Abstract
The present study investigated the inhibitory effects and the associated mechanism of the compound 25-OH-PPD (PPD) on cardiac hypertrophy, fibrosis and inflammation. The signaling pathways associated with diabetic mellitus cardiomyopathy (DMCM) were investigated using a rat model. DMCM Sprague-Dawley rats were induced by injection of streptozotocin. The animals were divided into 5 groups as follows: Normal group (NG group), diabetic group, PPD treatment group, PPD/LY294002 group (inhibitor of PI3K/Akt) and PPD/LiCl group [inhibitor of glycogen synthase kinase (GSK) 3β]. The studies were carried out during the 12 weeks following induction of diabetes and the levels of plasma brain natriuretic peptide (BNP), creatine phosphokinase isoenzyme (CK-MB) were measured. In addition, the volume of myocardial collagen fraction (CVF) was tested. The expression levels of the inflammatory cytokines, including transforming growth factor beta 1 (TGF-β1), connective tissue growth factor (CTGF), cell adhesion molecules α-smooth muscle actin (α-SMA) and vascular adhesion molecule 1 (VCAM-1) and associated signaling proteins (Akt, GSK-3β) were measured by biochemical analyses. The levels of BNP and CK-MB, the volume of CVF, the expression levels of TGF-β1, CTGF, α-SMA and VCAM-1 in the diabetic group were higher compared with those of the normal control group (P<0.05). Conversely, the levels of these molecules were significantly decreased in the PPD treatment groups (P<0.05). The aforementioned effects were partially eliminated in the PPD/LY294002 and PPD/LiCl groups. In addition, PPD treatment significantly increased the expression levels of p-Akt and decreased the levels of phosphorylated GSK-3β compared with those of the DMCM group (P<0.05). The data demonstrated that the protective effects of 25-OH-PPD against DMCM may be attributed to the PI3k/Akt/GSK-3β signaling pathway, via the suppression of the α-SMA/VCAM axis and the downregulation of TGF-β1 and CTGF expression.
Keywords: 25-OH-PPD, diabetic cardiomyopathy, cardiac fibrosis, PI3K/Akt/glycogen synthase kinase 3 β, transforming growth factor β 1
Introduction
Diabetes mellitus (DM) is an endocrine metabolic disease that affects different organs of the body and is considered the leading cause of mortality in adults worldwide (1). DM patients are prone to develop multiple cardiovascular complications, including coronary heart disease, cardiomyopathy (DMCM) and chronic heart failure (2,3). DMCM is the major complication of DM that occurs in the heart and is responsible for significant alterations in the myocardial structure and function of patients with DM. On average, 40-60% of DM patients will develop DMCM after suffering DM for 10 years (4). DM is one of the major causes of mortality worldwide and DMCM is the major chronic complication of DM that leads to morbidity and mortality in diabetic patients. Therefore, its prevention and treatment is crucial for DM patients (5,6).
The use of anti-diabetic drugs has been previously employed for the treatment of DMCM. However, these compounds were reported as ineffective and their application was associated with cardiovascular adverse reactions (7). Therefore, additional novel therapeutic strategies are necessary for the treatment of this disease (8). A previous study highlighted that conventional western medicine combined with traditional Chinese medicine could be used to treat DMCM (9). At present, it has been shown that Panax Notoginseng (PNS) exhibits therapeutic effects in the heart tissues of diabetic subjects (10).
PNS is a widely used traditional Chinese medicine extracted from the Sanqi or Tianqi plants. This agent exhibits a wide range of pharmacological and biochemical effects and can be used to treat specific diseases, such as cardiovascular and inflammatory disease, bleeding or pain due to injury, as well as trauma (11). Several chemical compounds and active ingredients have been isolated from PNS, including saponins, flavonoids and cyclopeptides. The compound 20(S)-25-OCH3-PPD (25-OH-PPD) was isolated by extraction from the leaves of PNS. PPD exhibited good therapeutic effects on cardiovascular diseases, notably as an adjunctive therapy in DMCM (12).
PPD is the active ingredient of the terpene-saponin fraction separated and isolated from the leaves of pseudo-ginseng (13). It has been reported to possess various types of pharmacological and biochemical effects on the cardiovascular and immune systems, including anti-inflammatory, anti-diabetic and anti-atherosclerotic actions (14). It has been previously confirmed that PPD exhibits a dose-dependent action. However, the exact mechanism regarding its therapeutic effects in DMCM is currently unclear.
Therefore, in the present study the therapeutic effects of PPD were evaluated with regard to the progression of DMCM by monitoring the inhibition of hypertrophy in cardiomyocytes and by investigating the associated mechanism mediated via the Akt/glycogen synthase kinase (GSK)-3β pathway. In the present study, the structure and function of a pathologic left ventricle was observed and compared with the levels of plasma brain natriuretic peptide (BNP) and with the volume of myocardial collagen fraction (CVF). The expression levels of inflammatory cytokines, including transforming growth factor beta 1 (TGF-β1) and connective tissue growth factor (CTGF), and of the cell adhesion molecules α-smooth muscle actin (α-SMA) and vascular adhesion molecule 1 (VCAM-1) were measured in order to estimate the effects of PPD on DMCM and the potential signaling mechanisms. Furthermore, the association of PPD with the Akt/GSK-3β signaling pathway was examined in the present study.
Materials and methods
Experimental animals and treatments
Experimental animal care was carried out according to the guide for the laboratory animals (15) and the present study was followed and approved by the Medical Ethics Committee of Jinzhou Medical University. The animals were kept at room temperature in 40-50% humidity and given access to normal light (12 h light/dark cycle). A total of 50 SD rats were used in this experiment: 12-week-old male rats weighing 200-220 g were provided by the Animal lab center of the Jinzhou Medical University, Jinzhou, China, SYXK: 2014-0002. The DM animal model was established in 40 SD rats by intraperitoneal injection of streptozotocin (cat. no. S0130; Sigma-Aldrich; Merck KGaA). STZ; 50 mg·kg-1 STZ in 0.1 M citrate buffer solution, pH 4.5). STZ was injected for three days to induce diabetes and the levels of blood glucose (BG) were tested using a Gluco-Meter (Accu-Chek Performa, Roche Diagnostics). Values of BG >200 mg·dl-1 were used as an indication of successful establishment of the model. The normal group (NG group) comprised 10 SD experimental animals treated with normal saline.
