Abstract
Cutaneous squamous cell carcinoma (cSCC) is the second most common form of skin cancer and is associated with cumulative UV-exposure. Studies have shown that prolonged voriconazole use promotes cSCC formation; however, the biological mechanisms responsible for the increased incidence remain unclear. Here, we show that voriconazole directly increases oxidative stress in human keratinocytes and promotes UV-induced DNA damage as determined by comet assay, 8-oxo-guanine immunofluorescence and mass spectrometry. Voriconazole treatment of human keratinocytes potentiates UV-induced apoptosis and activation of the p38 MAP kinase and 53BP1 UV-stress response pathways. The p38 MAP kinase activation promoted by voriconazole exposure can be mitigated by pretreating keratinocytes with N-acetylcysteine.Voriconazole increases oxidative stress in keratinocytes by directly inhibiting catalase leading to lower intracellular NADPH levels and the triazole moieties in voriconazole are critical for inhibiting catalase. Furthermore, voriconazole is shown to promote UV-induced dysplasia in an in vivo model. Together, these data demonstrate that voriconazole potentiates oxidative stress in UV-irradiated keratinocytes through catalase inhibition. Use of antioxidants may mitigate the pro-oncogenic effects of voriconazole.
Keywords: Voriconazole, oxidative stress, squamous cell carcincoma, triazole antifungal agents
Introduction
UV-induced skin cancer is the most common human cancer worldwide with an estimated 5.4 million cases in the United States (US) alone (1). Of these cases, cutaneous squamous cell carcinoma (cSCC) is second most common with approximately 670,000 lesions per year; incidence is expected to reach epidemic proportions because they frequently occur in the aged and immunosuppressed populations which are projected to grow (1). Within the immunosuppressed population, organ transplant recipients (OTRs) have a particularly high incidence of cSCC approaching a 60-fold increased risk of developing cSCCs. cSCCs in OTRs commonly present as multiple lesions with significant morbidity, which will increase with increasing numbers of OTRs (2-5).
OTRs typically need immunosuppressive medications, which can require prophylaxis against life-threatening infections such as aspergillosis. Approved by the FDA in 2002, voriconazole (Vcz) is a second generation broad spectrum, triazole anti-fungal agent effective in prophylaxis and treatment of aspergillosis, candidemia in non-neutropenic patients, and fusarium species fungal organisms (6-9). Vcz has become first-line therapy for invasive aspergillosis because it does not induce potentially fatal multi-organ toxicity associated with other systemic antifungal agents such as amphotericin. Due to its excellent anti-fungal spectrum and patient tolerance, Vcz is widely used for long-term prophylaxis in susceptible immunosuppressed populations, including transplant patients (10).
In 2010, chronic Vcz therapy was associated with an increased incidence of invasive cSCC (11-16). Although chronic immunosuppression, age, and cumulative UV exposure promote cSCC development, Vcz was identified as an independent risk factor for cSCC, especially in transplant populations (17-23). The short duration of immunosuppression prior to the appearance of cSCC, young age of onset, location of cSCC in sun-exposed skin, and frequency of cSCC tumors, all support a role for Vcz in promoting UV-induced carcinogenesis.
Although there is a growing body of epidemiologic data, little is known regarding the biological mechanisms that Vcz exploits to drive UV-induced cSCC formation. Clinically, cSCCs in Vcz-treated patients develop in sun-exposed sites, indicating that both UV radiation and Vcz are required to promote tumor formation. One study has suggested that the N-oxide metabolite of Vcz when exposed to UVB produces a photo-product, which in addition to the N-oxide metabolite, can absorb UVA and promote oxidative stress, potentially leading to DNA damage and an increased incidence of cSCC (24). However, the photo-product has yet to be identified (24). Also, this study suggests that the parental drug does not promote skin cancer formation.
In contrast, the data presented demonstrate that Vcz has direct effects on human keratinocytes and on skin in an in vivo model. Vcz decreases keratinocyte proliferation and increases UV-induced DNA-damage and apoptosis. We show that Vcz and voriconazole N-oxide (Vcn) inhibit catalase, a key anti-oxidative enzyme, resulting in higher levels of intracellular reactive oxygen species (ROS), evidenced by elevated 8-deoxoguanine (8-oxodG) levels in keratinocytes. Vcz promotes intracellular oxidative stress by decreasing cellular levels of NADPH and activates p38 MAP kinases and p53BP1 UV-induced stress responses in human keratinocytes. Antifungal agents lacking triazole moieties, such as terbinafine, do not inhibit catalase.
The data also show that Vcz selectively promotes ROS-mediated DNA-damage but not levels of UVB-induced cyclopyrimidine dimer formation or its repair. UV-dependent oxidative stress responses in keratinocytes promoted by Vcz can be inhibited by pre-treating keratinocytes with the antioxidant N-acetylcysteine. In vivo experiments using the K14-Fyn Y528F murine skin cancer model demonstrate that Vcz promotes formation of UV-induced precancerous lesions resembling actinic keratoses. Collectively, these data define a new mechanism where Vcz, as well as Vcn, inhibit catalase, raising intracellular levels of UV-associated oxidative stress and DNA damage in keratinocytes to promote skin carcinogenesis. Our data indicate that use of antioxidants may minimize the risk of Vcz-induced cSCCs in patients.
Methods
Cell culture and Vcz treatment:
Primary cultures of human keratinocytes (PHKs) were obtained from neonatal foreskins through the Penn Dermatology Skin Biology and Diseases Resource-based Center Core B. Additional details are provided in supplemental data.
UV irradiation:
PHKs were washed in HBSS lacking phenol red and then exposed to 25 mJ/cm2 UV irradiation supplied by FS20 T12 lamps filtered with cellulose triacetate to remove wavelengths below 290 nm (51). Spectral output is described in supplemental data.
Comet assay:
UV-induced DNA damage was evaluated using the Trevigen Comet Assay™ ESII electrophoresis system (Trevigen Inc., Gaithersburg, MD). Additional details are in supplemental data.
Antibodies:
Information regarding antibodies used for quantitative western blotting and DNA damage analysis are provided in supplemental data.
Western blotting:
Protein lysates as previously described (52) were analyzed using automated capillary electrophoresis on a Wes™ (Protein Simple, San Jose, CA) with up to 1 μg of protein loaded per capillary at a concentration of 0.2 μg/μl.
CPD quantification assay:
Cyclobutane pyrimidine dimers (CPD) were quantified based on an assay previously described (53). Details provided in supplemental data.
Immunohistochemistry/Immunofluorescence:
Standard techniques were used with details provided in supplemental data.
NADPH assay:
A detailed description of this assay is provided in supplemental data.
8-oxo-dG quantification by liquid chromatography and mass spectrometry:
Genomic DNA isolation and the LC-MS were performed as previously described (37, 54). Additional details provided in supplemental data.
Catalase assay:
Purified catalase was obtained from the Amplex Red Catalase Assay Kit (ThermoFisher) and the assay is detailed in supplemental data.
