Abstract
The urgent need to start anti-infective therapeutic interventions in suspected sepsis, and the lack of specific time-critical diagnostic information often lead to the widespread administration of broad-spectrum antimicrobial therapies, increasing the risk of unwanted patient harms and contributing to rising pathogen antimicrobial resistance. Nanotechnology, which involves engineering at the nanoscale, allows for the bespoke development of diagnostic solutions with multi-functionality and high sensitivity that has the potential to help provide time-critical information to make more accurate diagnoses and treatment decisions for sepsis. Nanotechnologies also have the potential to improve upon the current strategies used for novel biomarker discovery. Here we describe some of the current limitations to identifying sepsis and explore the potential role for nanotechnology solutions.
Keywords: Sepsis, nanotechnology, diagnosis, infection, biomarkers
Introduction
Despite advances in health technology and its delivery, the mortality rate associated with sepsis remains at around 35%,1 in part due to difficulties in recognising sepsis early and instigating appropriate treatment.2 Recognising sepsis in the early stages remains a challenge as the characteristic clinical signs and symptoms are seen in a variety of acute non-infective insults as well as common self-limiting infections.3
The current clinical paradigm in suspecting sepsis is to recognise that a patient is unwell with new organ dysfunction, and to consider if an infection could be the cause. Commonly available non-specific acute phase reactants such as C-reactive protein (CRP) and procalcitonin (PCT) may be used as an adjunct to clinical acumen; however, in patients presenting with tissue injuries such as following major surgery, polytrauma or burns, a complex clinical picture may obscure a co-developing episode of sepsis.
The last few years have seen a rapid rise in the number and variety of health technologies at a nanoscale (nanotechnologies), some of which have the potential to impact on the recognition of sepsis. Nanomedicine, as the application of nanoscale technologies in healthcare, exploits changes in material properties that occur at the nanoscale (1–100 nm in a single dimension). Engineering diagnostic systems of this scale provide the potential for rapid, multi-functional diagnostic solutions to complex disease processes such as sepsis.4 In this article, we highlight some of the main clinical challenges associated with identifying sepsis and we review how the most promising nanotechnologies may help provide solutions. The overall aim of this article is to help identify key hypotheses to test for the translation of nanoscale technologies in the management of patients with sepsis.
Diagnosing infection
Currently, microbiological cultures from biofluids such as blood and urine are the gold standard for pathogen identification. They offer useful information but are limited by long incubations times, making them unsuitable for rapid diagnostics. A significant proportion of patients (around 40%) with sepsis are blood culture-negative,5 often as a result of antibiotic administration prior to culture sampling, low concentrations of pathogen colony-forming units, or atypical pathogens that are not recognised by standard laboratory analysis.5
Polymerase chain reaction (PCR) is an established technique that multiplies DNA sequences found within biofluids (most often blood in the setting of undifferentiated sepsis) across several orders of magnitude to allow detection of target pathogen DNA sequences. PCR has been used for some time in microbiological laboratories to accelerate time-consuming steps in traditional culture-based amplification, providing faster pathogen identification.6
The use of multiple simultaneous PCR reactions (multiplex PCR) to amplify pathogenic DNA direct from patient blood samples is a potential solution to time delays associated with traditional microbiological cultures. Pathogenic DNA in the blood can be multiplied significantly faster and compared against a pre-set library of known common bacterial and fungal DNA sequences;7 however, this technique does not provide functional information about microbial antibiotic susceptibility. There are also technical limitations to using PCR in this setting. Due to the potential for high laboratory analytical sensitivity, any contamination could lead to the production of false-positive results due to amplification of contaminant DNA. The primers used in multiplex PCR are necessarily broad, as there is significant diversity in the potential causative pathogens, and thus common pathogenic sequences are targeted by the primers. Host or contaminative DNA that are similar, but not identical to the target sequence can bind to the primer, resulting in a false positive.8 It is therefore essential to ensure ultra-pure reaction reagents.9
There is an urgent need to develop technologies capable of identifying infection quickly, limiting the administration of antimicrobials to patients with a sterile pathology. Technologies that can reliably identify infective pathogens would allow for antimicrobials to be used with greater precision; improving outcomes, reducing the burden of broad-spectrum antimicrobials and limiting the progression of multi-drug-resistant organisms.
