Abstract
Purpose
We hypothesized that longitudinal changes in corneal nerve morphology would differ between the central cornea and inferior whorl in relation to other measures of diabetic neuropathy.
Methods
Thirty patients with diabetes (age: 54.08 ± 15.86, duration: 23.95 ± 14.2, HbA1c: 7.51 ± 1.37) and 19 age-matched healthy controls (age: 49.47 ± 13.25) underwent assessment of neuropathy disability score (NDS), vibration perception threshold (VPT), cold (CPT) and warm (WPT) perception thresholds, peroneal motor nerve conduction velocity (PMNCV), corneal nerve fiber density (CNFD), branch density (CNBD), fiber length (CNFL), inferior whorl length (IWL), and the average of CNFL and IWL (ANFL) at baseline and after 1 to 8 years.
Results
In patients with diabetes, between baseline and follow-up, there was a significant reduction in CNBD (57.72 ± 30.08 vs. 44.04 ± 23.69; P = 0.02), CNFL (21.77 ± 5.19 vs. 15.65 ± 4.7; P < 0.0001), IWL (24.69 ± 8.67 vs. 14.23 ± 6.13; P < 0.0001), ANFL (23.26 ± 5.53 vs. 15.09 ± 4.48; P < 0.0001), and WPT (43.56 ± 4.43 vs. 40.78 ± 4.93; P = 0.01), and an increase in VPT (12.9 ± 8.96 vs. 13.78 ± 8.99; P = 0.02). There was no significant change in CNFD (27.12 ± 8.2 vs. 25.43 ± 7.11; P = 0.2), NDS (3.38 ± 3.35 vs. 2.61 ± 2.8; P = 0.08), CPT (17.7 ± 10.59 vs. 22.45 ± 9.23; P = 0.06), or PMNCV (42.4 ± 4.21 vs. 42.16 ± 6.3; P = 0.2).
Conclusions
There is evidence of corneal nerve loss in patients with diabetes, particularly at the inferior whorl during follow-up.
Keywords: corneal nerves, diabetes mellitus, corneal confocal microscopy
Corneal confocal microscopy (CCM) is a rapid noninvasive ophthalmic imaging technique to quantify corneal nerve morphology,1 and corneal nerve loss is related to the severity of diabetic neuropathy.2,3 We have recently demonstrated good diagnostic ability of CCM in a large cohort of patients with diabetic neuropathy.4 The corneal sub-basal nerve plexus is comprised of unmyelinated nerve fibers, which course from the peripheral cornea to the central cornea, and then radiate to the more distal inferior whorl (IW).5,6 Most studies have shown a loss of corneal nerves in the central cornea.3,7,8 However, more recent studies in experimental animals9 and patients with diabetes10–13 have shown a reduction in corneal nerves at the IW in patients without diabetic peripheral neuropathy (DPN) and an association with the severity of painful diabetic neuropathy and poorer quality of life.11 Furthermore, the IW is an anatomically distinct structure, which allows more accurate assessment of longitudinal changes in the sub-basal nerve plexus.12
Previously, very few studies have assessed change in corneal nerve morphology longitudinally.8,14–16 In the current study, we have compared longitudinal changes in corneal nerve morphology in the central cornea and at the IW in relation to other measures of neuropathy.
Methodology
Study Subjects
Thirty patients with type 1 (n = 21) or type 2 (n = 9) diabetes and 19 age- and sex-matched healthy participants underwent detailed assessment of peripheral neuropathy and CCM at baseline and follow-up while receiving standard of care for diabetes management. We hypothesized that longitudinal changes in corneal nerve morphology would differ between the central cornea and IW in relation to other measures of diabetic neuropathy.
The follow-up visit for each participant ranged between 1 and 8 years, with an average 3.6 ± 1.3 years for patients with diabetes and 5.0 ± 1.75 years for controls. Patients with a history of or any other cause of neuropathy (malignancy, deficiency of B12 or folate, chronic renal, liver failure, connective tissue or systemic disease), current or active diabetic foot ulceration, previous corneal trauma or systemic disease that affects the cornea, surgery and a history of or current contact lens wear were excluded from the study. Each participant provided informed consent, and the research adhered to the tenets of the Declaration of Helsinki and was approved by Greater Manchester East Research Ethics Committee.
