Abstract
The marine-derived fungus Aspergillus fumigatus MF071, isolated from sediment collected from the Bohai Sea, China, yielded two new compounds 19S,20-epoxy-18-oxotryprostatin A (1) and 20-hydroxy-18-oxotryprostatin A (2), in addition to 28 known compounds (3–30). The chemical structures were established on the basis of 1D, 2D NMR and HRESIMS spectroscopic data. This is the first report on NMR data of monomethylsulochrin-4-sulphate (4) and pseurotin H (10) as naturally occurring compounds. Compounds 15, 16, 20, 23, and 30 displayed weak antibacterial activity (minimum inhibitory concentration: 100 μg/mL). Compounds 18 and 19 exhibited strong activity against S. aureus (minimum inhibitory concentration: 6.25 and 3.13 μg/mL, respectively) and E. coli (minimum inhibitory concentration: 6.25 and 3.13 μg/mL, respectively). A genomic data analysis revealed the putative biosynthetic gene clusters ftm for fumitremorgins, pso for pseurotins, fga for fumigaclavines, and hel for helvolinic acid. These putative biosynthetic gene clusters fundamentally underpinned the enzymatic and mechanistic function study for the biosynthesis of these compounds. The current study reported two new compounds and biosynthetic gene clusters of fumitremorgins, pseurotins, fumigaclavines and helvolinic acid from Aspergillus fumigatus MF071.
Keywords: Aspergillus fumigatus, genome mining, chemical diversity, antimicrobial activity, biosynthetic gene cluster, prenyltransferase
1. Introduction
It has been demonstrated that marine-derived microbes have become one of the most important sources of pharmacologically active metabolites [1,2,3,4,5]. Under extreme marine conditions, such as high salinity, high pressure, low temperature, and extreme pressures, microbes have evolved unique physiological and chemical capabilities to survive and proliferate [6]. Marine natural products (MNPs) display a wide range of structural diversity and remarkable pharmaceutically relevant bioactivities, including antibacterial, antiviral, anticancer, and anti-inflammatory properties [7,8,9]. Fungi derived MNPs are the largest category among all marine sources (bacteria, cyanobacteria, algae, sponges, invertebrate, and mangroves), with the average number of compounds in 2018 increased by 85% compared with the previous three years (2015–2017) [8].
The increasing rediscovery rate of known compounds through high-throughput screening (HTS) has led to a decline in natural product research, whereas both hospital and community-associated infectious pathogens with antimicrobial resistance (AMR) are spreading rapidly [10]. With technological advances in microbial genome sequencing and the development of bioinformatics tools [11], genome mining approaches have revealed a hidden reservoir of untapped biosynthetic gene clusters (BGCs) [12,13]. Recent studies of large-scale and meta-genome mining have highlighted unprecedented capabilities for biosynthesis of diverse novel classes of active natural products from microbes [14,15,16].
In the course of searching for bioactive metabolites produced from marine-derived fungi, a marine microbe library and a microbial crude extracts library were constructed and screened for various biological activities [17,18,19]. One strain, Aspergillus fumigatus MF071, was highlighted for a chemical constituent study based on HPLC chemical profiling and NMR fingerprinting analyses. Meanwhile, the genome of MF071 was sequenced for the metabolic potential analysis. The genome-inspired chemical constituents study and biological activities investigation of an extract of MF071 led to the isolation of 30 compounds, including the two new compounds 19S,20-epoxy-18-oxotryprostatin A (1) and 20-hydroxy-18-oxotryprostatin A (2), along with seven active compounds. The NMR data of two compounds, monomethylsulochrin-4-sulphate (4) and pseurotin H (10), are also reported here for the first time. The isolated compounds include the structural classes of indole alkaloids, polyketide and non-ribosomal peptide hybrids, terpenoids, and polyketides. An analysis of genome sequences revealed BGCs and the biosynthetic pathway of these compounds. The activities of isolated compounds against Mycobacterium smegmatis, Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa were also evaluated.
2. Results
2.1. Characterization and Identification of Strain MF071
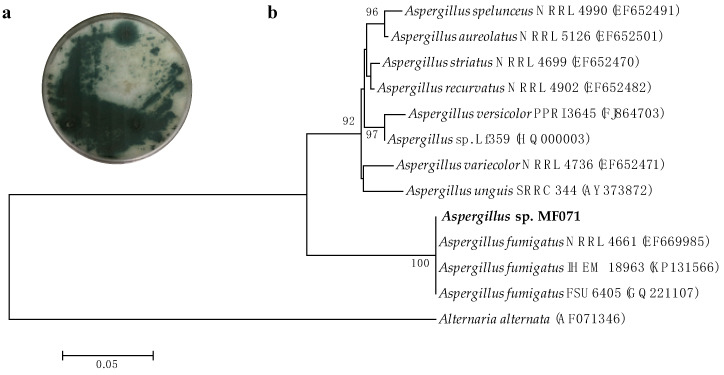
The identification of MF071 was performed based on the morphology and phylogenetic analysis. After incubation at 28 °C for ten days, strain MF071 formed colonies on a PDA plate with characteristic hyphal structures (Figure 1a) [20]. The ITS gene region of ribosomal DNA of the strain was PCR-amplified and sequenced. The phylogenetic tree (Figure 1b) constructed from the ITS gene sequence indicated that MF071 belonged to the genus of Aspergillus with the highest similarity to A. fumigatus (99.82%, accession number: EF669985). The nucleotide sequence of the ITS gene has been deposited in GenBank (accession no. MN700176). The MF071 strain has been deposited at Dr. Zhang’s Laboratory, East China University of Science and Technology.
Figure 1.
Morphology and phylogenetic tree of Aspergillus fumigatus MF071. (a) Colony characteristics of A. fumigatus MF071 grown on potato dextrose agar at 28 °C for 10 days; (b) Neighbor-joining tree of A. fumigatus MF071 based on 18S sequences. Numbers at nodes indicate levels of bootstrap support (%) based on a neighbor-joining analysis of 1000 resampled datasets; only values >50 % are shown. National Center for Biotechnology Information (NCBI) accession numbers are provided in parentheses. The Bar represents 0.05 nucleotide substitutions per site.
2.2. Structure Elucidation of the Isolated Compounds
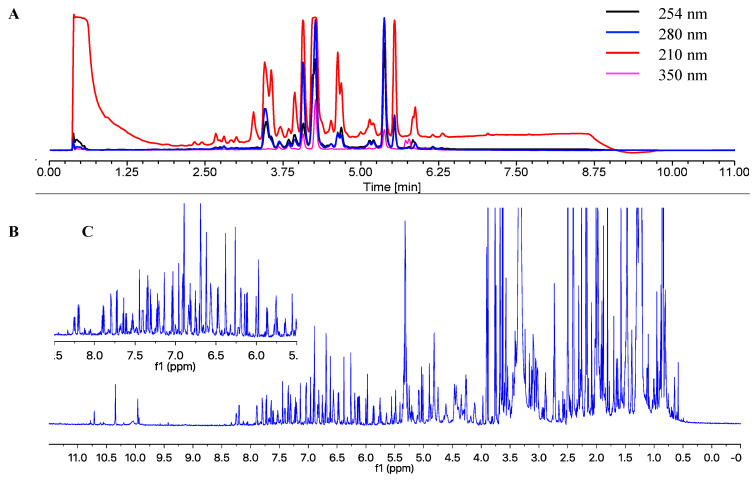
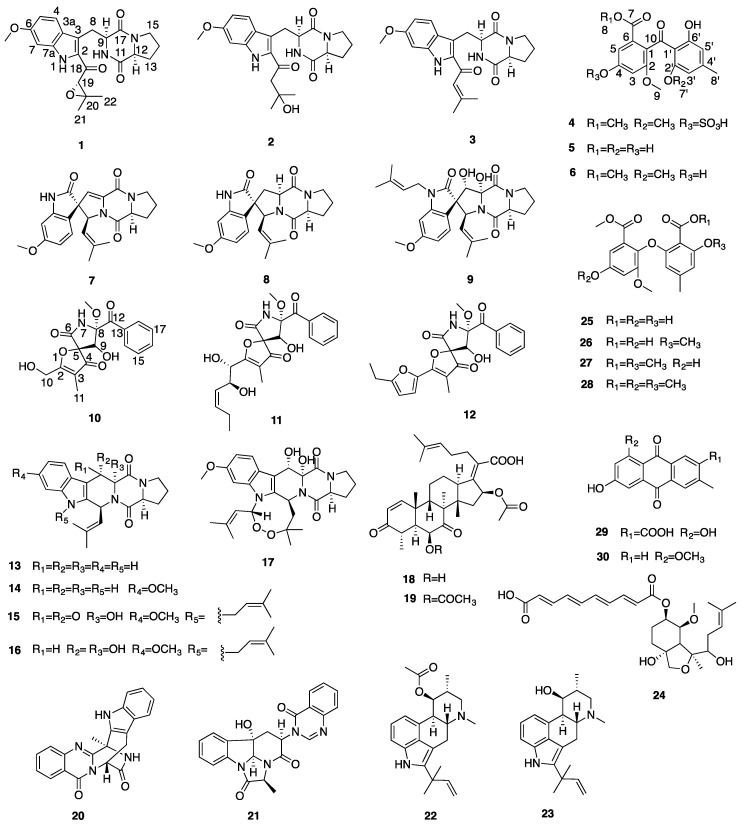
The chemical profiling of the MF071 extract obtained from rice medium fermentation was analysed using HPLC and 1H NMR (Figure 2). The 1H NMR displaying a wide range of signals indicated the chemical diversity of the extract. The MF071 extract was fractionated and purified, as detailed in Materials and Methods. Thirty compounds were identified, including two new compounds 19S,20-epoxy-18-oxotryprostatin A (1) and 20-hydroxy-18-oxotryprostatin A (2). The NMR data of the compounds monomethylsulochrin-4-sulphate (4), and pseurotin H (10) (Figure 3) are also reported.
Figure 2.
