Abstract
Simple Summary
Bovine milk contains proteins, minerals (e.g., calcium), vitamins (A, B, D, E and K), bioactive compounds and signaling molecules, as short non-coding RNA, either floating or contained in exosomes. Milk compositions and the milk-derived exosomes’ (EXO) cargo varies in relation to the health status of the mammary gland and the lactation stage. Stress, immune-depression and mastitis generate a differential microRNA (miRNA) expression, and consequently a modulation of the local immune cell response. This review aims to discuss the possible application of EXO cargo as a biomarker of animal resilience, and as a phenotype for genomic studies.
Abstract
Recent advances in ruminants’ milk-derived exosomes (EXO) have indicated a role of microRNAs (miRNAs) in cell-to-cell communication in dairy ruminants. The miRNAs EXO retain peculiar mechanisms of uptake from recipient cells, which enables the selective delivery of cargos, with a specific regulation of target genes. Although many studies have been published on the miRNAs contained in milk, less information is available on the role of miRNAs EXO, which are considered stable over time and resistant to digestion and milk processing. Several miRNAs EXO have been implicated in the cellular signaling pathway, as in the regulation of immune response. Moreover, they exert epigenetic control, as extenuating the expression of DNA methyltransferase 1. However, the study of miRNAs EXO is still challenging due to the difficulty of isolating EXO. In fact, there are not agreed protocols, and different methods, often time-consuming, are used, making it difficult to routinely process a large number of samples. The regulation of cell functions in mammary glands by miRNAs EXO, and their applications as genomic markers in livestock, is presented.
Keywords: bovine milk, exosomes, miRNA, ruminants, genome, mastitis
1. Introduction
Prokaryote and eukaryote cells release extracellular vesicles (EVs) as part of their normal physiology, and as a result of acquired abnormalities [1]. The cells of multicellular organisms use different ways to communicate, including EVs, which play a unique role [2]. A particularly interesting subtype of EVs is exosomes, the research into which is rapidly growing.
Exosomes are membrane-bound vesicles with a size range of about 40 to 160 nm in diameter (average about 100 nm), and are round or cup-shaped when observed under transmission electron microscope [1]. Indeed, exosomes are generated in a process that involves double invagination of the plasma membrane. The inward budding of late endosomes produces multivesicular bodies (MVBs), containing intraluminal vesicles that can fuse with the cell membrane and are released into the extracellular environment. Once released into extracellular space, these nanovesicles are termed exosomes [3,4]. Exosome secretion requires the sequential assembly of four endosomal sorting complex required for transport (ESCRT) complexes on the endosomal membrane.
A clear classification of EVs is difficult [5,6], but exosomes have some distinctive features, such as enzymatic and transport differences. These features distinguish exosomes in milk, here referred to as EXO, from milk fat globule membranes, which also have a plasma membrane origin [7].
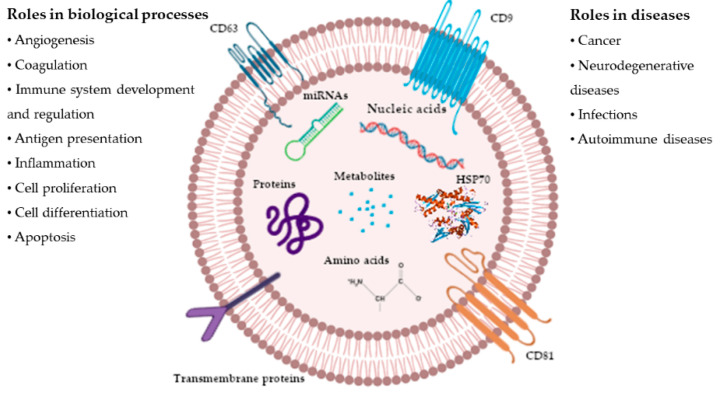
The final content of exosomes is produced by subsequent interactions with other intracellular vesicles and organelles, contributing to their diversity. Depending on the cellular origin, exosomes can contain many cell constituents, including nucleic acids, such as messanger RNA (mRNA), microRNAs (miRNAs), ribosomal RNA (rRNA), long noncoding RNA (lncRNA), transfer RNA (tRNA), as well as, variably, DNA, lipids, metabolites and proteins (Figure 1). The differences in exosome contents and their involvements in different biological processes represent a fingerprint of the donor cell [8,9]. Then, the different contents of exosomes can be delivered to a recipient cell, whose biological response can be effectively altered [1]. In addition, exosomes secreted by cells are found in various biological fluids such as blood, plasma, tears, semen, saliva, urine, cerebrospinal fluid, epididymal fluid, amniotic fluid, tumor ascites, bronchoalveolar lavage fluid, synovial fluid and milk [2,10,11]. As result, exosomes are used as biomarkers for the diagnosis of various diseases [1,12,13].
Figure 1.
Simplified representation of the roles and contents of exosomes. Exosomes are extracellular nanovesicles generated by all cells, and they carry nucleic acids, proteins, lipids and metabolites. They are mediators of near- and long-distance intercellular communication in health and disease (modified from [1]).
Exosomes, through their cargos, play significant roles in many biological processes, such as angiogenesis, coagulation, cell proliferation and differentiation, apoptosis, antigen presentation and inflammation [10]. Moreover, exosome cargos influence physiological and pathological processes in various diseases, including cancer, neurodegenerative diseases, infections and autoimmune diseases. Concerning the immune system, exosome cargos allow the exchange of antigens, or major histocompatibility complex–peptide complexes, between antigen-bearing cells and antigen-presenting cells [3]. Exosomes can also induce tolerance against allergens, the inhibition and activation of natural killer cells, and the differentiation, proliferation and apoptosis of T regulatory cells [8]. In cancer, they may contain molecules leading to adverse effects on recipient cells. In fact, exosomes derived from tumor cells have been associated with accelerated tumor growth [14,15] and invasiveness [16]. Indeed, exosomes containing tumor-specific RNAs and proteins are used as biomarkers for cancer diagnosis [10] and, by proteomic analysis, they indicate whether the cell of origin was epithelial-like or mesenchymal-like [1,17].
Exosomes can contain higher concentrations of miRNAs compared to the origin cells, indicative of selective wrapping [18]. miRNAs are a class of single-stranded noncoding RNAs about 22 nucleotides long, which induce post-transcriptional gene silencing by binding the 3′UTR (untranslated region) of target genes. They are generated in the cell cytoplasm, by the ribonuclease III Dicer, from nuclear precursor transcripts. Subsequently, they cause degradation mediated by proteins of the Argonaute family (Ago), or the translational repression of totally or partially complementary mRNAs [19]. Short sequence motifs of miRNAs controlling their loading into exosomes have been described [20]. These short non-coding RNAs can regulate up to 60% of gene expression at the post-transcriptional level [21,22]. Thereby, they regulate gene expression, and the usual consequence is the downregulation of protein expression [23]. In this way, miRNAs control a wide range of cellular functions, such as cell differentiation, organogenesis [24], proliferation and cell death, and regulate several aspects of health and diseases [25,26], fat storage, hematopoiesis and immunity [27]. Authors have reported that miRNAs constitute the majority of the RNA contained in these vesicles [2,28,29]. However, the stoichiometry analysis of miRNAs in exosomes, performed by Chevillet et al. [30], indicated that the copy numbers of these molecules are very low, and a reconsideration of the mechanism of exosome-mediated miRNAs communication deserves further investigation.
Milk contains many compounds [31,32,33], and is also the primary source of nutrition for newborn mammals; in fact, it is the only food that ensures infant development and health in short-time and long-time ways [34]. Breastfeeding is associated with significant health benefits, including protection against infection, obesity, diabetes and cancer [35,36]. Moreover, breast milk plays an important role in the development of a child’s immune system [37], and helps the infant’s intestine to develop, which becomes able to absorb milk and is prepared for weaning [38]. Milk from cattle [39,40], goats [41], humans [42] and rodents [43] also includes different biologically active EXO that explicate their functions in a manner dependent on their miRNA contents. Interestingly, the most abundant miRNAs EXO are found among humans, swine, cows and pandas [44]. Golan-Gerstl et al. [41] claim that 95% of the miRNAs expressed in human milk is also expressed in bovine and goat milk, and that bovine milk pasteurization does not seem to destroy miRNAs. Moreover, 24 validated targets were shared among conserved miRNAs, and these targets were involved in immunomodulatory functions [44]. miRNAs EXO can be transferred from mother to infant because they are packed into EXO, which protects them from degradation and from the infant gastrointestinal environment [45,46]; in fact, miRNAs EXO are stable in very acidic conditions (pH 1) [47]. Indeed, miRNAs EXO can cross the intestinal barrier, and are able to modify the gene expression of recipient cells [48,49]. EXO facilitate the absorption of miRNAs into the intestine through endocytosis, influencing the immune system, as the intestine is one of the major immune organs [38]. The incorporation of cow and goat milk EXO into human colon cancer cells [50], intestinal cells [40], kidney cells and peripheral blood mononuclear cells [41] has been reported. Hence, EXO make miRNAs stable, bioaccessible, bioavailable and bioactive [27].
