Abstract
Purpose
Inflammation, hyaluronan production, and adipogenesis are the main pathological events leading to Graves’ orbitopathy (GO). Guggulsterone (GS), a phytosterol found in the resin of the guggul plant, is a well-known treatment for several inflammatory disorders, such as arthritis, obesity, and hyperlipidemia. Here we investigated the effects of GS treatment on GO pathology.
Methods
Using primary cultures of orbital fibroblasts from GO patients and non-GO controls, we examined the effects of GS on hyaluronan production and the production of proinflammatory cytokines induced by interleukin (IL)-1β, using real-time reverse transcription-polymerase chain reaction analysis, western blots, and enzyme-linked immunosorbent assays. Further, adipogenic differentiation was evaluated by quantification of Oil Red O staining and assessment of protein levels of peroxisome proliferator activator gamma (PPARγ), CCAAT-enhancer-binding proteins (C/EBP) α and β, and sterol regulatory element-binding protein-1 (SREBP-1).
Results
Treatment with noncytotoxic concentrations of GS resulted in the dose-dependent inhibition of IL-1β-induced inflammatory cytokines, including IL-6, IL-8, MCP-1, and COX-2, at both mRNA and protein levels. The hyaluronan level was also significantly suppressed by GS. Moreover, GS significantly decreased the formation of lipid droplets and expression of PPARγ, C/EBP α/β, and SREBP-1 in a dose-dependent manner. GS pretreatment attenuated the phosphorylation of nuclear factor-kappa B induced by IL-1β.
Conclusions
Our data show significant inhibitory effects of GS on inflammation, production of hyaluronan, and adipogenesis in orbital fibroblasts. To our knowledge, this is the first in vitro preclinical evidence of the therapeutic effect of GS in GO.
Keywords: Graves’ orbitopathy, guggulsterone, inflammation, hyaluronan, adipogenesis
Graves’ orbitopathy (GO) is an autoimmune inflammatory manifestation of Graves’ hyperthyroidism.1 Its clinical manifestations are largely variable, including severe pain, ocular discomfort, retraction of the eyelid, ocular protrusion, and periorbital edema.2 Activation of the thyroid-stimulating hormone receptor (TSHR) and insulin-like growth factor 1 receptor, colocated in thyroid follicular cells and orbital fibroblasts, mediate the production of proinflammatory cytokines and chemokines. These cause massive infiltration of T and B cells, excessive hyaluronan accumulation, and orbital adipogenesis, inducing globe proptosis.1
GO is difficult to manage owing to its diverse clinical progression and multiple causative factors. High-dose glucocorticoids have been the primary treatment option in active inflammatory states3; however, they are associated with several limitations, namely side effects, such as Cushing syndrome, diabetes, hypertension, osteoporosis, and liver failure.4 Additionally, glucocorticoids might cause increased susceptibility to infection and adrenal insufficiency. Moreover, they do not treat symptoms, such as proptosis, eyelid edema, and diplopia, in a large proportion of GO patients. Despite the remarkable advances in drug development for chronic autoimmune diseases, major drawbacks of the drugs for the treatment of GO are observed owing to an incomplete understanding of its pathophysiology.
Guggulsterone (GS) [4,17(20)-pregnadiene-3,16-dione], a phytosterol present in the natural product gugulipid, which is derived from gum resin (Commiphora mukul), has been used as an effective herbal medicine to treat atherosclerosis, rheumatism, hyperlipidemia, obesity, and cancer.5–10 C. mukul has been shown to have a hypolipidemic effect through lowering total and LDL cholesterol levels and triglycerides. It has also recently been used as a dietary supplement to lower cholesterol and treat arthritis in the United States. GS was identified as the main bioactive constituent responsible for the therapeutic effects of C. mukul.8 Evidence has shown that the potential anti-inflammatory effects of GS are mediated by the suppression of the nuclear factor-kappa B (NF-κB) signaling pathway and/or production of its dependent inflammatory cytokines. Additionally, several studies have shown that GS inhibits adipocyte differentiation, induces lipolysis in mature adipocytes,11 suppresses LDL oxidation,9 and activates adipocyte beiging.12 Here we aimed to explore the potential regulatory effect of GS with regard to the main pathological events of GO in primary cultures of orbital fibroblasts from GO patients.
Materials and Methods
Cell Culture
Orbital tissue specimens were collected from the surgical waste of GO patients during orbital decompression surgery for exophthalmos (n = 7; 3 men, 4 women; aged 21–69 years). Healthy non-GO orbital tissue samples were collected during orbital wall fracture reduction or evisceration from patients without a history or clinical evidence of any thyroid disease (n = 5; 2 men, 3 women; aged 23–75 years) (Table). All recruitment was done at the Department of Ophthalmology, Severance Hospital, Seoul, Korea. Healthy controls were also age- and sex-matched. The study protocol was approved by the institutional review board of Severance Hospital, Yonsei University College of Medicine, Seoul, Korea (IRB 4-2019-0397), and complied with the guidelines of the Declaration of Helsinki. Written informed consent was obtained from all participants. All GO patients were in a euthyroid state for a minimum of 3 months presurgery with a ≤3 clinical activity score at surgery.13 None of the GO patients had received radiotherapy or high-dose steroid treatment for a minimum of 3 months prior to surgery.
Table.
Clinical Information of Patient Samples Used for all In Vitro Studies
| Age (y) | Sex | CAS | Smoker | Duration of GO (y) | Proptosis R/L (mm) | Surgery Performed |
|---|---|---|---|---|---|---|
| GO patients | ||||||
| 44 | F | 0/7 | N | 11 | 21/21 | Decompression |
| 61 | F | 1/7 | N | 2 | 23/23 | Decompression |
| 45 | F | 0/7 | N | 4 | 26/26 | Decompression |
| 69 | M | 1/7 | Y | 1.8 | 19/19 | Decompression |
| 21 | F | 0/7 | N | 8 | 22/22 | Decompression |
| 29 | M | 0/7 | Y | 4 | 23.5/23.5 | Decompression |
| 54 | M | 4/7 | Y | 0.5 | 18/19 | Decompression |
| Non-GO control subjects | ||||||
| 23 | M | N/A | N | N/A | N/A | Evisceration |
| 75 | M | N/A | N | N/A | N/A | Evisceration |
| 35 | F | N/A | Y | N/A | N/A | Orbital wall fracture |
| 44 | F | N/A | N | N/A | N/A | Orbital wall fracture |
| 47 | F | N/A | N | N/A | N/A | Orbital wall fracture |
CAS, clinical activity scores; Y, yes; N, No; N/A, not applicable; F, female; M, male; R, right eye; L, left eye.
