Abstract
Purpose
To investigate the association between balance and gait measures with fall rates in glaucoma patients.
Methods
Balance and gait were measured for 239 participants with glaucoma or suspected glaucoma. Daily falls were evaluated over 24 months. Annual accelerometer trials captured average daily steps. Multivariable negative binomial models evaluated balance and gait associations with average daily steps and rates of falls per time or step, as well as whether balance and gait parameters mediated the association between integrated visual field (IVF) sensitivity and falls.
Results
Average age was 70.5 years (SD = 7.6), and 22% of the participants had moderate to severe visual field damage. Over the first 12 months of the follow-up, the cumulative probability of falling one or more times was 44.8%, and the cumulative probability of falling two or more times was 17.7%. Gait deficits were associated with fewer daily steps (P < 0.03), but no balance parameters were (P > 0.19). Worse balance was associated with a higher rate of falls per year and step (P < 0.03). No gait measures were associated with the rate of falls per year (P > 0.17). More time in double support and greater swing time variability were associated with higher falls per step, and higher velocity and faster cadence were associated with fewer falls per step (P < 0.05 for all). Neither gait nor balance measures mediated the relationship between visual field damage and fall rates. IVF remained an independent predictor of falls per step (rate ratio = 1.36 to 1.48; P < 0.001 to P < 0.005) in multivariable models including individual balance/gait parameters.
Conclusions
Although balance and gait measures are associated with fall rates, they do not explain why persons with greater visual field damage fall more frequently, suggesting the importance of other potential factors such as hazard perception.
Keywords: gait, balance, falls, glaucoma
Older adults with balance problems are among those at the highest risk for falling,1,2 and poor balance leads to not only more falls but also poor confidence, manifesting as greater fear of falling.3,4 Likewise, slower walking speed and greater stride-to-stride variability are associated with higher rates of falling in older populations.5–7
Although previous work suggests that balance and gait deficits both contribute to a higher risk of falls, it is less understood how balance and gait relate to falls in populations with concomitant vision loss. Persons with visual impairment are more likely to fall when compared with their normally sighted counterparts.8–10 This association has been speculated to result from balance changes that have been noted in individuals with visual field (VF) damage11,12 or from changes in gait (slower walking speeds, shorter stride lengths, and greater gait variability) that reflect either a dangerous walking pattern or an attempt by the individual to adopt a safer walking style because of worse stability.13–16 However, no prior work has simultaneously analyzed balance, gait, and falls in persons with VF damage to determine which balance and gait features are most relevant to falls in this population and whether these features account for the higher rate of observed falls.
The literature thus far has evaluated falls exclusively as a rate over time. Although the rate of falls per period of time is of clear importance, safety and a healthy active lifestyle can be difficult to attain simultaneously. Specifically, more physical activity (e.g., walking) confers numerous health benefits, including greater strength, less frailty, an improved mood, better overall quality of life, and lower mortality, but it also exposes the individual to more opportunities for falling.17–20 As such, balance and gait features may not show a relationship to the rate of falls over time if these same features also are found in individuals with diminished physical activity, as has been observed previously.21–25 The healthiest way to avoid falls is to fall less per step taken and not to simply restrict activity, suggesting that it is important to identify and address factors associated with a higher rate of falls per step in addition to factors associated with a higher rate of falls per year.26 This is particularly relevant when analyzing gait features that predispose individuals to falling only when the individual is walking.
To better establish the relationship between balance/gait features and falls in persons with VF damage, we set forth to answer the following questions: (1) Are balance and gait parameters associated with less physical activity? (2) Which balance and gait measures increase the risk of falls in patients with glaucoma? (3) Do the associations between balance/gait measures and falls differ when assessing the rate of falls over time (falls/year) versus falls per activity (falls/step)? (4) Does poor balance or gait account for the association between VF loss and falls in glaucoma? (5) Is the impact of balance and gait measures on fall rates uniform across the spectrum of glaucoma severity? We hypothesized that the balance and gait features predisposing individuals to a higher rate of falls over time would differ from the features predisposing individuals to a higher rate of falls per step, and that balance and gait features would mediate the association between VF damage and higher fall rates.
Methods
Study Design and Study Population
The Falls in Glaucoma Study was a prospective longitudinal cohort of patients with glaucoma or suspected glaucoma. Participants were recruited between September 2013 and March 2015 from the Glaucoma Center of Excellence at The Johns Hopkins Wilmer Eye Institute. Inclusion criteria were as follows: (1) baseline age of 57 years or older (i.e., turning 60 by study completion); (2) diagnosis of suspect glaucoma or glaucoma not secondary to another systemic condition (e.g., neovascular or uveitic glaucoma); (3) residence within a 60-mile radius from the Wilmer Eye Institute; and (4) ability to perform VF testing. Exclusion criteria included: (1) presence of visually significant concurrent eye disease; (2) any ocular or non-ocular surgery in the past 2 months; (3) any hospitalization in the past month; (4) confinement to a bed or wheelchair; and (5) history of stroke or other neurological disorders causing VF damage. All study procedures were approved by The Johns Hopkins Institutional Review Board, and all subjects signed written informed consents. The described research was performed in accordance with the tenets of the Declaration of Helsinki.
Falls Data Collection
Falls data used in this analysis were collected over the first 24 months in the study for each participant. At the baseline visit, falls were defined for study participants as unintentionally coming to rest on the ground or a lower level and were further illustrated with an instructional video.27 Subjects were provided paper calendars on which they recorded falls, and they returned the calendar data monthly via mail or email. If fall calendars were not received, participants were contacted by phone and/or email to collect up to 3 months of missing data; data not collected within a 3-month period were recorded as missing.