Animals groups
The aforementioned 40 diabetic rats were randomly allocated into four groups as follows: DM model group, PPD-treatment group [it has been shown in a previous study that PPD exhibited a dose-dependent mechanism of action (13)], PPD/LY294002 group (LY294002, inhibitor of PI3K/Akt, 10 µmol·l-1). The PPD/LiCl-treated group (LiCl, inhibitor of GSK-3β, 20 µmol·l-1). PPD was administered daily at dosages of 5 mg·kg-1 intraperitoneally for 12 weeks. LY294002 and LiCl were administered daily intravenously. The tested animals in the NG and DM groups were administered with the same volume of normal saline as the rats in the PPD groups. The rats of each group were housed under suitable temperature and humidity conditions for 12 weeks. The DM rats of each different group were provided high-fat and high-sugar diet (18% fat). The rats in each group were weighed and non-fasting BG was measured every week in order to determine the successful establishment of the model. The tested animals were anesthetized with urethane (intraperitoneally, 1 g·kg-1) following 12 weeks of the appropriate treatment. The left cardiac functions were examined in order to prove the presence of cardiomyopathy in the diabetic rats.
Observation of myocardial levels of creatine phosphokinase isoenzyme (CK-MB), BNP and CVF
Following examination of the left cardiac functions, the rats were anaesthetized with urethane (intraperitoneally, 1 g kg-1), the skin and fascia of the chest were cut, the thorax was opened and the heart removed. The tissues were washed with PBS solution (0.01 mol·l-1). The extraction and separation of plasma was performed in order to assess the levels of the blood biochemical indices by the biochemical analyzer RA50 Semi auto (Bayer AG). The levels of CK-MB and BNP were also measured in each group. The heart tissues were cut into sections, then the samples were treated with 10% polyformaldehyde for 72 h for fixation, different concentrations of alcohol for dehydration (70, 80, 90, and 100%, each step for 2 min) and xylene to make them transparent (2 times, 2 min). Samples were then paraffin embedded and uniform intermittent sections were obtained at a thickness of 5 µm, each of these steps were performed at room temperature 25˚C. Cardiac collagen and paravascular collagen tissues were stained using 0.1% Ponceau red at 4˚C stained for 5 min. The volume of CVF was assessed by the random selection of five fields (CVF=the collagen area divided by total area x100%).
Measurement of the levels of α-SMA and VCAM-1
To investigate the inhibitory effects of 25-OH-PPD on myocardial hypertrophy in DMCM rats, the expression levels of α-SMA and VCAM-1 were investigated since these factors were shown to be involved in the proliferation and hypertrophy of myocardial tissues. The contents of cardiac α-SMA and VCAM-1 in different groups were tested by ELISA kits (cat. no. PV951; Beyotime Institute of Biotechnology). The determination was performed according to the manufacturer's instructions (BD Opt-EIA ELISA Set, BD Biosciences). The contents of α-SMA and VCAM-1 were measured in picogram per milliliter (pg ml-1).
Detection of TGF-β1 and CTGF mRNA levels
TRIzol reagent (Invitrogen, Thermo Fisher Scientific, Inc.) and the QIA-gen RNA kit (Qiagen GmbH) were used to collect and purify total RNA. 100 mg heart tissue was added into 1 ml Trizol reagent, and stored at -80˚C. Subsequently, 0.2 ml of chloroform was added at room temperature, shaken for 15 sec, centrifuged for 15 min at 12,000 x g at 4˚C, and the supernatant was then kept at 25˚C for 3 min. A total of 0.6 ml isopropanol was added to the supernatant, the mixture was gently mixed and after 10 min at 20˚C, the mixture was centrifuged at 12,000 x g, for 10 min at 4˚C. The supernatant was discarded, 1 ml 75% ethanol was added to wash the RNA precipitate and the mixture was centrifuged at 7,500 x g, for 5 min at 4˚C. The RNA samples were almost completely dried at 25˚C and store at -80˚C. When ready to use, the samples were dissolved in Depc Water (10 min at 55-60˚C, dried in vacuum for 5-10 min at 25˚C, and then the concentration of RNA was measured at 260 nm, and stored at -80˚C. The Prime script cDNA synthesis kit (Bio-Rad Laboratories, Inc.) was used for reverse transcription of total RNA. Reverse transcription synthesis cDNA. The OD280 value was determined by UV spectrophotometer after adding 2 µl total RNA into 98 µl sterilized water. DNA Eraser (1 µl) + total RNA (7 µl) + DNA Eraser buffer (2 µl), 42˚C for 2 min. After centrifuged at 7,500 x g, for 10 min at 4˚C, the mixture was placed in the PCR machine at 37˚C for 15 min, followed by 85˚C for 5 sec. The samples were cooled on ice and stored at -20˚C. The sequence of the TGF-β1 forward primer was 5'-GAGGGGGAGGAGGAGTGGGA-3' and the reverse primer was 5'-CCGGGTAGCGATCGAGTGTC-3'. The product length was 169 bp. CTGF forward primer was 5'-GCAAATAGCCTGTCAATCTC-3' and the reverse primer was 5'-TCCATAAAAATCTGGCTTGT-3'. The product length was 414 bp. β-actin was used as the internal control and its forward primer was 5'-GTGGGCCGCTCTAGGCACCAA-3' and the reverse primer was 5'-CTCTTTGATGTCACGCACATTTC-3'. Reaction conditions were as follows: 95˚C, 5 min, pre-denaturation; followed by 94˚C, 30 sec, denaturation; 61˚C, 20 sec, annealing; 72˚C, 20 sec, extension; 72˚C, 7 min, final extension, for 40 cycles; Subsequently at 4˚C, thermal insulation. CTGF reaction conditions: 95˚C, 5 min, pre-denaturation; followed by 94˚C, 30 sec, denaturation; 60˚C, 20 sec, annealing; 72˚C, 30 sec, extension; 72˚C, 7 min, final extension, for 40 cycles; Subsequently at 4˚C, thermal insulation. PCR was carried out for 40 cycles using the Bio-Rad iCycle iQ Real Time Detection System. The results were quantitative analysis by the 2-ΔΔCT method.
