Abstract
Central centrifugal cicatricial alopecia (CCCA) is a scarring alopecia that primarily affects women of African descent. Although histologic features of CCCA have been described, the pathophysiology of this disease remains unclear. To better understand the components of CCCA pathophysiology, we evaluated the composition of the inflammatory infiltrate, the distribution of Langerhans cells, and the relationship between fibrosis and perifollicular vessel distribution. Our data indicate that CCCA is associated with a CD4-predominant T-cell infiltrate with increased Langerhans cells extending into the lower hair follicle. Fibroplasia associated with follicular scarring displaces blood vessels away from the outer root sheath epithelium. These data indicate that CCCA is an inflammatory scarring alopecia with unique pathophysiologic features that differentiate it from other lymphocytic scarring processes.
Introduction
Central Centrifugal Cicatricial Alopecia (CCCA) is a scarring alopecia of unclear etiology that primarily affects women of African descent. It classically presents with hair loss beginning at the vertex of the scalp and spreading centrifugally.1 Histolopathologic features associated with CCCA include premature desquamation of the inner root sheath, compound follicular structures (polytrichia) with perifollicular fibrosis and naked hair shafts with a foreign-body inflammatory reaction in fibrous streamers.2-9 CCCA along with lichen planopilaris (LPP) and variants (frontal fibrosing alopecia [FFA], pseudopelade of Brocq, Graham-Little syndrome), and keratosis follicularis spinulosa, are classified as a lymphocyte-predominant scarring alopecias.10, 11 Although histologic features for lymphocytic inflammatory scarring alopecias have been proposed, the composition of the inflammatory infiltrates associated with these disorders have not been thoroughly analyzed using immunohistochemistry (IHC). An IHC study of LPP has indicated that the infiltrate at the level of the infundibulum is predominantly CD8+ cytotoxic T-cells, and prior work from our group found a similar CD8 predominance in the lymphocytic infiltrates in FFA.12, 13 However, to date, the composition of the inflammatory infiltrate in CCCA remains unstudied.
The goal of this study is to characterize the composition and distribution of the inflammatory cells in CCCA. In addition, the impact of peri-follicular fibrosis was also evaluated on the blood vessel distribution to the fibrous sheath. The data obtained indicate that CCCA is a CD4-predominant inflammatory alopecia associated with alterations in the Langerhans cells distribution and vascular pattern. These data help distinguish CCCA from other lymphocyte-predominant scarring alopecias and provide new insights regarding pathogenesis and potential therapies.
Materials and Methods
The use of de-identified archived biopsy specimens was approved by the University of Pennsylvania School of Medicine Institutional Review Board under protocol #808225. We retrospectively evaluated 4-mm punch biopsy specimens obtained from 18 African-American female patients who were clinically and histologically diagnosed with CCCA. The specimens were sectioned horizontally and stained with hematoxylin and eosin (H&E), trichrome, CD3, CD4, CD8, CD1a, and CD31.
Biopsy specimens of CCCA with active inflammation and scarring were selected for study. Biopsy specimens with horizontal sections through the upper isthmus region and were preferentially selected for review.
A hair follicle was determined to be “affected” if it showed lymphocytic perifollicular inflammation and at least two of the following: 1) premature desquamation of the inner root sheath, 2) mucinous fibroplasia with or without perifollicular fibrosis, 3) eccentric thinning of the outer root sheath. An unaffected follicle lacked these features.
Each specimen was examined under light microscopy and the following information was obtained from affected and unaffected follicles: (1) the number of perifollicular and intrafollicular CD3+ and CD8+ cells and (2) the number of CD1a+ cells in the outer root sheath below the level of the sebaceous glands per unit area. The number of CD4+ cells was calculated by subtracting the number CD8+ cells from the number of CD3+ cells. The percentage of perifollicular CD3+ cells and the CD4+:CD8+ ratio were calculated for affected and unaffected follicles. Using a micrometer eyepiece, we measured the thickness of perifollicular fibrosis in sections stained with trichrome, as well as the distance between the outer root sheath and closest blood vessels and vessel clusters (CD31+). For this study, closest blood vessels were defined as a cluster of at least 3 luminal structures composed of CD31+ cells, whose distance between one another did not exceed the diameter of an individual luminal structure. A blood vessel is a CD31+ vessel with a lumen not in a cluster.