Physical Activity Data Collection
Physical activity was measured at baseline (immediately before collection of falls data) and every subsequent study year using a medical grade omnidirectional accelerometer (Respironics, Inc., Murraysville, PA, USA). Every year, participants were instructed to wear the accelerometer for 7 days to collect data that would be used to estimate steps taken for the upcoming year.28
Subjects were instructed to clip an accelerometer to their belt, pants, or skirt in the morning, wear it for the entire day, and take it off in the evening before going to bed. To maximize compliance, subjects received two or more morning reminder calls during the week they wore their devices. Accelerometers recorded activity data for each minute, and steps were aggregated to the day level. Total accelerometer wear time was calculated by summing: (1) the number of hours between the first minute of non-zero counts after midnight and the last minute of non-zero counts before 4 AM, and (2) the number of hours between the first minute with non-zero count data after 4 AM and the last minute with non-zero count data prior to midnight. Days demonstrating fewer than 8 hours of wear time were excluded. Average steps per day were projected over the period of a year until the next accelerometer trial was performed.28 For individuals who had fewer than 4 valid days of physical activity data for any given year (6.3% of study years), data from the closest year with the 4 valid days were projected over that year, as prior work has suggested that 4 days of activity data are sufficient to estimate long-term activity patterns.28
Balance Evaluation
Balance was evaluated using the Opal Kinematic system (APDM, Inc., Portland, OR, USA), consisting of a set of lightweight motion sensors worn on the arm, leg, and torso and designed to directly measure movements in real time by capturing acceleration in various spatial planes. Sensors evaluated movement during balance tests with known consequences for health outcomes such as falls.29,30 In one set of tests, the Instrumental Clinical Test of Sensory Integration and Balance,31 patients stood on a foam surface with their eyes open and were asked to maintain an upright standing position with their arms crossed and feet approximately shoulder-width apart for 30 seconds. Inter-foot distance was standardized by placing a wooden block between the patient's feet which was removed prior to testing initiation. Prior work identified different measurements of postural sway to be associated with falls in elderly32–36; here we also examine postural sway as a risk factor for falls evaluating (1) root mean square (RMS) sway, (2) total sway, (3) ellipse sway, and (4) jerk. The specific meaning and derivation of these measurements are described in detail in Table 1.
Table 1.
List of Studied Balance and Gait Parameters
| Parameters | Units | Explanation |
|---|---|---|
| Balance | ||
| RMS sway | m/s2 | RMS of the acceleration vector length; larger sway values reflect worse balance |
| Jerk | m2/s5 | Time derivative of acceleration, which reflects the amount of active postural corrections; higher jerk values reflect worse balance |
| Total sway | m2/s4 | Total area bounded by the sway acceleration vectors occurring within the transverse plane (normalized to test duration) |
| Ellipse sway | m2/s4 | Area of the ellipse encompassing 95% of the sway acceleration vectors occurring within the transverse plane |
| Gait | ||
| Velocity | cm/s | Distance traveled divided by ambulation time |
| Cadence | steps/min | Step rate, defined as the average number of steps taken per minute |
| Base of support | cm | Distance between the heel center of the dominant foot and the line of progression created by the prior and subsequent heel strikes in the non-dominant leg |
| Stride length | cm | Distance between the heel centers of two consecutive footfalls of the dominant leg |
| Double-support percent cycle time | % | Percentage of stride time during which both feet are contacting the ground |
| Stride velocity | cm/s | Stride length divided by the stride time |
| Stride time | s | Time elapsed between the first contact with the mat of two consecutive footfalls of the dominant foot |
| Stance time | s | Time elapsed between the first contact and the last contact with the mat for a single footfall of the dominant foot |
| Swing time | s | Time elapsed between the last contact of the dominant leg footfall to the first contact of the next dominant leg footfall with the mat |
Gait Evaluation
Gait data were collected at the baseline clinical evaluation (immediately preceding the collection of fall data) using the GAITRite Electronic Walkway (CIR Systems, Inc., Franklin, NJ, USA).37–40 The GAITRite system measures temporal and spatial gait parameters via an electronic walkway. Participants’ gait measurements were collected barefoot during normal-paced walk. Participants wore their normal distance eyeglasses and were instructed to walk at their natural pace for 4 full lengths of the mat (back and forth two times, with a short pause in between each walk). The following gait parameters were used in this analysis based on suggestions from prior research that they may be relevant to falls: velocity, cadence, base of support, stride length, and percent of the stride time in double support.5,6,41–44 Additionally, stride-to-stride variability has been associated with fall rates in prior work and was assessed by calculating the coefficients of variation (ratio of the standard deviation to the mean multiplied by 100) for stride length, stride velocity, stride time, stance time, and swing time.6,7,45 Gait measurements used in analyses are described in further detail in Table 1. For comparison purposes, all balance and gait metrics were converted to z-score units as follows: (individual patient value – average study sample value)/(study sample standard deviation).
Visual Assessment
VF tests were performed on the Humphrey Field Analyzer II (Carl Zeiss Meditec, Inc., Dublin, CA, USA) as described by Odden et al.46 Tests were obtained at the baseline study visit or at a recent clinic visit (median time = 2.5 months). One glaucoma specialist (PYR) screened VFs for reliability to verify the absence of artifacts (i.e., rim or lid defects) and consistency with prior test results. Sensitivities of spatially corresponding points in the right eye and the left eye were integrated by picking the maximum sensitivities between the two eyes.47–49 These sensitivities were then unlogged to derive raw sensitivity values, arithmetically averaged across all points, and then transformed back to dB values to obtain the mean sensitivity for the integrated VF (IVF). Visual acuity was assessed using a back-lit Early Treatment Diabetic Retinopathy Study chart placed at 4-m distance, and patients were instructed to wear their distance eyeglasses (if used). Letters read were converted to logMAR values for analysis. Contrast sensitivity was evaluated using the Mars Letter Contrast Sensitivity Test (Mars Perceptrix Corporation, Chappaqua, NY, USA) with participants wearing their usual eyeglasses. Contrast sensitivity results were expressed in log units (logCS).
Evaluation of Covariates
Standardized questionnaires were employed to collected age, gender, and race. Comorbid illnesses were assessed using a questionnaire that asked subjects if they had been diagnosed with any of 15 distinct comorbid illnesses: arthritis, broken or fractured hip, back problems, history of heart attack, history of angina/chest pain, congestive heart failure, peripheral vascular disease, high blood pressure, diabetes, emphysema, asthma, stroke, Parkinson's disease, cancer other than skin cancer, and history of vertigo or Meniere's disease.50 Total comorbidities were summed. Participants with more than five comorbid illnesses (n = 9) were reclassified as having five comorbidities. Medication information was collected by directly observing medication bottles when possible, or otherwise by patient report. Patients were classified as having polypharmacy if they took five or more daily prescription medications, excluding eye drops.51 To measure grip strength, participants were asked to sit in a chair, extend their dominant hand parallel to the floor, and squeeze a Jamar Hand Dynamometer (Sammons Preston, Bolingbrook, IL, USA) as hard as possible. This was repeated three times. To measure leg strength, participants were seated in a chair and a microFET2 Dynamometer (Hoggan Scientific LLC, West Jordan, UT, USA) was placed just above a knee of the participant. Participants were asked to flex their leg from the hip for 5 seconds against the device. This was repeated twice for each leg. The maximum strength for both grip and leg was recorded in kilograms.