N-acetyl-cysteine rescue assay:
Details of N-acetylcysteine rescue experimenta are provided in supplemental data.
In vivo UV experiments using K14-Fyn Y528F mice:
K14-Fyn Y528F mice were treated with Vcz and UV irradiated as described in supplemental data.
Statistical analysis:
p values for pair-wise analysis of data sets with a single experimental variable were performed using a T-test for the means.
Results
Voriconazole inhibits growth and promotes UV-induced apoptosis in human keratinocytes:
To determine the impact of Vcz on PHK growth, cells were exposed to vehicle only or 25 μM of either Vcz or fluconazole (Fcz) for up to 6 days. Fcz is a triazole anti-fungal agent similar to Vcz, but is not known to be associated with an increased risk of cSCC. A concentration of 25 μM was selected as this is a serum concentration achieved during treatment for fungal infections (25). PHKs exposed to 25 μM Vcz with and without UV demonstrated decreased cell growth that was statistically significant five days post-UV exposure compared with vehicle-treated cells (p <0.00001). Although Fcz exposure also inhibited cell growth compared to vehicle-treated cells (p = 0.009), the magnitude of growth inhibition induced by Vcz was more significant than Fcz (p = 0.00018) (Fig. 1A). The data also indicate that Vcz and Vcz + UV inhibit PHK growth; but inhibition is greater with Vcz +UV at day 3 (p = 0.0001), suggesting that growth suppression by Vcz and UV may be cooperative. Inhibited keratinocyte growth can be associated with DNA damage, impaired nucleotide synthesis, or senescence suggesting potential biological effects of Vcz (26, 27). Fcz-treated cells manifested slower growth compared to control cells albeit to a lesser degree than Vcz-treated PHKs; this observation may be related to the lack of cSCC induction seen in patients.
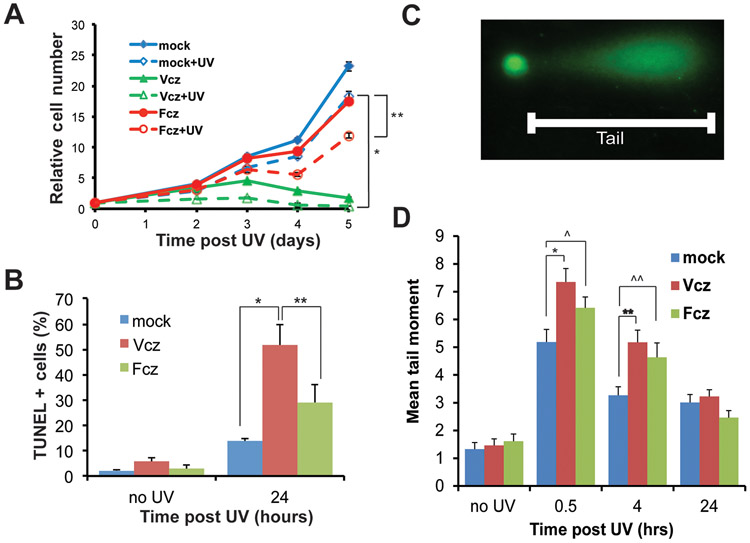
Figure 1. Voriconazole inhibits growth and promotes UV-induced apoptosis and DNA damage of primary human keratinocytes (PHKs) .
PHKs were cultured with 25 μM Voriconazole (Vcz), 25 μM Fluconazole (Fcz), or vehicle for 24 hours, then subjected to UV irradiation (25 mJ/cm2). A) Cell numbers were assayed and normalized to 1 at time 0. At five days post-UV, mock vs Vcz, * p < 0.00001 and mock vs Fcz, ** p = 0.009. Vcz vs Vcz + UV. p = 0.0001 B) At 24 hours post-UV, control and irradiated PHKs were subjected to TUNEL staining and the percentage of positive cells was determined, Vcz vs. mock p < 0.00001, Fcz vs. mock p = 0.019. C) Representative ‘comet tail’ consisting of fragmented DNA which was quantitated as described. D) Vcz and Fcz induce significantly larger tail moments at 0.5 and 4 hours post-UV. Mock vs Vcz at 0.5 hours, *p = 0.002; mock vs Vcz at 4 hours, **p = 0.0004. Mock vz Fcz at 0.5 hours, ^p = 0.0466; mock vs Fcz at 4 hours, ^^p = .0224. Number of nuclei evaluated per condition at time points shown in Table S1. Error bars represent SEM. N=3.
In parallel experiments, PHKs were exposed to 25 mJ/cm2 of UV radiation and the percentage of TUNEL positive cells was determined. As expected, UV exposure increased the percentage of TUNEL positive cells in all conditions (Fig. 1B). However, the combination of Vcz and UV irradiation markedly increased the percentage of TUNEL positive cells (51.8 +/− 8.2 %) in keratinocytes compared to UV alone (13.7+/− 1.2%) (p<0.00001). Although UV-induced apoptosis typically is considered an anti-oncogenic mechanism, it can also serve as a barometer for the level of DNA damage by sublethal activation of the caspase cascade in a larger numbers of surviving cells (28). These data suggest that Vcz-treated PHKs incur higher levels of UV-induced cellular damage resulting in more apoptosis. Fcz exposure and UV irradiation also increased the percentage of TUNEL positive cells (29.1 +/− 7.0) compared to control cells (p= 0.019) but, again, at lower levels than Vcz, similar to the growth inhibition data in Fig. 1A.
Voriconazole promotes UV-induced DNA damage:
To determine if Vcz promotes UV-induced DNA damage, the cellular levels of DNA strand breaks were determined using the comet assay. PHKs were cultured with Vcz (25 μM) for 48 hours and assessed for DNA damage at various time points after exposure to 25 mJ/cm2 UV. After UV exposure, Vcz treatment induced significantly higher levels of DNA strand breaks compared to controls (Fig 1 C and D). The level of DNA strand breaks was similar for all experimental conditions in the absence of UV, indicating that Vcz alone does not promote DNA strand breaks under assay conditions. To address whether the increased DNA damage is specific to Vcz, parallel cultures were treated with Fcz (25 μM) for 48 hours and exposed to UV. The data show that Fcz, like Vcz, promotes UV-dependent DNA strand breaks to similar levels as Vcz under the same experimental conditions (Fig 1D). The rate of decreasing ‘tail moments’ over time was not siginificantly different between the Vcz and Fcz conditions (−0.15 vs −0.16) suggesting that DNA repair activity did not differ between the two conditions.
Voriconazole promotes UV-induced stress responses and Src kinase activation:
To quantify UV-induced stress responses, phospho-p38 MAPK (pp38 MAPK) levels were measured by performing quantitative western blotting on cytoplasmic protein lysates from treated PHK cultures. Phosphorylation of p38MAPK is an early UV-induced response in PHKs that is proportional to the level of UV-induced stress (29). The data indicate that Vcz-treated PHKs demonstrate enhanced UV-induced activation of p38MAPK at 4 hours post-UV (Figs 2A and B). While pp38MAPK levels returned to baseline at 24 hours post-UV in control cells, Vcz-treated cells showed persistent activation of p38MAPK. These data support the conclusion that Vcz-treatment potentiates UV-induced stress signaling with activation of the p38 UV-activated stress response pathway in PHKs.
