Abstract
An overview of the current system for referrals and management of severe respiratory failure in the United Kingdom. We outline the history of severe respiratory failure centres, the process of retrieving a patient for veno-venous extra corporeal membrane oxygenation and highlight some common difficulties and pitfalls when referring these critically unwell patients
Keywords: Extracorporeal membrane oxygenation, referral, retrieval, severe respiratory failure, UK
The following review will focus on the indications and application of extracorporeal membrane oxygenation (ECMO) for severe respiratory failure (SRF) in adults in the United Kingdom. Other patient groups and indications for ECMO, including paediatric and neonatal patients, acute severe heart failure, bridge to transplant and refractory cardiac arrest are beyond the scope of this article.
History and organisation
Extra-corporeal membrane oxygenation (ECMO) has been utilised in healthcare since the 1970s although it is only within the last decade that its use has become more widespread for the treatment of severe respiratory failure or acute respiratory distress syndrome (ARDS) in adults.1 The first adult case report for survival in ECMO was published in 1972 by Hill et al.2 Over the following decades, there were several trials of ECMO in adult patients with ARDS.3,4 The results of these trials were negative and experience remained limited to a few specialist centres around the world.
In the UK, a team established in Glenfield Hospital in Leicester first provided ECMO supported by Heart Link Children's Charity in 1989. Although initially used for a paediatric population to support, they subsequently used the technology for adult patients with ARDS. The evidence base for the current system in the UK was created by the Glenfield team who published both their observational data and undertook the first randomised trial of ECMO for severe respiratory failure, the CESAR study.5–7
The CESAR study investigated the safety, efficacy and cost-effectiveness of ECMO in adult patients with severe respiratory failure, randomising patients to either transfer to a centre with access to ECMO and consideration for ECMO therapy (Glenfield) or to conventional ventilation. Patients were allocated in a 1:1 ratio and included if they had severe but potentially reversible respiratory failure. All the patients randomised to the ECMO arm were retrieved by Glenfield's own transfer team although none of them were transferred on ECMO therapy. Patients in the control arm remained in their referring hospital or were transferred to a severe respiratory failure centre on conventional ventilation (either pressure control mode or high frequency oscillatory ventilation) if possible.
Of those patients transferred to Glenfield, 76% actually received ECMO therapy, with the remainder continuing on conventional ventilation. Patients were commenced on ECMO at Glenfield if they did not respond to optimally titrated ventilation and PEEP settings within 12 h of admission.
The measured primary outcome was death or severe disability at six months following randomisation. A statistically significant survival benefit was demonstrated for patients transferred to an ECMO centre versus those remaining at their referral hospital on conventional ventilation (63% vs. 47%, p = 0.03). In CESAR, more patients received low tidal volume/low pressure (lung protective) ventilation in the ECMO centre (93% of intervention arm) than in the ARDS centres (70% of control arm), due in part to the ability to manage refractory hypoxia and hypercapnoea with extracorporeal support and it is possible that this contributes to the observed mortality difference. This may also reflect the value of strict lung protective ventilation strategies that can be employed in the setting of the expertise of an ECMO centre.
Further to the CESAR study, the recent EOLIA trial did not show conclusive benefit for ECMO in ARDS when comparing severe ARDS patients randomised to standard therapy (including prone position, inhaled nitric oxide, recruitment manoeuvres and lung protective ventilation) or ECMO. This trial appeared to be underpowered to detect the 20% absolute risk reduction the authors had anticipated and there was significant crossover from the control arm to the intervention arm. However, there was a trend towards a clinical benefit from ECMO above standard care, especially if used earlier rather than later.8
Two other factors have had significant impact on the structure of ECMO services in the UK. The first was the Influenza A (H1N1) pandemic of 2009–11 in which there were a large number of young, previously well patients with profound ARDS. These patients were shown to have a survival benefit from ECMO in the UK using data from propensity matched studies.9 The second factor was the development of miniaturised, percutaneously inserted systems capable of facilitating the transport of patients receiving ECMO therapy.10,11 The latter allowed the application of ECMO on a regionalised or networked basis with trained teams delivering care over a wide geographical area 24 h a day.12 NHS England Highly Specialised Services undertook a commissioning exercise in 2011 which resulted in five English hospitals being designated as ECMO centres for adult severe respiratory failure, an additional Scottish centre (Aberdeen) was commissioned as a satellite centre by NHS Scotland.13 Currently, in addition to The University Hospitals of Leicester (Glenfield), these are Guys and St. Thomas' Hospital (London), The Royal Brompton and Harefield (London), Royal Papworth Hospital (Cambridge) and the University Hospital of South Manchester (Wythenshawe). Glenfield is commissioned to provide ECMO for Scotland with Aberdeen acting as an additional satellite centre. Referrals from Wales and Northern Ireland are managed on ad hoc arrangements with English centres. There is now a well-established, robust service for provision of ECMO within the UK, with up to 27 available beds with additional surge capacity. Centres work together in times of increased numbers of referrals in a co-ordinated manner. This surge activity is overseen by NHS England Highly Specialised Commissioning and the NHS England Emergency Preparedness and Resilience Response (EPRR) team. The co-ordinated working results in the centres determining which centre will accommodate a patient at a given time. The process also works during potential major incidents (for example the Grenfell Tower fire where there was the potential for multiple patients developing respiratory failure which required ECMO). The process underpinning this is regular centralised communication through both the use of a bed-state tool and teleconferences between NHS England and the commissioned centres. The frequency of communication can be escalated during predicted surge periods and can be activated by any centre or NHS England during major incidents. Should any increase in activity occur, there is a planned and structured approach to bed management which includes phases in bed state: <13 pre-surge; 13–21 surges; 22–27 escalations; and >27 critical. At each stage, various actions are required by NHS England and the centres (e.g. cancellation of elective surgery). The current system does appear to provide adequate national coverage – since the inception of the service, in every case where ECMO has been deemed appropriate, a bed has been found in one of the commissioned providers.
