SUMMARY
Recent advancements in the recognition and understanding of both malignant and benign chronic diseases have continued to evolve. The role of IP as a diagnostic and therapeutic tool has become increasingly important given the scope of having a positive impact on diseases and conditions that previously were saturated in their treatment approaches.
Progress in systemic treatment options for malignant disease has resulted in a greater drive toward optimization of symptomatic patients through bronchoscopic intervention in order to enable access to anticancer therapy. Similarly, increased screening and potential identification of early lung cancers combined with newer non-invasive curative therapies drive the need to access and sample pulmonary lesions as early as possible. For this, advanced diagnostic approaches are vital and may in the future offer combination with therapeutic interventions and the delivery of local therapy within the lung parenchyma without the need for other invasive sampling or treatment. IP has been established as critical to optimal diagnosis and management of thoracic disorders and its role is likely to evolve as more therapeutic interventions are evaluated.
Keywords: Bronchoscopy, Interventional pulmonology, Lung cancer
INTRODUCTION
The past decade has seen somewhat of a shift away from diagnostic thoracic surgery due largely to a rapid development in technologies in interventional pulmonology (IP). Multidisciplinary thoracic cancer diagnosis and treatment involving IP, thoracic surgery, and oncology are increasingly adopted approaches. Increased focus on lung cancer screening and early detection, in particular, to aid diagnosis of peripheral pulmonary lesions (PPLs) has meant that the incidence of detection is likely to rise. Determining etiology is essential, because lesions between 0.8 cm and 2 cm have an 18% prevalence of malignancy, whereas those above 2 cm have a risk that climbs to 50%.1,2 Therefore, timely and accurate location, sampling, and diagnosis of PPL through a minimally invasive, less morbid approach increasingly are vital.
Computed tomography (CT)-guided biopsy often is performed to investigate PPLs but suffers somewhat from certain limitations. Anatomic accessibility, size of lesion (<10 mm associated with lower diagnostic yield), and patient comorbidities all play a role in candidacy. This technique also carries a risk of pneumothorax, reported overall at approximately 15% to 20% but with a heterogeneous risk profile as high as 60% in biopsies of lesions under 20 mm.3,4
Surgical biopsy still has a role to play, although this has diminished over the past decade.5 The main advantage remains conferring high diagnostic yield but with higher perioperative risk stratification than IP techniques. Mediastinoscopy anatomically is disadvantaged due to reduced ability to access posteriorly located subcarinal nodes and lower hilar stations (11R & 11L). Additionally, proceeding straight to surgery in PPLs is not straightforward either because it has been demonstrated that lobectomy without prior histologic confirmation of malignancy is associated with benign pathology in up to a third of cases.6–8 This review, therefore, focuses on current indications and techniques for advanced diagnostic and therapeutic bronchoscopy available in concert with traditional thoracic surgery in the management of benign and malignant thoracic disease.
ADVANCED DIAGNOSTIC BRONCHOSCOPY
Convex Endobronchial Ultrasound
Endobronchial ultrasound (EBUS) and transbronchial needle aspiration (TBNA) revolutionized the approach to lung cancer staging and diagnosis of mediastinal disease. Use of a convex linear ultrasound array positioned at the distal end of a flexible bronchoscope allows visualization of mediastinal lymph structures outside of the airway and real-time sampling (Fig. 1). This technique is associated with a high sensitivity, with tissue acquisition shown to be sufficient for immunohistochemistry and molecular analysis for targetable mutations.9
Fig. 1.
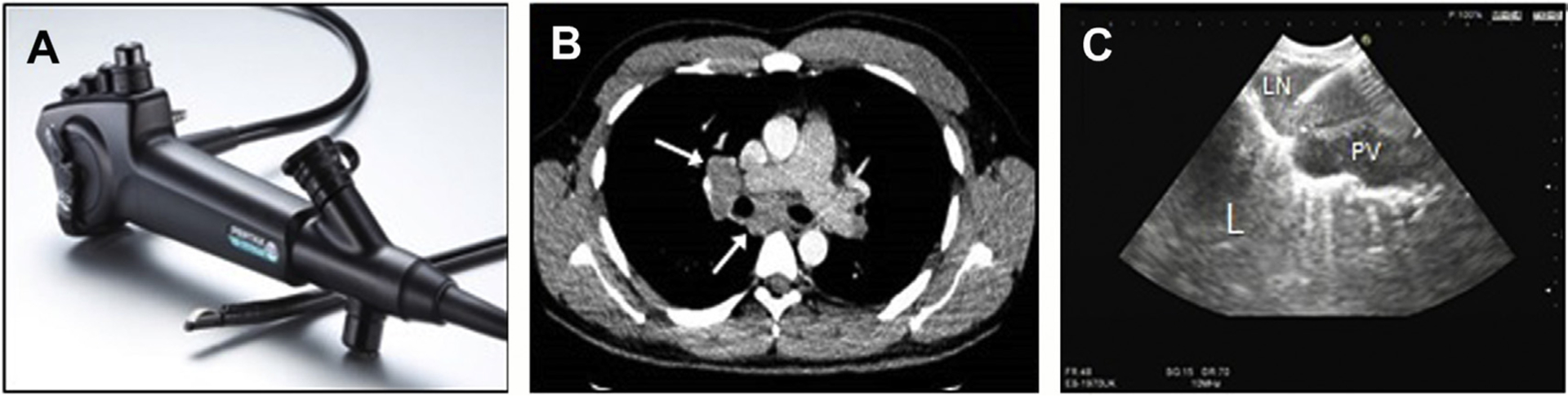
EBUS-TBNA. (A) EBUS bronchoscope with convex ultrasound array at tip. (B) CT chest demonstrating hilar and mediastinal lymphadenopathy in a 28-year-old patient with systemic weight loss and fatigue. (C) Ultrasound image at bronchoscopy showing EBUS needle in hilar lymph node (LN), adjacent pulmonary vessel (PV), and lung (L) with pleural enhancement and comet tail appearance. (Courtesy of Pentax Medical, Tokyo, Japan.)
Prior to the advent of EBUS, staging of disease was based on CT, PET imaging, and surgical sampling. CT imaging alone, however, has a sensitivity of 55% for detecting mediastinal lymph node metastasis, and 40% of CT-diagnosed lymph nodes considered malignant are actually benign whereas conversely, 20% of those under 10 mm are proved metastatic.10,11 Fluorodeoxyglucose (FDG)-PET improves on this and currently is the gold standard for detection of extrapulmonary metastases. While conferring 85% sensitivity for mediastinal lymph node metastasis, it does carry a poor specificity for large mediastinal lymph nodes12,13; 20% of enlarged FDG-avid lymph nodes are attributable to nonmalignant disease.
The landmark multicenter randomized ASTER trial compared surgical staging to EBUS/endoscopic ultrasound followed by surgical staging.14 The trial demonstrated greater sensitivity for mediastinal nodal metastases (94% in the EBUS/endoscopic ultrasound group vs 77% in the surgical group) and fewer unnecessary thoracotomies. In another study, compared with mediastinoscopy alone, diagnostic accuracy using EBUS was superior, 91% versus 78%.15 It must be acknowledged that this may well be in part be due to nonaccessible posteriorly located subcarinal lymph nodes via mediastinoscopy rather than overall decreased accuracy. Furthermore, there has been an effect on timing of progression through patient care pathways to treatment with EBUS. In a UK trial of 133 patients with stages I–IIIa disease, median time to treatment was shorter in those who underwent EBUS-TBNA instead of conventional diagnosis and staging, 14 days versus 29 days, respectively, with a post hoc analysis showing an increase in median survival in non–small cell lung cancer (NSCLC) patients who underwent EBUS (Fig. 2).16
Fig. 2.
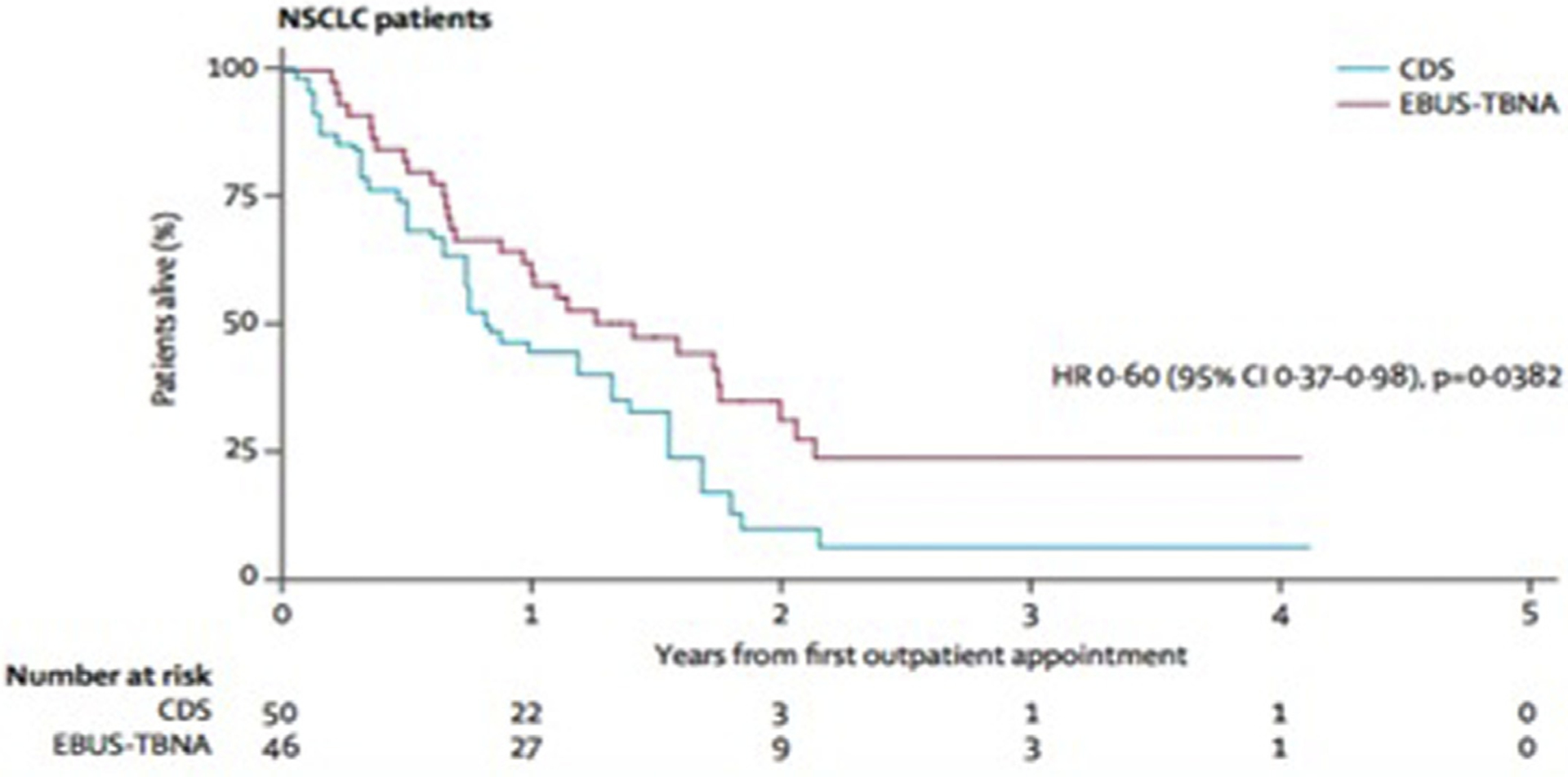
Survival outcomes in NSCLC patients who underwent conventional staging and diagnosis versus EBUS-TBNA staging. (From Navani N, Nankivell M, Lawrence DR, et al. Lung cancer diagnosis and staging with endobronchial ultrasound-guided transbronchial needle aspiration compared with conventional approaches: an open-label, pragmatic, randomised controlled trial. Lancet Respir Med. 2015;3(4):287; with permission.)
Peripheral Pulmonary Lesions
Fluoroscopy
Fluoroscopic imaging during bronchoscopy may adjunctively improve the diagnostic yield when targeting PPLs (Fig. 3). In a meta-analysis of 18 studies encompassing 1687 patients, use of fluoroscopy with bronchoscopy was associated with a 60% diagnostic success rate versus 45% when carried out via bronchoscopy alone.17 The presence of a bronchus leading directly to a PPL on CT scan, on-site pathology assessment, and lesion size greater than 3 cm were associated with a higher diagnostic yield.
