Abstract
Background
Randomized and observational studies have previously reported inconsistent results for the direct association between statin therapy and 25, hydroxyvitamin D [25(OH)D] levels. Thus, the present study aimed to examine the relationship between statin use and 25(OH)D and its metabolites concentrations in a large nationally representative sample of older adults.
Methods
This study was conducted using data from the National Health and Nutrition Examination Survey. Participants were asked to show the medication containers of all the products used in the previous 30 days, and the prescription of statins was defined on the three-level nested therapeutic classification scheme of Cerner Multum’s Lexicon. General linear models adjusted for potential confounders were created to compare 25(OH)D concentrations between older adults taking statins and those who did not.
Results
A total of 6,261 participants with a mean age of 69.5 years comprised the study sample. Of those, 40.2% were taking statins with a median length of therapy of 5 years. Adjusted mean 25(OH)D3 and 25(OH)D levels were 3.3 and 4.4 nmol/L higher among participants taking statins than those who did not, respectively. Moreover, this association was consistently seen regardless of the duration of therapy and particularly in subjects taking simvastatin, atorvastatin, or rosuvastatin. In subgroup analyses according to BMI categories and vitamin D intake, higher 25(OH)D levels were also seen among statin users. By contrast, this association was attenuated among those with a daily vitamin D between 400 and 800 and >800 IU.
Conclusion
Older adults on statin therapy had significantly higher serum 25(OH)D concentrations. Additional research should be conducted to define the mechanism of this association and determine if the pleiotropic effects attributed to statins may be mediated by vitamin D.
Keywords: statins, vitamin d levels, older adults
Introduction
3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins) have been widely used as a lipid-lowering therapy in the primary and secondary prevention of atherosclerotic cardiovascular disease [1-3]. A recent analysis of the National Health and Nutrition Examination Survey (NHANES) described that prescription of cholesterol-lowering drugs increased with age from 17.4% in adults aged 40-59 to 47.6% in adults aged 75 years and older during 2011-2012. Moreover, among adults prescribed cholesterol-lowering medication, 83% used a statin alone [4].
Although the precise mechanism of statin therapy on 25(OH)D metabolism is not fully clarified, researchers have postulated that since 7-dehydrocholesterol is the common precursor of vitamin D and cholesterol, the inhibition of 3-hydroxy-3-methylglutaryl-coenzyme A reductase may increase 7-dehydrocholesterol levels providing an adequate substrate for the synthesis of 25-hydroxyvitamin D [25(OH)D] [5]. The occupation of the active site of the CYP3A4 enzyme by statins may also account for increased serum 25(OH)D levels [6]. Previously, few observational studies and randomized controlled trials reported conflicting results regarding the effect of statins on 25(OH)D concentrations. Some studies described higher 25(OH)D concentrations among statin users, whereas others did not [7-15]. Nevertheless, these studies were limited by small sample size, subjects with acute coronary syndrome, individual generic statins, diabetics hospitalized, and postmenopausal women with osteopenia [7,9,11,14-15]. Therefore, the purpose of this study was to examine the relationship between statin use and 25(OH)D concentrations in a nationally representative sample of older adults.
Materials and methods
The NHANES is a biannual cross-sectional study conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention. The purpose of the NHANES is to collect data about the health, nutritional status, and health behaviors of the noninstitutionalized civilian resident population of the U.S. Information about the analysis and reporting guidelines of the NHANES are described elsewhere [16].
Characteristics of participants
The demographics file provides individual, family, and household level information on the following topics: The six-month time period when the examination was performed, demographic information about the household reference person, and other selected demographic information, such as gender, age, race/Hispanic origin, and education. The Department of Health and Human Services guidelines were used as the poverty measure to calculate the ratio of family income to poverty.