Expressions of AKT and p-AKT
The expression levels of AKT and p-AKT in the heart tissues of different rats were determined. The BSA protein assay was used to quantify protein levels. 100 mg myocardial specimens were added to a 1.5 ml EP tube. A total of 200 µl RIPA buffer solution (Thermo Fisher Scientific, Inc.) was added and then crushed by ultrasound. The specimens were left to rest for 30 min and centrifuged for 25 min at 4˚C at 12,000 x g/min. The proteins were extracted from tissue homogenates and 50 µg aliquots were subjected to SDS-PAGE (7.5%) and transferred onto nitrocellulose membranes. The initial voltage used was 90 V. The transfer was initially performed for 40 min and subsequently adjusted to 110 V for 90 min. The membrane was incubated with the following primary antibodies, which were all diluted to 1:500 in TBS: Rabbit anti-rat monoclonal antibodies targeting Akt (cat. no. AA326; Beyotime Institute of Biotechnology Co., Ltd.), phosphorylated (p)-Akt (cat. no. AA329; Beyotime Institute of Biotechnology), GSK-3β (cat. no. AG751, Beyotime Institute of Biotechnology Co., Ltd) and p-GSK-3β (cat. no. AG753; Beyotime Institute of Biotechnology). Incubations were maintained at 4˚C overnight before rinsing with TTBS 3 times, each time for 3 min. The membrane was incubated with goat anti-rabbit alkaline phosphatase labeled anti-second antibodies (cat. no. A0239; Beyotime Biotechnology Co., Ltd.; 1:500) at room temperature for 1-2 h. Subsequently, the films were washed with TTBS 3 times, each time for 10 min. The NBT/BCIP color kit (cat. no. C3206; Beyotime Biotechnology Co., Ltd.) developing solution was then added and the reaction was stopped. In the presence of alkaline phosphatase, BCIP is hydrolyzed to produce a highly reactive product that reacts with NBT to form an insoluble dark blue NBT-formazan. The protein bands were analyzed using the ImageQuant LAS GEL imaging system (GE Healthcare Life Sciences Co., Ltd.; 4000 biomolecular imager). This imaging software was used to analyze the band gray values and calculate the relative protein expression levels. Protein expression were applied for analysis using phosphorylated Akt, (p)-Akt, GSK-3β, p-GSK-3β and β-actin antibodies by Quantitative analysis.
Statistical analysis
Statistical analysis was performed with SPSS version 14.0 statistics software (SPSS, Inc.) and the values were expressed as the mean ± SD. one-way ANOVAs followed by post-hoc LSD or Tukey's tests of multiple comparisons were used for statistical analysis. P<0.05 was considered to indicate a statistically significant difference..
Results
25-OH-PPD reduces the expression levels of CK-MB, BNP and CVF in diabetic rats
In the present study, PPD increased the body weight and reduced the blood glucose levels in DM rats. The levels of CK-MB, BNP and the volume of CVF were all significantly increased in the DM groups, including the PPD, PPD/LY294002 and PPD/LiCl groups compared with those noted in the NS control group (P<0.05). However, the opposite effects were noted by 25-OH-PPD treatment and significant reductions in the levels of CK-MB, BNP and the volume of CVF were evident in diabetic animals compared with those of the DMCM group (P<0.05). The effects of 25-OH-PPD on the aforementioned results were partially reduced in the presence of LY294002 and LiCl, whereas an increase in the levels of CK-MB, BNP and CVF was noted in the PPD/LY294002 and PPD/LiCl groups (P<0.05) compared with the PPD alone group (Fig. 1A-C).
Figure 1.
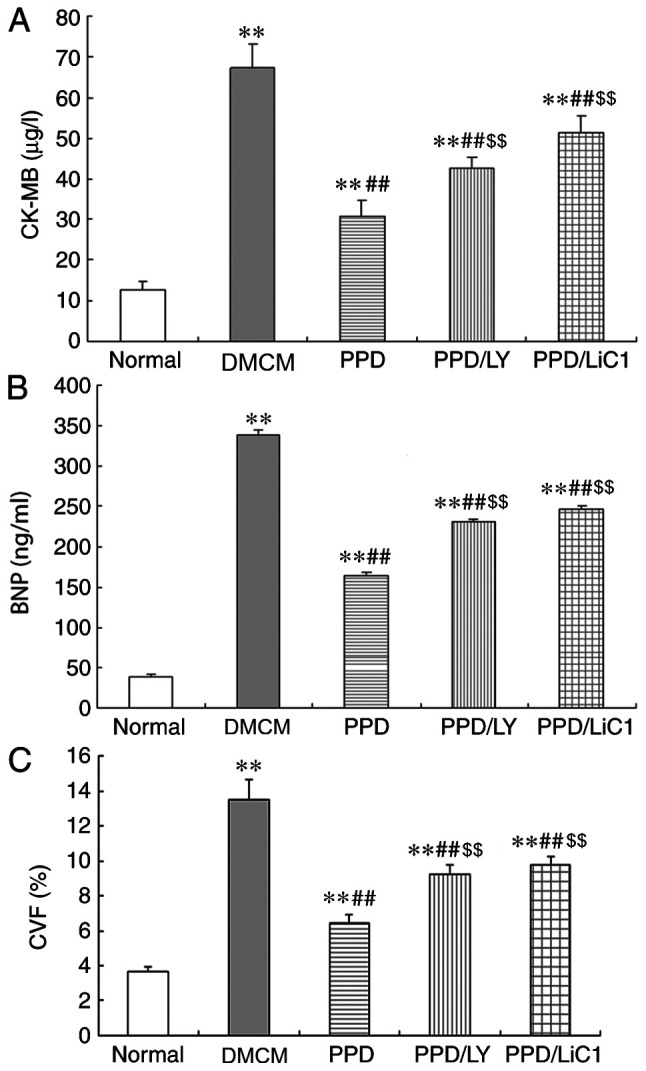
Effects of PPD on CK-MB, BNP levels and on CVP in DMCM rats. (A) CK-MB levels were increased in DMCM rats, following PPD treatment of the rats. (B) Effects of PPD on BNP levels in DMCM rats. CK-MB levels were increased in the DMCM groups. PPD reduced the levels of BNP. (C) Effects of PPD on CVF in DMCM rats. PPD reduced the levels of CK-MB. The values are presented as the mean ± SD, n=10. **P<0.01 vs. the Normal group; ##P<0.01 vs. the DMCM group; $$P<0.01 vs. the PPD group. PPD, 25-OH-PPD; CK-MB, creatine phosphokinase isoenzyme; BNP, brain natriuretic peptide; CVP, myocardial collagen fraction; DMCM, diabetic cardiomyopathy.
25-OH-PPD reduces the expression levels of α-SMA and VCAM-1
In the present study, the expression levels of α-SMA and VCAM-1 were significantly increased in DM animals compared with those noted in the NS group (P<0.05). In addition, 25-OH-PPD caused a significant reduction in the levels of α-SMA and VCAM-1 in the diabetic rats (P<0.01). The effects of PPD on α-SMA and VCAM-1 were partially attenuated in the LY294002 and LiCl groups (P<0.05). The expression levels of α-SMA and VCAM-1 were increased in the PPD/LY294002 and PPD/LiCl groups (P<0.05; Fig. 2A and B).