The standard ECMO circuit
The standard ECMO circuit consists of an access cannula (23–31 French Gauge), tubing carrying deoxygenated blood drawn by the centrifugal pump, a membrane oxygenator through which oxygen is passed to allow oxygenation and carbon dioxide removal and a return cannula (21–23 French Gauge) which completes the circuit back to the patient by further tubing. Intensivist cannulation is usually percutaneous, with potential approaches involving multiple single lumen cannulae or a single double lumen cannula.11 The UK providers use different cannula configurations including bi-femoral vein access and return, having the access cannula tip positioned in the inferior vena cava (IVC) and the return in the right atrium; femoral access with a jugular return cannula placed in the superior vena cava (SVC) or a single double lumen cannula can be placed in the SVC via the right internal jugular (RIJ) vein.
For patients with respiratory failure and an adequate cardiac output, veno-venous (VV) ECMO is used. In VV ECMO, blood is driven by the centrifugal pump, through the membrane oxygenator. Within the membrane, blood passes counter current to 100% oxygen at operator determined flow rates (so called “sweep gas”). Once it has passed through the oxygenator, blood is returned to the central veins/right atrium under positive pressure where it mixes with venous blood returning to the heart (Figure 1 for an example circuit). Care must be taken to ensure good cannula position and to minimise ‘recirculation’. Recirculation occurs when oxygenated blood returning from the oxygenator via the return cannula is immediately taken up via the access cannula (usually due to inadvertent proximity of access and return cannulae), providing no systemic benefit to the patient.
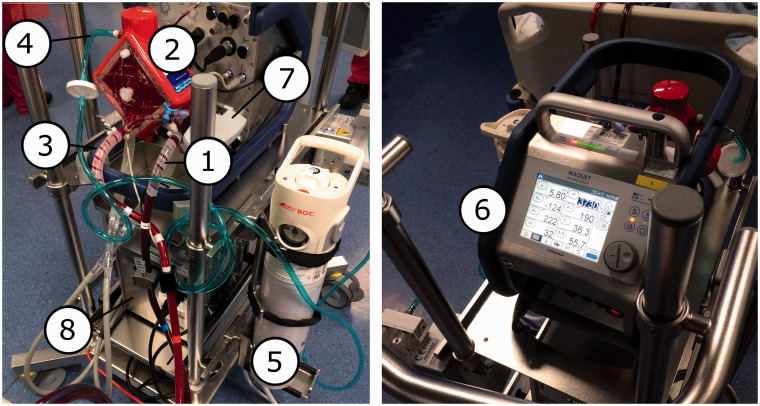
Figure 1.
Access limb of circuit (de-oxygenated side). 2. Oxygenator. 3. Return limb (oxygenated side). 4. Sweep gas pipeline. 5. Reserve/emergency oxygen supply. 6. Control console, front aspect. 7. Control console, rear aspect. 8. Heater-cooler unit. Circuit shown is the Maquet Cardiohelp.