Statistical Analysis
Negative binomial regression models were used to evaluate the association between balance/gait parameters and the average number of steps taken per day. Negative binomial regression models were also used to identify variables associated with a higher rate of falls over the first 24 months of collected falls data for each patient. Two sets of models were run. In the first set of models, rates of falls over time were considered, with calendar years defined as the offset. In the second set of models, rates of falls per step were modeled, with imputed steps over the study period (as judged by the 1-week accelerometer trials) taken as the offset.
Balance and gait variables were considered as the primary explanatory variables of fall rates. The ability of balance and gait to mediate the relationship between VF damage and falls rates was analyzed in models also including IVF sensitivity as an independent variable. Additional models incorporated an interaction term between IVF sensitivity and individual balance and gait parameters to evaluate whether the impact of balance or gait measures on fall rates varies across the spectrum of glaucoma severity. Only balance and gait metrics that had a statistically significant association with falls per step in multivariable models were chosen for further exploration in interaction and mediation models.
Integrated visual field, age, gender, race, polypharmacy, and number of medical comorbidities were included as covariates in all models based on their prior association with falls and balance and gait parameters.26,51,52 All analyses were conducted using Stata 14.0 (StataCorp LLC, College Station, TX, USA).
Results
Description of Study Population
Falls calendar data were gathered, and balance, gait, and vision testing was completed by 239 study participants. Roughly half (48%) of the participants were female, about one-third (29%) were black, one-fifth (20%) lived alone, and the average age was 70.5 years (Table 2). Fifty-two participants (22%) were glaucoma suspects, and 187 (78%) had a glaucoma diagnosis. Most participants (65%) had more than one comorbid illness, and 33% used five or more systemic prescription medications. Median IVF sensitivity was 28 dB (interquartile range [IQR], 26.0–29.7; normal value, 31 dB). On average, participants took 3987 steps/day at the baseline. Over the 12 months of follow-up, the cumulative probability of falling one or more times was 44.8%, and the cumulative probability for falling two or more times was 17.7%.
Table 2.
Study Population Characteristics
| Demographics | Value (N = 239) |
|---|---|
| Age (y), mean ± SD | 70.5 ± 7.6 |
| Black race, n (%) | 69 (28.9) |
| Female gender, n (%) | 115 (48.1) |
| Employed, n (%) | 86 (36.0) |
| Lives alone, n (%) | 47 (19.7) |
| Education, n (%) | |
| Less than high school | 7 (2.9) |
| High school | 29 (12.2) |
| Some college | 32 (13.5) |
| Bachelor's degree | 59 (24.8) |
| More than bachelor's degree | 112 (46.6) |
| Health | |
| Comorbid illnesses (>1), n (%) | 156 (65.3) |
| Polypharmacy, n (%)* | 79 (33.1) |
| Body mass index (kg/m2), mean ± SD | 27.3 ± 5.1 |
| Grip strength (kg), mean ± SD | 31.7 ± 10.4 |
| Lower body strength (kg), mean ± SD | 17.7 ± 6.0 |
| Vision | |
| IVF sensitivity (dB), median (IQR) | |
| All subjects (N = 239) | 28.0 (26.0, 29.7) |
| Glaucoma suspects (n = 52) | 28.9 (27.5, 30.2) |
| Manifest glaucoma (n = 187) | 27.7 (25.8, 29.6) |
| MD better eye, median (IQR)† | –2.6 (–5.4, –0.7) |
| MD better eye ≥ –6 dB, n (%) | 186 (78) |
| MD better eye > –12 dB and < –6 dB, n (%) | 27 (11) |
| MD better eye ≤ –12 dB, n (%) | 26 (11) |
| MD worse eye, median (IQR) | –5.7 (–12.9, –2.8) |
| Better-eye acuity (logMAR), median (IQR) | 0.06 (–0.02, 0.16) |
| Binocular (logCS), median (IQR) | 1.72 (1.64, 1.76) |
IQR, interquartile range; MD, mean deviation.
*Polypharmacy is five or more prescription medications, excluding eye drops.
†Mild loss is MD ≥ –6 dB, moderate loss is MD > –12 dB and < –6 dB, and severe loss is MD ≤ –12 dB.
Evaluating the Association Between Balance and Gait Parameters with Physical Activity
Separate multivariable models were created to evaluate the association between each individual balance and gait parameter with baseline physical activity (average steps per day) (Fig. 1). No balance measure was associated with the amount of daily activity (P > 0.2 for all). Among the gait measures evaluated, increased velocity (rate ratio [RR] = 1.27 per 1 z-score unit; 95% confidence interval [CI], 1.16–1.38), cadence (RR = 1.10 per 1 z-score unit; 95% CI, 1.02–1.20), and stride length (RR = 1.31 per 1 z-score unit; 95% CI, 1.19–1.43) were associated with more steps per day, whereas a greater percent of the cycle time spent in double support (RR = 0.79 per 1 z-score unit; 95% CI, 0.73–0.86) and greater variability in gait (evaluated with coefficients of variation for different gait metrics) were associated with fewer steps per day. Base of support was not found to be associated with average steps per day (P = 0.12).
Figure 1.
Association of individual balance and gait parameters with physical activity at baseline in multivariable models accounting for visual field damage. A positive z-score unit increment represents a higher numeric value for the analyzed metric. The RR values presented here come from separate multivariable models that controlled for visual field damage, age, race, gender, comorbidities, and polypharmacy. pct, percent cycle time; RR, rate ratio.