Fig. 3.
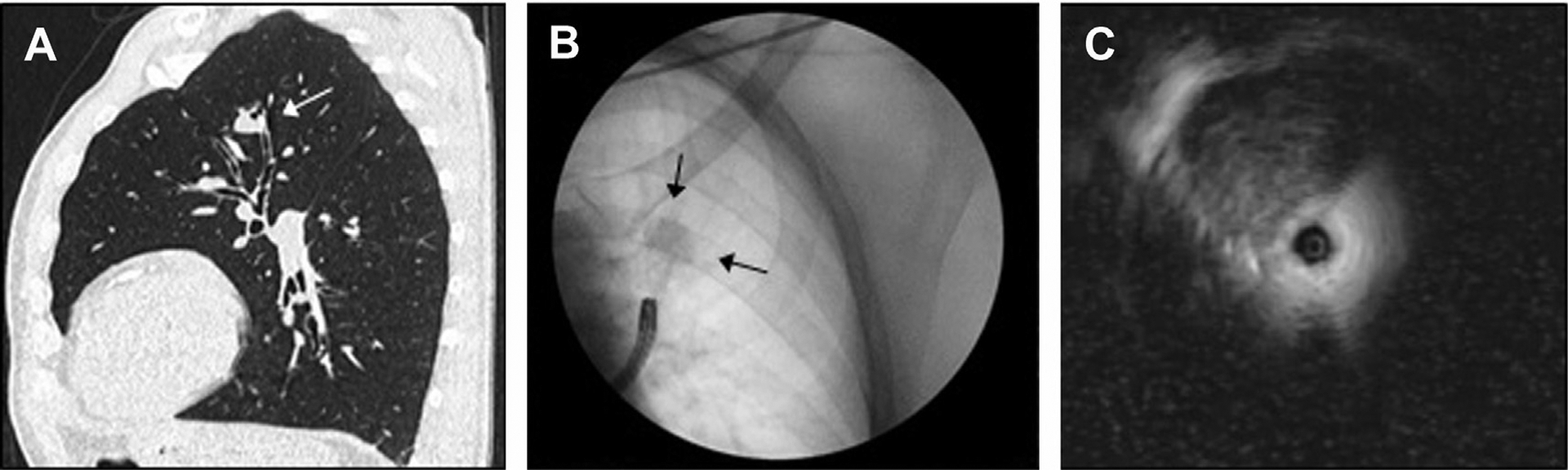
Fluoroscopy and radial EBUS. (A) Left upper lesion with bronchus sign in a renal transplant patient on immunosuppression. (B) Bronchoscopy with fluoroscopy to help target the lesion and subsegmental airway. (C) Ultrasound image obtained on radial EBUS showing lesion between the 10-o’clock and 1-o’clock positions.
Cone-beam computed tomography
The cone beam-CT (CBCT) method involves the use of an x-ray C-arm scanner, which rotates in real time during the procedure around a patient to produce a CT image. Although the image quality is not that of a diagnostic CT, it is sufficient to allow identification of a PPL and bronchoscopic equipment aimed at targeting this.18 CBCT in conjunction with other approaches has been shown effective, with a reported diagnostic yield of up to 84% in diagnosing PPLs.19
Radial endobronchial ultrasound
The radial EBUS technique consists of a flexible catheter with an oscillating ultrasound probe at its tip, providing a 360o assessment of a distal airway. This may be effective particularly in PPLs where a bronchus sign is present (see Fig. 3).20–22 Reported diagnostic yields range from 58% to 88% in the literature. One meta-analysis reviewed 16 studies involving 1420 patients undergoing radial EBUS-guided bronchoscopy for investigation of a PPL. The sensitivity rate for detection was 0.73 (0.70–0.76).23 A subsequent larger meta-analysis of 57 different studies and 7872 PPLs found a diagnostic yield rate of 70.6%, with the presence of malignancy, bronchus sign, or lesion greater than 2 cm associated with a higher success rate.24 The presence of the probe within a lesion rather than adjacent to it also was more favorable.
Electromagnetic navigational bronchoscopy
Distal targeting of a PPL with a bronchus sign can be limited by inaccessibility due to the physical dimensions of a bronchoscope. Electromagnetic navigational bronchoscopy is a method that can be used to overcome this to diagnose lesions, sample lymph nodes, site treatment catheters (eg, radiotherapy), and place fiducial markers to assist prospective surgery.25 Prebronchoscopy planning CT and virtual reconstruction can be synchronized with live bronchoscopy to provide navigation (Fig. 4). An electromagnetic plate beneath the plate permits a smaller steerable working channel to be advanced from the bronchoscope once the latter’s limitations are reached to distally access a lesion. The NAVIGATE study demonstrated electromagnetic navigational bronchoscopy is a safe and effective modality in diagnosing PPLs (radial-EBUS assisted in some cases), and to help site fiducial markers for surgery or stereotactic radiotherapy.26,27
Fig. 4.
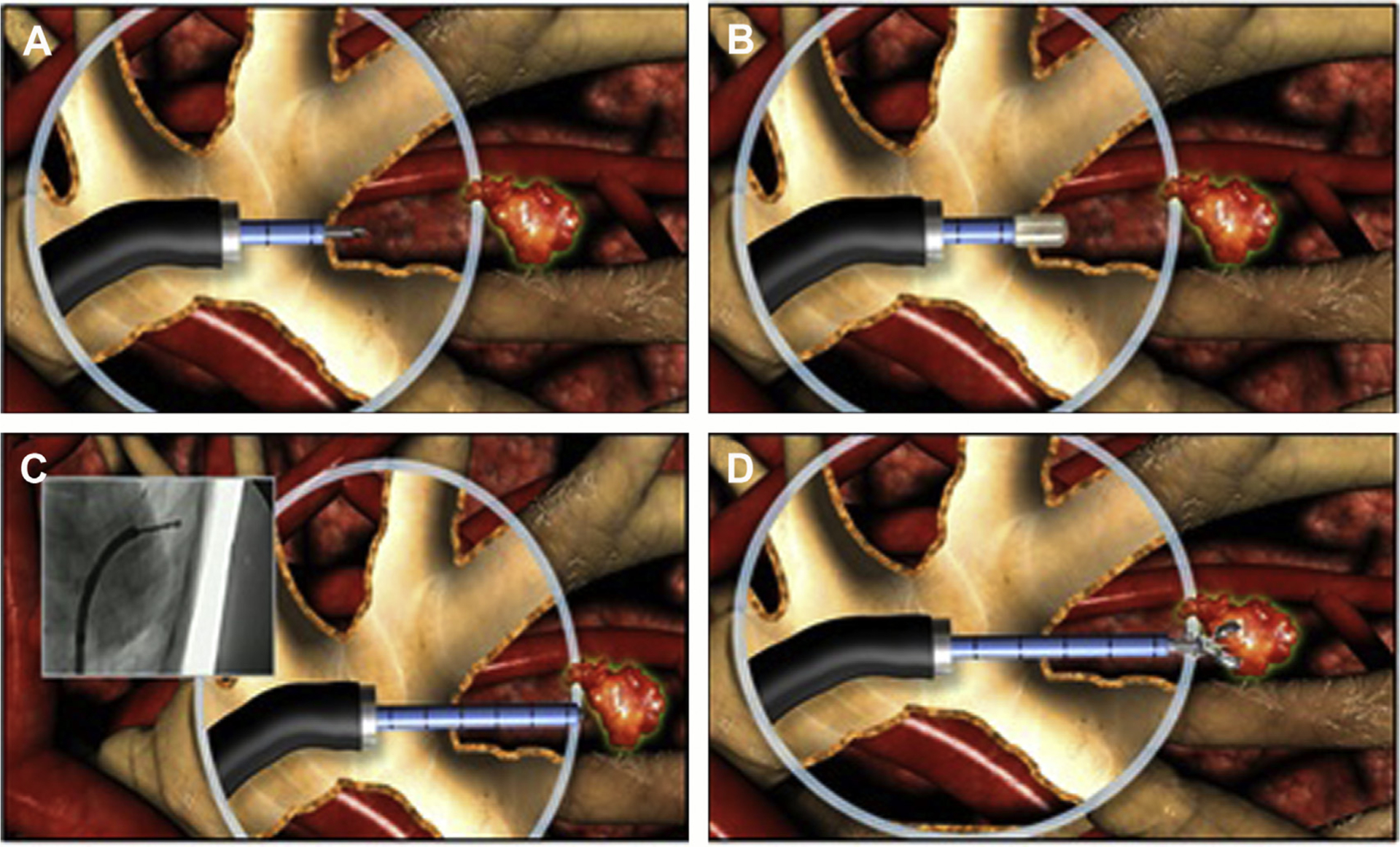
Bronchoscopic transparenchymal nodule access (BTPNA). (A) Initial puncture through a predetermined target in an airway wall. (B) Dilatation of the access point to establish the beginning of a tract. (C) Careful tunneling creating a tract toward the target parenchymal lesion. (D) Biopsy and sampling of peripheral parenchymal lesion. (Courtesy of Broncus Medical Inc., San Jose, CA.)
Bronchoscopic transparenchymal nodule access
The novel approach, bronchoscopic transparenchymal nodule access, employs augmented fluoroscopy after preprocedural planning of a path toward a peripheral parenchymal lesion through an airway wall. It does not rely on the need for a bronchus sign because the procedure involves puncturing an airway wall, dilation, and passage of a catheter through a tract toward a lesion and subsequently sampling to be performed (Fig. 5). A pilot human study in 12 patients demonstrated adequate diagnostic sampling was achieved in 83% of cases.28 A further small study of 6 patients demonstrated a malignancy diagnosis rate of 100% in the 5 individuals able to undergo the procedure.29 A multicenter trial across 9 sites aiming to recruit 200 patients currently is under way.
Fig. 5.

Autofluorescence imaging (AFI) and NBI. (A, B) Mucosal abnormality (arrow) in superior lingula on white light and abnormal fluorescence; biopsies confirming carcinoma in-situ recurrence. (C, D) New apical left lower lobe lesion in the same patient with corresponding abnormal fluorescence; biopsy demonstrated severely dysplastic epithelium and surgical resection confirmed a new invasive squamous cell carcinoma. (E) Left main bronchus mucosa under light bronchoscopy after previous laser therapy for endobronchial carcinoid. (F) Appearances under NBI, which show cluster of microvessels; biopsy confirming residual carcinoid disease.
Robotic bronchoscopy
Robotic bronchoscopy represents potentially the most advanced future platform on the horizon, with the possibility of a thinner, more flexible bronchoscope potentially being able to navigate more distally throughout the bronchial tree to interrogate disease.30,31 A small feasibility and safety study assessing its use in investigation of pulmonary lesions in a 15-patient cohort successfully was reported without adverse events encountered.32
Autofluorescence bronchoscopy
Autofluorescence bronchoscopy (AFB) can be used to detect preinvasive malignant disease by utilizing the differences between red light and green light absorption demonstrated by abnormal and normal epithelium.33–35 In abnormal mucosa, there is an increase in fluorophores, which absorb and emit fluorescence when irradiated with light (see Fig. 5).36 It is associated with a 1.4-fold to6.3-fold increase in sensitivity in detecting preinvasive disease compared with white light alone, and a meta-analysis showed a pooled sensitivity of 85% for white light bronchoscopy (WLB) combined with AFB versus 43% for WLB alone.37 A key aspect to consider, however, is that AFB has a lower specificity than WLB because nonspecific airway changes often also can lead to abnormal fluorescence.38
Narrow band imaging (NBI) demonstrates similar advantages and is helpful in detecting changes, such as vessel growth, tortuosity, and microvascular patterns associated with angiogenesis, which develop during early phases of premalignant disease (see Fig. 5).39,40 This is achieved through emission of blue light and green light bandwidths. The former is absorbed by superficial capillaries in the mucosa and the latter by submucosal blood vessels. The combined effect allows more detailed assessment of the mucosa for signs of angiogenesis, which may accompany premalignant changes or early invasive disease.40–42 In 1 meta-analysis of 6 studies, NBI demonstrated improved sensitivity compared with WLB (86% vs 70%) and specificity (81% vs 66%) in detecting early premalignant or invasive airway disease.43
Both modalities can prove effective in screening for disease recurrence in cases of previous carcinoma in situ at a surgical margin or in individuals with previously proved dysplastic airway lesions (eg, smokers), which may evolve over time. They also can be used to assess for response to treatment after previous direct endobronchial management, such as debulking and laser therapy.
