Abstract
Background:
Meniscal tears are traditionally classified into traumatic versus degenerative tears. Although this classification plays a major role in clinical decision making, no consensus exists on the exact definition of a traumatic or degenerative tear, and the histopathological basis for this classification is unclear.
Purpose:
To assess the histological degree of meniscal degeneration in patients with a traumatic meniscal tear, as compared with intact meniscal tissue and osteoarthritic meniscal tissue.
Study Design:
Descriptive laboratory study.
Methods:
Traumatically torn meniscal tissue was collected during arthroscopic partial meniscectomy. As a control group, intact meniscal tissue was used from transfemoral amputations or direct postmortem dissections. Meniscal tissue from osteoarthritic knees was obtained during total knee replacement surgery. Meniscal tissue was processed, stained, and histologically analyzed with the Pauli scoring system (range, 0-18), comprising the subdomains surface integrity, cellularity, collagen organization, and matrix staining. Scoring was performed by 2 independent observers, blinded to condition, region, and patient data of the meniscus.
Results:
The traumatic meniscal tear group contained 43 patients (34 men; median age, 29 years; median body mass index [BMI], 24 kg/m2); the intact meniscal tissue group, 8 patients (3 men; median age, 58 years; median BMI, 30 kg/m2); and the osteoarthritic group, 14 patients (4 men; median age, 66 years; median BMI, 28 kg/m2). After adjustment for sex, age, and BMI, patients with a traumatic meniscal tear had a significantly higher histological score than patients with intact meniscal tissue (2.7-point difference; P = .035). Histological score between the traumatic and osteoarthritic groups was not different.
Conclusion:
Traumatically torn menisci possess a higher degree of degeneration than intact menisci. Our results suggest that patients with a traumatic meniscal tear may already have had a certain degree of meniscal degeneration. These findings potentially challenge the classic view of traumatic versus degenerative meniscal tears.
Clinical Relevance:
Our findings provide a better understanding of the tissue condition of a torn meniscus. This knowledge may help clinicians decide on choice of treatment and may lead to new perspectives to prevent knee osteoarthritis in patients with a torn meniscus.
Keywords: meniscus, meniscal tear, degeneration, arthroscopy, meniscectomy
A symptomatic meniscal tear is the most common knee injury, affecting 0.6 to 8 per 1000 patients each year depending on their activity level.7,8,12,20,24,32,33 A torn meniscus can lead to pain, disability, and lower quality of life.28 In the longer term, a meniscal tear is an important risk factor for the development and progression of knee osteoarthritis (OA).11,16,28 Previously reported incidence rates for meniscal injury in physically active populations have ranged between 0.33 and 0.61 per 1000 person-years.
Meniscal tears are traditionally considered traumatic or degenerative, referring to their onset. Currently, this classification is based on medical history, trauma mechanism, patient age, and magnetic resonance imaging indicating a specific tear pattern. This classification of meniscal pathology is essential in clinical decision making: traumatic meniscal tears are mostly treated by arthroscopic partial meniscectomy or repair,4,17 whereas nonoperative management is the first choice for degenerative tears.3,4 Classifying meniscal tears into traumatic versus degenerative tears can, however, be challenging, as there is no consensus on how exactly to define degenerative and traumatic tears. In some cases, traumatic tears are caused by a very minor trauma (eg, walking stairs); in other cases, degenerative tears are incidentally found in asymptomatic healthy knees.15,21 Moreover, studies have shown that traumatic meniscal tears may result from early degenerative disease processes.35,44 Thus, differentiation between these types is not as straightforward as it may seem.
In soft tissues other than the meniscus, the role of tissue condition in pathophysiological processes has been studied before. For instance, degenerative changes were already present in ruptured Achilles tendons.1,10,25,26,29 These findings have led to the view that degeneration of a tendon can cause it to rupture in the absence of an abnormal movement or force, representing the so-called continuum theory.9,13 The continuum theory explains the pathological basis of the heterogeneity between healthy and degenerative tissue eventually leading to tendinopathy or rupture.9 This rationale might be extended to meniscal tissue. Hence, it is highly relevant to increase knowledge on tissue condition of a torn meniscus. This knowledge could begin to establish a pathophysiologic basis for the classification of meniscal tears. To date, there are no studies published evaluating the degree of histological degeneration in traumatically torn meniscal tissue (TM). Moreover, no research has compared traumatic and intact meniscal tissue (IM) regarding histological degeneration. Histological research has so far mainly focused on osteoarthritic and degenerative menisci.38
Our aim was to investigate the histological degree of degeneration in TM as compared with IM as a control group. Osteoarthritic menisci were used as reference for a degenerative state of the meniscus. We hypothesized that TM would show a higher degree of histological degeneration than IM. The secondary aim was to identify patient-related factors that are associated with a higher degree of degeneration. To our knowledge, this is the first study comparing histological degeneration between traumatic meniscal tissue and intact menisci.