Evaluating the Association Between Balance and Gait Parameters with Fall Rates
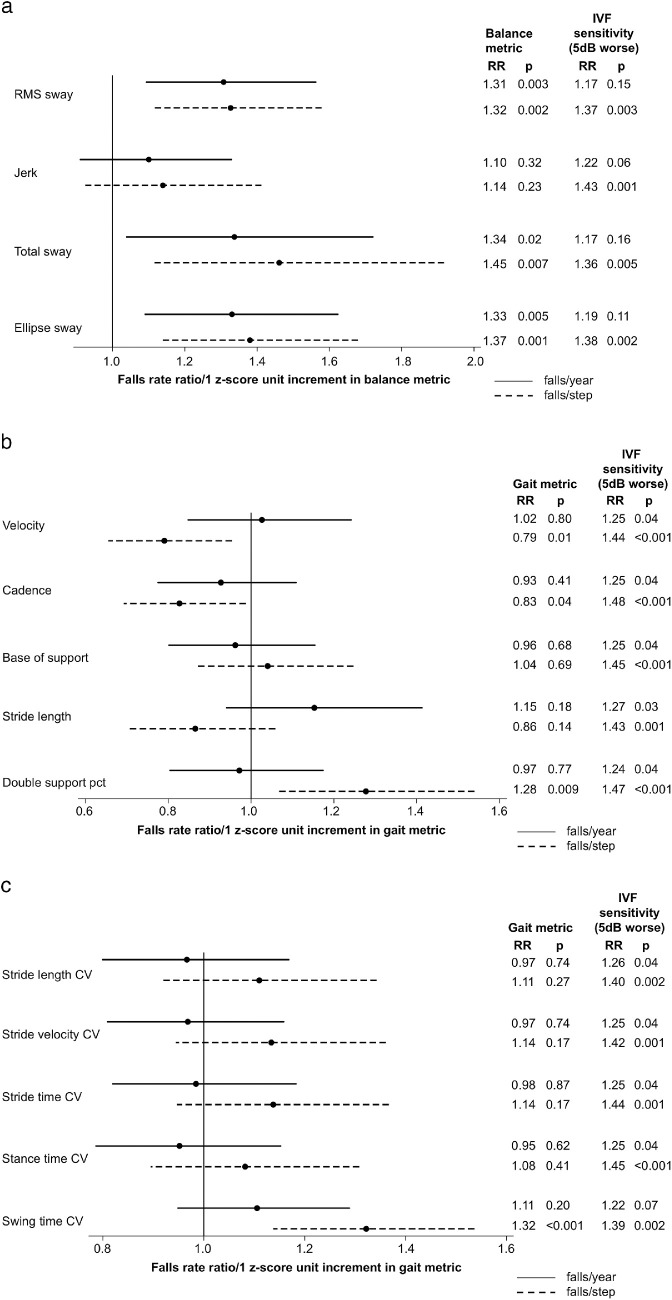
Separate multivariable models were created to evaluate the association between individual balance and gait parameters and fall rates. Fall rates were judged in two ways: (1) as falls/year, and (2) as falls/step (Fig. 2). Among the balance measures evaluated, worse RMS, total, and ellipse sways were each significantly associated with a higher rate of falls/year (P < 0.03 for all) and with a higher rate of falls/step (P < 0.008 for all) (Fig. 2A). Worse jerk was not significantly associated with a higher rate of falls/year or falls/step (P > 0.2 for both) (Fig. 2A).
Figure 2.
Association of individual balance and gait parameters with time- and step-adjusted fall rates in multivariable models accounting for visual field damage. (a) Balance parameters; (b) gait parameters; and (c) gait coefficients of variation. RRs for individual balance and gait parameters and IVF sensitivity are derived from the models that contain each individual balance or gait parameter, IVF sensitivity, and other covariates. Positive z-score unit increments represent a higher numeric value for the analyzed metric. The RRs presented here come from separate multivariable models that controlled for visual field damage, age, race, gender, comorbidities, and polypharmacy.
None of the gait parameters was associated with falls/year (P > 0.17 for all). However, higher gait velocity (RR = 0.79 per 1 z-score unit; 95% CI, 0.65–0.95) and faster cadence (RR = 0.83 per 1 z-score unit; 95% CI, 0.69–0.99) were associated with fewer falls/step. A larger percentage of time spent in double support was associated with a more falls/step (RR = 1.28 per 1 z-score unit; 95% CI, 1.06–1.54) (Fig. 2B). Neither base of support nor stride length was associated with falls/step (P > 0.13 for both).
Metrics of gait variability were not associated with falls/year (P > 0.19 for all) (Fig. 2C). Greater variability in swing time was observed to be associated with a higher rate of falls/step (RR = 1.32 per 1 z-score unit; 95% CI, 1.14–1.54), although variability in other gait metrics was not associated with a higher rate of falls/step (P >0.16 for all), (Fig. 2C).
Balance and gait metrics significantly associated with the falls/step were next evaluated in a single multivariable model to identify which of those variables have the strongest association with falls/step (Table 3). In this model, the coefficient of variation for a swing time (RR = 1.23 per 1 z-score unit; 95% CI, 1.04–1.45) was the only variable among the balance and gait metrics that remained significantly associated with falls/step in the multivariable model. Velocity and ellipse sway demonstrated high variance inflation factors (VIFs) (4.5 and 13.4, respectively) and were excluded from the model that contained the other balance and gait parameters.
Table 3.