Several studies have shown that over 60% of miRNAs EXO are related to immune regulation [51]. Human breast milk is rich in T cell-regulating miRNAs [52], and in miR-181 and miR-155, which induce B-cell differentiation [53]. This can explain why infants that are fed on breast milk, compared to infants fed on infant formula, have a reduced incidence of digestive problems, and can be more protected against gastrointestinal and respiratory infections [38].
Similar to the case with milk, bovine colostrum is rich in the common and most abundant proteins (caseins, β-lactoglobulin and α-lactalbumin), but also in monocyte differentiation antigen CD14 (CD14), glycosylation-dependent cell adhesion molecule 1 (GLYCAM1), xanthine dehydrogenase/oxidase (XDH/XO), lactadherin (MFGE8) and clusterin (CLU) [54]. Some of these proteins are implied in the modulation of the immune response of the offspring. Furthermore, colostrum contains large amounts of EXO enriched in proteins and in miRNAs (e.g., miR-223) responsible for immunity regulation [55,56]. It is likely that the miRNAs EXO of colostrum, and those delivered to the calf with milk during weaning, play an essential role in organism development and immune response [57]. Whether this will influence animal wellbeing later during maturity deserves further investigation.
Because EXO show very intriguing aspects, their isolation and characterization deserves particular attention, since agreed protocols are lacking and different methods, often time-consuming, are used, limiting the possibility of applying them to a large number of samples.
2. Isolation Methodologies
Isolation of exosomes is the first challenging step to face in biomedical research, or in any other application field. Since the exosomes have very small dimensions, their isolation turns out to be a difficult process. The choice of the most appropriate method depends on the chemical and physical properties of the biological matrix, which can be an obstacle to the extraction of the nanovesicles. Bovine milk contains caseins, and therefore usual techniques for isolating EXO must be modified accordingly (Table 1).
Table 1.
Types of isolation technique used for milk exosomes (EXO).
| Basic Technique | Method |
|---|---|
| Ultracentrifugation | Differential centrifugation |
| Density gradient ultracentrifugation | |
| Isoelectric Precipitation | |
| Microfluidics | Size exclusion (also magnetics activated) |
| Immunological method | Enzyme-linked Immunosorbent Assay (ELISA) |
2.1. Ultracentrifugation
Ultracentrifugation (UC) is the conventional and most utilized method, due to its applicability in pelleting lipoproteins, extravesicular protein complexes, aggregates and other contaminants, but it is not suitable for EXO isolation from clinical samples, because it is time-consuming, labor-intensive, requires costly instrumentations and includes multiple centrifugation steps [58].
According to Li et al. [59], two types of UC methods exist: analytical and preparative. The first one is used to understand the physicochemical properties of materials and the molecular interactions of polymeric materials, while the latter aims to fractionate biological elements (microorganisms, viruses, organelles and EVs). In particular, differential UC and density gradient UC are the two preparative election techniques for isolating EXO from milk. The second one gives the purest EXO population when compared to the other type of UC, according to Gurunathan et al. [60]. This method was also supported by Hata et al. [61], who obtained, through sucrose density-gradient ultracentrifugation, a suitable recovery of microvesicles from bovine milk to proceed with RNA isolation.
Yamada et al. [62] stated that density gradient UC is the most appropriate method for isolating high-quality EXO with intact morphology. Therefore, this method can be used to examine the relation of the exosomal proteins to the physiological or pathological status of the host, without any involvement or contamination of the other free proteins in milk. On the contrary, Vaswani et al. [63] obtained a population of nanovesicles from fresh bovine milk through differential UC, which they then enriched through size exclusion chromatography (SEC), or filtered through a 0.22-µm membrane [48], before the execution of the downstream application.
2.2. Isoelectric Precipitation
Milk contains many proteins (β-lactoglobulin, serum albumin, casein, etc.) that hinder EXO isolation and purification. In particular, the large quantity of caseins, which are the major milk proteins, accounting for more than 80% of total bovine milk proteins (in contrast to 35% of human breast milk proteins [64]), hinders the direct use of conventional methods for the isolation of bovine milk EXO. As such, some scientists contrived a specific method for removing caseins through isoelectric precipitation (IP). Yamauchi et al. [65] reported that the acidification of defatted bovine milk to pH 4.6, by adding HCl 6N, facilitates the precipitation of proteins, and improves the following steps of EXO isolation. Rahman et al. [66] compared two different acidification protocols by the addition of HCl or acetic acid to defatted milk. It was shown that neither acid condition alters EXO cargo, but both produce a rough surface and partial degradation of the EXO membrane proteins. Even Somiya et al. [67], before acidifying purchased defatted milk by adding acetic acid 1N, pre-warmed it, and then ultrafiltered the sample. However, even there is still no univocal protocol for isolating the EXO from milk, acidification seems to be the most suitable step for removing caseins (Table 2). To improve the isolation of EXO from raw milk, recently, Li et al. [68] suggested freezing the purified whey at −80 °C prior to the ultracentrifugation, in order to eliminate cell debris and apoptotic bodies, which can hinder the purity of the final pellet. The protocol that we have used for EXO isolation is reported in Table 3. This method allows us to obtain a satisfactory yield of EXO with high purity (Figure 2 and Figure 3).
Table 2.
Comparison of main steps for exosome isolation from milk with ultracentrifugation, with or without isoelectric precipitation.
| Steps | [67] | [65] | [66] | ||||
|---|---|---|---|---|---|---|---|
| AA/UC Method | C/UC Method | UC Method | IP Method | AA Method | UC Method | IP Method | |
| Centrifugation to defat milk | X 1 | - | 2000 g at 4 °C, 20 min | 2000 g at 4 °C, 20 min | 2000 g at 4 °C, 20 min | 2000 g at 4 °C, 20 min | |
| Distilled water addition | - | - | - | 1:1 | - | - | 1:1 |
| Warming | 10 min at 37 °C | - | - | 10 min at 37 °C | 10 min at 37 °C | - | 10 min at 37 °C |
| Casein precipitation | |||||||
| Acidification | Milk/Acetic acid (1:1), 5 min RT | - | - | HCl 6N to adjust pH 4.6 | Milk/Acetic acid (1:1), 5 min RT | - | HCl 6N to adjust pH 4.6 |
| Centrifugation | 10,000 g at 4 °C, 10 min | - | - | 5000 g RT, 20 min | 5000 g RT, 20 min | - | 5100 g RT, 20 min |
| Filtration | 0.22 μm | - | - | 1.0, 0.45, 0.2 μm | 1.0, 0.45, 0.2 μm | - | 1.0, 0.45, 0.2 μm |
| Ultracentrifugation | 210,000 g at 4 °C, 70 min | 13,000 g at 4 °C, 30 min; | 12,000 g at 4 °C, 60 min | - | - | 12,000 g at 4 °C, 60 min; | - |
| 100,000 g at 4 °C, 60min; | 35,000 g at 4 °C, 60 min | 35,000 g at 4 °C, 60 min; | |||||
| 130,000 g at 4 °C, 60 min | 70,000 g at 4 °C, 3 h | 75,000 g at 4 °C, 3 h | |||||
| Filtration | - | 1.0, 0.45, 0.2 μm | - | - | 1.0, 0.45, 0.2 μm | - | |
| Pellet resuspension with Phosphate-Buffered Saline (PBS) and ultracentrifugation | 210,000 g at 4 °C, 70 min | ||||||
X 1 Defatted purchased milk; AA/UC = Acetic acid/ultracentrifugation; C/UC = Centrifugation/ultracentrifugation; UC = Ultracentrifugation; IP = Isoelectric precipitation; AA = Acetic acid; RT = room temperature.
Table 3.
Proposed method for isolating exosomes from raw milk.
| Steps | IP Modified Method |
|---|---|
| Centrifugation to defat milk | 2000 g at 4 °C, 10 min |
| Centrifugation to remove cells and cell debris | 12,000 g at 4 °C, 40 min |
| Distilled water addition | 1:1 |
| Warming | 37 °C, 10 min |
| Casein precipitation: | |
| Acidification | HCl 6N to adjust pH 4.6 |
| Centrifugation | 5000 g RT, 20 min |
| Freezing | −80 °C, overnight |
| Filtration | 1.0, 0.45, 0.2 μm |
| Ultracentrifugation | 100,000 g at 4 °C, 1 h |
| Pellet resuspension | 0.1M PBS pH 7.4 |
Figure 2.