The typical ECMO circuit can run at a maximum pump rate of around 5–6 L/min. Consequently, the circuit can only capture a proportion of cardiac output (especially in a high output state). There is therefore mixing of blood in the right atrium as oxygen-rich blood which is returned from the circuit mixes with deoxygenated blood returning to the right atrium. In order to provide systemic arterial oxygen saturations of at least 90%, the effective blood flow via the circuit (total minus the recirculation fraction) should be at least 60% of cardiac output.14
Indications
The indications for referral to an ECMO centre are severe, potentially reversible, acute respiratory failure with a lung injury score (Murray score – a composite score assessing four domains of severity – chest X-ray findings, PEEP setting, compliance and oxygenation) of 3 or more or an arterial pH <7.20 due to uncompensated hypercapnoea. The most common cause for admission is primary respiratory infection (bacterial or viral pneumonia, Figure 3); however, other conditions including severe asthma, aspiration pneumonitis, persistent bronchopleural fistula, thoracic trauma, acute interstitial lung disease (e.g. eosinophilic pneumonia) and pulmonary vasculitis also occur. It is important to discuss patients with the ECMO centre as contra-indicated co-morbidities change frequently. For example – in the past, patients with certain co-morbidities were declined ECMO due to prognostic pessimism; however, many of these previous contra-indications have changed. In particular, patients with significant obesity are accepted providing cannulation can be undertaken; patients with HIV are not excluded if the cause of their respiratory failure is deemed reversible (e.g. Pneumocystis jirovecii);15–17 patients with active bleeding, including intracranial haemorrhage are no longer contra-indicated as they can be safely managed using heparin-free techniques.18
There are very few absolute contraindications to ECMO. At present it is generally agreed that patients with viral haemorrhagic fever should not be offered ECMO.19 There are, however, patients in whom ECMO is more likely to offer benefit and those in whom ECMO will not necessarily improve their likelihood of survival. For example, patients with haematological malignancy, especially if they have already received chemotherapy or bone marrow transplantation, have very poor outcomes if they require ECMO.20,21 Hence, discussion with the severe respiratory failure centre is essential to understand whether or not the individual patient is likely to benefit from ECMO. Patients in whom ECMO is of minimal benefit include patients with active malignancy and patients with significant, chronic progressive respiratory disease.22 The latter group of patients may be considered for urgent lung transplant, although ECMO as a bridge to lung transplant is not currently routinely commissioned in the UK. Another group where appropriateness is commonly questioned is in patients who have had a previous cardiac arrest without demonstrable neurological recovery – in this case an individual assessment will need to be made and may include additional prognostic information such as neurone specific enolase, somatosensory evoked potentials, computed tomography (CT) and electro-encephalography (EEG).
Referring for VV ECMO
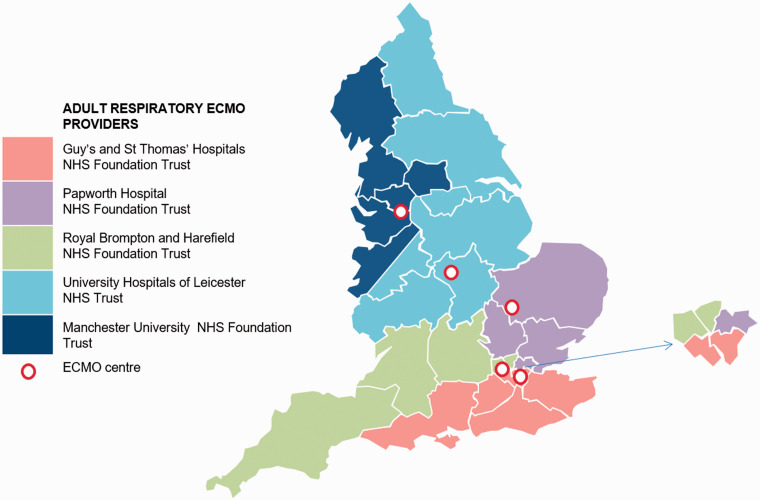
Referring hospitals are broken up into five regions in England (Figure 2). Each hospital has a different referral phone number and process although some have now changed to an online referral system. Key information required to facilitate decision making includes patient demographics, NHS number and review of organ function, severity of respiratory failure including ventilatory strategy and arterial blood gas analysis, management undertaken to date and co-morbidities. It is also important to include information to calculate the Lung Injury Score (Murray score) – positive end expiratory pressure (PEEP), dynamic compliance, chest X-ray and PaO2 to FiO2 ratio.
Figure 2.
Geographical territories covered by the five English severe respiratory failure centres. The commissioned service in England is available to patients from Scotland, Wales and Northern Ireland. Source: NHS England.
It is thought that the benefit from ECMO occurs when early referral (as soon as possible after meeting criteria, certainly less than a week) is undertaken.8,23,24 Furthermore, patients have a greater survival when the care before ECMO includes strict attention to known lung protective ventilatory strategies including low tidal volume (6 mL/kg ideal body weight)/airway pressure (Plateau pressure ≤ 30 cmH2O)) ventilation, prone positioning, neuromuscular blockade, and attention to appropriate PEEP and fluid balance.8,25–30 Where these have not been undertaken, the severe respiratory failure centre will commonly advise use of these strategies prior to consideration of ECMO unless they are contra-indicated. Earlier referral offers several advantages: early advice on alternative techniques, a clear, early opinion on potential suitability for ECMO, appropriately timed access to ECMO for the patient, and knowledge about the total number of potential patients allows planning for retrieval teams. This is especially valuable during times of high service demand (such as winter influenza season), where ECMO centres commonly cross-cover one another when there is demand-supply mismatch and the more the centres know about their potential workload, the better able they are to respond in an appropriate and timely fashion. Early referral also avoids the patient remaining on potentially injurious ventilation for a prolonged period of time.