Association Between Balance and Gait Metrics and Rates of Falls/Step, Multivariable Models
| Metric | Falls/Step, (Models with Single Gait OR Balance Metric + Covariates) RR (95%CI) | Falls/Step, (Model with All Shown Gait AND Balance Metrics + Covariates) RR (95%CI) |
|---|---|---|
| Gait metrics (per 1 z-score unit increase) | ||
| Velocity | 0.79 (0.65–0.95) | Not included* |
| Cadence | 0.83 (0.69–0.99) | 0.94 (0.77–1.16) |
| Double-support percent cycle time | 1.28 (1.06–1.54) | 1.17 (0.93–1.48) |
| Swing-time CV | 1.32 (1.14–1.54) | 1.23 (1.04–1.45) |
| Balance metrics (per 1 z-score unit increase) | ||
| RMS sway | 1.32 (1.11–1.57) | 1.22 (0.94–1.57) |
| Total sway | 1.45 (1.11–1.90) | 1.10 (0.82–1.47) |
| Ellipse | 1.37 (1.13–1.67) | Not included* |
| Covariates | ||
| IVF sensitivity per 5-dB decrement | 1.44 (1.18–1.77) † | 1.35 (1.09–1.66) |
| Age (5 y older) | 1.18 (1.05–1.32) † | 1.13 (1.01–1.27) |
| Gender (male vs. female) | 0.69 (0.49–0.97) † | 0.67 (0.47–0.96) |
| Race (black vs. white) | 0.57 (0.37–0.88) † | 0.59 (0.39–0.90) |
| Comorbidities (1 additional comorbidity) | 1.17 (1.04–1.33)† | 1.16 (1.03–1.31) |
| Polypharmacy (≥5 vs. <5 medications) | 1.35 (0.92–1.99)† | 1.50 (1.02–2.20) |
Bold entries represent significant values (P < 0.05). CV, coefficient of variation.
*Due to high variance inflation factors for velocity and ellipse sway, those two variables were not used in the regression model that included all of the balance and gait metrics. Covariates controlled for in all of the models were IVF sensitivity, age, race, gender, comorbidities, and polypharmacy.
†RRs are from the model with velocity as an explanatory variable, but they are similar to the RRs from the models where other balance and gait metrics were used as explanatory variables.
Evaluating Balance and Gait as Potential Mediators Between the Association of VF Damage and Fall Rates
In multivariable models not including either balance or gait parameters, IVF sensitivity was significantly associated with falls/step (RR = 1.44 per 5-dB decrement in sensitivity; 95% CI, 1.17–1.76) but not with falls/year (RR = 1.22 per 5-dB decrement in sensitivity; 95% CI, 0.99–1.51). The observed associations between IVF sensitivity and falls/step remained strong and unchanged in multivariable models including each of the individual balance, gait, or gait variability parameters (RR = 1.36–1.48; P < 0.001 to P < 0.005) (Figs. 2A–2C), suggesting that neither gait nor balance mediates the association between VF damage and fall rates.
Evaluating Interactions Between Balance and Gait Measures and VF Damage with Respect to Fall Rates
Additional regression models were evaluated to determine if any balance of gait parameters demonstrated a disproportionately stronger effect on rates of falls/step in individuals with worse VF damage (i.e., lower IVF sensitivity) (Table 4). In these models, IVF sensitivity was not observed to interact with any individual gait or balance parameter with regard to the rate of falls/step (Table 4), suggesting that the effect of these balance and gait features did not differ across the spectrum of VF damage.
Table 4.
Modeling of Potential Interactions Between Visual Field Sensitivity and Selected Balance and Gait Parameters with Regard to Rates of Falls/Step
| Variable | Interval | RR (95% CI) |
|---|---|---|
| Balance | ||
| RMS sway | ||
| IVF sensitivity | 5-dB decrement | 1.38 (1.12–1.71) |
| RMS sway | 1 z-score increase | 1.32 (1.11–1.57) |
| IVF sensitivity × RMS sway | 0.95 (0.75–1.20) | |
| Total sway | ||
| IVF sensitivity | 5-dB decrement | 1.36 (1.10–1.69) |
| Total sway | 1 z-score increase | 1.48 (1.12–1.96) |
| IVF sensitivity × total sway | 0.94 (0.72–1.21) | |
| Ellipse sway | ||
| IVF sensitivity | 5-dB decrement | 1.40 (1.14–1.72) |
| Ellipse sway | 1 z-score increase | 1.38 (1.14–1.67) |
| IVF sensitivity × ellipse sway | 0.90 (0.67–1.20) | |
| Gait | ||
| Velocity | ||
| IVF sensitivity | 5-dB decrement | 1.42 (1.15–1.76) |
| Velocity | 1 z-score increase | 0.79 (0.66–0.95) |
| IVF sensitivity × velocity | 0.94 (0.77–1.16) | |
| Cadence | ||
| IVF sensitivity | 5-dB decrement | 1.45 (1.18–1.79) |
| Cadence | 1 z-score increase | 0.83 (0.69–0.99) |
| IVF sensitivity × cadence | 1.12 (0.91–1.38) | |
| Double-support percent cycle time | ||
| IVF sensitivity | 5-dB decrement | 1.47 (1.19–1.80) |
| Double-support percent cycle time | 1 z-score increase | 1.28 (1.06–1.54) |
| IVF sensitivity × double-support percent cycle time | 1.03 (0.84–1.26) | |
| Swing time CV | ||
| IVF sensitivity | 5-dB decrement | 1.39 (1.13–1.70) |
| Swing time CV | 1 z-score increase | 1.31 (1.11–1.55) |
| IVF sensitivity × swing time CV | 1.02 (0.84–1.23) | |
Bold entries represent significant values (P < 0.05).
Discussion
In patients with a range of VF damage, several balance and gait parameters were associated with falls, although the parameters identified depend on whether falls were evaluated as a rate over time (falls/year) or as a rate over activity (falls/step). Although balance parameters had similar time- and step-adjusted fall rates, several gait parameters were associated with falls per step but not with falls per time, suggesting that these parameters mark individuals who are “dangerous walkers” but not necessarily frequent fallers, given their lower levels of physical activity. Further, neither gait nor balance parameters interacted with IVF sensitivity, suggesting that deficits in balance and gait likely produce higher fall rates in all older adults and that there is no disproportionate difference in risk of falling due to changes in balance and gait when comparing individuals with greater VF damage (worse IVF sensitivity) with those with less VF damage. Finally, VF damage remained associated with a higher rate of falls even after accounting for balance and gait measures, suggesting that VF damage itself may be the primary reason for more falls or that other factors besides balance and gait defects (i.e., poor hazard perception) account for the higher rate of falls in persons with VF damage.