HPLC spectrum (A), 1H NMR spectrum (B), and 1H NMR expansion of MF071 extract.
Figure 3.
Structures of compounds isolated from Aspergillus fumigatus MF071.
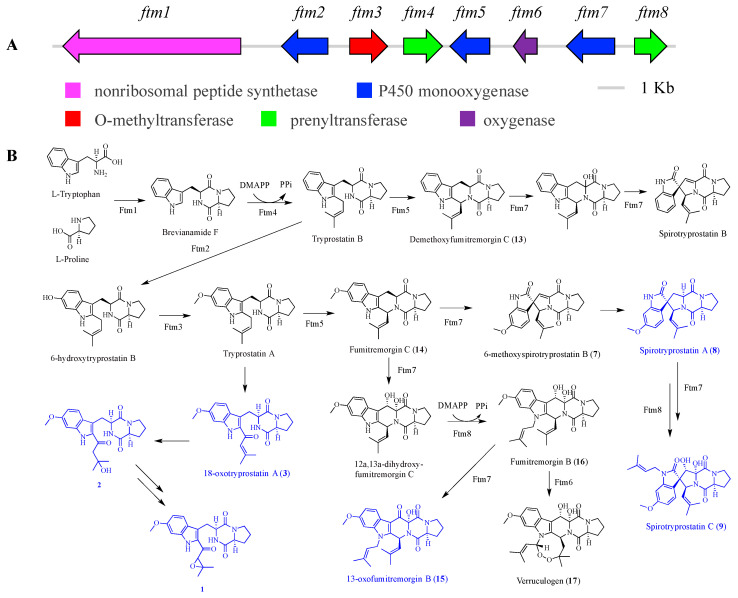
Compound 1 was isolated as a yellow powder. Its HRESIMS revealed a molecular ion peak of m/z 434.1689 for [M + Na]+ (calculated for C22H25N3O5 Na, 434.1686), indicating a molecular formula of C22H25N3O5 (Figure S1). The UV spectrum of 1 showed maximal absorbance at 260 nm and 350 nm in MeOH. The 1H, 13C and 2D NMR (Table 1 and Figures S2–S4) revealed the presence of three aromatic methine carbons (δC/δH 123.0/7.68, d, J = 8.9 Hz; 94.1/6.89, d, J = 2.3 Hz; 112.5/6.77, dd, J = 8.9 and 2.3 Hz); one methoxyl group at δH 3.81 (δC 55.6); two methyl groups at δH 1.48 (δC 24.7, C-21) and δH 1.17 (δC 18.6, C-22); four methylene groups (δC/δH 26.0/3.62 and 3.32, m; 45.5/3.39 and 3.28, m; 28.1/2.07 and 1.72, m; 22.6/1.75, m); three methine groups at δH 4.42 (δC 56.1, C-9), δH 4.36 (δC 63.9, C-19), and δH 4.16 (δC 58.9, C-12); and three carbonyl groups (δC 187.2, C-18; δC 167.2, C-11; δC 165.9, C-17).These NMR data suggested compound 1 as a tryprostatin derivative [21], including the moieties of the indole, diketopiperazine, and prenyl part. In comparison with the NMR data of 18-oxotryprostatin A (3) [22], the HMBC correlations from H-21 (δH 1.48) and H-22 (δH 1.17) to C-19 (δC 63.9) and C-20 (δC 61.8), and a degree of unsaturation suggested the presence of an epoxy moiety between C-19 and C-20. Thus, the planar structure of 1 was assigned as depicted (Table 1 and Figure 3). The relative configuration of compound 1 was determined by its biogenetic origin and DFT NMR calculation. The genome sequences of MF071 revealed that compound 1 had the same biosynthetic pathway as 18-oxotryprostatin A (3) (Figure 4B). Considering the same biogenetic origin, the relative configurations of positions C-9 and C-12 in compound 1 were proposed to be the same as those in 18-oxotryprostatin A (3). The 13C NMR chemical shifts of two possible isomers with 19S,20-epoxy and 19R,20-epoxy configurations were calculated using DFT. A DP4 probability analysis of calculated and experimental data allowed the determination of the configuration of 1 as 19S,20-epoxy-18-oxotryprostatin A (Table S1).
Table 1.
1H (800 MHz) and 13C (200 MHz) NMR data of compounds 1 and 2.
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δC, Type | δH, Mult (J in Hz) | δC, Type | δH, Mult (J in Hz) | |
| 1 | 11.57 s | |||
| 2 | 131.7, C | 133.8, C | ||
| 3 | 119.8, C | 119.2, C | ||
| 3a | 122.4, C | 122.3, C | ||
| 4 | 123.0, CH | 7.68 d (8.9) | 122.7, CH | 7.64 d (8.9) |
| 5 | 112.5, CH | 6.77 dd (8.9, 2.3) | 112.2, CH | 6.74 dd (8.9, 2.3) |
| 6 | 159.8, C | 159.3, C | ||
| 7 | 94.1, CH | 6.89 d (2.3) | 94.1, CH | 6.88 d (2.3) |
| 7a | 138.7, C | 138.0, C | ||
| 8 | 26.0, CH2 | 3.62 m, 3.32 m | 25.5, CH2 | 3.61 dd (14.2, 4.9), 3.27 m |
| 9 | 56.1, CH | 4.42 t (6.5) | 56.6, CH | 4.36 t (6.1) |
| 10 | 7.45 s | 7.42 s | ||
| 11 | 167.2, C | 167.0, C | ||
| 12 | 58.9, CH | 4.16 t (8.0) | 58.8, CH | 4.14 t (8.0) |
| 13 | 28.1, CH2 | 2.07 m, 1.72 m | 28.0, CH2 | 2.06 m, 1.73 m |
| 14 | 22.6, CH2 | 1.75 m | 22.7, CH2 | 1.75 m |
| 15 | 45.5, CH2 | 3.39 m, 3.28 m | 45.3, CH2 | 3.38 m, 3.32 m |
| 17 | 165.9, C | 166.0, C | ||
| 18 | 187.2, C | 194.1, C | ||
| 19 | 63.9, CH | 4.36 s | 52.9, CH2 | 3.05 d (13.9), 3.02 d (13.9) |
| 20 | 61.8, C | 70.1, C | ||
| 21 | 24.7, CH3 | 1.48 s | 30.3, CH3 | 1.25 s |
| 22 | 18.6, CH3 | 1.17 s | 30.3, CH3 | 1.25 s |
| 6-OCH3 | 55.6, CH3 | 3.81 s | 55.6, CH3 | 3.80 s |
Figure 4.
Organization of the fumitremorgin-type indole alkaloids BGC (ftm) (A), and proposed biosynthetic pathways for tryprostatins, spirotryprostatins, and fumitremorgins (B).
The HRESIMS spectrum of compound 2 exhibited an [M + Na]+ ion at m/z 436.1841 (calculated for C22H27N3O5Na, 436.1843), corresponding to a molecular formula of C22H27N3O5 (Figure S5). The UV spectrum of 2 showed maximal absorbance at 260 nm and 342 nm in MeOH. By comparing the 1H and 2D NMR data (Table 1, Figures S6–S9) of 2 with those of 1, it was evident that 2 possessed the same skeleton as 1. The only differences included the absence of the epoxy moiety and the presence of one hydroxyl group. The HMBC correlation from H-19 (δH 3.05) to C-20 (δC 70.1), C-21 (δC 30.3), C-22 (δC 30.3), and C-18 (δC 194.1) indicated that the hydroxyl group was attached to C-20 and the methylene group (C-19) was attached to C-20 and C-18. On the basis of the above analysis, the structure of 2 was determined (Table 1 and Figure 3).
The structure of compound 4 can be found in the SciFinder database, however no literature and NMR data were reported for this compound. Here, we provide the NMR assignment of the compound. Compound 4 had the molecular formula C18H18O10S, as established from the [M − H]− peak on HRESIMS (Figure S10) (m/z 425.0535 [M − H]−, calculated 425.0548). The 13C NMR spectrum showed 18 carbon signals. The 1H and 13C NMR spectra, together with 2D NMR (Table 2 and Figures S11–S14) revealed the presence of four aromatic methine carbons (δC/δH 112.4/7.43, d, J = 2.1 Hz; 108.3/7.06, d, J = 2.1 Hz; 110.2/6.39, s; 103.7/6.27, s); three methoxyl groups at δH 3.66 (δC 52.3, C-8), δH 3.65 (δC 56.2, C-9), and δH 3.31 (δC 55.9, C-7′); and one methyl group at 2.26 (δC 22.0, C-8′). In addition to the above carbon signals, there were ten sp2 hybridized quaternary carbons, including an α, β-unsaturated carbonyl group (C-10) and an ester group (C-7) at δC 199.1 and 165.5, four oxygenated aromatic carbons (δC 163.4, C-6′, δC 160.9, C-2′, δC 155.8, C-2, δC 154.1, C-4), and four aromatic carbons (δC 148.2, C-4′, δC 129.4, C-1, δC 127.1, C-6, δC 109.9, C-1′). These NMR data suggested that compound 4 could be a monomethylsulochrin derivative [23]. The molecular formula obtained from HRESIMS suggested the presence of one additional sulphate group. The sulphate group was determined to be attached to C-4 by comparing the NMR data with 6 (Table 2 and Figure 3).
Table 2.