Prone ventilation
Prone position ventilation is of proven benefit when undertaken for at least 16 h per day and accompanied by conventional lung protective ventilation strategies.8,25 However, ventilation in the prone position can be difficult to manage in centres with limited experience in the technique due to the potential complications. The severe respiratory failure centres often provide training at annual referrers' days and share protocols of care with referring centres to increase the comfort with the technique. It is of course important to realise that not all patients are suitable for ventilation in the prone position including patients with significant haemodynamic instability, spinal and thoracic trauma and intra-abdominal pathology to name but a few. Patient improvement generally occurs over a few hours with a steady improvement in PaO2 to FiO2 ratio, dynamic compliance and sputum mobilisation. For patients who either deteriorate or fail to improve, re-discussion with the severe respiratory failure centre is warranted to reconsider ECMO.
PEEP titration
Although a strategy of higher PEEP improves the outcomes for a population with severe respiratory failure, in certain situations it may be deleterious.26 For example, use of high PEEP may compromise cardiac output by inhibiting venous return. Furthermore, for patients with predominantly restrictive, non-recruitable pathology, excessive PEEP may increase dead space and therefore worsen oxygenation. Hence, it is important to titrate PEEP to an individual patient's respiratory mechanics using approaches such as optimising dynamic compliance or the PEEP with optimal gas exchange. Manoeuvres including PEEP escalation and de-escalation may assist in this regard.
Fluid balance
Although patients with severe respiratory failure have an improved outcome when they are managed with a fluid restrictive strategy, it is also clear that many patients with severe respiratory failure also have severe septic shock.27 Consequently, the fluid strategy which is optimal for the lungs may not necessarily be optimal for the circulation and vice versa. A balance must be achieved between the two and if the septic circulation cannot be adequately resuscitated due to the severity of lung injury, consideration should be given to the use of ECMO in this circumstance.31
Further to this, the SRF centre will often request that an echocardiograph be undertaken for the patient. Understanding of the functionality of the left and right side of the heart, in addition to any valvulopathy, will allow for appropriate choice of ongoing therapy. In some circumstances it may aid in the decision to offer additional therapy and to consider hybrid cardiac and respiratory ECMO (beyond the scope of this article).
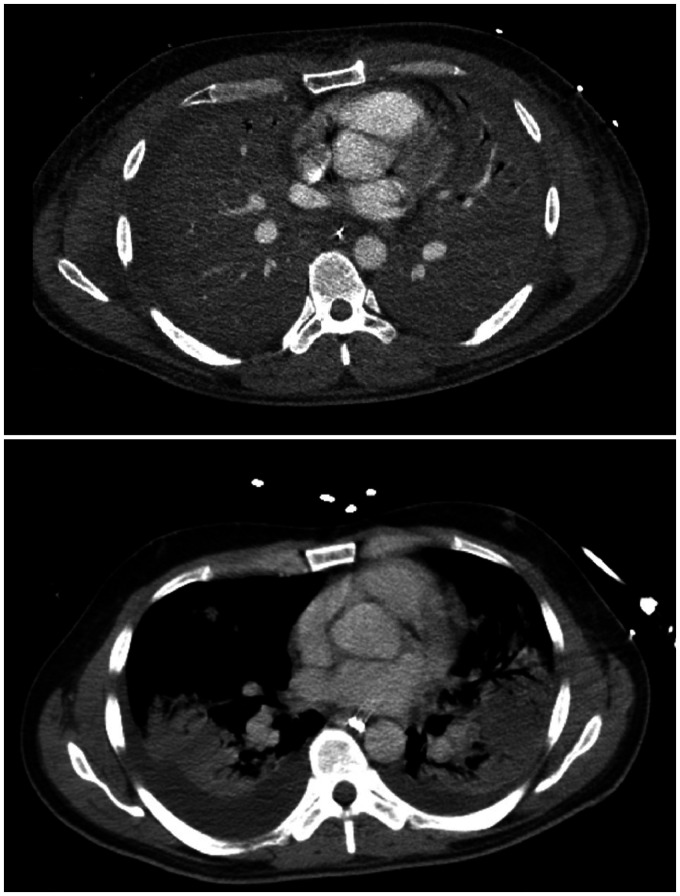
Figure 3.
CT imaging of a severe respiratory failure patient. The upper image shows a patient with group-A streptococcal septic shock on day of referral (6 h post cannulation), with complete opacification of both lungs. The image below shows the same patient after 10 days on VV-ECMO, with recovery and improvement in aeration. Images used with patient consent.
The referral pathway and decision to retrieve
Patients who are referred to the SRF service have one of four possible outcomes:
They are declined transfer due to futility.
They are accepted in principle but remain at the referring centre with ongoing surveillance.
They are retrieved using conventional ventilation.
They are retrieved on ECMO.