Although the falls literature has embraced the evaluation of falls over time as the primary measure of falls, we argue that this approach paints an incomplete picture, as individuals who are more likely to fall while walking may become less active, given that falls often result in fear of falling, which has been associated with lower levels of physical activity.22,25 Just as analyses of driving risks have focused on crash rate per mile as opposed to crash rate per year,53 we argue that rates of falling should account for the amount of walking performed. Indeed, we observed that several gait parameters (velocity, cadence, percent of stride time in double support, and variability in swing time) showed no association with falls per time but were significantly associated with falls per step, indicating that they identify individuals who are more likely to fall when walking. As would be expected, these same gait parameters were associated with lower levels of physical activity, such as fewer daily steps (average daily steps at the baseline in this cohort are comparable to general populations of similar age reported in other studies54,55). Given that gait factors are most relevant to falls while actually engaged in walking, it seems particularly relevant to evaluate gait factors as a risk factor for falls in models that evaluate the risk of falling per step. One potential criticism of analyzing falls per step as an outcome is that associations between a risk factor and this outcome may partially reflect associations between the risk factor and the offset (rate denominator). Although true, we would argue that any rate outcome inherently consists of a numerator and denominator, and the best rate denominator should not be chosen based on its being unassociated with the risk factors of interest but rather because it places the individual at risk for the event (rate numerator) occurring. We believe that steps subject the individuals to a risk of falling much more directly than calendar time.
Balance metrics, on the other hand, showed similar associations with falls per time and falls per step, reflecting the curious lack of association between balance metrics and daily physical activity. In other words, participants who had poor balance did not seem to lower their daily activity. The absence of lower levels of physical activity with poor balance in this cohort explains the similarities in the association between the balance metrics and rate of falls per step or per time. Our finding that balance is associated with a greater rate of falls is in accordance with several prior papers that have demonstrated an association between poor balance and more falls over time.32,34,36 Although various balance measures have been used in these prior studies, our study employing the Opal Kinematic system identified RMS sway, total sway, and ellipse sway as being the most relevant metrics for falls, and jerk was less relevant. Of note, no interaction was noted between VF damage and balance measures with regard to fall rates, suggesting that our results may apply equally to persons across the spectrum of VF damage, including those with little to no VF damage.
Velocity, cadence, percent of the stride time spent in double support, and swing time variability were found to be associated with the greater risk of falling per step, but not per time, in our study population, reflecting the fact that these gait parameters were also associated with lower levels of physical activity. Although several gait parameters studied here have been associated with fall rates over time in prior studies, we were not able to replicate these findings in the current study.6,7,41 Possibilities for these distinct findings may include differences in the study population with regard to their social and demographic parameters or each population's tendency to lower (or not lower) their activity in response to the threat of falling. Moreover, it remains possible that previous gait parameters associated with falls per time in prior studies would have been associated even more strongly with falls per step if activity levels were assessed. The association of these gait parameters with more falls per step does not necessarily imply that they play a causative role in falling. Rather, these gait changes may reflect a more cautious gait designed to avoid falls and that increasing velocity or cadence or decreasing double support time or swing time variability would lead to an even greater rate of falling. Although these gait parameters are associated with a greater rate of falls per step in our study population, like the balance measures discussed above, they do not demonstrate an interaction with the degree of VF damage, suggesting that they are not any more relevant to falls in persons with VF damage as opposed to persons without VF damage.
Visual field damage was found to be an independent predictor of the rate of falls, and balance and gait metrics did not appear to mediate this relationship. The failure of either balance or gait to mediate the association between VF damage and fall rates suggests that other measures likely account for falls in this population. One compelling possibility is hazard detection, as VF damage may preclude the ability to properly detect environmental hazards that could lead to a fall. Although these hazards could in theory be minimized, particularly in the home environment where the individual has the greatest control, our prior work has shown that among patients with glaucoma individuals with greater VF damage did not differ in the level of lighting or the number of fall-related hazards in their homes compared with those with less VF damage.56 Also, even though balance and gait measures do not mediate the association between VF damage and fall rates, it remains possible that improving balance may decrease fall rates, particularly as such programs have been demonstrated to prevent falls in older adults.57–59
Limitations of this study include the fact that balance and gait were assessed with barefoot patients, which is not necessarily representative of the conditions these patients encounter on a daily basis. Further, balance was tested on a single foam surface even though patients are likely to encounter a variety of surfaces (e.g., tile, grass) on a daily basis. Gait was measured only under controlled conditions on a flat surface without any obstructions on the path and in good ambient lighting, which is not representative of the environment in which individuals usually walk. Thus, our results might be underestimating the importance of gait to falls. Finally, there is the possibility of recruitment bias into the study. We evaluated the differences between the participants recruited for the study and those who were study eligible in the glaucoma clinic at the Wilmer Eye Institute, described in more detail elsewhere.26 We observed that our study participants were of similar race, age, gender, and visual field severity as the study eligible individuals. On the other hand, our study participants were more likely to report falling in the past 12 months (42% vs. 23%; P < 0.001). However, it is unclear if such bias would alter the relationships between balance and gait with fall rates described here.
Our findings highlight the importance of assessing physical activity when determining balance and gait parameters as risk factors for falls. Whereas balance and gait were clearly important predictors of falls in the current study, they did not contribute to fall risk more in persons with more advanced visual field damage, nor did they explain the association between visual field damage and the risk of falling. Future research should specifically address other potential causes of falls (e.g., hazard perception) that, in conjunction with changes in balance and gait, could more fully explain the relationship between visual field damage and falls among glaucoma patients. A proper understanding of the factors leading to falls in persons with VF damage can help optimize strategies to prevent falls in this high-risk population.
Acknowledgments
Supported by National Institutes of Health Grant No. EY022976.