Transbronchial lung cryobiopsy
Transbronchial lung biopsies have long been hindered by small tissue acquisition and crush artifact distorting analysis. Use of a flexible cryotherapy catheter probe inserted through a bronchoscope to obtain larger transbronchial lung cryobiopsies (TBLCBs) has shown increasing promise.44 Acquired tissue samples are larger (5–10 mm), with architecture maintained without distortion, allowing more accurate analysis.45 Surgical lung biopsies (SLBs), although conferring high diagnostic rates, are associated with a 30-day mortality rate of 2% in cases of video-assisted thoracoscopic biopsies and 43% in open lung biopsies.46 TBLCB also can be performed as a day-case procedure under monitored anesthesia care, thereby reducing risk and minimizing hospital inpatient stay. The main risks are pneumothorax (12%) and significant airway bleeding (39%), the latter managed through use of an endobronchial balloon blocker at sampling to occlude a segmental airway temporarily.47
Ravaglia and colleagues48 compared 150 patients who underwent SLB versus 297 who underwent TBLCB. Mortality rates and length of admission were higher in surgical patients, with diagnostic yield rates of 99% (SLB) and 82.8% (TBLCB). A retrospective review of 117 patients with undiagnosed interstitial lung disease compared outcomes in 58 who underwent TBLCB versus 59 who had SLB. Diagnostic confidence was similar in both groups, at 63% in the TBLCB group and 65% in the SLB group.49 Contrastingly, a prospective 2-center analysis in 2019 of 21 patients who underwent sequential TBLCB followed by SLB of the same anatomic region noted only a 48% concordance in diagnosis between sampling methods.50 More recently, however, the COLDICE study, carried out across 9 tertiary interstitial lung disease centers in Australia, pointed to a 70.8% diagnostic agreement between TBLCB and SLB, with this as high as 95% in high-probability/definite diagnosis cases when reviewed by a specialist multidisciplinary team.51
TBLCB has a lower risk profile than surgical profile but its role in the diagnostic algorithm of patients with interstitial lung disease remains to be clarified.
THERAPEUTIC BRONCHOSCOPY FOR CENTRAL AIRWAY OBSTRUCTION
Central airway obstruction (CAO) is defined specifically as obstruction of the central airways, including the trachea, main bronchi, and bronchus intermedius. Symptoms, including cough, dyspnea, wheeze, and stridor, often develop late and may be misdiagnosed as asthma or small airways disease. Late presentation is associated with high morbidity and mortality; hence, clinician awareness is key to prevent potential respiratory failure and asphyxia. Prompt clinical assessment, peak flow measurement (as an indicator of proximal airway airflow), and cross-sectional imaging are paramount to enabling rapid diagnosis and guide management, which often manifests as emergency rescue measures.
CAO can be classified as malignant, in the context of cancer, or as benign/nonmalignant, for example, stricture secondary to vasculitis. A majority of cases are due to primary lung carcinoma, where approximately 20% of patients develop clinically significant CAO. Squamous cell carcinomas account for most of these, but rarer forms, such as adenocystic carcinomas, which classically can affect the central airways, should be noted. The types of CAO are outlined in Fig. 6. An additional factor to consider when assessing CAO is the presence of viable lower airways and distal lung, which may influence whether intervention is appropriate.
Fig. 6.
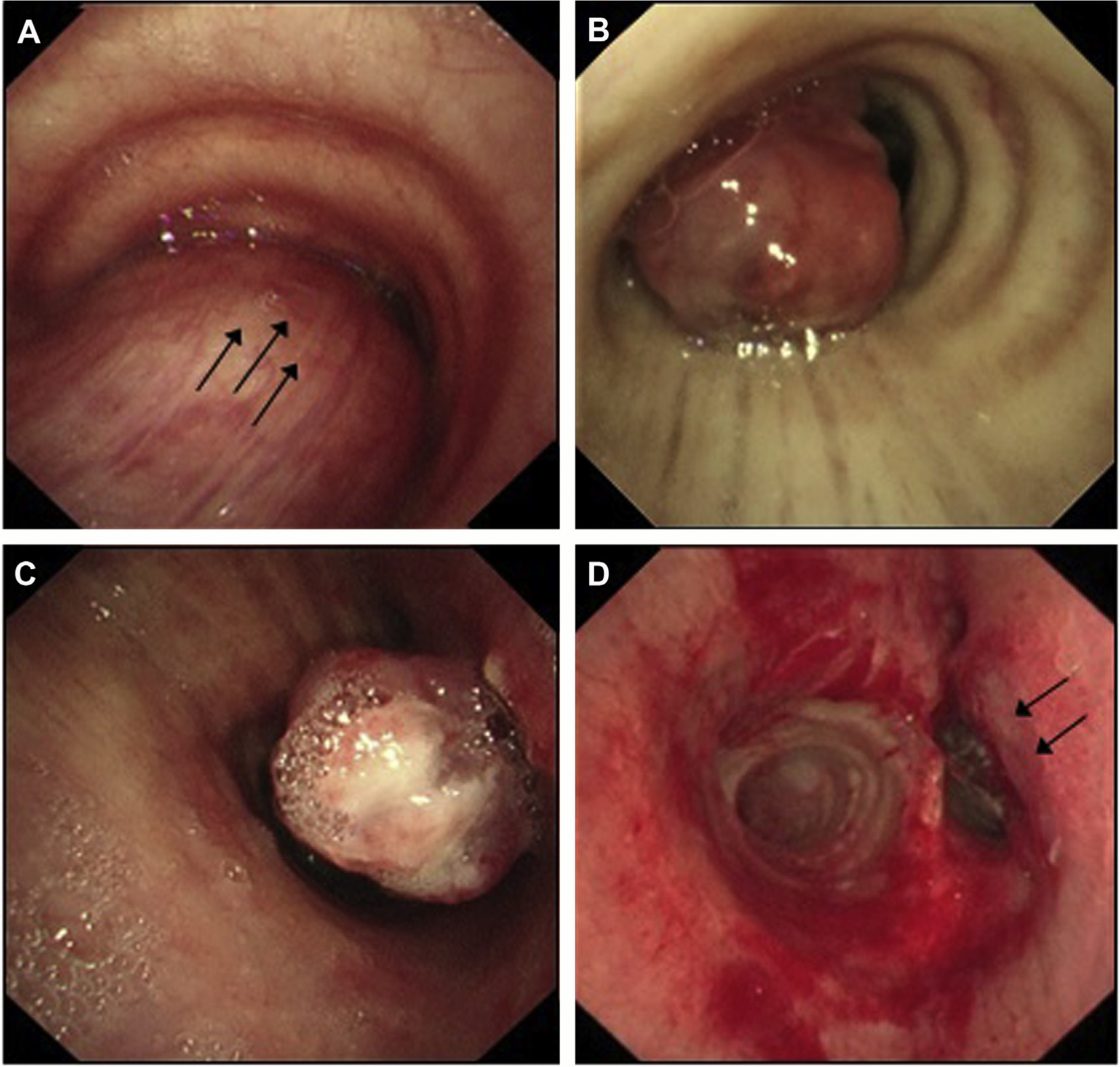
Types of CAO. (A) Extrinsic compression due to malignant disease outside of the trachea (posteriorly in this case). (B) Intraluminal obstruction secondary to a tracheal tumor within the airway lumen. (C, D) Mixed obstruction, best seen postdebulking (cryoextraction and laser) with endoluminal tumor and left tracheal wall extrinsic compression.
Laser Photoresection
Laser ablation is an important tool in the management of CAO, most importantly endoluminal disease. It can be used to vaporize, coagulate, devascularize, and debulk airway lesions. In benign disease, a laser also can be used in conjunction with balloon dilatation therapy to treat strictures by cutting superficial fibrotic airway bands. The effects of laser therapy are more immediate and, therefore, can be very helpful in the context of palliative disease control and symptom relief. There are several types of lasers, each with different characteristics that in turn may guide their role (Table 1); however, Nd:YAG often is the type used most commonly in bronchoscopy.52
Table 1.
Types of lasers and their respective properties
| Laser | Wavelength, nm | Penetration Depth | Vaporization | Coagulation | Cutting |
|---|---|---|---|---|---|
| APC | 516 | 1–2 mm | – | ++ | + |
| KTP | 532 | 0.8 mm | ++ | ++ | + |
| Diode | 808 | 8–10 mm | + | ++ | +++ |
| Nd:YAG | 1060 | 4–6 mm | +++ | +++ | + |
| Thulium | 1940 | 0.4 mm | ++ | ++ | +++ |
| CO2 | 10,600 | 0.3 mm | + | – | +++ |
Plus and minus symbols refer to level of activity where −, refers to no activity and +++, refers to significant activity.
Bronchoscopic application can be performed through flexible or rigid bronchoscopy and is done best under general anesthesia. Given the potential depth penetration, it is important that application ensures it is fired toward the airway lumen and not perpendicular to the wall (Fig. 7). Protective eyewear is mandatory to avoid retinal injury to operators and health care professionals. The most concerning risk is endobronchial fire, with a Fio2 below 40% strictly advised in all cases when delivering therapy within the bronchial tree.53 Short efficient time usage is important for this reason and often improves with operator experience. Other complications include bleeding, airway perforation, pneumothorax, hemorrhage, infection, and fistula formation.
Fig. 7.
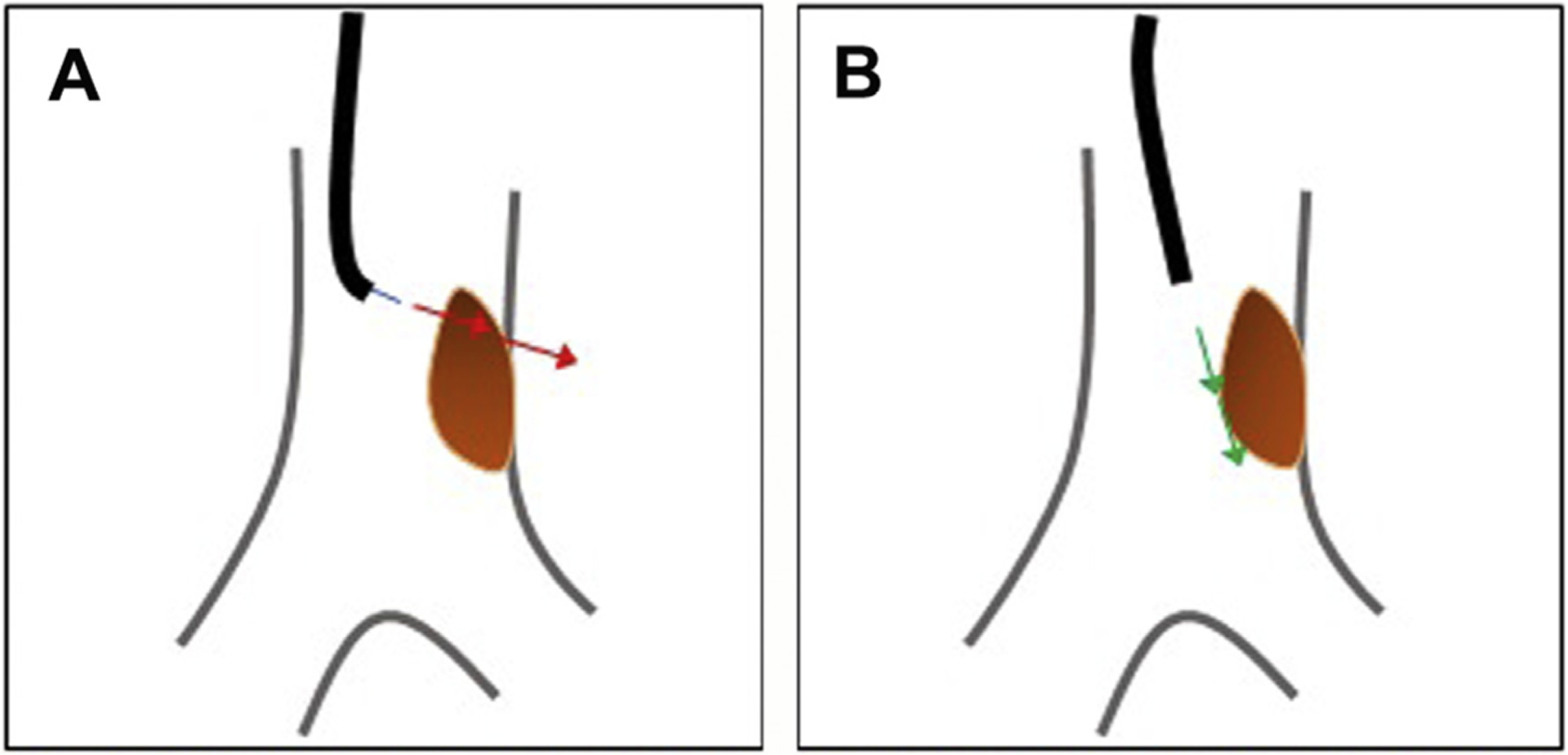
Endobronchial laser therapy. (A) Incorrect positioning of laser, raising risk of airway perforation through airway wall. (B) Correct laser application with beam firing at lesion but in direction of airway lumen distally.