Patients in group one are commonly referred and indeed it is important to do so. Much like other specialty referrals, the severe respiratory failure centre has a thorough understanding of patients who will and will not benefit from ECMO. The co-morbidities and acute presentations which are thought to be reversible change with time and experience. A good example is severe sepsis with multiple organ failure which several years ago was considered to be a contra-indication to ECMO, whereas now there is increasing evidence of excellent survival in this group.31 Another reason for refusal of patients in group one is the application of long duration, injurious ventilation with consequent fibrosis.
Group two commonly comprise patients early in the course of their disease where clinicians appropriately refer early to ensure that the patient is visible to the SRF centre, either for advice or simply to allow for planning in case of deterioration. Advice commonly given at this time is to support the local multidisciplinary team in applying lung protective strategies, especially ventilation in the prone position. Patients in this group should be followed up by the severe respiratory failure centre at a time agreed with the referring team. It should also be made clear at the time of initial discussion whether or not the patient would be deemed suitable for ECMO in the case of non-improvement/deterioration.
Group three is gradually reducing in the annual figures published by NHS England. The commonest reasons for conventional retrieval are that either the patient has started to respond to therapies following on-site review by the severe respiratory failure centre team but requires ongoing input or that the referral centre is less experienced with approaches such as prone position ventilation. These patients are retrieved if it is felt they will still benefit from management in the SRF centre and they still have the potential to require ECMO. Given the maturity of the service and progressive uptake of lung protective ventilation strategies across UK hospitals, this group is steadily reducing.
If it is apparent that no further optimisation is possible or appropriate and that ECMO is the best course of action, the SRF centre will despatch a retrieval team. This team will usually consist of an ECMO consultant, an ECMO specialist nurse and a perfusionist, in addition to ambulance crew and trainee doctors/nurses/perfusionists.
Retrieving and managing an ECMO patient
Prior to the retrieval team's arrival, the referring team needs to undertake the management of the patient as well as expediting the commencement of ECMO in accordance with the usual practice from that centre. The ECMO providers will provide any information that they deem necessary to facilitate this, for example the provision of blood products, theatre availability and ancillary equipment. They should also make arrangements for the patient's family to be present at the time of the arrival of the retrieval team so the procedure can be discussed. The referring team should also prepare the patient notes for transfer and make available any radiology images to the receiving centre.
On arrival the retrieval team will undertake their own assessment of the patient, which will include review of all clinical details/history as well as available laboratory, radiology and echocardiography investigations. The team will also usually have a family discussion to gain assent for ECMO and to explain the process and destination hospital. Written information will usually also be made available. Cannulation for ECMO is commonly undertaken in the operating theatres of the referring hospital.
Some ECMO providers use vascular ultrasound and fluoroscopy, while others use ultrasound and echocardiography to determine cannula placement. Peri-procedural antibiotics and heparin may be given. Central venous puncture (femoral or jugular) for cannulation is commonly achieved using real-time ultrasound. Once the veins are cannulated then guidewire position is confirmed with fluoroscopy or echocardiography to ensure correct placement. Once the ECMO practitioner is satisfied with the position of the guidewires, the skin is dilated by use of increasing size dilators fed over the guidewire using the Seldinger technique.10 The ECMO circuit is then connected and ECMO commenced under the guidance of the retrieval perfusionist.
There are significant physiological changes which occur during the process of cannulation. The patient has commonly been moved from ICU to the operating theatres and either the act of ventilator disconnection or movement has potential for lung de-recruitment and further hypoxaemia/hypercapnoea. Additionally, the patient is commonly nursed at 30–45° head up in ICU and during cannulation is most commonly nursed lying flat. This too has the potential to cause progressive respiratory deterioration. Although uncommon, the patient may also bleed during cannulation. Following connection of the circuit and commencement of ECMO, there is commonly a transient period of hypotension due to the priming volume (750 mL), prime temperature (often lower than core temperature), and a relative bradycardia due to this lower prime temperature. Following the transient hypotension, as the respiratory acidosis and systemic oxygenation improve, there is improvement in acute pulmonary hypertension and consequently global cardiac output. There is also improvement in systemic vascular resistance as the milieu interieur improves. This effectively results in a period where the vasopressors need rapid reduction in order to maintain relative normotension. These elements need to be managed by the anaesthetist from the referring centre in collaboration with the ECMO consultant. Once established on ECMO, the patient will be transported back to the ECMO centre by road or air transport.
Once returned to the ECMO centre, patients will undergo a series of investigations to reach a final diagnosis and exclude complications. In Guy's and St Thomas' Hospital, all patients undergo a CT head/thorax/abdomen/pelvis, bronchoscopy with a wide panel of microbiological investigations (including a 16S-PCR), an echocardiogram and a vasculitic/autoimmune screen. An integral part of the management of patients with SRF is the use of chest CT. This provides vital information on the potential underlying diagnosis. If the patient is sufficiently stable, referring centres are encouraged to obtain chest CT images early in the disease course of these patients. However, for many patients the relative risk of transfer to CT is significant and although thoracic CT does provide crucial information, it is uncommon for it to provide information which will have prevented the application of ECMO through an acute change in management strategy.