Clinical and Peripheral Neuropathy Assessment
Body mass index (BMI), blood pressure (BP), (glycated hemoglobin) HbA1c, and lipid profile were assessed in each participant. Neuropathy disability score (NDS) was used to quantify vibration, pinprick, temperature perception, and presence or absence of ankle reflexes.17 Vibration perception threshold (VPT) was evaluated using a Neurothesiometer (Scientific Laboratory Supplies, Wilford, Nottingham, UK), and cold perception threshold (CPT) and warm perception threshold (WPT) were tested on the dorsolateral aspect of the nondominant foot (S1) using a TSA-II NeuroSensory Analyser (Medoc, Ltd., Ramat-Yishai, Israel). Electrodiagnostic studies were undertaken by a consultant neurophysiologist using a Dantec “Keypoint” system (Dantec Dynamics Ltd., Bristol, UK) equipped with DISA temperature regulator to keep the limb temperature at 32°C to 35°C, and peroneal motor nerve conduction velocity (PMNCV) was tested.
Ophthalmic Assessment
Examinations of the anterior ocular segment using slit-lamp biomicroscopy, and CCM examination using laser scanning CCM HRT III (Heidelberg Retinal Tomograph III Rostock Cornea Module; Heidelberg Engineering, Heidelberg, Germany) were performed for both eyes according to our established protocol.10,18
Corneal Nerves
We selected six images (three per eye) from the central sub-basal nerve plexus and four images from the IW region and manually quantified corneal nerve morphology using CCMetrics (University of Manchester, Manchester, UK). Images were selected by a single expert in a masked fashion taking into account the quality, depth, and variability using an established protocol.10,19 We quantified five corneal nerve parameters: corneal nerve fiber density (CNFD: total number of main nerves per square millimeter) (no./mm2), corneal nerve branch density (CNBD: total number of branches per square millimeter) (mm/mm2), corneal nerve fiber length (CNFL: total length of main nerves and nerve branches per square millimeter) (mm/mm2), inferior whorl length (IWL: total length of nerves per square millimeter) (mm/mm2), and average nerve fiber length (ANFL = CNFL + IWL/2) (mm/mm2).
Statistical Analyses
The analysis was carried out using SPSS Version 22.0 for Macintosh (IBM Corporation, Armonk, NY, USA). The Shapiro-Wilk test was employed to assess whether the data were normally distributed. Paired and independent t-tests (Wilcoxon matched-pair signed-rank test and Mann-Whitney U test for nonparametric, respectively) were used to assess differences between the groups. The Pearson correlation coefficient (Spearman for nonparametric) was calculated to assess the correlations between different variables. All data are expressed as mean ± SD. P < 0.05 was considered significant. The sample size calculation was measured using G*Power software Version 3.1.9.4 for Macintosh (Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany). With the effect size of 0.5, α of 0.05, and β of 80%, a minimal sample size of 27 subjects was calculated to establish a change. The graphs were created using GraphPad Prism Version 7.0c for Macintosh (GraphPad Software, La Jolla, CA, USA).
Results
Clinical and Neuropathy Assessment
Baseline
Patients with diabetes and healthy controls were comparable for age (54.08 ± 15.86 vs. 49.47 ± 13.25; P = 0.2), sex (P = 0.6), ethnicity (P = 0.2), and BMI (P = 0.1). The mean duration of follow-up in controls was 5.0 ± 1.75 years, and for patients with diabetes it was 3.6 ± 1.3 years (Table 1). High-density lipoprotein (HDL; P = 0.8), systolic (P = 0.2) and diastolic (P = 0.8) BP were comparable between patients with diabetes and controls. HbA1c was higher and total cholesterol and low-density lipoprotein (LDL; all P < 0.0001) and triglycerides (P = 0.04) were lower in diabetic patients compared with controls. NDS, VPT, and WPT were higher and CPT and PMNCV were lower in patients with diabetes compared with controls (all P = 0.001).
Table 1.