1H (800 MHz) and 13C (200 MHz) NMR data of compounds 4 and 6.
| Position | 4 | 6 | ||
|---|---|---|---|---|
| δC, Type | δH, Mult (J in Hz) | δC, Type | δH, Mult (J in Hz) | |
| 1 | 129.4, C | 125.8, C | ||
| 2 | 155.8, C | 156.6, C | ||
| 3 | 108.3, CH | 7.06 d (2.1) | 103.2, CH | 6.69 d (2.2) |
| 4 | 154.1, C | 158.1, C | ||
| 5 | 112.4, CH | 7.43 d (2.1) | 107.2, CH | 6.89 d (2.2) |
| 6 | 127.1, C | 128.0, C | ||
| 7 | 165.5, C | 165.8, C | ||
| 8 | 52.3, CH3 | 3.66 s | 52.1, CH3 | 3.62 s |
| 9 | 56.2, CH3 | 3.65 s | 56.0, CH3 | 3.63 s |
| 10 | 199.1, C | 199.4, C | ||
| 1’ | 109.9, C | 110.1, C | ||
| 2’ | 160.9, C | 160.8, C | ||
| 3’ | 103.7, CH | 6.27 s | 103.5, CH | 6.26 s |
| 4’ | 148.2, C | 147.8, C | ||
| 5’ | 110.2, CH | 6.39 s | 110.1, CH | 6.38 s |
| 6’ | 163.4, C | 163.3, C | ||
| 7’ | 55.9, CH3 | 3.31 s | 55.9, CH3 | 3.33 s |
| 8’ | 22.0, CH3 | 2.26 s | 21.9, CH3 | 2.26 s |
| 6′-OH | 12.90 s | 12.95 s | ||
| 4-OH | 10.05 s | |||
The structure of compound 10 is given in one article, however, no NMR data were reported [24]. Here, we provide the NMR assignment of the compound. The molecular formula C17H17NO7 was assigned to 10 based on the HRESIMS molecular ion peak at m/z = 370.0895 ([M + Na]+, calculated 370.0897) (Figure S15). The 13C NMR spectrum showed 17 carbon signals. The 1H and 13C NMR spectra, together with 2D NMR (Table 3 and Figures S16–S19) revealed the presence of a monosubstituted benzene ring (2H at δH 8.25, dd, J = 8.4 and 1.2 Hz; 2H at δH 7.52, dd, J = 8.4 and 7.4 Hz; 1H at δH 7.67, tt, J = 7.4 and 1.2 Hz), one oxymethine group at δH 4.39 (δC 74.9, C-9), one oxymethylene group at δH 4.42 (δC 57.1, C-10), two methyl groups: one oxygenated (δC/δH 51.6/3.24) and one allylic (δC/δH 5.2/1.65), and eight quaternary carbons at δC 196.6 (C-4), 196.4 (C-12), 186.0 (C-2), 166.4 (C-6), 133.4 (C-13), 110.1 (C-3), 92.5 (C-8), and 91.4 (C-5). Based on these characteristic structural features and a comprehensive database search, the compound was identified as a pseurotin A derivative, with a side chain replaced by a hydroxymethyl group [25] (Figure 3). The NMR data were assigned unambiguously (Table 3).
Table 3.
1H (800 MHz) and 13C (200 MHz) NMR data of compound 10.
| Position | δC, Type | δH, Mult (J in Hz) | Position | δC, Type | δH, Mult (J in Hz) |
|---|---|---|---|---|---|
| 1 | 11 | 5.2, CH3 | 1.65 s | ||
| 2 | 186.0, C | 12 | 196.4, C | ||
| 3 | 110.1, C | 13 | 133.4, C | ||
| 4 | 196.6, C | 14 | 130.3, CH | 8.25 dd (8.4, 1.2) | |
| 5 | 91.4, C | 15 | 128.4, CH | 7.52 dd (8.4, 7.4) | |
| 6 | 166.4, C | 16 | 133.8, CH | 7.67 tt (7.4, 1.2) | |
| 7 | 9.92 s | 17 | 128.4, CH | 7.52 dd (8.4, 7.4) | |
| 8 | 92.5, C | 18 | 130.3, CH | 8.25 dd (8.4, 1.2) | |
| 9 | 74.9, CH | 4.39 s | 8-OCH3 | 51.6, CH3 | 3.24 s |
| 10 | 57.1, CH2 | 4.42 d (3.0) |
Other known compounds were identified as 18-oxotryprostatin A (3) [22], sulochrin (5) [26], monomethylsulochrin (6) [23], 6-methoxyspirotryprostatin B (7) [22], spirotryprostatin A (8) [27], spirotryprostatin C (9) [28], pseurotin A (11) [25], azaspirofuran A (12) [29], demethoxyfumitremorgin C (13) [30], fumitremorgin C (14) [28], 13-oxofumitremorgin B (15) [31], fumitremorgin B (16) [28], verruculogen (17) [28], helvolinic acid (18) [32], helvolic acid (19) [33], fumiquinazolines J (20) [34], chaetominine (21) [35], fumigaclavine C (22) [36], 9-deacetylfumigaclavine C (23) [36], fumagiringillin (24) [37], asterric acid (25), circinophoric acid (26) [38], dimethyl 2,3′-dimethylosoate (27) [23], methylated asterric acid (28) [39], endocrocin (29) [40], and questin (30) [41]. Their 1H and 13C NMR data were identical to those reported in the literature.
2.3. Biological Activities
All compounds were evaluated in vitro for antibacterial activities against M. smegmatis, S. aureus, E. coli, and P. aeruginosa, except for compounds 1, 2, and 4 because of limited amounts (Table 4). Compounds 15, 16, 20, 23, and 30 were active against certain test strains, showing weak activity with a shared minimum inhibitory concentration (MIC) value of 100 μg/mL. Compounds 18 and 19 exhibited strong activities against S. aureus (6.25 and 3.13 μg/mL, respectively) and E. coli (6.25 and 3.13 μg/mL, respectively). Other compounds were inactive at concentrations up to 100 μg/mL.
Table 4.
Antibacterial activity of identified compounds.
| Compounds | Pathogenic Bacteria (MIC, μg/mL) | |||
|---|---|---|---|---|
| M. smegmatis | S. aureus | E. coli | P. aeruginosa | |
| 15 | >100 | 100 | >100 | >100 |
| 16 | 100 | 100 | 100 | 100 |
| 18 | >100 | 6.25 | 6.25 | >100 |
| 19 | 100 | 3.13 | 3.13 | >100 |
| 20 | 100 | 100 | >100 | >100 |
| 23 | 100 | >100 | >100 | >100 |
| 30 | >100 | 100 | 100 | >100 |
2.4. Proposed BGCs and Biosynthetic Pathway
The putative secondary metabolite BGCs of MF071 were predicted based on the antiSMASH results and a further detailed sequence analysis. We report here the BGCs for fumitremorgins (ftm), pseurotins (pso), fumigaclavines (fga), and helvolinic acid (hel) and associated biosynthetic pathways.
Fumitremorgins BGC ftm consists of eight genes, encoding one nonribosomal peptide synthetase, three cytochrome P450 monooxygenases, two prenyltransferases, one O-methyltransferase, and one oxygenase (Figure 4A, Table S2). Based on the deduced function of each gene in predicted BGC (ftm, accession no. MT424560), the biosynthetic pathway of tryprostatins (1–3), spirotryprostatins (7–9), and fumitremorgins (13–17) was proposed with the initial catalysis of L-tryptophan, L-proline, and dimethylallyl diphosphate (Figure 4B) [42,43]. The catalytic evidence for the production of compounds 1–3, 8, 9, and 15 are still to be uncovered.
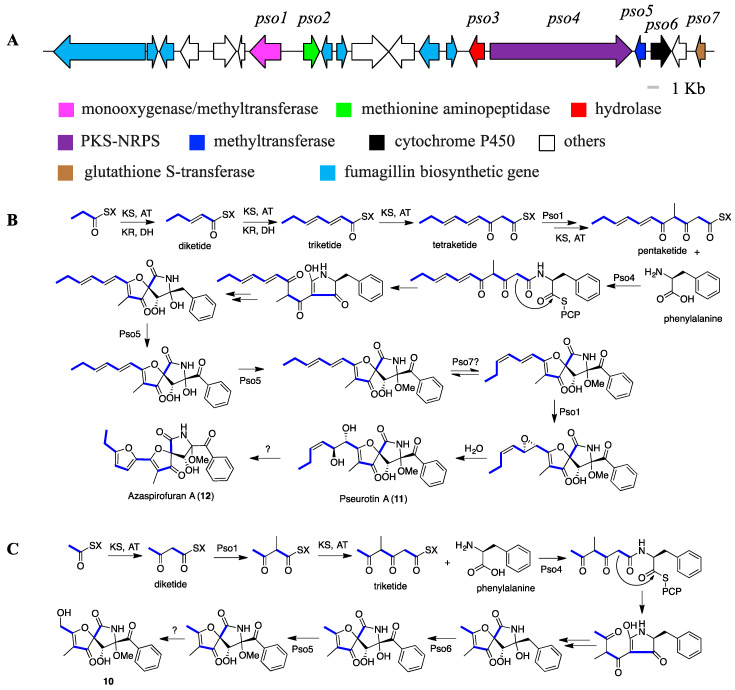
When we analyzed the BGC of pseurotins from MF071, one PKS-NRPS hybrid cluster was found (pso, accession no. MT424563). Unexpectedly, the pso gene cluster intertwined with biosynthetic genes involved in the formation of fumagillins (Figure 5A) [44]. On the basis of the putative function of each gene through the BLASTp analysis (Table S3), the biosynthetic pathway of pseurotins (10–12), one type of compound with an unusual heterospirocyclic γ-lactam feature, was proposed, starting with the condensation of one propionate (acetate for compound 10), four malonates (two malonates for compound 10), one L-methionine, and one L-phenylalanine (Figure 5) [45,46]. Notably, this is the first report of compound 10 as a natural product. Compared with the biosynthesis of most pseurotin derivatives, a different polyketide biosynthetic pathway was proposed for compound 10 (Figure 5C).
Figure 5.
Organization of the pseurotins biosynthetic gene clusters (BGC) (pso) (A), and proposed biosynthetic pathways for pseurotin A and azaspirofuran A (B), and compound 10 (C).