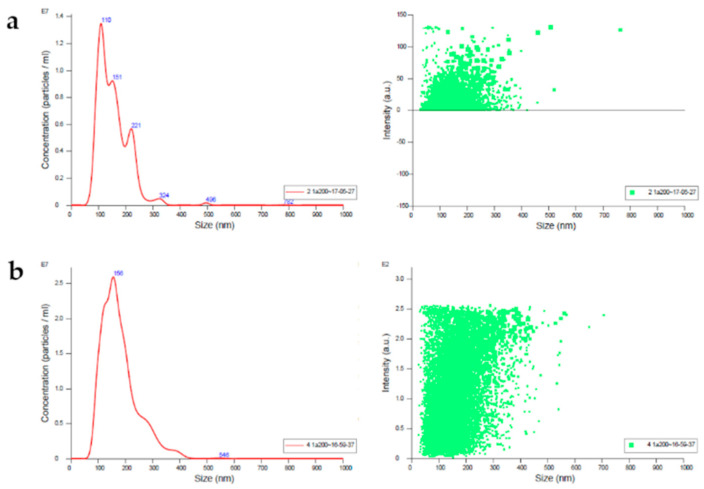
Quantification and size distribution of isoelectric precipitated and isolated milk exosomes, recorded through Nanosight (Malvern Panalytical, Malvern, UK) under light scatter mode. Red line indicates the signals recorded and depicts the extracellular vesicles (EVs) distribution in the sample, and the green spots indicate the intensity of the signals in an arbitrary unit (a.u.). (a) The sample has a concentration of 3.32 × 109 particles/mL and denotes different EVs population, with peaks at 110, 151, 221, 324, 496 and 782 nm. The mean size is 178.4 nm and the mode is 155.4 nm; (b) The sample has a concentration of 1.34 × 109 particles/mL and denotes only an EVs population with a peak at 156 nm, and with an irrelevant peak at 546 nm. The mean size is 154.2 nm and the mode is 109.3 nm.
Figure 3.
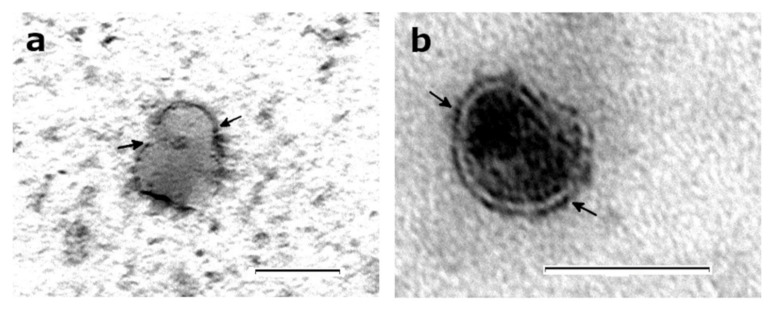
Exosomes were isolated from bovine milk according to the proposed method, and stained with antibodies (a) CD63 and (b) HSC70, coupled with a 10-nm gold particle. Images were taken using magnification 25,000× [(a), scale bar 200 nm] and 92,000× [(b), scale bar 100 nm].
Care must be taken in isolating milk EXO, but also in the downstream applications. Usually, the pellet has to be suspended in a buffer (e.g., Phosphate-Buffered Saline (PBS)0.1M) when EXO characterization is performed, but if miRNAs-derived EXO isolation is required, resuspension in a lysis buffer is necessary [69].
2.3. Immuno-Affinity Purification
Exosomes contain, and expose on their surface, a lot of characteristic proteins. Immunoaffinity techniques exploit the interaction between exosomal proteins (antigen) and their antibodies [68] to obtain a final pure concentration. Exosomal samples derived from different biofluids (e.g., urine, saliva, milk) contain a mix of other biological components and proteins, which increases the difficulty of obtaining a pure population of EXO. To overcome this issue, IP techniques take advantage of the exosome-specific antibodies, such as anti-CD9, anti-CD63 and anti-CD81.
Furthermore, magnetic beads, covalently coated with streptavidin, allow a more stable bond between antigens and any biotinylated capture antibody. In a comparison study between different methods, starting from cell culture media, Greening et al. [70] determined that the immuno-affinity magnetic technique, enriched for EXO and exosome-associated proteins, is at least twofold more efficient than the other methods used. Immuno-affinity methods with magnetic beads seem to be even more efficient, increasing capture and purity of the EXO from human serum samples, as reported in Yoo et al. [71].
2.4. Microfluidics-Based Isolation Techniques
In addition to the usual isolation methods, new techniques have been developed to deal with the problems of time-consuming steps, expensive lab machinery and high purity final pellets. These systems are based on the common separation determinants (size, density, immuno-affinity), but with the addition of innovative sorting mechanisms, e.g. acoustic, electrophoretic and electromagnetic manipulation, or viscoelastic flow. According to their procedures, these techniques can be divided into two categories: trapping by immune-affinity, sieving (e.g., nanoporous membranes), and trapping EXO on porous structures (e.g., nanowire-on-micropillars) [72].
Chen et al. [39] developed the first immune affinity microfluidic device for the capture of EXO, based on the bond of CD63, from serum samples. Kanwar et al. [73] obtained a pure population of EXO by using an Exo-chip platform, which also allowed EXO quantification by the fluorescent assay method. These methods are quicker than ultracentrifugation, and require low volumes of samples and reagents.
Im et al. [74] designed an on-chip nano plasmonic EXO sensor (nPLEX), in which surface plasmon resonance (SPR, a technique based on the oscillations of electrons at an interface stimulated by incident light) is used through nanohole arrays patterned on a metal film.
A collection of EXO was obtained by directly sieving whole blood through a membrane as a size-exclusion filter, but this method offered a low recovery of EVs [75].
Regarding the trapping of EXO on porous structures, Wang et al. [76] devised a ciliated micropillar plate that formed a microporous silicon nano-wire, which was able to selectively trap particles in the range of 40–100 nm, facilitating the selective collection of intact phospholipidic “EXO-like” vesicles. Unfortunately, the device was not validated with clinical samples, and no analysis of cargo protein or RNA was done.
3. Characterization of EXO
All body fluids produced by animal organisms contain EVs coming from different tissues. When analyzing exosomes isolated from body fluids, characterization is a crucial phase in discriminating exosomes from other families of EVs. An accurate method of characterization is necessary to clearly understand the roles of exosomes and other EVs in physiology and pathology [77]. So far, there is no gold standard technique for characterizing exosomes via all their physical and bio-molecular properties. The choice of the characterization protocol relies on sample type, isolation method and the application of isolated exososmes. In the case of bovines, different exosomes-containing body fluids have been deeply analyzed, including colostrum and milk [78,79,80,81,82], serum and plasma [78,83,84,85,86,87,88], urine [78,89], and saliva [78].Through comparative studies of different bovine biological fluids, a recent study reported that plasma and milk collected from cows have a significantly higher abundance of EXO, in comparison to their urine and saliva [78].
3.1. Size Characterization of EXO
One method for characterizing and discriminating EXO from EVs is flow cytometry (FC) [90,91]. In FC, a cell/vesicle suspension is forced through a laser beam, which scatters when it is crossed by a cell/vesicle, producing a fluorescent signal detected by a series of detectors. Despite the fact that this method is widely preferred, conventional FC protocols are challenged by the small size of EXO, due to the detection limit of flow cytometers, which is around 100 nm. This has resulted in the implementation of EXO-specific protocols, as described in several papers [91,92,93,94].
Nanoparticle tracking analysis (NTA) identifies, by illuminating with a laser beam, EVs in suspension, and detects their position with a dark-field microscope [95]. Although particles smaller than 200 nm are below the canonical light microscopy resolution limit, laser scattered light produced by suspended vesicles can be seen by the image sensor of a camera, allowing visualization of the vesicle’s position [96]. Since vesicles in suspension are characterized by Brownian motion, NTA tracks their movement in real time, and measures their mean velocity, which, being dependent on their size, can produce an absolute calculation of their diameter [97]. Most NTA instruments are now equipped with fluorescence detection, coupling NTA, with the possibility of phenotyping vesicles and EXO that react with fluorescently labeled antibodies [77], with a lower risk of artefacts [98]. NTA is greatly applicable in the rapid assessment of EXO size, refractive index and concentration [96]. NTA detects EXO in the 50–250 nm range (Figure 2), which overcomes the detection limit of FC [77,96,99].
3.2. Protein- and Microscopy-Based Characterization
Marker-based EXO characterization is an experimental procedure based on the detection of EXO-specific proteins through Western blotting and/or electron microscopy-coupled immunoreaction. The protein characterization of EVs from different origins is analyzed by sodium dodecyl sulphate—polyacrylamide gel electrophoresis (SDS-PAGE) separation, followed by protein staining, immunoblotting or proteomic analysis [100]. Immunogold labeling in transmission electron microscopy (TEM) characterizes the biochemical properties of EXO surface proteins (Figure 3). All steps are carried out on EXO directly bound to formvar carbon-coated nickel grids [90,101].