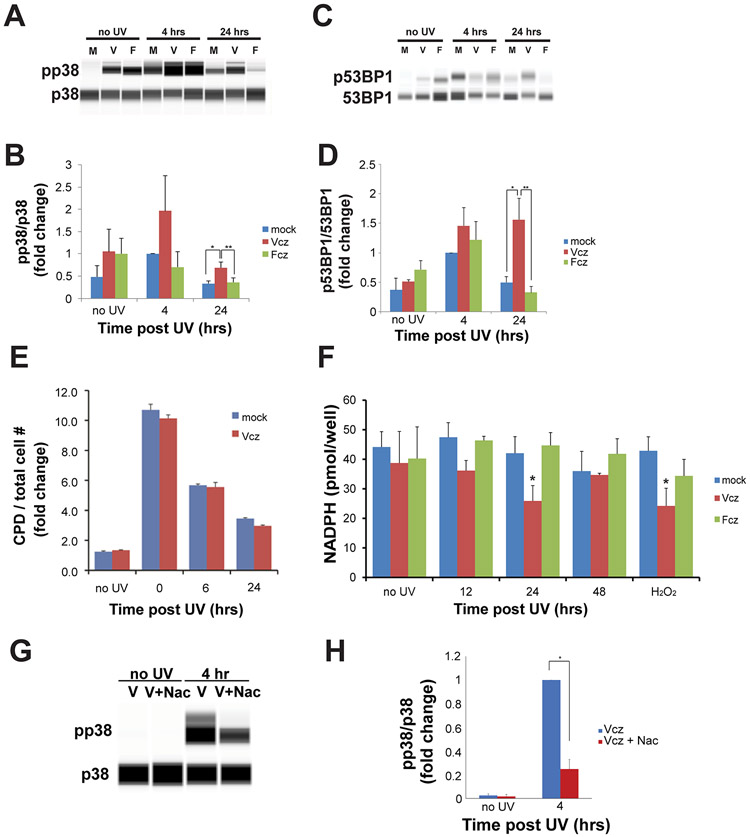
Figure 2. Voriconazole activates p38MAPK and p53BP1 in a UV-dependent manner but does not alter CPD formation. Voriconazole reduces cellular NADPH levels post-UV and activation of p38MAPK is inhibited by N-acetylcysteine.
PHKs were incubated with Vcz, Fcz, or vehicle for 24 hours; then, irradiated with UV as in Fig 1. Protein lysates were collected prior to UV, 4, or 24 hours after UV exposure. p38, pp38, 53BP1, and p53BP1 levels were determined by quantitative western blot analysis. A) Representative quantitative western blot for pp38 and p38. B) Graphical representation of pp38/p38 levels, normalized to mock at 4 hours. Error bars represent SEM. N = 3. Mock vs. Vcz at 24 hours, *p = .04; mock vs. Fcz at 24 hours, **p = .04. C) Representative quantitative western blot for p53BP1 and 53BP1. D) Graphical representative of p53BP1/53BP1 levels, normalized to mock at 4 hours. Error bars represent SEM. N = 5. Mock vs Vcz at 24 hours, * p = 0.009 and Vcz vs Fcz at 24 hours, ** p = 0.005. E) PHKs were fixed immediately after UV exposure (0 hour), and 6 or 24 hours after UV. CPD positive cells were quantified by performing quantitative in-cell western blotting; fluorescent signals were normalized to the total cell number. CPD levels under both conditions did not differ significantly. Error bars represent SEM. N=3. F) PHKs were harvested prior to UV irradiation or at the indicated time points and assayed for levels of NADPH. Hydrogen peroxide was the positive control. Assays were performed in triplicate for three independent experiments. Error bars represent SEM. Asterisks indicate p < 0.01 compared to mock at same time point. G) PHKs were treated with Vcz (25 μM) and a subset of PHKs were also treated with Nac (5 mM) one hour prior to UV irradiation with 25 mJ/cm2. PHKs were harvested prior to UV irradiation and at four hours post-UV. Protein lysates were subjected to quantitative western blotting for p38 and pp38. Representative western blot image for pp38/p38. H) Graphical representation of pp38/p38 levels. Error bars represent SEM. N = 3. Vcz vs Vcz + Nac at 4 hours, * p = 0.0004.
In response to DNA damage, 53-binding protein 1 (53BP1) becomes phosphorylated at Ser 1778, and translocates to the nucleus to form DNA repair complexes (30, 31). If Vcz promotes UV-induced DNA damage, then keratinocytes exposed to this drug should have increased levels of nuclear p53BP1 post-UV irradiation. To test this, PHKs were cultured with either Vcz or Fcz for 48 hours prior to UV exposure, and nuclear protein extracts were collected at 0, 4, and 24 hours post-UV exposure. Quantitative western blotting of nuclear lysates for p53BP1 demonstrated increased levels of nuclear p53BP1 at 24 hours only in Vcz treated cells (Figs 2C and D). The data indicate that pretreatment of PHKs with Vcz produces a prolonged post-UV DNA-damage response associated with nuclear accumulation of p53BP1.
Quantitative western blotting for activated and total Src kinases demonstrates that keratinocytes treated with Vcz and UV have higher levels of Src kinase activation than cells treated with Vcz or UV alone (Fig. S3). These data indicate that part of the mechanism for Vcz promoting cSCC may involve activation of Src kinases.
Voriconazole does not induce or inhibit repair of UV-induced CPD:
Cyclobutane pyrimidine dimers (CPD) are DNA damage related to the higher energy UVB photons (32). The effect of Vcz and Fcz on UV-induced CPD in PHKs was determined using a quantitative in-cell western blot technique. Vcz had no effect on the level of CPD produced by 25 mJ/cm2 UVB/A (Fig 2E), and did not have a significant effect on the repair of CPD mutations post-irradiation as well (Fig 2E). These data indicate that Vcz does not promote the acquisition or impact the repair of UVB-induced CPD in PHKs. These data raise the hypothesis that Vcz may promote UVA-induced DNA damage which is associated with oxidative stress.
NADPH is decreased in Vcz-treated human keratinocytes post-UV:
UV irradiation is known to promote oxidative stress and DNA damage in keratinocytes (33, 34). We determined if Vcz would increase UV-induced oxidative stress by assaying levels of NADPH, a key reducing agent. Results show that Vcz treatment lowered levels of NADPH and increased NADP+ post-UV radiation at 24 hours to a level similar to that induced by Vcz + hydrogen peroxide treatment (Fig 2F). These data support the hypothesis that Vcz promotes intracellular keratinocyte oxidative stress by decreasing NADPH levels in a UV-dependent manner.
