Abstract
Objective:
To investigate the association between biomarkers of inflammation and metabolic dysregulation and cancer mortality by obesity status.
Methods:
Data from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) cohort was used to examine the associations between baseline biomarkers of inflammation (IL-6, IL-8, IL-10, and CRP) and metabolism (adiponectin, resisting and lipoprotein (a)) with cancer mortality among 1,822 participants cancer-free at baseline. Weighted Cox proportional hazard regression with the robust sandwich method was used to estimate the hazard ratios and 95% confidence intervals (CIs) adjusting for baseline covariates and stratified by BMI (normal, overweight/obese) given the significant interaction between biomarkers and BMI (P<0.1).
Results:
During a mean follow-up of 8 years, there were statistically significant associations between cancer mortality and being in the highest vs. lowest tertile of IL-6 (HR: 5.3; 95% CI: 1.6, 17.8), CRP (HR: 3.4; 95% CI: 1.0, 11.2) and resistin (HR: 3.7; 95% CI: 1.2, 11.2) among participants with normal BMI. IL-6 was also associated with a 3-fold (HR: 3.5; 95% CI: 1.5, 8.1) increased risk of cancer mortality among participants with overweight/obesity; however, neither CRP nor resistin was significantly associated with cancer mortality in this group.
Conclusions:
Higher baseline inflammatory and metabolic biomarkers were associated with significantly increased risk of cancer mortality after adjusting for baseline risk factors and the associations varied by BMI. Cancer patients may benefit from interventions that modulate inflammatory and metabolic biomarkers.
Keywords: inflammatory cytokines, metabolic biomarkers, cancer mortality, obesity
1. Introduction
The established risk factors for cancer mortality include obesity, physical inactivity, smoking, and alcohol use [1–4]. These risk factors are associated with chronic inflammation and metabolic dysregulation, and growing evidence links these mechanisms with development, progression and mortality of cancer [5–7]. Obesity, specifically, has long been associated with increased risk of cancer mortality [8], and studies indicate every 5 kg/m2 increase in BMI increases the risk of cancer mortality by about 10% [9]. Furthermore, obesity is associated with adipose tissue dysfunction and chronic low-grade inflammation that leads to worse prognosis in cancer patients. However, there is also evidence of adipose tissue inflammation and protumorigenic consequences in some lean individuals[10]. Metabolism-related biomarkers [11–13] such as adiponectin, resistin, and lipoprotein (a) (Lp(a)), and inflammation-related cytokines [5, 6] such as interleukin (IL)-6, IL-8, IL-10 and C-reactive protein (CRP) have been shown to reflect a fertile, pro-tumorigenic inflammatory microenvironment that promotes tumor initiation, angiogenesis, and metastasis [14].
Recent studies, including by our group, suggests that metabolic health status may be a more clinically and epidemiologically important risk factor for cancer risk and mortality than obesity alone [15, 16]. Increased risk of cancer mortality has been observed in normal BMI individuals with metabolic dysregulation, an association not consistently observed among obese individuals who are metabolically healthy- a phenomenon termed ‘metabolic healthy obesity’ [15]. While the prevalence of obesity has increased significantly among US adults[17], it is important to better delineate the role of obesity, metabolic dysregulation and chronic inflammation in cancer risk. This helps to improve risk prediction and stratification, target appropriate clinical and interventions strategies based on precise biomarkers, and reduce the reliance on the crude measure of BMI as a predictor of cancer mortality risk. The aim of the present study was to investigate the role of pre-diagnostic metabolic and inflammatory biomarkers in the risk of cancer mortality by obesity status and to assess whether racial disparities exist in this association given the higher risk of cancer mortality and higher prevalence of obesity and associated conditions among Blacks.
2. Material and Methods
2.1. Study participants
Data for this study was obtained from the Reasons for Geographic and Racial Disparities in Stroke (REGARDS) study. The REGARDS study is a prospective cohort study of Black and White participants recruited nationally in the United States between 2003 and 2007, with oversampling of Blacks and residents of the Stroke Belt (South Carolina, North Carolina, Tennessee, Georgia, Louisiana, Arkansas, Mississippi, and Alabama). Detailed data on demographics, health behaviors, and history of comorbid conditions were collected at baseline using a computer-assisted telephone interview. Blood sample collection after 10–12 hours overnight fasting, echocardiography, and physical measurements including height and weight were conducted during initial in-home visits by trained staff following informed consent. Overall, 30,239 participants aged ≥45 years at baseline, 55% female, 42% Black, and 50% from the Stroke Belt region were recruited. The REGARDS study is ongoing and it is described in detail elsewhere [18, 19]. In the present analysis, 1,822 individuals who were cancer-free at baseline and selected into a sub-cohort with available inflammatory and metabolic biomarker data were included. The sub-cohort was randomly selected from the stratified random sample defined by equal distribution by race (Black/White), sex, and age (20% from each 10-year interval from 45 to 64, 25% from each 10-year interval from 65 to 84, and 10% from those over 84 years old) and region (50% from Stroke Belt) using study weights created based on the inverse probability of being selected. REGARDS participants were followed-up every 6 months for hospitalizations and for any medical event, and medical events or deaths were ascertained using death certificates, medical records, and/or interviewed proxies to determine the causes of the death. All participating institutional review boards approved the REGARDS study.
2.2. Exposure variables
The main exposure variables of interest in this study were inflammatory biomarkers- IL-6, IL-8, IL-10, and CRP, and metabolic biomarkers- adiponectin, resistin, and Lp(a). Biomarkers were analyzed from blood samples that were collected during the baseline in-home visit, centrifuged, separated and shipped overnight on gel ice packs to the central laboratory (Laboratory for Clinical Biochemistry Research at the University of Vermont). Ultra-sensitive ELISA (Quantikine HS Human IL-6 Immunoassay, R&D Systems, Minneapolis, MN) was used to measure IL-6 (minimum detectable dose /sensitivity=0.031 pg/ml and inter-assay coefficient of variation=6.3%) and no significant cross-reactivity was observed. The Human Serum Adipokine Panel B LINCOplex Kit (Linco Research, Inc., St. Charles, MO) was used to measure IL-8 (sensitivity=0.20 pg/ml, intra-assay coefficient of variation ranged from 1.4% to 7.9%, and inter-assay-coefficient of variation was <21%) and no significant cross-reactivity was observed. Milliplex MAP Human Cardiovascular Disease Panel 3 (Millipore Corporation, Billerica, MA) run as a singleplex assay was used to measure IL-10 (sensitivity=0.30 pg/ml and the average analytical coefficient of variation =8.09%) and no significant cross-reactivity was observed. CRP was measured in special plasma in batches during enrollment utilizing a validated high-sensitivity particle-enhanced immunonephelometric assay on the BN II nephelometer (N High Sensitivity CRP, Dade Behring Inc., Deerfield, IL), (sensitivity=0.16 μg/ml, intra-assay-coefficient of variation ranged from 2.3% to 4.4%, and inter-assay coefficient of variation=2.1% to 5.7%) and no significant cross-reactivity was observed. Adiponectin and resistin were measured using the Human Serum Adipokine Panel A LINCOplex Kit (Linco Research, Inc., St. Charles, MO) (for adiponectin: sensitivity=80.3 pg/ml, inter-assay coefficient of variation ranged from 5.68 to 8.20%, and for resistin: sensitivity=4.5 pg/ml and inter-assay coefficient of variation ranged from 8.04% to 9.42%) and no significant cross-reactivity was observed. Lp(a) was measured with the BN II nephelometer utilizing a particle-enhanced immunonephelometric assay (N Latex Lipoprotein-a, Siemens Healthcare Diagnostics, Deerfield, IL) (sensitivity=0.002 g/L, inter-assay coefficient of variation ranged from 6.10% to 10.28%). For LP(a), no cross-reactivity with apolipoprotein B (<1%) and plasminogen (<5%) was observed.