Demographics and Neuropathy Assessment in Healthy Controls and Patients with Diabetes at Baseline and Follow-Up
| Controls | Controls | Patients | Patient | |||
|---|---|---|---|---|---|---|
| Baseline | Follow-Up | P Value | Baseline | Follow-Up | P Value | |
| Age (y) | 49.47 ± 13.25 | NA | 54.08 ± 15.86 | NA | ||
| Sex (F/M) | (7/12) | (11/19) | ||||
| Ethnicity (Asian/European) | (4/15) | (3/27) | ||||
| Duration of diabetes (y) | 0 ± 0 | NA | 23.95 ± 14.2 | NA | ||
| Duration of follow-up (y) | 5.0 ± 1.75 (1–8 y) | 3.6 ± 1.3 (1–6 y) | ||||
| HbA1c (%) | 5.37 ± 0.42 | 5.28 ± 0.24 | 0.08 | 7.51 ± 1.37‡ | 7.14 ± 1.07 | 0.2 |
| BMI (kg/m2) | 25.76 ± 3.66 | 27.26 ± 3.02 | 0.8 | 28.81 ± 6.69 | 28.34 ± 5.8 | 0.08 |
| Cholesterol (mmol/mL) | 4.98 ± 0.86 | 4.65 ± 0.83 | 0.05 | 3.95 ± 0.81‡ | 3.73 ± 0.63 | 0.02 |
| HDL (mmol/mL) | 1.53 ± 0.31 | 1.61 ± 0.3 | 0.5 | 1.5 ± 0.5 | 1.47 ± 0.49 | 0.2 |
| Triglycerides(mmol/mL) | 1.58 ± 0.94 | 1.37 ± 0.51 | 0.7 | 1.17 ± 0.58* | 1.32 ± 0.94 | 0.4 |
| LDL (mmol/mL) | 2.74 ± 0.76 | 2.55 ± 0.66 | 0.1 | 1.94 ± 0.57† | 1.59 ± 0.49 | 0.02 |
| Systolic BP (mm Hg) | 120.21 ± 16.49 | 121 ± 16.56 | 0.9 | 126.2 ± 15.16 | 128.56 ± 17.32 | 0.9 |
| Diastolic BP (mm Hg) | 68.95 ± 10.64 | 68 ± 7.37 | 0.9 | 68.63 ± 7.85 | 65.88 ± 8.63 | 0.08 |
| VPT (V) | 6.83 ± 5.53 | 6.67 ± 4.21 | 0.5 | 12.9 ± 8.96† | 13.78 ± 8.99 | 0.02 |
| CPT foot (°C) | 27.08 ± 4.33 | 22.53 ± 7.37 | 0.1 | 17.7 ± 10.59‡ | 22.45 ± 9.23 | 0.06 |
| WPT foot (°C) | 39.27 ± 3.97 | 41.38 ± 5.28 | 0.4 | 43.56 ± 4.43† | 40.78 ± 4.93 | 0.01 |
| PMNCV (m/s) | 48.22 ± 4.1 | 47.25 ± 4 | 0.3 | 42.4 ± 4.21‡ | 42.16 ± 6.3 | 0.2 |
| NDS (0–10) | 0 | 0 | 0.1 | 3.38 ± 3.35† | 2.61 ± 2.8 | 0.08 |
All data are presented as mean ± SD. NA, not available.
P < 0.05 compared with controls baseline.
P < 0.01 compared with controls baseline.
P < 0.0001 compared with controls baseline.
Controls Baseline Versus Follow-Up
There was a reduction in total cholesterol (P = 0.05), but no change in BMI (P = 0.8), HDL (P = 0.5), LDL (P = 0.1), triglycerides (P = 0.7), or systolic and diastolic BP (both P = 0.9) between baseline and follow-up. NDS (P = 0.1), VPT (P = 0.5), CPT (P = 0.1), WPT (P = 0.4), and PMNCV (P = 0.3) did not change between baseline and follow-up (Table 1).
Diabetic Patients Baseline Versus Follow-Up
There was a significant reduction in total cholesterol (P = 0.02) and LDL (P = 0.02), but no change in BMI (P = 0.08), HbA1c (P = 0.2), HDL (P = 0.2), triglycerides (P = 0.4), systolic (P = 0.9) or diastolic (P = 0.08) BP. There was a significant increase in VPT (P = 0.02) (change per year 1.12 ± 2.75 V), but decrease in WPT (P = 0.01) (change per year –0.33°C ± 2.19°C), with no change in NDS (P = 0.08), CPT (P = 0.06), or PMNCV (P = 0.2) (Table 1) .