Nanotechnology solutions: Magnetic nanoparticles
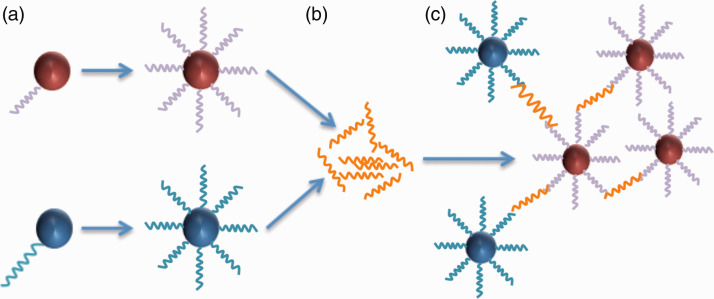
The use of magnetic nanoparticles has been explored in a number of innovative ways to provide rapid identification of bloodstream pathogens. One solution has been used to develop a T2 magnetic resonance (T2MR) diagnostic platform, which uses superparamagnetic iron oxide nanoparticles (SPIONs) that have been functionalised with short sequences of a specific DNA (targeted oligonucleotides – Figure 1). These oligonucleotides bind to Candida species within whole blood, forming agglomerates that can be detected via a T2MR biosensing platform. Different magnetic nanoparticles are functionalised with different oligonucleotides, enabling the T2MR sensor to differentiate between Candida species. This technology is able to detect five clinically relevant Candida species within 3 h, without the need for blood cultures.10 The T2MR technology has been used to design automated diagnostic platforms, T2Dx, and T2Candida panels, which have been approved by the U.S. Food and Drug Administration (FDA). The first extensive multicentre clinical trial of the T2Candida panel demonstrated an overall specificity and sensitivity of 98.1% and 91.0%, respectively, with an average time to species identification of 4.4 ± 1.0 h.11 A similar technology has been developed for bacterial detection and identification, this time using magnetic nanoparticles that have been functionalised with oligonucleotides designed to specifically target bacterial 16S ribosomal RNA. A micro-nuclear magnetic resonance system detects the change in signal output due to agglomeration, and has been able to accurately and differentially detect and phenotype a large pool of 13 bacterial species within 2 h.12 The first clinical trial of the T2Bacteria Panel has recently been published, with promising results. Across 140 samples from 129 patients with suspected blood stream infection (BSI), the sensitivity and specificity were 89% and 98%, respectively for identifying patients who had clinical indicators of BSI, regardless of blood culture result. The negative predictive value of the technology was 99.8%. The mean time to a negative result was 6.1 ± 1.5 h, whereas the mean time to species identification was 5.5 ± 1.4 h.13
Figure 1.
Schematic demonstrating T2MR detection of SPION-DNA nanoparticle agglomerates. (a) The SPION is functionalised with oligonucleotide probes. For each fungal DNA target, two SPIONs are generated. (b) Fungal DNA is amplified by PCR. (c) As the probes bind to the target DNA, the nanoparticles agglomerate. The degree of agglomeration is detected by T2MR and increases with fungal DNA concentration.
Magnetic microparticles (MMPs) have been developed to isolate pathogens and pathogen-associated molecular patterns from blood samples by attaching an engineered version of mannose-binding lectin (a protein that binds a wide range of pathogens and PAMPs) to the MMPs surface.14 The MMPs are added to a blood sample, and bind to pathogens or their released PAMPs. The MMPs are extracted via a magnetic field, and the presence of pathogens or PAMPs is quantified by a modified enzyme-linked immunosorbant assay (ELISA). This technology has been trialled using samples obtained from patients with sepsis against two control groups; healthy volunteers and patients admitted with traumatic injuries. Diagnostic accuracy was reported at 87%.14 This technology has also been incorporated into an extra-corporeal circuit. MMPs were added in a rodent sepsis model, with blood passing through a microfluidic apparatus surrounded by an electromagnetic field. Pathogens and PAMPs bound to MMPs were eliminated, and cleansed blood was returned.15 In the model following injection of a lethal dose of LPS (p < 0.02), there was a noticeable improvement of various physiological responses (breathing rate, temperature and restoration of circulating leukocyte counts), with survival increasing from 14% to 89%.