N-acetylcysteine pre-treatment inhibits UV-induced p38 activation by Vcz:
UVB/A induce activation of p38 in human keratinocytes through increased oxidative stress (35). The data indicate that Vcz-treatment of keratinocytes potentiates the level of UV-induced activation of p38 consistent with elevated intracellular oxidative stress. To confirm that Vcz-treatment potentiates UV-induced activation of p38 by increasing intracellular oxidative stress, we performed a rescue experiment where a subset of PHKs were pre-treated with N-acetylcysteine (Nac) and then subjected to 25 mJ/cm2 of UV radiation; protein lysates from these cells were subjected to quantitative western blotting for p38 and pp38. The western blot analysis demonstrates that pre-treatment of PHKs with Nac prior to UV irradiation decreases UV-induced activation of p38 by approximately 80% (Fig 2G and H, p = 0.0004). These data support the hypothesis that Vcz treatment of PHKs increases levels of UV-induced oxidative stress leading to higher levels of p38 activation which can be mitigated by the antioxidant Nac.
Triazole antifungal agents increase 8-oxoguanine (8-oxo-dG) levels in human keratinocytes:
Keratinocytes exposed to UV irradiation demonstrate increased 8-oxo-dG formation secondary to increased ROS, which generates a genomic G to T mutation (36). PHKs were exposed to Vcz, Fcz, or vehicle for 24 hours, and then four hours later subjected to analysis of 8-oxo-dG levels using liquid chromatography/time-of-flight mass spectrometry (LC/MS) as previously described (37). Exposure of PHKs to Vcz and Fcz raised cellular levels of 8-oxo-dG confirming that Vcz and Fcz promote oxidative stress (Fig 3A).
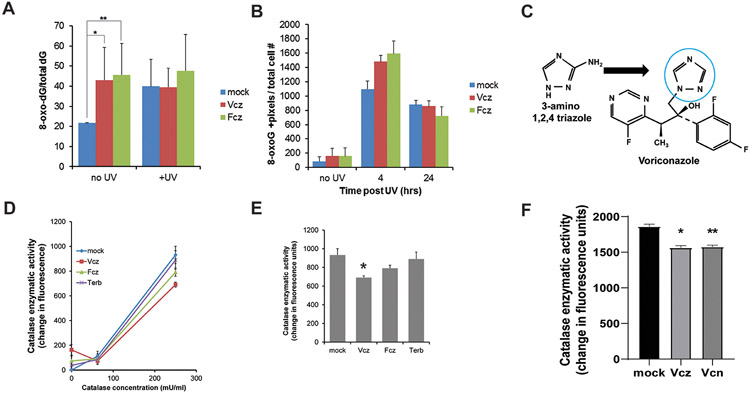
Figure 3. Triazole antifungal agents promote UV-induced 8-oxo-dG formation in vitro by inhibiting catalase.
PHKs were cultured with 25 μM Vcz, 25 μM Fcz, or vehicle for 24 hours and then UV irradiated (25 mJ/cm2). A) PHKs were assayed for cellular 8-oxo-dG using liquid chromatography/mass spectrometry. In the absence of UV, PHKs exposed to Vcz and Fcz contained higher levels of 8-oxo-dG than controls. Error bars show SEM. N=3. Mock vs. Vcz, *p = 0.04, mock vs. Fcz, **p = 0.05, mock vs mock + UV, p = 0.03. Post-UV, levels of 8-oxo-dG plateau in all conditions. B) Immunofluorescent staining for 8-oxo-dG was performed on the PHKs subjected to the experimental conditions described in A. 8-oxo-dG positive staining signal was determined using image analysis software. Error bars show SEM. N=3. At four hours, mock vs Vcz, *p = 0.01, and mock vs Fcz, **p = 0.03. C) Structural comparison of 3-amino 1,2,4 triazole, a triazole known to inhibit catalase, and the triazole moiety in voriconazole (blue circle) (38). D) Vcz inhibits catalase enzymatic activity as determined using a fluorescent in vitro assay described in methods. Fcz weakly inhibits catalase while terbinafine did not inhibit catalase significantly compared to controls. Error bars show SEM. N=3. E) Graphical representation of catalase assay data from the 250 mU/mL concentration of enzyme. Mock vs Vcz, * p = 0.01, mock vs Fcz, p = 0.07, Vcz vs Fcz, p = 0.03, mock vs terbinafine, p = 0.35. F) Graphical representation of catalase assay data from the 250 mU/mL concentration of enzyme 30 minute time point. Error bar is SEMMock vs Vcz, * p = 0.011, mock vs Vcn, **p = 0.013, Vcz vs Vcn, p = 0.38.
A parallel set of PHKs were subjected to 25 mJ/cm2 UV radiation and assayed for 8-oxoguanine. However, these cells demonstrated uniformly higher levels of 8-oxo-dG suggesting that UV irradiation of PHKs may have saturated the assay (Fig 3A). To overcome this limitation, we quantified 8-oxo-dG using immunofluorescent staining. In the absence of UV, Vcz- and Fcz-treated cells had a higher mean staining level of 8-oxo-dG; however, this difference was not found to be statistically significant (Fig 3B). At four hours post-UV irradiation, there was a significant increase in the level of 8-oxo-dG staining in all conditions, and the Vcz- and Fcz-treated cells demonstrated higher levels of 8-oxo-dG staining post-UV exposure compared to control cells (mock vs Vcz, p=0.01; mock vs Fcz, p=0.03). By twenty-four hours, the level of 8-oxo-dG staining had decreased in all conditions from the four hour timepoint (Fig 3B).
Assaying intracellular 8-oxo-dG using LC/MS confirms that both Vcz and Fcz raise intracellular 8-oxo-dG levels under culture conditions. Immunofluorescence for 8-oxo-dG demonstrates that UV irradiated Vcz- and Fcz-treated PHKs result in higher levels of 8-oxo-dG post-UV. These data support the hypothesis that Vcz and Fcz increase keratinocyte ROS-dependent DNA damage in the setting of UV irradiation.
Voriconazole but not terbinafine inhibits catalase:
Prior research in goldfish brain demonstrated that triazoles can increase cellular oxidative stress by inhibiting catalase (38). Since Vcz and Fcz are triazole antifungal agents, we hypothesized that Vcz and Fcz could increase intracellular oxidative stress by inhibiting catalase (Fig 3C). To test this hypothesis, we designed an in vitro catalase assay to determine if Vcz or Fcz inhibit catalase activity. Terbinafine, an anti-fungal agent lacking triazole structures, was a negative control. Purified, recombinant catalase was incubated with Vcz, Fcz, or terbinafine as described and catalase enzymatic activity was assayed via production of a fluorescent substrate. At the 250 mU/ml enzyme concentration, catalase enzymatic activity was inhibited by Vcz and weakly by Fcz compared to control and terbinafine (mock vs Vcz, p = 0.014, mock vs Fcz, p = 0.071, mock vs terbinafine, p = 0.353) (Figs 3D and E). These data show that Vcz directly inhibits catalase and demonstrate how Vcz increases oxidative stress. Note that Vcz was a more potent inhibitor than Fcz. In this assay, triazole moieties were required for catalase inhibition as terbinafine did not inhibit the enzyme.