EXO biomarkers are listed on www.exocarta.org [12] according to how many times they have been identified. CD9 is the most identified tetraspanin in EXO. It is involved in cell motility, adhesion and fusion [102], and its exosomal enrichment has been observed in EXO [103,104].The tetraspanin CD63 is also one of the most used markers, since it is particularly enriched in the intraluminal membrane of multivesicular bodies [105]. The detection of CD63 by Western blotting and immunogold has been extensively used in bovine EXO characterization [65,101,103]. Further, the tetraspanin CD81 is a selective exosomal biomarker in the milk of woman and cows [106]. The heat shock cognate 70-kDa protein (HSC70) and heat shock 60-kDa protein (HSP60) are molecular chaperones whose enrichment has been observed in the EXO generated by multiple tissues. HSC70 has been shown to be enriched in bovine EXO following immunogold analysis [101]. HSP60 is normally expressed in cytosol and mitochondria, but it can also be uptaken in EXO. A role in cell-to-cell communication has been attributed to EXO enriched in HSP60 and HSP60 since they are a Toll-like receptor ligands [107]. HSP60-enriched EXO have also been identified in bovine milk, making this chaperone another interesting biomarker for EXO characterization [108]. The minimum information required for the study of extracellular vesicles is reported by Théry et al. [6].
With respect to the antibody-dependent microscopical characterization of EXO, atomic force microscopy (AFM) presents great advantages. AFM’s principle relies on the interaction between the probing tip of the instrument and the surface of the sample, which is probed by a cantilever ending in a sharp tip. When the cantilever is very close to the sample, the atomic forces of the two interact, and this produces a deflection of the cantilever, which is then recorded by a laser-photodiode system [109]. Sample preparation and image acquisition are very simple, since atomic force microscopes work under ambient conditions, and EVs can be imaged and do not require further downstream isolation procedures [110,111]. Moreover, the EVs binding specific antibodies can be used to detect the presence of specific proteins with a better resolution than immunogold labeling. EXO analysis is simplified by the fact that AFM reaches a sub-nm resolution, at which it is possible to appreciate the morphology of a single EXO, and to count EXO in a label-free and unbiased fashion. The high resolution power of AFM also allows the quali–quantitative assessment of surface exosomal molecules [112].
4. Roles of miRNA EXO in Metabolism and Health
Numerous studies have been reported on milk-derived EXO, of human and laboratory animal origin, with both in vitro and in vivo approaches, but less information is available on the EXO in dairy ruminants. In humans, the molecular composition of milk EXO depends on the physiological status of the mother [2], and largely on her lifestyle, lactation stage and contact with allergens [113].
Since a strong relationship between the lactation curve and the plethora of pathways involved in the mammary gland exists, the specific cargo of the EXO can change during the different phases of the lactation cycle. Many of the miRNAs EXO originate from the mammary epithelium [114,115], and the modulation of miRNAs expression in mammary cells impacts the milk profile composition. It has been found that miR-221 is expressed differentially during mammary development, and regulates the lipid metabolism of the mammary cells of mice [116]. It has recently been stated that the miRNAs profile in goat milk exosomes changes during lactation, affecting milk fatty acids (FA) content through transcriptome modifications in the mammary epithelial cells [117,118]. For instance, miR-27a and miR-183 have been shown to promote the content of unsaturated FAs and medium chain FAs [117,118]. The likely mechanism underlying the variations in the FA profile of goat milk is the silencing of key genes involved in lipid metabolism. Lin et al. [117] stated that miR-27a downregulates stearoyl-CoA desaturase (SCD), while Chen et al. [118] found that the silencing action that miR-183 exerts on the MST1 gene leads to the upregulation of adipose triglyceride lipase (ATGL), carnitine palmitoyltransferase 1 (CPT1) and SCD [119].
The EXO protein and miRNA content play a role in modulating the inflammatory response and the development of the host [27,120,121,122,123]. Of note, miRNAs with immune-related activities are abundant in breast milk EXO [124]. Little information is available for cattle, but a change in the composition of the proteins [125] and nucleic acids [126] of EXO occurs during the inflammatory response to pathogens in the mammary gland. Stressful conditions can modify miRNAs derived from milk EXO as well. During the relocation of dairy cows in early lactation, 15 miRNAs were found to be differentially expressed [101]. Therefore, milk miRNA profiling could be useful for the early diagnosis of infections, and for monitoring the immune status of dairy cows [27]. According to Munagala et al. [48], a large copy number of genes and miRNAs, related to bovine mammary glands and immune function, have been found. Furthermore, miRNAs from EXO have been demonstrated to play a role in mouse thymic regulatory T (Treg) cell differentiation [38,51,127,128]. In mouse models, EXO have anti-inflammatory power, and further prevent and decrease the symptoms of rheumatoid arthritis [129]. Moreover, the immunomodulatory activity of mice, after oral administration of EXO, has exhibited an increase in M1 macrophage polarization and inflammatory cytokine secretion (IL-6, TNFα, IL12 and IL23) [130].
Most miRNAs derived from milk EXO are involved in immune functions, such as miR-148 and let-7a. In particular, miR-148 appears to be a negative regulator of the innate immune response, and of the antigenic presentation function in mouse dendritic cells [131]. Furthermore, miR-148a is up-regulated during the cell differentiation [132], targeting DNA methyltransferase 3b [133] and DNA methyltransferase 1 [134] genes in humans. Both genes are involved in tumor development, proliferation and metastasis, and are down-regulated if miR-148a is up-regulated, demonstrating that this miRNA has a tumor-suppressing effect. This feature has raised concerns about the impact of recurrent milk consumption on the epigenetic regulation of the human genome [135,136]. Of note, miR-148a is the one of the most highly expressed miRNAs in bovine and goat milk, before and after pasteurization [41]. Let-7a inhibits Th17 cell differentiation through the downregulation of IL-6 secretion [137]. Interestingly, these miRNAs and others that are mostly expressed in milk EXO, are highly conserved among species [27] and, considering their immune regulatory role, can be candidates of mastitis biomarkers. The early diagnosis and effective treatment of cow mastitis can guarantee a healthy growth of dairy cattle and a qualified production of dairy products, contributing to reducing and/or improving the use of antibiotics on farms [138,139]. In the milk from mammary glands naturally infected by Staphylococcus aureus, another three miRNAs (miR-378, bta-miR-185 and miR-146b) were found to be significantly up-regulated [140]. The target genes of bta-miR-378 and miR-185 have been validated, and their role has been associated to health parameters [80].
5. miRNA EXO as a Genomic Markers
The RumimiR database curated by Bourdon et al. [140] contains a collection of miRNAs from three species of dairy ruminants (cattle, goat and sheep), obtained from different tissues and animal statuses. The authors reported miRNAs differences in the expression level not only between species, but also among breeds. Variations of miRNomes among breeds are recognized as expression quantitative trait loci (miR-e-QTL) [141], which paves the way for their use in breeding programs for polygenic complex traits. In turn, miRNAs contain genomic information that can assist researchers to discover gene functions and to provide a better understanding the regulatory mechanisms of gene expression [142].
Focusing on ruminants’ mammary glands, several studies have underpinned the participation of miRNAs in the regulation of lactation signaling pathways, physiological processes, and health [143] and milk traits [144,145].
In a comparison between Montbéliarde and Holstein lactating cows, Billa et al. [146] found 11 up-regulated and 11 down-regulated miRNAs in the mammary gland tissue, suggesting that their expression level is related to the milk, protein, lactose and fat yields being higher in Holstein than in Montbéliarde cows. The six most differentially expressed miRNAs were miR-100, miR-146b, miR-186, miR-30e-5p, miR-25 and miR-16a. In particular, miR-100 and miR-146b target mammary metabolism through the mTOR signaling pathway, and their differential expression has been related to milk yield. Furthermore, miR-100, together with miR-186, is also related to epithelial-to-mesenchymal transition, controlling the remodeling and development of mammary glands. Again, miR-100, together with miR-30e-5p, miR-25 and miR-16a, targets lipid metabolism by promoting milk fat synthesis and miR-186 regulates glucose uptake. Indeed, Le Guillou et al. [147] compared the milk miRNomes of Normande and Holstein cows, which differed in milk yield and composition, finding 182 miRNAs, differentially regulated, that are involved in lipid metabolism and mammary structure.
Since miRNAs EXO have a role in cell-to-cell communications, their association with complex traits would indicate that they could be useful as genomic markers, and not as just markers of mammary gland function.