Methods
Patients and Data Collection
In this study, meniscal tissue in 3 conditions was collected from 3 groups: TM, IM, and degenerative meniscal tissue (DM).
TM was collected during arthroscopic partial meniscectomy. Inclusion criteria were as follows: age <45 years, history of a traumatic event in the past 6 months followed by clinical knee complaints, magnetic resonance imaging–proven meniscal tear without signs of knee OA, and an indication for arthroscopic partial meniscectomy. As a control group, IM was collected during acute transfemoral amputation or direct postmortem dissection after lethal incidents. Inclusion criteria were as follows: age between 18 and 70 years, no history of knee injury, and no signs of knee OA with macroscopic inspection of the knee. Menisci from patients with vascular or inflammatory diseases involving the knee or with systemic diseases were excluded. Inclusion criteria for DM were age >18 years, radiographically confirmed knee OA, and an indication for total knee replacement surgery. DM was used as reference standard.
Data were collected on patient characteristics: age, sex, and body mass index (BMI). In the TM group, additional information was collected on time interval between trauma and surgery, as well as associated anterior cruciate ligament (ACL) rupture. The meniscal tissue was obtained with implicit consent as waste material (MEC-2004-322 and MEC-2015-180). The patients had the right to refuse, as stated by the guidelines of the Dutch Federation of Biomedical Scientific Societies.
Meniscal Tissue Processing
Directly after harvesting, meniscal tissue was stored in formaldehyde 4%. Tissue was processed within 1 to 3 days after surgery in a standardized way, according to the method of Pauli et al.38 Meniscal tissue was cut in 2 planes into 5-mm samples. The sagittal (ie, vertical) cut, oriented perpendicular to the circumferential collagen bundles, provided an overview of the meniscal surface and matrix composition. The horizontal plane, at a 30° angle relative to the tibial plateau, revealed the longitudinal organization of the collagen bundles and matrix morphology (Figure 1). Samples were further processed by dehydration and infiltration with paraffin, followed by cutting into 6-µm sections with a microtome (Leica RM-2135; Leica Microsystems). Subsequently, the sections were stained with hematoxylin and eosin (Sigma) to assess surface integrity and cellularity. Additionally, safranin O–fast green and picrosirius red (Sigma) stainings were used to analyze proteoglycan content of the meniscus and collagen fiber organization, respectively.
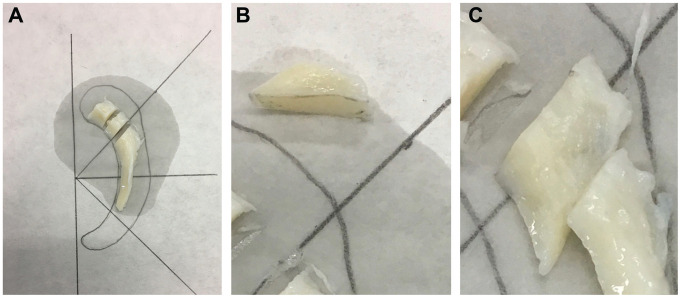
Figure 1.
(A) Meniscal tissue after resection of a medial bucket-handle tear in a 24-year-old man. (B) Sagittal plane cut from the posterior horn. (C) Horizontal plane cut from the posterior horn.
Histological Analysis
All meniscal sections were scored with the validated histological scoring system for meniscal degeneration developed by Pauli et al38 (Appendix Table A1, available in the online version of this article). The Pauli score contains the following subdomains: surface integrity (tibial surface, femoral surface, and inner rim), cellularity, collagen organization, and matrix staining intensity. A score ranging from 0 to 3 (depending on the degree of degeneration) is given to each subdomain, resulting in a total histological score ranging from 0 to 18. Scoring was performed independently by 2 authors (M.A.W. and S.M.E.), blinded to condition, region of the meniscal tissue, and patient data. Three weeks after initial evaluation, 1 researcher (M.A.W.) performed a second scoring. The latter scores were exclusively used to calculate intraobserver reliability.
Statistical Analysis
Baseline characteristics for each group (TM, IM, and DM) were collected and tested for normality via a Shapiro-Wilk test. Depending on data distribution, differences in continuous data among groups were assessed with the 1-way analysis of variance or Kruskal-Wallis test, followed by post hoc Wilcoxon rank sum analysis with Bonferroni correction. Categorical data were analyzed with a Fisher exact test with Bonferroni correction for pairwise comparisons. Inter- and intraobserver reliability of histological scoring is expressed as intraclass correlation coefficients (ICCs). A 2-way mixed model based on absolute agreement for single measures was used. Reliability is regarded as excellent if ICC >0.75.27
To investigate and correct for potential confounding variables within the groups, a multiple linear regression model was designed. Based on existing literature on factors associated with meniscal degeneration or OA, the variables age, sex, and BMI36,38,42 were included in a forced-entry analysis. Univariate linear regression analysis was performed within the TM group on the variables time interval between trauma and surgery and associated ACL rupture to assess their association with histological scores. In case an association was found for those variables, they were included in the multivariate model.