Corneal Confocal Microscopy
Baseline
CNFD (no/mm2) (27.12 ± 8.2 vs. 31.37 ± 5.31; P = 0.004), CNBD (no/mm2) (57.72 ± 30.08 vs. 87.53 ± 29.69; P = 0.002), CNFL (mm/mm2) (21.77 ± 5.19 vs. 26.07 ± 6.42; P = 0.03), IWL (mm/mm2) (24.69 ± 8.67 vs. 33.6 ± 10.11; P = 0.003), and ANFL (mm/mm2) (23.26 ± 5.53 vs. 31.04 ± 6.54; P = 0.01) were significantly lower in patients with diabetes compared with controls (Table 2, Fig. 1).
Table 2.
Corneal Nerve Parameters in Healthy Controls and Patients with Diabetes at Baseline and Follow-Up
| Controls Baseline | Controls Follow-Up | P Value | Patients Baseline | Patients Follow-Up | P Value | |
|---|---|---|---|---|---|---|
| CNFD (no/mm2) | 31.37 ± 5.31 | 31.02 ± 5.4 | 0.9 | 27.12 ± 8.2† | 25.43 ± 7.11 | 0.2 |
| CNBD (no/mm2) | 87.53 ± 29.69 | 68.49 ± 24.95 | 0.02 | 57.72 ± 30.08† | 44.04 ± 23.69 | 0.02 |
| CNFL (mm/mm2) | 26.07 ± 6.42 | 21.08 ± 4.52 | 0.01 | 21.77 ± 5.19* | 15.65 ± 4.7 | <0.0001 |
| IWL (mm/mm2) | 33.6 ± 10.11 | 29.79 ± 8.93 | 0.1 | 24.69 ± 8.67† | 14.23 ± 6.13 | <0.0001 |
| ANFL (mm/mm2) | 31.04 ± 6.54 | 26.11 ± 8.02 | 0.01 | 23.26 ± 5.53‡ | 15.09 ± 4.48 | <0.0001 |
All data are presented as mean ± SD.
P < 0.05 compared with controls baseline.
P < 0.01 compared with controls baseline.
P < 0.0001 compared with controls baseline.
Figure 1.
CCM images of the central cornea (first row) and IW (second row) in a healthy control at baseline (A, E) and follow-up (B, F), and an age-matched patient with diabetes at baseline (C, G) and follow-up (D, H). Scale bar: 50 pixels.
Controls Baseline Versus Follow-Up
There was a significant reduction in CNBD (P = 0.02), CNFL (P = 0.01), and ANFL (P = 0.01), but no change in CNFD (P = 0.9) or IWL (P = 0.1) between baseline and follow-up visits (Table 2, Fig. 2).
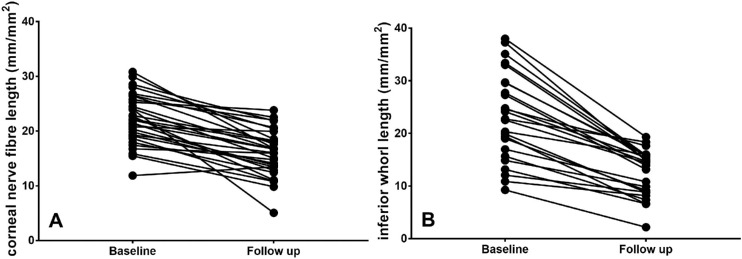
Figure 2.
(A) CNFL and (B) IWL in patients with diabetes at baseline and follow-up (each dot represents a single patient).
Diabetic Patients Baseline Versus Follow-Up
There was a significant reduction in CNBD (P = 0.02), CNFL (P < 0.0001), IWL (P < 0.0001), and ANFL (P < 0.0001), but no change in CNFD (P = 0.2) between baseline and follow-up visits (Table 2, Fig. 2).
Corneal Nerve Parameter Change per Year
To measure the average rate of corneal nerve loss, we divided the change between the baseline and follow-up visit by the time between the baseline and follow-up visit. The change per year in CNFL (mm/mm2) (2.08 ± 2.02 vs. 0.5 ± 3.35; P = 0.02), ANFL (mm/mm2) (2.52 ± 1.51 vs. 0.67 ± 2.27; P < 0.0001), and IWL (mm/mm2) (3.5 ± 2.91 vs. 0.68 ± 2.21; P < 0.0001) was significantly higher in patients with diabetes compared with healthy controls (Table 3). The intraclass correlations showed good consistency between the changes per year in CNFL and IWL in patients with diabetes (intraclass correlation coefficient, 0.78; 95% confidence interval, 0.56–0.88; P < 0.0001).
Table 3.
