To the Editor:
Recent attempts have been made to define abnormal pulmonary pressure during exercise (1). However, it is unclear whether exercise pulmonary arterial hypertension (ePAH)—defined here by 1) normal mean pulmonary artery pressure (mPAP) at rest, 2) elevation of mPAP (>30 mm Hg) with exercise, and 3) normal left ventricular filling pressures during exercise—represents a pathologic change of the pulmonary vasculature. Indeed, no resting measurement to date has revealed any anatomic or physiological abnormality in patients with ePAH.
Here, we applied positron emission tomography (PET) imaging using the 13NN-saline bolus method (2) to investigate vascular changes in subjects with ePAH. We hypothesized that pulmonary perfusion imaging conducted at rest before and after eliminating pulmonary vascular tone with inhaled nitric oxide (iNO) and hyperoxia would distinguish characteristic perfusion patterns among patients with ePAH and PAH, and control subjects. Some of the results of this study have been previously reported in the form of an abstract (3).
Methods
We studied five control subjects, four subjects with ePAH, and four subjects with PAH who underwent intravenous-bolus 13NN-saline functional PET imaging of pulmonary perfusion in the supine position, using previously described methods (2, 4, 5). Briefly, the total spatial heterogeneity of perfusion [CV2Qtotal = (SD of perfusion/average perfusion)2] was assessed at baseline and while the subjects were breathing 30 ppm iNO and oxygen (O2 + iNO) to eliminate resting vascular tone, thereby leaving structural abnormalities as the primary driver of perfusion differences among the subjects. The additive components of CV2Qtotal included the contributions of 1) the vertical (dorsoventral) gradient in perfusion (CV2Qvgrad), 2) the axial (craniocaudal) gradient in perfusion (CV2Qzgrad), and 3) the residual heterogeneity (CV2Qr). The residual heterogeneity in perfusion (CV2Qr) was calculated by subtracting the heterogeneity caused by both the vertical gradient and the axial gradient in perfusion from the total heterogeneity (CV2Qr = CV2Qtotal − CV2Qvgrad − CV2zgrad). The remaining CV2Qr was subsequently binned into contributions calculated at different image resolutions (length scales) (2, 6). Overall, small length scales starting at 10 mm correspond to variation among volumes of approximately 1 ml (5 acinar units), whereas large length scales correspond to variation among larger regions such as segments and subsegments.
We first conducted a one-way ANOVA and then tested simultaneous comparisons with multiple-hypothesis testing correction using the Tukey-Kramer method (SAS for Windows, version 9.4; SAS Institute Inc.). Statistical significance was set at P < 0.05.
Results
The baseline characteristics of the study population are displayed in Table 1. All of the subjects were nonsmokers without prior cardiovascular disease and without evidence of lung disease by high-resolution computed tomography. No subjects required oxygen therapy. Subjects with ePAH had normal resting mPAP (mean, 18 [range, 15–22] mm Hg) and pulmonary capillary wedge pressure (PCWP 9 [7–11] mm Hg), and an elevated mPAP with exercise (36 [33–38] mm Hg). Subjects with ePAH had a mean pulmonary vascular resistance [(mPAP − PCWP)/cardiac output] during exercise of 154.6 (136–168) dyn · s/cm5. According to recent European Respiratory Society standards for assessing pulmonary hemodynamics during exercise, all subjects with ePAH had a total pulmonary resistance (mPAP/cardiac output) of >3.0 (1) Wood units during exercise while maintaining a PCWP of <20 mm Hg, except for one subject who had a total pulmonary resistance of 2.3 Wood units.
Table 1.