Since the N-oxide metabolite of Vcz has been shown to promote oxidative stress and contains an azole moiety, we evaluated the ability of voriconazole N-oxide (Vcn) to inhibit catalase using the enzyme assay (24). Vcn inhibits catalase almost to the same degree as Vcz (Fig 3F). These data indicate that a component of the oxidative stress associated systemic administration of Vcz may be due to Vcn.
Voriconazole promotes oncogenic signaling and UV-induced dysplasia in vivo:
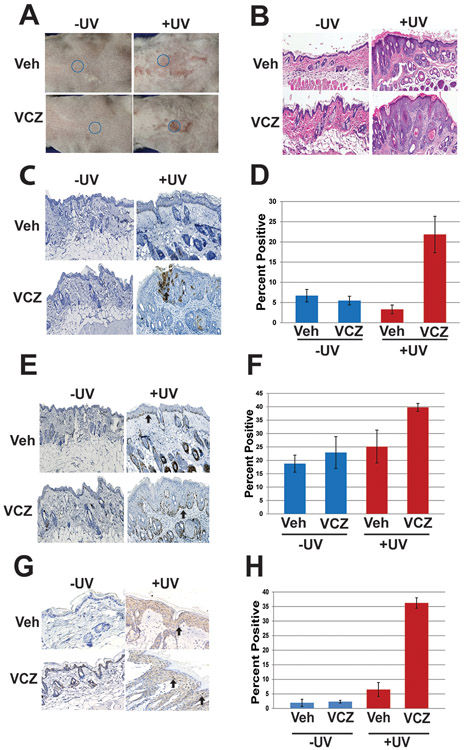
The in vitro studies on human keratinocyteys indicate that Vcz promotes UV-induced oxidative stress and ROS-dependent DNA damage. To determine if Vcz directly promotes UV-induced skin cancer in vivo, the drug was applied topically to skin of 10–12 week old K14-Fyn Y528F transgenic mice, an in vivo model of cSCC that also produces precancerous lesions (see methods for details)(39). Topical application of Vcz was necessary to determine the biological effect of the parent compound in mice since the drug is inactivated rapidly by cytochrome activity in the gut and liver (40). Parallel cohorts of mice were UV-irradiated three times per week at 200mJ/cm2 for eight weeks as described. Mice were photographed weekly, and during this time, multiple scaly plaques developed only in the UV irradiated cohorts (Fig 4 A and B). The thickest keratotic lesions were biopsied from the irradiated mice and the analogous areas were biopsied in controls (Fig. 4A and B). These biopsies revealed epidermal hyperplasia in both UV-irradiated cohorts with more prominent hyperplasia and dysplasia in the Vcz+UV cohort compared to Veh+UV cohort (Fig. 4B and Sup. Fig. 2). Biopsies from the non-irradiated Veh and Vcz cohorts appeared unremarkable and did not demonstrate any significant clinical or histologic differences at 8 weeks (Fig. 4B). To determine if Vcz promotes oncogenic signaling, immunohistochemistry for activated Src kinases was performed on representative specimens from each mouse (41). The percentage of keratinocytes with detectable membrane and/or cytoplasmic staining for activated Src kinases was most prominent in the Vcz + UV compared to the other cohorts (Fig 4C and D). Immunostaining for Ki-67 was performed to determine the proliferative index of the epidermis in representative biopsies. The epidermal Ki-67 index was approximately 1.6-fold higher in the Vcz + UV samples compared to Veh + UV (p = 0.0005; Fig 4E and F). The levels of phosphorylated 53BP1, a component of the nuclear DNA repair complex, was assessed in all biopsy specimens since UVB induced DNA damage promotes nuclear localization of the 53BP1 DNA repair protein (42). The Vcz+UV samples demonstrated higher levels of nuclear p53BP1 staining compared to Veh+UV (Fig 4G and H), and minimal nuclear staining of p53BP1 was detected in the non-irradiated specimens. Together, these data indicate that Vcz can promote UV-induced precancerous lesions by increasing oncogenic signaling, keratinocyte proliferation and epidermal dysplasia.
Figure 4. Voriconazole promotes UV-induced dysplasia in vivo.
K14-Fyn Y528F female transgenic mice were assigned to four cohorts and treated topically with vehicle, Vcz (25 μM), vehicle + UV and Vcz + UV as described, N=3 mice per cohort. Representative photographs and biopsies were taken from mice at indicated times and subjected to histology and immunohistochemistry to detect Ki-67, activated Src kinases and activated 53BP1. Representative biopsies from 8 week time point. A) Representative back skin photographs of mice; blue circle indicates biopsy site at 8 weeks selected for thickest area by palpation. B) Representative photomicrographs of skin histology showing increased hyperplasia and dysplasia in the Vcz + UV cohort. C and D) Representative photomicrographs and graphical data of immunohistochemistry for activated Src-family tyrosine kinases. Highest staining levels were detected in the Vcz + UV samples. Black arrow indicates representative positive staining. Error bars show SEM. Veh + UV vs. Vcz + UV, p = 0.02. E and F) Representative photomicrographs and graphical data of immunohistochemistry for Ki-67. The Vcz + UV cohort demonstrated the highest positive staining index. Black arrow indicates representative positive nuclear staining. Error bars show SEM. Veh + UV vs. Vcz + UV, p = 0.0005. G and H) Representative photomicrographs and graphical data of immunohistochemistry for phosphorylated 53BP1. Black arrows indicate representative positive granular staining in the nucleus. The Vcz + UV cohort demonstrated the highest levels of positive staining. Error bars show SEM. Veh vs. Vcz + UV, p = 7.9 × 10−6, Veh +UV vs Vcz + UV. p = 6.8 × 10-5.
Discussion:
cSCC, the second most common human cancer, is associated with UV exposure which promotes oxidative stress and DNA damage (1, 43, 44). Multiple epidemiologic studies show that chronic Vcz exposure is an independent risk factor for cSCC and that Vcz-induced cSCCs occur preferentially in sun-exposed skin. These observations suggest that Vcz synergizes with UV irradiation/oxidative stress to promote tumor formation (14, 45). Since Vcz/Vcn produce cSCCs in exclusively UV-exposed skin, these compounds likely require the additional oxidative stress and DNA damage provided by UVB/A to accelerate tumorigenesis.