2.3. Covariates
The study covariates included baseline demographic variables such as age (continuous), gender (male/female), education (college graduate, some college, high school graduate or less than high school), race (Black/White), and income (≥ $75K, $35K-$74K, $20K-$34K, < $20K or refused). Additionally, analyses included baseline data on exercise (≥ 4 times/week, 1–3 times/week or none), BMI (kg/m2), smoking (current, past, or never smoker), alcohol intake (heavy, moderate, or none), comorbidity score (number of comorbidities-score ranging from 0 to 7), regular aspirin use (yes/no), and statin use (yes/no).
2.4. Cancer Mortality
Cancer mortality was identified through semi-annual telephone follow-up, death information from participants’ proxies, linkages with the Social Security Death Index (SSDI) and the National Death Index (NDI). Date of death was confirmed using death certificates, SSDI and/or NDI. A committee of experts adjudicated the cause of death using all available information as recommended by national guidelines [20]. Follow-up data for this analysis was available through December 31, 2015.
2.5. Statistical Analysis
Chi-squared test was used to compare baseline categorical participants’ characteristics by BMI category i.e. obese/overweight (BMI≥25 kg/m2) and normal BMI (BMI=18.5–24.9 kg/m2), and t-test was used to compare continuous variables by BMI category. Weighted Cox proportional hazard regression analysis was used to compare the risk of cancer mortality by the levels of metabolic and inflammatory biomarkers stratified by BMI category in models sequentially adjusted for potential confounding variables. Robust sandwich estimation method was used to estimate the confidence intervals around the hazard ratio. The crude model included each of the main exposure variable (IL-6, IL-8, IL-10, CRP, adiponectin, leptin, resistin or Lp(a)) and age. Model 1 further adjusted for sex, BMI, education, income, and race. Model 2 (the main analytical model) further adjusted for exercise. Model 3 additionally adjusted for alcohol, smoking, aspirin and statin use, and comorbidity score. Since the continuous exposure variables were not normally distributed, they were log-transformed. Statistical interactions between log-transformed biomarker levels and BMI were tested using the likelihood ratio test and the interaction p-values ≤0.1 were considered statistically significant. In addition, participants were ranked into tertiles according to the levels of biomarkers and the highest tertiles were compared to the lowest for the risk of cancer mortality. The associations between the exposure variables and mortality were considered statistically significant if the 95% confidence intervals (95% CIs) do not include the null value (1.0) or if the p-values are ≤0.05, and for interaction terms, if the p-values are ≤0.1. The interactions between BMI and each biomarker in the non-stratified crude models were statistically significant at α=0.1, therefore results from BMI stratified models were presented. Participants were censored at the date of death, loss to follow-up or December 31, 2015, whichever happened first. All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA.)
3. Results
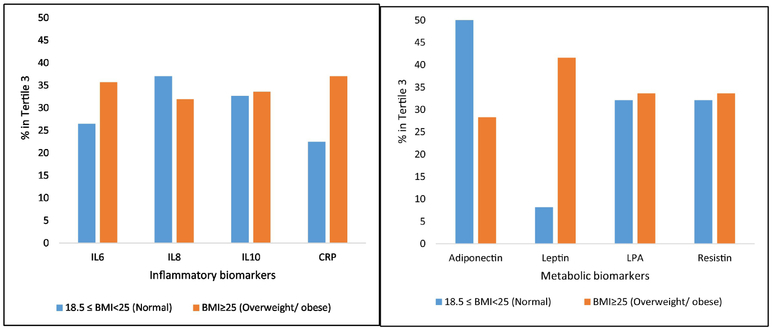
At baseline, compared with participants with normal BMI, those with overweight or obesity were younger, more likely to be male and Blacks, have less than high school education, and a higher average comorbidity score (all p-values <0.05, Table 1). Participants with normal BMI tended to have higher IL-8 and adiponectin levels, while those with overweight or obesity tended to have higher CRP, IL-6, and Lp(a) (p-value<0.05). The distribution of IL-10 and resistin were not significantly different between BMI categories, Table 2. A higher proportion of participants with normal BMI tended to be in the highest tertile of IL-6 and CRP than the participants with overweight/obesity, Figure 1. A higher proportion of the participants with normal BMI also tended to be in the highest tertile of adiponectin than those with overweight/obesity while those with overweight/obesity tended to be in the highest tertile of leptin than those with normal BMI, Figure 1.
Table 1.
Baseline characteristics of REGARDS participants by BMI category
| BMI Categories | |||
|---|---|---|---|
| Normal (18.5–24.9 kg/m2) | Overweight or Obese (≥25kg/m2) | P-value | |
| Participants n | 454 | 1368 | |
| Weighted Participants n | 6402 | 19904 | |
| Age at baseline, mean (SE)§ | 70.3 (12.0) | 66.4 (10.2) | <0.0001 |
| Race (Blacks)%Ψ | 28.4 | 45.7 | <0.0001 |
| Male Gender, % | 42.4 | 46.9 | 0.0001 |
| Education < High School, % | 10.0 | 12.9 | 0.003 |
| Income <$20,000, % | 14.7 | 16.7 | 0.501 |
| No Exercise Activity, % | 34.0 | 32.5 | 0.740 |
| BMI (kg/m2), Median (IQR)γ | 23.2 (2.3) | 30 (6.4) | |
| Current Smoking Status, % | 18.0 | 12.7 | 0.353 |
| Heavy Alcohol Consumption, % | 6.0 | 3.3 | 0.558 |
| Medication Use, % | |||
| NSAIDs - Aspirin | 43.9 | 45.1 | 0.836 |
| Statins | 28.6 | 35.9 | 0.243 |
| Comorbid Conditions, % | |||
| Atrial fibrillation | 12.2 | 8.0 | 0.030 |
| Chronic lung disease | 6.7 | 9.0 | 0.516 |
| Coronary artery disease | 16.5 | 16.6 | 0.551 |
| Deep vein thrombosis | 4.5 | 6.6 | <0.0001 |
| Diabetes | 9.9 | 27.5 | <0.0001 |
| Dyslipidemia | 50.6 | 63.6 | <0.0001 |
| Hypertension | 44.7 | 63.8 | <0.0001 |
| Myocardial infarction | 13.2 | 12.8 | 0.685 |
| Peripheral artery disease | 1.1 | 1.4 | 0.827 |
| Stroke | 7.6 | 4.6 | 0.081 |
| Comorbidity Score, mean (SE) | 1.9 (1.4) | 2.3 (1.4) | <0.0001 |
Results are mean and standard deviation (SD)
Results are percent
Results are median and interquartile ranges
Table 2.
