Abstract
Background:
Several antiretroviral therapy (ART) classes have been associated with increased myocardial infarction (MI) risk. Cardiovascular disease (CVD) in people living with HIV (PLWH) on integrase strand transfer inhibitors (INSTI) has not been examined. Here we aim to examine this.
Setting:
Retrospective cohort design study
Methods:
We used the IBM®MarketScan® databases for U.S. commercially insured and Medicaid covered adults to identify PLWH newly initiated on ART between Jan 1, 2008 and Dec 30, 2015. Major adverse cardiac event (MACE), a composite of acute MI, ischemic stroke, coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI), was the primary outcome. We used calendar-time specific probability-weighted Cox proportional hazards models to estimate hazard ratios (HR) and 95% confidence intervals (CI) for the association between INSTI use and MACE. We used propensity score weighting methods to account for potential confounding.
Results:
20,242 new ART initiators were identified. 5,069 (25%) PLWH initiated INSTI-based regimens. 203 MACE events occurred; acute MI 16 (0.32%) vs 66 (0.43%), stroke 24 (0.47%) vs 54 (0.36), CABG 2 (0.04%) vs 9 (0.06%), PCI 7 (0.14%) vs 25 (0.16%) of INSTI users vs non-users. INSTI-based ART was associated with significantly lower risk of MACE events (HR 0.79; 95% CI 0.64, 0.96) compared to non-INSTI-based regimens.
Conclusion:
In this cohort, INSTI-based regimens were associated with a 21% decreased risk of incident CVD. These finding require validation in other cohorts and with longer follow up.
Keywords: HIV, Integrase Strand transfer inhibitors, antiretroviral therapy, cardiovascular disease, major adverse cardiovascular events
Introduction
People living with HIV (PLWH) are at greater risk of cardiovascular disease (CVD), in particular myocardial infarction (MI)1,2. Like those without HIV, traditional risk factors for CVD, such as smoking, hypertension, and dyslipidaemia, independently predict risk of MI in PLWH3,4. Additionally, both HIV and antiretroviral therapy (ART) effect risk through multiple mechanisms5,6. ART has been associated with lipid abnormalities, a risk factor for CVD 2,7. However, lipid abnormalities do not explain increased rates of MI with all antiretroviral agents, for example, abacavir8.
Dyslipidemia has not been a notable feature of integrase strand transfer inhibitors (INSTI)-based ART. Furthermore, a more favorable lipid profile is generally seen when PLWH on PI-based therapy are switched to raltegravir9-11. Data on long-term effect of INSTIs on lipid parameters as well as on the potential cardiovascular implications of improved lipid profiles on the risk of major cardiovascular events is limited. In fact, at present knowledge is limited about the effect of INSTI use on risk of CVD.
Using administrative data, we sought to estimate the effect of INSTI-based versus non-INSTI-based regimens on the risk of major adverse cardiac events (MACE) in a contemporary cohort of U.S. adults with HIV who initiated ART using methods to account for possible confounding bias due to changes in INSTI-based regimens use over time (i.e., channeling bias)12.
Methods
Data source
We used the IBM MarketScan® Commercial Database (2007-2017) and the Medicaid Multi-State Database (2010-2017) (IBM Watson Health™, Ann Arbor, MI). These databases contain individual-level healthcare information on health insurance enrollment, inpatient and outpatient diagnoses and procedures, and outpatient pharmacy-dispensed medications for commercially-insured persons from participating employers and health plans and individuals covered by Medicaid from U.S states submitting data. The study was considered not human subjects research by our institutional review board.
Study design and population
Using a retrospective cohort study design, we identified adults newly initiated on ART from Jan 1, 2008 through Dec 30, 2015. We defined new ART initiators as persons who had at least six months continuous enrollment (i.e., baseline period) prior to initiation of stable ART. Stable ART was defined as 1) being consistently in possession of a particular set of medication classes constituting an ART regimen without having a period of 60 days or more in which a component of the regimen was missing; and 2) a medication possession ratio (the percentage of time a person has access to medications) exceeding 80% for at least 180 days13. We defined an ART regimen as a regimen that contained 1) two nucleoside reverse transcriptase inhibitors (NRTIs) plus an INSTI, or a protease inhibitor (PI), or a non-nucleoside reverse transcriptase inhibitor (NNRTI), or an entry inhibitor, or a fusion inhibitor 2) a PI plus an INSTI, or a PI and an NNRTI or 3) an NRTI plus a PI and INSTI or NNRTI. We defined an ART regimen switch, and thus the termination of a stable regimen, as the addition, or removal of, a class of ART. In-class substitutions were not considered a new regimen. We excluded persons with a history of MACE events prior to the start of the first stable regimen. To allow for the potential beneficial effect of an ART regimen to manifest we excluded those who had MACE events in the first 90 days after ART initiation.
