Abstract
Purpose
Pathological neovascularization and fibrosis are common pathological changes of many retinal diseases, such as proliferative retinopathy (PR) and age-related macular degeneration (AMD). Treatment modalities for these pathological changes are limited. The purpose of the present study was to test the effects of palmitoylethanolamide (PEA), an endocannabinoid mimetic amide, on retinal neovascularization and fibrosis and to determine its molecular mechanism of action.
Methods
A rat Müller cell line (rMC-1), a mouse model of oxygen-induced retinopathy (OIR), and the very-low-density lipoprotein receptor (VLDLR) knockout mouse model were used. PEA was intraperitoneally injected or orally administrated in animal models. Inflammation and profibrotic changes were evaluated by western blot analysis. Glial fibrillary acidic protein (GFAP) and peroxisome proliferator-activated receptor alpha (PPARα) were measured by RT-PCR and western blot analysis.
Results
Profibrotic changes were present in OIR and Vldlr−/− retinas. PEA significantly alleviated inflammation and inhibited neovascularization in OIR and Vldlr−/− retinas and suppressed profibrotic changes in OIR and Vldlr−/− retinas. Moreover, PEA potently suppressed Müller gliosis in these retinas. In rMC-1 cells, PEA suppressed Müller gliosis, reduced inflammatory cytokines, and attenuated profibrotic changes. Further, both mRNA and protein levels of PPARα were elevated in the retina under PEA treatment, and the effects of PEA were abolished in Pparα−/− OIR mice.
Conclusions
PEA reduced retinal neovascularization and fibrotic changes and suppressed Müller gliosis in experimental PR and neovascular AMD by activating PPARα. PEA may be a potential treatment for retinopathies with pathological neovascularization and fibrosis.
Keywords: palmitoylethanolamide, neovascularization, retinal fibrosis, proliferative retinopathy, neovascular age-related macular degeneration
Pathological neovascularization and subsequent fibrosis are common pathologies in many retinal diseases, such as proliferative retinopathy (PR) and age-related macular degeneration (AMD).1 In developed countries, retinal neovascularization and fibrosis are considered to be the most common pathological changes in the retina that cause vision loss.2 Following retinal injury, the release of chemotactic agents, proinflammatory cytokines, and vascular endothelial growth factor (VEGF) induces proliferation of endothelial cells and promotes the formation of new vessels. Meanwhile, the recruitment of inflammatory cells in the retina initiates a reparative process and facilitates synthesis and remodeling of new extracellular matrix (ECM). If the reparative process is not successful, the deposition of excess ECM may disturb the retinal architecture, interrupt proper cellular interaction, and cause retina contraction. Currently, the therapeutic strategies for retinal neovascularization and fibrosis are limited. The exploration of new therapeutic treatments will greatly benefit patients with neovascular and fibrotic retinopathies.
It has been reported that palmitoylethanolamide (PEA), an endocannabinoid mimetic amide, has anti-inflammatory and anti-analgesic effects in the central nervous system (CNS).3 Recent studies have explored the application of PEA in glaucoma and diabetic retinopathy; for example, topical PEA suppresses the ocular surface inflammation associated with the long-term use of hypotensive eye drops in glaucoma patients.4 PEA attenuates retinal inflammation and reduces the expressions of the proinflammatory factors in streptozotocin-induced diabetic rats.5 However, the effects of PEA on retinal neovascularization are unknown.
In addition to the anti-inflammatory properties of PEA, antifibrotic effects for PEA have also been reported. A prior study identified that PEA inhibits liver fibrosis in a carbon tetrachloride-induced mouse model.6 In another study, PEA was found to reduce lung inflammation, pulmonary damage, and lung fibrosis in a mouse model of idiopathic pulmonary fibrosis.7 However, the effects of PEA on retinal fibrosis have not yet been investigated.
Large amounts of PEA are produced and secreted by glial cells in the brain.8 Studies have shown that PEA inhibits reactive astrogliosis induced by β-amyloid peptide in cultured astrocyte cells.9 A recent study also reported that PEA has therapeutic effects for Alzheimer's disease and brain gliosis, indicating a possible protective role of PEA in gliosis.10 Müller cells are glial-like cells in the retina that play an important role in maintaining retinal hemostasis.11 Müller cell activation has been associated with initiation and development of many retinal diseases, including PR and AMD.12,13 Considering the suppressive effects of PEA on gliosis of the CNS, we hypothesize that PEA may also suppress retinal Müller cell activation.
In the present study, we evaluated the anti-angiogenic and antifibrotic effects of PEA in the retina using a mouse model of oxygen-induced retinopathy (OIR), an animal model for proliferative retinopathy,14 and the very-low-density lipoprotein receptor knockout (Vldlr−/−) mouse, an animal model of neovascular AMD.15 We identified profibrotic changes in OIR and Vldlr−/− retinas, suggesting the utility of these models for studying early retinal fibrotic changes. We also found that the Müller cells activated in these models were suppressed by PEA. Further, we confirmed the inhibitory effects of PEA on Müller cell activation using a cell culture model system. The mechanisms of action of PEA in the retina were also investigated. Our findings suggest that PEA inhibits retinal neovascularization and profibrotic changes and suppresses Müller cell activation in animal models of PR and neovascular AMD. PEA is a putative novel treatment for retinopathies with pathological angiogenesis and fibrosis.
Materials and Methods
Animals
C57BL/6J mice were purchased from the Laboratory Animal Center of Xiamen University. Peroxisome proliferator-activated receptor alpha (PPARα) knockout (Pparα−/−) mice and VLDLR knockout (Vldlr−/−) mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). In this study, Vldlr−/− mice and control mice for Vldlr−/− mice (littermate wild-type mice) were obtained by crossing C57BL/6J and Vldlr−/− mice. All animals were housed in a specific-pathogen-free facility and maintained in 12-hour light/12-hour dark cycles. All procedures with animals in this study were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The animal protocols were approved by the Xiamen University Experimental Animal Ethics Committee.
Cell Culture
The rat Müller cell line (rMC-1) cells were maintained in Dulbecco's Modified Eagle Medium basal medium supplemented with 10% fetal calf serum (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) and 10% penicillin streptomycin solution (Gibco).
Oxygen-Induced Retinopathy Model and PEA Treatment
Neonatal C57BL/6J mice (or Pparα−/-mice) were exposed to 75% oxygen for 5 days from postnatal day 7 (P7) to P12.16 At P12, the mice were returned to room air (21% oxygen). As described previously,17 PEA was dissolved in solution: 0.01% dimethyl sulfoxide, 10% Tween 80 (Sigma-Aldrich, St. Louis, MO, USA), 20% polyethylene glycol 400, and 69.9% physiological saline. To investigate the effects of PEA on pathological angiogenesis, PEA (30 mg/kg) was injected intraperitoneally in OIR mice from P12 to P17 and in Vldlr−/− mice from P12 to P26 daily. OIR mice (at P17) and Vldlr−/− mice (at P26) were sacrificed, and retinas were dissected for further experiments. To study the effects of PEA on fibrotic changes in Vldlr−/− mice, PEA (30 mg/kg) was administrated to 2-month-old Vldlr−/− mice by oral gavage for 30 days, and eyecups were collected for further experiments at P90.