Distribution of inflammatory and metabolic biomarkers among study participants
| BMI Category | |||
|---|---|---|---|
| Normal (18.5–24.9 kg/m2) | Overweight or Obese (≥25 kg/m2) | P-value | |
| Inflammatory Biomarkers (n=1822) | |||
| IL-6 (pg/ml)* | 2.2 (1.9) | 3.1 (2.4) | <0.0001 |
| IL-8 (pg/ml)* | 2.7 (1.8) | 2.5 (1.8) | 0.013 |
| IL-10 (pg/ml)* | 9.5 (7.1) | 9.1 (7.1) | 0.58 |
| CRP (mg/L)§ | 3.5 (6.5) | 4.8 (7.1) | <0.0005 |
| Metabolic Biomarkers (n=1733) | |||
| Adiponectin (ng/ml)* | 14568 (20982.2) | 10072 (20904.2) | <0.0001 |
| Resistin (pg/ml)* | 23.1 (20) | 24.3 (12.1) | 0.69 |
| Lp(a) (mg/dl)* | 0.13 (0.3) | 0.20 (0.40) | 0.045 |
Abbreviation: CRP, C-reactive protein; IQR, inter quartile range; IL, interleukine; Lp(a), lipoprotein (a); SD, standard deviation
Median (IQR)
Results are mean and SD
Figure 1:
Distribution of inflammatory (A) and metabolic (B) biomarkers by BMI in REGARDS BMI in kg/m2.
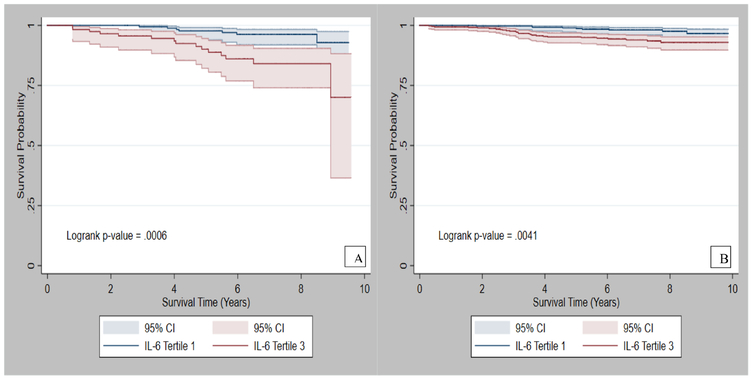
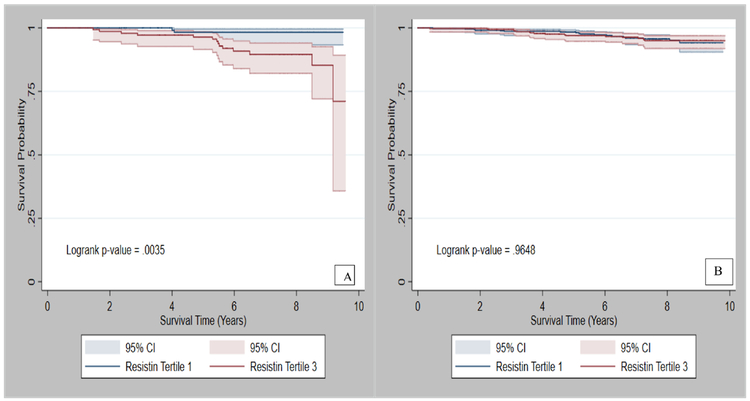
The average follow-up time in the REGARDS sub-cohort was 8 years (SD=3.3). Figures 2 and 3 present the survival probabilities by tertiles of IL-6 and resistin, respectively. Among participants with normal BMI, participants in the highest vs. lowest tertile of IL-6 had a 5-fold (HR: 5.3, 95% CI: 1.6–17.8) increased risk of cancer mortality. Similar results were observed for log-transformed values of IL-6. In addition, those in the highest tertile of CRP had over a 3-fold (HR: 3.4; 95% CI: 1.0, 11.2) increased risk of cancer mortality (Table 3), while those in the highest tertile of resistin had a nearly 4-fold increased risk of cancer mortality (HR: 3.7; 95% CI: 1.2, 11.2), (Table 4). The interactions between the exposure variables and BMI were statistically significant, p-values were <0.05, Table 3 and 4. Among participants with overweight/obesity, the highest tertile of IL-6 was associated with more than a 3-fold increased risk of cancer mortality (HR: 3.5; 95% CI: 1.5, 8.1), (Table 3). However, CRP and resistin were not significantly associated with cancer mortality in this group (Table 4).
Figure 2:
Survival probability of cancer patients with A) normal BMI, and B) overweight/obesity by tertiles of IL-6.
Figure 3:
Survival probability of cancer patients with A) normal BMI and B) overweight/obese by tertiles of resistin.
Table 3.