Outcomes and Covariates
All outcomes and covariates were defined using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis and procedure codes and Current Procedural Terminology, 4th Edition (CPT-4) codes as appropriate. The outcome, MACE, was a composite of MI, ischemic stroke, coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI). Supplementary Table 1 includes codes and restrictions used to define outcomes.
A wide range of baseline covariates were identified using all available claims data prior to initiation of a stable ART regimen, including standard Elixhauser comorbidities as well as other important comorbidities outlined in supplementary table 2.
Statistical analysis
We used a calendar time-specific propensity score model to account for possible confounding bias due to changes in the use of INSTI-based regimens over time (i.e., channeling bias)14,15. We used multivariable logistic regression to estimate propensity scores, with the propensity scores representing the likelihood of initiating an INSTI-based regimen. For the calendar time-specific propensity score, we segmented ART initiation into four distinct time segments (2008/2009, 2010/2011, 2012/2013, 2014/2015) and fitted separate propensity score models for each two-year time segment. The propensity score models included potential predictors of MACE outcomes and INSTI use including age (modeled using restricted quadratic splines), gender, underlying comorbidities, and CVD medications (complete list of included variables in Supplementary Table 2).
We transformed the propensity scores into inverse-probability of treatment (IPT) weights,16 computed as 1/(propensity score) for INSTI initiators and 1/(1-propensity score) for non-INSTI initiators. The data sets were then re-combined to create the full study cohort. We trimmed the propensity scores at the 0.5 and 99.5 percentiles (Supplementary figure 1). To evaluate confounding control, we assessed the balance of baseline covariates between exposure groups within the unweighted and weighted populations using standardized mean differences, with differences of 10% or greater indicating imbalance17 (supplementary table 3).
To examine the relationship between INSTI use and MACE, we used Cox proportional hazards models to estimate hazard ratios (HR) and 95% confidence intervals (CI). Individuals were followed from the date of stable ART regimen until the first occurrence of any MACE outcome or a censoring event. A censoring event was considered the earliest of 90 days post-ART regimen switch, health plan disenrollment, or end-of-study on December 31, 2017. Censoring did not occur immediately at regimen switch but rather 90 days later, as events in this timeframe could potentially be related to the index ART regimen.
Sensitivity analyses
We conducted a sensitivity analysis in which a binary variable indicating use of abacavir in the first stable regimen was included in the model.
Results
Between Jan 1, 2008 through Dec 30, 2015, we identified 20,242 new ART initiators, including 5,069 (25%) initiators of INSTI-based regimens (raltegravir 1,658 (32.7%), elvitegravir 2,475 (48.8%), and dolutegravir 936 (18.5%)). In accordance with guideline changes, the annual proportion initiating INSTI-based regimes increased significantly over time, from 4.3% in 2008 to 60.6% in 2015 (Supplementary figure 2).
Of the 15,173 non-INSTI-based ART initiators, 11,080 (73.0%) initiated NNRTIs and 4,096 (27.0%) initiated PIs. Abacavir was included in 853 (16.8%) in the INSTI group and 978 (6.5%) in the non-INSTI group. Tenofovir was included in 4,146 (81.8%) in the INSTI group and 13,950 (91.9%) in the non-INSTI group. Tenofovir disoproxil fumarate accounted for 99.6% of tenofovir use.
Overall, the median age of new ART initiators was 40.5 (interquartile range (IQR) 32, 49) years, 79.2% were male, and 16.4% were Medicaid-insured. Comorbidities were common, including hypertension (2,368 (11.6%)) and diabetes mellitus (1,141 (5.6%)). Almost 20% were receiving lipid lowering agents (4,028) and 3,648 (17.9%) had documented tobacco use. The prevalence of comorbidities was generally higher in the INSTI initiators (Table 1). Of note, the difference in the proportion of INSTI-initiators with a CVD risk factor (composite of hypertension, diabetes, tobacco and lipid lowering agent use) was greater in the earlier study period (2008-2012) compared to the later period (2013-2016). Among INSTI users, 51.6% had one or more CVD risk factors compared to 45% among non-INSTI users in the early study period, compared to 50.1% vs 51.1%, respectively, from 2013-2016 (Mantel-Haenszel p=<0.001 for the interaction with time). This is consistent with more channeling of individuals with one or more CVD risk factors to INSTIs in the earlier time period.