Corneal Nerve Parameter Changes per Year in Healthy Controls and Patients with Diabetes
| Controls Change Per Year | Patients Change Per Year | P Value | |
|---|---|---|---|
| CNFD (no/mm2) | 0.07 ± 0.85 | 0.28 ± 2.2 | 0.1 |
| CNBD (no/mm2) | 3.08 ± 9.21 | 4.19 ± 7.95 | 0.9 |
| CNFL (mm/mm2) | 0.5 ± 3.35 | 2.08 ± 2.02 | 0.02 |
| ANFL (mm/mm2) | 0.67 ± 2.27 | 2.52 ± 1.51 | <0.0001 |
| IWL (mm/mm2) | 0.68 ± 2.21 | 3.5 ± 2.91 | <0.0001 |
This table shows the average reduction per year. All data are presented as mean ± SD.
Discussion
There is currently no consensus as to which corneal nerve parameter has optimal sensitivity and specificity for the diagnosis and assessment of progression or benefit after therapeutic intervention in diabetic neuropathy.20,21 Earlier studies suggested that CNFD20,21 may be optimal in the diagnosis of DPN, whereas others have shown that CNFL is a more reliable parameter for both diagnosis and prediction of the development of diabetic neuropathy.22–24 However, longitudinal studies ideally require quantification of the same corneal nerves over time, and as there is significant heterogeneity in the central corneal nerve network, it can be difficult to capture the same area.25
Quantification of corneal nerve morphology at the IW has several distinct advantages as it assesses an anatomically more distal part of the sub-basal plexus compared with the central nerves,6 and is therefore more likely to be affected by a dying back neuropathy and it is also a unique landmark for longitudinal studies. At baseline, all corneal nerve parameters in the central and IW region were reduced in patients with diabetes compared with healthy controls, in agreement with previous studies.10–12,19 Furthermore, in patients with diabetes a change in more distal nerve fibers as captured by CNBD, CNFL, IWL, and ANFL showed a significant reduction at follow-up, whereas CNFD, a more proximal part of the corneal nerve plexus, did not change, indicating that these more distal nerve parameters are more capable of capturing change in CCM over time. Pritchard et al.8 have also previously demonstrated a reduction in CNFL and CNBD, with no change in CNFD in patients with type 1 diabetes. We also demonstrated a significant reduction in CNFL in healthy controls, consistent with the study of Dehghani et al.15 who reported a slight reduction in CNFL over 36 months. More recently, we have shown that corneal nerve loss has the greatest predictive value for the development of DPN.26
As expected, we also show that the rate of annual decline in CNFL, IWL, and ANFL was significantly higher in patients with diabetes compared with controls.27 Our previous study also showed a two- to three-fold greater reduction in IWL compared with CNFL in patients with DPN compared with healthy controls.11
All other accepted measures of diabetic neuropathy differed from healthy controls. However, over time whereas the controls showed no change, diabetic patients showed a small but significant worsening in VPT with no change in nerve conduction, whereas NDS and CPT showed trends for improvement and WPT improved significantly. This suggests that these measures have significant limitations when assessing longitudinal change, especially in small cohorts of patients with diabetes, especially in early phase 2 clinical trials.
To our knowledge, this is the first longitudinal study comparing corneal nerve loss in the central and IW regions. Our study shows that corneal nerve loss at baseline and over time is greater in the IW compared with the central cornea in controls and patients with diabetes. The reduction in CNFL in control subjects in the present study was greater than that reported by Sharma et al.,28 but our controls were older, and we assessed CNFL manually.
Cardiovascular risk factors, including glycemic control, BP, and lipids, have been associated with the development of diabetic neuropathy.29 A large body of experimental and clinical data suggests an important link between dyslipidemia and diabetic neuropathy.30 A reduction in corneal nerves has been associated with increasing age, HbA1c, and a lower HDL.14 We have also recently shown that an improvement in corneal nerve morphology after pancreas and kidney transplantation was associated with an improvement in HbA1c and triglycerides.31 The ADDITION-Denmark study showed that LDL and cholesterol are risk factors for the development of DPN.32 In the present study, we showed a significant improvement in total cholesterol and LDL attributed to statin treatment, but there was a reduction in all CCM parameters at follow-up.
Conclusions
Our study shows greater corneal nerve loss at the IW at follow-up compared with baseline. Furthermore, the minimal change or indeed improvement in accepted measures of diabetic neuropathy questions the utility of these tests in longitudinal and therapeutic studies of diabetic neuropathy.