Lectin Staining
Lectin staining of the flat-mounted retinas was conducted as described previously.18 Briefly, eyeballs were fixed with 4% paraformaldehyde for 1 hour. Retinas were dissected and then incubated with 0.5% Triton X-100 (Sigma-Aldrich) at 4°C overnight. After three washes with PBS, the retinas were incubated with isolectin GS-IB4 (Thermo Fisher Scientific) overnight at room temperature. Retinas were washed and flat-mounted for microscopy. Quantification of neovascularization vaso-obliteration was conducted as described previously.19
Immunofluorescent Staining
Retinal cryosections were fixed in acetone for 10 minutes and then incubated with antibody against glial fibrillary acidic protein (GFAP) (Abcam, Cambridge, United Kingdom) at 4°C overnight. After three washes with PBS, retinal sections were further incubated with Alexa Fluor 488-conjugated IgG (Thermo Fisher Scientific) for 1 hour. The sections were mounted with mounting medium (Vector Laboratories, Burlingame, CA, USA) and photographed with a fluorescent microscope (DM2500; Leica Microsystems, Wetzlar, Germany).
TUNEL Assay
The TUNEL assay was performed on retinal cryosections using the DeadEnd Fluorometric TUNEL System (Promega, Madison, WI, USA) according to the manufacturer's instructions.
Western Blot Analysis
Western blot analysis was conducted as previously described.20 Antibodies for GFAP, intercellular adhesion molecule 1 (ICAM-1), tumor necrosis factor alpha (TNF-α), and transforming growth factor beta (TGF-β) were obtained from Proteintech (Chicago, IL, USA). Antibodies for VEGF, TGF-β receptor II (TGF-βRII), Smad2/3, and fibronectin were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Antibodies for alpha smooth muscle actin (α-SMA) and β-actin were obtained from Sigma-Aldrich. An antibody for phospho-Smad2/3 (P-Smad2/3) was purchased from Cell Signaling (Danvers, MA, USA).
RNA Extraction and Quantitative RT-PCR Assay
Total RNA was extracted from retinas using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). Reverse transcription was performed according to the manufacturer's protocol (TaKaRa Bio, Inc., Shiga, Japan). The procedure for quantitative RT-PCR was described previously.21
Statistical Analysis
Prism 6 software (GraphPad, San Diego, CA, USA) was used to calculate a one-way ANOVA or a Student's t-test for statistical analyses. All values are expressed as mean ± SEM. At least three independent measurements were conducted for each assay. A value of P < 0.05 was considered statistically significant.
Results
PEA Attenuates Inflammation and Suppresses Neovascularization in OIR Retinas
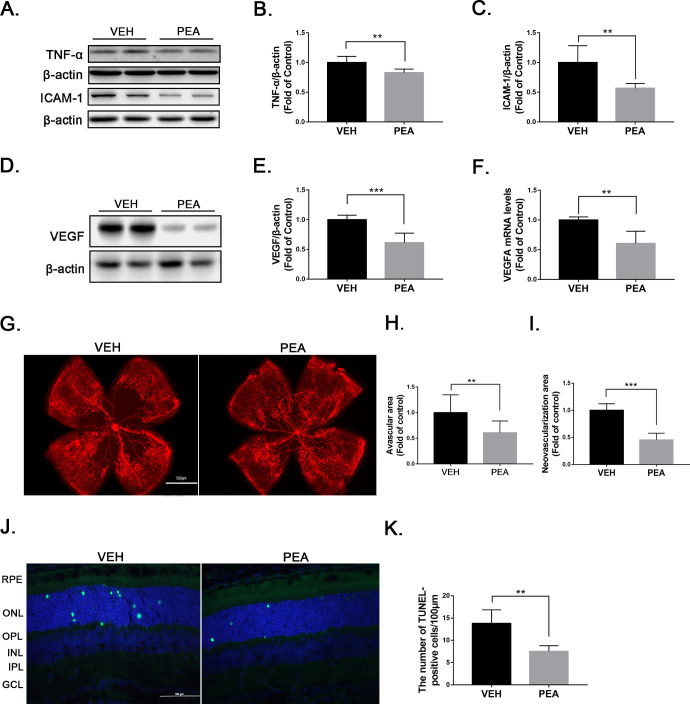
To test the therapeutic effects of PEA in pathological retinal neovascularization, we used the OIR mouse model, a commonly used animal model of proliferative retinopathy.14 Multiple studies have demonstrated that levels of inflammatory cytokines such as TNF-α, ICAM-1, and VEGF are increased in OIR retinas.22,23 In the present study, PEA treatment significantly reduced protein levels of the inflammatory cytokines TNF-α and ICAM-1 in OIR retinas (Figs. 1A–1C). Also, both mRNA and protein levels of VEGF were significantly decreased in PEA-treated OIR retinas compared to OIR retinas treated with vehicle (VEH) (Figs. 1D–1F). Lectin staining of flat-mounted OIR retinas revealed that PEA treatment decreased retinal areas of vaso-obliteration and neovascularization (Figs. 1G–1I), suggesting an anti-angiogenic effect of PEA in OIR retinas. Further, apoptotic cells in retinal sections were evaluated by TUNEL staining (Fig. 1J). PEA reduced apoptotic cells in OIR retinas (Fig. 1K), suggesting neuroprotective effects of PEA in this context. Taken together, these results indicate that PEA treatment attenuates retinal inflammation, neovascularization, and neuronal cell death in OIR retinas.
Figure 1.
Anti-inflammatory and anti-angiogenic effects of PEA in OIR. (A) Representative images of western blotting for TNF-α and ICAM1 in OIR retinas treated with VEH or PEA. Protein levels of (B) TNF-α and (C) ICAM-1 were quantified by densitometry and normalized to β-actin levels (n = 6). (D) Representative images of western blotting for VEGF in OIR retinas treated with VEH or PEA. (E) Protein level of VEGF was quantified by densitometry and normalized to β-actin levels (n = 6). (F) Quantitative RT-PCR of Vegfa mRNA expression in VEH-OIR and PEA-OIR groups (n = 5). (G) Retinas from VEH- and PEA-treated OIR mice were flat-mounted and stained with isolectin GS-IB4 (red). Areas of (H) retinal vaso-obliteration and (I) neovascularization were quantified in VEH- and PEA-treated OIR retinas (n = 8). (J) Retinal cryosections from VEH- and PEA-treated OIR mice were stained with TUNEL (green) and 4′,6-diamidino-2-phenylindole (DAPI) (blue). Representative images are shown. (K) Apoptotic cells were quantified and compared in VEH- and PEA-treated OIR mice. Data are presented as mean ± SEM; **P < 0.01, ***P < 0.001.