6. Conclusions
Exosomes are a distinctive cell-to-cell communication mechanism, which can deliver molecules able to influence, in multiple ways, the biological function of the recipient cell. Among these molecules, miRNAs EXO represent an intriguing regulatory system, involved in the biology of the mammary glands, specifically with regard to immune responses and metabolic processes. Whether the miRNome EXO can be used as a genetic marker in lactating ruminants is still unresolved, and needs to be deeply investigated. Moreover, efforts are required to define standardized and agreed-upon methodological procedures for EXO isolation, in order to gain a deeper insight into their possible application in livestock production sciences.
Author Contributions
Writing original draft preparation, formal analysis, investigation, M.C. (Michela Cintio); writing original draft preparation, formal analysis and editing, E.S.; writing original draft preparation, formal analysis and editing, G.P.; writing original draft preparation, formal analysis and editing, T.M.; writing, review and editing, supervision, project administration, B.S.; writing, review and editing; investigation, M.C. (Monica Colitti); performed TEM analyses and reviewed the paper. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported financially by the Department of Agricultural, Food, Environmental and Animal Sciences: University of Udine (MIPAAF BEST MILK).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Kalluri R., LeBleu V.S. The biology, function, and biomedical application of exosomes. Science. 2020;367:eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sedykh S.E., Burkova E.E., Purvinsh L.V., Klemeshova D.A., Ryabchikova E.I., Nevinsky G.A. Milk exosomes: Isolation, biochemistry, morphology, and perspectives of use. In: De Bona A.G., Reales-Calderon J.A., editors. Extracellular Vesicles and Their Importance in Human Health. 1st ed. IntechOpen; London, UK: 2020. pp. 1–28. [Google Scholar]
- 3.Rani S., O’Brien K., Kelleher F.C., Corcoran C., Germano S., Radomski M.W., Crown J., O’Driscoll L. Isolation of exosomes for subsequent mRNA, microRNA, and protein profiling. Methods Mol. Biol. 2011;784:181–195. doi: 10.1007/978-1-61779-289-2_13. [DOI] [PubMed] [Google Scholar]
- 4.Keller S., Sanderson M.P., Stoeck A., Altevogt P. Exosomes: From biogenesis and secretion to biological function. Immunol. Lett. 2006;107:102–108. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Lötvall J., Hill A.F., Hochberg F., Buzás E.I., Di Vizio D., Gardiner C., Gho Y.S., Kurochkin I.V., Mathivanan S., Quesenberry P., et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reinhardt T.A., Lippolis J.D., Nonnecke B.J., Sacco R.E. Bovine milk exosome proteome. J. Proteomics. 2012;75:1486–1492. doi: 10.1016/j.jprot.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Lässer C., Eldh M., Lötvall J. Isolation and characterization of RNA-containing exosomes. J. Vis. Exp. 2012;59:e3037. doi: 10.3791/3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barile L., Vassalli G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol. Therapeut. 2017;174:63–78. doi: 10.1016/j.pharmthera.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Gurunathan S., Kang M.H., Jeyaraj M., Qasim M., Kim J.H. Review of isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 2019;8:307. doi: 10.3390/cells8040307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin J., Xu Q. Functions and application of exosomes. Acta Pol. Pharm. 2014;71:537–543. [PubMed] [Google Scholar]
- 12.Keerthikumar S., Chisanga D., Ariyaratne D., Al Saffar H., Anand S., Zhao K., Samuel M., Pathan M., Jois M., Chilamkurti N., et al. ExoCarta: A web-based compendium of exosomal cargo. J. Mol. Biol. 2016;428:688–692. doi: 10.1016/j.jmb.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pathan M., Fonseka P., Chitti S.V., Kang T., Sanwlani R., Van Deun J., Hendrix A., Mathivanan S. Vesiclepedia 2019: A compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acid Res. 2019;47:D516–D519. doi: 10.1093/nar/gky1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skog J., Würdinger T., Van Rijn S., Meijer D.H., Gainche L., Sena-Esteves M., Curry W.T., Jr., Carter B.S., Krichevsky A.M., Breakefield X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumor growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu C., Yu S., Zinn K., Wang J., Zhang L., Jia Y., Kappes J.C., Barnes S., Kimberly R.P., Grizzle W.E., et al. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J. Immunol. 2006;176:1375–1385. doi: 10.4049/jimmunol.176.3.1375. [DOI] [PubMed] [Google Scholar]
- 16.Ginestra A., La Placa M.D., Saladino F., Cassarà D., Nagase H., Vittorelli M.L. The amount of proteolytic content of vesicles shed by human cancer cell lines correlates with their in vitro invasiveness. Anticancer Res. 1998;18:3433–3437. [PubMed] [Google Scholar]
- 17.Wen S.W., Lima L.G., Lobb R.J., Norris E.L., Hastie M.L., Krumeich S., Möller A. Breast cancer-derived exosomes reflect the cell-of-origin phenotype. Proteomics. 2019;19:e1800180. doi: 10.1002/pmic.201800180. [DOI] [PubMed] [Google Scholar]
- 18.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 19.Kim V.N. MicroRNA biogenesis: Coordinated cropping and dicing. Nat. Rev. Mol. Cell Biol. 2005;6:376–385. doi: 10.1038/nrm1644. [DOI] [PubMed] [Google Scholar]
- 20.Villarroya-Beltri C., Gutiérrez-Vázquez C., Sánchez-Cabo F., Pérez-Hernández D., Vázquez J., Martin-Cofreces N., Martinez-Herrera D.J., Pascual-Montano A., Mittelbrunn M., Sánchez-Madrid F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis B.P., Burge C.B., Bartel D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 22.Friedman R.C., Kai-How F.K., Burge C.B., Bartel D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartel D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 24.Laurent L.C. MicroRNAs in embryonic stem cells and early embryonic development. J. Cell Mol. Med. 2008;12:2181–2188. doi: 10.1111/j.1582-4934.2008.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stahlhut E., Slack F.J. The role of microRNAs in cancer. Yale J. Biol. Med. 2006;79:131–140. [PMC free article] [PubMed] [Google Scholar]
- 26.Feng J., Xing W., Xie L. Regulatory roles of microRNAs in diabetes. Int. J. Mol. Sci. 2016;17:1729. doi: 10.3390/ijms17101729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benmoussa A., Provost P. Milk microRNAs in health and disease. Compr. Rev. Food. Sci. Food Saf. 2019;18:703–722. doi: 10.1111/1541-4337.12424. [DOI] [PubMed] [Google Scholar]
- 28.Yu S., Zhao Z., Xu X., Li M., Li P. Characterization of three different types of extracellular vesicles and their impact on bacterial growth. Food. Chem. 2019;272:372–378. doi: 10.1016/j.foodchem.2018.08.059. [DOI] [PubMed] [Google Scholar]
- 29.De Toro J., Herschlik L., Waldner C., Mongini C. Emerging roles of exosomes in normal and pathological conditions: New insights for diagnosis and therapeutic applications. Front. Immunol. 2015;6:203. doi: 10.3389/fimmu.2015.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chevillet J.R., Kang Q., Ruf I.K., Briggs H.A., Vojtech L.N., Hughes S.M., Cheng H.H., Arroyo J.D., Meredith E.K., Gallichotte E.N., et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc. Natl. Acad. Sci. USA. 2014;111:14888–14893. doi: 10.1073/pnas.1408301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colitti M. Expression of NGF, BDNF and their high-affinity receptors in ovine mammary glands during development and lactation. Histochem. Cell Biol. 2015;144:559–570. doi: 10.1007/s00418-015-1360-0. [DOI] [PubMed] [Google Scholar]
- 32.Van Hooijdonk A.C., Kussendrager K.D., Steijns J.M. In vivo antimicrobial and antiviral activity of components in bovine milk and colostrum involved in non-specific defense. Br. J. Nutr. 2000;84:S127–S134. doi: 10.1017/S000711450000235X. [DOI] [PubMed] [Google Scholar]