Data on use of laser therapy often are retrospective and not randomized or controlled. Furthermore, it often is used in a multimodality approach with other therapies at the time of bronchoscopy. In 1 study comparing Nd:YAG laser therapy in conjunction with external beam radiotherapy versus control (external beam radiotherapy alone), it was seen that use of additional laser therapy improved survival.54 A case series assessing laser therapy alone, brachytherapy alone, and both together observed a longer median survival time in the combined cohort, 264 days, versus 111 days and 115 days, respectively.55 Laser therapy also has been shown effective in treating endobronchial carcinoid disease, negating the need for invasive surgery.56–58
Argon Plasma Photocoagulation
Argon plasma photocoagulation (APC) first was described in the treatment of gastrointestinal bleeding in 1981 and subsequently has been expanded in its uses to various settings.59 It uses nonionized argon gas to which a voltage electrical current is applied once it is injected on to a target area. This leads to ionization of the argon gas, generating a monopolar current in the target tissue and subsequent heat generation.60,61 Its small-depth penetration means it is best suited for treatment of superficial disease and is effective in achieving hemostasis at mucosal surfaces. Due to the movement of the argon gas around bends, it can be effective to treat disease bifurcation points or distal locations within the bronchial tree. The risks related to APC are similar to those of laser photoresection; Laser therapy has also been shown to be effective in treating endobronchial disease, negating the need for invasive surgery.62,63
Small cases series have shown that APC is safe and effective in treating obstructive airway lesions, both malignant and benign.64–66 It also has been shown to provide benefit in conjunction with chemotherapy in treatment of tuberculosis with evidence of airway lesions.67 In 1 case series of 364 patients who underwent bronchoscopy for treatment of airway tumors, APC was shown effective in hemostasis control, stent recanalization, and tumor debulking.64
Electrocautery
This is a contact modality that uses an electrical probe to direct a monopolar current in to tissue to cause vaporization and coagulation. The probe tip can come in different forms, such as a snare, to loop a polypoid lesion root, or a knife, to cut through fibrotic webs in a stricture.61 The depth penetration is more superficial compared with a laser, with the risks similar except that no eye protection is required for operation.
Electrocautery has been shown a safe and effective therapy in treating CAO.61,65,68,69 In a case series of 94 patients who underwent bronchoscopy with electrocautery for malignant or benign disease, endoscopic improvement was seen in 94% of cases along with 78% radiological improvement in luminal patency on CT and, similarly, lung aeration on both CT (63%) and radiograph (43%).68 A study by Boxem and colleagues70 showed electrocautery demonstrated comparable efficacy to Nd:YAG laser therapy in the palliative treatment of symptomatic airway obstruction and was significantly more cost effective.
Cryotherapy and Cryoextraction
Conventional cryotherapy involves repeated free-thaw cycles and can be used to treat airway stenosis, granulation tissue recurrence, and early-grade airway lesions.71 Freezing cells to sub-zero temperatures using liquid nitrogen induces cell death by damaging blood vessels, causing ischemia. Formation of ice crystals creates an osmotic gradient, driving water out of cells, resulting in cell rupture.
In acute CAO due to endoluminal disease, debulking of exophytic lesions can be performed using cryoextraction (Fig. 8A, B). This allows for larger pieces of tissue to be removed, enabling rapid more efficient debulking.72 The cryoprobe is positioned on to a target area of tumor before freezing. While the probe tip is adhered to tumor, both it and scope are retracted, shearing off larger chunks of tissue. It is effective, with a review of 16 case series demonstrating an overall success rate for significant recanalization of approximately 80%.73
Fig. 8.
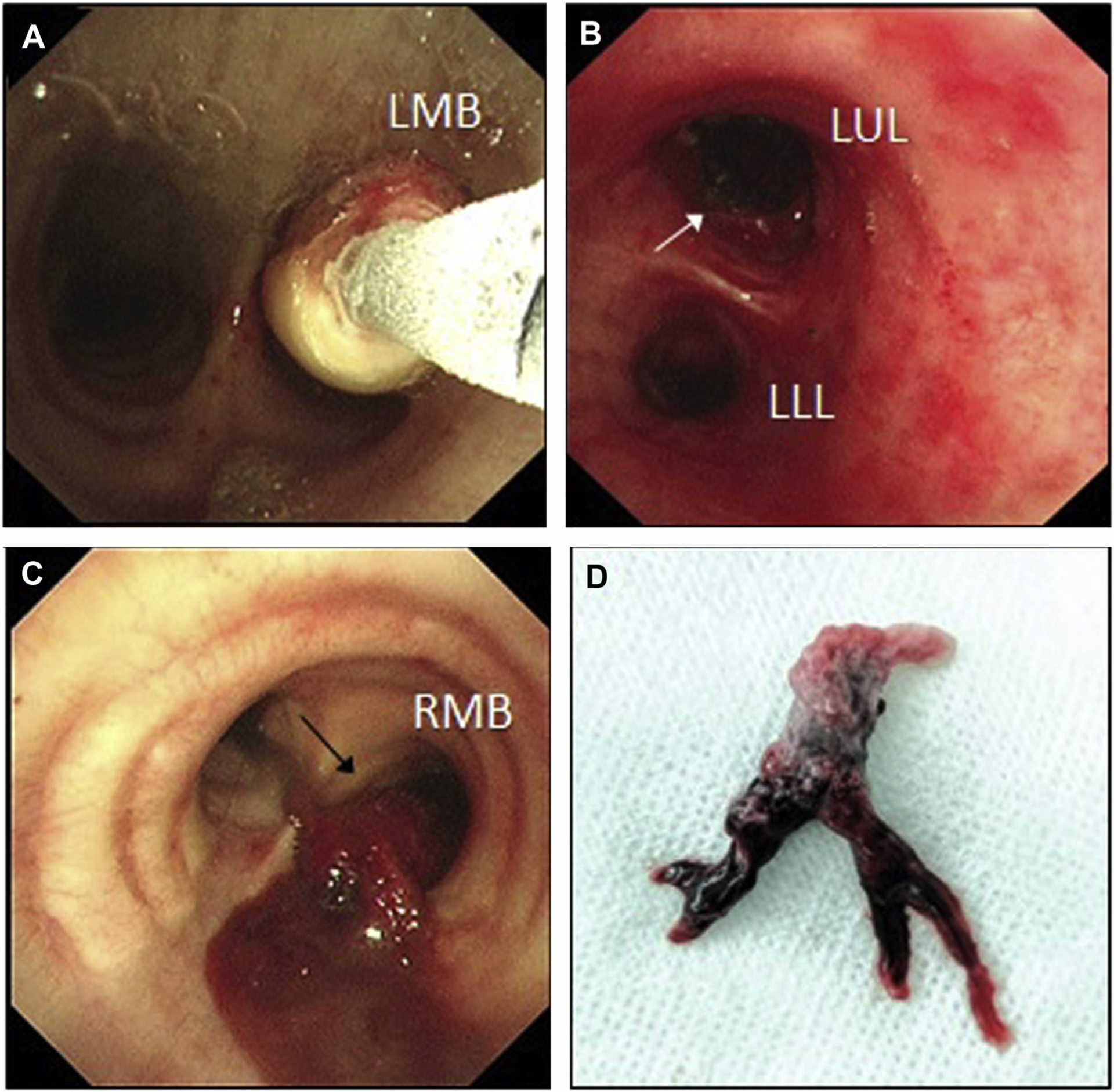
Bronchoscopic cryoextraction. (A) Left main bronchus (LMB) obstruction due to a lesion within the airway; cryoprobe seen freezing on to surface of lesion to begin debulking. (B) Successful cryoextraction of the airway lesion to its origin in the left upper lobe (LUL). (C) Visible partially organized clot originating from right main bronchus (RMB) and flowing over main carina in to left lung in an intensive care unit patient with massive hemoptysis. (D) Sample of clot extracted from subsegmental airway using cryoprobe, which demonstrates an airway cast appearance.
Another potential role for cryotherapy is the removal of endobronchial clots causing ventilatory failure secondary to airway obstruction in patients presenting with massive hemoptysis (Fig. 8C, D). Often, such patients are critically unwell in an intensive care unit, with mechanical ventilation struggling to compensate. Cryotherapy can be used to remove clots and restore airflow to subsegmental airways, leading to a rapid improvement in clinical state.74
BRONCHOSCOPIC RADIOFREQUENCY AND MICROWAVE ABLATION
Experience in percutaneous ablation of lung lesions is well documented in the literature. The RAPTURE trial demonstrated the effectiveness in using radiofrequency ablation (RFA) to treat nonoperable pulmonary or oligometastatic lesions.75 Microwave ablation (MWA) offers better physical properties with more consistent ablation fields, reduced heat sink effect, and less distortion due to tissue impedance. In one meta-analysis of 53 studies and more than 3000 patients, percutaneous application of both modalities in the treatment of pulmonary lesions demonstrated similar efficacy rates, 86% versus 81.1%.76 The drawbacks of percutaneous approaches, however, remain, in particular, the risk of pneumothorax, which, although quoted at approximately 15% to 20% in some studies, has been observed as high as 50% in others. This has led in some part to a growing interest regarding potential bronchoscopic delivery of thermal ablation to target and treat peripheral lung lesions.
A seminal study on the use of bronchoscopic RFA was carried out on lungs from anesthetized sheep (N = 6) to assess safety and efficacy, followed-up by a similar porcine-based study assessing MWA in the same context.77,78 The first human pilot evaluated CBCT bronchoscopy and RFA performed in 10 individuals with T1N0M0 lung cancer who subsequently had surgical resection, which demonstrated promising results with no adverse events noted.79 A similar 20-patient study was carried out by a Chinese research group assessing use of RFA delivered via bronchoscope to treat primary lung tumors, of 24-mm median size. In this group, patients included had T1–2aN0M0 NSCLC, with local tumor control recorded as 82.6% on CT follow-up and 61% 5-year survival.80 Given the potential promise, this represents an interesting new prospect in the realm of IP. Table 2 outlines the current trials in progress and the imaging modalities used by the corresponding groups.
Table 2.
Current radiofrequency and microwave in progress registered on clinicaltrials.gov
| Ablative Modality | Postablation Resection? | Number of Participants | Navigational Method | Region | Clinical Trials Identifier | Sponsor |
|---|---|---|---|---|---|---|
| RFA | No | 50 | ENB | China | NCT03009630 | Shanghai Chest Hospital |
| RFA | Yes | 10 | BTPNA | China | NCT03272971 | Bronchus Medical; Uptake Medical |
| RFA/MWA | No | 60a | ENB | China | NCT02972177 | Shanghai Chest Hospital |
| MWA | No | 30 | CBCT | USA | NCT03713099 | Ethicon |
| MWA | No | 30 | CBCT | UK | NCT03569111 | Medtronic-MITG |
| MWA | No | 60 | ENB | China | NCT04005157 | Shanghai Chest Hospital |
Abbreviations: BTPNA, bronchoscopic transparenchymal nodule access; ENB, electromagnetic navigational bronchoscopy.
30 patients in per each modality.