Disclosure: A. Mihailovic, None; R.M. De Luna, None; S.K. West, None; D.S. Friedman, None; L.N. Gitlin, None; P.Y. Ramulu, None
References
- 1. Thurman DJ, Stevens JA, Rao JK, Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: assessing patients in a neurology practice for risk of falls (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2008; 70: 473–479. [DOI] [PubMed] [Google Scholar]
- 2. Wolfson L, Judge J, Whipple R, King M. Strength is a major factor in balance, gait, and the occurrence of falls. J Gerontol A Biol Sci Med Sci. 1995; 50: 64–67. [DOI] [PubMed] [Google Scholar]
- 3. Maki B, Holliday P, Topper A. Fear of falling and postural performance in the elderly. J Gerontol. 1991; 46: M123–M131. [DOI] [PubMed] [Google Scholar]
- 4. Vellas BJ, Wayne SJ, Romero LJ, Baumgartner RN, Garry PJ. Fear of falling and restriction of mobility in elderly fallers. Age Ageing. 1997; 26: 189–193. [DOI] [PubMed] [Google Scholar]
- 5. Shimada H, Kim H, Yoshida H, et al.. Relationship between age-associated changes of gait and falls and life-space in elderly people. J Phys Ther Sci. 2010; 22: 419–424. [Google Scholar]
- 6. Maki BE. Gait changes in older adults: predictors of falls or indicators of fear? J Am Geriatr Soc. 1997; 45: 313–320. [DOI] [PubMed] [Google Scholar]
- 7. Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001; 82: 1050–1056. [DOI] [PubMed] [Google Scholar]
- 8. Legood R, Scuffham P, Cryer C. Are we blind to injuries in the visually impaired? A review of the literature. Inj Prev. 2002; 8: 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lord SR, Dayhew J. Visual risk factors for falls in older people. J Am Geriatr Soc. 2001; 49: 508–515. [DOI] [PubMed] [Google Scholar]
- 10. Ivers RQ, Cumming RG, Mitchell P, Attebo K. Visual impairment and falls in older adults: the Blue Mountains Eye Study. J Am Geriatr Soc. 1998; 46: 58–64. [DOI] [PubMed] [Google Scholar]
- 11. Kotecha A, Richardson G, Chopra R, Fahy RT, Garway-Heath DF, Rubin GS. Balance control in glaucoma. Invest Ophthalmol Vis Sci. 2012; 53: 7795–7801. [DOI] [PubMed] [Google Scholar]
- 12. Black AA, Wood JM, Lovie-Kitchin JE, Newman BM. Visual impairment and postural sway among older adults with glaucoma. Optom Vis Sci. 2008; 85: 489–497. [DOI] [PubMed] [Google Scholar]
- 13. Hallemans A, Ortibus E, Truijen S, Meire F. Development of independent locomotion in children with a severe visual impairment. Res Dev Disabil. 2011; 32: 2069–2074. [DOI] [PubMed] [Google Scholar]
- 14. Nakamura T. Quantitative analysis of gait in the visually impaired. Disabil Rehabil. 2009; 19: 194–197. [DOI] [PubMed] [Google Scholar]
- 15. Turano K, Rubin G, Quigley H. Mobility performance in glaucoma. Invest Ophthalmol Vis Sci. 1999; 40: 2803–2809. [PubMed] [Google Scholar]
- 16. Tomomitsu M, Alonso A, Morimoto E, Bobbio TG, Greve JM. Static and dynamic postural control in low-vision and normal-vision adults. Clinics. 2013; 68: 517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Penedo FJ, Dahn JR. Exercise and well-being: a review of mental and physical health benefits associated with physical activity. Curr Opin Psychiatr. 2005; 18: 189–193. [DOI] [PubMed] [Google Scholar]
- 18. Vogel T, Brechat P-H, Leprêtre P-M, Kaltenbach G, Berthel M, Lonsdorfer J. Health benefits of physical activity in older patients: a review. Int J Clin Pract. 2009; 63: 303–320. [DOI] [PubMed] [Google Scholar]
- 19. Warburton D, Nicol C, Bredin S. Health benefits of physical activity: the evidence. Can Med Assoc J. 2006; 174: 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taylor A, Cable N, Faulkner G, Hillsdon M, Narici M, Van Der Bij AK. Physical activity and older adults: a review of health benefits and the effectiveness of interventions. J Sport Sci. 2004; 22: 703–725. [DOI] [PubMed] [Google Scholar]
- 21. Rochat S, Büla CJ, Martin E, et al.. What is the relationship between fear of falling and gait in well-functioning older persons aged 65 to 70 years? Arch Phys Med Rehabil. 2010; 91: 879–884. [DOI] [PubMed] [Google Scholar]
- 22. Fletcher PC, Guthrie DM, Berg K, Hirdes JP. Risk factors for restriction in activity associated with fear of falling among seniors within the community. J Patient Saf. 2010; 6: 187–191. [DOI] [PubMed] [Google Scholar]
- 23. Fletcher PC, Hirdes JP. Restriction in activity associated with fear of falling among community-based seniors using home care services. Age Ageing. 2004; 33: 273–279. [DOI] [PubMed] [Google Scholar]
- 24. Guthrie DM, Fletcher PC, Berg K, Williams E, Boumans N, Hirdes JP. The role of medications in predicting activity restriction due to a fear of falling. J Aging Health. 2012; 24: 269–286. [DOI] [PubMed] [Google Scholar]
- 25. Deshpande N, Metter JE, Bandinelli S, Lauretani F, Windham BG, Ferrucci L. Psychological, physical, and sensory correlates of fear of falling and consequent activity restriction in the elderly. Am J Phys Med Rehabil. 2008; 87: 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ramulu PY, Mihailovic A, West SK, Friedman DS, Gitlin LN. What is a falls risk factor? Factors associated with falls per time or per step in individuals with glaucoma. J Am Geriatr Soc. 2019; 67: 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Davalos-Bichara M, Lin FR, Carey JP, et al.. Development and validation of a falls-grading scale. J Geriatr Phys Ther. 2013; 36: 63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Togo F, Watanabe E, Park H, et al.. How many days of pedometer use predict the annual activity of the elderly reliably? Med Sci Sports Exerc. 2008; 40: 1058–1064. [DOI] [PubMed] [Google Scholar]
- 29. Boulgarides LK, McGinty SM, Willett JA, Barnes CW. Use of clinical and impairment-based tests to predict falls by community-dwelling older adults. Phys Ther. 2003; 83: 328–339. [PubMed] [Google Scholar]
- 30. Chu L, Chi I, Chiu A. Incidence and predictors of falls in the Chinese elderly. Ann Acad Med Singapore. 2005; 34: 60–72. [PubMed] [Google Scholar]