Baseline Characteristics
| Parameter | Control (n = 5) | ePAH (n = 4) | PAH (n = 4) | P Value |
|---|---|---|---|---|
| Age, yr | 41.2 (22–60) | 53.5 (44–62) | 47.3 (22–64) | 0.52 |
| Sex, F | 1 | 2 | 3 | 0.31 |
| Race, white | 5 | 3 | 3 | 0.55 |
| Height, cm | 170.9 (160–181.6) | 169.2 (167.6–180.3) | 170.7 (167.6–174) | 0.96 |
| Weight, kg | 86 (72.6–134.6) | 88 (68–107) | 77.3 (56.2–119.7) | 0.85 |
| Years of symptoms | – | 4.2 (1–10) | 8.4 (7–10) | 0.19 |
| FEV1% predicted | 103.7 (90–117) | 87.9 (76–97) | 82.9 (66–95) | 0.12 |
| FVC, % predicted | 105.8 (100–115) | 91.2 (83–118) | 85.6 (64–110) | 0.24 |
| FEV1/FVC | 79 (70–87) | 72 (67–89) | 80 (63–86) | 0.50 |
| mPAP, rest, mm Hg | – | 18 (15–22) | 41.6 (33–63) | 0.01 |
| PVR, rest, dyn · s/cm5 | – | 122.7 (81–143) | 442 (322–548) | 0.001 |
| PCWP, rest, mm Hg | – | 9.3 (7–11) | 10 (7–16) | 0.65 |
| mPAP, exercise, mm Hg | – | 36 (33–38) | – | – |
| PVR, exercise, dyn · s/cm5 | – | 154.6 (136–168) | – | – |
| PCWP, exercise, mm Hg | – | 14.3 (11–18) | – | – |
| 6MWD, m | – | 485.2 (366–595) | 463.2 (382–670) | 0.083 |
Definition of abbreviations: 6MWD = 6-minute-walk distance; ePAH = exercise pulmonary arterial hypertension; mPAP = mean pulmonary arterial pressure; PAH = primary pulmonary arterial hypertension; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance.
Continuous variables are represented as mean (minimum–maximum). Categorical variables are represented as counts.
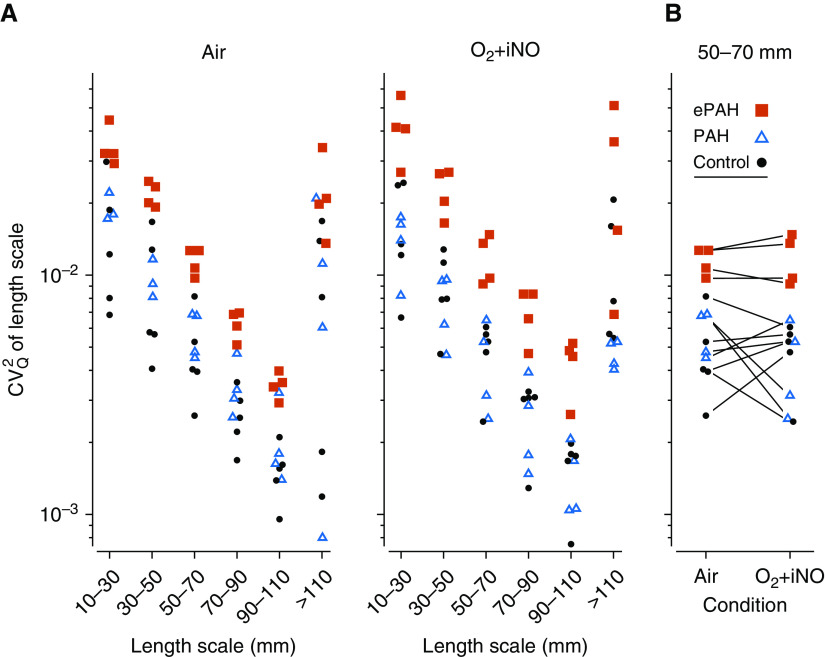
13NN-saline functional PET imaging revealed perfusion characteristics that distinguished all groups. For instance, subjects with PAH had significantly attenuated vertical perfusion gradients (Qvgrad) compared with subjects with ePAH and their control counterparts while breathing O2 + iNO (Qvgrad 10−2 cm−1, PAH 2.51 ± 2.06 vs. ePAH 6.96 ± 0.62 vs. control 7.37 ± 0.86, PANOVA < 0.001). Subjects with ePAH, on the other hand, had perfusion gradients similar to those observed in control subjects but had significantly greater CV2Qtotal than both subjects with PAH and control subjects at baseline (CV2Qtotal baseline, ePAH 0.15 ± 0.04 vs. PAH 0.08 ± 0.02 vs. control 0.09 ± 0.04, PANOVA < 0.02), with further discrimination while breathing O2 + iNO (CV2Qtotal breathing O2 + iNO, ePAH 0.21 ± 0.04 vs. PAH 0.06 ± 0.03 vs. control 0.13 ± 0.03, PANOVA < 0.0002). After we subtracted the perfusion gradients, we found that the greater CV2Qr in subjects with ePAH was driving this increased CV2Qtotal. Furthermore, subjects with ePAH demonstrated greater perfusion heterogeneity across the components of CV2Qr, or different image resolutions (length scale spectrum 10–110 mm) (PANOVA < 0.01 for all length scales) compared with their control and PAH counterparts (Figure 1). After further adjustment for regional lung density by high-resolution computed tomography, these differences in perfusion within and between groups persisted.