However, the mechanism responsible for Vcz-associated cSCC remains unclear. Prior work suggests that a UVB-induced photoproduct of Vcz with Vcn promote oxidative stress and inhibits terminal differentiation of keratinocytes (24), but this implicated photoproduct has not been yet identified. In this study, we present novel data showing that the parent compound Vcz has direct effects on human and murine keratinocytes that are pro-carcinogenic in the UV setting. Vcz inhibits keratinocyte growth and this is potentiated by UV irradiation, indicating that the parent compound alters keratinocytes independent of UVB/A (Fig 1A). UV-induced apoptosis is also increased with Vcz, which is consistent with the exposed cells incurring more significant DNA damage for a given UV dose. Additional data show that Vcz potentiates UV-induced DNA damage in the comet assay (Figs 1C and D), and induces stress that is potentiated by UV irradiation leading to increased activation of p38 MAP kinases and p53BP1 (Fig 2A-D), markers of UV-induced cellular stress and DNA damage, respectively. The capacity of Vcz to promote intracellular oxidative stress was confirmed by pre-incubating keratinocytes with the antioxidant N-acetylcysteine prior to UV-irradiation, which mitigated Vcz’s impact on UV-induced activation of p38 (Figs 2G and H).
To better understand how Vcz promotes UV-induced DNA damage, we conducted experiments to determine what form of UV-induced DNA damage is promoted by Vcz. The data show that Vcz does not alter acquisition or repair of UVB-induced CPD (Fig 2E), but instead incites ROS-dependent DNA damage, reflected by elevated intracellular levels of UV-induced 8-oxo-dG determined by immunofluorescence of nuclear 8-oxo-dG. Mass spectroscopy also indicates that Vcz raises 8-oxo-dG in resting keratinocytes (Figs. 3A and B). Additional experiments also show that Vcz decreases intracellular levels of the reducing agent NADPH (Fig 3B). Collectively, these data show that Vcz directly increases oxidative stress in PHKs, which potentiates ROS-mediated, UV-induced DNA damage.
Phosphorylated 53BP1 serves as a biomarker of DNA damage since it translocates to the nucleus to form DNA repair complexes (46). The levels of nuclear p53BP1 on western blotting and number of p53BP1-positive nuclear foci correlate with DNA damage. The higher levels of p53BP1 seen on western blotting in Vcz-treated keratinocytes 24 hours post-UV indicates that these cells have higher levels of DNA damage than control or Fcz-treated cells. These data support the hypothesis that Vcz-induced inhibition of catalase is associated with elevated ROS levels and DNA damage in keratinocytes.
53BP1 has been shown to be phosphorylated on multiple, highly conserved serine and threonine residues post-UV irradiation by ATR (46). The ATR-Chk1 pathway is a key regulator of apoptosis in UV-treated keratinocytes and it will be important to determine if Vcz regulates UV-induced activation of ATR-Chk1 (47). Activation of p38 and ATR are biomarkers for UVA-induced ROS and subsequent DNA damage; the increased activation of p38 noted in Vcz-treated keratinocytes is consistent with the hypothesis that Vcz exposure promotes UV-induced, ROS-mediated cells damage in keratinocytes (48).
In order to identify the mechanism for increased oxidative stress, Vcz contains a triazole ring, and a prior study in goldfish showed that triazole-containing compounds promoted oxidative stress in the brain by inhibiting catalase activity (38). To determine if Vcz inhibited catalase activity, we designed an in vitro assay using anti-fungal agents with triazole rings such as Vcz and Fcz, and terbinafine, an anti-fungal agent lacking a triazole ring. Our data show that Vcz, and to a lesser degree Fcz, inhibited the catalytic activity of catalase whereas terbinafine did not (Figs 3 C-E). Furthremore, prior studies indicate Vcn can also promote oxidative stress, which may be related to its azole moiety. We tested this hypothesis by conducting a catalase inhibition assay using Vcz and Vcn; and Vcn is shown to inhibit catalase as efficiently as its parent compound. These data indicate that Vcz, as well as Vcn, increases intracellular oxidative stress by inhibiting catalase and that its triazole moiety may plan an important role.
Long-term Vcz use for prophylaxis is associated with increased risk of cSCC in sun-exposed skin but Fcz is not (13-16, 28). Although Fcz also contains an azole moiety, our data suggest that Fcz does not promote UV-induced cell responses and DNA damage as prominently as Vcz. Also, clinical dosing of Fcz is not typically long term so there is scant data regarding its long term effects on the skin of patients. However, a recent study shows that long term use (>28 days) of Fcz causes xerosis or alopecia in 16% of patients (49). Therefore, the shorter-term dosing of Fcz may lessen the risk of cSCCs in patients taking this drug. Moving forward, it may be prudent to conduct catalase-inhibition assays on new azole-containing antifungal agents to determine their relative potential for inducing oxidative-stress compared to Vcz.
To corroborate our in vitro findings, we tested the ability of Vcz to promote UV-induced carcinogenesis in vivo by applying the drug topically to the skin of K14-Fyn Y528F mice followed by UV irradiation. In mice, hepatic cytochromes 3A4, 2B9 and 2B19 are primarily responsible for metabolizing Vcz into the N-oxide form (Vcn) (40). Gene expression data indicates that cyp3A4 is not significantly expressed in murine P5 postnatal skin, whereas 2B9 and 2B19 are expressed at very low levels (www.hair-gel.net). These data indicate the production of Vcn from Vcz is less efficient with topical drug application than with systemic administration. Therefore, the effects of topical Vcz seen in irradiated mice are likely due to the parental compound; however, we cannot completely exclude some contribution from Vcn. Topical Vcz promoted formation of epithelial lesions with features of actinic keratoses in association with UV irradiation, which are well recognized as precancerous lesions (Figs 4 A, B and Sup Fig 2). Topical Vcz also enhanced UV-induced epithelial hyperproliferation, Src kinase activity and DNA damage as indicated by higher nuclear p53BP1 (Figs 4 C-H). Since Vcz activates Src kinases in UV-treated keratinocytes, one potential approach to preventing Vcz-induced cSCCs could be to apply topical tyrosine kinase inhibitors to sun-exposed skin (50).
Together, these new data show that Vcz increases levels of UV-induced oxidative stress and DNA damage both in vitro and in vivo. The oncogenic effects of Vcz are associated with its ability to inhibit catalase, a key anti-oxidative enzyme, and to promote oxidative stress in keratinocytes. Therefore, our findings indicate that the Vcz parent compound manifests pro-oncogenic effects independent of its N-oxide metabolites and provide new mechanistic insights regarding the promotion of cSCC associated with this drug. These observations suggest that in vitro catalase assays may be helpful in determining the relative catalase inhibitory activity of triazole antifungal agents which could help stratify the relative risk of a particular agent for developing cSCCs with long-term use. In addition, our data also signify a possible therapeutic role for antioxidant use in patients treated with long term Vcz.
Supplementary Material
Acknowledgements:
This work was supported in part by the following grants: R01 CA165836 (PI-JTS), P30-CA016520 (PI-Dang, Program Co-Director-JTS), P30 AR069589–01 (SBDRC, PI- Cotsarelis, Core Director-JTS, Co-Director of Core-TWR), R01 CA163566 (PI-TWR), P30ES013508 and P30CA016520 (PI-IAB), K08 EY025742 (PI-VL), Dermatology Foundation CDA Award to M. Gober. We thank Ms. Yerin Kweon for administrative assistance.