Hazard ratios (HR) and 95% confidence intervals (CI) for cancer mortality by baseline inflammatory biomarkers
| BMI categories | |||||
|---|---|---|---|---|---|
| Normal (18.5≤BMI<25 kg/m2) | Overweight or Obese (BMI≥25 kg/m2) | pint with BMI | |||
| Log Transformed | T3§ | Log Transformed | T3 | ||
| IL-6 (pg/ml), N (Cases) | 454 (29) | 120 (15) | 1368 (54) | 488 (27) | |
| Weighted N (Cases) | 6402 (371) | 1136 (195) | 19904 (937) | 6310 (492) | |
| Crude | 3.3 (1.5, 6.7)** | 5.5 (1.8, 16.4)** | 2.8 (1.6, 4.7)** | 3.2 (1.5, 6.7)* | <0.0001 |
| Model 1 | 3.0 (1.4, 6.3)** | 4.2 (1.5, 11.9)** | 2.8 (1.6, 5.0)** | 3.4 (1.4, 7.0)** | 0.007 |
| Model 2 | 3.2 (1.5, 7.0)** | 5.3 (1.6, 17.8)** | 3.0 (1.7, 5.4)** | 3.5 (1.5, 8.1)** | 0.007 |
| Model 3 | 2.1 (0.8, 5.4) | 2.4 (0.6, 9.0) | 2.6 (1.4, 4.9)** | 2.9 (1.2, 6.6)* | 0.716 |
| IL-8 (pg/ml), N (Cases) | 454 (29) | 168 (12) | 1368 (54) | 436 (25) | |
| Weighted N (Cases) | 6402 (371) | 2101 (187) | 19904 (937) | 6228 (552) | |
| Crude | 1.1 (0.7, 1.8) | 1.8 (0.6, 5.2) | 1.4 (1.0, 2.2)** | 1.5 (0.8, 3.1)* | 0.047 |
| Model 1 | 0.9 (0.5, 1.7) | 1.5 (0.5, 4.2) | 1.4 (0.9, 2.3) | 1.4 (0.7, 2.8) | 0.049 |
| Model 2 | 0.9 (0.5, 1.7) | 1.5 (0.5, 4.1) | 1.4 (0.9, 2.3) | 1.4 (0.7, 2.9) | 0.043 |
| Model 3 | 0.9 (0.4, 1.9) | 1.1 (0.3, 4.4) | 1.2 (0.7, 2.2) | 1.3 (0.6, 2.8) | 0.710 |
| IL-10 (pg/ml), N (Cases) | 454 (29) | 148 (9) | 1368 (54) | 459 (22) | |
| Weighted N (Cases) | 6402 (371) | 2307 (148) | 19904 (937) | 6230 (367) | |
| Crude | 0.7 (0.5, 1.1) | 0.7 (0.3, 1.7) | 1.0 (0.6,1.5) | 1.2 (0.6, 2.2) | 0.006 |
| Model 1 | 0.8 (0.5,1.2) | 0.7 (0.3, 1.9) | 0.9 (0.6, 1.6) | 1.0 (0.5, 2.0) | 0.021 |
| Model 2 | 0.7 (0.5, 1.2) | 0.7 (0.2, 2.1) | 0.9 (0.6, 1.5) | 1.0 (0.5, 2.0) | 0.016 |
| Model 3 | 0.6 (0.4, 1.1) | 0.6 (0.2, 2.6) | 0.9 (0.5, 1.6) | 0.8 (0.4, 1.7) | 0.636 |
| CRP (mg/L), N (Cases) | 678 (31) | 102 (8) | 1368 (54) | 506 (20) | |
| Weighted N (Cases) | 6402 (371) | 1217 (92) | 19904 (937) | 6987 (364) | |
| Crude | 1.7 (1.2, 2.4)** | 3.6 (1.2, 10.4)** | 1.1 (0.8, 1.4) | 1.1 (0.6, 2.2) | 0.0003 |
| Model 1 | 1.6 (1.1, 2.2)** | 3.5(1.1, 11.4)* | 1.1 (0.8, 1.4) | 1.2 (0.6, 2.4) | 0.009 |
| Model 2 | 1.6 (1.1, 2.2)** | 3.4 (1.0, 11.2)* | 1.1 (0.8, 1.5) | 1.3 (0.6, 2.8) | 0.008 |
| Model 3 | 1.6 (1.1, 2.2)* | 4.2 (1.1,16.4)* | 1.2 (0.9, 1.6) | 1.6 (0.7, 3.5) | 0.412 |
Crude model included the exposure and age.
Model 1: Adjusted for age, gender, education, race, and income.
Model 2: Additionally adjusted for exercise
Model 3: Additionally adjusted for smoking status, alcohol use, aspirin use, statin, and comorbidity score
T1: 1st tertile, was the reference category; T3: 3rd tertile
Bold indicates significance at 0.05 alpha level.
p-values were ≤0.01;
p-values were ≤0.05 while the rest of p-values were >0.05.
pint, p for the interaction of the log form with BMI. The p-values are from type 3 tests. P for the interaction is considered significant at alpha=0.1.
Table 4.
Hazard ratios (HR) and 95% confidence intervals (CI) for cancer mortality by baseline metabolic biomarkers
| BMI categories | |||||
|---|---|---|---|---|---|
| Normal (18.5 ≤ BMI <25 kg/m2) | Overweight or Obese (BMI ≥25 kg/m2) | pint with BMI | |||
| Weighted N (case) | Log-Transformed | T3§ | Log Transformed | T3 | |
| Adiponectin (pg/ml), N (Cases) | 418 (31) | 207 (19) | 1315 (55) | 178 (214) | |
| Weighted N (Cases) | 5664 (322) | 2713 (191) | 18694 (938) | 2563 (102) | |
| Crude | 1.0 (0.7, 1.5) | 0.9 (0.4, 2.3) | 0.9 (0.6, 1.3) | 0.9 (0.5, 1.6) | 0.029 |
| Model 1 | 1.2 (0.8, 1.8) | 1.7 (0.6, 4.9) | 1.1 (0.8, 1.7) | 1.3 (0.6, 2.6) | 0.025 |
| Model 2 | 1.3 (0.9, 1.9) | 2.1 (0.7, 6.0) | 1.1 (0.7, 1.6) | 1.3 (0.6, 2.7) | 0.014 |
| Model 3 | 1.4 (0.9, 2.2) | 2.3 (0.5, 9.4) | 1.1 (0.7, 1.7) | 1.3 (0.6, 2.9) | 0.141 |
| Lipoprotein a (mg/dl), N (Cases) | 418 (31) | 134 (10) | 1315 (55) | 220 (9) | |
| Weighted N (Cases) | 5664 (322) | 1467 (99) | 18694 (938) | 3339 (170) | |
| Crude | 0.8 (0.5, 1.3) | 1.2 (0.5, 2.8) | 0.9 (0.7, 1.2) | 0.8 (0.5, 1.7) | 0.008 |
| Model 1 | 0.7 (0.4, 1.1) | 0.8 (0.3, 2.2) | 0.9 (0.6, 1.2) | 0.8 (0.4, 1.7) | 0.082 |
| Model 2 | 0.7 (0.4, 1.2) | 0.9 (0.3, 2.6) | 0.9 (0.6, 1.2) | 0.8 (0.4, 1.8) | 0.066 |
| Model 3 | 1.0 (0.5, 1.9) | 1.7 (0.5, 6.3) | 0.9 (0.7, 1.3) | 1.0 (0.4, 2.3) | 0.712 |
| Resistin (pg/ml), N (Cases) | 418 (31) | 134 (15) | 1315 (55) | 224 (8) | |
| Weighted N (Cases) | 5664 (322) | 1528 (112) | 18694 (938) | 3025 (148) | |
| Crude | 2.5 (1.1, 5.7)* | 3.7 (1.3, 11.0)* | 0.8 (0.5, 1.5) | 0.7 (0.4, 1.5) | 0.001 |
| Model 1 | 2.4 (1.1, 5.3)* | 4.0 (1.3, 11.7)* | 1.0 (0.5, 1.8) | 0.8 (0.4, 1.6) | 0.012 |
| Model 2 | 2.3 (1.0, 5.0)* | 3.7 (1.2, 11.2)* | 1.0 (0.5, 1.8) | 0.7 (0.3, 1.5) | 0.008 |
| Model 3 | 2.5 (0.9, 7.0) | 8.7 (1.9, 39.9)** | 1.0 (0.5, 2.0) | 0.9 (0.4, 1.8) | 0.001 |
Crude model included the exposure and age.