All statistical analysis included 2-tailed tests. A P value <.05 indicated statistical significance. SPSS (version 21.0; IBM Corporation) was used for all analysis.
Results
Patient Characteristics
In total, 65 meniscal tissue samples were analyzed: 43 traumatic meniscal tear samples, 8 intact menisci, and 14 degenerative menisci. Given the limited sample sizes in each group, most data were nonparametric and described as median with interquartile range. Baseline characteristics per group are summarized in Table 1. Statistically significant differences in age were encountered between the TM and IM groups (P = .001) and the TM and DM groups (P < .001). The median BMI in the TM group (23.6 kg/m2) was significantly lower than the BMI of the DM group (28.4 kg/m2) (P = .043). BMI in the IM group (29.7 kg/m2) was not significantly different from the TM or DM group. A significantly higher percentage of male samples was present in the TM group than in the DM group (79% vs 29%) (P = .001).
Table 1.
Characteristics of Patients in the Traumatic, Intact, and Degenerative Groups a
| Traumatic Meniscal Tear (n = 43) | Intact Meniscus (n = 8) | Degenerative Meniscus (n = 14) | P Value b | P Value c | |
|---|---|---|---|---|---|
| Age at surgery, y | 29.0 (21.5-40.2) | 58.0 (53.8-64.0) | 66.1 (62.6-70.0) | .001 | <.001 |
| Body mass index, kg/m2 | 23.6 (21.7-25.8) | 29.7 (22.3-35.9) | 28.4 (24.3-32.3) | .181 | .043 |
| Sex: male | 34 (79.1) | 3 (37.5) | 4 (28.6) | .044 | .001 |
| Meniscal region examined | |||||
| Medial posterior horn | 28 (65.1) | 8 (100) | 12 (85.7) | ||
| Medial midbody | 6 (14.0) | ||||
| Lateral posterior horn | 7 (16.3) | 1 (7.1) | |||
| Lateral midbody | 1 (2.3) | ||||
| Lateral anterior horn | 1 (2.3) | ||||
| Unknown | 1 (7.1) | ||||
| Time between injury and surgery, wk | 12.6 (5.7-29.7) | ||||
| History of ACL rupture | 17 (39.5) | ||||
| Tear type | |||||
| Vertical (bucket-handle, radial, flap) | 40 (93) | ||||
| Bucket-handle | 23 (53) | ||||
| Horizontal | 1 (2.3) | ||||
| Unknown | 2 (4.7) |
Continuous data are presented as median (interquartile range) and categorical data as No. (%). ACL, anterior cruciate ligament.
Statistical significant difference between the traumatic and intact groups, P < .05.
Statistical significant difference between the traumatic and osteoarthritic groups, P < .05.
Histological Analysis
Representative histological images illustrating the range of scores are presented in Figure 2. The mean ± SD histological score in the TM group was 4.4 ± 2.2; the IM group, 3.2 ± 1.6; and the DM group, 7.1 ± 2.9 (Figure 3).
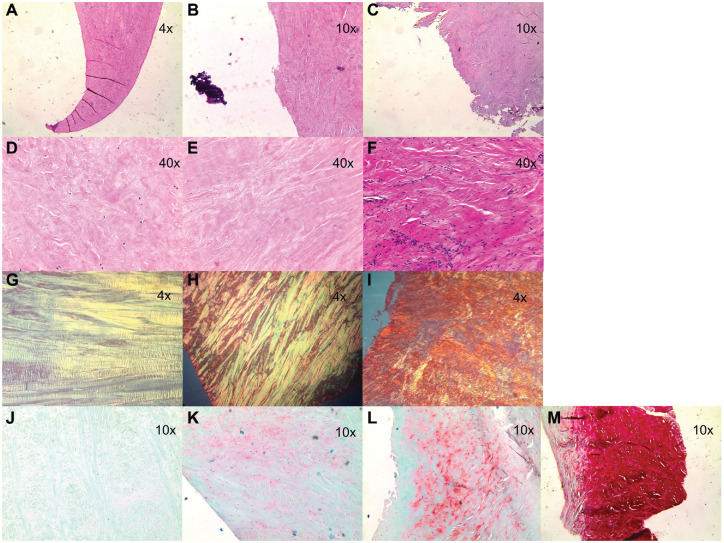
Figure 2.