Once established on ECMO, the goal is the treatment of the underlying disease process, use of appropriate supportive therapies and lung rest. The application of ECMO allows the avoidance of high plateau pressures and the use of ultra-low tidal volume ventilatory strategies. A frequently employed strategy is the use of PEEP 10–15 cmH2O, maintenance of a plateau pressure of no more than 25 cmH2O and a low respiratory rate (10–15).32 The major problem with this strategy is that tidal ventilation is commonly lower than anatomical dead space and complete lung collapse occurs giving a high requirement for respiratory physiotherapy.33
Some patients referred for ECMO with severe respiratory failure will have a severe but short disease process, particularly influenza (10–14 days) or asthma (3–5 days). Others will have a longer-term course and if suitable, be extubated on ECMO or require a tracheostomy and prolonged ventilator weaning. Patients who progress to fibrosis either following ARDS or due to a defined acute interstitial lung disease tend to fall into the latter category and may require extensive rehabilitation, mobilisation and long periods on ECMO (weeks to months) before pulmonary recovery is adequate. As the underlying disease process resolves, the contribution of the ECMO circuit to systemic oxygenation/carbon dioxide removal diminishes and patients approach the point where ECMO can be ceased, the cannulae removed and the access sites are commonly sutured (which may be removed two weeks post decannulation).
The team is multi-disciplinary in nature, consisting of consultant intensivists, ECMO specialist nurses, physiotherapists and perfusionists. Additionally, cases are often discussed at weekly meetings in the presence of experts experienced in management of inflammatory lung conditions, such as a respiratory physician, rheumatologists and chest specialist radiologists. This is especially useful in more complex cases such as in organising pneumonias and diffuses alveolar haemorrhage, where high-dose immunosuppression may be collaboratively considered.
Once the patient has been decannulated and has no further requirements for them to remain at the SRF centre then contact is made with the original referring centre with view to repatriation. This contact is generally made a day or two after decannulation providing there are no other complex issues. The patient is then back-transferred once a critical care bed is available. Following repatriation centres will commonly offer a follow-up clinic appointment within three to six months. A common complication post ECMO is deep vein thrombosis (DVT) and all patients have lower limb Doppler ultrasounds post decannulation.34,35 If a DVT is identified, it is advised they receive three months of low molecular weight heparin therapy and are referred to the local anticoagulation service. Stitches from the cannula sites should be removed at 12 days and any surrounding wound infection treated as per local hospital guidelines (though this is rare). Significant psychological and physical symptoms are commonplace following the critical illness associated with ECMO and patients may require ongoing psychological and financial support, although many can return to their previous level of functioning.36 Access to appropriate support services can be accessed through the SRF centre's follow-up clinic. In the unusual situation where a patient who has been repatriated to their referring hospital deteriorates again then further contact should be made to the SRF centre. Consideration for repeat of ECMO therapy will be made based upon the causative disease process and likely reversibility, although a second run of ECMO for any one individual patient is very unusual.
Governance
It is important to appreciate the stringent standards under which the commissioned ECMO centres operate. All centres are required to meet the commissioning standards which include elements relating to staffing, 24/7 provision of care, ancillary services, audit and quality improvement. Centres are obliged to share outcomes with referrers, patients/families, commissioners and other centres. Centres are also obliged to report all outcomes to the Extracorporeal Life Support Organisation (ELSO) to allow international benchmarking. All commissioned UK centres exceed international outcomes. In the UK, survival to discharge of the last 700 consecutive patients over three years who required VV ECMO support was 81%.37 Additionally, commissioned centres have a six monthly audit meeting where outcomes and agreed audit criteria are presented. Commissioned centres have also undergone review by the NHS England and have also successfully undergone peer review of the service.
Difficult decisions will routinely be discussed within a centre by the consultants as well as between centres. Furthermore, lessons learnt from case selection particularly around cohorts of patients who do or do not benefit from ECMO are discussed at the biannual ECMO meeting. This has resulted in a progressive expansion in the groups of patients in whom ECMO is recognised to provide a benefit, for example intracranial haemorrhage, acute pulmonary haemorrhage and trauma.18,38,39
The future for ECMO
ECMO for severe respiratory failure has become an established therapy in England with five centres providing equitable access to the population 24 h a day, using mobile ECMO to facilitate retrievals and safe patient transfer. Future potential commissioning pathways include ECMO as a bridge to lung transplantation and ECMO as supportive therapy for acute cardiogenic shock. In this latter patient group, survival is predominantly predicted by the time to commencement of veno-arterial (VA) ECMO and reversibility of the underlying pathology.40 At present there is no commissioned pathway for ECMO for acute cardiogenic shock, outside of the limited number of patients who require ECMO as a bridge to transplantation. There is also growing interest in low flow extracorporeal support for carbon dioxide removal (ECCO2R) which is conceptually similar to renal replacement therapy.41–43 Potential applications, which are currently the subject of research studies, include patients with exacerbations of chronic obstructive pulmonary disease and in facilitating lung protective ventilation.44 Should the evidence support these approaches, it is likely that this technology will become relatively widely available and utilised.