Primary cultures of orbital fibroblasts were established as previously described.14 Minced tissue explants were placed in plastic culture dishes, with penicillin (100 U/mL), gentamycin (20 µg/mL), and Dulbecco's modified Eagle's medium (DMEM) containing 20% fetal bovine serum (FBS), to promote fibroblast proliferation. The cells were maintained in two 80-mm flasks containing DMEM, antibiotics, and 10% FBS. Monolayers, treated with trypsin/EDTA, were serially passaged and stored in liquid nitrogen. Cells between the third and fifth passages were used for experiments.
Reagents
E-GS was obtained from Sigma–Aldrich, Inc. (St. Louis, MO, USA). Oil Red O was purchased from Sigma–Aldrich, Inc. DMEM, penicillin, gentamicin, and FBS were acquired from Hyclone Laboratories, Inc. (Logan, UT, USA). Recombinant human interleukin (IL)-1β was obtained from R&D Systems (Minneapolis, MN, USA). Anti-CCAAT-enhancer-binding protein (C/EBP) α and β, anti-sterol regulatory element-binding protein-1 (SREBP-1), anti-peroxisome proliferator activator gamma (PPARγ), and anti-β-actin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against extracellular signal-regulated kinase (ERK), phosphorylated ERK, Akt, phosphorylated Akt, c-Jun NH(2)-terminal kinase (JNK), phosphorylated JNK, NF-κB, phosphorylated NF-κB, p38, and phosphorylated p38 were all purchased from Cell Signaling Technology (Beverly, MA, USA). Phosphorylated levels of proteins were normalized to their respective total protein levels for each phosphorylation analysis.
Cell Viability
Orbital fibroblast viability was examined by assessing 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) reduction to formazan. Post-GS treatment at different concentrations (5–50 µM) for 24 and 48 hours, both GO and non-GO cells were washed twice with PBS and incubated for 3 hours with 5 mg/mL MTT solution at 37°C. After solubilization of the converted dye with dimethyl sulfoxide, absorbance was measured at 560 nm using a microplate reader (EL 340 Bio Kinetics Reader; Bio-Tek Instruments, Winooski, VT, USA), with background subtraction at 630 nm.
Quantitative Real-Time PCR
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA); 1 µg of RNA was used for first-strand cDNA synthesis, according to the manufacturer's protocol (#639543; Takara Bio, Inc., Shiga, Japan). Using a thermocycler (ABI StepOnePlus Real-Time PCR; Applied Biosystems, Foster City, CA, USA) with the TaqMan Gene Expression Master Mix (Applied Biosystems, Foster City, CA) and the suggested PCR conditions, the resulting cDNA amplification was performed to quantitatively determine the level of gene transcript in the samples. All PCRs were carried out in triplicate using Hs00985639_m1 for IL-6, Hs00174103_m1 for IL-8, Hs00234140_m1 for monocyte chemotactic protein (MCP)-1, Hs00153133_m1 for COX-2, and H299999905_m1 for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as primers.
The amount of cytokine mRNA normalized to GAPDH was calculated and expressed as fold changes in the threshold cycle (Ct) value relative to the control group using the 2−∆∆Ct method.15
Western Blot
Equal amounts of protein (50 µg) were separated by 10% SDS polyacrylamide gel electrophoresis. The resolved proteins were transferred to nitrocellulose membranes and incubated overnight with primary antibodies at 4°C. Immunoreactive bands were detected using horseradish peroxidase–conjugated secondary antibodies. Using an enhanced chemiluminescence solution, membranes were then visualized after exposure to x-ray film (GE Healthcare, Piscataway, NJ, USA) or an image reader (LAS-4000 mini; Fuji Photo Film, Tokyo, Japan). Densitometric quantification of each immunoreactive band was performed using ImageJ software (National Institutes of Health, Bethesda, MD, USA); values were normalized to β-actin levels in the same sample.
Adipogenesis
Fibroblasts were incubated for 10 days in a differentiation medium of serum-free DMEM supplemented with 17 µM pantothenic acid, 33 µM biotin, 10 µg/mL transferrin, 1 µM insulin (Boehringer-Mannheim, Mannheim, Germany), 0.2 nM T3, and 0.2 µM carbaprostaglandin (cPGI2; Calbiochem, La Jolla, CA, USA), according to a previously published protocol.16 For the first 4 days of cell growth, the differentiation medium included 10 µM dexamethasone and 0.1 mM isobutylmethylxanthine (IBMX). From day 5 onward, dexamethasone and IBMX were excluded. Starting on the first day of differentiation, rosiglitazone (10 µM; Cayman, Ann Arbor, MI, USA), a PPARγ agonist, or 10 ng/mL IL-1β or both were added for heightened adipogenesis stimulation. To assess the influence of GS on adipogenesis, cells were treated with GS (5, 10, or 25 µM) for the entire differentiation period of 10 days, with media changes every 2 to 3 days.
Oil Red O Stain
Using Oil Red O, cells were stained in accordance with the procedure described by Green and Kehinde.17 Six milliliters of 0.5% Oil Red O stock solution in isopropanol was mixed with 4 mL distilled water and stored at room temperature for 1 hour. The solution was then poured through a 0.2-µm filter and added to cells that had been washed with PBS and fixed with 10% formalin at 4°C for 1 hour. After 1 hour at room temperature, the cell–Oil Red O solution mixture was inspected and photographed using a light microscope (Olympus BX60; Olympus Corp., Melville, NY, USA). To quantify lipid accumulation, cell-bound Oil Red O was solubilized with isopropanol, followed by measuring the optical density of the solution at absorbance 490 nm using a spectrophotometer.
Enzyme-Linked Immunosorbent Assay (ELISA)
Commercially accessible ELISA kits (R&D Systems, Minneapolis, MN, USA) were used to determine hyaluronan levels in the culture supernatant (diluted 1:100) of confluent orbital fibroblasts, according to the manufacturer's instructions. A hyaluronan standard curve was used to calculate the concentration in the samples. The mean value of triplicate samples was reported.
Statistical Analyses
At least three cell strains from different individuals were used in all performed experiments, and sample assays were done in duplicate. Experimental results are shown as the mean ± SD calculated from normalized measurements. ANOVA or the Student's t-test was used to determine statistical significance (P < 0.05) using SPSS version 20.0 (IBM Corporation, Armonk, NY, USA).