Whilst there appears to be early promise with these technologies, further translational research will be required to evaluate clinical applicability as well as cost effectiveness
Identifying organ dysfunction
The Sepsis-3 consensus definitions emphasise organ dysfunction as an integral component of recognising sepsis.16 Detecting organ dysfunction at the bedside can be challenging as compensatory mechanisms may result in some organs appearing to function normally despite huge physiological insults, with dysfunction only becoming apparent at a comparatively later stage.
The 2016 Surviving Sepsis guidelines encourage the use of the sequential organ failure assessment (SOFA) score as a means of identifying organ dysfunction in sepsis.17 As the SOFA score requires a period of 24 h to observe and calculate the nadir in organ function, it is not appropriate as an early indicator of organ dysfunction. To address this, the surviving sepsis guidelines recommend using the quick SOFA (qSOFA) tool; a 3-point scoring system designed to help clinicians identify patients at greater risk of mortality outside of intensive care. When used as a screening tool to identify patients who may have sepsis, qSOFA is more specific than the systemic inflammatory response syndrome (SIRS) score it replaced,18 although its sensitivity is lower.19 Early warning scores such as the National Early Warning Score-2 (NEWS2) are increasingly used in hospitals. These scores measure a variety of physiological variables and when compared to qSOFA and SIRS scores, they may be of more value in helping clinicians identify and prognosticate patients with sepsis.19,20
The qSOFA, SIRS and NEWS systems provide a quick non-invasive means of identifying unwell patients; however, they provide no information on the function of specific organs. In a patient who has been identified as being compromised, in order to more fully assess their clinical situation, blood tests of specific organ function are taken. Serum bilirubin and creatinine are often used as markers of end organ function; however, due to compensatory mechanisms that try to preserve regional blood flow to major organs, these markers are acutely elevated only relatively late in response to sepsis.21,22 Patients may also have pre-morbid conditions which may result in these markers being comparatively high, or low, as the patient’s baseline, confounding the recognition of dysfunction. Experimental rodent models have indicated that liver function is impacted early in sepsis, with phosphatidylinositol-3-kinase (PI3K) signalling playing a crucial role in the development of sepsis-induced cholestasis. Increases in PI3K signalling significantly preceded plasma elevation of traditional markers, providing early evidence of hepatic dysfunction.23 Similarly, a murine model of renal dysfunction in sepsis has shown that damage to the renal endothelium occurs within hours of a septic insult.24 Neutrophil gelatinase-associated lipocalin (NGAL) is produced by damaged nephron epithelium and is a potential early marker of acute kidney injury (AKI), with elevation in the blood detectable within 3 h of injury.25 Within the critical care setting, NGAL levels on admission to ICU were able to predict who would develop AKI.26
Detecting specific markers of organ dysfunction as soon as possible is essential to identifying patients who are deteriorating, allowing for earlier intervention and guiding resource allocation. Highly sensitive analytical techniques, with very low limits of detection will play an important role in identifying the deteriorating patient earlier than ever.