- 33.Sgorlon S., Fanzago M., Guiatti D., Gabai G., Stradaioli G., Stefanon B. Factors affecting milk cortisol in mid lactating dairy cows. BMC Vet. Res. 2015;11:259. doi: 10.1186/s12917-015-0572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andreas N.J., Kampmann B., Mehring Le-Doare K. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015;91:629–635. doi: 10.1016/j.earlhumdev.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 35.Gale C., Logan K.M., Santhakumaran S., Parkinson J.R., Hyde M.J., Modi N. Effect of breastfeeding compared with formula feeding on infant body composition: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012;95:656–669. doi: 10.3945/ajcn.111.027284. [DOI] [PubMed] [Google Scholar]
- 36.Amitay E.L., Keinan-Boker L. Breastfeeding and childhood leukemia incidence. JAMA Pediatr. 2015;169:1071–1072. doi: 10.1001/jamapediatrics.2015.2643. [DOI] [PubMed] [Google Scholar]
- 37.Verhasselt V., Milcent V., Cazareth J., Kanda A., Fleury S., Dombrowicz D., Glaichenhaus N., Julia V. Breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nat. Med. 2008;14:170–175. doi: 10.1038/nm1718. [DOI] [PubMed] [Google Scholar]
- 38.Kosaka N., Izumi H., Sekine K., Ochiya T. MicroRNA as a new immune-regulatory agent in breast milk. Silence. 2010;1:7. doi: 10.1186/1758-907X-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen C., Skog J., Hsu C.H., Lessard R.T., Balaj L., Wurdinger T., Carter B.S., Breakefield X.O., Toner M., Irimia D. Microfluidic isolation and transcriptome analysis of serum microvesicles. Lab. Chip. 2010;10:505–511. doi: 10.1039/B916199F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Izumi H., Tsuda M., Sato Y., Kosaka N., Ochiya T., Iwamoto H., Namba K., Takeda Y. Bovine milk exosomes contain microRNA and mRNA and are taken up by human macrophages. J. Dairy Sci. 2015;98:2920–2933. doi: 10.3168/jds.2014-9076. [DOI] [PubMed] [Google Scholar]
- 41.Golan-Gerstl R., Elbaum Shiff Y., Moshayoff V., Schecter D., Leshkowitz D., Reif S. Characterization and biological function of milk-derived miRNAs. Mol. Nutr. Food Res. 2017;61:201700009. doi: 10.1002/mnfr.201700009. [DOI] [PubMed] [Google Scholar]
- 42.Lässer C., Alikhani V.S., Ekström K., Eldh M., Paredes P.T., Bossios A., Sjöstrand M., Gabrielsson S., Lötvall J., Valadi H. Human saliva, plasma and breast milk exosomes contain RNA: Uptake by macrophage. J. Transl. Med. 2011;9:9. doi: 10.1186/1479-5876-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Izumi H., Kosaka N., Shimizu T., Sekine K., Ochiya T., Takase M. Time-dependent expression profiles of microRNA and mRNA in rat milk whey. PLoS ONE. 2014;9:e88843. doi: 10.1371/journal.pone.0088843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Herwijnen M.J.C., Driedonks T.A.P., Snoek B.L., Kroon A.M.T., Kleinjan M., Jorritsma R., Pieterse C.M.J., Hoen E.N.M.N., Wauben M.H.M. Abundantly present miRNAs in milk-derived extracellular vesicles are conserved between mammals. Front. Nutr. 2018;5:81. doi: 10.3389/fnut.2018.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lonnerdal B., Du X., Liao Y., Li J. Human milk exosomes resist digestion in vitro and are internalized by human intestinal cells. FASEB J. 2015;29:121–123. [Google Scholar]
- 46.Shandilya S., Rani P., Onteru S.K., Singh D. Small interfering RNA in milk exosomes is resistant to digestion and crosses the intestinal barrier in vitro. J. Agric. Food Chem. 2017;65:9506–9513. doi: 10.1021/acs.jafc.7b03123. [DOI] [PubMed] [Google Scholar]
- 47.Izumi H., Kosaka N., Shimizu T., Sekine K., Ochiya T., Takase M. Bovine milk contains microRNA and messenger RNA that are stable under degradative conditions. J. Dairy Sci. 2012;95:4831–4841. doi: 10.3168/jds.2012-5489. [DOI] [PubMed] [Google Scholar]
- 48.Munagala R., Aqil F., Jeyabalan J., Gupta R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016;371:48–61. doi: 10.1016/j.canlet.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manca S., Upadhyaya B., Mutai E., Desaulniers A.T., Cederberg R.A., White B.R., Zempleni J. Milk exosomes are bioavailable and distinct microRNA cargos have unique tissue distribution patterns. Sci. Rep. 2018;8:11321. doi: 10.1038/s41598-018-29780-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolf T., Baier S.R., Zempleni J. The intestinal transport of bovine milk exosomes is mediated by endocytosis in human colon carcinoma Caco-2 cell and rat small intestinal IEC-6 cells. J. Nutr. 2015;14:2201–2206. doi: 10.3945/jn.115.218586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Q., Li M., Wang X., Li Q., Wang T., Zhu Q., Zhou X., Wang X., Gao X., Li X. Immune-related microRNAs are abundant in breast milk exosomes. Int. J. Biol. Sci. 2012;8:118–123. doi: 10.7150/ijbs.8.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Q.J., Chau J., Ebert P.J., Sylvester G., Min H., Liu G., Braich R., Manoharan M., Soutschek J., Skare P., et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 53.Vigorito E., Perks K.L., Abreu-Goodger C., Bunting S., Xiang Z., Kohlhaas S., Das P.P., Miska E.A., Rodriguez A., Bradley A., et al. microRNA-155 regulates the generation of immunoglobulin class-switched plasma cells. Immunity. 2007;27:847–859. doi: 10.1016/j.immuni.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang L., Boeren S., Hageman J.A., van Hooijdonk T., Vervoort J., Hettinga K. Bovine milk proteome in the first 9 days: Protein interactions in maturation of the immune and digestive system of the newborn. PLoS ONE. 2015;10:e0116710. doi: 10.1371/journal.pone.0116710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samuel M., Chisanga D., Liem M., Shivakumar K., Sushma A., Ching-Seng A., Adda G.C., Versteegen E., Markandeya J., Suresh M. Bovine milk-derived exosomes from colostrum are enriched with proteins implicated in immune response and growth. Sci. Rep. 2017;7:5933. doi: 10.1038/s41598-017-06288-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Admyre C., Johansson S.M., Qazi K.R., Filen J.J., Lahesmaa R., Norman M., Neve E.P., Scheynius A., Gabrielsson S. Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 2007;179:1969–1978. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- 57.Van Hese I., Goossens K., Vandaele L., Opsomer G. Invited review: MicroRNAs in bovine colostrum-Focus on their origin and potential health benefits for the calf. J. Dairy Sci. 2020;103:1–15. doi: 10.3168/jds.2019-16959. [DOI] [PubMed] [Google Scholar]
- 58.Witwer K.W., Buzás E.I., Bemis L.T., Bora A., Lässer C., Lötvall J., Nolte-’t Hoen E.N., Piper M.G., Sivaraman S., Skog J., et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles. 2013;2:20360. doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li P., Kaslan M., Lee S.H., Yao J., Gao Z. Progress in EXO isolation techniques. Theranostics. 2017;7:789–804. doi: 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gurunathan S., Marash M., Weinberger A., Gerst J.E. t-SNARE phosphorylation regulates endocytosis in yeast. Mol. Biol. Cell. 2002;13:1594–1607. doi: 10.1091/mbc.01-11-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hata T., Murakami K., Nakatani H., Yamamoto Y., Matsuda T., Aoki N. Isolation of bovine milk-derived microvesicles carrying mRNAs and microRNAs. Biochem. Biophys. Res. Commun. 2010;396:528–533. doi: 10.1016/j.bbrc.2010.04.135. [DOI] [PubMed] [Google Scholar]
- 62.Yamada T., Inoshima Y., Matsuda T., Ishiguro N. Comparison of methods for isolating exosomes from bovine milk. J. Vet. Med. Sci. 2012;74:1523–1525. doi: 10.1292/jvms.12-0032. [DOI] [PubMed] [Google Scholar]
- 63.Vaswani K., Koh Y.Q., Almughlliq F.B., Peiris H.N., Mitchell M.D. A method for the isolation and enrichment of purified bovine milk exosomes. Reprod. Biol. 2017;17:341–348. doi: 10.1016/j.repbio.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 64.Lonnerdal B. Nutritional and physiologic significance of human milk proteins. Am. J. Clin. Nutr. 2003;77:1537S–1543S. doi: 10.1093/ajcn/77.6.1537S. [DOI] [PubMed] [Google Scholar]
- 65.Yamauchi M., Shimizu K., Rahman M., Ishikawa H., Takase H., Ugawa S., Okada A., Inoshima Y. Efficient method for isolation of exosomes from raw bovine milk. Drug Dev. Ind. Pharm. 2019;45:359–364. doi: 10.1080/03639045.2018.1539743. [DOI] [PubMed] [Google Scholar]