Results
Effect of GS on Viability of Orbital Fibroblasts
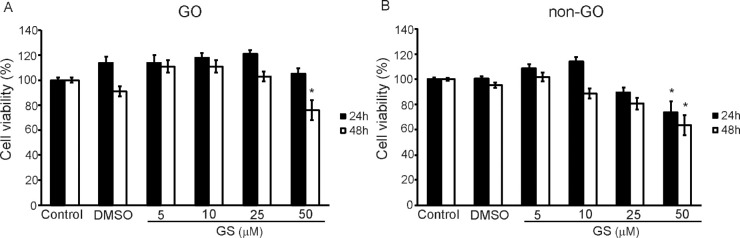
GO and non-GO fibroblasts were treated with various doses of GS (0–50 µM) for 24 or 48 hours. After treatment, the number of live cells was determined by MTT analysis, and the noncytotoxic concentration of GS was determined. When cells were treated with up to 25 µM of GS for 24 and 48 hours, no cytotoxicity was observed in GO and non-GO cells (Fig. 1). However, 50 µM of GS for 48 hours lowered cell viability to less than 75% in both cell lines, whereas 50 µM of GS for 24 hours did not affect cell viability in GO cells. Thus we determined the maximal noncytotoxic concentration of GS to be 25 µM for 24 hours in GO and non-GO cells; this concentration was used in the subsequent assays.
Figure 1.
Effect of GS on the viability of orbital fibroblasts. Orbital fibroblasts from (A) GO and (B) non-GO subjects were primarily cultured in 24 well-culture plates (1 × 104 cells per well) and treated with various concentrations (0–50 µM) of GS for 24 hours (black columns) and 48 hours (white columns). MTT assays, used to evaluate viability, were performed in duplicate using cells (n = 3 for GO and non-GO samples) from different donors. Results are presented as percentages of untreated control values and shown as the means ± SD.
Effect of GS on IL-1β-Induced Proinflammatory Cytokine Expression
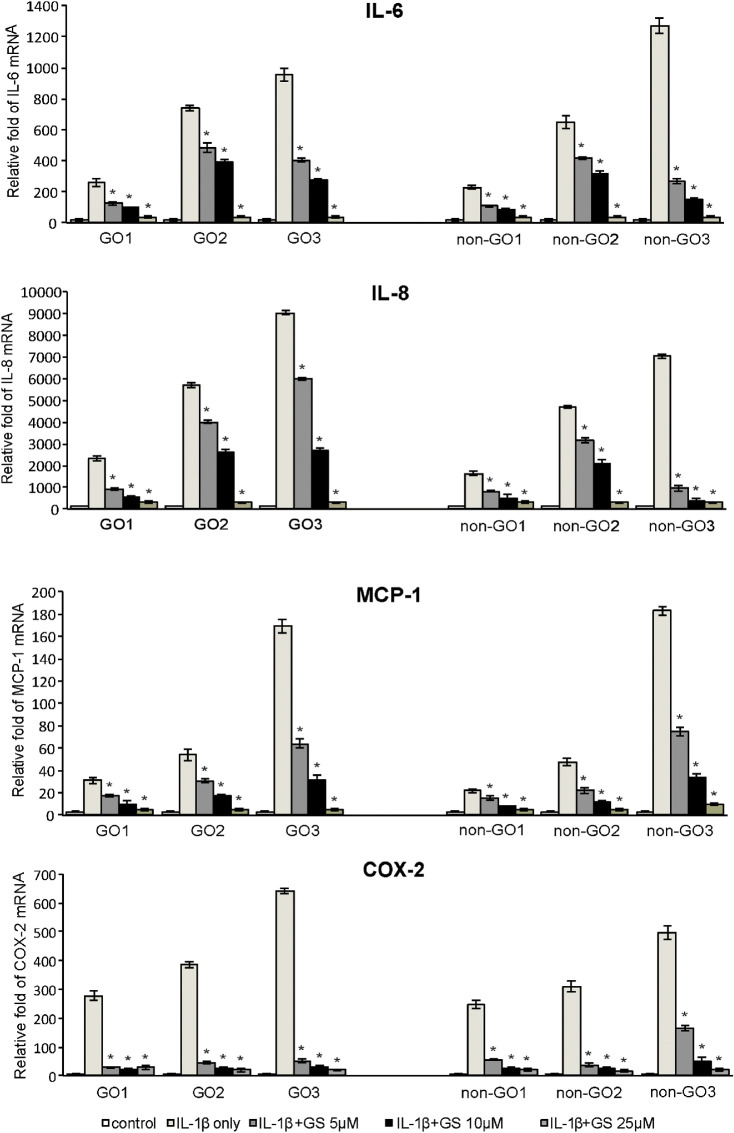
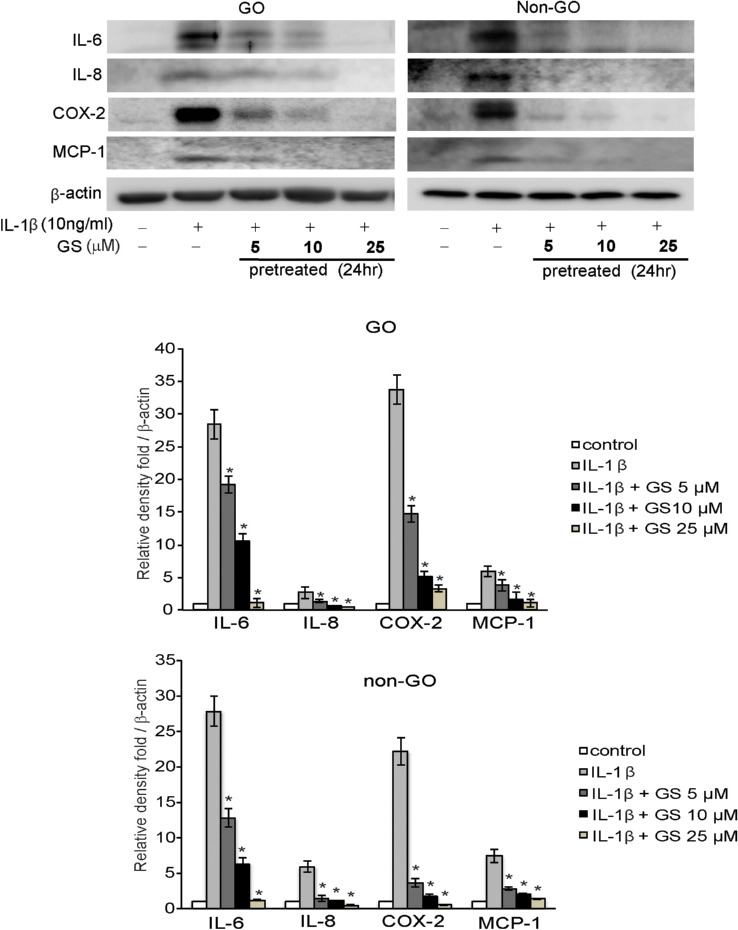
IL-1β stimulates cytokine and chemokine production in GO.18 In one study, when orbital fibroblasts were activated by IL-1β, extremely high levels of cytokines, including IL-6, were released and adipocyte differentiation was enhanced.14 Based on these findings, we examined cytokine production in response to IL-1β treatment. Prior to 24 hours pretreatment with GS (5, 10, and 25 µM), both GO and non-GO cells were cultured to near confluence. Thereafter, cells were exposed to IL-1β (10 ng/mL) for 16 hours, prior to analysis of mRNA cytokine expression, including IL-6, IL-8, MCP-1, and COX-2, using real-time PCR. IL-1β significantly increased the expression of all cytokines; however, 24 hours pretreatment with GS decreased this expression in both GO and non-GO cells in a dose-dependent manner (Fig. 2). Western blot analyses exhibited similar results, showing significant dose-dependent GS-mediated inhibition of the IL-1β-boosted levels of IL-6, IL-8, MCP-1, and COX-2 proteins, in both GO and non-GO cells (Fig. 3).