Footnotes
Disclosure of Potential Conflicts of Interest:
The authors declare no conflicts of interests
References:
- 1.Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol 2010: 146: 283–287. [DOI] [PubMed] [Google Scholar]
- 2.Jenni D, Hofbauer GF. Keratinocyte cancer and its precursors in organ transplant patients. Curr Probl Dermatol 2015: 46: 49–57. [DOI] [PubMed] [Google Scholar]
- 3.Sandoval M, Ortiz M, Diaz C, Majerson D, Molgo M. Cutaneous manifestations in renal transplant recipients of Santiago, Chile. Transplant Proc 2009: 41: 3752–3754. [DOI] [PubMed] [Google Scholar]
- 4.Garrett G L, Blanc P D, Boscardin J, et al. Incidence of and Risk Factors for Skin Cancer in Organ Transplant Recipients in the United States. JAMA dermatology 2017: 153: 296–303. [DOI] [PubMed] [Google Scholar]
- 5.Kim C, Cheng J, Colegio OR. Cutaneous squamous cell carcinomas in solid organ transplant recipients: emerging strategies for surveillance, staging, and treatment. Semin Oncol 2016: 43: 390–394. [DOI] [PubMed] [Google Scholar]
- 6.Arikan S, Rex JH. New agents for the treatment of systemic fungal infections--current status. Expert Opin Emerg Drugs 2002: 7: 3–32. [DOI] [PubMed] [Google Scholar]
- 7.Herbrecht R Improving the outcome of invasive aspergillosis: new diagnostic tools and new therapeutic strategies. Ann Hematol 2002: 81 Suppl 2: S52–53. [PubMed] [Google Scholar]
- 8.Muijsers RB, Goa KL, Scott LJ. Voriconazole: in the treatment of invasive aspergillosis. Drugs 2002: 62: 2655–2664; discussion 2665–2656. [DOI] [PubMed] [Google Scholar]
- 9.Walsh TJ, Pappas P, Winston DJ, et al. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N Engl J Med 2002: 346: 225–234. [DOI] [PubMed] [Google Scholar]
- 10.Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus Amphotericin B for Primary Therapy of Invasive Aspergillosis. N Engl J Med 2002: 347: 408–415. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim SF, Singer JP, Arron ST. Catastrophic squamous cell carcinoma in lung transplant patients treated with voriconazole. Dermatol Surg 2010: 36: 1752–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cowen EW, Nguyen JC, Miller DD, et al. Chronic phototoxicity and aggressive squamous cell carcinoma of the skin in children and adults during treatment with voriconazole. J Am Acad Dermatol 2010: 62: 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunel AS, Fraisse T, Lechiche C, Pinzani V, Mauboussin JM, Sotto A. Multifocal squamous cell carcinomas in an HIV-infected patient with a long-term voriconazole therapy. Aids 2008: 22: 905–906. [DOI] [PubMed] [Google Scholar]
- 14.Cowen EW, Nguyen JC, Miller DD, et al. Chronic phototoxicity and aggressive squamous cell carcinoma of the skin in children and adults during treatment with voriconazole. J Am Acad Dermatol 2010: 62: 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ibrahim SF, Singer JP, Arron ST. Catastrophic squamous cell carcinoma in lung transplant patients treated with voriconazole. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al ] 2010: 36: 1752–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanacker A, Fabre G, Van Dorpe J, Peetermans WE, Maes B. Aggressive cutaneous squamous cell carcinoma associated with prolonged voriconazole therapy in a renal transplant patient. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2008: 8: 877–880. [DOI] [PubMed] [Google Scholar]
- 17.Singer J P, Boker A, Metchnikoff C, et al. High cumulative dose exposure to voriconazole is associated with cutaneous squamous cell carcinoma in lung transplant recipients. J Heart Lung Transplant 2012: 31: 694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wojenski DJ, Bartoo GT, Merten JA, et al. Voriconazole exposure and the risk of cutaneous squamous cell carcinoma in allogeneic hematopoietic stem cell transplant patients. Transpl Infect Dis 2015: 17: 250–258. [DOI] [PubMed] [Google Scholar]
- 19.Epaulard O, Villier C, Ravaud P, et al. A multistep voriconazole-related phototoxic pathway may lead to skin carcinoma: results from a French nationwide study. Clin Infect Dis 2013: 57: e182–188. [DOI] [PubMed] [Google Scholar]
- 20.Feist A, Lee R, Osborne S, Lane J, Yung G. Increased incidence of cutaneous squamous cell carcinoma in lung transplant recipients taking long-term voriconazole. J Heart Lung Transplant 2012: 31: 1177–1181. [DOI] [PubMed] [Google Scholar]
- 21.Hamandi B, Fegbeutel C, Silveira FP, et al. Voriconazole and squamous cell carcinoma after lung transplantation: A multicenter study. Am J Transplant 2018: 18: 113–124. [DOI] [PubMed] [Google Scholar]
- 22.Kolaitis NA, Duffy E, Zhang A, et al. Voriconazole increases the risk for cutaneous squamous cell carcinoma after lung transplantation. Transpl Int 2017: 30: 41–48. [DOI] [PubMed] [Google Scholar]
- 23.Mansh M, Binstock M, Williams K, et al. Voriconazole Exposure and Risk of Cutaneous Squamous Cell Carcinoma, Aspergillus Colonization, Invasive Aspergillosis and Death in Lung Transplant Recipients. Am J Transplant 2016: 16: 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ona K, Oh DH. Voriconazole N-oxide and its ultraviolet B photoproduct sensitize keratinocytes to ultraviolet A. The British journal of dermatology 2015: 173: 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lat A, Thompson GR 3rd. Update on the optimal use of voriconazole for invasive fungal infections. Infect Drug Resist 2011: 4: 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang ES, Iwata K, Ikami K, et al. Aldose reductase in keratinocytes attenuates cellular apoptosis and senescence induced by UV radiation. Free Radic Biol Med 2011: 50: 680–688. [DOI] [PubMed] [Google Scholar]
- 27.Kuehne A, Emmert H, Soehle J, et al. Acute Activation of Oxidative Pentose Phosphate Pathway as First-Line Response to Oxidative Stress in Human Skin Cells. Mol Cell 2015: 59: 359–371. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, He Y, Li F, et al. Caspase-3 promotes genetic instability and carcinogenesis. Mol Cell 2015: 58: 284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chouinard N, Valerie K, Rouabhia M, Huot J. UVB-mediated activation of p38 mitogen-activated protein kinase enhances resistance of normal human keratinocytes to apoptosis by stabilizing cytoplasmic p53. Biochem J 2002: 365: 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rappold I, Iwabuchi K, Date T, Chen J. Tumor suppressor p53 binding protein 1 (53BP1) is involved in DNA damage-signaling pathways. The Journal of cell biology 2001: 153: 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng L, Li N, Li Y, et al. Cell cycle-dependent inhibition of 53BP1 signaling by BRCA1. Cell Discov 2015: 1: 15019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tommasi S, Denissenko MF, Pfeifer GP. Sunlight induces pyrimidine dimers preferentially at 5-methylcytosine bases. Cancer Research 1997: 57: 4727–4730. [PubMed] [Google Scholar]