Model 1 adjusted for age, gender, education, race, and income.
Model 2 additionally adjusted for exercise.
Model 3 additionally adjusted for smoking status, alcohol use, aspirin use, statin, and comorbidity score
T1: 1st tertile was the reference category; T3: 3rd tertile
Bold indicates significance at 0.05 alpha level.
p-values were ≤0.01;
p-values were ≤0.05 while the rest of p-values were >0.05.
pint, p for the interaction of the log form with BMI. The p-values are from type 3 tests. Pfor the interaction is considered significant at alpha=0.1.
In race-stratified analyses (Table 5), IL-6 was significantly associated with higher risk of cancer mortality among Black participants with normal BMI (HR: 4.7; 95% CI: 1.3, 16.9) and overweight/obesity (HR: 3.3; 95% CI: 1.6, 7.0), and among White participants with overweight/obesity (HR: 3.1; 95% CI: 1.3, 7.5). Furthermore, CRP was significantly associated with an increased risk of cancer mortality, but only among White participants with normal BMI (HR: 1.9; 95% CI: 1.0, 3.4). The interactions between BMI and the exposure variables remained among Black participants (p-values<0.05) but disappeared among White participants (p-values>0.1), Table 5. In a sensitivity analysis excluding those who died of cancer within 6 months from baseline, the associations remained consistent for all biomarkers evaluated (data not shown).
Table 5.
Hazard ratios (HR) and 95% confidence intervals (CI) for cancer mortality by inflammatory and metabolic biomarkers by race
| BMI categories | |||
|---|---|---|---|
| Normal BMI (18.5 ≤ BMI <25 kg/m2) | Ove rweight or Obese (BMI ≥25 kg/m2) | pint with BMI | |
| Log-Transformed§ | Log Transformed§ | ||
| Black Participants (N=798, cancer death=40) | |||
| IL-6 | |||
| Model 1 | 3.6(1.2, 10.6)* | 3.4 (1.6, 7.0)** | 0.008 |
| Model 2 | 4.7 (1.3, 16.9)* | 3.3 (1.6, 7.0)* | 0.021 |
| Model 3 | 5.9 (1.5, 23.1)* | 3.0 (1.3, 6.9)* | 0.156 |
| CRP | |||
| Model 1 | 1.4 (0.9, 2.0) | 1.3 (0.9, 2.0) | 0.039 |
| Model 2 | 1.4 (0.9, 1.9) | 1.3 (0.9, 2.0) | 0.043 |
| Model 3 | 1.3 (0.7, 2.2) | 1.3 (0.9, 2.0) | 0.396 |
| Resistin | |||
| Model 1 | 4.4 (0.6, 31.4) | 1.3 (0.7, 2.3) | 0.007 |
| Model 2 | 4.2 (0.7, 26.9) | 1.3 (0.7, 2.3) | 0.013 |
| Model 3 | 8.5 (2.0, 35.8)** | 1.3 (0.7, 2.5) | 0.008 |
| White Participants (N=1024, cancer death=46) | |||
| IL-6 | |||
| Model 1 | 2.6 (0.8, 9.1) | 2.4 (1.0, 5.7)* | 0.269 |
| Model 2 | 3.0 (0.8, 12.0) | 3.1 (1.3, 7.5)* | 0.278 |
| Model 3 | 1.0 (0.20, 4.6) | 2.7 (1.1, 6.4)* | 0.649 |
| CRP | |||
| Model 1 | 1.8(1.1, 3.1)* | 0.9 (0.6, 1.3) | 0.115 |
| Model 2 | 1.9 (1.0, 3.4)* | 0.9 (0.6, 1.4) | 0.131 |
| Model 3 | 1.7 (1.2, 2.5)** | 1.0 (0.6, 1.5) | 0.571 |
| Resistin | |||
| Model 1 | 1.3 (0.3, 5.5) | 0.4 (0.1, 1.6) | 0.625 |
| Model 2 | 1.3 (0.3, 6.0) | 0.4 (0.1, 1.8) | 0.454 |
| Model 3 | 3.6 (0.5, 24.2) | 0.4 (0.1, 2.5) | <0.0001 |
Crude model included the exposure and age.
Model 1 adjusted for age, gender, education, and income
Model 2 additionally adjusted for exercise
Model 3 further adjusted for comorbidity scores, smoking, alcohol, aspirin, and statin use
Biomarkers (except CRP) were log-transformed due to non-normal distributions
Bold indicates significance at 0.05 alpha level.
p-values were ≤0.01;
p-values were ≤0.05 while the rest of p-values were >0.05.
pint, p for the interaction of the log form with BMI. The p-values are from type 3 tests.P for the interaction is considered significant at alpha=0.1
4. Discussion
In prospective REGARDS cohort, after adjusting for study covariates, higher baseline IL-6, CRP, and resistin were significantly associated with increased risk of cancer mortality among participants with normal BMI, while higher IL-6 was also associated with increased risk of cancer mortality among participants with overweight/obesity. When stratified by race, IL-6 remained significantly associated with higher risk of cancer mortality among Blacks regardless of BMI, but only among Whites with overweight/obesity. To our knowledge, this is the first study to simultaneously evaluate the independent associations of inflammatory and metabolic biomarkers with cancer mortality across levels of obesity.