In the Mobile Examination Center, BMI was calculated as body weight in kilograms divided by height in meters squared and then rounded to one decimal place. Questionnaires on alcohol consumption and cigarette smoking were also administered. As previously reported, the physical activity questionnaire is based on the Global Physical Activity Questionnaire and includes questions related to leisure-time activities. The reported number of days and time (in minutes) spent performing vigorous or moderate leisure-time physical activity in the previous week were calculated, and according to the 2008 Physical Activity Guidelines for Americans, participants were categorized as physically active, insufficiently active, or physically inactive [17]. Moreover, participants were asked about their general health, which was categorized as good to excellent and fair to poor. The diagnosis of diabetes was established if participants reported a physician diagnosis of diabetes or had an HbA1c ≥6.5% [18]. Cardiovascular disease was defined based on an affirmative response to any one of four questions about doctor-diagnosed heart attack, angina, coronary heart disease, or stroke [4]. Similarly, participants were considered to have high cholesterol if they affirmatively responded to the question “Have you ever been told by a doctor or other health professional that your blood cholesterol level was high?”
Vitamin D intake
Since the NHANES cycle 2007-2008, vitamin D intake has been collected from the types and amounts of foods and beverages consumed during the 24-hour period prior to the interview. All NHANES participants responding to the dietary recall interview were also eligible for the dietary supplement questions. The participant’s use of vitamin D supplements was reported as a mean vitamin D dosage over the course of 30 days prior to the household interview. Data were routinely examined for discrepancies and erroneous entries [19].
25(OH)D and its Metabolite Concentrations
The CDC developed the standardized liquid chromatography-tandem mass spectrometry (LC-MS/MS) to measure 25(OH)D3, 25(OH)D2, and total 25(OH)D levels. For the LC-MS/MS method, total 25(OH)D (in SI units of nmol/L) was defined as the sum of 25(OH)D3 and 25(OH)D2. However, due to rounding off, the sum of 25(OH)D3 and 25(OH)D2 will not necessarily be equal to the 25(OH)D. This method has better analytical specificity and sensitivity compared to immunoassay methods and fixed analytical goals for imprecision (≤10%) and bias (≤5%) [20].
Statin prescription
During the household interview, participants were asked if they have taken medications in the past 30 days for which they needed a prescription. Those who answer “yes” were asked to show the interviewer the medication containers of all the products used. For each drug reported, the interviewer recorded the product’s complete name from the container. Prescription medications were classified based on the three-level nested therapeutic classification scheme of Cerner Multum’s Lexicon. Cholesterol-lowering medications were identified using the second level of drug category code 19, and the third level of drug category code 173 was used to identify statins [4]. The following drugs were included in the present analysis: lovastatin, pravastatin, simvastatin, fluvastatin, atorvastatin, cerivastatin, rosuvastatin, and pitavastatin.
Statistical analysis
The characteristics of the study population were stratified according to statin therapy and compared using the chi-squared test and the t-test for categorical and continuous variables, respectively. General linear models adjusted for the six-month study period, age, gender, race/ethnicity, education, income, BMI, smoking status, alcohol use, physical activity, self-reported health status, diabetes, and vitamin D intake were created to compare 25(OH)D2, 25(OH)D3, and total 25(OH)D concentrations between statin users with their non-user counterparts. Similarly, the independent associations between generic statins, the duration of statin therapy (< 2, 3-6, and >6 years) and 25(OH)D concentrations were explored. Since a small number of participants (n = 5) were prescribed fluvastatin, cerivastatin, or pitavastatin, these drugs were excluded from this analysis. Moreover, in subgroup analyses, the modifying effects of BMI categories and vitamin D intake (< 400, 400-800, and >800 IU) on 25(OH)D levels were examined according to statin therapy status. Statistical analyses were performed using SPSS Complex Sample software, V.17 (SPSS Inc, Chicago, Illinois, USA) to incorporate constructed weights for the combined survey cycles and obtain unbiased, national estimates representative of the older U.S. population. A P-value <0.05 was considered statistically significant. Of 7,859 participants aged 60 years and older in the NHANES cycles 2007-2008 through 2013-2014, those with missing data on 25(OH)D concentrations (n = 990), BMI (n = 514), and dietary vitamin D (n = 945) were excluded from the analysis. In general, participants with missing data were more likely to be examined during the winter months, female, had less than high school education, physically inactive, and self-reported fair to poor health.