Figure 1.
Subjects with ePAH had increased perfusion heterogeneity across image resolutions (length scales). The residual heterogeneity of perfusion (CV2Qr) is a component of the total spatial heterogeneity (CV2Qtotal), which also includes the vertical (dorsoventral) gradient in perfusion (CV2Qvgrad) and the axial (craniocaudal) gradient in perfusion (CV2Qzgrad), so that CV2Qr = CV2Qtotal − CV2Qvgrad − CV2zgrad. The residual heterogeneity in perfusion (CV2Qr) was then split into components of heterogeneity captured at different image resolutions (length scales) (2, 6). The contribution of specific image resolution ranges to CV2Qr was calculated as the difference between two successive filter sizes, which is demonstrated in this figure for all subjects with ePAH and PAH, and control subjects. (A) The variation in perfusion at different image resolutions (length scales) revealed that subjects with ePAH had significantly greater perfusion heterogeneity at all image resolutions at baseline. This perfusion profile further discriminated subjects with ePAH from those with PAH and control subjects during O2 + iNO. (B) Paired perfusion heterogeneity at the medium-sized image resolutions (length scale, CV2Q50–70) demonstrated that subjects with ePAH had greater perfusion heterogeneity than those with PAH and control subjects both before and during O2 + iNO. CV2Q50–70 = squared coefficient of variation in perfusion quantifying the heterogeneity at the length scale of 50–70 mm; ePAH = exercise pulmonary arterial hypertension; O2 +iNO = inhaled nitric oxide with balance gas oxygen; PAH = pulmonary arterial hypertension.
Discussion
PET imaging revealed two unique perfusion characteristics in subjects with ePAH at rest. First, these subjects demonstrated a perfusion pattern characterized by increased spatial heterogeneity in perfusion across image resolutions (length scale spectrum 10–110 mm) at rest that distinguished them from both subjects with PAH and control subjects. Second, subjects with ePAH maintained a normal resting vertical gradient in perfusion that was responsive to vasodilation, similar to what was observed in their control counterparts but distinct from subjects with PAH.
We show for the first time that subjects with ePAH at rest have distinct perfusion characteristics at baseline and during pulmonary vasodilation (O2 + iNO). Subjects with ePAH demonstrated greater total perfusion heterogeneity (CV2Qtotal) than control subjects and subjects with PAH. An examination of the components of CV2Qtotal revealed that subjects with ePAH had resting vertical and axial gradients and perfusion redistribution in response to O2 + iNO similar to what was observed in control subjects, indicating low resting pulmonary vascular pressures and a normal vascular response to gravitational forces. However, subjects with ePAH demonstrated greater residual perfusion heterogeneity (CV2Qr) and greater heterogeneity regardless of image resolution or length scale spectrum (10–110 mm) than control subjects and subjects with PAH. Our observations suggest that subjects with ePAH have a higher spatial heterogeneity in perfusion across different sizes of anatomical and functional units among the branching regions of the vascular tree at rest, ranging from acinar-sized units represented at smaller image resolutions to segmental-sized units represented at larger image resolutions.
Although our findings are compelling, a key limitation should be considered. This was a small pilot study, but our observations of attenuated vertical perfusion gradients in subjects with PAH are consistent with prior observations (7). Additionally, accentuation of perfusion differences with O2 + iNO further corroborates baseline perfusion differences, because if our result were due to chance alone, we would not have expected a systematic strengthening of the finding.
To date, no resting structural or functional measurement has been able to predict the symptomatic increases in mPAP at exercise that have been demonstrated in patients with ePAH (8). Although individuals with ePAH and PAH both have objective manifestations of exercise intolerance, including signs of increased right ventricular afterload and right ventricular/pulmonary vascular uncoupling (8, 9), the distinct mechanisms that are responsible for an increase in pulmonary artery pressure during exercise in ePAH have been unclear. Dynamic pulmonary vasoconstriction has been previously implicated (9, 10); however, in comparison with control subjects and subjects with PAH, our findings show the presence of a distinct vascular pathology at rest in subjects with ePAH, represented by increased perfusion heterogeneity across image resolutions (length scale spectrum 10–110 mm). Whether this pattern heralds permanent vascular remodeling cannot be determined by our data, and a longitudinal study will be required to definitively evaluate whether this pathology relates to an intermediate PAH phenotype (10) or whether this unique perfusion pattern represents a distinct disease process.