Obesity has been well studied in relation to the risk of cancer mortality; however, estimates of the risk associated with higher BMI have been inconsistent due to the limitations in the validity of BMI as a measure of adiposity, and due to differences in the type and pattern of adiposity. This has led to a renewed interest in identifying biomarkers that may be less vulnerable to measurement error and can more reliably predict cancer risk. Recent studies have also shown that independent of obesity, metabolic health status is a key risk factor for cancer. Significant associations have been observed between high blood pressure [21], dyslipidemia, and type 2 diabetes [22–25] with cancer risk and mortality. Furthermore, obesity is associated with low-grade chronic inflammation, which is independently associated with cancer risk [26, 27]. Therefore, it is likely that obesity induces measurable changes in metabolism and inflammatory biomarkers associated with risk of cancer. It is also likely that non-obese individuals with higher levels of certain risk-associated biomarkers may be at higher risk of cancer. However, studies evaluating the impact of obesity in modifying the association between biomarkers of inflammation and metabolic dysregulation are limited. In particular, given that obesity has been evaluated well as a risk factor for cancer incidence and mortality, the emphasis to date has been on identifying biomarkers of this association in obese individuals. Our finding suggests that chronic inflammation and metabolic dysregulation in normal BMI individuals may substantially increase their risk of cancer mortality.
We observed a robust association between IL-6 and cancer mortality in both obese and non-obese participants after adjusting for socio-demographics, physical activity, and comorbidities. The association became attenuated and non-significant, although still a 2-fold increased risk, among normal BMI but not obese individuals when adjusted for behavioral risk factors (smoking, alcohol) and statin use. In contrast, estimates for overweight/obese participants became stronger after adjusting for the behavioral risk factors and statin use. We also observed significantly increased cancer mortality risk with higher CRP levels in normal BMI participants in the fully adjusted models. These findings suggest that regardless of BMI, certain markers of inflammation may influence the risk of cancer mortality. In addition, the results suggest that while behavioral factors may explain the increased risk associated with IL-6 in individuals with normal BMI but not overweight/obese, the association between CRP and cancer mortality risk in normal BMI individuals regardless of behavioral risk factors highlights a potentially important role for chronic inflammation. Past studies have also observed independent and significant associations of high levels of IL-6 [28–30], CRP [31–34], and obesity [35–37] with cancer mortality. Our study adds to this body of work by showing that the association between inflammatory biomarkers and cancer mortality varies by BMI. Mechanistically, it has been suggested that a chronic inflammatory microenvironment promotes tumor cell motility, invasion, epithelial to mesenchymal transition and metastasis which in turn lead to poor prognosis [38]. IL-6 promotes tumorigenesis, angiogenesis, invasiveness, and metastasis, and inhibits apoptosis [29, 39]. IL-6 also protects cancer cells from therapy-induced DNA damage and oxidative stress by facilitating the repair and induction of counter-signaling pathways, thus increasing cancer mortality [39].
We found a consistent association between higher baseline resistin, a metabolism biomarker, and increased risk of cancer mortality, but only among participants with normal BMI. This association remained statistically significant after adjusting for behavioral risk factors. Of note, mean resistin was not significantly different by BMI categories. Thus, the differences in the association between resistin and cancer mortality by BMI may be attributable to the interaction between resistin and BMI and may not be due to resistin alone. Resistin has been studied as a potential missing link between obesity, chronic inflammation, and cancer [40] due to its association with increased low-density lipoprotein production and inflammatory response. A previous study has found a direct association between resistin and cancer mortality [41], and studies have documented that resistin promotes insulin resistance [42], chronic low-grade inflammation [43], and tumor cells adhesion to endothelium-a critical step for metastasis [44, 45]. The differences by BMI in the risk of cancer mortality associated with resistin may be at least partially explained in that resistin may not increase the risk any further in the obese individuals who already may have chronic inflammation. However, in individuals with normal BMI who might otherwise be without chronic inflammation, having high resistin may lead to chronic inflammation and thus worsen the prognosis of cancer. Lp(a), another metabolism-related biomarker, is involved in cholesterol transport, wound healing and tissue repair-pathways that are co-opted by cancer cells to enhance invasion and metastasis. We did not observe a significantly increased risk of cancer mortality associated with Lp(a) or adiponectin in the current study, although there was a suggestion of increased risk for adiponectin. Studies with larger sample sizes may be needed to definitively evaluate the role of adiponectin and Lp(a) in cancer mortality among obese and non-obese individuals; it is possible that these associations are easier to detect in lean individuals.
The association between baseline biomarkers and risk of cancer mortality also varied by race. For instance, IL-6 was associated with an increased risk of cancer mortality among normal BMI and overweight/obese Blacks, but only among overweight/obese Whites. CRP was associated with increased risk of cancer mortality among normal BMI Whites, but not among Blacks despite those with overweight/obesity having significantly higher mean CRP levels. This difference might be partially explained by the fact that obese/overweight participants might already have other risk factors that cumulatively increase the risk of cancer mortality and inflammation due to CRP may not significantly increase the risk while in those with normal BMI there may be fewer factors to mask the role of chronic inflammation. In addition, a higher proportion of those with normal BMI died of cancer than the proportion of overweight/obese participants that died of cancer. In a previous study, IL-6 was associated with increased cancer mortality in both Blacks and White participants [46]. Our analysis was likely underpowered given the limited sample sizes to detect racial differences in cancer mortality risk. None of the interaction terms between inflammatory and metabolic biomarkers on cancer mortality risk were statistically significant, likely due to the small sample size and the limited number of events. Future studies with larger, racially diverse prospective cohorts, or including a larger subset of this cohort, are needed to definitively evaluate racial differences in the independent and synergistic associations of inflammatory and metabolic biomarkers in cancer mortality risk. The finding from this study improves our understanding of the role of metabolic health status and obesity in cancer outcomes and informs race-specific cancer prevention interventions.
The strengths of the study include the consistent results in the sensitivity analysis that excluded those who died of cancer within six months from baseline and the use of a large biracial prospective cohort with detailed baseline measures of the biomarkers of interest and covariates. REGARDS participants were recruited nationally across the US, although residents of the stroke belt were over-sampled, thus improving the generalizability of the study results. This study also has some limitations relevant to the interpretation of the results. First, the mean follow-up period was 8 years. This also resulted in a limited number of events especially in stratified analysis, leading to reduced power to detect significant associations especially in the race-stratified and interaction analyses. Second, since REGARDS was originally designed to evaluate stroke outcomes, there is currently no data on cancer incidence. While cost-effective and convenient, the methods used to determine the assay levels in this study such as the BN II nephelometer, Milliplex MAP Human Cardiovascular Disease Panel 3, and Human Serum Adipokine Panel B LINCOplex Kit may have low sensitivities, especially for LP(a); however, in this study, they had satisfactory sensitivities with MDD well below the 1% for each biomarker except for LP(a), and the methods have been used previously and had similar coefficients of variations as in our study [47, 48]. Larger cohorts of racially diverse participants using different assay measurement methods are needed to confirm these findings and potentially identify other relevant biomarkers that may vary by BMI and race.