Nanotechnology solutions: Gold nanoparticles
Gold nanoparticles (AuNPs) exhibit unique optical properties as a result of a phenomenon called surface plasmon resonance (SPR). In this phenomenon, incidence light reflects off of colloidal AuNPs in a manner determined by the size and surface properties of the nanoparticle. Any changes in the surface refractive index will alter the SPR. AuNPs conjugated with antibodies will therefore shift the SPR when the analyte is bound to the antibody.27 Plasmonic sensors based on gold nanoparticle growth have already shown to be able to detect single molecules in serum by the naked eye.28
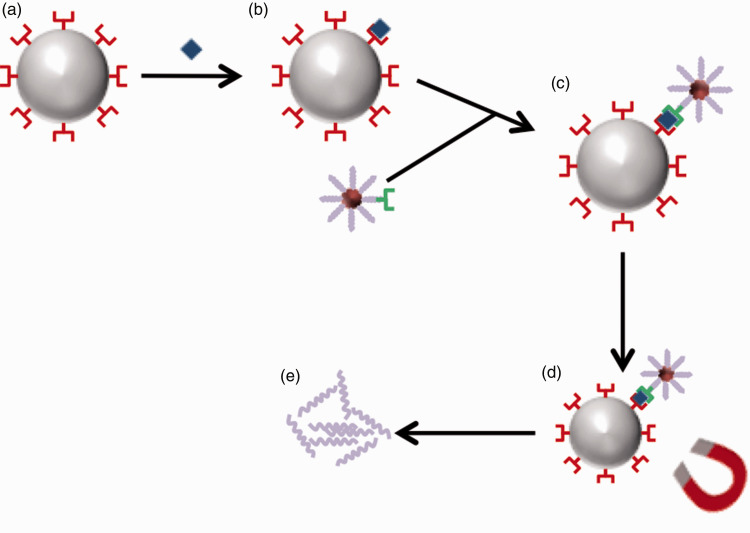
AuNPs have also been used as part of a bio-barcode system to detect ultralow plasma concentrations of prostate-specific antigen (330 femto-g/mL) after radical prostatectomy. A bio-barcode system employs both MMPs that have been functionalised with targeted monoclonal antibodies, and AuNPs that have been dual-functionalised with oligonucleotides (short DNA sequences assigned to a specific analyte of interest) and a target polyclonal antibody. The target analyte then binds to both the poly and monoclonal antibodies forming an AuNP-analyte-MMP complex. A magnetic field then removes the complex from the sample fluid and the oligonucleotide undergoes PCR after removal from the AuNP (Figure 2). This technique can be several orders of magnitude more sensitive than the standard enzyme-linked immunosorbant assays (ELISA).29,30 Whilst this technique has yet to be used within sepsis diagnostics, coated AuNP bio-barcodes have been investigated in detecting early HIV infection, with a 1000-fold increase in sensitivity over conventional ELISA assays.31 With pathogens and their constituents (surface proteins, microbial DNA) only being present in very low concentrations, the use of AuNP bio-barcodes could prove a useful technique for increasing detection sensitivity.
Figure 2.
The process of biobarcode analysis. (a) An MMP is conjugated with monoclonal antibodies. (b) Analyte of interest binds to monoclonal antibody. (c) MMP-analyte complex binds to polyclonal antibody of AuNP. (d) Magnetic field separates the MMP-analyte-AuNP complex from sample. (e) De-conjugation of specific oligonucleotides occurs prior to PCR analysis.
Recognising immune dysregulation
A dysregulated immune response to an infection is the mechanistic cause of organ dysfunction and failure in sepsis; however, like the variety of causative pathogens, there is also variation in the host response. Recently, the Molecular Diagnosis and Risk Stratification in Sepsis (MARS) study group published data identifying four genetic endotypes (MARS-1 to MARS-4) in patients with sepsis,32 with significant variations in survival between these groups. Patients of the MARS-1 endotype had consistently worse mortality, and notably decreased expression of genes involved in innate and adaptive immune functions, whereas those of the MARS-3 endotype have a lower risk of mortality and had an increase in the expression of genes involved in T-cell function. A systematic review of the genomic expression in the host response during sepsis revealed that the expression of traditional cytokine markers of inflammation was highly variable, challenging the traditional two-phase model of a septic response.33
There is clear evidence of the wide heterogeneity of the septic response and it is clear that clinical trials that homogenise patients despite differences in the causative mechanisms and host responses are likely to produce equivocal results.34 Consequently, the failure of clinical trials to demonstrate a clear benefit of treatments such as activated protein C35 or glucocorticoids36 may in part be due to regarding these patients as part of a single well-defined disease population, where in fact there may be subgroups of patients, or periods within a septic response where these treatments are beneficial. It is becoming increasingly clear that managing sepsis patients as part of a homogenised population is inappropriate,37–39 and that in order to maximise outcomes treatments will need to be tailored to suit the individual needs of a patient.