Representative examples of histological findings. Histological scores by subdomain, left to right: (A-C) surface integrity (0-2), (D-F) cellularity (0-2), (G-I) collagen organization (0-2), (J-M) matrix staining (0-3).
Figure 3.
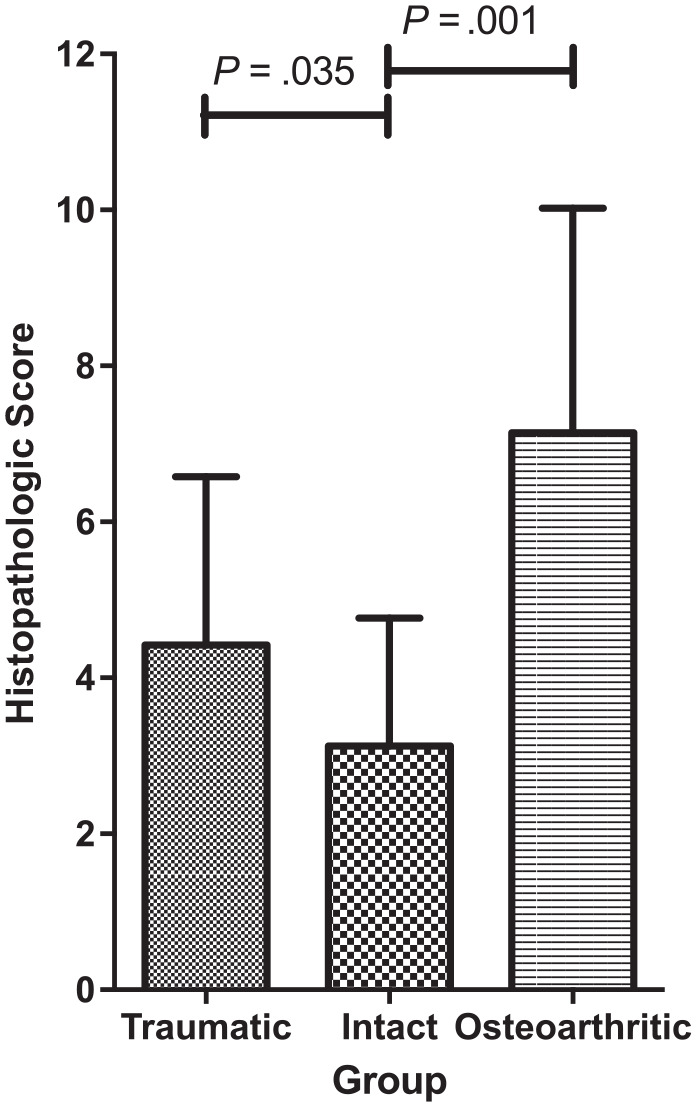
Histopathological score based on scoring system of Pauli et al38 in the traumatic, intact, and degenerative (osteoarthritic) meniscus groups. After correction for age, sex, and body mass index, statistically significant differences in histological scores were found among the groups. Data are shown as mean (SD).
Interobserver reliability for histological scoring based on absolute agreement was excellent (ICC, 0.95; 95% CI, 0.86-0.99). Similar findings were observed regarding intraobserver reliability (ICC, 0.96; 95% CI, 0.90-0.98).
Multivariate Analysis on Histological Scores
To identify and adjust for potential confounders among the groups, a multivariate linear regression analysis was performed (Table 2). After adjustment for age, sex, and BMI, menisci in the TM group showed a statistically significant higher histological score as compared with the IM group (2.7 points higher, P = .035). Furthermore, the adjusted histological score for the DM group was significantly higher than for the IM group (P = .001). The histological score of the TM group did not, after adjustment, differ from the DM group. BMI appeared to be independently associated with histological score. An increase in BMI by 1 unit resulted in an increase of 0.16 in histological score (P = .04) (Figure 4). The histological score and the variables age and sex were not associated.
Table 2.
Multivariate Analysis of Risk Factors on Histological Score a
| Regression Coefficient | 95% CI | P Value | |
|---|---|---|---|
| Age at time of surgery | 0.02 | −0.04 to 0.09 | .497 |
| Sex: female | −0.88 | −2.22 to 0.45 | .190 |
| Body mass index | 0.16 | 0.07 to 0.32 | .040 |
| Trauma group | 2.73 | 0.20 to 5.27 | .035 |
| Osteoarthritic group | 3.97 | 1.65 to 6.29 | .001 |
The intact vital meniscal tissue group is the reference group in the analysis. Bold indicates P < .05.
Figure 4.