In conclusion, biomarkers of inflammation (IL-6 and CRP) and metabolism (resistin) were significantly associated with increased risk of cancer mortality independent of obesity, suggesting that both pathways may play a synergistic role in creating a favorable microenvironment for tumorigenesis and prognosis. This supports the need for improved risk stratification based on metabolic health and/or inflammatory biomarkers regardless of BMI or obesity status in predicting cancer outcomes. Interventions targeting the inflammatory and metabolic biomarkers may improve cancer survival.
Funding
The REGARDS study was supported by cooperative agreement U01-NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org. This analysis was supported by award R01-NR012726 from the National Institute for Nursing Research, UL1-RR025777 from the National Center for Research Resources, K08HL096841 and R01HL080477 from the National Heart, Lung, and Blood Institute, and by grants from the Center for Clinical and Translational Science and the Lister Hill Center for Health Policy of the University of Alabama at Birmingham. Dr. Akinyemiju was supported by grant K01TW010271 from the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. Representatives of the funding agencies have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data.
Abbreviations:
- BMI
Body Mass Index
- IL-6
: Interleukin-6
- IL-8
Interleukin-8
- IL-10
Interleukin-10
- CRP
C-Reactive Protein
- Lp(a)
Lipoprotein (a)
- REGARDS
Reasons for Geographic and Racial Disparities in Stroke
- SSDI
Social Security Death Index
- NDI
National Death Index
- HR
Hazard Ratio
- CI
Confidence Interval
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
REFERENCES
- 1.Yang M, Kenfield SA, Van Blarigan EL, Batista JL, Sesso HD, Ma J, Stampfer MJ, et al. , Dietary patterns after prostate cancer diagnosis in relation to disease-specific and total mortality. Cancer Prev Res (Phila), 2015. 8(6): p. 545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orlich MJ, Singh PN, Sabate J, Fan J, Sveen L, Bennett H, Knutsen SF, et al. , Vegetarian dietary patterns and the risk of colorectal cancers. JAMA Intern Med, 2015. 175(5): p. 767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang B, Jacobs EJ, Gapstur SM, Stevens V, and Campbell PT, Active smoking and mortality among colorectal cancer survivors: the Cancer Prevention Study II nutrition cohort. J Clin Oncol, 2015. 33(8): p. 885–93. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari P, Licaj I, Muller DC, Kragh Andersen P, Johansson M, Boeing H, Weiderpass E, et al. , Lifetime alcohol use and overall and cause-specific mortality in the European Prospective Investigation into Cancer and nutrition (EPIC) study. BMJ Open, 2014. 4(7): p. e005245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trabert B, Eldridge RC, Pfeiffer RM, Shiels MS, Kemp TJ, Guillemette C, Hartge P, et al. , Pre-diagnostic circulating inflammation markers and endometrial cancer risk in the Prostate, Lung, Colorectal and Ovarian Cancer (PLCO) Screening Trial. Int J Cancer, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epplein M, Xiang YB, Cai Q, Peek RM Jr., Li H, Correa P, Gao J, et al. , Circulating cytokines and gastric cancer risk. Cancer Causes Control, 2013. 24(12): p. 2245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng T, Lyon CJ, Bergin S, Caligiuri MA, and Hsueh WA, Obesity, Inflammation, and Cancer. Annu Rev Pathol, 2016. 11: p. 421–49. [DOI] [PubMed] [Google Scholar]
- 8.Kolb R, Sutterwala FS, and Zhang W, Obesity and cancer: inflammation bridges the two. Curr Opin Pharmacol, 2016. 29: p. 77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, et al. , Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet, 2009. 373(9669): p. 1083–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iyengar NM, Gucalp A, Dannenberg AJ, and Hudis CA, Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. Journal of Clinical Oncology, 2016. 34(35): p. 4270–4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chong DQ, Mehta RS, Song M, Kedrin D, Meyerhardt JA, Ng K, Wu K, et al. , Prediagnostic Plasma Adiponectin and Survival among Patients with Colorectal Cancer. Cancer Prev Res (Phila), 2015. 8(12): p. 1138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duggan C, Irwin ML, Xiao L, Henderson KD, Smith AW, Baumgartner RN, Baumgartner KB, et al. , Associations of insulin resistance and adiponectin with mortality in women with breast cancer. J Clin Oncol, 2011. 29(1): p. 32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oba J, Wei W, Gershenwald JE, Johnson MM, Wyatt CM, Ellerhorst JA, and Grimm EA, Elevated Serum Leptin Levels are Associated With an Increased Risk of Sentinel Lymph Node Metastasis in Cutaneous Melanoma. Medicine (Baltimore), 2016. 95(11): p. e3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colotta F, Allavena P, Sica A, Garlanda C, and Mantovani A, Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis, 2009. 30(7): p. 1073–1081. [DOI] [PubMed] [Google Scholar]
- 15.Akinyemiju T, Moore JX, Pisu M, Judd SE, Goodman M, Shikany JM, Howard VJ, et al. , A Prospective Study of Obesity, Metabolic Health, and Cancer Mortality. Obesity (Silver Spring), 2018. 26(1): p. 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feliciano EMC, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Dannenberg AJ, Kwan ML, et al. , Metabolic Dysfunction, Obesity, and Survival Among Patients With Early-Stage Colorectal Cancer. Journal of Clinical Oncology, 2016. 34(30): p. 3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hales CM, Carroll MD, Fryar CD, and Ogden CL, Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS Data Brief, 2017(288): p. 1–8. [PubMed] [Google Scholar]
- 18.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, et al. , The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology, 2005. 25(3): p. 135–43. [DOI] [PubMed] [Google Scholar]
- 19.Colantonio LD, Gamboa CM, Kleindorfer DO, Carson AP, Howard VJ, Muntner P, Cushman M, et al. , Stroke symptoms and risk for incident coronary heart disease in the REasons for Geographic And Racial Differences in Stroke (REGARDS) Study. International journal of cardiology, 2016. 220: p. 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halanych JH, Shuaib F, Parmar G, Tanikella R, Howard VJ, Roth DL, Prineas RJ, et al. , Agreement on cause of death between proxies, death certificates, and clinician adjudicators in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Am J Epidemiol, 2011. 173(11): p. 1319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beebe‐Dimmer JL, Dunn RL, Sarma AV, Montie JE, and Cooney KA, Features of the metabolic syndrome and prostate cancer in African‐American men. Cancer, 2007. 109(5): p. 875–881. [DOI] [PubMed] [Google Scholar]