Conclusion
ECMO for severe respiratory failure has a robust referral and retrieval system within the UK with excellent outcomes when compared internationally. The indications are consistently expanding as the knowledge base grows and clinicians are encouraged to refer early for specialist advice.
Referral details
Guy's and St Thomas' Hospital: 020 7188 2511 University Hospitals of Leicester (Glenfield): 0300 300 3200 Royal Brompton and Harefield Hospital: 020 7351 8585 Papworth Hospital: 01480 830541 University Hospital of South Manchester (Wythenshawe): 07837 541143
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Paden ML, SA Conrad, PT Rycus, et al. Extracorporeal life support organization registry report 2012. ASAIO J 2013; 59: 202–210. [DOI] [PubMed] [Google Scholar]
- 2.Hill JD, O'Brien TG, Murray JJ, et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med 1972; 286: 629–634. [DOI] [PubMed] [Google Scholar]
- 3.Gattinoni L, Pesenti A, Mascheroni D, et al. Low-frequency positive pressure ventilation with extracorporeal CO2-removal in severe acute respiratory failure. JAMA 1986; 256: 881–886. [PubMed] [Google Scholar]
- 4.Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 1994; 149: 295–305. [DOI] [PubMed] [Google Scholar]
- 5.Peek GJ, Clemens F, Elbourne D, et al. CESAR: conventional ventilatory support vs extracorporeal membrane oxygenation for severe adult respiratory failure. BMC Health Serv Res 2006; 6: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peek GJ, Killer HM, Sosnowski MA, et al. Modular extracorporeal life support for multiorgan failure patients. Liver 2002; 22: 69–71. [DOI] [PubMed] [Google Scholar]
- 7.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009; 374: 1351–1363. [DOI] [PubMed] [Google Scholar]
- 8.Combes A, Hajage D, Capellier D, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med 2018; 378: 1965–1975. [DOI] [PubMed] [Google Scholar]
- 9.Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 2011; 306: 1659–1668. [DOI] [PubMed] [Google Scholar]
- 10.Brodie D, Bacchetta M. Extracorporeal membrane oxygenation in adults. N Engl J Med 2011; 365: 1905–1914. [DOI] [PubMed] [Google Scholar]
- 11.Burns J, Cooper E, Salt G, et al. Retrospective observational review of percutaneous cannulation for extracorporeal membrane oxygenation. ASAIO J 2016; 62: 325–328. [DOI] [PubMed] [Google Scholar]
- 12.Sherren PB, Shepherd SJ, Glover GW, et al. Capabilities of a mobile extracorporeal membrane oxygenation service for severe respiratory failure delivered by intensive care specialists. Anaesthesia 2015; 70: 707–714. [DOI] [PubMed] [Google Scholar]
- 13.Barrett NA, Camporota L, Langrish CJ, et al. Severe respiratory failure in the UK. JICS 2013; 14: 114–119. [Google Scholar]
- 14.Schmidt M, Tachon G, Devilliers C, et al. Blood oxygenation and decarboxylation determinants during venovenous ECMO for respiratory failure in adults. Intensive Care Med 2013; 39: 838–846. [DOI] [PubMed] [Google Scholar]
- 15.Cawcutt K, Gallo De Moraes A, Lee S, et al. The use of ECMO in HIV/AIDS with pneumocystis jirovecii pneumonia: a case report and review of the literature. ASAIO J 2014; 60: 606–608. [DOI] [PubMed] [Google Scholar]
- 16.De Rosa F, Fanelli V, Corcione S, et al. Extra corporeal membrane oxygenation (ECMO) in three HIV-positive patients with acute respiratory distress syndrome. BMC Anesthesiol 2014; 14: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capatos G. ECMO in the HIV population. Qatar Med J 2017; 2017: 45.
- 18.Lockie C, Gillon S, Barrett N, et al. Severe respiratory failure, extra corporeal membrane oxygenation and intracranial haemorrhage. Crit Care Med 2017; 45: 1642–1649. [DOI] [PubMed] [Google Scholar]
- 19.Fortenberry J, Peek G, Maclaren G, et al. Position statement on the use of extracorporeal life support in patients with ebola virus disease from the steering committee, Extracorporeal Life Support Organization. 2014, October, www.elso.org/EbolaStatement.aspx (accessed, 17 August 2019).