ENDOBRONCHIAL BRACHYTHERAPY
Delivery of focused intraluminal high-dose radiotherapy to a tumor bed along an airway wall can be effective in treating, arresting, or slowing disease progression and thereby preventing pending obstruction and collapse (Fig. 9).81–83 This can apply to malignant disease, such as cancer, and nonmalignant but progressive disease, such as airway amyloidosis. Gamma rays from focused radiotherapy (typically iridium) cause DNA chain breaks, triggering cell apoptosis and reduce proliferation. The therapeutic effect takes up to 4 weeks to 6 weeks, making it more suitable for localized slowly progressive disease rather than acute obstruction.71,84 Mediastinitis, esophagitis, and fistula formation are key specific complications of note as well as massive hemoptysis, which may occur after treatment of upper lobe airway lesions with close proximity to pulmonary vessels.
Fig. 9.
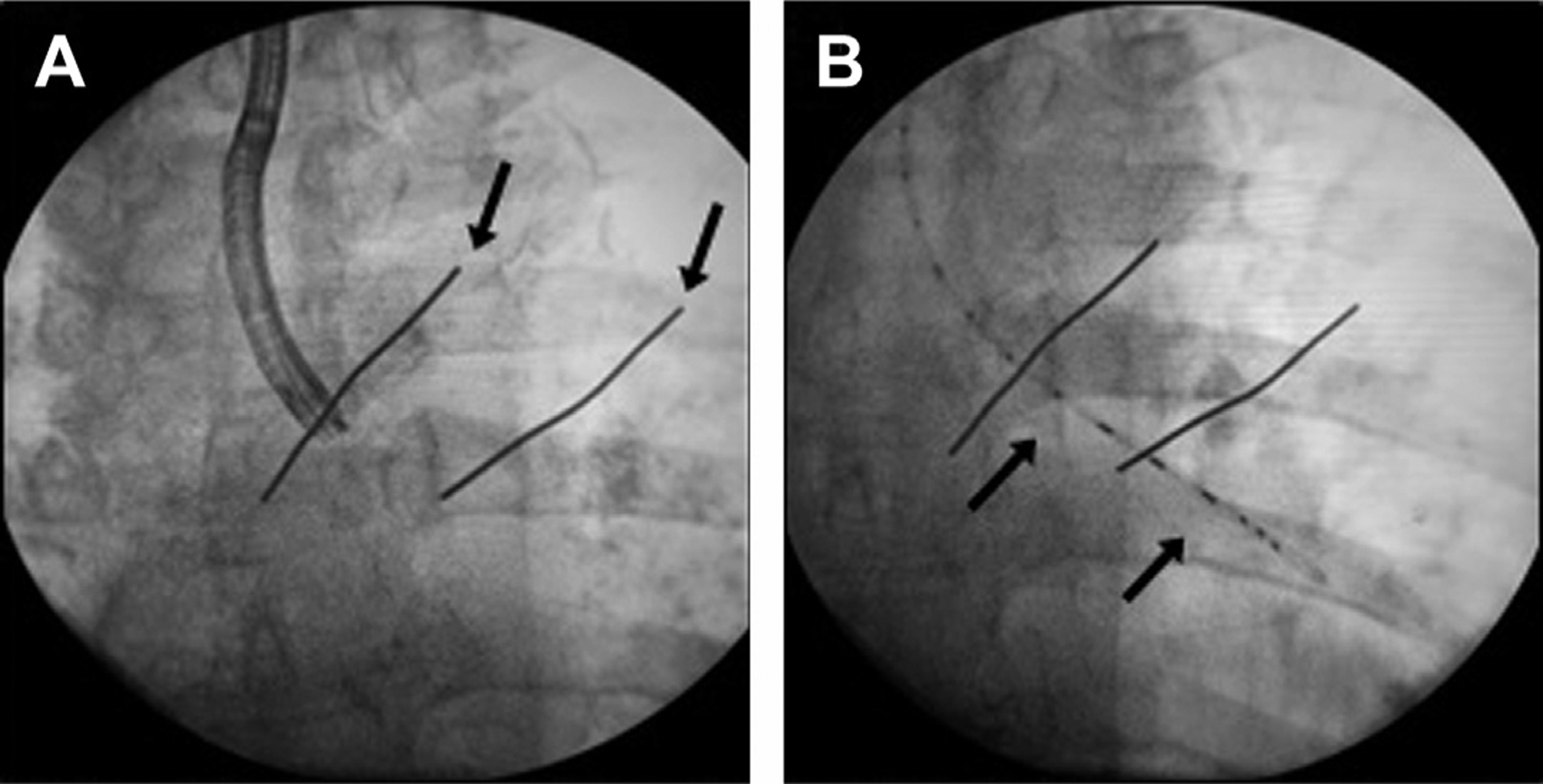
Intraluminal brachytherapy. (A) Bronchoscopic marking of proximal and distal margins of intended airway for treatment; zones then marked superficially (arrows) on the patient’s chest with skin markers. (B) Brachytherapy catheter (arrows) inserted using the bronchoscope and positioned carefully in the intended airway segment.
Studies have shown brachytherapy is effective in helping treat clinical symptoms in progressive palliative disease.81,82,84 In 1 study, 81 patients underwent high-dose endobronchial brachytherapy for malignant disease, of which 76 were lung primary and 5 metastatic.81 Clinical symptoms of cough, hemoptysis, dyspnea, and stridor were seen to improve, with 84.53% of patients experiencing a complete clinical response and only 1 out of 24 continuing to experience hemoptysis post-treatment. A retrospective review of 88 patients over a 13-year period in Massachusetts saw similar responses and noted survival benefits were best seen in metastatic (nonthoracic origin) lesions, followed by NSCLC.
Although some studies suggest it may be used effectively in combination with external beam radiotherapy or chemotherapy, a Cochrane review in 2012 suggested against this, citing insufficient evidence to suggest brachytherapy combined with external beam radiotherapy or chemotherapy or Nd:YAG laser ablation was more effective.85
PHOTODYNAMIC THERAPY
The use of a photosensitizer administered systematically and retained within tumor cells to allow application of targeted light therapy approximately 2 days later to induce tumor necrosis is well recognized. The first reported use of photodynamic therapy (PDT) to treat endobronchial lung cancer was in 1980 in a patient who declined surgery and underwent successful ODT with compete remission for the next 4 years. Following this, several reports have outlined its use in treating early and advanced endobronchial disease.86–88
PDT can be carried out under topical anesthetic, but general anesthesia often is more preferable. Recognized side effects include skin photosensitivity, hemoptysis, cough, and transient dyspnea postprocedure. Repeat bronchoscopy 2 days to 3 days after illumination is advised to assess for possible airway swelling and débride postnecrosis tissue that may cause distal airway obstruction. This is more common in exophytic bulky disease rather than mucosal early grade lesions.
In a review of 24 reported case series of PDT to treat either advanced exophytic disease or early-grade lesions, 12 studies outlined use of PDT in symptomatic advanced endoluminal lesions, causing greater than 50% obstruction.89 Treatment was associated with almost complete symptom relief and correlated with improved spirometry. Factors influencing response related to stage of disease at time of treatment and performance status (>2 associated with a poorer outcome).90,91 In patients with early-stage bronchial carcinoma, complete response was reported in more than 70% cases. Cure rates were higher in lesions less than 1 cm in diameter, with some newer photosensitizing agents demonstrating better antitumor properties and outcomes for lesions above 1 cm. Addition of adjuvant therapies, such as external beam radiotherapy, was associated with an improved response rate.
PDT also has been shown effective in treating nonbronchogenic endobronchial metastases, with acute relief of hemoptysis and dyspnea seen in 85% of patients in 1 case series.92 The 30-day mortality, however, was 22% but a majority of cases were renal cell carcinomas, which are notoriously vascular and hazardous to treat endobronchially.
BRONCHOPLASTY AND INTRALESIONAL STEROID INJECTION
Airway strictures can cause significant compromise in patients with high symptom burden. They may develop in the context of active disease, such as vasculitis; after previous airway insults, such as tuberculosis; or as a late complication of previous therapies, for example, PDT and surgery. Management of such strictures can be challenging and is guided best by symptoms, spirometry, and evidence of downstream complications, such as recurrent infections, high sputum loads, or lobar collapse.
Studies have shown that bronchoscopic balloon dilatation is an effective method to treat airway strictures (Fig. 10).93–95 In cases of high tracheal lesions, advice from a specialist airway otorhinolaryngology team should be sought, and, in some cases, performing bronchoscopy under suspension laryngoscopy may be beneficial.96 Strictures may be managed through a combination of approaches, such as laser, brachytherapy, and cautery.97,98 In cases of airway vasculitis, systemic immunosuppression also should be addressed in addition to local treatment of airway disease.
Fig. 10.
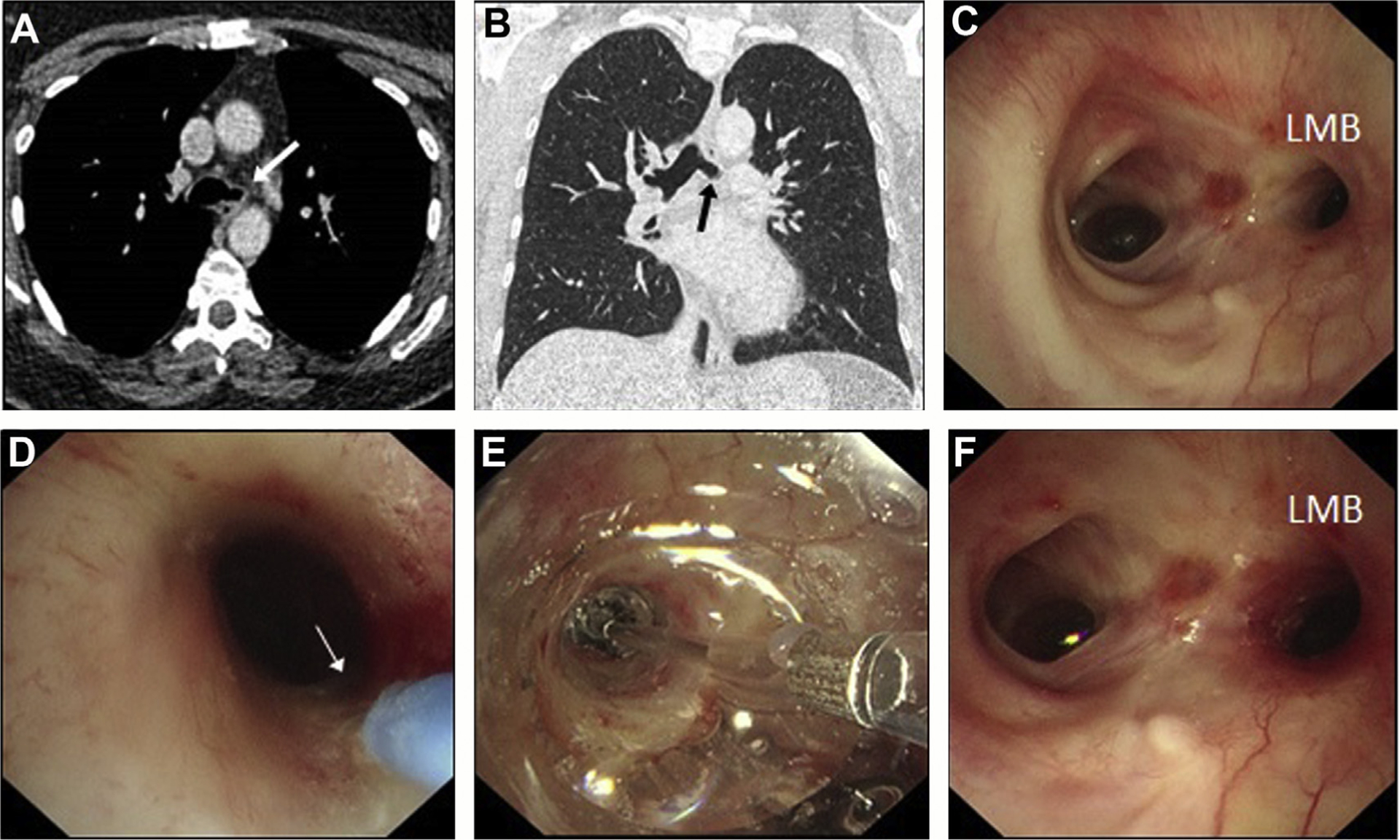
Balloon bronchoplasty and intra-airway steroid injection. (A–C) Left main bronchus (LMB) stricture seen on CT and on bronchoscopy in a patient with vasculitis. (D) Injection of methylprednisolone into the airway wall to deliver local anti-inflammatory therapy. (E) Noncutting balloon dilatation performed to stretch open the stricture. (F) Postintervention endobronchial appearances showing improved luminal diameter of the LMB orifice.