ECM Remodeling and Profibrotic Changes in OIR Retinas
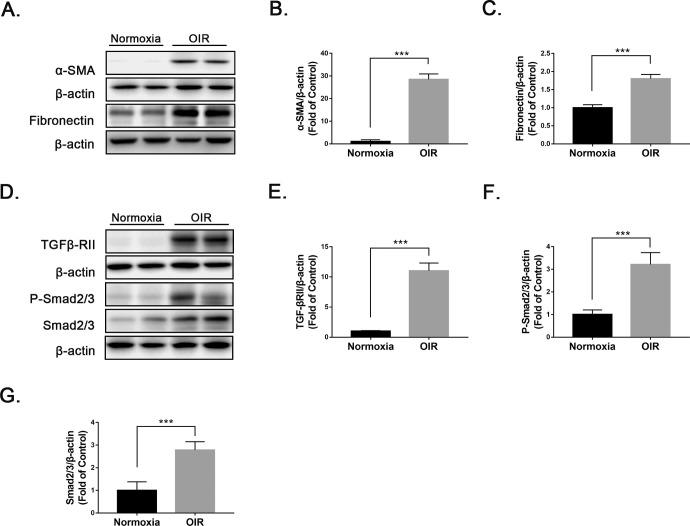
Prior studies have demonstrated that pathological angiogenesis is accompanied by remodeling of the ECM.24,25 Under pathological conditions, excessive deposition of ECM can lead to fibrotic diseases and organ dysfunction.26 To determine if early fibrotic changes were present in the OIR retinas, we measured the profibrotic marker α-SMA and a marker of ECM protein, fibronectin, in OIR retinas and normoxic retinas using western blot analysis (Fig. 2A). Protein levels of α-SMA and fibronectin were significantly upregulated in OIR retinas relative to normoxic retinas (Figs. 2B, 2C). In addition, protein levels of several key components of the TGF-β/Smad2/3 signaling pathway, TGF-βRII, P-Smad2/3, and Smad2/3 were significantly upregulated in OIR retinas compared with normoxic retinas (Figs. 2D–2G). Taken together, these results indicate the presence of ECM remodeling and profibrotic changes in OIR retinas.
Figure 2.
Presence of ECM remodeling and profibrotic changes in OIR retinas. (A–C) Protein levels of (A, B) α-SMA and (A, C) fibronectin were measured by western blot analysis and quantified by densitometry in wild-type (WT) and OIR retinas at P17 (n = 6). (D–G) Similarly, protein levels of (D, E) TGFβ-RII, (D, F) P-Smad2/3, and (D, G) Smad2/3 were measured by western blot analysis and quantified by densitometry (n = 6). Data are presented as mean ± SEM; ***P < 0.001.
PEA Inhibits Profibrotic Changes in OIR Retinas
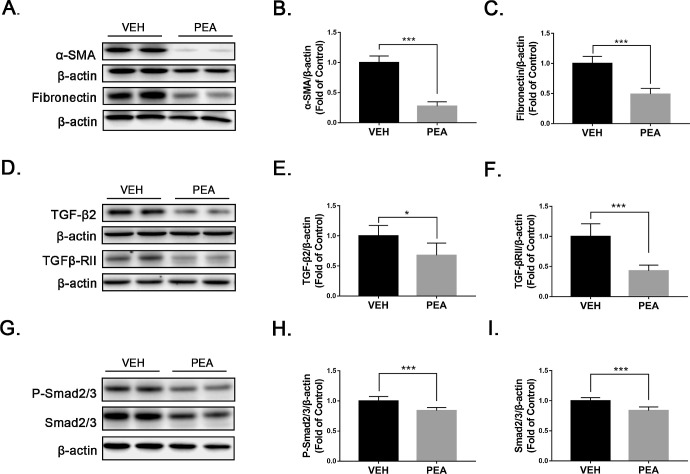
Next, we tested whether PEA had protective effects against profibrotic changes in OIR retinas. Levels of α-SMA, fibronectin, and several components of the TGF-β/Smad2/3 pathway were measured by western blot analysis. PEA significantly suppressed upregulation of α-SMA (Figs. 3A, 3B) and fibronectin (Figs. 3A, 3C) in OIR retinas. Further, PEA treatment reversed upregulation of TGF-β2 (Figs. 3D, 3E), TGF-βRII (Figs. 3D, 3F), P-Smad2/3 (Figs. 3G, 3H), and Smad2/3 (Figs. 3G, 3I) in OIR retinas. Taken together, these results demonstrate that PEA suppresses profibrotic changes in OIR retinas.
Figure 3.
Antifibrotic effects of PEA in OIR retinas. (A–C) Protein levels of (A, B) α-SMA and (A, C) fibronectin were measured by western blot analysis and quantified by densitometry in VEH- and PEA-treated OIR retinas (n = 6). (D–I) Similarly, protein levels of (D, E) TGF-β2, (D, F) TGFβ-RII, (G, H) P-Smad2/3, and (G, I) Smad2/3 were measured by western blot analysis and quantified by densitometry (n = 6). Data are presented as mean ± SEM; *P < 0.05, ***P < 0.001.
PEA Attenuates Inflammation and Suppresses Neovascularization in Vldlr−/− Retinas
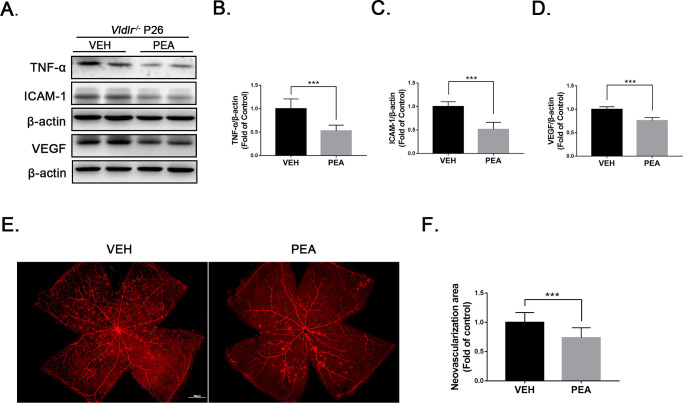
We further assessed the anti-inflammatory and anti-angiogenic effects of PEA in the Vldlr−/− mouse, a mouse model of neovascular AMD.15,27 Prior studies have demonstrated that protein levels of TNF-α, ICAM-1, and VEGF are elevated in Vldlr−/− retinas compared to wild-type (WT) retinas.28 After 2 weeks of treatment with PEA, protein expressions of the proinflammatory factors TNF-α (Figs. 4A, 4B), ICAM-1 (Figs. 4A, 4C), and VEGF (Figs. 4A, 4D) were significantly decreased in PEA-treated Vldlr−/− retinas compared with VEH-treated Vldlr−/− retinas. Lectin staining of whole-mounted retinas revealed less neovascular areas (Figs. 4E, 4F), suggesting that PEA suppresses pathological neovascularization in Vldlr−/− retinas.