Figure 2.
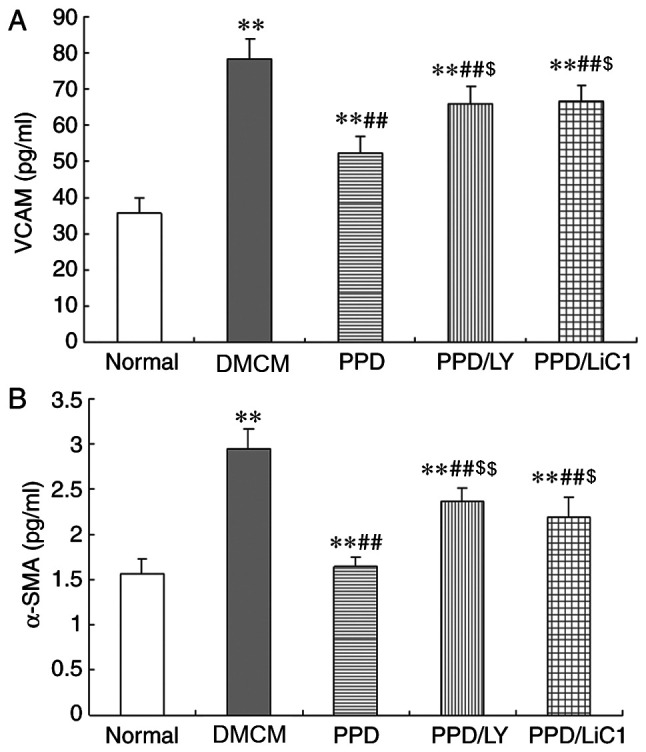
Effects of PPD on VCAM-1 and α-SMA levels in DMCM rats. (A) PPD reduced the levels of VCAM-1. VCAM-1 levels were increased in each DMCM group. VCAM-1 levels were partially increased in the PPD/LY294002 and PPD/LiCl groups. (B) Effects of PPD on α-SMA in DMCM rats. PPD reduced the levels of α-SMA. α-SMA levels were increased in each DMCM group and were partially increased in the PPD/LY294002 and PPD/LiCl groups. The values are presented as mean ± standard deviation, n=10. **P<0.01 vs. the Normal group; ##P<0.01 vs. the DMCM group; $P<0.05 and $$P<0.01 vs. the PPD group. PPD, 25-OH-PPD; VCAM-1, vascular adhesion molecule 1; α-SMA, α-smooth muscle actin; DMCM, diabetic cardiomyopathy; TGF-β1, transforming growth factor beta 1; CTGF, connective tissue growth factor.
25-OH-PPD reduces the expression levels of TGF-β1 and CTGF
The data indicated that the expression levels of TGF-β1 and CTGF were enhanced in the DM and PPD treatment groups, whereas they were increased in the PPD/LY294002 and PPD/LiCl groups. These were higher than those noted in the control NS group (P<0.05). In the present study, it was shown that PPD decreased both TGF-β1 and CTGF expression levels in DMCM rats and that these effects were partially weakened by the administration of LY294002 and LiCl (P<0.01 compared with treatment of PPD alone). In addition, the expression levels of TGF-β1 and CTGF were higher in the LY294002 and LiCl groups compared with those noted following single treatment of the animals with 25-OH-PPD (Figs. 3 and 4).
Figure 3.
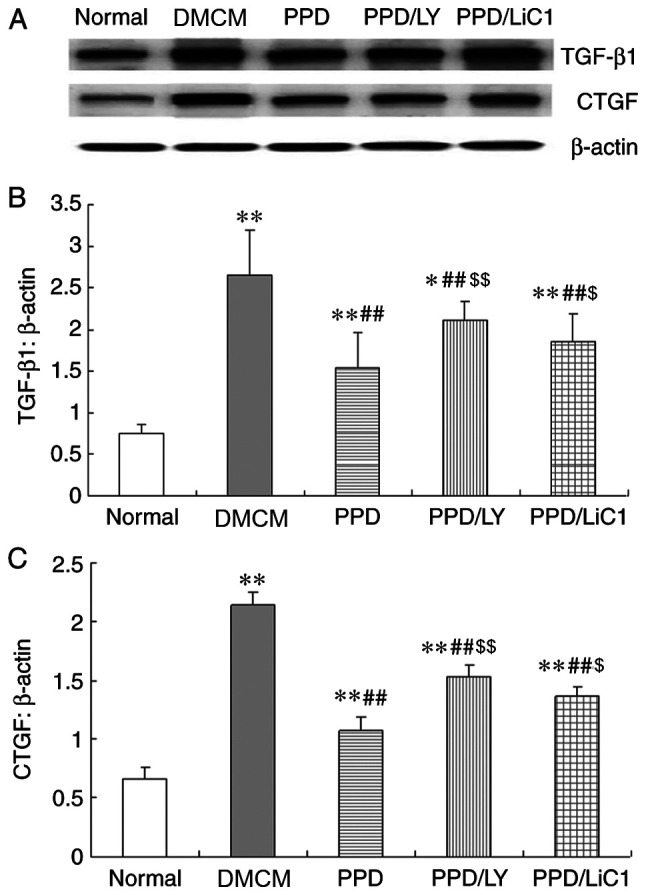
PPD reduced (A and B) TGF-β1 and (A and C) CTGF (expression levels in the heart tissues of diabetic rats. The values are presented as the mean ± SD. *P<0.05, **P<0.01 vs. the Normal group; ##P<0.01 vs. the DMCM group; $P<0.05 and $$P<0.01 vs. the PPD group. PPD, 25-OH-PPD; TGF-β1, transforming growth factor beta 1; CTGF, connective tissue growth factor; DMCM, diabetic cardiomyopathy.
Figure 4.