Results
A total of 6,261 participants with a mean age of 69.5 years comprised the sample size. Of these, 40.2% reported used statins and the median duration of therapy was 5 years. Table 1 shows the characteristics of participants stratified according to statin therapy. Overall, statin therapy was higher in men, non-Hispanic whites, high-school graduates, former smokers, and those with fair to poor health compared with those who did not. Moreover, as expected, statins were more frequently prescribed among diabetics and those with cardiovascular disease. In general, mean 25(OH)D2 and total 25(OH)D concentrations were significantly higher among statin users than their non-user counterparts.
Table 1. Characteristics of the participants according to statin therapy.
RIP, Ratio of family income to poverty; AA, Associates of Arts
Parenthesis represents standard error of the estimates.
| Statin users (n = 2,456) | Non-users (n = 3,805) | P value | |
| Six-month period, % | 0.816 | ||
| Nov 1st to Apr 30th | 38.7 (3.4) | 38.3 (3.4) | |
| May 1st to Oct 31th | 61.3 (3.4) | 61.7 (3.4) | |
| Age (years), mean | 70.4 (0.2) | 69.0 (0.1) | <0.0001 |
| Gender, % | <0.0001 | ||
| Male | 51.0 (1.1) | 42.0 (0.8) | |
| Female | 49.0 (1.1) | 58.0 (0.8) | |
| Race/ethnicity, % | <0.05 | ||
| Hispanics | 6.2 (0.9) | 7.8 (1.0) | |
| Non-Hispanic white | 81.1 (1.3) | 80.3 (1.5) | |
| Non-Hispanic black | 7.7 (0.8) | 8.0 (0.8) | |
| Others | 5.0 (0.6) | 3.9 (0.4) | |
| BMI (kg/m2), mean | 29.8 (0.1) | 28.4 (0.1) | <0.0001 |
| Education, % | <0.05 | ||
| Less than high school | 21.8 (1.5) | 19.4 (1.2) | |
| High school graduate | 25.7 (1.4) | 22.4 (0.9) | |
| Some college or AA degree | 28.4 (1.4) | 29.1 (1.0) | |
| College graduate or above | 24.1 (1.4) | 29.1 (1.6) | |
| RIP, % | 0.101 | ||
| <1.00 | 8.6 (0.7) | 9.7 (0.6) | |
| ≥1.00 | 91.4 (0.7) | 90.3 (0.6) | |
| Smoking status, % | 0.0001 | ||
| Never | 45.2 (1.5) | 51.7 (1.1) | |
| Former | 44.5 (1.4) | 37.5 (0.9) | |
| Current | 10.8 (0.7) | 10.8 (0.7) | |
| Alcohol use, % | 0.470 | ||
| Yes | 70.1 (1.4) | 69.0 (1.4) | |
| No | 29.9 (1.4) | 31.0 (1.4) | |
| Physical activity status, % | 0.342 | ||
| Inactive | 56.3 (1.6) | 56.6 (1.4) | |
| <150 min/week | 14.7 (0.9) | 16.2 (0.8) | |
| ≥150 min/week | 29.0 (1.4) | 27.2 (1.3) | |
| General health condition, % | <0.05 | ||
| Good to excellent | 75.6 (1.2) | 80.2 (0.9) | |
| Fair to poor | 24.4 (1.2) | 19.8 (0.9) | |
| Diabetes, % | <0.0001 | ||
| Yes | 33.2 (1.2) | 15.9 (0.9) | |
| No | 66.8 (1.2) | 84.1 (0.9) | |
| High cholesterol, % | <0.0001 | ||
| Yes | 82.2 (0.9) | 38.9 (1.1) | |
| No | 17.8 (0.9) | 61.1 (1.1) | |
| Cardiovascular disease, % | <0.0001 | ||
| Yes | 66.0 (1.8) | 34.0 (1.8) | |
| No | 33.7 (1.0) | 66.3 (1.0) | |
| Vitamin D intake (IU), % | <0.05 | ||
| <400 | 43.5 (1.4) | 48.5 (0.9) | |
| 400-800 | 22.1 (0.9) | 21.2 (1.1) | |
| >800 | 34.4 (1.2) | 30.3 (1.1) | |
| 25(OH)D (nmol/L), mean | |||
| Total 25(OH)D | 79.0 (1.0) | 75.4 (0.8) | <0.05 |
| 25(OH)D3 | 71.5 (1.0) | 69.8 (0.9) | 0.081 |
| 25(OH)D2 | 7.5 (0.7) | 5.6 (0.4) | <0.05 |
As shown in Table 2, after adjustment for potential confounders, 25(OH)D3 and 25(OH)D levels were on average 3.1 and 4.4 nmol/L higher among older adults taking statins than those who did not, respectively. In contrast, a non-significant 1.4 nmol/L difference in 25(OH)D2 levels was seen among older adults on statin therapy.