Results
Overall, we found that both affected and unaffected hair follicles in CCCA biopsy specimens had a CD3+ T-cell infiltrate that was primarily perifollicular as opposed to intrafollicular (in the outer root sheath). There was no significant difference in the ratio of perifollicular to intrafollicular CD3+ T-cells when comparing between affected and unaffected follicles. (Table 1)
Table 1. Distribution of CD3+ Cells:
A primarily perifollicular CD3+ T-cell infiltrate was seen in both affected and unaffected follicles
| Affected Follicles | Unaffected Follicles | |
|---|---|---|
| Perifollicular CD3+ cells | 92.94% (n = 39) | 92.02% (n = 12) |
| Intrafollicular CD3+ cells | 7.06% (n = 29) | 7.98% (n = 6) |
The lymphocytic infiltrate was predominantly comprised of CD4+ cells for both affected and unaffected follicles, with an average CD4:CD8 ratio of 5:1 for both groups. This CD4 predominance was seen in both the perifollicular and intrafollicular inflammatory infiltrates. The predominance was more pronounced with the perifollicular lymphocytes, with an average CD4:CD8 ratio of 5.3:1 and 4.3:1 for affected and unaffected follicles, respectively. The intrafollicular CD4:CD8 ratio was less pronounced, at 2.5:1 and 1.3:1 for affected and unaffected follicles respectively, but still tended towards a CD4 predominance in the T-cell infiltrate. (Figure 1)
Figure 1. CD4:CD8 T-cell ratios in follicles of CCCA:
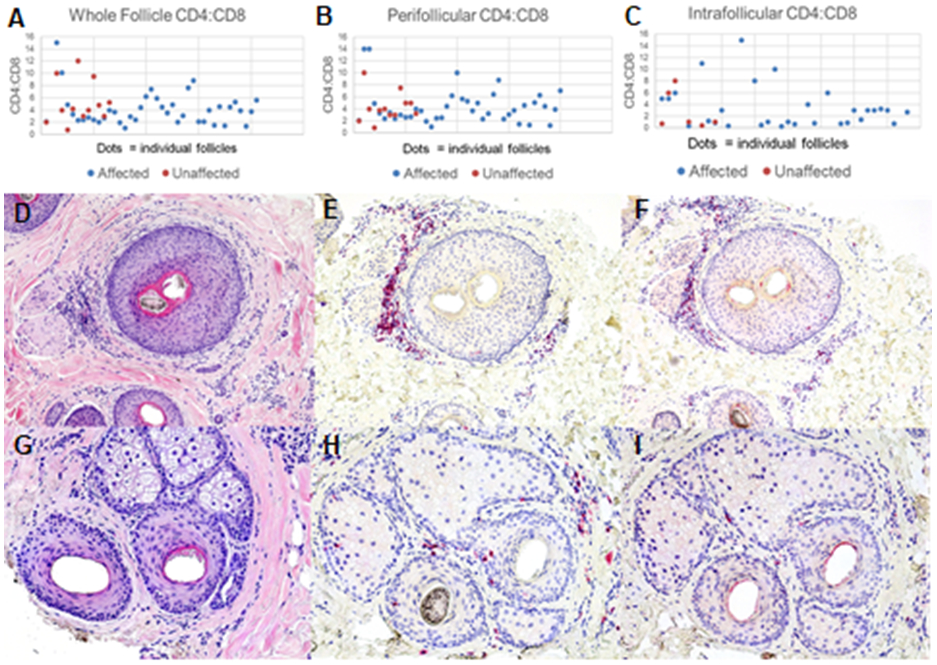
Both affected (blue dots) and unaffected (red dots) follicles showed an elevated CD4:CD8 T-cell ratio (A). The elevated CD4:CD8 ratio was seen in both the perifollicular (B) and intrafollicular (C) infiltrates of these follicles. A representative affected follicle is shown on H&E (D), as well as CD3 (E), and CD8 (F) immunostaining, also demonstrating a CD4 T-cell predominance. This is also seen in a representative unaffected follicle (G: H&E, H: CD3, I: CD8). Photomicrographs 100x, D and G-H&E, E, F, H and I-hematoxylin.