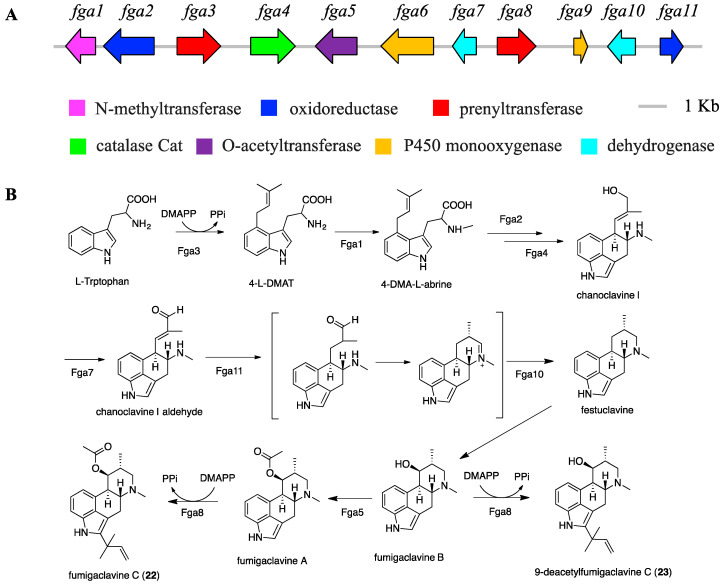
Bioinformatic analysis identified fga BGC (accession no. MT424562) as the putative biosynthetic cluster for fumigaclavines, consisting of 11 open reading frames (ORFs) and spanning 23 kb of genomic DNA (Figure 6A). The function of each gene was proposed by BLASTp analysis against the NCBI database using an amino acid sequence (Table S4). The biosynthetic pathway of fumigaclavines (22 and 23) was also proposed (Figure 6). The enzymatic catalysis of prenylation at position C4 of indole by Fga3, methylation by Fga1, acetylation by Fga5, and prenylation at position 2 of indole by Fga8 have been proven by genetic approaches [47,48,49]. However, the formation of the D ring of the tetracyclic ergoline and the catalytic mechanism of the tert-prenylation at position C2 are still unclear.
Figure 6.
Organization of the fumigaclavines BGC (fga) (A), and proposed biosynthetic pathway for fumigaclavine C and 9-deacetylfumigaclavine C (B).
A bioinformatic analysis and literature search also revealed the BGC of helvolinic acid (hel, accession no. MT424561) (Figure S20A, Table S5), and the biosynthetic pathway of helvolinic acid (18) and helvolic acid (19) was proposed (Figure S20B) [50].
3. Discussion
Marine derived fungi are still important sources for the discovery of new bioactive natural products. The current study presents a genome-inspired metabolic mining of marine fungus A. fumigatus MF071, which led to the discovery of diverse BGCs and 30 compounds, including two new compounds and two known compounds with NMR data reported for the first time. Evaluation of antibacterial activity showed that compounds 18 and 19 exhibited strong activity against S. aureus and E. coli. A bioinformatic analysis of the genome sequences of MF071 revealed large numbers of secondary metabolite gene clusters. Careful inspection and analysis of the sequences revealed the BGCs for fumitremorgins (ftm), pseurotins (pso), fumigaclavines (fga), and helvolinic acid (hel). The putative BGC prediction fundamentally underpinned the enzymatic and mechanistic function for the biosynthesis of these compounds.
Fumitremorgins, pseurotins, and fumigaclavines were most frequently isolated from A. fumigatus strains. However, some of them were also reported from taxonomically close species, such as 18-oxotryprostatin A (3), 6-methoxyspirotryprostatin B (7) from A. sydowi [22], azaspirofuran A (12) from A. sydowi D2–6 [29], fumigaclavine I from A. terreus [51]. Helvolinic acid (18) was also isolated from Corynascus setosus and M. anisopliae [32,52]. Fumitremorgin B (16) showed weak activity against M. smegmatis, S. aureus, E. coli, P. aeruginosa in our in vitro assay. It was also reported with antifungal activity against a variety of phytopathogenic fungi, which could be involved in fighting against invasion by other pathogens [53]. Our research also showed the strong activities of helvolinic acid (18) and helvolic acid (19) against S. aureus (6.25 and 3.13 μg/mL, respectively) and E. coli (6.25 and 3.13 μg/mL, respectively). Previous studies revealed that helvolic acid (19) exhibited in vitro antimycobacterial activity against M. tuberculosis H37Ra [54], antitrypanosomal activity against Trypanosoma brucei brucei [55], and antimalarial activity against multidrug resistant Plasmodium falciparum [56]. No cytotoxic activity against normal cell lines and broad biological activity indicated the potential of helvolic acid for drug development [56].
Pseurotins have a unique heterospirocyclic furanone-lactam structure. They are produced by hybrid PKS/NRPS and other tailing enzymes, and exhibit a broad range of biological activities. However, the compounds showed no antibacterial activity in our screening at concentrations up to 100 μg/mL, which did not agree with the results of antibacterial activity against E. coli (ATCC 25922), P. aeruginosa (ATCC 27853), S. aureus (ATCC 25923) from Pinheiro et al. [57] It is likely that different bacterial strains contributed to the different results, as pseurotin A was also reported to have no activity against S. aureus (ATCC 6538) and S. aureus [58,59]. The mechanism of the biosynthesis of the unusual spiro-ring structural feature of pseurotins has remained uncharacterized. We propose that it could be formed by isomerization and hydroxylation. Previous research showed the physically intertwined supercluster genes for the biosynthesis of both pseurotin A and fumagillin. The gene fumR regulates the production of pseurotin A and fumagillin. It was intriguing that the presence of genes in the cluster which were similar to fumagillin targets conferred the strain resistance to fumagillin [60]. However, fumagillin was not isolated from the extract of MF071, possibly due to the low yield.
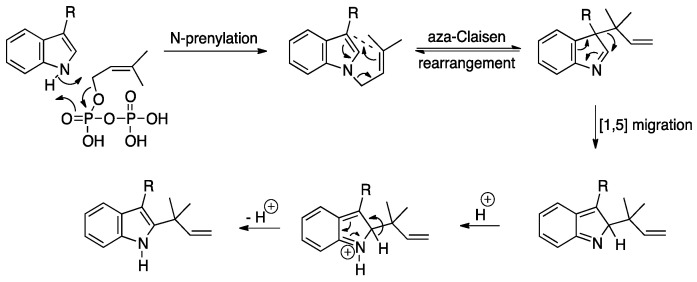
Several putative prenyltransferases were identified in MF071 for the incorporation of one prenyl moiety in the biosynthesis of fumitremorgins and fumigaclavines, such as Ftm4, Ftm8, Fga3, and Fga8. Fga8 catalyzed a “reverse” prenylation of fumigaclavine A with the 2-(1,1-dimethylallyl) moiety connected to the indole system at the 2-position (Figure 6B). The whole genome sequence analysis of MF071 revealed two additional prenyltransferases. Gene deletion experiments or heterologous expression have revealed the function of most “reverse” prenyltransferase genes, such as lxc from Lyngbya majuscule, notF from Aspergillus sp., anaPT from Neosartorya fischeri, brePT from Aspergillus versicolor, and cdpC2PT/cdpNPT from A. nidulans [61,62,63,64,65]. However, the mechanism of the enzymatic catalysis of these “reverse” prenyltransferases has not been fully revealed. The amino acid sequence alignment of Fga8 with the above-mentioned prenyltransferases gave a relatively low similarity value (20%-30%), indicating that Fga8 could be a potential new prenyltransferase. A possible mechanism of the tert-prenylation at position C2 by Fga 8 was that the prenyltransferase Fga8 initially alkylates the nitrogen atom of the indole. The resulting N-(3,3-dimethylallyl) indole then undergoes an aza-Claisen rearrangement to give the rearranged 3-(1,1-dimethylallyl)indole, followed by a [1,5]-alkyl shift and aromatization to give the corresponding 2-substituted indole (Figure 7).
Figure 7.
Proposed mechanism for the tert-prenylation of indoles at the C-2 position.
We require more information to test the substrate specificity of these prenyltransferases, as prenylations or tert-prenylations of indole could occur at positions N1, C2, C3, C4, C5, C6, and C7 (Figure S21). Interestingly, the brevianamide F could be catalyzed by both FtmB (“regular” prenyltransferase) and NotF to produce tryprostatin B and deoxybrevianamide E, respectively [42,62]. CdpC3PT from A. nidulans has been reported to catalyse the formation of N1-regularly, C2-, and C3- reverse-prenylated derivatives [65]. Further protein structure research could be of importance to confirm this prenylation mechanism.
The current study reports 30 compounds and BGCs of fumitremorgins, pseurotins, fumigaclavines and helvolinic acid, whereas the prediction of MF071 metabolic potential gave large numbers of BGCs. A preliminary blast analysis showed the presence of BGCs for pyripyropene A, neosartoricin B, gliotoxin, trypacidin, xanthocillin, fumisoquin, ferricrocin, 1,8-dihydroxynaphthalene, and many others. However, biosynthetic genes are often silent or transcribed at very low levels under certain conditions, which makes the detection difficult. As the condition used for fermentation is quite different from the native environment (high salinity, oligotrophy, microbial competition, temperature variation, etc.), the chemical profile could be different from that of the extract from rice medium fermentation. Approaches for the activation of these silent BGCs such as OSMAC, microbial co-culture or heterologous expression of unknown clusters could be carried out to further expand the structure classes.
In conclusion, we isolated 30 compounds from A. fumigatus MF071, including two new compounds 1 and 2. The NMR data of two compounds, monomethylsulochrin-4-sulphate (4) and pseurotin H (10), are also reported here for the first time. Compounds 18 and 19 exhibited strong activities against S. aureus and E. coli. BGCs of fumitremorgins, pseurotins, fumigaclavines and helvolinic acid and biosynthetic pathways were proposed. The mechanism for the tert-prenylation of indoles by prenyltransferase was also discussed.