Figure 4.
Anti-angiogenic effects of PEA in Vldlr−/− retinas. (A) Protein levels of TNF-α, ICAM-1 and VEGF were measured by western blot analysis in the eyecups of Vldlr−/− mice treated with VEH or PEA. (B) TNF-α, (C) ICAM-1, and (D) VEGF levels were quantified by densitometry (n = 6). (E) Retinas from VEH- and PEA-treated Vldlr−/− mice were flat-mounted and stained with isolectin GS-IB4 (red). (F) Areas of neovascularization were quantified (n = 8). Data are presented as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001.
PEA Suppresses Fibrosis in Vldlr−/− Retinas
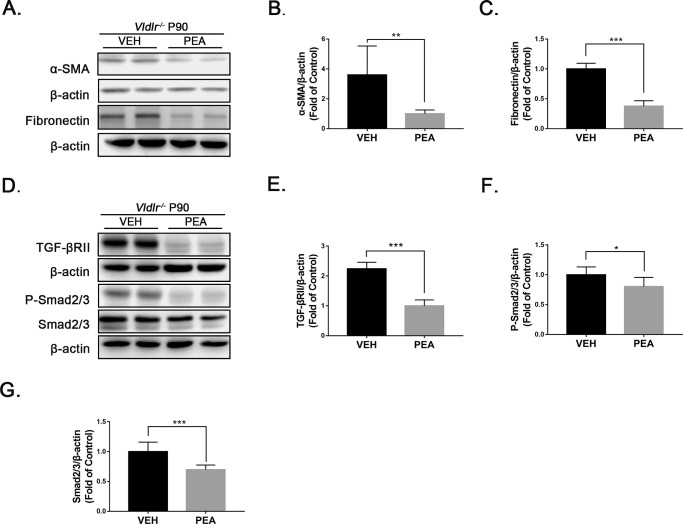
Similarly, we evaluated the antifibrotic effects of PEA in Vldlr−/− mice. At P90, the retinas of Vldlr−/− mice exhibited increased levels of TGF-β2, α-SMA, and fibronectin compared to age-matched WT controls, indicating the presence of fibrosis (Supplementary Fig. S1). After 1 month of PEA treatment, protein levels of α-SMA and fibronectin were decreased in PEA-treated Vldlr−/− retinas (Figs. 5A–5C). The levels of TGF-βRII (Figs. 5D, 5E), P-Smad2/3 (Figs. 5D, 5F), and Smad2/3 (Figs. 5D, 5G) were significantly decreased in PEA-treated Vldlr−/− retinas compared with VEH-treated Vldlr−/− retinas, suggesting antifibrotic effects of PEA in the context of the Vldlr−/− model.
Figure 5.
Antifibrotic effects of PEA in Vldlr−/− retinas. (A) Protein levels of α-SMA and fibronectin were measured by western blot analysis in the eyecups of Vldlr−/− mice treated with VEH or PEA. (B) α-SMA and (C) fibronectin were quantified by densitometry (n = 6). (D) Similarly, protein levels of TGF-βRII, P-Smad2/3, and Smad2/3 were measured by western blot analysis, and (E) TGF-βRII, (F) P-Smad2/3, and (G) Smad2/3 were quantified by densitometry (n = 6). Data are presented as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001.
PEA Inhibits Gliosis in OIR and Vldlr−/− Retinas
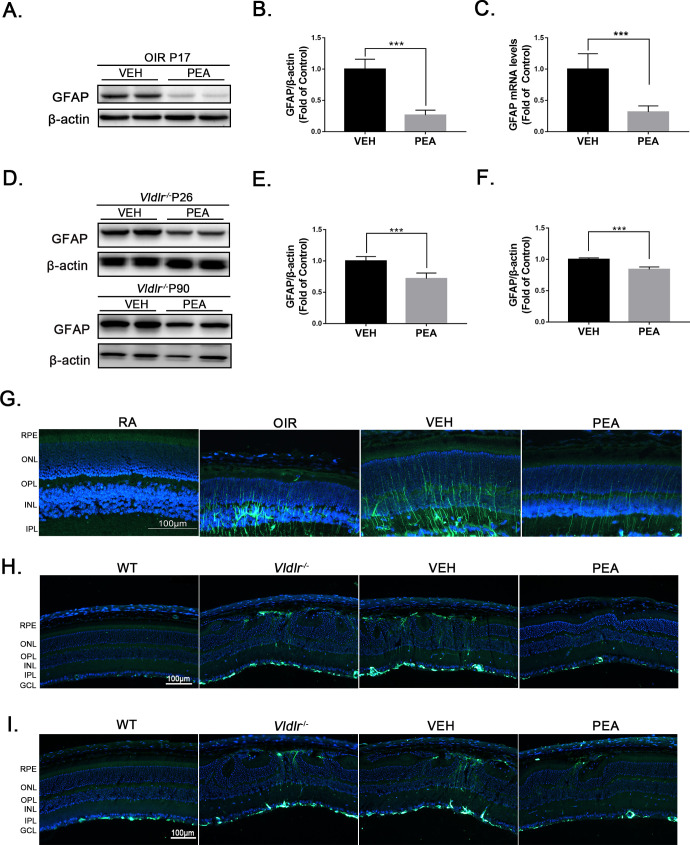
Müller cells are the major glial cells in the retina; under stress conditions, massive retinal gliosis may occur.29 Because it has been reported that PEA inhibits reactive gliosis in the CNS,9,10 we hypothesized that PEA could potentially inhibit Müller cell activation (gliosis) in the retina. GFAP, an indicator of glial activation, is dramatically increased in OIR retinas compared with normoxic retinas.20 After PEA treatment, both mRNA and protein levels of GFAP were significantly decreased in OIR retinas (Figs. 6A–6C), suggesting that PEA suppresses gliosis in the context of OIR. In Vldlr−/− retinas, protein levels of GFAP were significantly increased relative to WT retinas at P26 (Supplementary Figs. S2A, S2B) and at P90 (Supplementary Figs. S2C, S2D). GFAP levels were significantly decreased in PEA-treated Vldlr−/− retinas relative to VEH-treated Vldlr−/− retinas at P26 (Figs. 6D, 6E) and at P90 (Figs. 6D, 6F). Moreover, GFAP immunostaining revealed much fewer activated Müller cells (green) in PEA-treated OIR retinas relative to control OIR retinas (Fig. 6G). Similarly, GFAP immunostaining showed fewer activated Müller cells (green) in Vldlr−/− retinas at P26 (Fig. 6H) and at P90 (Fig. 6I) that were treated with PEA compared with retinas treated with VEH. Taken together, these results demonstrate that PEA inhibits Müller cell activation and suggest that the anti-angiogenic and antifibrotic effects of PEA could be due in part to suppression of Müller cell activation.