Table 1.
Baseline study characteristics
| Characteristics | Non-INSTI-based regimens n=15,173 |
INSTI-based regimens n=5,069 |
p-value |
|---|---|---|---|
| Age in years (median, IQR) | 41 (32, 49) | 40 (30, 49) | <0.0001 |
| Males | 11,985 (79.0%) | 4,050 (79.9%) | 0.17 |
| Medicaid insured | 2,359 (15.6%) | 1,004 (19.8%) | <0.0001 |
| Hypertension | 1,677 (11.1%) | 691 (13.6%) | <0.0001 |
| Diabetes mellitus | 806 (5.3%) | 335 (6.6%) | 0.0005 |
| Tobacco use | 2650 (17.5%) | 998 (19.7%) | 0.0004 |
| Lipid lowering therapy | 2,962 (19.5%) | 1,065 (21.0%) | 0.022 |
| Drug use | 770 (5.1%) | 352 (6.9%) | <0.0001 |
| Hepatitis B infection | 322 (2.1%) | 122 (2.4%) | 0.23 |
| Hepatitis C infection | 570 (3.8%) | 204 (4%) | 0.39 |
| Depression | 661 (4.4%) | 325 (6.4%) | <0.0001 |
INSTI, integrase strand transfer inhibitor; Unweighted standardized mean differences were <0.1, with the exceptions of tobacco use, Hepatitis C infection, and Medicaid coverage.
In the unweighted population, INSTI-based ART initiators were more likely to have documented tobacco use, and to have Medicaid coverage (Table 1). The distribution of observed covariates was well-balanced after propensity-score weighting (Supplementary Table 3).
The number of individual and composite MACE events, censoring events, and person-time at risk are presented in supplementary table 4. The median duration of follow-up was 515 (IQR 263, 882) days for the INSTI-based initiators and 524 (IQR 262, 1,026) days for the non-INSTI group. Overall MACE events occurred in 203 persons (1.0%), including 49 (1.0%) events in the INSTI group and 154 (1.0%) in the non-INSTI group (supplementary table 4). The majority of these outcomes were MI (0.4%) and stroke (0.4%), followed by PCI (0.16%) and coronary artery bypass (0.05%).
The unadjusted and weighted hazard ratios are presented in Figure 1. In the unadjusted analysis, there was no difference in risk of MACE events between initiators of INSTI or non-INSTI-based regimens (HR 1.10; 95% CI 0.80, 1.52). In the primary model, weighted using the calendar-time specific propensity score, there was a 21% decreased risk of MACE associated with INSTI-based therapy compared to non-INSTI-based therapy (HR 0.79; 95% CI 0.64, 0.96) (Figure 1).
Figure 1.
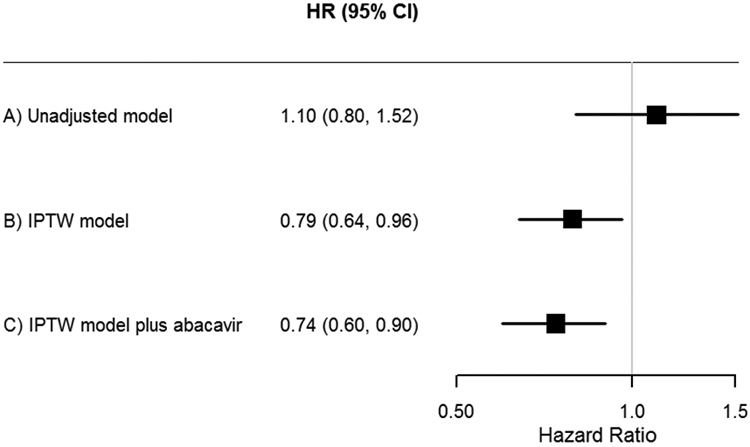
Hazard ratio estimates of overall MACE events associated with INSTI-based regimens versus non-INSTI-based regimens using calendar-time inverse probability of treatment-weighted models.