Table 2. The association between statin therapy and 25(OH)D levels and its metabolites among older adults.
Model 1 adjusted for study period, age, gender, race/ethnicity, education, income, BMI, smoking status, alcohol use, and physical activity.
Model 2 adjusted for model 1 and self-reported health status, diabetes, and vitamin D intake.
| Vitamin D concentrations | Statin users (SE) | Non-users (SE) | Mean difference (SE) | P-value |
| 25(OH)D3 (nmol/L) | ||||
| Mean | 71.5 (1.0) | 69.8 (0.9) | 1.7 (0.9) | 0.081 |
| Model 1 | 72.9 (0.9) | 69.1 (0.8) | 3.8 (0.9) | <0.0001 |
| Model 2 | 72.4 (0.8) | 69.3 (0.7) | 3.1 (0.8) | <0.05 |
| 25(OH)D2 (nmol/L) | ||||
| Mean | 7.5 (0.7) | 5.6 (0.4) | 1.9 (0.6) | <0.05 |
| Model 1 | 7.5 (0.7) | 5.7 (0.4) | 1.8 (0.6) | <0.05 |
| Model 2 | 7.2 (0.7) | 5.8 (0.4) | 1.4 (0.6) | 0.054 |
| Total 25(OH)D (nmol/L) | ||||
| Mean | 79.0 (1.0) | 75.4 (0.8) | 3.6 (1.0) | <0.05 |
| Model 1 | 80.5 (1.0) | 74.8 (0.7) | 5.7 (0.9) | <0.0001 |
| Model 2 | 79.6 (0.8) | 75.2 (0.7) | 4.4 (1.0) | <0.0001 |
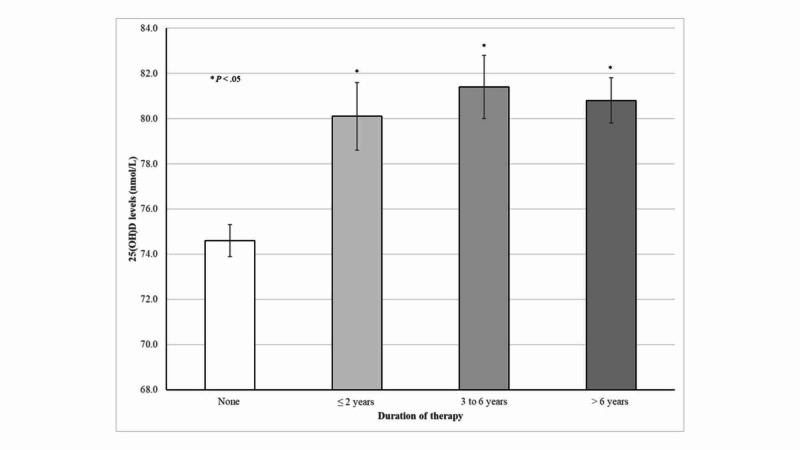
As shown in Figure 1, adjusted mean 25(OH)D concentrations were consistently higher among older adults taking statins irrespective of the duration of treatment. For instance, those taking statins >6 years had on average 6.2 nmol/L higher 25(OH)D concentrations than those who did not.
Figure 1. 25(OH)D concentrations according to duration of statin therapy among older adults.