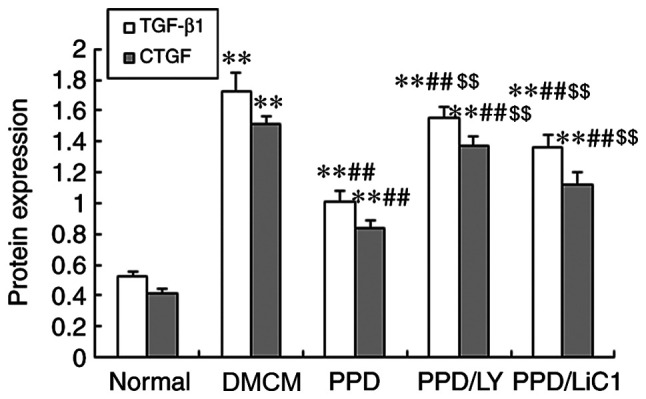
Effects of PPD on TGF-β1 and CTGF levels. PPD reduced the expression levels of TGF-β1 and CTGF in the heart tissues of diabetic rats. The values are presented as mean ± SD, n=10. **P<0.01 vs. the Normal group; ##P<0.01 vs. the DMCM group; $$P<0.01 vs. the PPD group. PPD, 25-OH-PPD; TGF-β1, transforming growth factor beta 1; CTGF, connective tissue growth factor; DMCM, diabetic cardiomyopathy.
25-OH-PPD activates the PI3k/Akt/GSK-3 β pathway
In the present study, the results demonstrated that the expression levels of Akt were significantly inhibited and that the expression levels of GSK-3β were increased in the heart tissues of DMCM rats. The expression levels of the GSK-3β pathway-associated proteins were monitored and the results indicated that Akt levels were reduced in the DMCM group. In contrast to these findings, 25-OH-PPD significantly enhanced Akt phosphorylation levels and decreased the expression levels of p-GSK-3β in diabetic myocardial tissues compared with those of the DMCM model group. In addition, p-Akt levels were suppressed and GSK-3β levels were increased in the PPD/LY294002 groups compared with those noted in the PPD group. In addition, single treatment of the animals with PPD resulted in a significant increase in the expression levels of p-Akt and a significant decrease in the expression levels of GSK-3β in the PPD with LiCl group (P<0.01) compared with those noted in the control group (Fig. 5A-B).
Figure 5.
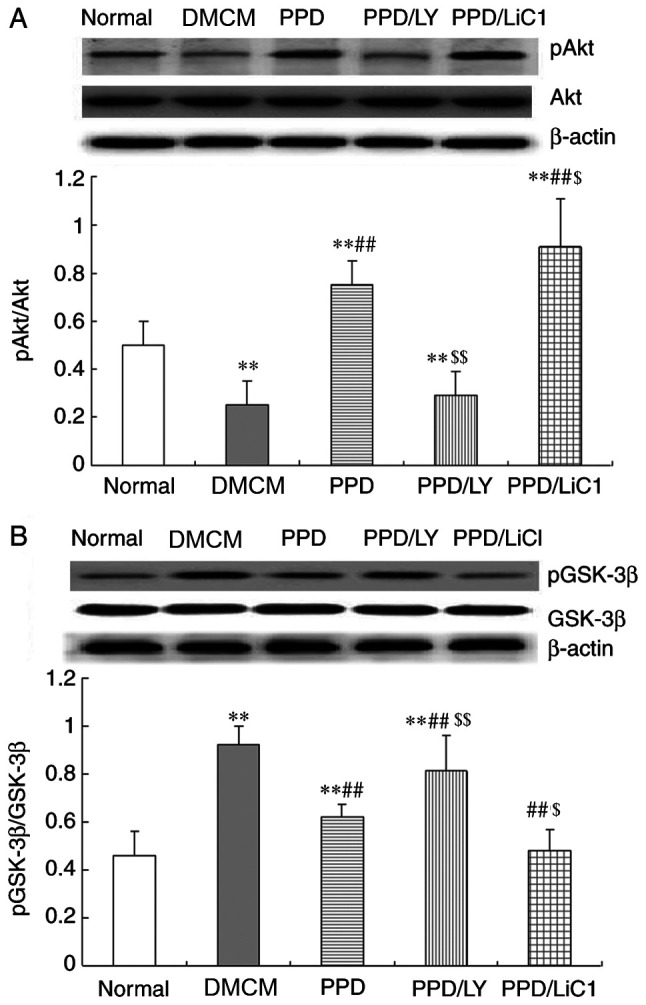
PPD activates the PI3k/Akt/GSK-3 β signaling pathway. PPD increased p-Akt levels and decreased the expression levels of p-GSK-3β. p-Akt levels were suppressed and GSK-3β levels were enhanced in the PPD/LY294002 group. The expression levels of p-Akt were increased, whereas the expression levels of GSK-3β were decreased in the PPD/LiCl group. (A) Western blotting of p-Akt and Akt levels. (B) Western blotting of p-GSK-3β and GSK-3β levels. The values are presented as mean ± SD, n=10. **P<0.01 vs. the NS group; ##P<0.01 vs. the DMCM group; $P<0.05 and $$P<0.01 vs. the PPD group. PPD, 25-OH-PPD; GSK-3 β, inhibitor of glycogen synthase kinase 3β; p-, phosphorylated; DMCM, diabetic cardiomyopathy.
Discussion
PNS serves as a traditional Chinese drug with several pharmacological effects. It is commonly applied in the treatment of specific diseases, such as diabetic retinopathy and bleeding disorders causing inhibition of bleeding and simultaneous prevention of thrombosis (16). Additional applications of this herbal product include improved wound healing, inhibition of bone and muscle tissue inflammation, edema and pain management and treatment of traumatic injury (17).
PNS contains several active compounds, including anti-oxidant flavonoids, oxygen-rich polysaccharides, ginsenosides, cyclopeptides and saponins, which are generally useful to enhance body tissue functions (18). Previous studies have shown that 25-OH-PPD is a new active ingredient separated and isolated from the leaves of pseudo-ginseng that contributes to cellular metabolism (13,14). This compound exhibits a positive outcome on cardiovascular health and regulates the immune system by demonstrating anti-inflammatory and anti-atherosclerotic activities (14,19). Therefore, it can also be used in the treatment of complications caused by DM. According to previous studies, 25-OH-PPD has been shown to regulate blood glucose levels and enhance insulin sensitivity in diabetes (13,14,19). Subsequent reduction in blood glucose levels may be applied for the treatment of non-insulin dependent diabetes mellitus (type 2, NIDDM). These results suggested that 25-OH-PPD could be used as a drug target for the treatment of insulin resistant and diabetic complications (10). However, it is still uncertain if its protective effects and molecular mechanism exhibit potential clinical applications for the treatment of DMCM.