CD1a+ cells were present in higher numbers within affected follicles than unaffected follicles, with an average of 4.0 CD1a+ cells in and around affected follicles vs 1.3 in and around unaffected follicles. The ratio of CD1a+ to CD3+ cells was also increased in affected follicles as opposed to unaffected follicles, at a ratio of 0.11 vs 0.01. (Table 2 and Figure 2)
Table 2. CD1a+ Cells:
A higher number of CD1a+ cells was seen in affected versus unaffected follicles. Affected follicles also demonstrated a higher CD1a:CD3 cell ratio.
| Affected Follicles | Unaffected Follicles | |
|---|---|---|
| Average CD1a+ Overall | 4.00 (n = 14) | 1.54 (n = 14) |
| Average CD1a+:CD3+ | 0.108 (n = 14) | 0.009 (n = 14) |
Figure 2: CD1a+ Cells Associated with Affected Follicles:
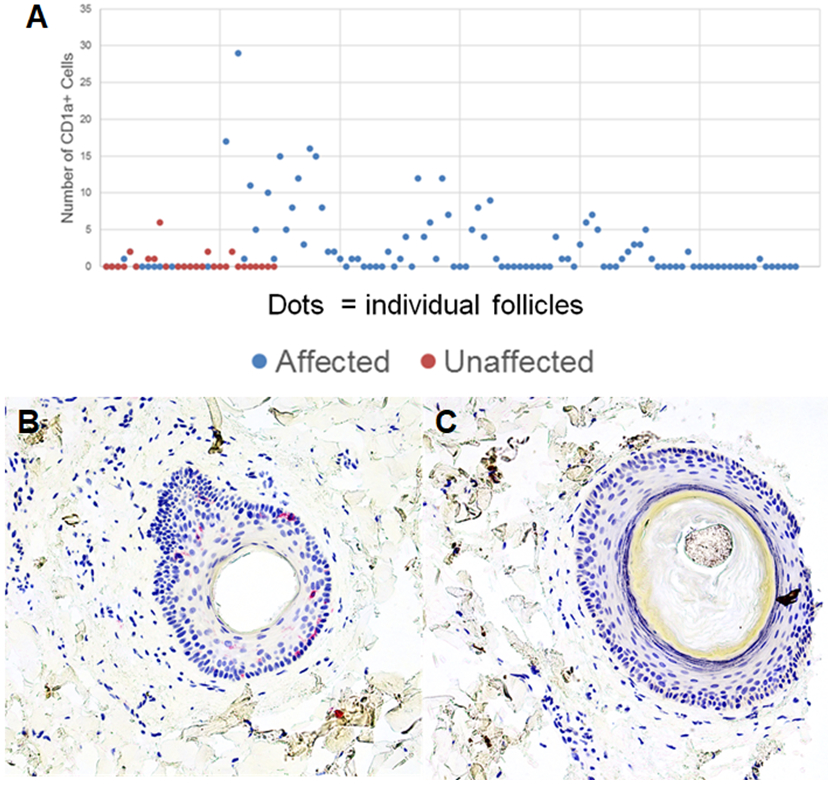
Affected and unaffected follicles were evaluated for the number of CD1a+ cells in the outer root sheath. Affected follicles have a higher number of CD1a+ cells than unaffected follicles (A). CD1a immunostaining of representative affected and unaffected follicle is seen in panels B and C, respectively. Photomicrographs 200x, B and C-hematoxylin.