Figure 6.
PEA inhibited gliosis in OIR and Vldlr−/− retinas. (A) Protein levels of GFAP in the retinas of OIR mice treated with VEH or PEA were measured by western blot analysis. (B) Protein levels of GFAP were quantified by densitometry and normalized to β-actin levels (n = 6). (C) Quantitative RT-PCR of Gfap gene expression in the retina of VEH- and PEA-treated OIR mice (n = 5). (D) Vldlr−/− mice at P14 were intraperitoneally injected with VEH or PEA for 2 weeks. Protein levels of GFAP were measured in Vldlr−/− retinas at P26. In addition, Vldlr−/− mice at P60 were orally administrated PEA or VEH for 30 days. Protein levels of GFAP were then measured in the retina of these Vldlr−/− mice at P90. (E) Levels of GFAP in Vldlr−/− retinas at P26 were quantified by densitometry (n = 6). (F) Levels of GFAP in Vldlr−/− retinas at P90 were quantified by densitometry (n = 6). (G) Representative images of immunostaining for GFAP in the retinal sections of OIR mice treated with VEH or PEA. (H) Representative images of immunostaining for GFAP in the retinal sections of Vldlr−/− mice at P26 treated with VEH or PEA. (I) Representative images of immunostaining for GFAP in the retinal sections of Vldlr−/− mice at P90 treated with VEH or PEA. Data are presented as mean ± SEM; ***P < 0.001.
PEA Suppresses Gliosis and Inflammation in rMC-1 Cells
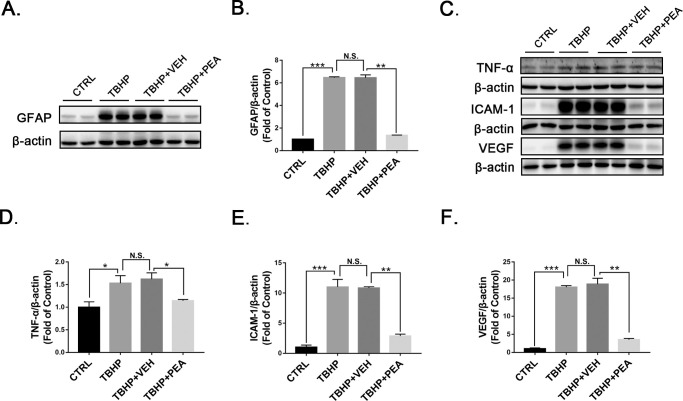
To further test the effects of PEA on the inhibition of Müller gliosis, we treated rMC-1 cells with tertiary-butyl hydrogen peroxide, a common inducer of cellular oxidative stress and inflammation.30 Protein levels of GFAP were significantly decreased in rMC-1 cells treated with PEA relative to those treated with VEH (Figs. 7A, 7B). Further, protein levels of TNF-α (Figs. 7C, 7D), ICAM-1 (Figs. 7C, 7E), and VEGF (Figs. 7C, 7F) were significantly decreased in rMC-1 cells treated with PEA. Taken together, the results indicate that PEA suppresses Müller cell activation and the release of inflammatory and pro-angiogenic cytokines.
Figure 7.
Effects of PEA in TBHP-treated rMC-1 cells. Representative images of western blotting for (A) GFAP and (C) TNF-α, ICAM-1, and VEGF in Müller cells treated with VEH or PEA in the presence of TBHP. Protein levels of (B) GFAP, (D) TNF-α, (E) ICAM-1, and (F) VEGF were quantified by densitometry and normalized to β-actin levels. Data are presented as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001. TBHP, tertiary-butyl hydrogen peroxide.
Antifibrotic Effects of PEA in TGF-β2-Treated rMC-1 Cells
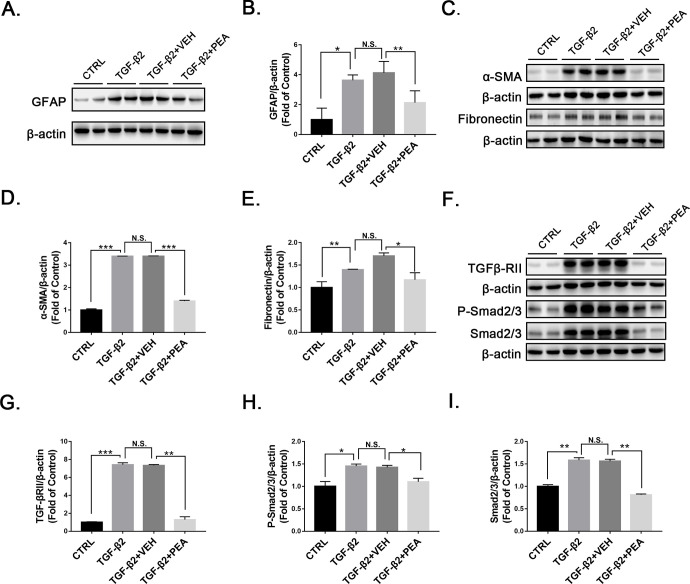
Müller cells are important sources of profibrotic cytokines.2,24 Müller cells secrete TGF-β2 to promote fibrosis in proliferative retinopathy.31 Thus, we used TGF-β2 to induce fibrosis and measured protein levels of profibrotic markers and signaling in Müller cells with and without PEA. Under TGF-β2 treatment, protein levels of GFAP were significantly upregulated in rMC-1 cells, which was significantly attenuated by PEA treatment (Figs. 8A, 8B). TGF-β2 induced expression of α-SMA and fibronectin in rMC-1 cells, which was suppressed by PEA treatment (Figs. 8C–8E). Similarly, TGF-β2 induced upregulation of TGF-βRII, which was reversed by PEA (Figs. 8F, 8G). In addition, PEA also reversed the TGF-β2-induced increases of P-Smad2/3 and Smad2/3 (Figs. 8F, 8H–8I) in rMC-1 cells, suggesting that PEA suppresses the TGF-β/Smad2/3 signaling pathway and alleviates profibrotic changes in Müller cells.
Figure 8.
Effects of PEA in TGF-β2-treated rMC-1 cells. (A) Representative images of western blotting for GFAP in rMC-1 cells treated with VEH or PEA in the presence of TGF-β2. (B) Protein levels of GFAP were quantified by densitometry and normalized by β-actin levels. Similarly, protein levels of (C, D) α-SMA and (C, E) fibronectin were measured and quantified. Further, components of TGF-β/Smad2/3 signaling, (F, G) TGF-βRII, (F, H) P-Smad2/3, and (F, I) Smad2/3 were measured and quantified (n = 6). Data are presented as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001.