- 33.Brem R, Guven M, Karran P. Oxidatively-generated damage to DNA and proteins mediated by photosensitized UVA. Free Radic Biol Med 2017: 107: 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brem R, Macpherson P, Guven M, Karran P. Oxidative stress induced by UVA photoactivation of the tryptophan UVB photoproduct 6-formylindolo[3,2-b]carbazole (FICZ) inhibits nucleotide excision repair in human cells. Scientific reports 2017: 7: 4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolfle U, Esser PR, Simon-Haarhaus B, Martin SF, Lademann J, Schempp CM. UVB-induced DNA damage, generation of reactive oxygen species, and inflammation are effectively attenuated by the flavonoid luteolin in vitro and in vivo. Free Radic Biol Med 2011: 50: 1081–1093. [DOI] [PubMed] [Google Scholar]
- 36.Hseu YC, Chou CW, Senthil Kumar KJ, et al. Ellagic acid protects human keratinocyte (HaCaT) cells against UVA-induced oxidative stress and apoptosis through the upregulation of the HO-1 and Nrf-2 antioxidant genes. Food Chem Toxicol 2012: 50: 1245–1255. [DOI] [PubMed] [Google Scholar]
- 37.Mesaros C, Arora JS, Wholer A, Vachani A, Blair I A. 8-Oxo-2’-deoxyguanosine as a biomarker of tobacco-smoking-induced oxidative stress. Free Radic Biol Med 2012: 53: 610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bagnyukova T V, Vasylkiv O Y, Storey K B, Lushchak V I. Catalase inhibition by amino triazole induces oxidative stress in goldfish brain. Brain research 2005: 1052: 180–186. [DOI] [PubMed] [Google Scholar]
- 39.Zhao L, Li W, Marshall C, et al. Srcasm inhibits Fyn-induced cutaneous carcinogenesis with modulation of Notch1 and p53. Cancer Res 2009: 69: 9439–9447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graybill JR, Najvar LK, Gonzalez GM, Hernandez S, Bocanegra R. Improving the mouse model for studying the efficacy of voriconazole. J Antimicrob Chemother 2003: 51: 1373–1376. [DOI] [PubMed] [Google Scholar]
- 41.Ayli EE, Li W, Brown TT, Witkiewicz A, Elenitsas R, Seykora JT. Activation of Src-family tyrosine kinases in hyperproliferative epidermal disorders. J Cutan Pathol 2008: 35: 273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uehara F, Miwa S, Tome Y, et al. Comparison of UVB and UVC effects on the DNA damage-response protein 53BP1 in human pancreatic cancer. J Cell Biochem 2014: 115: 1724–1728. [DOI] [PubMed] [Google Scholar]
- 43.Parekh V, Seykora JT. Cutaneous Squamous Cell Carcinoma. Clin Lab Med 2017: 37: 503–525. [DOI] [PubMed] [Google Scholar]
- 44.Schmitt J, Haufe E, Trautmann F, et al. Is UV-exposure acquired at work the most important risk factor for cutaneous squamous cell carcinoma? Results of the population-based case-control study FB-181. The British journal of dermatology 2017. [DOI] [PubMed] [Google Scholar]
- 45.Vadnerkar A, Nguyen MH, Mitsani D, et al. Voriconazole exposure and geographic location are independent risk factors for squamous cell carcinoma of the skin among lung transplant recipients. J Heart Lung Transplant 2010: 29: 1240–1244. [DOI] [PubMed] [Google Scholar]
- 46.Jowsey P, Morrice NA, Hastie CJ, McLauchlan H, Toth R, Rouse J. Characterisation of the sites of DNA damage-induced 53BP1 phosphorylation catalysed by ATM and ATR. DNA Repair (Amst) 2007: 6: 1536–1544. [DOI] [PubMed] [Google Scholar]
- 47.Heffernan TP, Kawasumi M, Blasina A, Anderes K, Conney AH, Nghiem P. ATR-Chk1 pathway inhibition promotes apoptosis after UV treatment in primary human keratinocytes: potential basis for the UV protective effects of caffeine. The Journal of investigative dermatology 2009: 129: 1805–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Girard P-M, Pozzebon M, Delacote F, Douki T, Smirnova V, Sage E. Inhibition of S-phase progression triggered by UVA-induced ROS does not require a functional DNA damage checkpoint response in mammalian cells. DNA Repair (Amst) 2008: 7: 1500–1516. [DOI] [PubMed] [Google Scholar]
- 49.Davis MR, Nguyen MH, Donnelley MA, Thompson Iii GR. Tolerability of long-term fluconazole therapy. J Antimicrob Chemother 2019: 74: 768–771. [DOI] [PubMed] [Google Scholar]
- 50.Yang X, Daifallah AEM, Shankar S, et al. Topical kinase inhibitors induce regression of cutaneous squamous cell carcinoma. Experimental dermatology 2019: 28: 609–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Learn DB, Beard J, Moloney SJ. The ultraviolet C energy emitted from FS lamps contributes significantly to the induction of human erythema and murine ear edema. Photodermatol Photoimmunol Photomed 1993: 9: 147–153. [PubMed] [Google Scholar]
- 52.Li W, Marshall C, Mei L, et al. Srcasm modulates EGF and Src-kinase signaling in keratinocytes. The Journal of biological chemistry 2005: 280: 6036–6046. [DOI] [PubMed] [Google Scholar]
- 53.Nishinaga M, Kurata R, Onishi K, Kuriyama K, Wakasugi M, Matsunaga T. Establishment of a microplate-formatted cell-based immunoassay for rapid analysis of nucleotide excision repair ability in human primary cells. Photochem Photobiol 2012: 88: 356–362. [DOI] [PubMed] [Google Scholar]
- 54.Mangal D, Vudathala D, Park JH, Lee SH, Penning TM, Blair IA. Analysis of 7,8-dihydro-8-oxo-2’-deoxyguanosine in cellular DNA during oxidative stress. Chem Res Toxicol 2009: 22: 788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Faergemann J, Zehender H, Denouel J, Millerioux L. Levels of terbinafine in plasma, stratum corneum, dermis-epidermis (without stratum corneum), sebum, hair and nails during and after 250 mg terbinafine orally once per day for four weeks. Acta Derm Venereol 1993: 73: 305–309. [DOI] [PubMed] [Google Scholar]
- 56.Billings PC, Sanzari JK, Kennedy AR, Cengel KA, Seykora JT. Comparative analysis of colorimetric staining in skin using open-source software. Experimental dermatology 2015: 24: 157–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.