AIRWAY STENTING
The first airway stent was inserted by Harkins99 in the management of benign tracheal stenosis. Over time, newer stents have been developed and trialed as understanding of bronchial dynamics and airway pathology increases. Table 3 outlines the variety of stents currently available. More commonly, self-expanding metallic airway stents (SEMAS) are often used and available in a covered design, which is removable if required in the future.
Table 3.
Types of airway stents and associated features
| Type | Features |
|---|---|
| Silicone | Most commonly used worldwide, able to withstand significant extrinsic forces but disrupts mucus clearance |
| Montgomery T-tube | Used for subglottic stenosis and requires a tracheal stoma, helpful in laryngeal and high tracheal obstruction, exchangeable/removable |
| Dumon | Studded wall design visible in radiograph, deployed using rigid bronchoscopy and available in Y-stent shape |
| Polyflex | Tungsten dotted markers for visibility on radiograph, less prone to granulation but more likely to migrate |
| Uncovered metallic | Useful in managing extrinsic disease only and not removable; therefore, only for limited expectancy cases Early variants were expandable through bronchoplasty |
| SEMASs | Made of itinoln alloy; expand postinsertion to final diameter through metal memory/retained elasticity. Prone to metal fatigue |
| AERO/alveolus | Fully covered nitinol SEMAS, easily removable. Useful in cases of temporary need for stenting to permit other treatment |
Impending critical airway obstruction, particularly due to extraluminal disease, may require immediate stenting to prevent asphyxia (Fig. 11). Symptoms often develop once obstruction is greater than 50%. Where employed, stents should be used as a bridging tool for consolidative therapies (eg, radiotherapy) to treat underlying disease and protect the bronchial tree. If successful, this may allow for stent removal at a later date. Airway stenting can remain unpredictable, however, and careful decision making should be undertaken when weighing up the potential benefits versus risks both short term and long term (Fig. 12, Table 4).
Fig. 11.
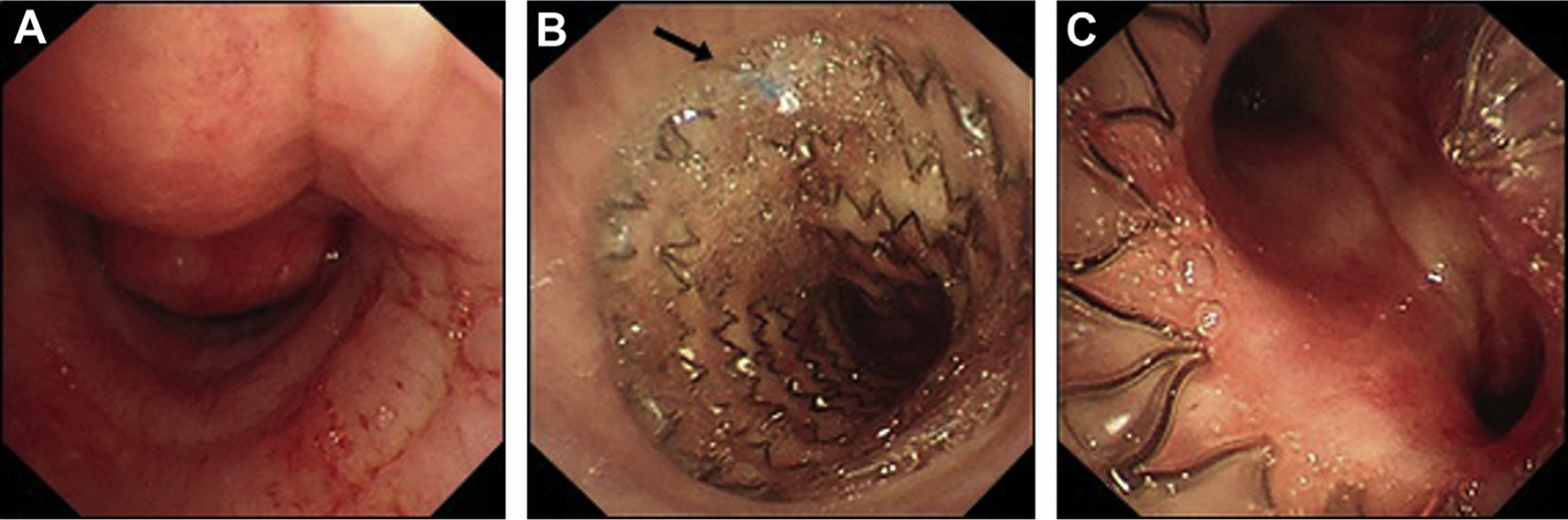
Tracheal obstruction and airway stenting in an elderly smoker presenting with cough and breathlessness. (A) Severe extrinsic compression of the lower trachea seen on bronchoscopy. (B) Covered SEMAS inserted, proximal end view showing blue purse string (arrow), which can be grasped using forceps to reposition or remove the stent. (C) View of distal end of stent with good patency of proximal main bronchi (patient also had mixed obstructive disease in the left main bronchus).
Fig. 12.
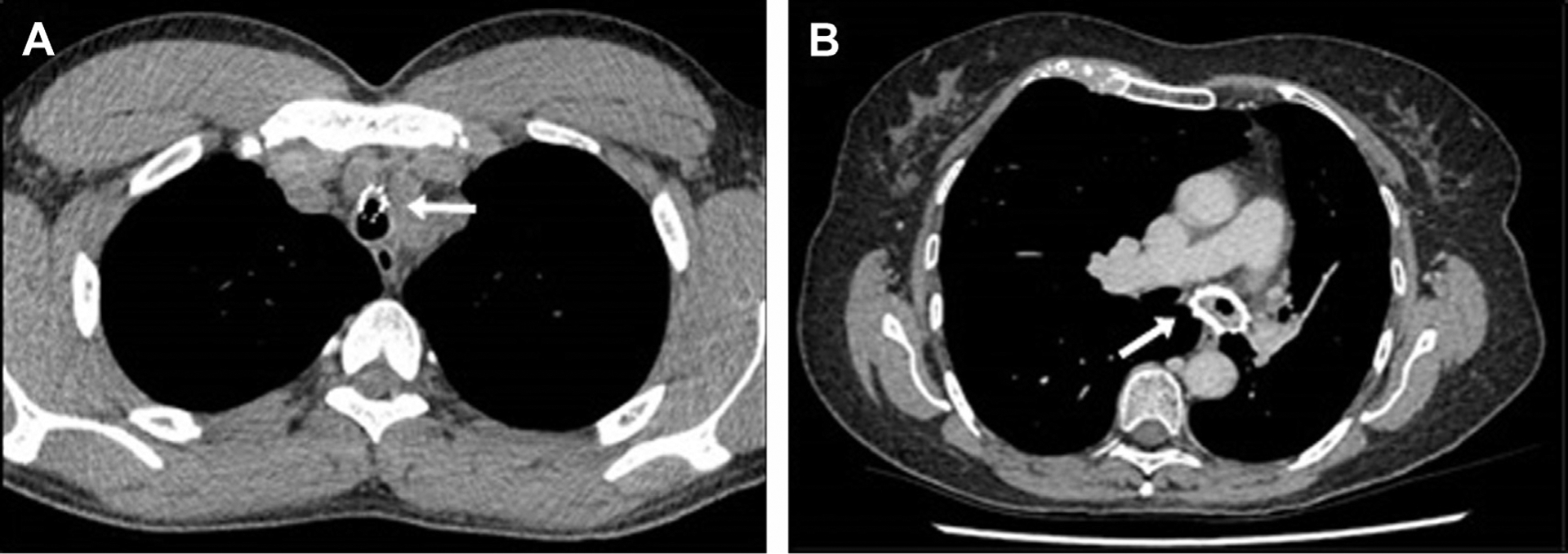
Airway stenting and complications. (A) Patient born with complete tracheal rings, which required surgical fracture as a neonate and bare metal stent insertion–stent fracture over time with partial migration toward great vessels in mediastinum. (B) Mucostasis and granulation tissue formation in a patient with a vasculitis-related bronchomalacia of the left main bronchus, which required previous stenting.
Table 4.
Potential complications of airway stents
| Mucostasis | Most common complication due to impaired mucociliary clearance and tendency for mucus impaction; long-term mucolytic therapy important |
| Granulation tissue formation | Localized inflammatory response, which may require debulking or steroid injection; most common at proximal and distal stent margins |
| Bacterial overgrowth | Biofilm formation common, associated with Staphyloccoccus and Pseudomonas, may require prolonged antibiotics or stent removal/replacement |
| Migration | More common with silicone stents and fully covered SEMASs Anterior wall suture insertion may be required if high risk |
| Fracture/fatigue | Rare but due to forces sustained during coughing; some alloys may be less resilient but newer nitinol alloy SEMAS show greater elasticity and durability |
| Bronchovascular fistula | Rare but possibly more common in specific stent cases with close proximity to hilar and pulmonary anatomy |
| Airway wall perforation | Less common, previously seen more so with bare metal and firstgeneration SEMASs |
Experience in endobronchial stent insertion, although extensively reported in case series, suffers from a lack of objective reported data. Therefore, information is collated from specialist center experiences and accounts of patient cohorts. In 1 large case series, 95% of patients experienced benefit from endobronchial stent insertion for CAO; however, complication rates were notably high, at 42%, with secretion blockage the most common.100 In 1 retrospective 7-year review of 924 patients who underwent silicone stenting for mixed etiology of CAO, the complication rate was 21%, with only 9% due to stent migration.101 There are numerous reports by groups detailing that stent insertion does help improve quality of life, breathlessness, and lung function but there is variation amongst clinical practice, stent choice, and complication report.
Tracheobronchomalacia and similar entities, such as excessive dynamic airway collapse, are causes of chronic breathlessness that continue to prove difficult to treat, with no consensus on whether stenting is effective. Stenting often is unpredictable and unlikely to prove effective in managing symptoms balanced against side effects and longer-term risks. In 1 study of 58 patients with tracheobronchomalacia who underwent endobronchial stenting, although initial subjective markers of breathlessness and quality of life were noted to improve, this was counterbalanced by no improvement in FEV1 and significant complications developing as early as 4 weeks postinsertion.102 Stenting trials with symptom control, improved dynamic CT findings, and spirometry allow for definitive surgical correction in many centers.103
Endobronchial stenting may be helpful outside of pending CAO. In cases of aerodigestive fistulas, often seen in locally advanced esophageal cancer, stenting can help seal a fistula and protect against recurrent aspiration and airway insult.104,105 There have been reports in the literature of stents used to close bronchopleural fistulae and to bridge anastomotic healing in cases of dehiscence.106,107
KEY POINTS.
Interventional pulmonology has evolved into a major field with a crucial role in patient care pathways.
Implementation of lung cancer screening has meant that more patients with peripheral lung nodules are detected and has increased the need for advanced and innovative bronchoscopic approaches.
Bronchoscopic intervention commonly is used to treat benign and malignant airway obstruction in order to improve symptoms and performance status, which may allow access to further therapies previously considered not appropriate.
There are several newer diagnostic and therapeutic bronchoscopic approaches now available in clinical practice and this review aims to provide a more detailed insight in to their utility.
DISCLOSURE
Dr S. Shaefi is supported by a Grant for Early Medical/Surgical Specialists’ Transition to Aging Research (GEMSSTAR) award (R03AG060179) from the National Institute on Aging and a Mentored Clinical Scientist Research Career Development Award from the National Institute of General Medical Sciences (K08GM134220). This work was undertaken partly at UCLH/UCL, which received a proportion of funding from the Department of Health NIHR Biomedical Research Centre funding stream. Dr N. Navani is supported by a Medical Research Council fellowship (MR/T02481X/1).