Blood vessels and CD31+ cells were located at a similar average distance from affected and unaffected follicles, at an average of 95μm and 90μm, respectively. However, blood vessel clusters were located at a farther distance from the outer root sheath in affected follicles, at an average length of 97.13 μm, as compared to unaffected follicles, at 64.51 μm. (Figure 3)
Figure 3. Average Blood Vessel Cluster Distance from Affected and Unaffected Follicles:
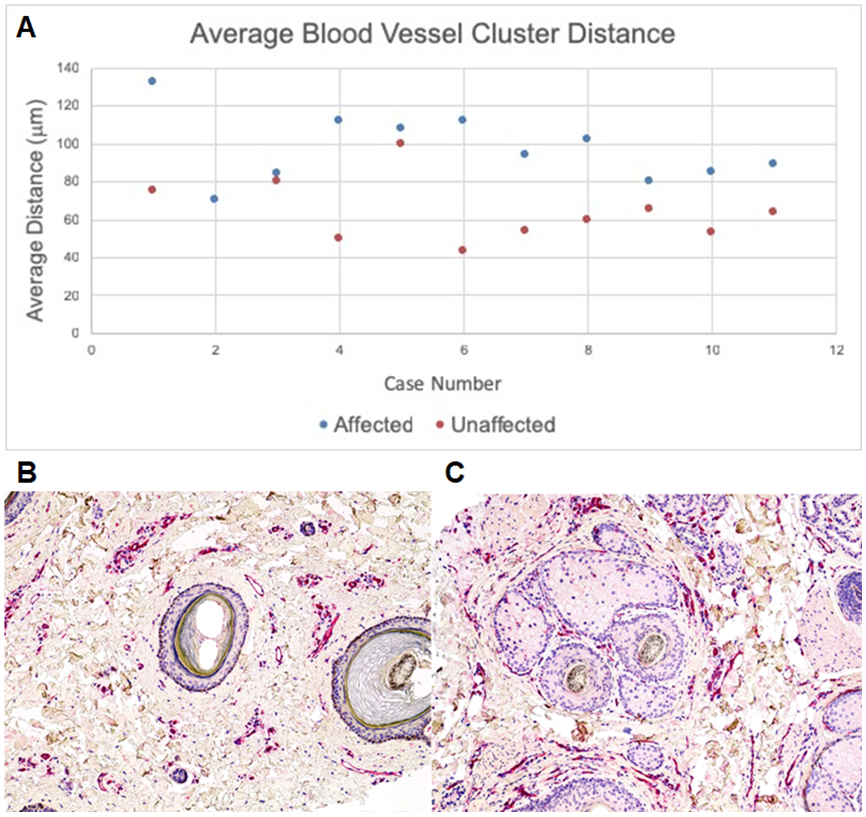
Blood vessel clusters were on average further away from the outer root sheath in affected follicles than unaffected follicles (A). CD31+ immunostaining of a respective affected (B) and unaffected follicle (C) demonstrate the visibly appreciable difference in blood vessel cluster distance.
Photomicrographs 100x, B and C-hematoxylin.
It was also observed that affected hair follicles demonstrating mucinous fibroplasia were noted to have an increased number of scattered, perifollicular CD31+ cells as opposed to unaffected and affected hair follicles with perifollicular lamellar fibrosis. (Figure 4)
Figure 4. CD31 staining in follicles with Mucinous Fibroplasia:
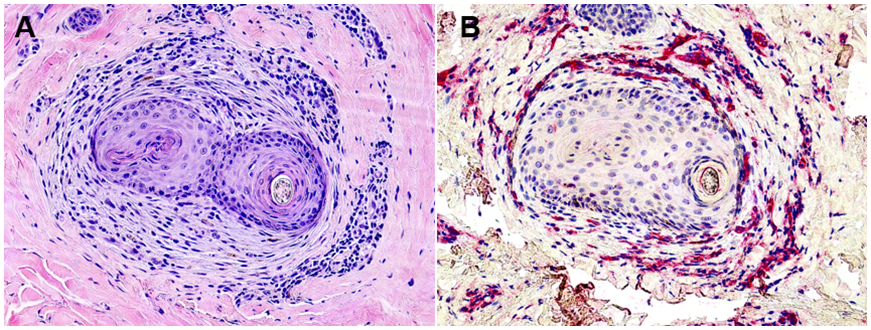
Affected follicles showing mucinous fibroplasia (A) demonstrated an increased number of single vessels and solitary scattered CD31+ cells (B). Photomicrographs 100x, A-H&E and B-hematoxylin.