MACE, major adverse cardiac event; INSTI, integrase strand transfer inhibitor; IPTW, inverse probability of treatment weighted; PS, propensity score. (a) unadjusted model (b) IPTW model using calendar time-specific PS (primary analysis). (c) IPTW model using calendar time-specific PS including additional variable (abacavir) (sensitivity analysis).
In the sensitivity analysis, in which abacavir was added as a covariate in the calendar-time specific inverse probability of treatment-weighted Cox proportional hazards model, INSTI-based therapy was associated with 26% decreased risk of MACE compared to non-INSTI-based treatment (HR 0.74; 95% CI 0.60, 0.90) (Figure 1). In this model, abacavir was associated with 2.6 fold-increased risk of MACE (CI 2.0, 3.5.
Discussion
In this study, we observed a lower risk of CVD, as measured by MACE events, among users of INSTI-based regimens, after accounting for demographic and clinical characteristics. Insured persons on INSTI-based regimens had higher prevalence of underlying conditions associated with increased risk of CVD than non-INSTI users, which we accounted for through use of inverse treatment weighting via the propensity score.
Prescription of INSTI-based ART increased during the study timeframe reflecting changes in prescribing guidelines over the last decade18. Now recommended for PLWH initiating ART, when INSTIs initially became available, and prior to their introduction into the guidelines as the regimen of choice, they were prescribed in persons with greater prevalence of medical comorbidities due to their good side effect profile and lack of interaction with other medications. We confirmed this in our data, with a higher rate of cardiovascular risk factors observed in people initiating INSTIs from 2008-2012 compared to 2013-2016. It was therefore not only important to account for imbalance in our groups overall using propensity scores, but necessary to account for changing prescribing patterns over time to avoid bias introduced by the change in guideline recommendations for INSTI use. In our unadjusted model, there was no effect of INSTI-regimens on CVD, likely due to a higher risk of MACE events in INSTI users due to their higher prevalence of CVD-related comorbidities. However, after weighting by the propensity score to balance covariates, INSTI use was associated with significantly lower risk of MACE events.
As mentioned, INSTIs have not been associated with increased rates of cardiovascular risk factors, such as dyslipidemia. However, a number of recent studies have indicated that INSTIs, in particular dolutegravir, may be associated with increased weight gain compared to other contemporary ART regimens19-21. Although obesity is an independent risk factor for CVD, it is unclear as of yet whether INSTI-induced weight gain is a significant predictor of CVD.
This study has several limitations. Firstly, our study is not a prospective randomized controlled trial. However, the majority of data relating to CVD with ART has not come from randomized controlled trials as the follow-up duration required for randomized trials makes them cost prohibitive. Our observational study design did not involve randomization of the exposure and therefore our effect estimates are subject to potential confounding by unobserved differences between exposure groups. However, we attempted to control for confounding through restriction of the study population to ART users, adjustment for a rich set of covariates, and use of calendar-specific propensity scores to account for possible channeling bias. Second, our study used administrative data, collected for administrative and reimbursement purposes, rather than for clinical research. This may impact the generalizability of the results to those with similar demographics. In addition, our eligibility criteria was based on continuous prescriptions of the same stable regimen rather than HIV RNA test results to confirm virological suppression. Also, some important clinical factors associated with CVD (e.g., body mass index and tobacco use) are potentially subject to misclassification due to under-coded in billing data. Family history was not included due to marked under-coding, although there was no difference in the proportions coded for family history between the groups. Finally, the overall rates of MACE events observed in this study may be lower than other cohorts because of the relatively young median age.
We report the first large-scale study examining the effect of INSTIs on risk of cardiovascular disease and demonstrate that as a class, INSTI use was associated with decreased risk of MACE outcomes. As the widespread use of these drugs continues, it will be important that the results of our study are verified in other large cohorts of PLWH and with individual INSTI drugs.
Supplementary Material
Acknowledgments
Conflicts of Interest and Source of Funding: This work was supported by an unrestricted institutional grant from Merck & Co. AMB is supported in part by a grant from the National Center for Advancing Translational Sciences (NCATS), NIH under award number KL2 TR002346. The Center for Administrative Data Research is supported in part by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR002345 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) and Grant Number R24 HS19455 through the Agency for Healthcare Research and Quality (AHRQ). WGP reports grants and personal fees from Merck and Co, and personal fees from Gilead Sciences. MAO reports grants and personal fees from Pfizer, and grants from Sanofi. JAO, JS and AMB report no conflicts.