Figure 2.
Effect of GS on IL-1β-induced IL-6, IL-8, MCP-1, and COX-2 mRNA expression in GO and non-GO fibroblasts. Orbital fibroblasts obtained from both GO (n = 3) and non-GO (n = 3) subjects were pretreated with GS for 24 hours prior to stimulation with 10 ng/mL IL-1β for 16 hours. IL-6, IL-8, MCP-1, and COX-2 mRNA levels were evaluated using RT-PCR. Results are presented as the mean ± SD fold mRNA expression (relative to the control gene GAPDH) from three independent experiments using cells from different individuals (*P < 0.05 vs. IL-1β-stimulated cells without GS pretreatment).
Figure 3.
Effect of GS on IL-1β-induced expression of IL-6, IL-8, MCP-1, and COX-2 proteins in GO and non-GO fibroblasts. Confluent orbital fibroblasts from GO (n = 3) and non-GO (n = 3) subjects were untreated or pretreated with various doses (5–25 µM) of GS for 24 hours before stimulation with 10 ng/mL IL-1β for 16 hours. Protein expression of IL-6, IL-8, MCP-1, and COX-2 was evaluated by western blot analyses. Results are presented as the mean density ratio (/β-actin) ± SD of three experiments from different cell samples, and bands are representative figures (*P < 0.05 vs. IL-1β-treated cells without GS pretreatment).
Effect of GS on Adipogenesis of GO Fibroblasts
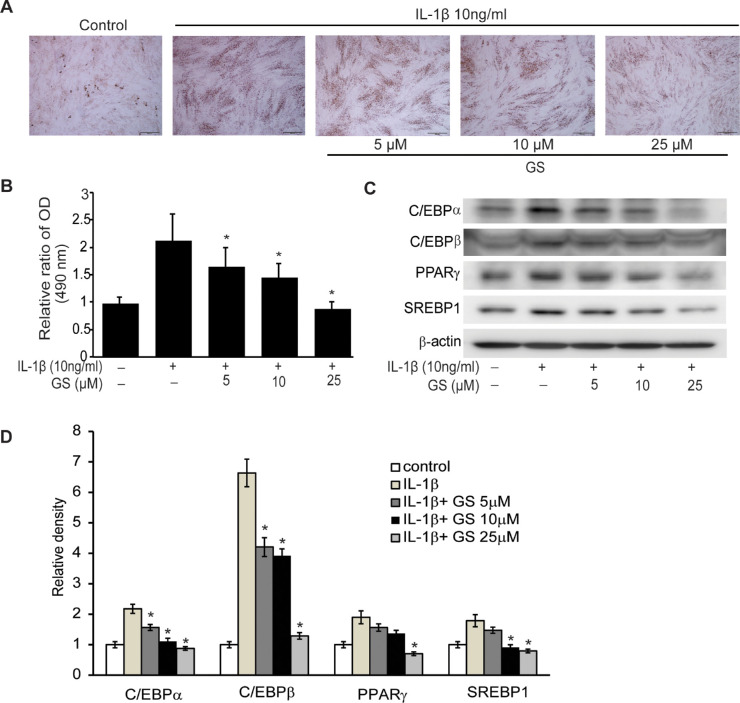
The primary cultured orbital fibroblasts from GO patients were incubated in adipogenic medium, with the addition of IL-1β, for 10 days to induce differentiation into adipocytes. Various concentrations of GS (5, 10, and 25 µM) were added at every time point of the media change during differentiation to assess the suppressive effect of GS on adipogenesis. The stellate shape of the orbital fibroblasts was lost, accompanied by reduced formation of intracellular lipid droplets. Microscopic examination post–Oil Red O staining revealed that GS dose-dependently reduced lipid accumulation. (Fig. 4A). This suggests that GS dose-dependently reduced lipid accumulation. Quantifying the Oil Red O–stained cell lysates identified the same pattern (Fig. 4B). Additionally, western blot analysis showed that GS suppressed the protein expression of adipogenic transcription factors, including PPARγ, C/EBPα, C/EBPβ, and SREBP-1 proteins (Fig. 4C).
Figure 4.
Effect of GS on adipocyte differentiation of GO fibroblasts. IL-1β (10 ng/mL) was added to the adipogenic medium to stimulate differentiation of fibroblasts into adipocytes, and various concentrations of GS (5–25 µM) were added during adipogenesis. (A) Cells were stained with Oil Red O and examined using a microscope at 40×. Adipocyte differentiation was prominently suppressed by increasing doses of GS. (B) Quantification of Oil Red O by measurements of optical density of cell lysates at 490 nm reflected the histochemical results. The results are presented as the mean optical density (%) ± SD of the three experiments using GO cells at 10 days of adipogenesis (*P < 0.05 vs. differentiated cells without GS treatment). (C) Protein expression of adipogenic transcriptional regulators, PPARγ, C/EBPα, C/EBPβ, and SREBP-1, was analyzed using western blotting at 10 days of differentiation. (D) The results of the quantification of the relative density of the western blot bands are shown. The data in the columns are the mean relative density ratio ± SD of three experiments, showing significant suppression of proteins by GS treatment in a dose-dependent manner (*P < 0.05 vs. IL-1β-treated cells without GS pretreatment).
Effect of GS on Hyaluronan Production
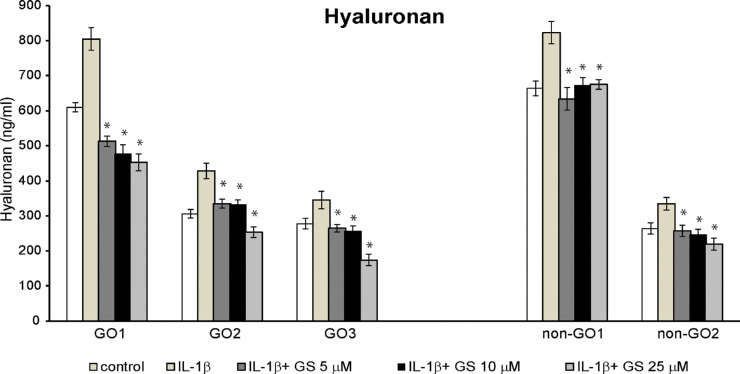
Hyaluronan accumulation in the connective tissue of the orbit leading to tissue remodeling is a distinctive histopathologic finding associated with GO.19 IL-1β can enhance the release of hyaluronan and the mRNA expression of three types of hyaluronan synthases (HAS 1-3).20 Here after pretreatment with 5 to 25 µM GS for 24 hours, the amount of released hyaluronan in the supernatant following IL-1β stimulation for 16 hours was measured using ELISA for both GO and non-GO cells. IL-1β-induced hyaluronan production was significantly suppressed by GS pretreatment in both cell types (Fig. 5, Supplementary Fig. S1).
