2000) Where do all the ions go? The cellular basis of differential ion accumulation in leaf cells. Trends Plant Sci 5: 465–470 [DOI] [PubMed] [Google Scholar]
- Koontz H, Biddulph O(1957) Factors affecting absorption and translocation of foliar applied phosphorus. Plant Physiol 32: 463–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopittke PM, de Jonge MD, Wang P, McKenna BA, Lombi E, Paterson DJ, Howard DL, James SA, Spiers KM, Ryan CG, et al. (2014) Laterally resolved speciation of arsenic in roots of wheat and rice using fluorescence-XANES imaging. New Phytol 201: 1251–1262 [DOI] [PubMed] [Google Scholar]
- Kopittke PM, Lombi E, van der Ent A, Wang P, Laird JS, Moore KL, Persson DP, Husted S(2020) Methods to visualize elements in plants. Plant Physiol 182: 1869–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange OL, Lösch R, Schulze ED, Kappen L(1971) Responses of stomata to changes in humidity. Planta 100: 76–86 [DOI] [PubMed] [Google Scholar]
- Läuchli A, Epstein E(1970) Transport of potassium and rubidium in plant roots: The significance of calcium. Plant Physiol 45: 639–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wang P, Lombi E, Cheng M, Tang C, Howard DL, Menzies NW, Kopittke PM(2018a) Absorption of foliar-applied Zn fertilizers by trichomes in soybean and tomato. J Exp Bot 69: 2717–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wang P, Lombi E, Wu J, Blamey FPC, Fernández V, Howard DL, Menzies NW, Kopittke PM(2018b) Absorption of foliar applied Zn is decreased in Zn deficient sunflower (Helianthus annuus) due to changes in leaf properties. Plant Soil 433: 309–322 [Google Scholar]
- Li C, Wang P, Menzies NW, Lombi E, Kopittke PM(2017) Effects of changes in leaf properties mediated by methyl jasmonate (MeJA) on foliar absorption of Zn, Mn and Fe. Ann Bot 120: 405–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wang P, van der Ent A, Cheng M, Jiang H, Lund Read T, Lombi E, Tang C, de Jonge MD, Menzies NW, et al. (2019) Absorption of foliar-applied Zn in sunflower (Helianthus annuus): Importance of the cuticle, stomata and trichomes. Ann Bot 123: 57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesche J, Martens HJ, Schulz A(2011) Symplasmic transport and phloem loading in gymnosperm leaves. Protoplasma 248: 181–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenço A, Rencoret J, Chemetova C, Gominho J, Gutiérrez A, Del Río JC, Pereira H(2016) Lignin composition and structure differs between xylem, phloem and phellem in Quercus suber L. Front Plant Sci 7: 1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald GK, Bennett EM, Potter PA, Ramankutty N(2011) Agronomic phosphorus imbalances across the world’s croplands. Proc Natl Acad Sci USA 108: 3086–3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell BG, Lepp NW, Phipps DA(1986) Vanadium uptake by higher plants: Some recent developments. Environ Geochem Health 8: 14–18 [DOI] [PubMed] [Google Scholar]
- Noack SR, McBeath TM, McLaughlin MJ(2010) Potential for foliar phosphorus fertilisation of dryland cereal crops: A review. Crop Pasture Sci 61: 659 [Google Scholar]
- Obersteiner M, Peñuelas J, Ciais P, van der Velde M, Janssens IA(2013) The phosphorus trilemma. Nat Geosci 6: 897–898 [Google Scholar]
- Peirce CAE, McBeath TM, Fernández V, McLaughlin MJ(2014) Wheat leaf properties affecting the absorption and subsequent translocation of foliar-applied phosphoric acid fertiliser. Plant Soil 384: 37–51 [Google Scholar]