A diagnostic platform designed to monitor an array of key pro and anti-inflammatory biomarkers would be able to monitor the pattern of an individual patient’s immune response allowing clinicians to identify the onset of sepsis. The real-time inflammatory profile of a patient could then help guide a personalised approach.
Nanotechnology solutions: Quantum dots
Quantum dots are semi-conductor nanocrystals comprised of atoms from groups II–VI, IV–VI or III–V in the periodic table.40 When stimulated they emit light of varying wavelengths, which can be tuned depending on the size and material of the crystal.41 Han et al.42 have developed a multiplex panel of different quantum-dot-tagged microbeads, which have been engineered to detect specific nucleic acid and protein sequences. This technology uses polymer beads that have targeting antibodies attached to the surface, while on the inside quantum dots are imbedded that fluoresce when the antibodies bind to their target. This multiplex panel allows for the parallel detection of specific analytes.
Quantum dot technology has also been incorporated into point-of-care (POC) settings. Lateral flow strip (LFS) analysis is a simple, quick, and robust technique that is widely used in POC diagnostics such as pregnancy tests. LFS assays which have been labelled with two different quantum dot-antibody conjugates, with different fluorescence emission spectra, have been able to simultaneously detect CRP and PCT with greater sensitivity than traditional analysis (down to 0.1µg/mL and 1 ng/mL, respectively).43
A multiplex panel of quantum dots or diagnostic packs with multiple quantum dot LFS assays that are engineered to detect specific inflammatory mediators may provide a real-time representation of the inflammatory response at the bedside, arming clinicians with information on which to base a personalised management plan.
Panels of biomarkers are being investigated as a method to diagnose sepsis,44,45 with some success.46 Clinical validation of these panels to elucidate the most effective combination of markers will be required ahead of their implementation within a quantum dot technology. If successful, however, these quantum dot panels will aid clinicians in diagnosing sepsis at the bedside.
Conclusion
Sepsis is a complex pathophysiological challenge with early recognition and treatment key to improving outcome. A central challenge is to quickly and accurately recognise infection as the cause of an acutely ill patient, limiting the wider use of antimicrobials to all patients with disordered physiology. Nanotechnology solutions to diagnostic challenges are continually being developed across the medical world, including in infection diagnostics. Further translational studies of relatively mature nanotechnologies will be required to allow evaluation of their clinical and cost-effectiveness against the current standard of care, while early collaboration of clinicians with material scientists will be crucial in guiding the development of novel nanotechnologies.
Acknowledgements
This work was part of a NIHR Academic Clinical Fellowship (AC) at the University of Manchester.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Andrew Claxton https://orcid.org/0000-0002-5252-1487
References
- 1.Daniels R. Surviving the first hours in sepsis: getting the basics right (an intensivist’s perspective). J Antimicrob Chemother 2011; 66: 11–23. [DOI] [PubMed] [Google Scholar]
- 2.Ferrer R, Martin-Loeches I, Phillips G, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour. Crit Care Med 2014; 42: 1749–1755. [DOI] [PubMed] [Google Scholar]