References
- 1.Alonso A, Barnes AE, Guest JL, Shah A, Shao IY, Marconi V. HIV Infection and Incidence of Cardiovascular Diseases: An Analysis of a Large Healthcare Database. J Am Heart Assoc. 2019;8(14):e012241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Halloran JA, Satchell CS, Mallon PWG. Dyslipidemia, atherosclerosis and cardiovascular disease: an increasingly important triad in an aging population living with HIV. Future Virol. 2013;8(10):1021–1034. [Google Scholar]
- 3.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petoumenos K, Reiss P, Ryom L, et al. Increased risk of cardiovascular disease (CVD) with age in HIV-positive men: a comparison of the D:A:D CVD risk equation and general population CVD risk equations. HIV Med. 2014. [DOI] [PubMed] [Google Scholar]
- 5.Tien PC, Choi AI, Zolopa AR, et al. Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. J Acquir Immune Defic Syndr. 2010;55(3):316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Worm SW, Sabin C, Weber R, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis. 2010;201(3):318–330. [DOI] [PubMed] [Google Scholar]
- 7.Ryom L, Lundgren JD, El-Sadr W, et al. Cardiovascular disease and use of contemporary protease inhibitors: the D:A:D international prospective multicohort study. Lancet HIV. 2018;5(6):e291–e300. [DOI] [PubMed] [Google Scholar]
- 8.O'Halloran JA, Dunne E, Tinago W, Denieffe S, Kenny D, Mallon PWG. Switching from abacavir to tenofovir disoproxil fumarate is associated with rises in soluble glycoprotein VI, suggesting changes in platelet-collagen interactions. AIDS. 2018;32(7):861–866. [DOI] [PubMed] [Google Scholar]
- 9.Sax PE, DeJesus E, Mills A, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet. 2012;379(9835):2439–2448. [DOI] [PubMed] [Google Scholar]
- 10.Markowitz M, Nguyen BY, Gotuzzo E, et al. Rapid and durable antiretroviral effect of the HIV-1 Integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: results of a 48-week controlled study. J Acquir Immune Defic Syndr. 2007;46(2):125–133. [DOI] [PubMed] [Google Scholar]
- 11.Quercia R, Roberts J, Martin-Carpenter L, Zala C. Comparative changes of lipid levels in treatment-naive, HIV-1-infected adults treated with dolutegravir vs. efavirenz, raltegravir, and ritonavir-boosted darunavir-based regimens over 48 weeks. Clin Drug Investig. 2015;35(3):211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneeweiss S, Gagne JJ, Glynn RJ, Ruhl M, Rassen JA. Assessing the comparative effectiveness of newly marketed medications: methodological challenges and implications for drug development. Clin Pharmacol Ther. 2011;90(6):777–790. [DOI] [PubMed] [Google Scholar]
- 13.Sikka R, Xia F, Aubert RE. Estimating medication persistency using administrative claims data. Am J Manag Care. 2005;11(7):449–457. [PubMed] [Google Scholar]
- 14.Hughes MD, Williams PL. Challenges in using observational studies to evaluate adverse effects of treatment. N Engl J Med. 2007;356(17):1705–1707. [DOI] [PubMed] [Google Scholar]
- 15.Mack CD, Glynn RJ, Brookhart MA, et al. Calendar time-specific propensity scores and comparative effectiveness research for stage III colon cancer chemotherapy. Pharmacoepidemiol Drug Saf. 2013;22(8):810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sturmer T, Wyss R, Glynn RJ, Brookhart MA. Propensity scores for confounder adjustment when assessing the effects of medical interventions using nonexperimental study designs. J Intern Med. 2014;275(6):570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed [03/24/2019]. [Google Scholar]
- 19.Norwood J, Turner M, Bofill C, et al. Brief Report: Weight Gain in Persons With HIV Switched From Efavirenz-Based to Integrase Strand Transfer Inhibitor-Based Regimens. J Acquir Immune Defic Syndr. 2017;76(5):527–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bourgi K, Rebeiro PF, Turner M, et al. Greater Weight Gain in Treatment Naive Persons Starting Dolutegravir-Based Antiretroviral Therapy. Clin Infect Dis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus Two Different Prodrugs of Tenofovir to Treat HIV. N Engl J Med. 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