- 22.Jaggers JR, Sui X, Hooker SP, LaMonte MJ, Matthews CE, Hand GA, and Blair SN, Metabolic Syndrome and Risk of Cancer Mortality in Men. European journal of cancer (Oxford, England : 1990), 2009. 45(10): p. 1831–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lund Håheim L, Wisløff TF, Holme I, and Nafstad P, Metabolic Syndrome Predicts Prostate Cancer in a Cohort of Middle-aged Norwegian Men Followed for 27 Years. American Journal of Epidemiology, 2006. 164(8): p. 769–774. [DOI] [PubMed] [Google Scholar]
- 24.Grundmark B, Garmo H, Loda M, Busch C, Holmberg L, and Zethelius B, The Metabolic Syndrome and the Risk of Prostate Cancer under Competing Risks of Death from Other Causes. Cancer Epidemiology Biomarkers & Prevention, 2010. 19(8): p. 2088–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gathirua-Mwangi WG, Monahan PO, Murage MJ, and Zhang J, Metabolic syndrome and total cancer mortality in the Third National Health and Nutrition Examination Survey. Cancer Causes Control, 2017. 28(2): p. 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pietrzyk L, Torres A, Maciejewski R, and Torres K, Obesity and Obese-related Chronic Low-grade Inflammation in Promotion of Colorectal Cancer Development. Asian Pac J Cancer Prev, 2015. 16(10): p. 4161–8. [DOI] [PubMed] [Google Scholar]
- 27.Harvey AE, Lashinger LM, and Hursting SD, The growing challenge of obesity and cancer: an inflammatory issue. Ann N Y Acad Sci, 2011. 1229: p. 45–52. [DOI] [PubMed] [Google Scholar]
- 28.Lin S, Gan Z, Han K, Yao Y, and Min D, Interleukin-6 as a prognostic marker for breast cancer: a meta-analysis. Tumori, 2015. 101(5): p. 535–41. [DOI] [PubMed] [Google Scholar]
- 29.Salgado R, Junius S, Benoy I, Van Dam P, Vermeulen P, Van Marck E, Huget P, et al. , Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer, 2003. 103(5): p. 642–6. [DOI] [PubMed] [Google Scholar]
- 30.Lippitz BE and Harris RA, Cytokine patterns in cancer patients: A review of the correlation between interleukin 6 and prognosis. Oncoimmunology, 2016. 5(5): p. e1093722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ko YJ, Kwon YM, Kim KH, Choi HC, Chun SH, Yoon HJ, Goh E, et al. , High-sensitivity C-reactive protein levels and cancer mortality. Cancer Epidemiol Biomarkers Prev, 2012. 21(11): p. 2076–86. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Zhong X, Cheng G, Zhao C, Zhang L, Hong Y, Wan Q, et al. , Hs-CRP and all-cause, cardiovascular, and cancer mortality risk: A meta-analysis. Atherosclerosis, 2017. 259: p. 75–82. [DOI] [PubMed] [Google Scholar]
- 33.Goyal A, Terry MB, Jin Z, and Siegel AB, C-reactive protein and colorectal cancer mortality in U.S. adults. Cancer Epidemiol Biomarkers Prev, 2014. 23(8): p. 1609–18. [DOI] [PubMed] [Google Scholar]
- 34.Wulaningsih W, Holmberg L, Ng T, Rohrmann S, and Van Hemelrijck M, Serum leptin, C-reactive protein, and cancer mortality in the NHANES III. Cancer Med, 2016. 5(1): p. 120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calle EE, Rodriguez C, Walker-Thurmond K, and Thun MJ Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. New England Journal of Medicine, 2003. 348(17): p. 1625–1638. [DOI] [PubMed] [Google Scholar]
- 36.Schmitz KH, Neuhouser ML, Agurs-Collins T, Zanetti KA, Cadmus-Bertram L, Dean LT, and Drake BF, Impact of obesity on cancer survivorship and the potential relevance of race and ethnicity. Journal of the National Cancer Institute, 2013. 105(18): p. 1344–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gallagher EJ and LeRoith D, Obesity and Diabetes: The Increased Risk of Cancer and Cancer-Related Mortality. Physiological Reviews, 2015. 95(3): p. 727–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilbert CA and Slingerland JM, Cytokines, obesity, and cancer: new insights on mechanisms linking obesity to cancer risk and progression. Annu Rev Med, 2013. 64: p. 45–57. [DOI] [PubMed] [Google Scholar]
- 39.Kumari N, Dwarakanath BS, Das A, and Bhatt AN, Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol, 2016. 37(9): p. 11553–11572. [DOI] [PubMed] [Google Scholar]
- 40.Dalamaga M, Resistin as a biomarker linking obesity and inflammation to cancer: potential clinical perspectives. Biomarkers in Medicine, 2014. 8(1): p. 107–118. [DOI] [PubMed] [Google Scholar]
- 41.Lee YC, Chen YJ, Wu CC, Lo S, Hou MF, and Yuan SS, Resistin expression in breast cancer tissue as a marker of prognosis and hormone therapy stratification. Gynecol Oncol, 2012. 125(3): p. 742–50. [DOI] [PubMed] [Google Scholar]
- 42.Jamaluddin MS, Weakley SM, Yao Q, and Chen C, Resistin: functional roles and therapeutic considerations for cardiovascular disease. British Journal of Pharmacology, 2012. 165(3): p. 622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al Hannan F and Culligan KG, Human resistin and the RELM of Inflammation in diabesity. Diabetology & Metabolic Syndrome, 2015. 7: p. 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JO, Kim N, Lee HJ, Lee YW, Kim SJ, Park SH, and Kim HS, Resistin, a fat-derived secretory factor, promotes metastasis of MDA-MB-231 human breast cancer cells through ERM activation. Scientific Reports, 2016. 6: p. 18923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsu WY, Chao YW, Tsai YL, Lien CC, Chang CF, Deng MC, Ho LT, et al. , Resistin induces monocyte-endothelial cell adhesion by increasing ICAM-1 and VCAM-1 expression in endothelial cells via p38MAPK-dependent pathway. J Cell Physiol, 2011. 226(8): p. 2181–8. [DOI] [PubMed] [Google Scholar]
- 46.Enewold L, Mechanic LE, Bowman ED, Zheng YL, Yu Z, Trivers G, Alberg AJ, et al. , Serum concentrations of cytokines and lung cancer survival in African Americans and Caucasians. Cancer Epidemiol Biomarkers Prev, 2009. 18(1): p. 215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Højbjerre L, Sonne MP, Alibegovic AC, Nielsen NB, Dela F, Vaag A, Bruun JM, et al. , Impact of physical inactivity on adipose tissue low-grade inflammation in first-degree relatives of type 2 diabetic patients. Diabetes care, 2011. 34(10): p. 2265–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee S-A, Kallianpur A, Xiang Y-B, Wen W, Cai Q, Liu D, Fazio S, et al. , Intra-individual Variation of Plasma Adipokine Levels and Utility of Single Measurement of These Biomarkers in Population-Based Studies. 2007. 16(11): p. 2464–2470. [DOI] [PubMed] [Google Scholar]