- 3.Vincent JL. The clinical challenge of sepsis identification and monitoring. PLoS Med 2016; 13: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herrmann IK. How nanotechnology-enabled concepts could contribute to the prevention, diagnosis and therapy of bacterial infections. Crit Care 2015; 19: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phua J, Ngerng W, See K, et al. Characteristics and outcomes of culture-negative versus culture-positive severe sepsis. Crit Care 2013; 17: R202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ziegler R, Johnscher I, Martus P, et al. Controlled clinical laboratory comparison of two supplemented aerobic and anaerobic media used in automated blood culture systems to detect bloodstream infections. J Clin Microbiol 1998; March: 657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dark PM, Dean P, Warhurst G. Bench-to-bedside review: the promise of rapid infection diagnosis during sepsis using polymerase chain reaction-based pathogen detection. Crit Care 2009; 13: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garlbyan L, Avashia N. Research techniques made simple: polymerase chain reaction (PCR). J Invest Dermatol 2013; 133: e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humphrey B, McLeod N, Turner C, et al. Removal of contaminant DNA by combined UV-EMA treatment allows low copy number detection of clinically relevant bacteria using pan-bacterial real-time PCR. PLoS One 2015; 10: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neely LA, Audeh M, Phung NA, et al. T2 magnetic resonance enables nanoparticle-mediated rapid detection of candidemia in whole blood. Sci Transl Med 2013; 5: 182ra54. [DOI] [PubMed] [Google Scholar]
- 11.Mylonakis E, Clancy CJ, Ostrosky-Zeichner L, et al. T2 magnetic resonance assay for the rapid diagnosis of candidemia in whole blood: a clinical trial. Clin Infect Dis 2015; 60: 892–899. [DOI] [PubMed] [Google Scholar]
- 12.Chung HJ, Castro CM, Im H, et al. A magneto-DNA nanoparticle system for target specific bacterial identification. Nat Nanotechnol 2013; 8: 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Angelis G, Posteraro B, De Carolis E, et al. T2Bacteria magnetic resonance assay for the rapid detection of ESKAPEc pathogens directly in whole blood. J Antimicrob Chemother 2018; 73: iv20–iv26. [DOI] [PubMed] [Google Scholar]
- 14.Cartwright M, Rottman M, Shapiro NI, et al. A broad-spectrum infection diagnostic that detects pathogen-associated molecular patterns (PAMPs) in whole blood. EBioMedicine 2016; 9: 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang JH, Super M, Yung CW, et al. An extracorporeal blood-cleansing device for sepsis therapy. Nat Med 2014; 20: 1211–1216. [DOI] [PubMed] [Google Scholar]
- 16.Singer M, Deutschman CS, Seymour CW, et al. The Third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315: 801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017; 43: 304–377. [DOI] [PubMed] [Google Scholar]
- 18.Gunn N, Haigh C, Thomson J. Triage of sepsis patients: SIRS or qSOFA – which is best? Emerg Med J 2016; 33: 909–910. [Google Scholar]
- 19.Askim Å, Moser F, Gustad LT, et al. Poor performance of quick-SOFA (qSOFA) score in predicting severe sepsis and mortality – a prospective study of patients admitted with infection to the emergency department. Scand J Trauma Resusc Emerg Med 2017; 25: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goulden R, Hoyle M-C, Monis J, et al. qSOFA, SIRS and NEWS for predicting inhospital mortality and ICU admission in emergency admissions treated as sepsis. Emerg Med J 2018; 35: 345–349. [DOI] [PubMed] [Google Scholar]
- 21.Honore PM, Jacobs R, Joannes-Boyau O, et al. Biomarkers for early diagnosis of AKI in the ICU: ready for prime time use at the bedside? Ann Intensive Care 2012; 2: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marshall JC, Cook DJ, Christou NV, et al. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med 1995; 23: 1638–1652. [DOI] [PubMed] [Google Scholar]
- 23.Recknagel P, Gonnert FA, Westermann M, et al. Liver dysfunction and phosphatidylinositol-3-kinase signalling in early sepsis: experimental studies in rodent models of peritonitis. PLoS Med 2012; 9: e1001338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu C, Chang A, Hack BK, et al. TNF-mediated damage to glomerular endothelium is an important determinant of acute kidney injury in sepsis. Kidney Int 2014; 85: 72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devarajan P. Review: neutrophil gelatinase-associated lipocalin: a troponin-like biomarker for human acute kidney injury. Nephrology 2010; 15: 419–428. [DOI] [PubMed] [Google Scholar]