The average width of the perifollicular fibrosis around affected hair follicles was 104 μm.
Discussion
CCCA is a lymphocytic scarring alopecia that is likely associated with environmental and genetic etiologies. A recent study demonstrated that mutations in the PADI3 gene may be associated with CCCA.14 The PADI3 gene is a peptidyl arginine deiminase that converts arginine into citrulline and plays an important role in hair shaft structure and inner root sheath (IRS) biology. Aberrant PADI3 function may be related to premature desquamation of the IRS which has been noted to be an early feature of CCCA histopathology.6
Overall, these data show that CCCA is associated with a CD4-predominant lymphocytic inflammatory infiltrate. This contrasts with both LPP and FFA, which have been shown to have CD8-predominant lymphocytic infiltrates 13,14. Evaluation of the T-cell infiltrate may be a useful way to distinguish CCCA from LPP and FFA in some cases when histologic features alone cannot be used to definitely distinguish between them.11-13
Although some studies have described relatively decreased numbers of CD8+ T lymphocytes in biopsy specimens showing scarring alopecia.15 These studies contained a mixture of biopsy specimens of LPP, CCCA, chronic cutaneous lupus erythematosus, and other causes of scarring alopecia at relatively low numbers, which could explain why a clear preponderance of CD8+ cells was not detected in any specific type of alopecia.
Our findings additionally highlighted that affected follicles had a higher number of CD1a+ Langerhans cells (LCs), as opposed to unaffected follicles. This would fit with the hypotheses of previous studies of scarring alopecia, specifically that an increased number of LCs localizing to the hair follicle, and specifically to the area of the bulge, could promote antigen presentation and future immunological attack of hair stem cells leading to subsequent follicular scarring. Other studies have similarly found that LCs are elevated in other forms of scarring alopecia, such as LPP, pointing to a similar destructive mechanism.
The average distance of blood vessels and CD31+ cells was not significantly different between affected and unaffected follicles; however, a difference was seen in the average distance of clusters of small vessels when comparing affected and unaffected follicles. This discrepancy could be explained by our observation that affected follicles showing mucinous fibroplasia, an early finding in the scarring process, showed an increased number of scattered small vessels and single CD31+ cells. In CCCA, the mucinous fibroplasia and perifollicular fibrosis may disrupt and fragment blood vessels in the fibrous sheath, leaving only small clusters of vessels more distant to the keratinocytes in the outer root sheath. This is represented graphically in figure 5.
Figure 5. Pictoral representation of changes in blood vessels surrounding CCCA follicles.
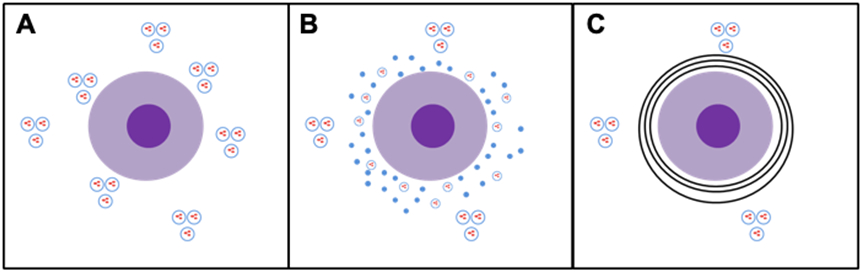
Unaffected follicles demonstrate a normal density of blood vessels clustered both close to and further from the follicle (A). As the follicle shows changes of mucinous fibroplasia, an increased number of single vessels and scattered solitary endothelial cells are seen (B). Once the follicle is fully affected and demonstrates fibrosis, only more distantly located blood vessel clusters remain (C).