REFERENCES
- 1.Leef JL, Klein JS. The solitary pulmonary nodule. Radiol Clin North Am 2002;40(1):123–43, ix. [DOI] [PubMed] [Google Scholar]
- 2.Midthun DE, Swensen SJ, Jett JR, et al. O-127 Evaluation of nodules detected by screening for lung cancer with low dose spiral computed tomography. Lung Cancer 2003;41:S40. [Google Scholar]
- 3.Ost D The solitary pulmonary nodule. N Engl J Med 2003;348:2535–42. [DOI] [PubMed] [Google Scholar]
- 4.Shulman L, Ost D. Advances in bronchoscopic diagnosis of lung cancer. Curr Opin Pulm Med 2007;13(4):271–7. [DOI] [PubMed] [Google Scholar]
- 5.Vyas KS, Davenport DL, Ferraris VA, et al. Mediastinoscopy: trends and practice patterns in the United States. South Med J 2013;106(10): 539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson DO, Weissfeld JL, Fuhrman CR, et al. The Pittsburgh Lung Screening Study (PLuSS): outcomes within 3 years of a first computed tomography scan. Am J Respir Crit Care Med 2008;178(9): 956–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swensen SJ, Jett JR, Hartman TE, et al. CT screening for lung cancer: five-year prospective experience. Radiology 2005;235(1):259–65. [DOI] [PubMed] [Google Scholar]
- 8.Pastorino U, Bellomi M, Landoni C, et al. Early lung-cancer detection with spiral CT and positron emission tomography in heavy smokers: 2-year results. Lancet 2003;362(9384):593–7. [DOI] [PubMed] [Google Scholar]
- 9.Navani N, Brown JM, Nankivell M, et al. Suitability of endobronchial ultrasound-guided transbronchial needle aspiration specimens for subtyping and genotyping of non-small cell lung cancer: a multicenter study of 774 patients. Am J Respir Crit Care Med 2012;185(12):1316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerr KM, Lamb D, Wathen CG, et al. Pathological assessment of mediastinal lymph nodes in lung cancer: implications for non-invasive mediastinal staging. Thorax 1992;47(5):337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silvestri GA, Gould MK, Margolis ML, et al. Non-invasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007;132(3 Suppl):178S–201S. [DOI] [PubMed] [Google Scholar]
- 12.Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143(5 Suppl): e211S–50S. [DOI] [PubMed] [Google Scholar]
- 13.McLoud TC, Bourgouin PM, Greenberg RW, et al. Bronchogenic carcinoma: analysis of staging in the mediastinum with CT by correlative lymph node mapping and sampling. Radiology 1992; 182(2):319–23. [DOI] [PubMed] [Google Scholar]
- 14.Annema JT, van Meerbeeck JP, Rintoul RC, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA 2010;304(20):2245–52. [DOI] [PubMed] [Google Scholar]
- 15.Ernst A, Anantham D, Eberhardt R, et al. Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J Thorac Oncol 2008;3(6):577–82. [DOI] [PubMed] [Google Scholar]
- 16.Navani N, Nankivell M, Lawrence DR, et al. Lung cancer diagnosis and staging with endobronchial ultrasound-guided transbronchial needle aspiration compared with conventional approaches: an open-label, pragmatic, randomised controlled trial. Lancet Respir Med 2015;3(4):282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mondoni M, Sotgiu G, Bonifazi M, et al. Transbronchial needle aspiration in peripheral pulmonary lesions: a systematic review and meta-analysis. Eur Respir J 2016;48(1):196–204. [DOI] [PubMed] [Google Scholar]
- 18.Casal RF, Sarkiss M, Jones AK, et al. Cone beam computed tomography-guided thin/ultrathin bronchoscopy for diagnosis of peripheral lung nodules: a prospective pilot study. J Thorac Dis 2018; 10(12):6950–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pritchett MA, Schampaert S, de Groot JAH, et al. Cone-beam CT with augmented fluoroscopy combined with electromagnetic navigation bronchoscopy for biopsy of pulmonary nodules. J Bronchol Interv Pulmonol 2018;25(4):274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hürter T, Hanrath P. Endobronchial sonography: feasibility and preliminary results. Thorax 1992; 47(7):565–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herth FJF, Ernst A, Becker HD. Endobronchial ultrasound-guided transbronchial lung biopsy in solitary pulmonary nodules and peripheral lesions. Eur Respir J 2002;20(4):972–4. [DOI] [PubMed] [Google Scholar]
- 22.Jacomelli M, Demarzo SE, Cardoso PFG, et al. Radial-probe EBUS for the diagnosis of peripheral pulmonary lesions. J Bras Pneumol 2016;42(4): 248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinfort DP, Khor YH, Manser RL, et al. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysis. Eur Respir J 2011;37(4):902–10. [DOI] [PubMed] [Google Scholar]
- 24.Ali MS, Trick W, Mba BI, et al. Radial endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions: A systematic review and meta-analysis. Respirol Carlton Vic 2017;22(3): 443–53. [DOI] [PubMed] [Google Scholar]
- 25.Khandhar SJ, Bowling MR, Flandes J, et al. Electromagnetic navigation bronchoscopy to access lung lesions in 1,000 subjects: first results of the prospective, multicenter NAVIGATE study. BMC Pulm Med 2017;17(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folch EE, Pritchett MA, Nead MA, et al. Electromagnetic navigation bronchoscopy for peripheral pulmonary lesions: one-year results of the prospective, multicenter NAVIGATE study. J Thorac Oncol 2019;14(3):445–58. [DOI] [PubMed] [Google Scholar]
- 27.Bowling MR, Folch EE, Khandhar SJ, et al. Fiducial marker placement with electromagnetic navigation bronchoscopy: a subgroup analysis of the prospective, multicenter NAVIGATE study. Ther Adv Respir Dis 2019;13 1753466619841234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herth FJF, Eberhardt R, Sterman D, et al. Bronchoscopic transparenchymal nodule access (BTPNA): first in human trial of a novel procedure for sampling solitary pulmonary nodules. Thorax 2015; 70(4):326–32. [DOI] [PubMed] [Google Scholar]
- 29.Harzheim D, Sterman D, Shah PL, et al. Bronchoscopic transparenchymal nodule access: feasibility and safety in an endoscopic unit. Respir Int Rev Thorac Dis 2016;91(4):302–6. [DOI] [PubMed] [Google Scholar]
- 30.Mondoni M, Sotgiu G. Bronchoscopic management of peripheral pulmonary lesions: robotic approach paves the way to the future. BMC Pulm Med 2019;19(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen AC, Gillespie CT. Robotic endoscopic airway challenge: REACH assessment. Ann Thorac Surg 2018;106(1):293–7. [DOI] [PubMed] [Google Scholar]
- 32.Rojas-Solano JR, Ugalde-Gamboa L, Machuzak M. Robotic bronchoscopy for diagnosis of suspected lung cancer: a feasibility study. J Bronchol Interv Pulmonol 2018;25(3):168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee P, van den Berg RM, Lam S, et al. Color fluorescence ratio for detection of bronchial dysplasia and carcinoma in situ. Clin Cancer Res 2009; 15(14):4700–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Wu J, Yang Y, et al. White light, autofluorescence and narrow-band imaging bronchoscopy for diagnosing airway pre-cancerous and early cancer lesions: a systematic review and meta-analysis. J Thorac Dis 2016;8(11):3205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lam B, Lam SY, Wong MP, et al. Sputum cytology examination followed by autofluorescence bronchoscopy: a practical way of identifying early stage lung cancer in central airway. Lung Cancer 2009; 64(3):289–94. [DOI] [PubMed] [Google Scholar]
- 36.Zaric B, Stojsic V, Sarcev T, et al. Advanced bronchoscopic techniques in diagnosis and staging of lung cancer. J Thorac Dis 2013;5(Suppl 4): S359–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun J, Garfield DH, Lam B, et al. The value of autofluorescence bronchoscopy combined with white light bronchoscopy compared with white light alone in the diagnosis of intraepithelial neoplasia and invasive lung cancer: a meta-analysis. J Thorac Oncol 2011;6(8):1336–44. [DOI] [PubMed] [Google Scholar]
- 38.Epelbaum O, Aronow WS. Autofluorescence bronchoscopy for lung cancer screening: a time to reflect. Ann Transl Med 2016;4(16):311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iftikhar IH, Musani AI. Narrow-band imaging bronchoscopy in the detection of premalignant airway lesions: a meta-analysis of diagnostic test accuracy. Ther Adv Respir Dis 2015;9(5):207–16. [DOI] [PubMed] [Google Scholar]
- 40.Shibuya K, Nakajima T, Fujiwara T, et al. Narrow band imaging with high-resolution bronchovideoscopy: a new approach for visualizing angiogenesis in squamous cell carcinoma of the lung. Lung Cancer Amst Neth 2010;69(2):194–202. [DOI] [PubMed] [Google Scholar]
- 41.Herth FJF, Eberhardt R, Anantham D, et al. Narrow-band imaging bronchoscopy increases the specificity of bronchoscopic early lung cancer detection. J Thorac Oncol 2009;4(9):1060–5. [DOI] [PubMed] [Google Scholar]
- 42.Advani M, Purohit G, Vyas S, et al. Comparison of diagnostic potential of narrow band imaging bronchoscopy over white light bronchoscopy in lung cancer. J Bronchol Interv Pulmonol 2018;25(2): 132–6. [DOI] [PubMed] [Google Scholar]
- 43.Zhu J, Li W, Zhou J, et al. The diagnostic value of narrow-band imaging for early and invasive lung cancer: a meta-analysis. Clinics (Sao Paulo) 2017;72(7):438–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Babiak A, Hetzel J, Krishna G, et al. Transbronchial cryobiopsy: a new tool for lung biopsies. Respir Int Rev Thorac Dis 2009;78(2):203–8. [DOI] [PubMed] [Google Scholar]
- 45.Griff S, Ammenwerth W, Schönfeld N, et al. Morphometrical analysis of transbronchial cryobiopsies. Diagn Pathol 2011;6:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han Q, Luo Q, Xie J-X, et al. Diagnostic yield and postoperative mortality associated with surgical lung biopsy for evaluation of interstitial lung diseases: A systematic review and meta-analysis. J Thorac Cardiovasc Surg 2015;149(5):1394–401. e1. [DOI] [PubMed] [Google Scholar]
- 47.Johannson KA, Marcoux VS, Ronksley PE, et al. Diagnostic yield and complications of transbronchial lung cryobiopsy for interstitial lung disease. A systematic review and metaanalysis. Ann Am Thorac Soc 2016;13(10):1828–38. [DOI] [PubMed] [Google Scholar]
- 48.Ravaglia C, Bonifazi M, Wells AU, et al. Safety and diagnostic yield of transbronchial lung cryobiopsy in diffuse parenchymal lung diseases: a comparative study versus video-assisted thoracoscopic lung biopsy and a systematic review of the literature. Respir Int Rev Thorac Dis 2016;91(3):215–27. [DOI] [PubMed] [Google Scholar]
- 49.Tomassetti S, Wells AU, Costabel U, et al. Bronchoscopic lung cryobiopsy increases diagnostic confidence in the multidisciplinary diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2016;193(7):745–52. [DOI] [PubMed] [Google Scholar]
- 50.Romagnoli M, Colby TV, Berthet J-P, et al. Poor concordance between sequential transbronchial lung cryobiopsy and surgical lung biopsy in the diagnosis of diffuse interstitial lung diseases. Am J Respir Crit Care Med 2019;199(10):1249–56. [DOI] [PubMed] [Google Scholar]
- 51.Troy LK, Grainge C, Corte TJ, et al. Diagnostic accuracy of transbronchial lung cryobiopsy for interstitial lung disease diagnosis (COLDICE): a prospective, comparative study. Lancet Respir Med 2019. 10.1016/S2213-2600(19)30342-X. [DOI] [PubMed] [Google Scholar]