- 26.De Geus HRH, Bakker J, Lesaffre EMEH, et al. Neutrophil gelatinase-associated lipocalin at ICU admission predicts for acute kidney injury in adult patients. Am J Respir Crit Care Med 2011; 183: 907–914. [DOI] [PubMed] [Google Scholar]
- 27.Lin H-Y, Chen C-T, Chen Y-C. Detection of phosphopeptides by localized surface plasma resonance of titania-coated gold nanoparticles immobilized on glass substrates. Anal Chem 2006; 78: 6873–6878. [DOI] [PubMed] [Google Scholar]
- 28.de la Rica R, Stevens MM. Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the naked eye. Nat Nanotechnol 2012; 7: 821–824. [DOI] [PubMed] [Google Scholar]
- 29.Nam JM, Wise AR, Groves JT. Colorimetric bio-barcode amplification assay for cytokines. Anal Chem 2005; 77: 6985–6988. [DOI] [PubMed] [Google Scholar]
- 30.Bao YP, Wei TF, Lefebvre PA, et al. Detection of protein analytes via nanoparticle-based bio bar code technology. Anal Chem 2006; 78: 2055–2059. [DOI] [PubMed] [Google Scholar]
- 31.Dong H, Liu J, Zhu H, et al. Two types of nanoparticle-based bio-barcode amplification assays to detect HIV-1 p24 antigen. Virol J 2012; 9: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scicluna BP, Van Vught LA, Zwinderman AH, et al. Classification of patients with sepsis according to blood genomic endotype: a prospective cohort study. Lancet Repair Med 2017; 5: 816–826. [DOI] [PubMed] [Google Scholar]
- 33.Tang BM, Huang SJ, McLean AS. Genome-wide transcription profiling of human sepsis: a systematic review. Crit Care 2010; 14: R237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalil AC, Sweeney DA. Should we manage all septic patients based on a single definition? an alternative approach. Crit Care Med 2018; 46: 177–180. [DOI] [PubMed] [Google Scholar]
- 35.Ranieri V, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med 2012; 366: 2055–2064. [DOI] [PubMed] [Google Scholar]
- 36.Venkatesh B, Finfer S, Cohen J, et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med 2018; 378: 797–808. [DOI] [PubMed] [Google Scholar]
- 37.Vincent JL. Individual gene expression and personalised medicine in sepsis. Lancet Respir Med 2016; 4: 242–243. [DOI] [PubMed] [Google Scholar]
- 38.Angus DC. The search for effective therapy for sepsis back to the drawing board? JAMA 2011; 306: 2614–2615. [DOI] [PubMed] [Google Scholar]
- 39.Singer M. Biomarkers in sepsis. Curr Infect Dis Rep 2013; 15: 413–420. [DOI] [PubMed] [Google Scholar]
- 40.Reiss P, Protière M, Li L. Core/shell semiconductor nanocrystals. Small 2009; 5: 154–168. [DOI] [PubMed] [Google Scholar]
- 41.Kim J, Mohamed MAA, Zagorovsky K, et al. State of diagnosing infectious pathogens using colloidal nanomaterials. Biomaterials 2017; 146: 97–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han M, Gao X, Su JZ, et al. Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules. Nat Biotechnol 2001; 19: 631–635. [DOI] [PubMed] [Google Scholar]
- 43.Qi X, Huang Y, Lin Z, et al. Dual-quantum-dots-labeled lateral flow strip rapidly quantifies procalcitonin and C-reactive protein. Nanoscale Res Lett 2016; 11: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kibe S, Adams K, Barlow G. Diagnostic and prognostic biomarkers of sepsis in critical care. J Antimicrob Chemother 2011; 66: 33–40. [DOI] [PubMed] [Google Scholar]
- 45.Limongi D, D’Agostini C, Ciotti M. New sepsis biomarkers. Asian Pac J Trop Biomed 2016; 6: 516–519. [Google Scholar]
- 46.Punyadeera C, Schneider EM, Verhaegh WF. A biomarker panel to discriminate between systemic inflammatory response syndrome and sepsis and sepsis severity. J Emerg Trauma Shock 2010; 3: 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]