- 66.Rahman M.M., Shimizu K., Yamauchi M., Takase H., Ugawa S., Okada A., Inoshima Y. Acidification effects on isolation of extracellular vesicles from bovine milk. PLoS ONE. 2019;14:e0222613. doi: 10.1371/journal.pone.0222613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Somiya M., Yusuke Y., Ochiya T. Biocompatibility of highly purified bovine milk-derived extracellular vesicles. J. Extracell. Vesicles. 2018;7:1440132. doi: 10.1080/20013078.2018.1440132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li B., Hock A., Wu R.Y., Minich A., Botts S.R., Lee C., Antounians L., Miyake H., Koike Y., Chen Y., et al. Bovine milk-derived exosomes enhance goblet cell activity and prevent the development of experimental necrotizing enterocolitis. PLoS ONE. 2019;14:e0211431. doi: 10.1371/journal.pone.0211431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McDonald M.K., Capasso K.E., Ajit S.K. Purification and microRNA profiling of exosomes derived from blood and culture media. J. Vis. Exp. 2013;76:e50294. doi: 10.3791/50294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Greening D.W., Xu R., Ji H., Tauro B.J., Simpson R.J. A protocol for exosome isolation and characterization: Evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods. Methods Mol. Biol. 2015;1295:179–209. doi: 10.1007/978-1-4939-2550-6_15. [DOI] [PubMed] [Google Scholar]
- 71.Yoo C.E., Kim G., Kim M., Park D., Kang H.J., Lee M., Huh N. A direct extraction method for microRNAs from exosomes captured by immunoaffinity beads. Anal. Biochem. 2012;431:96–98. doi: 10.1016/j.ab.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 72.Liga A., Vliegenthart A.D.B., Oosthuyzen W., Dear J.W., Kersaudy-Kerhoas M. Exosome isolation: A microfluidic road-map. Lab. Chip. 2015;15:2388–2394. doi: 10.1039/C5LC00240K. [DOI] [PubMed] [Google Scholar]
- 73.Kanwar S.S.S., Dunlay C.J., Simeone D.M., Nagrath S. Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab Chip. 2014;14:1891–1900. doi: 10.1039/C4LC00136B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Im H., Shao H., Park Y.I., Peterson V.M., Castro C.M., Weissleder R., Lee H. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat. Biotechnol. 2014;32:490–495. doi: 10.1038/nbt.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davies R.T., Kim J., Jang S.C., Choi E.J., Gho Y.S., Park J. Microfluidic filtration system to isolate extracellular vesicles from blood. Lab Chip. 2012;12:5202–5210. doi: 10.1039/c2lc41006k. [DOI] [PubMed] [Google Scholar]
- 76.Wang Z.H.J., Wu D., Fine J., Schmulen Y., Hu B., Godin J.X.J.Z., Liu X. Ciliated micropillars for the microfluidic-based isolation of nanoscale lipid vesicles. Lab Chip. 2013;13:2879–2882. doi: 10.1039/c3lc41343h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carnell-Morris P., Tannetta D., Siupa A., Hole P., Dragovic R. Analysis of extracellular vesicles using fluorescence nanoparticle tracking analysis. In: Kuo W.P., Jia S., editors. Extracellular Vesicles Methods and Protocols. Humana Press; New York, NY, USA: 2017. pp. 153–173. [DOI] [PubMed] [Google Scholar]
- 78.Koh Y.Q., Peiris H.N., Vaswani K., Meier S., Burke C.R., Macdonald K.A., Roche J.R., Almughlliq F., Arachchige B.J., Reed S., et al. Characterization of exosomes from body fluids of dairy cows. J. Anim. Sci. 2017;95:3893–3904. doi: 10.2527/jas2017.1727. [DOI] [PubMed] [Google Scholar]
- 79.Vaswani K., Mitchell M.D., Holland O.J., Koh Y.Q., Hill R.J., Harb T., Davies P.S.W., Peiris H. A method for the isolation of exosomes from human and bovine milk. J. Nutr. Metab. 2019;2019:5764740. doi: 10.1155/2019/5764740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ma S., Tong C., Ibeagha-Awemu E.M., Zhao X. Identification and characterization of differentially expressed exosomal microRNAs in bovine milk infected with Staphylococcus aureus. BMC Genomics. 2019;20:934. doi: 10.1186/s12864-019-6338-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Özdemir S. Identification and comparison of exosomal microRNAs in the milk and colostrum of two different cow breeds. Gene. 2020;743:144609. doi: 10.1016/j.gene.2020.144609. [DOI] [PubMed] [Google Scholar]
- 82.Brown B.A., Zeng X., Todd A.R., Barnes L.F., Winstone J.M.A., Trinidad J.C., Novotny M.V., Jarrold M.F., Clemmer D.E. Charge detection mass spectrometry measurements of exosomes and other extracellular particles enriched from bovine milk. Anal. Chem. 2020;92:3285–3292. doi: 10.1021/acs.analchem.9b05173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Almughlliq F.B., Koh Y.Q., Peiris H.N., Vaswani K., McDougall S., Graham E.M., Burke C.R., Mitchell M.D. Effect of exosomes from plasma of dairy cows with or without an infected uterus on prostaglandin production by endometrial cell lines. J. Dairy Sci. 2017;100:9143–9152. doi: 10.3168/jds.2017-13261. [DOI] [PubMed] [Google Scholar]
- 84.Crookenden M.A., Walker C.G., Peiris H., Koh Y.Q., Almughlliq F., Vaswani K., Reed S., Heiser A., Loor J.J., Kay J.K., et al. Effect of circulating exosomes from transition cows on Madin-Darby bovine kidney cell function. J. Dairy Sci. 2017;100:5687–5700. doi: 10.3168/jds.2016-12152. [DOI] [PubMed] [Google Scholar]
- 85.Koh Y.Q., Peiris H.N., Vaswani K., Almughlliq F.B., Meier S., Burke C.R., Roche J.R., Reed C.B., Arachchige B.J., Reed S., et al. Proteome profiling of exosomes derived from plasma of heifers with divergent genetic merit for fertility. J. Dairy Sci. 2018;101:6462–6473. doi: 10.3168/jds.2017-14190. [DOI] [PubMed] [Google Scholar]
- 86.Almughlliq F.B., Koh Y.Q., Peiris H.N., Vaswani K., McDougall S., Graham E.M., Burke C.R., Arachchige B.J., Reed S., Mitchell M.D. Proteomic content of circulating exosomes in dairy cows with or without uterine infection. Theriogenology. 2018;114:173–179. doi: 10.1016/j.theriogenology.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 87.Almughlliq F.B., Koh Y.Q., Peiris H.N., Vaswani K., Holland O., Meier S., Roche J.R., Burke C.R., Crookenden M.A., Arachchige B.J., et al. Circulating exosomes may identify biomarkers for cows at risk for metabolic dysfunction. Sci. Rep. 2019;9:13879. doi: 10.1038/s41598-019-50244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao G., Guo S., Jiang K., Zhang T., Wu H., Qiu C., Deng G. MiRNA profiling of plasma-derived exosomes from dairy cows during gestation. Theriogenology. 2019;130:89–98. doi: 10.1016/j.theriogenology.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 89.Sinlapadeelerdkul T., Sonoda H., Uchida K., Kitahara G., Ikeda M. Release of urinary aquaporin-2-bearing extracellular vesicles is decreased in pregnant Japanese Black cattle. J. Vet. Med. Sci. 2019;81:1609–1615. doi: 10.1292/jvms.19-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Théry C., Amigorena S., Raposo G., Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006;3:22. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 91.Nolan J.P., Duggan E. Analysis of individual extracellular vesicles by flow cytometry. In: Hawley T.S., Hawley R.G., editors. Flow Cytometry Protocols. 4th ed. Humana Press; New York, NY, USA: 2018. pp. 79–92. [DOI] [PubMed] [Google Scholar]
- 92.Van der Pol E., van Gemert M.J., Sturk A., Nieuwland R., van Leeuwen T.G. Single vs. swarm detection of microparticles and exosomes by flow cytometry. J. Thromb. Haemost. 2012;10:919–930. doi: 10.1111/j.1538-7836.2012.04683.x. [DOI] [PubMed] [Google Scholar]
- 93.Nolte-’t Hoen E.N., van der Vlist E.J., Aalberts M., Mertens H.C., Bosch B.J., Bartelink W., Mastrobattista E., van Gaal E.V., Stoorvogel W., Arkesteijn G.J., et al. Quantitative and qualitative flow cytometric analysis of nanosized cell-derived membrane vesicles. Nanomedicine. 2012;8:712–720. doi: 10.1016/j.nano.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Van der Vlist E.J., Nolte-’t Hoen E.N., Stoorvogel W., Arkesteijn G.J., Wauben M.H. Fluorescent labeling of nano-sized vesicles released by cells and subsequent quantitative and qualitative analysis by high-resolution flow cytometry. Nat. Protoc. 2012;7:1311–1326. doi: 10.1038/nprot.2012.065. [DOI] [PubMed] [Google Scholar]