Supplementary Material
Footnotes
Supported by an unrestricted grant from United Therapeutics. The sponsor was not involved in the study design, analysis, or reporting of study findings.
Author Contributions: Study design: E.G.K., M.K., R.C., and R.S.H. Data acquisition: P.K., V.J.K., E.G.K., J.R.-L., K.A.H., M.K., D.M.S., J.G.V., R.C., T.W., and R.S.H. Data analysis: P.K., V.J.K., T.W., and R.S.H. Data interpretation: P.K., T.W., and R.S.H. Manuscript drafting and editing: P.K., V.J.K., T.W., and R.S.H. Final manuscript approval: P.K., V.J.K., E.G.K., J.R.-L., K.A.H., M.K., D.M.S., A.B.W., J.G.V., R.C., T.W., and R.S.H. Agreement to be accountable for the work: P.K., V.J.K., E.G.K., J.R.-L., K.A.H., M.K., D.M.S., A.B.W., J.G.V., R.C., T.W., and R.S.H.
Originally Published in Press as DOI: 10.1164/rccm.201810-1899LE on February 27, 2019
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Kovacs G, Herve P, Barbera JA, Chaouat A, Chemla D, Condliffe R, et al. An official European Respiratory Society statement: pulmonary haemodynamics during exercise. Eur Respir J. 2017;50:1700578. doi: 10.1183/13993003.00578-2017. [DOI] [PubMed] [Google Scholar]
- 2.Vidal Melo MF, Winkler T, Harris RS, Musch G, Greene RE, Venegas JG. Spatial heterogeneity of lung perfusion assessed with (13)N PET as a vascular biomarker in chronic obstructive pulmonary disease. J Nucl Med. 2010;51:57–65. doi: 10.2967/jnumed.109.065185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohli P, Winkler T, Kelly VJ, Gladysheva E, Kone M, Hibbert K, et al. EIPAH and PAH subjects have different regional perfusion patterns after vasodilation. Am J Respir Crit Care Med. 2018;197:A4375. [Google Scholar]
- 4.Venegas JG, Winkler T, Musch G, Vidal Melo MF, Layfield D, Tgavalekos N, et al. Self-organized patchiness in asthma as a prelude to catastrophic shifts. Nature. 2005;434:777–782. doi: 10.1038/nature03490. [DOI] [PubMed] [Google Scholar]
- 5.Kelly VJ, Hibbert KA, Kohli P, Kone M, Greenblatt EE, Venegas JG, et al. Hypoxic pulmonary vasoconstriction does not explain all regional perfusion redistribution in asthma. Am J Respir Crit Care Med. 2017;196:834–844. doi: 10.1164/rccm.201612-2438OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motta-Ribeiro GC, Hashimoto S, Winkler T, Baron RM, Grogg K, Paula LFSC, et al. Deterioration of regional lung strain and inflammation during early lung injury. Am J Respir Crit Care Med. 2018;198:891–902. doi: 10.1164/rccm.201710-2038OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones AT, Hansell DM, Evans TW. Quantifying pulmonary perfusion in primary pulmonary hypertension using electron-beam computed tomography. Eur Respir J. 2004;23:202–207. doi: 10.1183/09031936.03.00033803. [DOI] [PubMed] [Google Scholar]
- 8.Naeije R, Vanderpool R, Dhakal BP, Saggar R, Saggar R, Vachiery J-L, et al. Exercise-induced pulmonary hypertension: physiological basis and methodological concerns. Am J Respir Crit Care Med. 2013;187:576–583. doi: 10.1164/rccm.201211-2090CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanderpool RR, Pinsky MR, Naeije R, Deible C, Kosaraju V, Bunner C, et al. RV-pulmonary arterial coupling predicts outcome in patients referred for pulmonary hypertension. Heart. 2015;101:37–43. doi: 10.1136/heartjnl-2014-306142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oudiz RJ, Rubin LJ. Exercise-induced pulmonary arterial hypertension: a new addition to the spectrum of pulmonary vascular diseases. Circulation. 2008;118:2120–2121. doi: 10.1161/CIRCULATIONAHA.108.819573. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.