In the present study, 25-OH-PPD reduced the levels of BNP and CK-MB following 12 weeks of administration to the diabetic animals. The volume of CVF was increased in the diabetic rats that exhibited DMCM and existent myocardial damage. These findings suggested that 25-OH-PPD was beneficial for the treatment of DM by reversing the complications of DMCM. This finding was consistent with a previous study that used diabetic rats in the presence of PPD and characterized the levels of collagen accumulation and the induction of cardiac hypertrophy and remodeling (11). DMCM is a major complication of DM and can induce cardiac dysfunction (dysfunction of sugar and fat metabolism) in diabetic patients (20). The major pathophysiological changes noted in DMCM include cardiomyocyte degeneration and necrosis with myocardial interstitial fibrosis, which in turn induces myocardial hypertrophy and remodeling (21). The pathophysiological changes induce myocardial remodeling and play an important role in the pathogenesis of DMCM (22). The results of the present study suggested that 25-OH-PPD could inhibit hypertrophy and reverse the remodeling of DMCM.
The data of the present study indicated that 25-OH-PPD could significantly reduce the levels of α-SMA and VCAM-1 in the diabetic rat model. VCAM-1 is an inflammatory mediator involved in obesity, diabetes and cardiovascular and inflammatory diseases. It also plays a key function in the progression of hypertrophy and cardiac remodeling in DMCM (23). In addition, α-SMA is the major factor which induces tissue and cell fibroblast differentiation by regulating these processes. α-SMA overexpression increases collagen contraction and promotes proliferative activity as demonstrated by a previous study (24). Inhibition of α-SMA expression may reduce development of tissue fibrosis, notably in acellular fibrotic lung scaffolds (25). Previous studies have suggested that the increased levels of α-SMA expression induced by high-glucose conditions are an important event in renal tubule-interstitial fibrosis, which is a clinical manifestation of diabetic nephropathy (26,27). In the present study, the results indicated that 25-OH-PPD-inhibited overexpression of α-SMA and that VCAM may serve as a potential therapeutic target for vascular injury and myofibroblast migration (28,29). In addition, the effects of 25-OH-PPD on α-SMA and VCAM-1 expression levels were partially suppressed in the presence of LY294002 and LiCl, suggesting that inhibition of the proliferative effects of 25-OH-PPD may be mediated via the PI3K/Akt/GSK3β signaling pathway in DMCM.
At present, the pathogenesis of DMCM is still unclear and its causes are believed to be multifactorial. Previous studies have shown that complications of DMCM involve hyperglycemia, fat metabolism disorders, inflammatory reactions, apoptosis and oxidative stress (5,30). High glucose levels can lead to enhanced expression levels of myocardial TGF-β1 and CTGF (31). However, 25-OH-PPD reduced the expression levels of these factors and its action was accompanied with the subsequent inhibition of cardiac fibrosis mediated by the inactivation of the PI3k/Akt/GSK-3β signaling pathway (32). It has been shown that the inflammatory cytokines, namely TGF-β1 and CTGF are highly expressed in diabetic cardiomyocytes, which results in the development of various biological processes including differentiation, extracellular matrix accumulation, cell proliferation, reconstitution, apoptosis and remodeling (33). TGF-β1 is a major inflammatory factor and a potent profibrotic cytokine (34). The levels of this cytokine were significantly increased by high blood glucose concentrations, which triggered tissue fibrosis. TGF-β1 promotes the synthesis and secretion of collagen by myocardial fibroblasts and induces myocardial hypertrophy (35). CTGF acts as a downregulation factor of TGF-β1 in this process (36). In the present study, the expression levels of TGF-β1 and CTGF in the diabetic group were significantly higher compared with the NG group, indicating that 25-OH-PPD could reduce TGF-β1 and CTGF levels in the DM rats suggesting its role in the inhibition of cardiac remodeling and in the treatment of DMCM.
The results of the present study further demonstrated that p-Akt levels were suppressed and that GSK-3β levels were enhanced in the PPD/LY294002 group compared with those in the PPD group. In addition, the expression levels of p-Akt were increased and those of GSK-3β were decreased in the PPD/LiCl group compared with the PPD group. The Akt signaling pathway promotes cellular survival by inhibiting the action of a series of target proteins in the apoptotic signaling pathway. Akt exhibits distinct key roles in regulating cardiovascular functions, such as blood pressure, regulation of myocardial systolic and diastolic functions, coronary angiogenesis and atherosclerosis (37). GSK-3β is the major substrate of the Akt-GSK-3β pathway that exerts specific physiological and biochemical functions in glycogen metabolism and plays a decisive role in diabetes-induced inflammation and fibrosis (38). Previous investigations have demonstrated that the Akt-GSK-3β signaling pathway plays an important function in diabetes-induced energy metabolic dysfunction and consequently in heart hypertrophy (39,40). In diabetes mellitus, the expression levels of p-Akt were decreased by free fatty acids and inflammatory cytokines, which led to the activation of GSK-3β (41,42). In addition, the data of the present study indicated that triciribine could partially reduce the actions of 25-OH-PPD on DMCM. These findings suggested that PPD prevented the development of DMCM via the p-Akt-GSK-β signaling pathway.
In summary, the results of the present study demonstrated that 25-OH-PPD could inhibit the progression of cardiac dysfunction, myocardial hypertrophy and inflammation in DMCM. The cell growth inhibitory mechanism of 25-OH-PPD was mediated by downregulation of TGF-β1 and CTGF expression via the PI3K-Akt-GSK-β signaling pathway. The present study demonstrated the application and mechanism of action of an effective therapeutic drug that can be used for the treatment of DMCM.
The present study provided evidence that 25-OH-PPD could suppress α-SMA/VCAM expression and downregulate the levels of the inflammatory cytokines, such as TGF-β1 and CTGF. In addition, it significantly improved cardiac functions and inhibited myocardial hypertrophy and inflammation in DMCM via the PI3k-Akt-GSK-3β signaling pathway.
Acknowledgements
We thank Dr Junxian (Department of pharmacy, Beijing friendship hospital of capital medical university) for expert technical assistance.