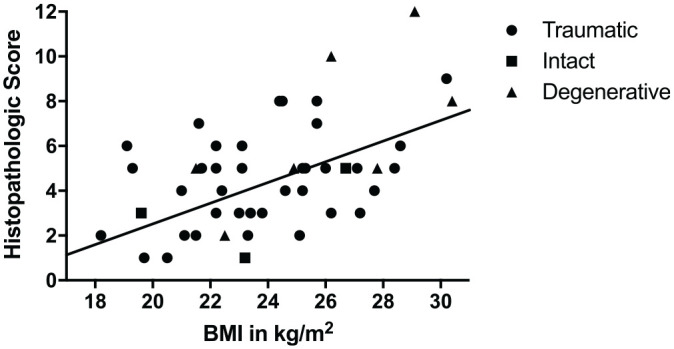
Scatter plot shows correlation between body mass index (BMI) and histopathological score. Each dot represents a unique sample.
Effect of ACL Rupture and Time Interval Between Trauma and Surgery on Degree of Degeneration in the TM Group
Univariate regression analysis showed an increase of 0.21 points in histological score for each unit in BMI increase (P = .03). No association was found between the histological score and the independent variables age at time of surgery, sex, meniscal region, time interval between trauma and surgery, and ACL rupture (Table 3).
Table 3.
Univariate Analysis of Risk Factors on Histologic Score in Traumatically Torn Meniscal Tissue Group a
| Regression Coefficient | 95% CI | P Value | |
|---|---|---|---|
| Age at time of surgery | −0.01 | −0.07 to 0.06 | .84 |
| Sex: female | −0.53 | −2.18 to 1.12 | .52 |
| Body mass index | 0.21 | 0.02 to 0.40 | .03 |
| Time from trauma to surgery | 0.00 | −0.02 to 0.02 | .98 |
| History of ACL rupture | 1.06 | −0.22 to 2.35 | .10 |
Bold indicates P < .05. ACL, anterior cruciate ligament.
Discussion
In this study, we assessed the degree of degeneration in TM, IM, and DM using a validated histological scoring system. Traumatic meniscal tear tissue showed a higher degree of degeneration than intact menisci. We identified BMI as an independent risk factor for meniscal degeneration. No clear association was observed between histological degree of degeneration and age, sex, or time interval between trauma and surgery.
To our knowledge, this is the first study that scored histological degeneration in different meniscal conditions. Most previous studies focused on a single feature of degeneration. Mesiha and colleagues36 reported that traumatic menisci had histological scores equal to those of degenerative menisci. The authors’ main outcome measure to assess degeneration was cellularity; low cellularity was observed in traumatic and degenerative tissue. In a study by Meister et al,35 safranin O matrix staining intensity of the meniscus (as a measure of the amount of glycosaminoglycans) was increased in patients with a traumatic meniscal tear.35 In degenerative menisci, the number of glycosaminoglycans was increased.34,43 Therefore, the observed safranin O staining intensity in our study is regarded as a sign of degeneration of the meniscus. Collagen organization was disturbed in our traumatic meniscal tear samples. Park et al37 earlier concluded that torn meniscal tissue showed less organization of the collagen bundles and a lower amount of collagen type I than intact meniscal tissue. Despite the fact that their research was done in a cohort of patients with knee OA, torn meniscal tissue showed more disturbance of collagen alignment when compared with patients without a tear. These findings may suggest that tissue quality plays an important role in the risk of a meniscal tear in the context of a traumatic event.
Much is unclear regarding the role of meniscal degeneration in traumatic meniscal tears. Currently, the most common view is that most traumatic meniscal tears are the result of a tibiofemoral rotational force as the knee moves from flexion to extension or vice versa while bearing weight.7 In addition, Snoeker et al42 showed that swimming was a risk factor for a meniscal tear, suggesting that a large force transmission is not necessary in the occurrence of a traumatic meniscal tear. These findings underscore that classifying meniscal tears is not as straightforward as it seems. A more plausible view may be the earlier-mentioned continuum theory: the condition alters from a healthy to a degenerative meniscus. In this view, the chance to get a traumatic meniscal tear (partly) depends on the preexisting degree of meniscal degeneration. That is, the more degeneration, the higher the risk of a torn meniscus in case of a traumatic event. The first clue for this theory was provided by the finding that older US military servicemembers showed a higher rate of meniscal injuries despite being exposed to the same activities and movements as their younger colleagues. Jones et al24 concluded that the higher rate of injuries was related to degeneration of the meniscus as age increased. Our findings challenge the classic view of traumatic versus degenerative meniscal tears and support the continuum theory in which degeneration of the meniscal tissue plays a major role in the risk of a meniscal tear.31
In Achilles tendon research, the role of degeneration in traumatic ruptures has been studied more. It is hypothesized that chronic degeneration is the underlying cause of an Achilles tendon rupture.13 The main degenerative changes observed in histological tendon samples are decreased cellularity, increased glycosaminoglycan content, and lack of collagen fiber organization.31 The degenerative theory in tendon ruptures could be extrapolated to meniscal tissue, implicating degenerative tissue as being more vulnerable to a rupture or tear.