PPARα-Dependent Anti-inflammatory and Antifibrotic Effects of PEA
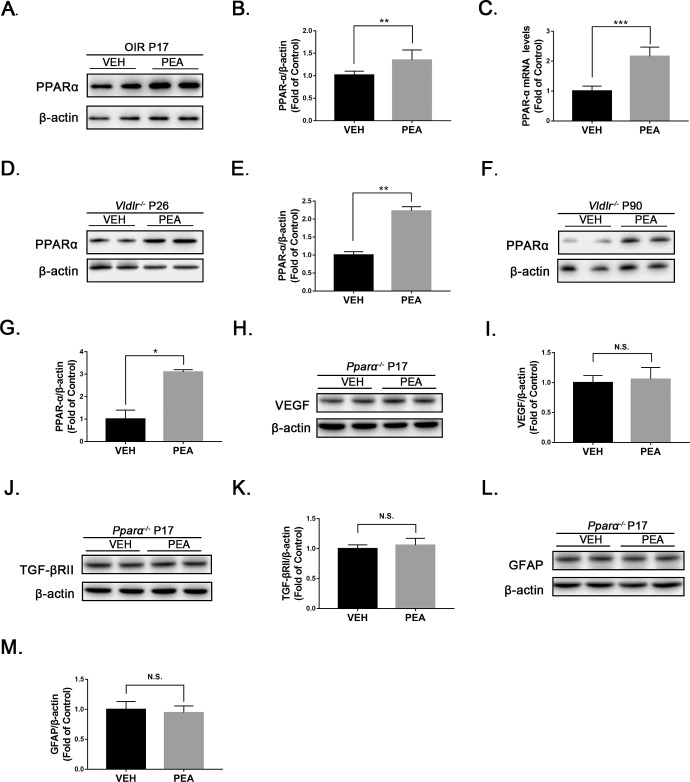
Prior studies have suggested that the therapeutic effects of PEA are modulated in part by PPARα activation.32,33 Therefore, we explored whether PEA inhibits retinal inflammation and fibrosis through activation of PPARα. Indeed, both mRNA and protein levels of PPARα were increased in PEA-treated OIR retinas relative to VEH-treated OIR retinas (Figs. 9A–9C). In addition, PPARα levels were elevated in Vldlr−/− retinas treated with PEA at P26 (Figs. 9D, 9E) and P90 (Figs. 9F, 9G). To further verify the specific role of PPARα in PEA-mediated suppression of inflammation and fibrosis, we subjected PPARα knockout (Pparα−/−) mice to OIR, treated them with PEA, and then measured protein levels of VEGF and TGF-βRII by western blot analysis (Figs. 9H, 9J). VEGF and TGF-βRII levels were not significantly changed by PEA in Pparα−/− OIR mice (Figs. 9I, 9K), and retinal expression of GFAP was similar for VEH-treated and PEA-treated Pparα−/− OIR mice (Figs. 9L, 9M). These results suggest that the protective effects of PEA in OIR are PPARα dependent.
Figure 9.
The effects of PEA were PPARα dependent. (A) Representative images of western blotting for PPARα in the retinas of OIR mice treated with VEH or PEA. (B) Protein levels of PPARα were quantified by densitometry and normalized to β-actin levels (n = 8). (C) Quantitative RT-PCR of Pparα mRNA expression in PEA- and VEH-treated OIR retinas (n = 5). (D–G) Western blot analysis of PPARα was performed in Vldlr−/− mice treated with PEA or VEH at (D, E) P26 and (F, G) P90 (n = 6). (H–M) Further, OIR was induced in Pparα−/− mice. At P17, protein levels of (H) VEGF, (J) TGF-βRII, and (L) GFAP were measured by western blot analysis in Pparα−/− OIR retinas under VEH or PEA treatment. Protein levels of (I) VEGF, (K) TGF-βRII, and (M) GFAP were quantified by densitometry and normalized to β-actin levels (n = 6). Data are presented as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Pathological retinal neovascularization and fibrosis commonly lead to significant vision loss, but effective treatment modalities for these pathologies are limited. In the present study, we demonstrated that PEA, an endocannabinoid mimetic amide, has anti-angiogenic and antifibrotic effects in animal models of PR and neovascular AMD (as summarized in Supplementary Fig. S3). This is the first study to report that α-SMA and fibronectin, as well as TGF-β/Smad2/3 signaling, are increased in OIR and Vldlr−/− retinas, suggesting the utility of these models for studying early retinal fibrotic changes. The present study is also the first to demonstrate that PEA treatment potently inhibits retinal gliosis, angiogenesis, and fibrosis in OIR and Vldlr−/− retinas, indicating that PEA may be a potential therapeutic drug for retinopathies with pathological neovascularization and fibrosis. Further, we found that the therapeutic effects of PEA in the retina are mediated in part by activation of PPARα.
Two animal models, the OIR mouse model and the Vldlr−/− mouse model, were used. OIR is a commonly used animal model for proliferative retinopathy.14 It is well characterized by retinal avascular regions and pathological neovascularization.16 However, there are no prior reports of fibrotic changes in this model. In the present study, we found that the profibrotic marker α-SMA and fibronectin, a marker of ECM remodeling, are significantly upregulated in OIR retinas. Meanwhile, profibrotic TGF-β/Smad2/3 signaling is also activated in OIR retinas. These findings indicate the possible new application of the OIR model to study early fibrotic changes in the retina, for which currently available animal models are lacking.
In Vldlr−/− mice, the retinal vessels extend from the retinal capillary plexus of the outer proximal layer into the avascular photoreceptor layer,34 mimicking the pathological process of retinal angiomatous proliferation in humans, which is present in approximately 10% of patients with newly diagnosed AMD.35–37 Thus, the Vldlr−/− mouse is considered to be an animal model of neovascular AMD.27,38 In the present study, we observed that the proinflammatory cytokines TNF-α, ICAM-1, and VEGF were upregulated in Vldlr−/− retinas, consistent with previous studies.27,28 Most importantly, we also identified that the profibrotic cytokines/markers TGF-β, α-SMA, and fibronectin were increased in these retinas, suggesting that the Vldlr−/− mouse could also be used to study retinal fibrotic changes.
PEA, a naturally occurring N-acylethanolamine, has protective effects in animal models of neurodegenerative diseases.8 PEA is abundant in the CNS and may originate from glial cells.39 In the eye, PEA is shown to be present in the retina and ciliary body,40 but the function of PEA in the eye is not fully understood. Prior studies have reported that PEA has beneficial effects in certain eye diseases, such as glaucoma41 and uveitis,42 due to the anti-inflammatory and neuroprotective properties of PEA. In this study, we explored the anti-angiogenic and antifibrotic effects of PEA in PR and AMD. PEA treatment reduced retinal expression of the inflammatory cytokines TNF-α, ICAM-1, and VEGF in OIR and Vldlr−/− mice. These results are consistent with previous studies in other systems that have also identified anti-inflammatory effects of PEA.5,42 In addition to its anti-inflammatory effects, we also demonstrated that PEA could suppress pathological neovascularization in the retina.