Figure 5.
Effect of GS on IL-1β-induced hyaluronan production in GO and non-GO cells. Hyaluronan release (ng/mL) subsequent to treatment with diverse concentrations of GS (5–25 µM) for 24 hours before stimulation with 10 ng/mL IL-1β for 16 hours was measured in GO (n = 3) and non-GO (n = 2) cell samples using ELISA. GS significantly suppressed IL-1β-induced hyaluronan production in a dose-dependent manner in both GO and non-GO cells. Data in the columns represent the mean ± SD of duplicate measurement in cells from independent subjects (*P < 0.05 vs. IL-1β-stimulated cells without GS pretreatment).
Effect of GS on Phosphorylation of Signal Transduction Pathways in IL-1β-Simulated Orbital Fibroblasts
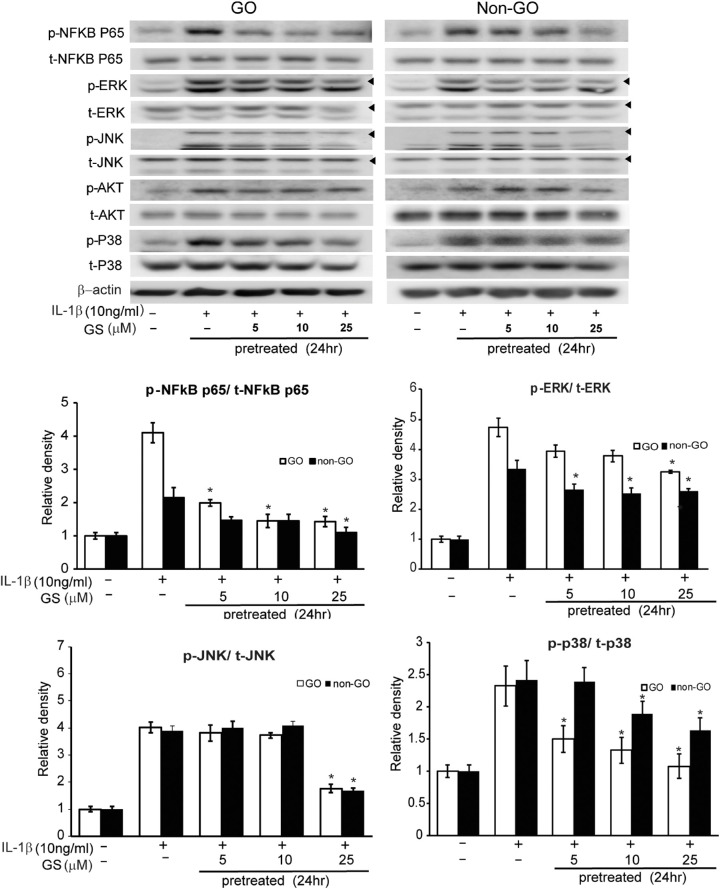
IL-1β elicited phosphorylation of not only NF-κB but also other multiple transcription factors, including ERK, JNK, AKT, and p38, in both GO and non-GO cells. Various concentrations of GS (5–25 µM) were used for 24 hour pretreatment before 1 hour IL-1β stimulation to explore the effect of GS on signaling pathways. Western blot analyses revealed the dose-dependent inhibitory effects of GS on the phosphorylation of NF-κB and mitogen-activated protein kinases (MAPK), including ERK, JNK, and p38, in cells from both GO and non-GO patients (Fig. 6).
Figure 6.
Effect of GS on intracellular signaling pathways in GO and non-GO cells. Cells from GO (n = 3, white column) and non-GO (n = 3, black column) were treated with various concentrations of GS (5–25 µM) for 24 hours prior to stimulation with 10 ng/mL IL-1β for 1 hour. GS cell lysates were analyzed by western blotting to evaluate phosphorylated NF-κB p65, ERK, JNK, Akt, and p38 protein expression. Treatment of GS significantly attenuated IL-1β-induced activation of p-NF-kB protein in both GO and non-GO cells in a dose-dependent manner. P-ERK, p-JNK, and p-p38 were significantly inhibited by GS in both GO and non-GO cells; however, p-Akt signaling was not altered by GS. The data in the columns are the mean relative density ratio (phosphorylated form/total form) ± SD of three experiments (*P < 0.05 vs. IL-1β stimulated cells without GS pretreatment).
Discussion
Gugulipid, an ethyl acetate extract of the guggul tree gum resin, has been used as a remedy for obesity, arthritis, and hyperlipidemia in Ayurvedic medicine for centuries.10 GS has aroused considerable interest as an active gugulipid component, exerting diverse pharmacologic reactions, including anti-inflammatory, hypolipidemic, antineoplastic, antioxidative, antidiabetic, and cardioprotective effects.9,21–26 In this study, we illustrate the significant ability of GS to suppress the elevated production of proinflammatory mediators in IL-1β-stimulated GO and non-GO fibroblasts. Additionally, GS attenuated adipogenesis and hyaluronan production. Adipocyte-specific transcription factors, including C/EBPα, C/EBPβ, PPARγ, and SREBP-1, were also downregulated by GS in a dose-dependent manner. Furthermore, GS inflicted suppressive effects on IL-1β-induced phosphorylation of NF-κB and MAP kinases, such as ERK, JNK, and p38, in both GO and non-GO cells. Our in vitro results indicate that GS may be a promising therapeutic drug as it significantly suppressed the main pathologic phenomena of GO.