4. Materials and Methods
4.1. General Experimental Procedures
NMR spectra were acquired at 25 °C on a Bruker Avance HDX 800 MHz spectrometer (Zürich, Switzerland) equipped with a TCI cryoprobe. The 1H and 13C chemical shifts were referenced to the DMSO-d6 solvent peaks at δH 2.50 and δC 39.52 ppm, respectively, and all deuterated solvents were from Cambridge Isotope Laboratories (CIL). Low resolution mass spectra were measured with a Thermo Ultimate 3000 system equipped with an AccucoreTM C18 column (2.6 μm, 150 × 2.1 mm), a diode-array detector (DAD), and an ESI mass spectrometer. HRESIMS measurements were obtained on a Bruker Maxis II ETD QTOF mass spectrometer (Bremen, Germany) and was calibrated with sodium trifluoroacetate. SINGLE StEP Silica Column™ and Sephadex LH-20 (GE Healthcare BioSciences AB) were used for fractionation. Reverse phase HPLC was performed on Thermo Ultimate 3000 system separation module with a DionexTM diode array detector (MA, USA). Optical rotations were determined on a Jasco P-1020 Polarimeter (10 cm cell) (Tokyo, Japan). All solvents used for extraction, chromatography, [α]D, and MS were Honeywell Burdick & Jackson HPLC grade (Muskegon, MI, USA), 0.1% formic acid (Sigma-Aldrich) was used in solvent system for LC-MS and 0.1% TFA (Sigma-Aldrich) was used in solvent system for HPLC. H2O was purified with Sartorius Arium®Pro VF ultrapure water system (Göttingen, Germany).
4.2. Microbial Strain Culture and Identification
The marine fungus Aspergillus fumigatus MF071 (MF071) was isolated from a sediment sample collected at a depth of 60 m from the Bohai Sea, China. Specifically, 1.0 g of sediment sample was added into 50 mL sterile centrifuge tube and suspended in 9 mL sterile artificial seawater (3.8% sea salt) under aseptic operation. An aliquot of 200 µL diluted suspension (1/10) was spread plated on the separation medium (1.0% peptone, 4.0% glucose, 1.5% agar, pH 6.0), supplemented with 0.5 mg/mL chloramphenicol and streptomycin, and 200 µL sterile artificial seawater was also spread, plated on another plate as control. The plate was incubated at 28 °C. The pure colony of MF071 was transferred to potato dextrose agar (PDA) medium for further lab experiments and cryogenic vials, with MF071 suspended in 25% glycerol stored at −80 °C. A DNA extraction of MF071 was carried out using CTAB (cetyltrimethylammonium bromide) as described previously [66]. The identification of strain MF071 was performed based on the morphological and 18S ribosomal DNA (rDNA) analyses. Multiple sequence alignments with 18S sequences of related species were carried out using CLUSTAL W [67]. A phylogenetic tree was constructed using the neighbor-joining method [68], as implemented in MEGA 5.0 [69]. Bootstrap values were generated by resampling 1000 replicates. The voucher specimen has been deposited at Dr. Zhang’s Laboratory, East China University of Science and Technology (strain no. MF071).
4.3. Genome Sequencing and Secondary Metabolite BGCs Analysis
Whole-genome sequencing of strain MF071 was conducted on PacBio RSII platform (Tianjin Biochip Corporation, Tianjin, China), with the single molecule real-time (SMRT) technique [70]. The data from a single SMRT sequencing cell were used directly for the assembly process. The raw reads were processed and assembled using the hierarchical genome assembly process (HGAP) to obtain the final genomic sequence [71]. Gene prediction of the draft genome assembly was performed using AUGUSTUS [72]. The prediction of secondary metabolite BGCs was carried out using antiSMASH online software (version 5.1.2) [73]. Each gene in the putative gene clusters was analyzed by BLASTp against the GenBank database [74].
4.4. Fermentation, Extraction, and Isolation
Strain MF071 was cultured on a PDA at 28 °C for seven days, and agar plugs (5-mm-diameter) were aseptically inoculated into three conical flasks (250 mL), each containing 50 mL of potato dextrose broth (PDB). The flasks were incubated at 28 °C on a rotary shaker at 200 rpm for five days to generate the seed cultures, which were distributed into 20 conical flasks (1000 mL), each containing 160 g of autoclaved rice and 240 mL distilled H2O. These cultures were statically fermented at 28 °C for thirty days. The fermentation products were extracted with EtOAc three times and concentrated in vacuo to give a crude extract (20 g).
A MF071 extract was fractionated on a Sephadex LH-20 column using MeOH and DCM (1:1), affording 11 fractions (F1-F11). F8 (20 mg) was purified by semi-preparative RP-HPLC using an Alltech Hyperprep C18 column (5 μm, 250 × 21.2 mm), eluting at a flow rate of 9.0 mL/min with a gradient elution of MeOH-H2O: 0-50 min, 10%/90% to 100%/0%, to obtain compound 5 (0.9 mg, tR = 21.5 min). F9 (4.8 mg) was subjected to Sephadex LH-20 column to give compound 4. F10 (23 mg) was purified by semi-preparative RP-HPLC on a Phenomenex Luna C18 column (5 μm, 250 × 10 mm), eluting at a flow rate of 4.0 mL/min with a gradient elution from 10% MeOH to 100% MeOH in 50 min, to obtain sub-fraction 20 (F20), compound 29 (1.4 mg, tR = 31.2 min), and 30 (1.3 mg, tR = 37.0 min). F20 was further purified using RP-HPLC, with a gradient elution from 40% MeOH to 65% MeOH in 25 min, to obtain compound 6 (0.1 mg, tR = 23.0 min).
F4 (6.2 g) was subjected to Single StEP Silica Column™, and eluted with gradient DCM and MeOH to afford 8 sub-fractions (F4A-F4H). F4D (280 mg) was purified using RP-HPLC on a Thermo Electron Betasil C18 column (5 μm, 150 × 21.2 mm), eluting at a flow rate of 9.0 mL/min, with a gradient elution from 10% MeOH to 100% MeOH in 50 min, to give fraction 35. Fraction 35 was further purified on a Phenomenex Luna C18 column (5 μm, 250 × 10 mm), eluting at a flow rate of 4.0 mL/min with 60% MeOH to obtain compound 25 (tR = 12.0 min). F4B was separated using RP-HPLC on a Thermo Electron Betasil C18 column (5 μm, 150 × 21.2 mm), eluting at a flow rate of 9.0 mL/min with a gradient elution from 10% MeOH to 100% MeOH in 50 min, to give six sub-fractions (F4B1- F4B6).
F4B2 (284 mg) was purified using RP-HPLC on a Phenomenex Luna C18 column (5 μm, 250 × 21.2 mm), eluting at a flow rate of 9.0 mL/min, with a gradient elution from 10% MeOH to 100% MeOH in 50 min, to give sub-fractions F4B2_F20 and F4B2_F27. F4B2_F20 was further purified using RP-HPLC on a Phenomenex Luna C18 column (5 μm, 250 × 10 mm), eluting at a flow rate of 4.0 mL/min, with 30% MeOH, to give compound 10 (0.9 mg, tR = 16.7 min) and 11 (tR = 30.2 min).
F4B3 (619 mg) was purified using RP-HPLC on a Phenomenex Luna C18 column (5 μm, 250 × 21.2 mm), eluting at a flow rate of 9.0 mL/min, with a gradient elution from 30% MeOH to 100% MeOH in 50 min, to give sub-fractions F4B3_F19, F4B3_F23 and F4B3_F28. F4B3_F19 was further purified using RP-HPLC on a Phenomenex Luna C18 column (5 μm, 250 × 10 mm), eluting at a flow rate of 4.0 mL/min, with a gradient 30% MeOH to 62% MeOH in 25 min, to give compound 26 (1.6 mg, tR = 22.2 min). F4B3_F23 was further purified using RP-HPLC on a Phenomenex Luna C18 column (5 μm, 250 × 10 mm), eluting at a flow rate of 4.0 mL/min, with 50% MeOH, to give compound 21 (2.7 mg, tR = 18.8 min). F4B3_F28 was further purified using RP-HPLC on a Phenomenex Luna C18 column (5 μm, 250 × 10 mm), eluting at a flow rate of 4.0 mL/min, with 45% MeOH, to give compound 8 (0.1 mg, tR = 9.7 min), 2 (tR = 11.9 min), 1 (tR = 12.7 min), 12 (0.1 mg, tR = 14.5 min), 28 (1.0 mg, tR = 16.4 min), 3 (1.8 mg, tR = 18.8 min).
F4B4 (373 mg) was purified using RP-HPLC on a Phenomenex Luna C18 column (5 μm, 250 × 21.2 mm), eluting at a flow rate of 9.0 mL/min, with a gradient elution from 30% MeOH, to 100% MeOH in 50 min to give sub-fractions F4B4_F30, F4B4_F34, F4B4_F39 and F4B4_F40. F4B4_F30 was further purified using RP-HPLC on a Phenomenex Luna C18 column (5 μm, 250 × 10 mm), eluting at a flow rate of 4.0 mL/min, with 45% MeOH, to give compound 7 (tR = 10.7 min). F4B4_F34 was further purified using RP-HPLC on a Phenomenex Luna C18 column (5 μm, 250 × 10 mm), eluting at a flow rate of 4.0 mL/min, with a gradient from 30% MeOH to 70% MeOH in 20 min, to give compound 23 (tR = 14.7 min) and 22 (tR = 17.1 min). F4B4_F39 was further purified using RP-HPLC on a Phenomenex Luna C18 column (5 μm, 250 × 10 mm), eluting at a flow rate of 4.0 mL/min, with 65% MeOH, to give compound 9 (tR = 17.5 min) and 24 (tR = 20.5 min). F4B4_F40 was further fractionated using Sephadex LH-20 to give compound 20.
F4B5 (355 mg) was subjected on a Sephadex LH-20 column using MeOH and DCM (1:1). Sub-fraction 26 was purified using RP-HPLC on a Phenomenex Luna C18 column (5 μm, 250 × 10 mm), eluting at a flow rate of 4.0 mL/min, with a gradient from 50% MeOH to 100% MeOH in 25 min, to give compound 15 (0.4 mg, tR = 16.1 min) and 19 (2.6 mg, tR = 19.9 min). Sub-fraction 33 was purified using RP-HPLC on a Phenomenex Luna C18 column (5 μm, 250 × 10 mm), eluting at a flow rate of 4.0 mL/min, with 45% MeOH, to give compound 27 (2.5 mg, tR = 5.1 min), 14 (1.0 mg, tR = 7.0 min), 13 (0.5 mg, tR = 7.5 min), 17 (1.1 mg, tR = 10.8 min), 18 (0.5 mg, tR = 12.5 min), and 16 (3.9 mg, tR = 13.1 min).