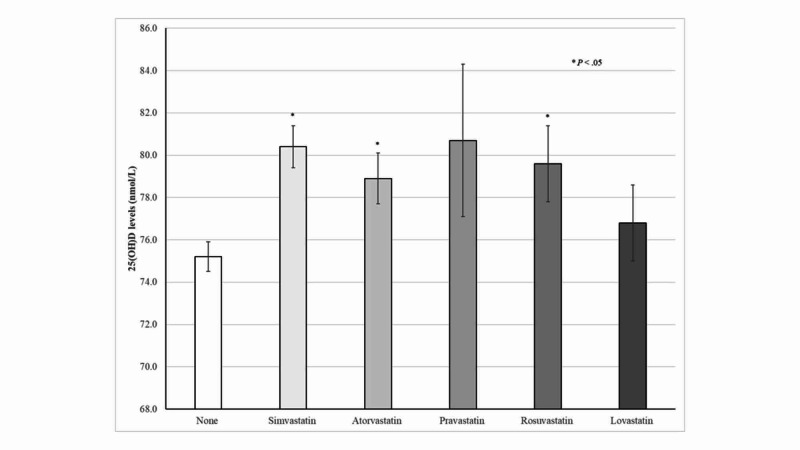
As shown in Figure 2, overall higher 25(OH)D concentrations were consistently seen among participants on any generic statin therapy. However, these associations were statically significant among subjects taking simvastatin, atorvastatin, or rosuvastatin.
Figure 2. 25(OH)D concentrations according to generic statin therapy among older adults .
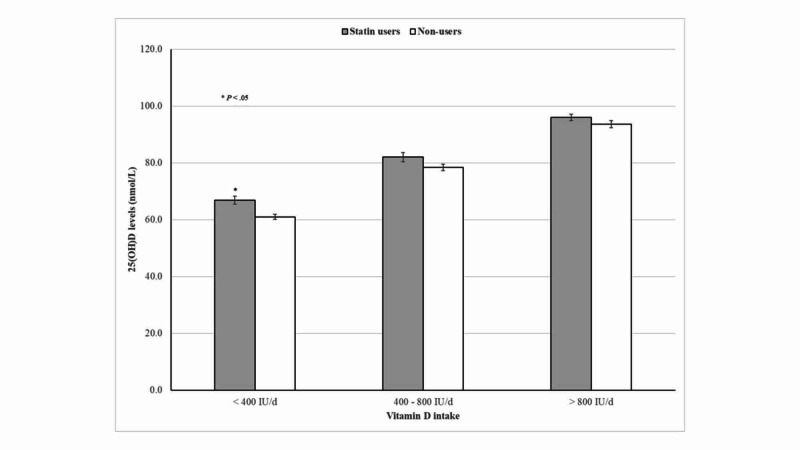
As shown in Figure 3, 25(OH)D concentrations progressively increased with an increase in vitamin D intake. However, no significant differences in 25(OH)D levels were seen between statin users compared with their non-user counterparts, particularly among participants taking vitamin D between 400 and 800 IU and >800 IU per day.
Figure 3. 25(OH)D concentrations according to statin therapy and vitamin D intake.
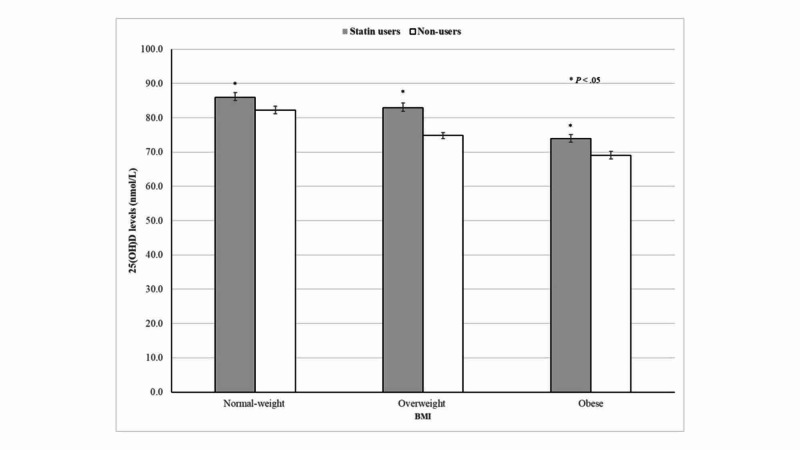
As expected, 25(OH)D concentrations were inversely correlated with BMI categories. Despite this evidence, higher 25(OH)D levels were seen among normal-weight, overweight, and obese participants on statin therapy than those who did not (Figure 4).
Figure 4. 25(OH)D concentrations according to statin therapy and BMI categories.