The effectiveness of GS in attenuating proinflammatory responses has been proven in a variety of inflammation-related disorders, including arthritis, colitis, gastritis, inflammatory bowel disease, pancreatic disease, and uveitis.25,27–29 We demonstrated that GS blocked proinflammatory cytokine production. This included the production of IL-6, IL-8, MCP-1, and COX-2, which are important factors in the inflammatory events of GO. A stimulatory TSHR antibody and TSH increase the expression of IL-6 in orbital fibroblasts.30 TSHR+ and CD40+ fibrocytes in the peripheral blood of patients with GO express IL-8, a proinflammatory chemoattractant molecule.31 Macrophage infiltration and MCP-1 expression are higher in Graves’ orbital fat than in control fat, which indicates the important role of macrophages in the pathogenesis of GO via the overexpression of MCP-1.32 COX-2 is also overexpressed in patients with GO and is associated with GO disease activity.33 In addition, we discovered that GS blocked the phosphorylation of the NF-κB signaling pathway and MAPK signaling in IL-1β-stimulated orbital fibroblasts. GS seems to have a universal effect on inhibiting the activation of NF-κB regardless of cell type, as demonstrated by numerous studies on a variety of cell types, including most tumor cells, LX-2 cells (human hepatic stellate cell line), mouse inner medullary collecting duct-3 cells, RAW 264.7 cells (a murine macrophage-like cell line), and intestinal epithelial cells.34–38
Various studies have been conducted to clarify the relationship between GS and components of the MAPK signaling pathway, including ERK, JNK, and p38.35,37,39–41 Unlike NF-κB, MAPK signals, such as ERK, JNK, and p38, react to GS differently in various cell types. In one study, GS had no effect on the LPS-induced phosphorylation of MAP kinase family members in RAW 264.7 cells.37 However, GS suppressed ERK and JNK phosphorylation, but not p38, in other cell types, such as murine pancreatic cells.39 In our study, GS significantly suppressed the phosphorylation of ERK, JNK, and p38 in both GO and non-GO orbital fibroblasts. A previous study showed a correlation between AKT signals and GO pathogenesis42; moreover, a recent study by our group showed that AKT inhibitors suppressed inflammation and adipogenesis in GO orbital fibroblasts.43 However, GS did not affect AKT signaling in this study, in contrast to the results of another study using human monocytic leukemia cells.40
Numerous studies have exemplified the beneficial effects of GS as an anti-obesity compound. GS treatment in 3T3-L1 adipocytes impaired differentiation of derived mature adipocytes to both lipolysis and apoptosis.11 Recently, it was reported that GS treatment in 3T3-L1 adipocytes increased mitochondrial density and markers of the brown adipocyte phenotype, such as UCP1.12 GS is the only known farnesoid X receptor (FXR) antagonist, a bile acid receptor expressed in adult mouse white adipose tissue and differentiated 3T3-L1 cells.44 In a study by Rizzo et al.,44 a potent and selective FXR ligand increased 3T3-L1 differentiation, which was reversed by GS. In this study, GS remarkably attenuated adipogenesis and downregulated the transcription of the adipocyte-specific proteins, PPARγ, C/EBP, and SREBP-1. The underlying mechanism of its anti-adipogenic effect may be associated with inhibition of the transcriptional cascades or partial modulation of the MAPK signaling pathway. Nevertheless, considering the presentation of excess adipose tissue formation as the cause of disfiguring proptosis in GO, phytosterols such as GS can be promising agents to avoid disease progression.
GS has been extensively studied regarding its hypolipidemic actions. Rats treated with 25 mg/kg body weight of GS for 10 days exhibited an approximately 30% reduction in serum cholesterol and triglycerides and showed a significant increase in hepatic LDL binding.45 Interestingly, several recent epidemiologic reports assert high serum cholesterol as a risk factor for GO.46,47 In a cross-sectional study by Sabini et al.,46 an increased risk of GO was observed in relation to the presence of total or LDL-cholesterol serum concentrations, and clinical activity score was found to be higher (P = 0.02) in patients with high total cholesterol. It is not yet known whether lowering cholesterol ameliorates GO pathogenesis; however, we may consider GS, a hypolipidemic phenolic compound, as a suitable treatment in patients with a poor lipid profile.
Tissue expansion due to excess hyaluronan accumulation within the bony orbits is considered to be the cause of GO progression. Our results also demonstrate the ability of GS, to inhibit the synthesis of hyaluronan in both GO and non-GO cells. The observed inhibitory effect of GS on hyaluronan and the proinflammatory response is not related to nonspecific drug cytotoxicity. A previous report presented contradictory data that GS can act as a hyaluronidase and collagenase inhibitor.48 The mechanism involving hyaluronan inhibition in orbital fibroblasts was not accurately determined. However, the blockade of inflammatory reactions downstream of IL-1β appears to be the main route.
Gugulipid, the ethyl acetate extract of gum guggul, was not associated with adverse hepatic or renal side effects or hematological dysfunction when administered at a 400 mg per day dose for 4 weeks.8,21,49 With regard to GS, no data are currently available on its bioavailability, metabolism, and pharmacokinetics in either animal models or humans. Clinical studies to identify the appropriate concentrations of GS and the possible side effects that may arise in effective plasma concentrations are yet to be conducted. However, GS, a plant steroid with chemical resemblance to glucocorticoids, may potentially be devoid of conventional glucocorticoid side effects, such as adrenal insufficiency and hyperglycemia. The current recommended dosage for GS supplements is 500 to 1000 mg 2 to 3 times a day, as doses of 2000 mg 3 times a day are correlated with a greater risk of hypersensitivity skin reactions.50 The development of GS formulae that can increase the absorption rate while reducing possible adverse events is needed. Additionally, further multicenter trials involving a large number of patients are needed to systematically test the efficacy of GS in GO. Considering that GO is a localized disease confined to the orbit, development of GS injections in a depot form may be useful; thus further research using disease-specific models is imperative.
The possible influence of GS on thyroid stimulation should also be studied. Although hormonal status does not directly correlate with the progression of GO, maintenance of euthyroid is important in the treatment of GO, as GO is inevitably prone to being influenced by hormonal status. It is reported that the mechanism involved in the lipid-lowering effect of GS is related to its thyroid stimulating action, and that GS administration to rats enhanced thyroid function and iodine uptake.51 It is still unclear whether a similar increase in thyroid function occurs with GS in humans; thus this also necessitates further study to clarify the effect of GS on thyroid function.
Conclusions
To our knowledge, we are the first to report that GS suppresses inflammatory reactions, hyaluronan production, and adipogenesis in primary cultured GO cells. Considering the complexity of the underlying pathogenesis of GO and the lack of clear understanding, phytochemicals such as GS, with multiple targets, could be effective alternatives for the treatment of GO. Although further studies to establish the clinical efficacy, safety, and bioavailability are required, we provide in vitro background evidence to suggest GS as a notable candidate for GO treatment.
Supplementary Material
Acknowledgments
The authors thank Editage (www.editage.co.kr) for English language editing.
Supported by the Bio and Medical Technology Development Program of the National Research Foundation funded by the Ministry of Science and Information Communication Technology (2016M3A9E9941746). The authors alone are responsible for the content and writing of the article.