Compound 1: yellow powder; UV (MeOH) λmax 260 and 350 nm; 1H and 13C NMR data, see Table 1; 2D NMR spectra, see Supplementary Figures S3–S4; HRESIMS m/z 434.1689 [M + Na]+ (calculated for 434.1686) (Figure S1).
Compound 2: yellow powder; UV (MeOH) λmax 260 and 342 nm; 1H and 13C NMR data, see Table 1; 2D NMR spectra, see Supplementary Figures S7–S9; HRESIMS m/z 436.1841 [M + Na]+ (calculated for 436.1843) (Figure S5).
Compound 4: yellow powder; UV (MeOH) λmax 291 nm; 1H and 13C NMR data, see Table 2; 2D NMR spectra, see Supplementary Figures S13–S14; HRESIMS m/z 425.0535 [M − H]− (calculated for 425.0548) (Figure S10).
Compound 10: yellow powder; UV (MeOH) λmax 260 nm; 1H and 13C NMR data, see Table 1; 2D NMR spectra, see Supplementary Figures S18–S19; HRESIMS m/z 370.0895 [M + Na]+ (calculated for 370.0897) (Figure S15).
4.5. DFT Theory and Calculation
The NMR chemical shift calculations were performed using density functional theory (DFT) in Gaussian 09. The preliminary conformational distribution search was performed by HyperChem Release 8.0 software. GaussView 5.0 was used to view the conformational structures and change the input file for calculation. All ground-state geometries were optimized at the B3LYP/6-31G(d) level, and the stable conformations obtained at the B3LYP/6-31G(d) level were further used in magnetic shielding constants at the B3LYP/6-311++G(2d,p) level [75]. The calculated chemical shift of each atom in each conformer was viewed by GaussView 5.0, and the final chemical shift of each atom was calculated from the Boltzmann distribution of each conformer. A DP4 probability analysis of the calculated and experimental chemical shifts was used to assign the stereochemistry [76].
4.6. Bioassays
The bioactivity of isolated compounds was tested against Mycobacterium smegmatis (ATCC 70084), Staphylococcus aureus (ATCC BAA-2312), Escherichia coli (ATCC 43887), and Pseudomonas aeruginosa (ATCC 10145), using a 96-well plate microdilution method, as previously reported [77,78,79]. The minimum inhibitory concentrations (MICs) were calculated as the minimum concentration of the compounds that inhibited visible growth. Isoniazid was used as a positive control in the activity screening against M. smegmatis with MIC value of 4 μg/mL. Gentamycin was used as a positive control in the activity screening against S. aureus, E. coli, and P. aeruginosa, with MIC values of 0.5 μg/mL, 0.03 μg/mL, 1.0 μg/mL, respectively. All the experiments were performed in triplicate.
Acknowledgments
J.H. thanks Griffith University for the provision of PhD scholarships (Griffith University Postgraduate Research Scholarship and the Griffith University International Postgraduate Research Scholarship).
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-3397/18/7/352/s1, Figures S1–S4: HRESIMS, 1D, and 2D NMR of 1, Figures S5–S9: HRESIMS, 1D, and 2D NMR of 2, Figures S10–S14: HRESIMS, 1D, and 2D NMR of 4, Figures S15–S19: HRESIMS, 1D, and 2D NMR of 10, Figure S20: Organization of the fusidane-type antibiotic helvolic acid BGC (hel) (A) and proposed biosynthetic pathways for helvolic acid and helvolinic acid (B), Figure S21: Representatives of prenylated indole alkaloids, Table S1: The calculated 13C NMR data for two possible isomers (19S) and (19R) of compound 1 and DP4 analysis, Table S2: Deduced functions of ORFs in fumitremorgins BGC (ftm) from MF071, Table S3: Deduced functions of ORFs in pseurotins BGC (pso) from MF071, Table S4: Deduced functions of ORFs in fumigaclavines BGC (fga) from MF071, Table S5: Deduced functions of ORFs in helvolic acid BGC (hel) from MF071.
Author Contributions
Conceptualization, Y.F. and R.J.Q.; investigation, J.H. and M.L., I.D.J.; resources, X.L. and L.Z.; writing—original draft preparation, J.H.; writing—review and editing, Y.F. and R.J.Q.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (31430002, 81573341, 21877038, 31720103901, 31320103911), Open Project Funding of the State Key Laboratory of Bioreactor Engineering, the 111 Project (B18022), the Fundamental Research Funds for the Central Universities (22221818014), the Shandong Taishan Scholar Award to L.Z. the Australian Research Council (DP160101429, LE140100119, LE120100170).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bugni T.S., Ireland C.M. Marine-derived fungi: A chemically and biologically diverse group of microorganisms. Nat. Prod. Rep. 2004;21:143–163. doi: 10.1039/b301926h. [DOI] [PubMed] [Google Scholar]
- 2.Liu M., El-Hossary E.M., Oelschlaeger T.A., Donia M.S., Quinn R.J., Abdelmohsen U.R. Potential of marine natural products against drug-resistant bacterial infections. Lancet Infect. Dis. 2019;19:e237–e245. doi: 10.1016/S1473-3099(18)30711-4. [DOI] [PubMed] [Google Scholar]
- 3.Ashforth E.J., Fu C., Liu X., Dai H., Song F., Guo H., Zhang L. Bioprospecting for antituberculosis leads from microbial metabolites. Nat. Prod. Rep. 2010;27:1709–1719. doi: 10.1039/c0np00008f. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L., Yan K., Zhang Y., Huang R., Bian J., Zheng C., Sun H., Chen Z., Sun N., An R., et al. High-throughput synergy screening identifies microbial metabolites as combination agents for the treatment of fungal infections. Proc. Natl. Acad. Sci. USA. 2007;104:4606–4611. doi: 10.1073/pnas.0609370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang A., Demain A.L. Natural Products: Drug Discovery and Therapeutical Medicine. Springer; New York, NY, USA: 2005. p. 382. [Google Scholar]
- 6.Rao T.E., Imchen M., Kumavath R. Marine Enzymes: Production and Applications for Human Health. Elsevier; North Andover, MA, USA: 2017. pp. 149–163. [DOI] [PubMed] [Google Scholar]
- 7.Petersen L.-E., Kellermann M.Y., Schupp P.J. Secondary Metabolites of Marine Microbes: From Natural Products Chemistry to Chemical Ecology. Springer; New York, NY, USA: 2020. pp. 159–180. [Google Scholar]
- 8.Carroll A.R., Copp B.R., Davis R.A., Keyzers R.A., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2019;36:122–173. doi: 10.1039/C8NP00092A. [DOI] [PubMed] [Google Scholar]
- 9.Wang Q., Song F., Xiao X., Huang P., Li L., Monte A., Abdel--Mageed W.M., Wang J., Guo H., He W., et al. Abyssomicins from the South China Sea deep--sea sediment Verrucosispora sp.: Natural thioether Michael addition adducts as antitubercular prodrugs. Angew. Chem. Int. Ed. 2013;52:1231–1234. doi: 10.1002/anie.201208801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu M., Grkovic T., Liu X., Han J., Zhang L., Quinn R.J. A systems approach using OSMAC, Log P and NMR fingerprinting: An approach to novelty. Synth. Syst. Biotechnol. 2017;2:276–286. doi: 10.1016/j.synbio.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medema M.H., Fischbach M.A. Computational approaches to natural product discovery. Nat. Chem. Biol. 2015;11:639. doi: 10.1038/nchembio.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han J., Zhang J., Song Z., Liu M., Hu J., Hou C., Zhu G., Jiang L., Xia X., Quinn R.J., et al. Genome-and MS-based mining of antibacterial chlorinated chromones and xanthones from the phytopathogenic fungus Bipolaris sorokiniana strain 11134. Appl. Microbiol. Biotechnol. 2019;103:5167–5181. doi: 10.1007/s00253-019-09821-z. [DOI] [PubMed] [Google Scholar]
- 13.Han J., Zhang J., Song Z., Zhu G., Liu M., Dai H., Hsiang T., Liu X., Zhang L., Quinn R.J., et al. Genome-based mining of new antimicrobial meroterpenoids from the phytopathogenic fungus Bipolaris sorokiniana strain 11134. Appl. Microbiol. Biotechnol. 2020;104:3835. doi: 10.1007/s00253-020-10522-1. [DOI] [PubMed] [Google Scholar]
- 14.Ju K.-S., Gao J., Doroghazi J.R., Wang K.-K.A., Thibodeaux C.J., Li S., Metzger E., Fudala J., Su J., Zhang J.K., et al. Discovery of phosphonic acid natural products by mining the genomes of 10,000 actinomycetes. Proc. Natl. Acad. Sci. USA. 2015;112:12175–12180. doi: 10.1073/pnas.1500873112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doroghazi J.R., Albright J.C., Goering A.W., Ju K.-S., Haines R.R., Tchalukov K.A., Labeda D.P., Kelleher N.L., Metcalf W.W. A roadmap for natural product discovery based on large-scale genomics and metabolomics. Nat. Chem. Biol. 2014;10:963. doi: 10.1038/nchembio.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan G., Xu Z., Guo Z., Ma M., Yang D., Zhou H., Gansemans Y., Zhu X., Huang Y., Zhao L.-X., et al. Discovery of the leinamycin family of natural products by mining actinobacterial genomes. Proc. Natl. Acad. Sci. USA. 2017;114:E11131–E11140. doi: 10.1073/pnas.1716245115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bian J., Song F., Zhang L. Strategies on the construction of high-quality microbial natural product library--a review. Wei Sheng Wu Xue Bao. 2008;48:1132–1137. [PubMed] [Google Scholar]