- Peirce CAE, McBeath TM, Priest C, McLaughlin MJ(2019) The timing of application and inclusion of a surfactant are important for absorption and translocation of foliar phosphoric acid by wheat leaves. Front Plant Sci 10: 1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson DP, Chen A, Aarts MGM, Salt DE, Schjoerring JK, Husted S(2016) Multi-element bioimaging of Arabidopsis thaliana roots. Plant Physiol 172: 835–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel B, Newville M(2005) ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for x-ray absorption spectroscopy using IFEFFIT. J Synchrotron Radiat 12: 537–541 [DOI] [PubMed] [Google Scholar]
- Ruban AV, Johnson MP, Duffy CDP(2012) The photoprotective molecular switch in the photosystem II antenna. Biochim Biophys Acta 1817: 167–181 [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012) Fiji: An open-source platform for biological-image analysis. Nat Methods 9: 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönherr J.(2006) Characterization of aqueous pores in plant cuticles and permeation of ionic solutes. J Exp Bot 57: 2471–2491 [DOI] [PubMed] [Google Scholar]
- Schreel JDM, Leroux O, Goossens W, Brodersen C, Rubinstein A, Steppe K(2020) Identifying the pathways for foliar water uptake in beech (Fagus sylvatica L.): A major role for trichomes. Plant J 103: 769–780 [DOI] [PubMed] [Google Scholar]
- Schreiber L, Schönherr J(2009) Water and Solute Permeability of Plant Cuticles. Springer, Berlin [Google Scholar]
- Schwartz A, Illan N, Assmann SM(1991) Vanadate inhibition of stomatal opening in epidermal peels of Commelina communis: Cl− interferes with vanadate uptake. Planta 183: 590–596 [DOI] [PubMed] [Google Scholar]
- Shen H, Chen J, Wang Z, Yang C, Sasaki T, Yamamoto Y, Matsumoto H, Yan X(2006) Root plasma membrane H+-ATPase is involved in the adaptation of soybean to phosphorus starvation. J Exp Bot 57: 1353–1362 [DOI] [PubMed] [Google Scholar]
- Storey R, Leigh RA(2004) Processes modulating calcium distribution in citrus leaves: An investigation using x-ray microanalysis with strontium as a tracer. Plant Physiol 136: 3838–3848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syers JK, Bekunda M, Cordell D, Corman J, Johnston J, Rosemarin A, Salcedo I(2011) Phosphorus and food production In UNEP Year Book 2011: Emerging Issues in our Global Environment. United Nations, Nairobi, Kenya, pp 35–45 [Google Scholar]
- Syers JK, Johnston AE, Curtin D(2008) Efficiency of Soil and Fertilizer Phosphorus Use: Reconciling Changing Concepts of Soil Phosphorus Behaviour with Agronomic Information. Food and Agriculture Organization of the United Nations, Rome [Google Scholar]
- Tracy AS, Jaswal JS, Angus-Dunne SJ(1995) Influences of pH and ionic strength on aqueous vanadate equilibria. Inorg Chem 34: 5680–5685 [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL(2003) Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol 157: 423–447 [DOI] [PubMed] [Google Scholar]
- Vincent JFV.(1983) The influence of water content on the stiffness and fracture properties of grass leaves. Grass Forage Sci 38: 107–114 [Google Scholar]
- Walsh KB, Sky RC, Brown SM(2005) The anatomy of the pathway of sucrose unloading within the sugarcane stalk. Funct Plant Biol 32: 367–374 [DOI] [PubMed] [Google Scholar]
- Wang WN, Tarafdar JC, Biswas P(2013) Nanoparticle synthesis and delivery by an aerosol route for watermelon plant foliar uptake. J Nanopart Res 15: 1417 [Google Scholar]
- Weis KG, Polito VS, Labavitch JM(1988) Microfluorometry of pectic materials in the dehiscence zone of almond (Prunus dulcis [Mill.] DA Webb) fruits. J Histochem Cytochem 36: 1037–1041 [DOI] [PubMed] [Google Scholar]
- Will S, Eichert T, Fernández V, Müller T, Römheld V(2012) Boron foliar fertilization of soybean and lychee: Effects of side of application and formulation adjuvants. J Plant Nutr Soil Sci 175: 180–188 [Google Scholar]
- Williams ML, Farrar JF, Pollock CJ(1989) Cell specialization within the parenchymatous bundle sheath of barley. Plant Cell Environ 12: 909–918 [Google Scholar]