This study explored the antifibrotic effects of PEA. The TGF-β/Smad2/3 pathway plays important roles in fibrotic disorders of the eye.43 The pathway is initiated by TGF-β binding to TGF-βRII, which phosphorylates TGF-βRI; the phosphorylated TGF-βRI triggers the phosphorylation of Smad2 and Smad3, which activates downstream gene expression and ultimately leads to tissue fibrosis.44 In OIR and Vldlr−/− retinas, PEA decreased protein levels of TGF-β2, TGF-βRII, P-Smad2/3, and Smad2/3, suggesting suppression of the TGF-β/Smad2/3 pathway in these contexts. Previous studies have shown that elevated levels of TGF-β increase the synthesis and deposition of ECM proteins, such as fibronectin, and induce expression of α-SMA.39,45 In this study, PEA decreased the levels of fibronectin and α-SMA in both animal models, further supporting the antifibrotic effects of PEA. Interestingly, the PEA levels in OIR retinas and Vldlr−/− retinas were not significantly changed compared to their controls (Supplementary Fig. S4). Because we have observed the beneficial effects of PEA treatment on OIR retinas and Vldlr−/− retinas, it is possible that the amounts of PEA needed in the retina are increased when mice are in stressful conditions.
Müller cells are the major glial cell type of the retina, accounting for 90% of the glial population.46 Müller cells produce pro-angiogenic and proinflammatory factors that may cause or exacerbate neovascularization, chronic retinal inflammation, and eventually cell death.12,13 In addition, Müller cells play a role similar to that of fibroblasts in non-CNS tissue and participle in the process of retinal fibrosis.13,24 In the present study, we demonstrated that PEA inhibits Müller gliosis in pathological retinal neovascularization. To further test the inhibitory effect of PEA in Müller gliosis, we investigated the effect of PEA using the cultured Müller cell line rMC-1. In rMC-1 cells, PEA inhibited Müller cell activation and reduced levels of proinflammatory cytokines and profibrotic markers. Our combined in vivo and in vitro findings suggest that the protective effects of PEA are mediated by suppression of Müller cell activation and the resultant release of proinflammatory and profibrotic cytokines.
Multiple studies have demonstrated the anti-inflammatory and anti-analgesic effects of PEA that occur through activation of PPARα.47,48 Consistent with previous studies in other systems, we also found that the anti-angiogenic and antifibrotic effects of PEA are mediated by PPARα and that these effects of PEA can be abolished in Pparα−/− mice, further supporting the notion that the protective effects of PEA are PPARα dependent.
In summary, we found that PEA suppressed retinal inflammation and neovascularization and reduced retinal fibrotic changes in experimental PR and neovascular AMDs. These findings suggest the potential utility of PEA for retinal neovascularization and retinal fibrosis. PEA is an endogenous amide with an excellent safety profile, and it has been used safely as an oral supplement.3 Further studies are warranted to evaluate the effects of PEA in other animal models of retinal fibrosis, potentially in small clinical trials.
Supplementary Material
Acknowledgments
The authors thank Vijay Sarthy of Northwestern University (Evanston, IL, USA) for providing the rMC-1 cell line. The authors also thank Elizabeth Pearsall of Massachusetts Eye & Ear (Boston, MA, USA) for her critical review of the manuscript.
Supported by grants from the National Science Foundation for Young Scientists of China (31807795) and the National Key R&D program of China (2018YFA0107302).
Disclosure: S. Ye, None; Q. Chen, None; N. Jiang, None; X. Liang, None; J. Li, None; R. Zong, None; C. Huang, None; Y. Qiu, None; J.-X. Ma, None; Z. Liu, None
References
- 1. Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012; 366: 1227–1239. [DOI] [PubMed] [Google Scholar]
- 2. Roy S, Amin S, Roy S. Retinal fibrosis in diabetic retinopathy. Exp Eye Res. 2016; 142: 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Keppel Hesselink JM, Costagliola C, Fakhry J, Kopsky DJ. Palmitoylethanolamide, a natural retinoprotectant: its putative relevance for the treatment of glaucoma and diabetic retinopathy. J Ophthalmol. 2015; 2015: 430596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Di Zazzo A, Roberti G, Mashaghi A, Abud TB, Pavese D, Bonini S. Use of topical cannabinomimetic palmitoylethanolamide in ocular surface disease associated with antiglaucoma medications. J Ocul Pharmacol Ther. 2017; 33: 670–677. [DOI] [PubMed] [Google Scholar]
- 5. Paterniti I, Di Paola R, Campolo M, et al.. Palmitoylethanolamide treatment reduces retinal inflammation in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2015; 769: 313–323. [DOI] [PubMed] [Google Scholar]
- 6. Ohara M, Ohnishi S, Hosono H, et al.. Palmitoylethanolamide ameliorates carbon tetrachloride-induced liver fibrosis in rats. Front Pharmacol. 2018; 9: 709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Di Paola R, Impellizzeri D, Fusco R, et al.. Ultramicronized palmitoylethanolamide (PEA-um(®)) in the treatment of idiopathic pulmonary fibrosis. Pharmacol Res. 2016; 111: 405–412. [DOI] [PubMed] [Google Scholar]
- 8. Esposito E, Cuzzocrea S.. Palmitoylethanolamide in homeostatic and traumatic central nervous system injuries. CNS Neurol Disord Drug Targets. 2013; 12: 55–61. [DOI] [PubMed] [Google Scholar]
- 9. Scuderi C, Esposito G, Blasio A, et al.. Palmitoylethanolamide counteracts reactive astrogliosis induced by β-amyloid peptide. J Cell Mol Med. 2011; 15: 2664–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cipriano M, Esposito G, Negro L, et al.. Palmitoylethanolamide regulates production of pro-angiogenic mediators in a model of β amyloid-induced astrogliosis in vitro. CNS Neurol Disord Drug Targets. 2015; 14: 828–837. [DOI] [PubMed] [Google Scholar]
- 11. Dyer MA, Cepko CL.. Control of Müller glial cell proliferation and activation following retinal injury. Nat Neurosci. 2000; 3: 873–880. [DOI] [PubMed] [Google Scholar]
- 12. Coughlin BA, Feenstra DJ, Mohr S. Müller cells and diabetic retinopathy. Vision Res. 2017; 139: 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bringmann A, Reichenbach A.. Role of Müller cells in retinal degenerations. Front Biosci. 2001; 6: E72–92. [DOI] [PubMed] [Google Scholar]
- 14. Stahl A, Connor KM, Sapieha P, et al.. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci. 2010; 51: 2813–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen Y, Hu Y, Lu K, Flannery JG, Ma JX. Very low density lipoprotein receptor, a negative regulator of the wnt signaling pathway and choroidal neovascularization. J Biol Chem. 2007; 282: 34420–34428. [DOI] [PubMed] [Google Scholar]