- 18.Bai C., Zhang Y., Zhao X., Hu Y., Xiang S., Miao J., Lou C., Zhang L. Exploiting a precise design of universal synthetic modular regulatory elements to unlock the microbial natural products in Streptomyces. Proc. Natl. Acad. Sci. USA. 2015;112:12181–12186. doi: 10.1073/pnas.1511027112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao Q., Tan G.-Y., Xia X., Zhang L. Learn from microbial intelligence for avermectins overproduction. Curr. Opin. Biotechnol. 2017;48:251–257. doi: 10.1016/j.copbio.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Van De Veerdonk F.L., Gresnigt M.S., Romani L., Netea M.G., Latgé J.-P. Aspergillus fumigatus morphology and dynamic host interactions. Nat. Rev. Microbiol. 2017;15:661. doi: 10.1038/nrmicro.2017.90. [DOI] [PubMed] [Google Scholar]
- 21.Cui C.-B., Kakeya H., Okada G., Onose R., Ubukata M., Takahashi I., Isono K., Osada H. Tryprostatins A and B, novel mammalian cell cycle inhibitors produced by Aspergillus fumigatus. J. Antibiot. 1995;48:1382–1384. doi: 10.7164/antibiotics.48.1382. [DOI] [PubMed] [Google Scholar]
- 22.Zhang M., Wang W., Fang Y., Zhu T., Gu Q., Zhu W. Cytotoxic alkaloids and antibiotic nordammarane triterpenoids from the marine-derived fungus Aspergillus sydowi. J. Nat. Prod. 2008;71:985–989. doi: 10.1021/np700737g. [DOI] [PubMed] [Google Scholar]
- 23.Liu R., Zhu W., Zhang Y., Zhu T., Liu H., Fang Y., Gu Q. A new diphenyl ether from marine-derived fungus Aspergillus sp. BF-2. J. Antibiot. 2006;59:362. doi: 10.1038/ja.2006.52. [DOI] [PubMed] [Google Scholar]
- 24.Ishikawa M., Ninomiya T., Akabane H., Kushida N., Tsujiuchi G., Ohyama M., Gomi S., Shito K., Murata T. Pseurotin A and its analogues as inhibitors of immunoglobuline E production. Bioorg. Med. Chem. Lett. 2009;19:1457–1460. doi: 10.1016/j.bmcl.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 25.Bloch P., Tamm C. Isolation and structure of pseurotin A, a microbial metabolite of Pseudeurotium ovalis Stolk with an unusual heterospirocyclic system. Helv. Chim. Acta. 1981;64:304–315. doi: 10.1002/hlca.19810640131. [DOI] [Google Scholar]
- 26.Natori S., Nishikawa H. Structures of osoic acids and related compounds, metabolites of Oospora sulphurea-ochracea v. BEYMA. Chem. Pharm. Bull. 1962;10:117–124. doi: 10.1248/cpb.10.117. [DOI] [PubMed] [Google Scholar]
- 27.Cui C.-B., Kakeya H., Osada H. Novel mammalian cell cycle inhibitors, spirotryprostatins A and B, produced by Aspergillus fumigatus, which inhibit mammalian cell cycle at G2/M phase. Tetrahedron. 1996;529:12651–12666. doi: 10.1016/0040-4020(96)00737-5. [DOI] [Google Scholar]
- 28.Wang F., Fang Y., Zhu T., Zhang M., Lin A., Gu Q., Zhu W. Seven new prenylated indole diketopiperazine alkaloids from holothurian-derived fungus Aspergillus fumigatus. Tetrahedron. 2008;64:7986–7991. doi: 10.1016/j.tet.2008.06.013. [DOI] [Google Scholar]
- 29.Ren H., Liu R., Chen L., Zhu T., Zhu W.M., Gu Q.Q. Two new hetero-spirocyclic γ-lactam derivatives from marine sediment-derived fungus Aspergillus sydowi D2–6. Arch. Pharm. Res. 2010;33:499–502. doi: 10.1007/s12272-010-0401-4. [DOI] [PubMed] [Google Scholar]
- 30.Cui C.-B., Kakeya H., Osada H. Novel mammalian cell cycle inhibitors, tryprostatins A, B and other diketopiperazines produced by Aspergillus fumigatus. J. Antibiot. 1996;49:534–540. doi: 10.7164/antibiotics.49.534. [DOI] [PubMed] [Google Scholar]
- 31.Fujimoto H., Fujimaki T., Okuyama E., Yamazaki M. Immunosuppressive constituents from an Ascomycete, Sordaria gondaensis. JSM Mycotoxins. 2000;50:93–99. doi: 10.2520/myco1975.50.93. [DOI] [Google Scholar]
- 32.Fujimoto H., Negishi E., Yamaguchi K., Nishi N., Yamazaki M. Isolation of new tremorgenic metabolites from an Ascomycete, Corynascus setosus. Chem. Pharm. Bull. 1996;44:1843–1848. doi: 10.1248/cpb.44.1843. [DOI] [Google Scholar]
- 33.Ratnaweera P.B., Williams D.E., de Silva E.D., Wijesundera R.L., Dalisay D.S., Andersen R.J. Helvolic acid, an antibacterial nortriterpenoid from a fungal endophyte, Xylaria sp. of orchid Anoectochilus setaceus endemic to Sri Lanka. Mycology. 2014;5:23–28. doi: 10.1080/21501203.2014.892905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng C., Li L., Zou J., Han T., Qin L. Identification of a quinazoline alkaloid produced by Penicillium vinaceum, an endophytic fungus from Crocus sativus. Pharm. Biol. 2012;50:129–133. doi: 10.3109/13880209.2011.569726. [DOI] [PubMed] [Google Scholar]
- 35.Jiao R.H., Xu S., Liu J.Y., Ge H.M., Ding H., Xu C., Zhu H.L., Tan R.X. Chaetominine, a cytotoxic alkaloid produced by endophytic Chaetomium sp. IFB-E015. Org. Lett. 2006;8:5709–5712. doi: 10.1021/ol062257t. [DOI] [PubMed] [Google Scholar]
- 36.Ge H.M., Yu Z.G., Zhang J., Wu J.H., Tan R.X. Bioactive alkaloids from endophytic Aspergillus fumigatus. J. Nat. Prod. 2009;72:753–755. doi: 10.1021/np800700e. [DOI] [PubMed] [Google Scholar]
- 37.Jiao W., Blunt J.W., Cole A.L., Munro M.H. Fumagiringillin, a new fumagillin derivative from a strain of the fungus Aspergillus fumigatus. J. Nat. Prod. 2004;67:1434–1437. doi: 10.1021/np049893p. [DOI] [PubMed] [Google Scholar]
- 38.Buttachon S., Zin W.W.M., Dethoup T., Gales L., Pereira J.A., Silva A.M., Kijjoa A. Secondary metabolites from the culture of the marine sponge-associated fungi Talaromyces tratensis and Sporidesmium circinophorum. Planta Med. 2016;82:888–896. doi: 10.1055/s-0042-103687. [DOI] [PubMed] [Google Scholar]
- 39.Ohashi H., Akiyama H., Nishikori K., Mochizuki J.-I. Asterric acid, a new endothelin binding inhibitor. J. Antibiot. 1992;45:1684–1685. doi: 10.7164/antibiotics.45.1684. [DOI] [PubMed] [Google Scholar]
- 40.Waser M., Lackner B., Zuschrader J., Müller N., Falk H. An efficient regioselective synthesis of endocrocin and structural related natural anthraquinones starting from emodin. Tetrahedron Lett. 2005;46:2377–2380. doi: 10.1016/j.tetlet.2005.02.061. [DOI] [Google Scholar]
- 41.Fujimoto H., Fujimaki T., Okuyama E., Yamazaki M. Immunomodulatory constituents from an ascomycete, Microascus tardifaciens. Chem. Pharm. Bull. 1999;47:1426–1432. doi: 10.1248/cpb.47.1426. [DOI] [PubMed] [Google Scholar]
- 42.Tsunematsu Y., Ishikawa N., Wakana D., Goda Y., Noguchi H., Moriya H., Hotta K., Watanabe K. Distinct mechanisms for spiro-carbon formation reveal biosynthetic pathway crosstalk. Nat. Chem. Biol. 2013;9:818. doi: 10.1038/nchembio.1366. [DOI] [PubMed] [Google Scholar]
- 43.Li S.-M. Genome mining and biosynthesis of fumitremorgin-type alkaloids in ascomycetes. J. Antibiot. 2011;64:45. doi: 10.1038/ja.2010.128. [DOI] [PubMed] [Google Scholar]
- 44.Wiemann P., Guo C.-J., Palmer J.M., Sekonyela R., Wang C.C., Keller N.P. Prototype of an intertwined secondary-metabolite supercluster. Proc. Natl. Acad. Sci. USA. 2013;110:17065–17070. doi: 10.1073/pnas.1313258110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maiya S., Grundmann A., Li X., Li S.M., Turner G. Identification of a hybrid PKS/NRPS required for pseurotin A biosynthesis in the human pathogen Aspergillus fumigatus. Chembiochem. 2007;8:1736–1743. doi: 10.1002/cbic.200700202. [DOI] [PubMed] [Google Scholar]
- 46.Tsunematsu Y., Fukutomi M., Saruwatari T., Noguchi H., Hotta K., Tang Y., Watanabe K. Elucidation of pseurotin biosynthetic pathway points to trans--acting C--methyltransferase: Generation of chemical diversity. Angew. Chem. Int. Ed. 2014;53:8475–8479. doi: 10.1002/anie.201404804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Unsöld I.A., Li S.M. Reverse prenyltransferase in the biosynthesis of fumigaclavine C in Aspergillus fumigatus: Gene expression, purification, and characterization of fumigaclavine C synthase FGAPT1. Chembiochem. 2006;7:158–164. doi: 10.1002/cbic.200500318. [DOI] [PubMed] [Google Scholar]
- 48.Liu X., Wang L., Steffan N., Yin W.B., Li S.M. Ergot alkaloid biosynthesis in Aspergillus fumigatus: FgaAT catalyses the acetylation of fumigaclavine B. Chembiochem. 2009;10:2325–2328. doi: 10.1002/cbic.200900395. [DOI] [PubMed] [Google Scholar]