BMI, body mass index
Discussion
The present findings indicate that serum 25(OH)3 and total 25(OH)D concentrations were significantly higher among statin users than their non-user counterparts. Notably, these associations were consistently seen after adjustment for potential confounders, irrespective of the duration of therapy, or generic statin prescribed. Our findings contrast with those from a recent systematic review and meta-analysis conducted by Mazidi et al., in which inconclusive effects of statins on 25(OH)D concentrations were reported. Indeed, the pooled estimate (weighted mean difference) of the effect of statin therapy on serum vitamin D from randomized clinical trials was 2.71 ng/ml and decreased by -0.70 ng/ml in observational studies. These contradictory findings were attributed to small sample sizes of the included studies and different doses and duration of vitamin D supplementation [5].
In subgroup analyses, we found that among participants with dietary vitamin D intakes between 400 and 800 IU or >800 IU per day, 25(OH)D concentrations did not significantly differ between participants on statin therapy and those who did not. However, increased 25(OH)D levels were seen among older adults on statin therapy and a daily intake of <400 IU per day. Since dietary vitamin D intake from food and supplements is the main determinant of vitamin D status in older adults, increased 25(OH)D levels among statin users were particularly seen among those with inadequate vitamin D intake [21]. In addition, Bischoff-Ferrari et al. in a pooled analysis of three double-blind randomized clinical trials suggested that lower 25(OH)D response to vitamin D supplementation may be a class effect of all statins, which differ according to vitamin D dosing regimens [13].
Notably, higher 25(OH)D concentrations were consistently seen among statin users, irrespective of the duration of therapy. Moreover, mean 25(OH)D concentrations minimally differed between statin therapy <2 years compared with their counterparts with >6 years. Thus, the present findings suggest that statins have a sustained effect on 25(OH)D levels, which was seen even at the beginning of therapy. Similarly, higher 25(OH)D concentrations were seen among participants according to individual generic statins use. This association was statistically significant among participants taking simvastatin, atorvastatin, or rosuvastatin. Indeed, subjects taking simvastatin and atorvastatin, the most frequently prescribed statins, had on average 5.2 and 3.7 nmol/L higher 25(OH)D levels compared with non-users, respectively. In contrast, Renjmark et al. in a small randomized controlled trial among healthy postmenopausal women reported no significant differences in plasma 25(OH)D levels between subjects treated with simvastatin 40 mg/day for a year compared with their counterparts receiving placebo [15]. Likewise, Mazidi et al. previously demonstrated in a randomized, double-blind, placebo-controlled trial lasting 30 days that simvastatin 40 mg/day did not have a significant effect on serum 25(OH)D levels [7]. Possible explanations for these contradictory results were attributed to unmeasured predictors of vitamin D status such as vitamin D intake and sun exposure [15]. Lower 25(OH)D concentrations have been consistently reported across different latitudes in obese subjects compared with their normal-weight counterparts, which have been related to limited sun exposure, decreased bioavailability of vitamin D in fat tissue, and lower vitamin D supplements use [22-25]. As expected, the present results also demonstrated an inverse relationship between BMI and 25(OH)D concentrations among older adults. Despite this evidence, normal-weight, overweight, and obese participants taking statins had significantly higher 25(OH)D concentrations than those who did not.
Of relevance, the beneficial effects of statin therapy on coronary artery disease have been described as not only aggressive reduction of circulating pro-atherogenic lipid particles but also to its pleiotropic effects, including improvement of endothelial function and anti-inflammatory and anti-oxidant actions, whose mechanism is presently undetermined [26]. Moreover, it has been postulated that the pleiotropic effects of statins may be mediated by vitamin D [9]. In fact, in agreement with this hypothesis, previous studies have demonstrated that lower 25(OH)D concentrations have been inversely associated with the progression of aortic calcification and myocardial infarction [27-28].
The present study has several limitations that should be mentioned. First, because of the NHANES cross-sectional design, these results do not necessarily infer causation. Second, the statin dosage was not available in the prescription medication section. Thus, the intensity of statin therapy on 25(OH)D concentrations may not be established. Third, sun exposure and sun-protective behavior characteristics, which may have a direct effect on the synthesis of 25(OH)D3, were not explored in the present analysis. Fourth, most sociodemographic and health variables were self-reported, which may have led to recall bias. Despite these limitations, this study clearly demonstrated that statin therapy is associated with increased 25(OH)D concentrations in a large nationally representative sample of older adults.