- 52.Cavaliere S, Venuta F, Foccoli P, et al. Endoscopic treatment of malignant airway obstructions in 2,008 patients. Chest 1996;110(6):1536–42. [DOI] [PubMed] [Google Scholar]
- 53.Khemasuwan D, Mehta AC, Wang K-P. Past, present, and future of endobronchial laser photoresection. J Thorac Dis 2015;7(Suppl 4):S380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Desai SJ, Mehta AC, VanderBrug Medendorp S, et al. Survival experience following Nd:YAG laser photoresection for primary bronchogenic carcinoma. Chest 1988;94(5):939–44. [DOI] [PubMed] [Google Scholar]
- 55.Jang TW, Blackman G, George JJ. Survival benefits of lung cancer patients undergoing laser and brachytherapy. J Korean Med Sci 2002;17(3): 341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dalar L, Ozdemir C, Abul Y, et al. Endobronchial treatment of carcinoid tumors of the lung. Thorac Cardiovasc Surg 2016;64(2):166–71. [DOI] [PubMed] [Google Scholar]
- 57.Neyman K, Sundset A, Naalsund A, et al. Endoscopic treatment of bronchial carcinoids in comparison to surgical resection: a retrospective study. J Bronchol Interv Pulmonol 2012;19(1): 29–34. [DOI] [PubMed] [Google Scholar]
- 58.Fuks L, Fruchter O, Amital A, et al. Long-term follow-up of flexible bronchoscopic treatment for bronchial carcinoids with curative intent. Diagn Ther Endosc 2009;2009:782961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goldman L, editor. The biomedical laser. New York: Springer; 1981. 10.1007/978-1-4612-5922-0. [DOI] [Google Scholar]
- 60.Grund KE, Storek D, Farin G. Endoscopic argon plasma coagulation (APC) first clinical experiences in flexible endoscopy. Endosc Surg Allied Technol 1994;2(1):42–6. [PubMed] [Google Scholar]
- 61.Bolliger CT, Sutedja TG, Strausz J, et al. Therapeutic bronchoscopy with immediate effect: laser, electrocautery, argon plasma coagulation and stents. Eur Respir J 2006;27(6):1258–71. [DOI] [PubMed] [Google Scholar]
- 62.Reddy C, Majid A, Michaud G, et al. Gas embolism following bronchoscopic argon plasma coagulation: a case series. Chest 2008;134(5): 1066–9. [DOI] [PubMed] [Google Scholar]
- 63.Shaw Y, Yoneda KY, Chan AL. Cerebral gas embolism from bronchoscopic argon plasma coagulation: a case report. Respir Int Rev Thorac Dis 2012;83(3):267–70. [DOI] [PubMed] [Google Scholar]
- 64.Reichle G, Freitag L, Kullmann HJ, et al. [Argon plasma coagulation in bronchology: a new method–alternative or complementary?]. Pneumol Stuttg Ger 2000;54(11):508–16. [DOI] [PubMed] [Google Scholar]
- 65.Farhat AA, Ragab M, Abd-Elzaher AH, et al. Bronchoscopic electrocauterization versus argon plasma coagulation as a palliative management for patients with bronchogenic carcinoma. Egypt J Chest Dis Tuberc 2015;64(1):243–8. [Google Scholar]
- 66.Morice RC, Ece T, Ece F, et al. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest 2001;119(3):781–7. [DOI] [PubMed] [Google Scholar]
- 67.Jin F, Mu D, Xie Y, et al. Application of bronchoscopic argon plasma coagulation in the treatment of tumorous endobronchial tuberculosis: historical controlled trial. J Thorac Cardiovasc Surg 2013; 145(6):1650–3. [DOI] [PubMed] [Google Scholar]
- 68.Wahidi MM, Unroe MA, Adlakha N, et al. The use of electrocautery as the primary ablation modality for malignant and benign airway obstruction. J Thorac Oncol 2011;6(9):1516–20. [DOI] [PubMed] [Google Scholar]
- 69.van Boxem TJ, Westerga J, Venmans BJ, et al. Tissue effects of bronchoscopic electrocautery: bronchoscopic appearance and histologic changes of bronchial wall after electrocautery. Chest 2000; 117(3):887–91. [DOI] [PubMed] [Google Scholar]
- 70.Boxem TV, Muller M, Venmans B, et al. Nd-YAG laser vs bronchoscopic electrocautery for palliation of symptomatic airway obstruction: a cost-effectiveness study. Chest 1999;116(4):1108–12. [DOI] [PubMed] [Google Scholar]
- 71.Vergnon J-M, Huber RM, Moghissi K. Place of cryotherapy, brachytherapy and photodynamic therapy in therapeutic bronchoscopy of lung cancers. Eur Respir J 2006;28(1):200–18. [DOI] [PubMed] [Google Scholar]
- 72.Hetzel M, Hetzel J, Schumann C, et al. Cryorecanalization: a new approach for the immediate management of acute airway obstruction. J Thorac Cardiovasc Surg 2004;127(5):1427–31. [DOI] [PubMed] [Google Scholar]
- 73.Lee S-H, Choi W-J, Sung S-W, et al. Endoscopic cryotherapy of lung and bronchial tumors: a systematic review. Korean J Intern Med 2011; 26(2):137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee H, Leem CS, Lee JH, et al. Successful removal of endobronchial blood clots using bronchoscopic cryotherapy at bedside in the intensive care unit. Tuberc Respir Dis (Seoul) 2014;77(4):193–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol 2008; 9(7):621–8. [DOI] [PubMed] [Google Scholar]
- 76.Yuan Z, Wang Y, Zhang J, et al. A meta-analysis of clinical outcomes after radiofrequency ablation and microwave ablation for lung cancer and pulmonary metastases. J Am Coll Radiol 2019;16(3): 302–14. [DOI] [PubMed] [Google Scholar]
- 77.Tsushima K, Koizumi T, Tanabe T, et al. Bronchoscopy-guided radiofrequency ablation as a potential novel therapeutic tool. Eur Respir J 2007;29(6): 1193–200. [DOI] [PubMed] [Google Scholar]
- 78.Ferguson J, Egressy K, Schefelker R, et al. Bronchoscopically-guided microwave ablation in the lung. Chest 2013;144(4):87A.23392731 [Google Scholar]
- 79.Tanabe T, Koizumi T, Tsushima K, et al. Comparative study of three different catheters for CT imaging-bronchoscopy-guided radiofrequency ablation as a potential and novel interventional therapy for lung cancer. Chest 2010;137(4):890–7. [DOI] [PubMed] [Google Scholar]
- 80.Koizumi T, Tsushima K, Tanabe T, et al. Bronchoscopy-guided cooled radiofrequency ablation as a novel intervention therapy for peripheral lung cancer. Respir Int Rev Thorac Dis 2015;90(1):47–55. [DOI] [PubMed] [Google Scholar]
- 81.Escobar-Sacristán JA, Granda-Orive JI, Gutiérrez Jiménez T, et al. Endobronchial brachytherapy in the treatment of malignant lung tumours. Eur Respir J 2004;24(3):348–52. [DOI] [PubMed] [Google Scholar]
- 82.Skowronek J Brachytherapy in the treatment of lung cancer - a valuable solution. J Contemp Brachytherapy 2015;7(4):297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nori D, Allison R, Kaplan B, et al. High dose-rate intraluminal irradiation in bronchogenic carcinoma. Technique and results. Chest 1993;104(4): 1006–11. [DOI] [PubMed] [Google Scholar]
- 84.Hardavella G, George J. Interventional bronchoscopy in the management of thoracic malignancy. Breathe (Sheff) 2015;11(3):202–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reveiz L, Rueda J-R, Cardona AF. Palliative endobronchial brachytherapy for non-small cell lung cancer. Cochrane Database Syst Rev 2012;(12): CD004284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moghissi K, Bond MG, Sambrook RJ, et al. Treatment of endotracheal or endobronchial obstruction by non-small cell lung cancer: lack of patients in an MRC randomized trial leaves key questions unanswered. Medical Research Council Lung Cancer Working Party. Clin Oncol (R Coll Radiol) 1999; 11(3):179–83. [DOI] [PubMed] [Google Scholar]
- 87.Usuda J, Kato H, Okunaka T, et al. Photodynamic therapy (PDT) for lung cancers. J Thorac Oncol 2006;1(5):489–93. [PubMed] [Google Scholar]
- 88.Usuda J, Ichinose S, Ishizumi T, et al. Management of multiple primary lung cancer in patients with centrally located early cancer lesions. J Thorac Oncol 2010;5(1):62–8. [DOI] [PubMed] [Google Scholar]
- 89.Moghissi K, Dixon K. Is bronchoscopic photodynamic therapy a therapeutic option in lung cancer? Eur Respir J 2003;22(3):535–41. [DOI] [PubMed] [Google Scholar]
- 90.McCaughan JS, Williams TE. Photodynamic therapy for endobronchial malignant disease: A prospective fourteen-year study. J Thorac Cardiovasc Surg 1997;114(6):940–7. [DOI] [PubMed] [Google Scholar]
- 91.Moghissi K, Dixon K, Stringer M, et al. The place of bronchoscopic photodynamic therapy in advanced unresectable lung cancer: experience of 100 cases1. Eur J Cardiothorac Surg 1999; 15(1):1–6. [DOI] [PubMed] [Google Scholar]
- 92.Litle VR, Luketich JD, Christie NA, et al. Photodynamic therapy as palliation for esophageal cancer: experience in 215 patients. Ann Thorac Surg 2003; 76(5):1687–92 [discussion: 1692–3]. [DOI] [PubMed] [Google Scholar]
- 93.Jabbardarjani HR, Kiani A, Sheikhi N, et al. Balloon bronchoplasty: case series. Tanaffos 2012;11(2): 42–8. [PMC free article] [PubMed] [Google Scholar]
- 94.Li S [Interventional management of benign airway stenosis]. Zhonghua Jie He He Hu Xi Za Zhi 2011; 34(5):329–32. [PubMed] [Google Scholar]
- 95.Shitrit D, Kuchuk M, Zismanov V, et al. Bronchoscopic balloon dilatation of tracheobronchial stenosis: long-term follow-up. Eur J Cardiothorac Surg 2010;38(2):198–202. [DOI] [PubMed] [Google Scholar]
- 96.Nouraei SAR, Mills H, Butler CR, et al. Outcome of treating airway compromise due to bronchial stenosis with intralesional corticosteroids and cutting-balloon bronchoplasty. Otolaryngol Head Neck Surg 2011;145(4):623–7. [DOI] [PubMed] [Google Scholar]
- 97.Rahman NA, Fruchter O, Shitrit D, et al. Flexible bronchoscopic management of benign tracheal stenosis: long term follow-up of 115 patients. J Cardiothorac Surg 2010;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Garg M, Gogia P, Manoria P, et al. Bronchoscopic management of benign bronchial stenosis by electrocautery and balloon dilatation. Indian J Chest Dis Allied Sci 2012;54(1):41–3. [PubMed] [Google Scholar]
- 99.Harkins WB. An endotracheal metallic prosthesis in the treatment of stenosis of the upper trachea. Ann Otol Rhinol Laryngol 1952;61(3):663–76. [DOI] [PubMed] [Google Scholar]
- 100.Wood DE. Airway stenting. Chest Surg Clin N Am 2001;11(4):841–60. [PubMed] [Google Scholar]
- 101.Dumon J-F, Cavaliere S, Diaz-Jimenez JP, et al. Seven-year experience with the dumon prosthesis. J Bronchol 1996;3(1):6–10. [Google Scholar]
- 102.Ernst A, Majid A, Feller-Kopman D, et al. Airway stabilization with silicone stents for treating adult tracheobronchomalacia: a prospective observational study. Chest 2007;132(2):609–16. [DOI] [PubMed] [Google Scholar]
- 103.Parikh M, Wilson J, Majid A, et al. Airway stenting in excessive central airway collapse. J Vis Surg 2017; 3:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Herth FJF, Peter S, Baty F, et al. Combined airway and oesophageal stenting in malignant airway-oesophageal fistulas: a prospective study. Eur Respir J 2010;36(6):1370–4. [DOI] [PubMed] [Google Scholar]
- 105.Zhou C, Hu Y, Xiao Y, et al. Current treatment of tracheoesophageal fistula. Ther Adv Respir Dis 2017; 11(4):173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.de Lima A, Holden V, Gesthalter Y, et al. Treatment of persistent bronchopleural fistula with a manually modified endobronchial stent: a case-report and brief literature review. J Thorac Dis 2018;10(10): 5960–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tufail M, Pannu K, Bhusari S, et al. Management of post-pneumonectomy bronchopleural fistula using a Y dumon tracheobronchial stent and a novel deployment technique - a case report. Am J Respir Crit Care Med 2017;2017:A1632. [Google Scholar]