- 49.Rigbers O., Li S.-M. Ergot alkaloid biosynthesis in Aspergillus fumigatus overproduction and biochemical characterization of a 4-dimethylallyltryptophan N-methyltransferase. J. Biol. Chem. 2008;283:26859–26868. doi: 10.1074/jbc.M804979200. [DOI] [PubMed] [Google Scholar]
- 50.Lv J., Hu D., Gao H., Kushiro T., Awakawa T., Chen G., Wang C., Abe I., Yao X. Biosynthesis of helvolic acid and identification of an unusual C-4-demethylation process distinct from sterol biosynthesis. Nat. Commun. 2017;8:1644. doi: 10.1038/s41467-017-01813-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen L., Zhu L., Luo Q., Li X., Xi J., Kong G., Song Y. Fumigaclavine I, a new alkaloid isolated from endophyte Aspergillus terreus. Chin. J. Nat. Med. 2015;13:937–941. doi: 10.1016/S1875-5364(15)30101-1. [DOI] [PubMed] [Google Scholar]
- 52.Yadav R., Rashid M.M., Zaidi N., Kumar R., Singh H. Secondary Metabolites of Metarhizium spp. and Verticillium spp. and Their Agricultural Applications. Springer; New York, NY, USA: 2019. pp. 27–58. [Google Scholar]
- 53.Li X., Zhang Q., Zhang A., Gao J. Metabolites from Aspergillus fumigatus, an endophytic fungus associated with Melia azedarach, and their antifungal, antifeedant, and toxic activities. J. Agric. Food Chem. 2012;60:3424–3431. doi: 10.1021/jf300146n. [DOI] [PubMed] [Google Scholar]
- 54.Sanmanoch W., Mongkolthanaruk W., Kanokmedhakul S., Aimi T., Boonlue S. Helvolic acid, a secondary metabolite produced by Neosartorya spinosa KKU-1NK1 and its biological activities. Chiang Mai J. Sci. 2016;43:483–493. [Google Scholar]
- 55.Ganaha M., Yoshii K., Ōtsuki Y., Iguchi M., Okamoto Y., Iseki K., Ban S., Ishiyama A., Hokari R., Iwatsuki M. In vitro antitrypanosomal activity of the secondary metabolites from the mutant strain IU-3 of the insect pathogenic fungus Ophiocordyceps coccidiicola NBRC 100683. Chem. Pharm. Bull. 2016;64:988–990. doi: 10.1248/cpb.c16-00220. [DOI] [PubMed] [Google Scholar]
- 56.Sawadsitang S., Mongkolthanaruk W., Suwannasai N., Sodngam S. Antimalarial and cytotoxic constituents of Xylaria cf. cubensis PK108. Nat. Prod. Res. 2015;29:2033–2036. doi: 10.1080/14786419.2015.1017724. [DOI] [PubMed] [Google Scholar]
- 57.Pinheiro E.A.A., Carvalho J.M., dos Santos D.C.P., Feitosa A.D.O., Marinho P.S.B., Guilhon G.M.S.P., de Souza A.D.L., da Silva F.M.A., Marinho A.M.D.R. Antibacterial activity of alkaloids produced by endophytic fungus Aspergillus sp. EJC08 isolated from medical plant Bauhinia guianensis. Nat. Prod. Res. 2013;27:1633–1638. doi: 10.1080/14786419.2012.750316. [DOI] [PubMed] [Google Scholar]
- 58.Xu X., Han J., Wang Y., Lin R., Yang H., Li J., Wei S., Polyak S.W., Song F. Two new spiro-heterocyclic γ-lactams from a marine-derived Aspergillus fumigatus strain CUGBMF170049. Mar. Drugs. 2019;17:289. doi: 10.3390/md17050289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wenke J., Anke H., Sterner O. Pseurotin A and 8-O-demethylpseurotin A from Aspergillus fumigatus and their inhibitory activities on chitin synthase. Biosci. Biotechnol. Biochem. 1993;57:961–964. doi: 10.1271/bbb.57.961. [DOI] [Google Scholar]
- 60.Guruceaga X., Perez-Cuesta U., Abad-Diaz de Cerio A., Gonzalez O., Alonso R.M., Hernando F.L., Ramirez-Garcia A., Rementeria A. Fumagillin, a mycotoxin of Aspergillus fumigatus: Biosynthesis, biological activities, detection, and applications. Toxins. 2020;12:7. doi: 10.3390/toxins12010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Edwards D.J., Gerwick W.H. Lyngbyatoxin biosynthesis: Sequence of biosynthetic gene cluster and identification of a novel aromatic prenyltransferase. J. Am. Chem. Soc. 2004;126:11432–11433. doi: 10.1021/ja047876g. [DOI] [PubMed] [Google Scholar]
- 62.Ding Y., Wet J.R.D., Cavalcoli J., Li S., Greshock T.J., Miller K.A., Finefield J.M., Sunderhaus J.D., McAfoos T.J., Tsukamoto S., et al. Genome-based characterization of two prenylation steps in the assembly of the stephacidin and notoamide anticancer agents in a marine-derived Aspergillus sp. J. Am. Chem. Soc. 2010;132:12733–12740. doi: 10.1021/ja1049302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yin W., Grundmann A., Cheng J., Li S. Acetylaszonalenin biosynthesis in Neosartorya fischeri identification of the biosynthetic gene cluster by genomic mining and functional proof of the genes by biochemical investigation. J. Biol. Chem. 2009;284:100–109. doi: 10.1074/jbc.M807606200. [DOI] [PubMed] [Google Scholar]
- 64.Yin S., Yu X., Wang Q., Liu X.-Q., Li S.-M. Identification of a brevianamide F reverse prenyltransferase BrePT from Aspergillus versicolor with a broad substrate specificity towards tryptophan-containing cyclic dipeptides. Appl. Microbiol. Biotechnol. 2013;97:1649–1660. doi: 10.1007/s00253-012-4130-0. [DOI] [PubMed] [Google Scholar]
- 65.Wunsch C., Mundt K., Li S.-M. Targeted production of secondary metabolites by coexpression of non-ribosomal peptide synthetase and prenyltransferase genes in Aspergillus. Appl. Microbiol. Biotechnol. 2015;99:4213–4223. doi: 10.1007/s00253-015-6490-8. [DOI] [PubMed] [Google Scholar]
- 66.Möller E., Bahnweg G., Sandermann H., Geiger H. A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies, and infected plant tissues. Nucleic Acids Res. 1992;20:6115. doi: 10.1093/nar/20.22.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Larkin M.A., Blackshields G., Brown N., Chenna R., McGettigan P.A., McWilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 68.Saitou N., Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 69.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eid J., Fehr A., Gray J., Luong K., Lyle J., Otto G., Peluso P., Rank D., Baybayan P., Bettman B., et al. Real-time DNA sequencing from single polymerase molecules. Science. 2009;323:133–138. doi: 10.1126/science.1162986. [DOI] [PubMed] [Google Scholar]
- 71.Chin C.-S., Alexander D.H., Marks P., Klammer A.A., Drake J., Heiner C., Clum A., Copeland A., Huddleston J., Eichler E.E., et al. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat. Methods. 2013;10:563. doi: 10.1038/nmeth.2474. [DOI] [PubMed] [Google Scholar]
- 72.Stanke M., Diekhans M., Baertsch R., Haussler D. Using native and syntenically mapped cDNA alignments to improve de novo gene finding. Bioinformatics. 2008;24:637–644. doi: 10.1093/bioinformatics/btn013. [DOI] [PubMed] [Google Scholar]
- 73.Blin K., Shaw S., Steinke K., Villebro R., Ziemert N., Lee S.Y., Medema M.H., Weber T. antiSMASH 5.0: Updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019;47:W81–W87. doi: 10.1093/nar/gkz310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnson M., Zaretskaya I., Raytselis Y., Merezhuk Y., McGinnis S., Madden T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008;36:W5–W9. doi: 10.1093/nar/gkn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lv Q., Fan Y., Tao G., Fu P., Zhai J., Ye B., Zhu W. Sekgranaticin, a SEK34b-granaticin hybrid polyketide from Streptomyces sp. 166#. J. Org. Chem. 2019;84:9087–9092. doi: 10.1021/acs.joc.9b01022. [DOI] [PubMed] [Google Scholar]
- 76.Smith S.G., Goodman J.M. Assigning stereochemistry to single diastereoisomers by GIAO NMR calculation: The DP4 probability. J. Am. Chem. Soc. 2010;132:12946–12959. doi: 10.1021/ja105035r. [DOI] [PubMed] [Google Scholar]
- 77.Wang J.F., Dai H.Q., Wei Y.L., Zhu H.J., Yan Y.M., Wang Y.H., Long C.L., Zhong H.M., Zhang L.X., Cheng Y.X. Antituberculosis agents and an inhibitor of the para--aminobenzoic acid biosynthetic pathway from hydnocarpus anthelminthica seeds. Chem. Biodivers. 2010;7:2046–2053. doi: 10.1002/cbdv.201000072. [DOI] [PubMed] [Google Scholar]
- 78.Taneja N.K., Tyagi J.S. Resazurin reduction assays for screening of anti-tubercular compounds against dormant and actively growing Mycobacterium tuberculosis, Mycobacterium bovis BCG and Mycobacterium smegmatis. J. Antimicrob. Chemother. 2007;60:288–293. doi: 10.1093/jac/dkm207. [DOI] [PubMed] [Google Scholar]
- 79.Song F., Ren B., Yu K., Chen C., Guo H., Yang N., Gao H., Liu X., Liu M., Tong Y., et al. Quinazolin-4-one coupled with pyrrolidin-2-iminium alkaloids from marine-derived fungus Penicillium aurantiogriseum. Mar. Drugs. 2012;10:1297–1306. doi: 10.3390/md10061297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.