A recent study showed a higher expression level of genes encoding matrix metalloproteinases 1 and 3 (MMP1 and MMP3) in traumatic meniscal tissue.31 Increased expression of MMP1 and MMP3 can lead to degradation of collagen fibers. Disrupted collagen organization is one of the determinants of degeneration of the meniscus. Moreover, COL1A1, a gene coding for the main protein in meniscal tissue collagen type 1,7,20,33 was less expressed in traumatic tears.31 Breakdown and less production of collagen type I fibers do not favor a healthy condition of the meniscus and may lead to disturbance in collagen organization. However, it is not clear whether these biologic responses were present before injury or occurred in response to injury.
In the present study, BMI was found to be independently correlated with the degree of meniscal degeneration. Previous clinical studies concluded that higher BMI is associated with a greater risk of a meniscal tear.2,11,19,42 The effect of BMI on degeneration of the meniscus can be explained by its biomechanical role. An increase in BMI results in a greater force transmission by the meniscus.20 Moreover, a higher BMI results in chronic inflammation, which could contribute to an increase in MMP production and degradation of collagen fibers.5,30 This could be a partial explanation of the degenerative changes that occur in meniscal tissue. BMI might be used as a predictive factor for the degree of degeneration of the meniscus.
Interestingly enough, we found no association between age and degeneration of the meniscus. This is contradictory to previous evidence in the literature.36,38 A possible explanation could be that the effect of BMI was not considered in most studies. As age increases, BMI increases as well.23,41,45 Therefore, BMI might be the correct explanation, instead of an increase in age. Our results support this idea. After univariate testing, an association was found between age and histological degeneration. However, adjusted for BMI, this association lost significance (data not shown).
Time interval between trauma and surgery was not associated with the degree of degeneration in the TM group. These findings suggest that degenerative changes did not change after the injury and were present before the injury occurred. These findings are consistent with those of a previous study,36 which reported no differences in cell density and histological score with respect to time interval between trauma and surgery. However, we acknowledge that we cannot comment on the degenerative changes before injury or over time without intrasubject longitudinal evaluation of the meniscal tissue composition. Moreover, previous studies showed that a meniscal tear has effects on other structures of the joint. This corresponds with the idea that a meniscal tear results in cartilage loss and thus accelerated development knee OA.14,22,39,40 The loss of cartilage is greater in patients with a resected part of the meniscus as compared with patients without resection.6 Englund et al14,18 showed that knees with meniscal tears on magnetic resonance imaging but without cartilage lesions were at higher risk of radiographic development of knee OA in later life than were those with intact menisci. This implies that visible meniscal damage occurs before visible cartilage changes. These findings warrant careful decision making in the choice of treatment and, in the case of arthroscopic partial meniscectomy, the timing of surgery.
Unfortunately, we included only 8 intact menisci owing to the low incidence of acute transfemoral amputations, especially in a younger population. Also, results on the subdomain score of surface integrity may be influenced by the meniscal tears affecting the meniscal surface. However, a macroscopically intact portion of the meniscus was cut without the edge of the tear, if possible.
In conclusion, we found a higher degree of meniscal degeneration in the menisci of patients with a traumatic meniscal tear than in intact menisci and no association between time interval between trauma and surgery and histological score. This implies that a meniscus with a higher degree of degeneration might have a higher chance of a meniscal tear during a traumatic event. A better understanding of the degeneration process in the meniscus might help clinicians decide on choice of treatment in the future. This knowledge may also lead to new perspectives to prevent knee OA in patients with a torn meniscus after a traumatic event. Future studies should explore the possibilities of longitudinal in vivo evaluation of degeneration, for instance with quantitative magnetic resonance imaging techniques.
Supplemental Material
Supplemental material, DS_10.1177_0363546520934766 for Traumatic Meniscal Tears Are Associated With Meniscal Degeneration by Marinus A. Wesdorp, Susanne M. Eijgenraam, Duncan E. Meuffels, Sita M.A. Bierma-Zeinstra, Gert-Jan Kleinrensink, Yvonne M. Bastiaansen-Jenniskens and Max Reijman in The American Journal of Sports Medicine
Acknowledgments
This study was supported by the departments of neuroscience and trauma surgery. These departments acquired cadaver knees and the facility for resecting meniscal tissue. The authors thank all orthopaedic surgeons and trauma surgeons of Erasmus MC, University Medical Center Rotterdam, for the collection of meniscal tissue. The authors are grateful for the technical assistance of Nicole Kops (Department of Orthopedic Surgery, Erasmus MC) with histological procedures.