Acknowledgments
Supported by the Manchester Biomedical Research Centre and the Greater Manchester Comprehensive Local Research Network. Also supported by the European Union Seventh Framework Programme FP7/2007-2013 (n°602273), National Institutes of Health (R01DK077903-0101; Bethesda, MD, USA), and Juvenile Diabetes Research Foundation International (27-2008-362). The authors alone are responsible for the content and writing of the article.
Author contributions: MF researched data, performed statistical analysis, and wrote the manuscript; AK researched data, performed statistical analysis, and wrote the manuscript; IP researched data; SA researched data; SD researched data; AM researched data; AJMB reviewed and revised the manuscript; NE reviewed and revised the manuscript; CGF reviewed and revised the manuscript; GL reviewed and revised the manuscript; HS reviewed and revised the manuscript; RAM designed the study and reviewed and revised the manuscript. RAM is the guarantor of this work and, as such, had full access of the data of the study and takes responsibility of the integrity of the data and the accuracy of the data analyses.
Disclosure: M. Ferdousi, None; A. Kalteniece, None; I. Petropoulos, None; S. Azmi, None; S. Dhage, None; A. Marshall, None; A.J.M. Boulton, None; N. Efron, None; C.G. Faber, None; G. Lauria, None; H. Soran, None; R.A. Malik, None
References
- 1. Oliveira Soto L. Morphology of corneal nerves using confocal microscopy. Cornea. 2001; 20: 374–3784. [DOI] [PubMed] [Google Scholar]
- 2. Petropoulos IN, Alam U, Fadavi H, et al.. Corneal nerve loss detected with corneal confocal microscopy is symmetrical and related to the severity of diabetic polyneuropathy. Diabetes Care. 2013; 36: 3646–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Petropoulos IN, Green P, Chan AW, et al.. Corneal confocal microscopy detects neuropathy in patients with type 1 diabetes without retinopathy or microalbuminuria. PLoS One. 2015; 10: e0123517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Perkins BA, Lovblom LE, Bril V, et al.. Corneal confocal microscopy for identification of diabetic sensorimotor polyneuropathy: a pooled multinational consortium study. Diabetologia. 2018; 61: 1856–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patel DV, McGhee CN. Mapping of the normal human corneal sub-basal nerve plexus by in vivo laser scanning confocal microscopy. Invest Ophthalmol Vis Sci. 2005; 46: 4485–4488. [DOI] [PubMed] [Google Scholar]
- 6. Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003; 76: 521–542. [DOI] [PubMed] [Google Scholar]
- 7. Malik RA, Kallinikos P, Abbott CA, et al.. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. 2003; 46: 683–688. [DOI] [PubMed] [Google Scholar]
- 8. Pritchard N, Edwards K, Dehghani C, et al.. Longitudinal assessment of neuropathy in type 1 diabetes using novel ophthalmic markers (LANDMark): study design and baseline characteristics. Diabetes Res Clin Pract. 2014; 104: 248–256. [DOI] [PubMed] [Google Scholar]
- 9. Davidson EP, Coppey LJ, Kardon RH, Yorek MA. Differences and similarities in development of corneal nerve damage and peripheral neuropathy and in diet-induced obesity and type 2 diabetic rats. Invest Ophthalmol Vis Sci. 2014; 55: 1222–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Petropoulos IN, Ferdousi M, Marshall A, et al.. The inferior whorl for detecting diabetic peripheral neuropathy using corneal confocal microscopy. Invest Ophthalmol Vis Sci. 2015; 56: 2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kalteniece A, Ferdousi M, Petropoulos I, et al.. Greater corneal nerve loss at the inferior whorl is related to the presence of diabetic neuropathy and painful diabetic neuropathy. Sci Rep. 2018; 8: 3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Utsunomiya T, Nagaoka T, Hanada K, et al.. Imaging of the corneal subbasal whorl-like nerve plexus: more accurate depiction of the extent of corneal nerve damage in patients with diabetes. Invest Ophthalmol Vis Sci. 2015; 56: 5417–5423. [DOI] [PubMed] [Google Scholar]
- 13. Pritchard N, Dehghani C, Edwards K, et al.. Utility of assessing nerve morphology in central cornea versus whorl area for diagnosing diabetic peripheral neuropathy. Cornea. 2015; 34: 756–761. [DOI] [PubMed] [Google Scholar]