Disclosure: B.R. Kim, None; J. Kim, None; J.E. Lee, None; E.J. Lee, None; J.S. Yoon, None
References
- 1. Khoo TK, Bahn RS. Pathogenesis of Graves’ ophthalmopathy: the role of autoantibodies. Thyroid. 2007; 17: 1013–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brent GA. Clinical practice. Graves’ disease. N Engl J Med. 2008; 358: 2594–2605. [DOI] [PubMed] [Google Scholar]
- 3. Bartalena L, Pinchera A, Marcocci C. Management of Graves’ ophthalmopathy: reality and perspectives. Endocr Rev. 2000; 21: 168–199. [DOI] [PubMed] [Google Scholar]
- 4. Weissel M, Hauff W. Fatal liver failure after high-dose glucocorticoid pulse therapy in a patient with severe thyroid eye disease. Thyroid. 2000; 10: 521. [DOI] [PubMed] [Google Scholar]
- 5. Wu J, Xia C, Meier J, Li S, Hu X, Lala DS. The hypolipidemic natural product guggulsterone acts as an antagonist of the bile acid receptor. Mol Endocrinol. 2002; 16: 1590–1597. [DOI] [PubMed] [Google Scholar]
- 6. Burris TP, Montrose C, Houck KA, et al.. The hypolipidemic natural product guggulsterone is a promiscuous steroid receptor ligand. Mol Pharmacol. 2005; 67: 948–954. [DOI] [PubMed] [Google Scholar]
- 7. Cui J, Huang L, Zhao A, et al.. Guggulsterone is a farnesoid X receptor antagonist in coactivator association assays but acts to enhance transcription of bile salt export pump. J Biol Chem. 2003; 278: 10214–10220. [DOI] [PubMed] [Google Scholar]
- 8. Deng R. Therapeutic effects of guggul and its constituent guggulsterone: cardiovascular benefits. Cardiovasc Drug Rev. 2007; 25: 375–390. [DOI] [PubMed] [Google Scholar]
- 9. Wang X, Greilberger J, Ledinski G, Kager G, Paigen B, Jurgens G. The hypolipidemic natural product Commiphora mukul and its component guggulsterone inhibit oxidative modification of LDL. Atherosclerosis. 2004; 172: 239–246. [DOI] [PubMed] [Google Scholar]
- 10. Sinal CJ, Gonzalez FJ. Guggulsterone: an old approach to a new problem. Trends Endocrinol Metab. 2002; 13: 275–276. [DOI] [PubMed] [Google Scholar]
- 11. Yang JY, Della-Fera MA, Baile CA. Guggulsterone inhibits adipocyte differentiation and induces apoptosis in 3T3-L1 cells. Obesity (Silver Spring). 2008; 16: 16–22. [DOI] [PubMed] [Google Scholar]
- 12. Miller CN, Samuels JS, Azhar Y, Parmar A, Shashidharamurthy R, Rayalam S. Guggulsterone activates adipocyte beiging through direct effects on 3T3-L1 adipocytes and indirect effects mediated through RAW264.7 macrophages. Medicines (Basel). 2019; 6: E22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mourits MP, Koornneef L, Wiersinga WM, Prummel MF, Berghout A, van der Gaag R. Clinical criteria for the assessment of disease activity in Graves’ ophthalmopathy: a novel approach. Br J Ophthalmol. 1989; 73: 639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yoon JS, Lee HJ, Choi SH, Chang EJ, Lee SY, Lee EJ. Quercetin inhibits IL-1beta-induced inflammation, hyaluronan production and adipogenesis in orbital fibroblasts from Graves’ orbitopathy. PLoS One. 2011; 6: e26261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 16. Kim SE, Lee JH, Chae MK, Lee EJ, Yoon JS. The role of sphingosine-1-phosphate in adipogenesis of Graves’ orbitopathy. Invest Ophthalmol Vis Sci. 2016; 57: 301–311. [DOI] [PubMed] [Google Scholar]
- 17. Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975; 5: 19–27. [DOI] [PubMed] [Google Scholar]
- 18. Chen B, Tsui S, Smith TJ. IL-1β induces IL-6 expression in human orbital fibroblasts: identification of an anatomic-site specific phenotypic attribute relevant to thyroid-associated ophthalmopathy. J Immunol. 2005; 175: 1310–1319. [DOI] [PubMed] [Google Scholar]
- 19. Smith TJ, Hoa N. Immunoglobulins from patients with Graves’ disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-I receptor. J Clin Endocrinol Metab. 2004; 89: 5076–5080. [DOI] [PubMed] [Google Scholar]
- 20. van Zeijl CJ, Fliers E, van Koppen CJ, et al.. Effects of thyrotropin and thyrotropin-receptor-stimulating Graves’ disease immunoglobulin G on cyclic adenosine monophosphate and hyaluronan production in nondifferentiated orbital fibroblasts of Graves’ ophthalmopathy patients. Thyroid. 2010; 20: 535–544. [DOI] [PubMed] [Google Scholar]
- 21. Urizar NL, Moore DD. GUGULIPID: a natural cholesterol-lowering agent. Annu Rev Nutr. 2003; 23: 303–313. [DOI] [PubMed] [Google Scholar]
- 22. Samudio I, Konopleva M, Safe S, McQueen T, Andreeff M. Guggulsterones induce apoptosis and differentiation in acute myeloid leukemia: identification of isomer-specific antileukemic activities of the pregnadienedione structure. Mol Cancer Ther. 2005; 4: 1982–1992. [DOI] [PubMed] [Google Scholar]
- 23. Wang W-C, Uen Y-H, Chang M-L, et al.. Protective effect of guggulsterone against cardiomyocyte injury induced by doxorubicin in vitro. BMC Complement Altern Med. 2012; 12: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chander R, Rizvi F, Khanna A, Pratap R. Cardioprotective activity of synthetic guggulsterone (E and Z-isomers) in isoproterenol induced myocardial ischemia in rats: a comparative study. Indian J Clin Biochem. 2003; 18: 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mencarelli A, Renga B, Palladino G, Distrutti E, Fiorucci S. The plant sterol guggulsterone attenuates inflammation and immune dysfunction in murine models of inflammatory bowel disease. Biochem Pharmacol. 2009; 78: 1214–1223. [DOI] [PubMed] [Google Scholar]
- 26. Sharma B, Salunke R, Srivastava S, Majumder C, Roy P. Effects of guggulsterone isolated from Commiphora mukul in high fat diet induced diabetic rats. Food Chem Toxicol. 2009; 47: 2631–2639. [DOI] [PubMed] [Google Scholar]
- 27. Xiao D, Singh SV.. z-Guggulsterone, a constituent of Ayurvedic medicinal plant Commiphora mukul, inhibits angiogenesis in vitro and in vivo. Mole Cancer Ther. 2008; 7: 171–180. [DOI] [PubMed] [Google Scholar]