Funding
The present study was supported by the Natural Foundation Guidance Plan of Liaoning Province (grant nos. 2019-ZD-0822 and 2019-ZD-0821) and the Key Research and Development Program Guidance Program Project of Liaoning Province (grant no. 2018225030).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
XL performed the experiments in the present study; FS prepared the animal models; CL was responsible for the statistics and writing the article, as well as organizing the study; YZ analyzed the immunohistochemistry data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This experiment was approved by the ethics committee of JinZhou Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Gregg EW, Cheng YJ, Srinivasan M, Lin J, Geiss LS, Albright AL, Imperatore G. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: An epidemiological analysis of linked national survey and vital statistics data. Lancet. 2018;16:2430–2440. doi: 10.1016/S0140-6736(18)30314-3. [DOI] [PubMed] [Google Scholar]
- 2.Jun JE, Lee SE, Choi MS, Park SW, Hwang YC, Kim JH. Clinical factors associated with the recovery of cardiovascular autonomic neuropathy in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2019;18:29–30. doi: 10.1186/s12933-019-0830-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahajan UB, Chandrayan G, Patil CR, Arya DS, Suchal K, Agrawal Y, Ojha S, Goyal SN. Eplerenone attenuates myocardial infarction in diabetic rats via modulation of the PI3K-Akt pathway and phosphorylation of GSK-3β. Am J Transl Res. 2018;10:2810–2821. [PMC free article] [PubMed] [Google Scholar]
- 4.Hölscher ME, Bode C, Bugger H. Diabetic cardiomyopathy: Does the type of diabetes matter? Int J Mol Sci. 2016;17(2136) doi: 10.3390/ijms17122136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furuya F, Ishii T, Kitamura K. Chronic inflammation and progression of diabetic kidney disease. Contrib Nephrol. 2019;198:33–39. doi: 10.1159/000496526. [DOI] [PubMed] [Google Scholar]
- 6.Lombardi C, Spigoni V, Gorga E, Dei Cas A. Novel insight into the dangerous connection between diabetes and heart failure. Herz. 2016;41:201–207. doi: 10.1007/s00059-016-4415-7. [DOI] [PubMed] [Google Scholar]
- 7.Huber SA. Viral myocarditis and dilated cardiomyopathy: Etiology and pathogenesis. Curr Pharm Des. 2016;22:408–426. doi: 10.2174/1381612822666151222160500. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida Y, Boren SA, Soares J, Popescu M, Nielson SD, Koopman RJ, Kennedy DR, Simoes EJ. Effect of health information technologies on cardiovascular risk factors among patients with diabetes. Curr Diab Rep. 2019;19(28) doi: 10.1007/s11892-019-1152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo X, Xue M, Li CJ, Yang W, Wang SS, Ma ZJ, Zhang XN, Wang XY, Zhao R, Chang BC. Protective effects of triptolide on TLR4 mediated autoimmune and inflammatory response induced myocardial fibrosis in diabetic cardiomyopathy. J Ethnopharmacol. 2016;193:333–344. doi: 10.1016/j.jep.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 10.Kitamura K, Takamura Y, Iwamoto T, Nomura M, Iwasaki H, Ohdera M, Murakoshi M, Sugiyama K, Matsuyama K, Manabe Y, et al. Dammarane-type triterpene extracts of Panax notoginseng root ameliorates hyperglycemia and insulin sensitivity by enhancing glucose uptake in skeletal muscle. Biosci Biotechnol Biochem. 2017;81:335–342. doi: 10.1080/09168451.2016.1246173. [DOI] [PubMed] [Google Scholar]
- 11.Hu S, Liu T, Wu Y, Yang W, Hu S, Sun Z, Li P1, Du S. Panax notoginseng saponins suppress lipopolysaccharide-induced barrier disruption and monocyte adhesion on bEnd.3 cells via the opposite modulation of Nrf2 antioxidant and NF-κB inflammatory pathways. Phytother Res. 2019;33:3163–3176. doi: 10.1002/ptr.6488. [DOI] [PubMed] [Google Scholar]
- 12.Jin X, Luo Y, Chen Y, Ma Y, Yue P, Yang M. Novel breviscapine nanocrystals modified by panax notoginseng saponins for enhancing bioavailability and synergistic anti-platelet aggregation effect. Colloids Surf B Biointerfaces. 2019;175:333–342. doi: 10.1016/j.colsurfb.2018.11.067. [DOI] [PubMed] [Google Scholar]
- 13.Yu J, Liu C, Li Z, Zhang C, Wang Z, Liu X. Inhibitory effects and mechanism of 25-OH-PPD on glomerular mesangial cell proliferation induced by high glucose. Environ Toxicol Pharmacol. 2016;44:93–98. doi: 10.1016/j.etap.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Shi X, Yu W, Yang T, Qiu S, Yao C, Feng Z, Wei W, Wu W, Guo D. Panax notoginseng saponins provide neuroprotection by regulating NgR1/RhoA/ROCK2 pathway expression, in vitro and in vivo. J Ethnopharmacol. 2016;190:301–312. doi: 10.1016/j.jep.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Liu C, Li J, Zhang X, Song F, Xu J. Urocortin attenuates myocardial fibrosis in diabetic rats via the Akt/GSK-3β signaling pathway. Endocr Res. 2016;41:148–157. doi: 10.3109/07435800.2015.1094489. [DOI] [PubMed] [Google Scholar]
- 16.Xie W, Meng X, Zhai Y, Zhou P, Ye T, Wang Z, Sun G, Sun X. Panax notoginseng saponins: A review of its mechanisms of antidepressant or anxiolytic effects and network analysis on phytochemistry and pharmacology. Molecules. 2018;17(940) doi: 10.3390/molecules23040940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loh YC, Tan CS, Ch'ng YS, Ng CH, Yeap ZQ, Yam MF. Mechanisms of action of Panax notoginseng ethanolic extract for its vasodilatory effects and partial characterization of vasoactive compounds. Hypertens Res. 2019;42:182–194. doi: 10.1038/s41440-018-0139-9. [DOI] [PubMed] [Google Scholar]
- 18.Chan YS, Wong JH, Ng TB. Bioactive proteins in Panax notoginseng roots and other panax species. Curr Protein Pept Sci. 2019;20:231–239. doi: 10.2174/1389203719666180612083650. [DOI] [PubMed] [Google Scholar]