- 31. Willis JR, Vitale SE, Agrawal Y, Ramulu PY. Visual impairment, uncorrected refractive error, and objectively measured balance in the United States. JAMA Ophthalmol. 2013; 131: 1049–1056. [DOI] [PubMed] [Google Scholar]
- 32. Maki B, Holliday P, Topper A. A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. J Gerontol. 1994; 49: M72–M84. [DOI] [PubMed] [Google Scholar]
- 33. Melzer I, Benjuya N, Kaplanski J. Postural stability in the elderly: a comparison between fallers and non-fallers. Age Ageing. 2004; 33: 602–607. [DOI] [PubMed] [Google Scholar]
- 34. Stalenhoef P, Diederiks, JP, Knottnerus J, Kesler AD, Crebolder HF. A risk model for the prediction of recurrent falls in community-dwelling elderly: a prospective cohort study. J Clin Epidemiol. 2002; 55: 1088–1094. [DOI] [PubMed] [Google Scholar]
- 35. Merlo A, Zemp D, Zanda E, et al.. Postural stability and history of falls in cognitively able older adults: The Canton Ticino study. Gait Posture. 2012; 36: 662–666. [DOI] [PubMed] [Google Scholar]
- 36. Thapa PB, Gideon P, Brockman KG, Fought RL, Ray WA. Clinical and biomechanical measures of balance fall predictors in ambulatory nursing home residents. J Gerontol A Biol Sci Med Sci. 1996; 51: M239–M246. [DOI] [PubMed] [Google Scholar]
- 37. Bilney B, Morris M, Webster K. Concurrent related validity of the GAITRite walkway system for quantification of the spatial and temporal parameters of gait. Gait Posture. 2003; 17: 68–74. [DOI] [PubMed] [Google Scholar]
- 38. Cutlip RG, Mancinelli C, Huber F, DiPasquale J. Evaluation of an instrumented walkway for measurement of the kinematic parameters of gait. Gait Posture. 2000; 12: 134–138. [DOI] [PubMed] [Google Scholar]
- 39. Nough AL, Batavia M, Chen FC, Kwon S, Ziai J. The validity and reliability of the GAITRite system's measurements: a preliminary evaluation. Arch Phys Med Rehabil. 2001; 82: 419–425. [DOI] [PubMed] [Google Scholar]
- 40. Webster KE, Wittwer JE, Feller JA. Validity of the GAITRite walkway system for the measurement of averaged and individual step parameters of gait. Gait Posture. 2005; 22: 317–321. [DOI] [PubMed] [Google Scholar]
- 41. Quach L, Galica AM, Jones RN, et al.. The nonlinear relationship between gait speed and falls: the Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly of Boston Study. J Am Geriatr Soc. 2011; 59: 1069–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Toulotte C, Thevenon A, Watelain E, Fabre C. Identification of healthy elderly fallers and non-fallers by gait analysis under dual-task conditions. Clin Rehabil. 2006; 20: 269–276. [DOI] [PubMed] [Google Scholar]
- 43. Gehlsen G, Whaley M.. Falls in the elderly: Part I, Gait. Arch Phys Med Rehabil. 1990; 71: 735–738. [PubMed] [Google Scholar]
- 44. Wolfson L, Whipple R, Amerman P, Tobin J. Gait assessment in the elderly: a gait abnormality rating scale and its relation to falls. J Gerontol. 1990; 45: M12–M19. [DOI] [PubMed] [Google Scholar]
- 45. Hausdorff JM, Edelberg HK, Mitchell SL, Goldberger AL, Wei JY. Increased gait unsteadiness in community-dwelling elderly fallers. Arch Phys Med Rehabil. 1997; 78: 278–283. [DOI] [PubMed] [Google Scholar]
- 46. Odden JL, Mihailovic A, Boland MV, Friedman DS, West SK, Ramulu PY. Evaluation of central and peripheral visual field concordance in glaucoma. Invest Ophthalmol Vis Sci. 2016; 57: 2797–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Crabb D, Viswanathan A, McNaught A, Poinoosawmy D, Fitzke FW, Hitchings RA. Simulating binocular visual field status in glaucoma. Br J Ophthalmol. 1998; 82: 1236–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nelson-Quigg J, Cello K, Johnson C. Predicting binocular visual field sensitivity from monocular visual field results. Invest Ophthalmol Vis Sci. 2000; 41: 2212–2221. [PubMed] [Google Scholar]
- 49. Mihailovic A, Swenor BK, Friedman DS, West SK, Gitlin LN, Ramulu PY. Gait implications of visual field damage from glaucoma. Transl Vis Sci Technol. 2017; 6: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ramulu PY, Maul E, Hochberg C, Chan ES, Ferrucci L, Friedman DS. Real-world assessment of physical activity in glaucoma using an accelerometer. Ophthalmology. 2012; 119: 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gnjidic D, Hilmer SN, Blyth FM, et al.. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol. 2012; 65: 989–995. [DOI] [PubMed] [Google Scholar]
- 52. Campbell A, Borrie M, Spears G. Risk factors for falls in a community-based prospective study of people 70 years and older. J Gerontol. 1989; 44: M112–M117. [DOI] [PubMed] [Google Scholar]
- 53. Evans L. Risks older drivers face themselves and threats they pose to other road users. Int J Epidemiol. 2000; 29: 315–322. [DOI] [PubMed] [Google Scholar]
- 54. Bassett DR Jr, Wyatt HR, Thompson H, Peters JC, Hill JO. Pedometer-measured physical activity and health behaviors in U.S. adults.Med Sci Sports Exerc. 2010; 42: 1819–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tudor-Locke C, Hart TL, Washington TL. Expected values for pedometer-determined physical activity in older populations. Int J Behav Nutr Phys Act. 2009; 6: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yonge AV, Swenor BK, Miller R, et al.. Quantifying fall-related hazards in the homes of persons with glaucoma. Ophthalmology. 2017; 124: 562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rubenstein LZ, Josephson KR.. Falls and their prevention in elderly people: what does the evidence show? Med Clin N Am. 2006; 90: 807–824. [DOI] [PubMed] [Google Scholar]
- 58. Kannus P, Sievänen H, Palvanen M, Järvinen T, Parkkari J. Prevention of falls and consequent injuries in elderly people. Lancet. 2002; 366: 1885–1893. [DOI] [PubMed] [Google Scholar]
- 59. Karlsson M, Magnusson H, von Schewelov T, Rosengren B. Prevention of falls in the elderly—a review. Osteoporos Int. 2013; 24: 747–762. [DOI] [PubMed] [Google Scholar]