Together, the data presented support a pathophysiologic mechanism of follicular destruction in CCCA that involves CD4+ T-cells and LCs. LCs localizing to affected follicles may respond to some form of follicular injury and sustain an inflammatory reaction involving CD4+ T-cells that ultimately leads to destruction of hair stem cells and their niche. This inflammatory infiltrate also promotes perifollicular mucinous fibroplasia and fibrosis that disrupts the follicular blood supply in the fibrous sheath likely leading to increased stress on follicular keratinocytes. It will be important to determine what recruits LCs to affected follicles in CCCA.
References:
- 1.Olsen EA, Callender V, McMichael A, et al. Central hair loss in African American women: incidence and potential risk factors. Journal of the American Academy of Dermatology. 2011;64(2):245–252. [DOI] [PubMed] [Google Scholar]
- 2.Callender VD, Wright DR, Davis EC, Sperling LC. Hair breakage as a presenting sign of early or occult central centrifugal cicatricial alopecia: clinicopathologic findings in 9 patients. Archives of Dermatology. 2012;148(9):1047–1052. [DOI] [PubMed] [Google Scholar]
- 3.Miteva M. A comprehensive approach to hair pathology of horizontal sections. The American Journal of Dermatopathology. 2013;35(5):529–540. [DOI] [PubMed] [Google Scholar]
- 4.Miteva M, Torres F, Tosti A. The 'eyes' or 'goggles' as a clue to the histopathological diagnosis of primary lymphocytic cicatricial alopecia. The British Journal of Dermatology. 2012;166(2):454–455. [DOI] [PubMed] [Google Scholar]
- 5.Miteva M, Tosti A. 'A detective look' at hair biopsies from African-American patients. The British Journal of Dermatology. 2012;166(6):1289–1294. [DOI] [PubMed] [Google Scholar]
- 6.Sperling LC, Sau P. The follicular degeneration syndrome in black patients. 'Hot comb alopecia' revisited and revised. Archives of Dermatology. 1992;128(1):68–74. [PubMed] [Google Scholar]
- 7.Bolduc C, Sperling LC, Shapiro J. Primary cicatricial alopecia: Other lymphocytic primary cicatricial alopecias and neutrophilic and mixed primary cicatricial alopecias. Journal of the American Academy of Dermatology. 2016;75(6):1101–1117. [DOI] [PubMed] [Google Scholar]
- 8.Herskovitz I, Miteva M. Central centrifugal cicatricial alopecia: challenges and solutions. Clinical, Cosmetic and Investigational Dermatology. 2016;9:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar UM, Yelikar BR. The spectrum of histopathological lesions in scarring alopecia: a prospective study. J Clin Diagn Res. 2013;7(7):1372–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olsen EA, Bergfeld WF, Cotsarelis G, et al. Summary of North American Hair Research Society (NAHRS)-sponsored Workshop on Cicatricial Alopecia, Duke University Medical Center, February 10 and 11, 2001. Journal of the American Academy of Dermatology. 2003;48(1):103–110. [DOI] [PubMed] [Google Scholar]
- 11.Galvez-Canseco A, Sperling L. Lichen planopilaris and frontal fibrosing alopecia cannot be differentiated by histopathology. Journal of Cutaneous Pathology. 2018;45(5):313–317. [DOI] [PubMed] [Google Scholar]
- 12.Mobini N, Tam S, Kamino H. Possible role of the bulge region in the pathogenesis of inflammatory scarring alopecia: lichen planopilaris as the prototype. Journal of Cutaneous Pathology. 2005;32(10):675–679. [DOI] [PubMed] [Google Scholar]
- 13.Ma SA, Imadojemu S, Beer K, Seykora JT. Inflammatory features of frontal fibrosing alopecia. Journal of Cutaneous Pathology. 2017;44(8):672–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malki L, Sarig O, Romano M-T, et al. Variant PADI3 in Central Centrifugal Cicatricial Alopecia. The New England journal of medicine. 2019;380(9):833–841. [DOI] [PubMed] [Google Scholar]
- 15.Pozdnyakova O, Mahalingam M. Involvement of the bulge region in primary scarring alopecia. J Cutan Pathol. October 2008;35(10):922–925. [DOI] [PubMed] [Google Scholar]