Footnotes
Submitted January 8, 2020; accepted April 6, 2020.
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Arner O, Lindholm A, Orell SR. Histologic changes in subcutaneous rupture of the Achilles tendon; a study of 74 cases. Acta Chir Scand. 1959;116(5-6):484-490. [PubMed] [Google Scholar]
- 2. Baker P, Coggon D, Reading I, Barrett D, McLaren M, Cooper C. Sports injury, occupational physical activity, joint laxity, and meniscal damage. J Rheumatol. 2002;29(3):557-563. [PubMed] [Google Scholar]
- 3. Beaufils P, Becker R, Kopf S, et al. Surgical management of degenerative meniscus lesions: the 2016 ESSKA meniscus consensus. Knee Surg Sports Traumatol Arthrosc. 2017;25(2):335-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beaufils P, Becker R, Kopf S, Matthieu O, Pujol N. The knee meniscus: management of traumatic tears and degenerative lesions. EFORT Open Rev. 2017;2(5):195-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage. 2013;21(1):16-21. [DOI] [PubMed] [Google Scholar]
- 6. Berthiaume M-J, Raynauld J-P, Martel-Pelletier J, et al. Meniscal tear and extrusion are strongly associated with progression of symptomatic knee osteoarthritis as assessed by quantitative magnetic resonance imaging. Ann Rheumatic Dis. 2005;64(4):556-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brindle T, Nyland J, Johnson DL. The meniscus: review of basic principles with application to surgery and rehabilitation. J Athl Train. 2001;36(2):160-169. [PMC free article] [PubMed] [Google Scholar]
- 8. Clayton RA, Court-Brown CM. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury. 2008;39(12):1338-1344. [DOI] [PubMed] [Google Scholar]
- 9. Cook JL, Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br J Sports Med. 2009;43(6):409-416. [DOI] [PubMed] [Google Scholar]
- 10. Davidsson L, Salo M. Pathogenesis of subcutaneous tendon ruptures. Acta Chir Scand. 1969;135(3):209-212. [PubMed] [Google Scholar]
- 11. Ding C, Martel-Pelletier J, Pelletier J-P, et al. Meniscal tear as an osteoarthritis risk factor in a largely non-osteoarthritic cohort: a cross-sectional study. J Rheumatol. 2007;34(4):776-784. [PubMed] [Google Scholar]
- 12. Draijer LW, Belo JN, Berg HF, Geijer RM, Goudswaard AN. De NHG-Standaard “Traumatische knieproblemen” (eerste herziening): samenvatting. Ned Tijdschr Geneeskd. 2010;154:A2225. [PubMed] [Google Scholar]
- 13. Egger AC, Berkowitz MJ. Achilles tendon injuries. Curr Rev Musculoskel Med. 2017;10(1):72-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Englund M. Meniscal tear—a feature of osteoarthritis. Acta Orthop Scand Suppl. 2004;75(312):1-45. [PubMed] [Google Scholar]
- 15. Englund M, Guermazi A, Gale D, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med. 2008;359(11):1108-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Englund M, Guermazi A, Roemer FW, et al. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: the Multicenter Osteoarthritis Study. Arthritis Rheum. 2009;60(3):831-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Englund M, Roemer FW, Hayashi D, Crema MD, Guermazi A. Meniscus pathology, osteoarthritis and the treatment controversy. Nat Rev Rheumatol. 2012;8(7):412-419. [DOI] [PubMed] [Google Scholar]
- 18. Englund M, Roos EM, Lohmander LS. Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis: a sixteen-year followup of meniscectomy with matched controls. Arthritis Rheum. 2003;48(8):2178-2187. [DOI] [PubMed] [Google Scholar]
- 19. Ford GM, Hegmann KT, White GL, Holmes EB. Associations of body mass index with meniscal tears. Am J Prev Med. 2005;28(4):364-368. [DOI] [PubMed] [Google Scholar]
- 20. Fox AJ, Wanivenhaus F, Burge AJ, Warren RF, Rodeo SA. The human meniscus: a review of anatomy, function, injury, and advances in treatment. Clin Anat. 2015;28(2):269-287. [DOI] [PubMed] [Google Scholar]
- 21. Greis PE, Bardana DD, Holmstrom MC, Burks RT. Meniscal injury: I. Basic science and evaluation. J Am Acad Orthop Surg. 2002;10(3):168-176. [DOI] [PubMed] [Google Scholar]
- 22. Hunter DJ, Zhang YQ, Niu JB, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54(3):795-801. [DOI] [PubMed] [Google Scholar]