- 28. Kalariya NM, Shoeb M, Reddy AB, Zhang M, van Kuijk FJ, Ramana KV. Plant sterol guggulsterone prevents endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2010; 51: 5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim JM, Kang HW, Cha MY, et al.. Novel guggulsterone derivative GG-52 inhibits NF-κB signaling in intestinal epithelial cells and attenuates acute murine colitis. Lab Invest. 2010; 90: 1004. [DOI] [PubMed] [Google Scholar]
- 30. Kumar S, Schiefer R, Coenen MJ, Bahn RS. A stimulatory thyrotropin receptor antibody (M22) and thyrotropin increase interleukin-6 expression and secretion in Graves’ orbital preadipocyte fibroblasts. Thyroid. 2010; 20: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Douglas RS, Mester T, Ginter A, Kim DS. Thyrotropin receptor and CD40 mediate interleukin-8 expression in fibrocytes: implications for thyroid-associated ophthalmopathy (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2014; 112: 26–37. [PMC free article] [PubMed] [Google Scholar]
- 32. Chen MH, Chen MH, Liao SL, Chang TC, Chuang LM. Role of macrophage infiltration in the orbital fat of patients with Graves’ ophthalmopathy. Clin Endocrinol (Oxf). 2008; 69: 332–337. [DOI] [PubMed] [Google Scholar]
- 33. Vondrichova T, de Capretz A, Parikh H, et al.. COX-2 and SCD, markers of inflammation and adipogenesis, are related to disease activity in Graves’ ophthalmopathy. Thyroid. 2007; 17: 511–517. [DOI] [PubMed] [Google Scholar]
- 34. Shishodia S, Aggarwal BB. Guggulsterone inhibits NF-kappaB and IkappaBalpha kinase activation, suppresses expression of anti-apoptotic gene products, and enhances apoptosis. J Biol Chem. 2004; 279: 47148–47158. [DOI] [PubMed] [Google Scholar]
- 35. Kim BH, Yoon JH, Yang JI, et al.. Guggulsterone attenuates activation and survival of hepatic stellate cell by inhibiting nuclear factor kappa B activation and inducing apoptosis. J Gastroenterol Hepatol. 2013; 28: 1859–1868. [DOI] [PubMed] [Google Scholar]
- 36. Kim D-G, Bae G-S, Jo I-J, et al.. Guggulsterone attenuated lipopolysaccharide-induced inflammatory responses in mouse inner medullary collecting duct-3 cells. Inflammation. 2016; 39: 87–95. [DOI] [PubMed] [Google Scholar]
- 37. Zhang J-H, Shangguan Z-S, Chen C, Zhang H-J, Lin Y. Anti-inflammatory effects of guggulsterone on murine macrophage by inhibiting LPS-induced inflammatory cytokines in NF-κB signaling pathway. Drug Des Devel Ther. 2016; 10: 1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cheon JH, Kim JS, Kim JM, Kim N, Jung HC, Song IS. Plant sterol guggulsterone inhibits nuclear factor-kappaB signaling in intestinal epithelial cells by blocking IkappaB kinase and ameliorates acute murine colitis. Inflamm Bowel Dis. 2006; 12: 1152–1161. [DOI] [PubMed] [Google Scholar]
- 39. Kim DG, Bae GS, Choi SB, et al.. Guggulsterone attenuates cerulein-induced acute pancreatitis via inhibition of ERK and JNK activation. Int Immunopharmacol. 2015; 26: 194–202. [DOI] [PubMed] [Google Scholar]
- 40. Shishodia S, Sethi G, Ahn KS, Aggarwal BB. Guggulsterone inhibits tumor cell proliferation, induces S-phase arrest, and promotes apoptosis through activation of c-Jun N-terminal kinase, suppression of Akt pathway, and downregulation of antiapoptotic gene products. Biochem Pharmacol. 2007; 74: 118–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gebhard C, Stämpfli SF, Gebhard CE, et al.. Guggulsterone, an anti-inflammatory phytosterol, inhibits tissue factor and arterial thrombosis. Basic Res Cardiol. 2009; 104: 285–294. [DOI] [PubMed] [Google Scholar]
- 42. Iyer S, Bahn R. Immunopathogenesis of Graves’ ophthalmopathy: the role of the TSH receptor. Best Pract Res Clin Endocrinol Metab. 2012; 26: 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ko J, Kim J-Y, Lee EJ, Yoon JS. Inhibitory effect of idelalisib, a selective phosphatidylinositol 3-kinase δ inhibitor, on adipogenesis in an in vitro model of Graves’ orbitopathy. Invest Ophthalmol Vis Sci. 2018; 59: 4477–4485. [DOI] [PubMed] [Google Scholar]
- 44. Rizzo G, Disante M, Mencarelli A, et al.. The farnesoid X receptor promotes adipocyte differentiation and regulates adipose cell function in vivo. Mol Pharmacol. 2006; 70: 1164–1173. [DOI] [PubMed] [Google Scholar]
- 45. Singh V, Kaul S, Chander R, Kapoor N. Stimulation of low density lipoprotein receptor activity in liver membrane of guggulsterone treated rats. Pharmacol Res. 1990; 22: 37–44. [DOI] [PubMed] [Google Scholar]
- 46. Sabini E, Mazzi B, Profilo MA, et al.. High serum cholesterol is a novel risk factor for graves' orbitopathy: results of a cross-sectional study. Thyroid. 2018; 28: 386–394. [DOI] [PubMed] [Google Scholar]
- 47. Lanzolla G, Sabini E, Profilo M, et al.. Relationship between serum cholesterol and Graves’ orbitopathy (GO): a confirmatory study. J Endocrinol Invest. 2018; 41: 1417–1423. [DOI] [PubMed] [Google Scholar]
- 48. Sumantran VN, Kulkarni AA, Harsulkar A, et al.. Hyaluronidase and collagenase inhibitory activities of the herbal formulation triphala guggulu. J Biosci. 2007; 32: 755–761. [DOI] [PubMed] [Google Scholar]
- 49. Agarwal R, Singh S, Saran R, et al.. Clinical trial of gugulipid–a new hypolipidemic agent of plant origin in primary hyperlipidemia. Indian J Med Res. 1986; 84: 626. [PubMed] [Google Scholar]
- 50. Szapary PO, Wolfe ML, Bloedon LT, et al.. Guggulipid for the treatment of hypercholesterolemia: a randomized controlled trial. JAMA. 2003; 290: 765–772. [DOI] [PubMed] [Google Scholar]
- 51. Tripathi YB, Malhotra O, Tripathi S. Thyroid stimulating action of Z-guggulsterone obtained from Commiphora mukul. Planta Med. 1984; 50: 78–80. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.