Conclusions
Older adults on statin therapy had significantly higher 25(OH)D concentrations than those who did not. Further research is needed to precisely define the mechanism of this association and determine whether the pleiotropic effects attributed to statins may be mediated by vitamin D.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained by all participants in this study
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. Shepherd J, Cobbe SM, Ford I, et al. https://pubmed.ncbi.nlm.nih.gov/7566020/ N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 2.The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. Sacks FM, Pfeffer MA, Moye LA, et al. https://pubmed.ncbi.nlm.nih.gov/8801446/ N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 3.2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Grundy SM, Stone NJ, Bailey AL, et al. https://www.ahajournals.org/doi/10.1161/CIR.0000000000000625. Circulation. 2019;139:1046–1081. doi: 10.1161/CIR.0000000000000624. [DOI] [PubMed] [Google Scholar]
- 4.Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003-2012. Gu Q, Paulose-Ram R, Burt VL, et al. https://pubmed.ncbi.nlm.nih.gov/25536410/#:~:text=During%202003%E2%80%932012%2C%20there%20was,in%20the%20past%2030%20days. NCHS Data Brief. 2014;177:1–8. [PubMed] [Google Scholar]
- 5.Effect of statins on serum vitamin D concentrations: a systematic review and meta-analysis. Mazidi M, Rezaie P, Vatanparast H, et al. Eur J Clin Invest. 2017;47:93–101. doi: 10.1111/eci.12698. [DOI] [PubMed] [Google Scholar]
- 6.Possible mechanisms of interaction between statins and vitamin D. Bhattacharyya S, Bhattacharyya K, Maitra A. QJM. 2012;105:487–491. doi: 10.1093/qjmed/hcs001. [DOI] [PubMed] [Google Scholar]
- 7.Simvastatin treatment does not affect serum vitamin D concentrations in patients with dyslipidemia: a Randomized double-blind placebo-controlled cross-over trial. Mazidi M, Rokni H, Sahebkar AH, et al. Int J Prev Med. 2016;8:80. doi: 10.4103/2008-7802.183652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vascular protective effects of statin-related increase in serum 25-hydroxyvitamin D among high-risk cardiac patients. Chan YH, Lau KK, Yiu KH, et al. https://www.researchgate.net/publication/269412977_Vascular_protective_effects_of_statin-related_increase_in_serum_25-hydroxyvitamin_D_among_high-risk_cardiac_patients. J Cardiovasc Med. 2015;16:51–58. doi: 10.2459/JCM.0000000000000026. [DOI] [PubMed] [Google Scholar]
- 9.Effects of atorvastatin on vitamin D levels in patients with acute ischemic heart disease. Pérez-Castrillón JL, Vega G, Abad L, et al. https://pubmed.ncbi.nlm.nih.gov/17398180/#:~:text=Atorvastatin%20treatment%20produced%20a%20statistically,atorvastatin%20increases%20vitamin%20D%20levels. Am J Cardiol. 2007;99:903–905. doi: 10.1016/j.amjcard.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 10.Increased levels of 25 hydroxyvitamin D and 1,25-dihydroxyvitamin D after rosuvastatin treatment: a novel pleiotropic effect of statins? Yavuz B, Ertugrul DT, Cil H, et al. Cardiovasc Drugs Ther. 2009;23:295–299. doi: 10.1007/s10557-009-6181-8. [DOI] [PubMed] [Google Scholar]
- 11.STATIN-D study: comparison of the influences of rosuvastatin and fluvastatin treatment on the levels of 25 hydroxyvitamin D. Ertugrul DT, Yavuz B, Cil H, et al. Cardiovasc Ther. 2011;29:146–152. doi: 10.1111/j.1755-5922.2010.00141.x. [DOI] [PubMed] [Google Scholar]
- 12.Statins and vitamin D. Aloia JF, Li-Ng M, Pollack S. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2075568/ Am J Cardiol. 2007;100:1329. doi: 10.1016/j.amjcard.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Statin use and 25-Hydroxyvitamin D blood level response to vitamin D treatment of older adults. Bischoff-Ferrari HA, Fischer K, Orav EJ, et al. J Am Geriatr Soc. 2017;65:1267–1273. doi: 10.1111/jgs.14784. [DOI] [PubMed] [Google Scholar]