- 23. Hutfless S, Maruthur NM, Wilson RF, et al. Strategies to prevent weight gain in adults: a systematic review. Am J Prev Med. 2013;45(6):e41-e51. [DOI] [PubMed] [Google Scholar]
- 24. Jones JC, Burks R, Owens BD, Sturdivant RX, Svoboda SJ, Cameron KL. Incidence and risk factors associated with meniscal injuries among active-duty US military service members. J Athl Train. 2012;47(1):67-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jozsa L, Kannus P. Histopathological findings in spontaneous tendon ruptures. Scand J Med Sci Sports. 1997;7(2):113-118. [DOI] [PubMed] [Google Scholar]
- 26. Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon: a controlled study of 891 patients. J Bone Joint Surg Am. 1991;73(10):1507-1525. [PubMed] [Google Scholar]
- 27. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769. [DOI] [PubMed] [Google Scholar]
- 29. Longo UG, Ronga M, Maffulli N. Acute ruptures of the achilles tendon. Sports Med Arthrosc Rev. 2009;17(2):127-138. [DOI] [PubMed] [Google Scholar]
- 30. Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121(6):2111-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Magnan B, Bondi M, Pierantoni S, Samaila E. The pathogenesis of Achilles tendinopathy: a systematic review. Foot Ankle Surg. 2014;20(3):154-159. [DOI] [PubMed] [Google Scholar]
- 32. Majewski M, Susanne H, Klaus S. Epidemiology of athletic knee injuries: a 10-year study. Knee. 2006;13(3):184-188. [DOI] [PubMed] [Google Scholar]
- 33. Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32(30):7411-7431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McDevitt CA, Webber RJ. The ultrastructure and biochemistry of meniscal cartilage. Clin Orthop Relat Res. 1990;252:8-18. [PubMed] [Google Scholar]
- 35. Meister K, Indelicato PA, Spanier S, Franklin J, Batts J. Histology of the torn meniscus: a comparison of histologic differences in meniscal tissue between tears in anterior cruciate ligament–intact and anterior cruciate ligament–deficient knees. Am J Sports Med. 2004;32(6):1479-1483. [DOI] [PubMed] [Google Scholar]
- 36. Mesiha M, Zurakowski D, Soriano J, Nielson JH, Zarins B, Murray MM. Pathologic characteristics of the torn human meniscus. Am J Sports Med. 2007;35(1):103-112. [DOI] [PubMed] [Google Scholar]
- 37. Park DY, Min BH, Choi BH, et al. The degeneration of meniscus roots is accompanied by fibrocartilage formation, which may precede meniscus root tears in osteoarthritic knees. Am J Sports Med. 2015;43(12):3034-3044. [DOI] [PubMed] [Google Scholar]
- 38. Pauli C, Grogan SP, Patil S, et al. Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthritis Cartilage. 2011;19(9):1132-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis Cartilage. 1995;3(4):261-267. [DOI] [PubMed] [Google Scholar]
- 40. Sharma L, Eckstein F, Song J, et al. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum. 2008;58(6):1716-1726. [DOI] [PubMed] [Google Scholar]
- 41. Sheehan TJ, DuBrava S, DeChello LM, Fang Z. Rates of weight change for black and white Americans over a twenty year period. Int J Obes Relat Metab Disord. 2003;27(4):498-504. [DOI] [PubMed] [Google Scholar]
- 42. Snoeker BA, Bakker EW, Kegel CA, Lucas C. Risk factors for meniscal tears: a systematic review including meta-analysis. J Orthop Sports Phys Ther. 2013;43(6):352-367. [DOI] [PubMed] [Google Scholar]
- 43. Sun Y, Mauerhan DR, Kneisl JS, et al. Histological examination of collagen and proteoglycan changes in osteoarthritic menisci. Open Rheumatol J. 2012;6:24-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Uysal M, Akpinar S, Bolat F, Cekin N, Cinar M, Cesur N. Apoptosis in the traumatic and degenerative tears of human meniscus. Knee Surg Sports Traumatol Arthrosc. 2008;16(7):666-669. [DOI] [PubMed] [Google Scholar]
- 45. Zheng Y, Manson JE, Yuan C, et al. Associations of weight gain from early to middle adulthood with major health outcomes later in life. JAMA. 2017;318(3):255-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0363546520934766 for Traumatic Meniscal Tears Are Associated With Meniscal Degeneration by Marinus A. Wesdorp, Susanne M. Eijgenraam, Duncan E. Meuffels, Sita M.A. Bierma-Zeinstra, Gert-Jan Kleinrensink, Yvonne M. Bastiaansen-Jenniskens and Max Reijman in The American Journal of Sports Medicine