- 20.Kang HS, Rhee CK, Lee HY, et al. Clinical outcomes of extracorporeal membrane oxygenation support in patients with hematologic malignancies. Korean J Intern Med 2015; 30: 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wohlfarth P, Wohlfarth P, Ullrich R, et al. Extracorporeal membrane oxygenation in adult patients with hematologic malignancies and severe acute respiratory failure. Crit Care 2014; 18: R20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gow KW, Lao OB, Leong T, et al. Extracorporeal life support for adults with malignancy and respiratory or cardiac failure: the extracorporeal life support experience. Am J Surg 2010; 199: 669–675. [DOI] [PubMed] [Google Scholar]
- 23.Rozencwaijg S, Pilcher D, Combes A, et al. Outcomes and survival prediction models for severe adult acute respiratory distress syndrome treated with extracorporeal membrane oxygenation. Crit Care 2016; 20: 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt M, Bailey M, Sheldrake J, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med 2014; 189: 1374–1382. [DOI] [PubMed] [Google Scholar]
- 25.Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013; 368: 2159–2168. [DOI] [PubMed] [Google Scholar]
- 26.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome: a randomized control trial. JAMA 2004; 299: 646–655. [DOI] [PubMed] [Google Scholar]
- 27.The National Heart, Lung and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-managmenet strategies in acute lung injury. N Engl J Med 2006; 354: 2564–2575.16714767 [Google Scholar]
- 28.Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015; 372: 747–755. [DOI] [PubMed] [Google Scholar]
- 29.The Acute Respiratory Distress Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342: 1301–1308. [DOI] [PubMed] [Google Scholar]
- 30.Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010; 363: 1107–1116. [DOI] [PubMed] [Google Scholar]
- 31.Vogel D, Murray J, Czapran A, et al. Veno-arterio-venous ECMO for septic cardiomyopathy: a single-centre experience. Perfusion 2018; 33: 57–64. [DOI] [PubMed] [Google Scholar]
- 32.Camporota L, Nicoletti E, Malafronte M, et al. International survey on the management of mechanical ventilation during ECMO in adults with severe respiratory failure. Minerva Anestesiol 2015; 81: 1170–1183. [PubMed] [Google Scholar]
- 33.Cork G, Barrett N, Ntoumenopoulos G. Justification for chest physiotherapy during ultra-protective lung ventilation and extra-corporeal membrane oxygenation: a case study. Physiother Res Int 2014; 19: 126–128. [DOI] [PubMed] [Google Scholar]
- 34.Menaker J, Tabatabai A, Rector R, et al. Incidence of cannula associated deep vein thrombosis after veno-venous extracorporeal membrane oxygenation. ASAIO J 2017; 63: 588–591. [DOI] [PubMed] [Google Scholar]
- 35.Cooper E, Burns J, Retter A, et al. Prevalence of venous thrombosis following venovenous extracorporeal membrane oxygenation in patients with severe respiratory failure. Crit Care Med 2015; 43: e581–e584. [DOI] [PubMed] [Google Scholar]
- 36.Galazzi A, Brambilla A, Grasselli G, et al. Quality of life of adult survivors after extra corporeal membrane oxygenation (ECMO). Dimens Crit Care Nurs 2018; 37: 12–17. [DOI] [PubMed] [Google Scholar]
- 37.Patel BV, Barrett NA, Vuylsteke A. ECMO for severe acute respiratory distress syndrome. N Engl J Med 2018; 379: 1090–1091. [DOI] [PubMed] [Google Scholar]
- 38.Simpson T, Ling C, Glover G, et al. Extra-corporeal membrane oxygenation and diffuse alveolar haemorrhage – a single centre case series and analysis of the ELSO database. Thorax 2014; 69: A195–A196. [Google Scholar]
- 39.Arlt M, Philipp A, Voelkel S, et al. Extracorporeal membrane oxygenation in severe trauma patients with bleeding shock. Resuscitation 2010; 81: 804–809. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt M, Burrell A, Roberts L, et al. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial -ECMO (SAVE)-score. Eur Heart J 2015; 36: 2246–2256. [DOI] [PubMed] [Google Scholar]
- 41.Moss C, Galtrey E, Camporota L, et al. A retrospective observational case series of low flow veno-venous extracorporeal carbon dioxide removal in patients with respiratory failure. ASAIO J 2016; 62: 458–462. [DOI] [PubMed] [Google Scholar]
- 42.Camporota L, Barrett N. Current applications for the use of extracorporeal carbon dioxide removal in critically ill patients. Biomed Res Int 2016; 2016: 9781695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taccone FS, Malfertheiner MV, Ferrari F, et al. Extracorporeal CO2 removal (ECCO2R) in critically ill patients: a systematic review. Minerva Anestesiol 2017; 83: 762–772. [DOI] [PubMed] [Google Scholar]
- 44.McNamee J, Gillies MA, Barrett NA, et al. pRotective vEntilation with veno-venouS lung assisT in respiratory failure (REST): a protocol for a multi centre randomised controlled trial of extracorporeal carbon dioxide removal in patients with acute hypoxaemic respiratory failure. J Intensive Care Soc 2017; 18: 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]