- 14.Lack of association of statin use with vitamin D levels in a hospital-based population of type 2 diabetes mellitus patients. Iqbal K, Islam N, Azam I, et al. Pak J Med Sci. 2018;34:204–208. doi: 10.12669/pjms.341.11977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simvastatin does not affect vitamin D status, but low vitamin D levels are associated with dyslipidemia: results from a randomized, controlled trial. Rejnmark L, Vestergaard P, Heickendorff L, et al. Int J Endocrinol. 2010;2010:957174. doi: 10.1155/2010/957174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Health and Nutrition Examination Survey: Analytic Guidelines, 2011-2012. [Oct;2019 ];https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx. https://wwwn.cdc.gov/nchs/data/nhanes/20112012/analyticguidelines/analytic. 2013 11:16. [PubMed] [Google Scholar]
- 17.Association between leisure-time aerobic physical activity and vitamin D concentrations among US older adults: the NHANES 2007-2012. Orces CH. Aging Clin Exp Res. 2019;31:685–693. doi: 10.1007/s40520-018-1031-9. [DOI] [PubMed] [Google Scholar]
- 18.Diagnosis and classification of diabetes mellitus. American Diabetes Association. Diabetes Care. 2010;33:62–69. [Google Scholar]
- 19.NHANES 2013-2014. [Jul;2020 ];https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2013 2020 27:2019. [Google Scholar]
- 20.Analytical Note for 25-Hydroxyvitamin D Data Analysis using NHANES III (1988-1994), NHANES 2001-2006, and NHANES 2007-2010 (October 2015) [Jul;2020 ];https://wwwn.cdc.gov/nchs/nhanes/vitamind/analyticalnote.aspx?h=/Nchs/Nhanes/2013-2014/VID_H.htm&t=VID_H%20Doc 2020 27:2019. [Google Scholar]
- 21.The prevalence of vitamin D deficiency and the determinants of 25(OH)D concentration in older Irish adults: Data from the Irish Longitudinal Study on Ageing (TILDA) Laird E, O'Halloran AM, Carey D, et al. J Gerontol A Biol Sci Med Sci. 2018;73:519–525. doi: 10.1093/gerona/glx168. [DOI] [PubMed] [Google Scholar]
- 22.Low circulating vitamin D in obesity. Liel Y, Ulmer E, Shary J, et al. Calcific Tissue Int. 1988;43:199–201. doi: 10.1007/BF02555135. [DOI] [PubMed] [Google Scholar]
- 23.Decreased bioavailability of vitamin D in obesity. Wortsman J, Matsuoka LY, Chen TC, et al. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 24.Comparison of prevalence of inadequate nutrient intake based on body weight status of adults in the United States: an analysis of NHANES 2001-2008. Agarwal S, Reider C, Brooks JR, et et. J Am Coll Nutr. 2015;34:126–134. doi: 10.1080/07315724.2014.901196. [DOI] [PubMed] [Google Scholar]
- 25.The association between body mass index and vitamin D supplement use among adults in the United States. Orces C. Cureus. 2019;11:5721. doi: 10.7759/cureus.5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Impact of high-dose statins on vitamin D levels and platelet function in patients with coronary artery disease. Verdoia M, Pergolini P, Rolla R, et al. https://pubmed.ncbi.nlm.nih.gov/28068529/ Thromb Res. 2017;150:90–95. doi: 10.1016/j.thromres.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 27.Association between blood vitamin D and myocardial infarction: a meta-analysis including observational studies. Huang J, Wang Z, Hu Z, et al. Clin Chim Acta. 2017;471:270–275. doi: 10.1016/j.cca.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 28.Low calcidiol levels and risk of progression of aortic calcification. Naves-Díaz M, Cabezas I, Barrio S, et al. Osteoporos Int. 2012;23:1177–1182. doi: 10.1007/s00198-011-1550-0. [DOI] [PubMed] [Google Scholar]