- 14. Dehghani C, Pritchard N, Edwards K, Russell AW, Malik RA, Efron N. Risk factors associated with corneal nerve alteration in type 1 diabetes in the absence of neuropathy: a longitudinal in vivo corneal confocal microscopy study. Cornea. 2016; 35: 847–852. [DOI] [PubMed] [Google Scholar]
- 15. Dehghani C, Pritchard N, Edwards K, et al.. Morphometric stability of the corneal subbasal nerve plexus in healthy individuals: a 3-year longitudinal study using corneal confocal microscopy. Invest Ophthalmol Vis Sci. 2014; 55: 3195–3199. [DOI] [PubMed] [Google Scholar]
- 16. Dehghani C, Pritchard N, Edwards K, et al.. Natural history of corneal nerve morphology in mild neuropathy associated with type 1 diabetes: development of a potential measure of diabetic peripheral neuropathy. Invest Ophthalmol Vis Sci. 2014; 55: 7982–7990. [DOI] [PubMed] [Google Scholar]
- 17. Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993; 36: 150–154. [DOI] [PubMed] [Google Scholar]
- 18. Tavakoli M, Malik RA. Corneal confocal microscopy: a novel non-invasive technique to quantify small fibre pathology in peripheral neuropathies. J Vis Exp. 2011; 47: 2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kalteniece A, Ferdousi M, Adam S, et al.. Corneal confocal microscopy is a rapid reproducible ophthalmic technique for quantifying corneal nerve abnormalities. PLoS One. 2017; 12: e0183040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ziegler D, Papanas N, Zhivov A, et al.. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes. 2014; 63: 2454–2463. [DOI] [PubMed] [Google Scholar]
- 21. Tavakoli M, Quattrini C, Abbott C, et al.. Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care. 2010; 33: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hertz P, Bril V, Orszag A, et al.. Reproducibility of in vivo corneal confocal microscopy as a novel screening test for early diabetic sensorimotor polyneuropathy. Diabet Med. 2011; 28: 1253–1260. [DOI] [PubMed] [Google Scholar]
- 23. Ahmed A, Bril V, Orszag A, et al.. Detection of diabetic sensorimotor polyneuropathy by corneal confocal microscopy in type 1 diabetes: a concurrent validity study. Diabetes Care. 2012; 35: 821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lovblom LE, Halpern EM, Wu T, et al.. In vivo corneal confocal microscopy and prediction of future-incident neuropathy in type 1 diabetes: a preliminary longitudinal analysis. Can J Diabetes. 2015; 39: 390–397. [DOI] [PubMed] [Google Scholar]
- 25. Lv Y, Zhao SZ.. What is the best strategy on detection of cornea neuropathy in people with diabetes? Recent advances in potential measurements. Diabetes Res Clin Pract. 2018; 142: 203–212. [DOI] [PubMed] [Google Scholar]
- 26. Edwards K, Pritchard N, Dehghani C, et al.. Corneal confocal microscopy best identifies the development and progression of neuropathy in patients with type 1 diabetes. J Diabetes Complications. 2017; 31: 1325–1327. [DOI] [PubMed] [Google Scholar]
- 27. Tesfaye S, et al.. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010; 33: 2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sharma S, Tobin V, Vas PRJ, Malik RA, Rayman G. The influence of age, anthropometric and metabolic variables on LDIFLARE and corneal confocal microscopy in healthy individuals. PLoS One. 2018; 13: e0193452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tesfaye S, Chaturvedi N, Eaton SE, et al.. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005; 352: 341–350. [DOI] [PubMed] [Google Scholar]
- 30. Eid S, Sas KM, Abcouwer SF, et al.. New insights into the mechanisms of diabetic complications: role of lipids and lipid metabolism. Diabetologia. 2019; 62: 1539–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Azmi S, Jeziorska M, Ferdousi M, et al.. Early nerve fibre regeneration in individuals with type 1 diabetes after simultaneous pancreas and kidney transplantation. Diabetologia. 2019; 62: 1478–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Andersen ST, Grosen K, Tankisi H, et al.. Corneal confocal microscopy as a tool for detecting diabetic polyneuropathy in a cohort with screen-detected type 2 diabetes: ADDITION-Denmark. J Diabetes Complications. 2018; 32: 1153–1159. [DOI] [PubMed] [Google Scholar]