- 16. Smith LE, Wesolowski E, McLellan A, et al.. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994; 35: 101–111. [PubMed] [Google Scholar]
- 17. Li Y, Zhou P, Chen H, et al.. Inflammation-restricted anti-inflammatory activities of a N-acylethanolamine acid amidase (NAAA) inhibitor F215. Pharmacol Res. 2018; 132: 7–14. [DOI] [PubMed] [Google Scholar]
- 18. Connor KM, Krah NM, Dennison RJ, et al.. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc. 2009; 4: 1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stahl A, Connor KM, Sapieha P, et al.. Computer-aided quantification of retinal neovascularization. Angiogenesis. 2009; 12: 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen Q, Qiu F, Zhou K, et al.. Pathogenic role of microRNA-21 in diabetic retinopathy through downregulation of PPARα. Diabetes. 2017; 66: 1671–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murray AR, Chen Q, Takahashi Y, Zhou KK, Park K, Ma JX. MicroRNA-200b downregulates oxidation resistance 1 (Oxr1) expression in the retina of type 1 diabetes model. Invest Ophthalmol Vis Sci. 2013; 54: 1689–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takahashi Y, Chen Q, Rajala RVS, Ma JX. MicroRNA-184 modulates canonical Wnt signaling through the regulation of frizzled-7 expression in the retina with ischemia-induced neovascularization. FEBS Lett. 2015; 589: 1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang B, Hu Y, Ma JX. Anti-inflammatory and antioxidant effects of SERPINA3K in the retina. Invest Ophthalmol Vis Sci. 2009; 50: 3943–3952. [DOI] [PubMed] [Google Scholar]
- 24. Friedlander M. Fibrosis and diseases of the eye. J Clin Invest. 2007; 117: 576–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fischer AJ, Omar G, Eubanks J, McGuire CR, Dierks BD, Reh TA. Different aspects of gliosis in retinal Müller glia can be induced by CNTF, insulin, and FGF2 in the absence of damage. Mol Vis. 2004; 10: 973–986. [PubMed] [Google Scholar]
- 26. Cox TR, Erler JT.. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011; 4: 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qiu F, Matlock G, Chen Q, et al.. Therapeutic effects of PPARα agonist on ocular neovascularization in models recapitulating neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017; 58: 5065–5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sun Y, Lin Z, Liu CH, et al.. Inflammatory signals from photoreceptor modulate pathological retinal angiogenesis via c-Fos. J Exp Med. 2017; 214: 1753–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guidry C. The role of Müller cells in fibrocontractive retinal disorders. Prog Retin Eye Res. 2005; 24: 75–86. [DOI] [PubMed] [Google Scholar]
- 30. Cai J, Wu M, Nelson KC, Sternberg P Jr Jones DP. Oxidant-induced apoptosis in cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1999; 40: 959–966. [PubMed] [Google Scholar]
- 31. Yafai Y, Iandiev I, Lange J, et al.. Müller glial cells inhibit proliferation of retinal endothelial cells via TGF-β2 and Smad signaling. Glia. 2014; 62: 1476–1485. [DOI] [PubMed] [Google Scholar]
- 32. Lo Verme J, Fu J, Astarita G, et al.. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005; 67: 15–19. [DOI] [PubMed] [Google Scholar]
- 33. Di Cesare Mannelli L, D'Agostino G, Pacini A, et al.. Palmitoylethanolamide is a disease-modifying agent in peripheral neuropathy: pain relief and neuroprotection share a PPAR-alpha-mediated mechanism. Mediators Inflamm. 2013; 2013: 328797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hu W, Jiang A, Liang J, et al.. Expression of VLDLR in the retina and evolution of subretinal neovascularization in the knockout mouse model's retinal angiomatous proliferation. Invest Ophthalmol Vis Sci. 2008; 49: 407–415. [DOI] [PubMed] [Google Scholar]
- 35. Lafaut BA, Aisenbrey S, Vanden Broecke C, Bartz-Schmidt KU. Clinicopathological correlation of deep retinal vascular anomalous complex in age related macular degeneration. Br J Ophthalmol. 2000; 84: 1269–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bermig J, Tylla H, Jochmann C, Nestler A, Wolf S. Angiographic findings in patients with exudative age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2002; 240: 169–175. [DOI] [PubMed] [Google Scholar]
- 37. Donati MC, Carifi G, Virgili G, Menchini U. Retinal angiomatous proliferation: association with clinical and angiographic features. Ophthalmologica. 2006; 220: 31–36. [DOI] [PubMed] [Google Scholar]
- 38. Joyal JS, Sun Y, Gantner ML, et al.. Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1. Nat Med. 2016; 22: 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Muccioli GG, Stella N. Microglia produce and hydrolyze palmitoylethanolamide. Neuropharmacology. 2008; 54: 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Matias I, Wang JW, Moriello AS, Nieves A, Woodward DF, Di Marzo V. Changes in endocannabinoid and palmitoylethanolamide levels in eye tissues of patients with diabetic retinopathy and age-related macular degeneration. Prostaglandins Leukot Essent Fatty Acids. 2006; 75: 413–418. [DOI] [PubMed] [Google Scholar]
- 41. Strobbe E, Cellini M, Campos EC. Effectiveness of palmitoylethanolamide on endothelial dysfunction in ocular hypertensive patients: a randomized, placebo-controlled cross-over study. Invest Ophthalmol Vis Sci. 2013; 54: 968–973. [DOI] [PubMed] [Google Scholar]
- 42. Impellizzeri D, Ahmad A, Bruschetta G, et al.. The anti-inflammatory effects of palmitoylethanolamide (PEA) on endotoxin-induced uveitis in rats. Eur J Pharmacol. 2015; 761: 28–35. [DOI] [PubMed] [Google Scholar]
- 43. Saika S, Yamanaka O, Sumioka T, et al.. Fibrotic disorders in the eye: targets of gene therapy. Prog Retin Eye Res. 2008; 27: 177–196. [DOI] [PubMed] [Google Scholar]
- 44. Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-β signaling in fibrosis. Growth Factors. 2011; 29: 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ignotz RA, Massague J.. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986; 261: 4337–4345. [PubMed] [Google Scholar]
- 46. Reichenbach A, Bringmann A.. New functions of Müller cells. Glia. 2013; 61: 651–678. [DOI] [PubMed] [Google Scholar]
- 47. LoVerme J, Russo R, La Rana G, et al.. Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-alpha. J Pharmacol Exp Ther. 2006; 319: 1051–1061. [DOI] [PubMed] [Google Scholar]
- 48. Paterniti I, Impellizzeri D, Crupi R, et al.. Molecular evidence for the involvement of PPAR-δ and PPAR-γ in anti-inflammatory and neuroprotective activities of palmitoylethanolamide after spinal cord trauma. J Neuroinflammation. 2013; 10: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.