- 19.Yin Z, Ma L, Xu J, Xia J, Luo D. Pustular drug eruption due to Panax notoginseng saponins. Drug Des Devel Ther. 2014;16:957–961. doi: 10.2147/DDDT.S67015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joubert M, Manrique A, Cariou B, Prieur X. Diabetes-related cardiomyopathy: The sweet story of glucose overload from epidemiology to cellular pathways. Diabetes Metab. 2019;45:238–247. doi: 10.1016/j.diabet.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Guo X, Sun W, Luo G, Xia J, Luo D. Panax notoginseng saponins alleviate skeletal muscle insulin resistance by regulating the IRS1-PI3K-AKT signaling pathway and GLUT4 expression. FEBS Open Bio. 2019;9:1008–1019. doi: 10.1002/2211-5463.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding W, Chang WG, Guo XC, Liu Y, Xiao DD, Ding D, Wang JX, Zhang XJ. Exenatide protects against cardiac dysfunction by attenuating oxidative stress in the diabeticmouse heart. Front Endocrinol (Lausanne) 2019;10(202) doi: 10.3389/fendo.2019.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shinde AV, Humeres C, Frangogiannis NG. The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim Biophys Acta. 2017;1863:298–309. doi: 10.1016/j.bbadis.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo SJ, Zhang P, Wu LY, Zhang GN, Chen WD, Gao PJ. Adenovirus-Mediated overexpression of septin 2 attenuates α-Smooth muscle actin expression and adventitial myofibroblast migration induced by angiotensin II. J Vasc Res. 2016;53:309–316. doi: 10.1159/000452413. [DOI] [PubMed] [Google Scholar]
- 25.Sun KH, Chang Y, Reed NI, Sheppard D. α-Smooth muscle actin is an inconsistent marker of fibroblasts responsible for force-dependent TGFβ activation or collagen production across multiple models of organ fibrosis. Am J Physiol Lung Cell Mol Physiol. 2016;310:L824–L836. doi: 10.1152/ajplung.00350.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang H, Zheng F, Dong X, Wu F, Wu T, Li H. Allicin inhibits tubular epithelial-myofibroblast transdifferentiation under high glucose conditions in vitro. Exp Ther Med. 2017;13:254–262. doi: 10.3892/etm.2016.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Chu HR, Guo Y, Liu JH, Li WP, Li H, Cheng M. The effects and mechanisms of high glucose on the phenotype transformation of rat vascular smooth muscle cells. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2015;31:458–461. (In Chinese) [PubMed] [Google Scholar]
- 28.Yu GI, Jun SE, Shin DH. Associations of VCAM-1 gene polymorphisms with obesity and inflammation markers. Inflamm Res. 2017;66:217–225. doi: 10.1007/s00011-016-1006-2. [DOI] [PubMed] [Google Scholar]
- 29.Weber ZA, Kaur P, Hundal A, Ibriga SH, Bhatwadekar AD. Effect of the pharmacist-managed cardiovascular risk reduction services on diabetic retinopathy outcome measures. Pharm Pract (Granada) 2019;17(1319) doi: 10.18549/PharmPract.2019.1.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang SQ, Li D, Yuan Y. Long-term moderate intensity exercise alleviates myocardial fibrosis in type 2 diabetic rats via inhibitions of oxidative stress and TGF-β1/Smad pathway. J Physiol Sci. 2019;69:861–873. doi: 10.1007/s12576-019-00696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdel-Hamid AAM, Firgany AEL. Favorable outcomes of metformin on coronary microvasculature in experimental diabetic cardiomyopathy. J Mol Histol. 2018;49:639–649. doi: 10.1007/s10735-018-9801-4. [DOI] [PubMed] [Google Scholar]
- 32.Zhao B, Guan H, Liu JQ, Zheng Z, Zhou Q, Zhang J, Su LL, Hu DH. Hypoxia drives the transition of human dermal fibroblasts to a myofibroblast-like phenotype via the TGF-β1/Smad3 pathway. Int J Mol Med. 2017;39:153–159. doi: 10.3892/ijmm.2016.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Zhao L, Jiao FZ, Zhang WB, Chen Q, Gong ZJ. Histone deacetylase inhibitor suberoylanilide hydroxamic acid alleviates liver fibrosis by suppressing the transforming growth factor-β1 signal pathway. Hepatobiliary Pancreat Dis Int. 2018;17:423–429. doi: 10.1016/j.hbpd.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Kim KK, Sheppard D, Chapman HA. TGF-β1 Signaling and tissue fibrosis. Cold Spring Harb Perspect Biol. 2018;2(a022293) doi: 10.1101/cshperspect.a022293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen JQ, Guo YS, Chen Q, Cheng XL, Xiang GJ, Chen MY, Wu HL, Huang QL, Zhu PL, Zhang JC. TGFβ1 and HGF regulate CTGF expression in human atrial fibroblasts and are involved in atrial remodelling in patients with rheumatic heart disease. J Cell Mol Med. 2019;23:3032–3039. doi: 10.1111/jcmm.14165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsai CC, Wu SB, Kau HC, Wei YH. Essential role of connective tissue growth factor (CTGF) in transforming growth factor-β1 (TGF-β1)-induced myofibroblast transdifferentiation from Graves' orbital fibroblasts. Sci Rep. 2018;8(7276) doi: 10.1038/s41598-018-25370-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Pan J, Liu D, Zhang M, Li X, Tian J, Liu M, Jin T, An F. Nicorandil alleviates apoptosis in diabetic cardiomyopathy through PI3K/Akt pathway. J Cell Mol Med. 2019;23:5349–5359. doi: 10.1111/jcmm.14413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng W, Shang X, Zhang C, Gao X, Robinson B, Liu J. The effects of carvedilol on cardiac function and the AKT/XIAP signaling pathway in diabetic cardiomyopathy rats. Cardiology. 2017;136:204–211. doi: 10.1159/000450825. [DOI] [PubMed] [Google Scholar]
- 39.Shang L, Pin L, Zhu S, Zhong X, Zhang Y, Shun M, Liu Y, Hou M. Plantamajoside attenuates isoproterenol-induced cardiac hypertrophy associated with the HDAC2 and AKT/GSK-3β signaling pathway. Chem Biol Interact. 2019;307:21–28. doi: 10.1016/j.cbi.2019.04.024. [DOI] [PubMed] [Google Scholar]
- 40.Wang CY, Li XD, Hao ZH, Xu D. Insulin-like growth factor-1 improves diabetic cardiomyopathy through antioxidative and anti-inflammatory processes along with modulation of Akt/GSK-3β signaling in rats. Korean J Physiol Pharmacol. 2016;20:613–619. doi: 10.4196/kjpp.2016.20.6.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X, Liu C, Zhang X, Zhao J, Xu J. Urocortin ameliorates diabetic cardiomyopathy in rats via the Akt/GSK-3β signaling pathway. Exp Ther Med. 2015;9:667–674. doi: 10.3892/etm.2015.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Q, Huang DD, Li DG, Chen B, Zhang LM, Yuan CL, Huang HH. Tetramethylpyrazine exerts a protective effect against injury from acute myocardial ischemia by regulating the PI3K/Akt/GSK-3β signaling pathway. Cell Mol Biol Lett. 2019;24(17) doi: 10.1186/s11658-019-0141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.