- 95.Van der Pol E., Coumans F., Varga Z., Krumrey M., Nieuwland R. Innovation in detection of microparticles and exosomes. J. Thromb. Haemost. 2013;11:36–45. doi: 10.1111/jth.12254. [DOI] [PubMed] [Google Scholar]
- 96.Buzás E.A., Gardiner C., Lee C., Smith Z.J. Single particle analysis: Methods for detection of platelet extracellular vesicles in suspension (excluding flow cytometry) Platelets. 2016;28:249–255. doi: 10.1080/09537104.2016.1260704. [DOI] [PubMed] [Google Scholar]
- 97.Dragovic R.A., Gardiner C., Brooks A.S., Tannetta D.S., Ferguson D.J., Hole P., Carr B., Redman C.W., Harris A.L., Dobson P.J., et al. Sizing and phenotyping of cellular vesicles using nanoparticle tracking analysis. Nanomedicine. 2011;7:780–788. doi: 10.1016/j.nano.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gleadle J., McNicholas K., Li J., Michael M., Rojas-Canales D. Nanoparticle tracking analysis of urine to detect exosomes can be confounded by albuminuria. J. Am. Soc. Nephrol. 2018;29:1784. doi: 10.1681/ASN.2018020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McNicholas K., Michael M.Z. Immuno-characterization of exosomes using nanoparticle tracking analysis. In: Hill A.F., editor. Exosomes and Microvesicles Methods and Protocols. 1st ed. Humana Press; New York, NY, USA: 2017. pp. 35–42. [DOI] [PubMed] [Google Scholar]
- 100.Raposo G., Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Colitti M., Sgorlon S., Licastro D., Stefanon B. Differential expression of miRNAs in milk exosomes of cows subjected to group relocation. Res. Vet. Sci. 2019;122:148–155. doi: 10.1016/j.rvsc.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 102.Böker K.O., Lemus-Diaz N., Rinaldi Ferreira R., Schiller L., Schneider S., Gruber J. The impact of the CD9 tetraspanin on lentivirus infectivity and exosome secretion. Mol. Ther. 2018;26:634–647. doi: 10.1016/j.ymthe.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang X., Tian F., Chen C., Feng Y., Sheng X., Guo Y., Ni H. Exosome-derived uterine microRNAs isolated from cows with endometritis impede blastocyst development. Reprod. Biol. 2019;19:204–209. doi: 10.1016/j.repbio.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 104.Qiao F., Ge H., Ma X., Zhang Y., Zuo Z., Wang M., Zhang Y., Wang Y. Bovine uterus-derived exosomes improve developmental competence of somatic cell nuclear transfer embryos. Theriogenology. 2018;114:199–205. doi: 10.1016/j.theriogenology.2018.03.027. [DOI] [PubMed] [Google Scholar]
- 105.Pols M.S., Klumperman J. Trafficking and function of the tetraspanin CD63. Exp. Cell Res. 2009;315:1584–1592. doi: 10.1016/j.yexcr.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 106.Blans K., Hansen M.S., Sørensen L.V., Hvam M.L., Howard K.A., Möller A., Wiking L., Larsen L.B., Rasmussen J.T. Pellet-free isolation of human and bovine milk extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles. 2017;6:1294340. doi: 10.1080/20013078.2017.1294340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gupta S., Knowlton A.A. HSP60 trafficking in adult cardiac myocytes: Role of the exosomal pathway. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H3052–H3056. doi: 10.1152/ajpheart.01355.2006. [DOI] [PubMed] [Google Scholar]
- 108.Huang T., Zhou C., Che Y., Zhang M., Ren W., Lei L. Exosomes derived from bovine mammary epithelial cells treated with transforming growth factor-β1 inhibit the proliferation of bovine macrophages. J. Interferon Cytokine Res. 2019;39:752–759. doi: 10.1089/jir.2019.0032. [DOI] [PubMed] [Google Scholar]
- 109.Szatanek R., Baj-Krzyworzeka M., Zimoch J., Lekka M., Siedlar M., Baran J. The methods of choice for extracellular vesicles (EVs) characterization. Int. J. Mol. Sci. 2017;18:1153. doi: 10.3390/ijms18061153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Biasutto L., Chiechi A., Couch R., Liotta L.A., Espina V. Retinal pigment epithelium (RPE) exosomes contain signaling phosphoproteins affected by oxidative stress. Exp. Cell Res. 2013;319:2113–2123. doi: 10.1016/j.yexcr.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Madhankumar A.B., Mrowczynski O.D., Patel S.R., Weston C.L., Zacharia B.E., Glantz M.J., Siedlecki C.A., Xu L.C., Connor J.R. Interleukin-13 conjugated quantum dots for identification of glioma initiating cells and their extracellular vesicles. Acta Biomater. 2017;58:205–213. doi: 10.1016/j.actbio.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 112.Sharma S., LeClaire M., Gimzewski J.K. Ascent of atomic force microscopy as a nanoanalytical tool for exosomes and other extracellular vesicles. Nanotechnology. 2018;29:132001. doi: 10.1088/1361-6528/aaab06. [DOI] [PubMed] [Google Scholar]
- 113.Torregrosa Paredes P., Gutzeit C., Johansson S., Admyre C., Stenius F., Alm J., Scheynius A., Gabrielsson S. Differences in exosome populations in human breast milk in relation to allergic sensitization and lifestyle. Allergy. 2014;69:463–471. doi: 10.1111/all.12357. [DOI] [PubMed] [Google Scholar]
- 114.Modepalli V., Kumar A., Hinds L.A., Sharp J.A., Nicholas K.R., Lefevre C. Differential temporal expression of milk miRNA during the lactation cycle of the marsupial tammar wallaby (Macropus eugenii) BMC Genomics. 2014;15:1012. doi: 10.1186/1471-2164-15-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Alsaweed M., Lai C.T., Hartmann P.E., Geddes D.T., Kakulas F. Human milk miRNAs primarily originate from the mammary gland resulting in unique miRNA profiles of fractionated milk. Sci. Rep. 2016;6:20680. doi: 10.1038/srep20680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chu M., Zhao Y., Yu S., Hao Y., Zhang P., Feng Y., Zhang H., Ma D., Liu J., Cheng M., et al. MicroRNA-221 may be involved in lipid metabolism in mammary epithelial cells. Int. J. Biochem. Cell Biol. 2018;97:118–127. doi: 10.1016/j.biocel.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 117.Lin X.Z., Luo J., Zhang L.P., Wang W., Shi H.B., Zhu J.J. miR-27a suppresses triglyceride accumulation and affects gene mRNA expression associated with fat metabolism in dairy goat mammary gland epithelial cells. Gene. 2013;521:15–23. doi: 10.1016/j.gene.2013.03.050. [DOI] [PubMed] [Google Scholar]
- 118.Chen Z., Shi H., Sun S., Luo J., Zhang W., Hou Y., Loor J.J. MiR-183 regulates milk fat metabolism via MST1 in goat mammary epithelial cells. Gene. 2018;646:12–19. doi: 10.1016/j.gene.2017.12.052. [DOI] [PubMed] [Google Scholar]
- 119.Fernández-Hernando C., Suárez Y., Rayner K.J., Moore K.J. MicroRNAs in lipid metabolism. Curr. Opin. Lipidol. 2011;22:86–92. doi: 10.1097/MOL.0b013e3283428d9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Benmoussa A., Ly S., Shan S.T., Laugier J., Boilard E., Gilbert C., Provost P. A subset of extracellular vesicles carries the bulk of microRNAs in commercial dairy cow’s milk. J. Extracell. Vesicles. 2017;6:1401897. doi: 10.1080/20013078.2017.1401897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen C., Xiang H., Peng Y.L., Peng J., Jiang S.W. Mature miR-183, negatively regulated by transcription factor GATA3, promotes 3T3-L1 adipogenesis through inhibition of the canonical Wnt/β-catenin signaling pathway by targeting LRP6. Cell Signal. 2014;26:1155–1165. doi: 10.1016/j.cellsig.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 122.Chen T., Xi Q.Y., Ye R.S., Cheng X., Qi Q.E., Wang S.B., Shu G., Wang L.N., Zhu X.T., Jiang Q.Y., et al. Exploration of microRNAs in porcine milk exosomes. BMC Genomics. 2014;15:100. doi: 10.1186/1471-2164-15-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Larssen P., Wik L., Czarnewski P., Eldh M., Löf L., Ronquist K.G., Dubois L., Freyhult E., Gallant C.J., Oelrich J., et al. Tracing cellular origin of human exosomes using multiplex proximity extension assays. Mol. Cell. Proteomics. 2017;16:502–511. doi: 10.1074/mcp.M116.064725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zheng Y., Chen K.L., Zheng X.M., Li H.X., Wang G.L. Identification and bioinformatics analysis of microRNAs associated with stress and immune response in serum of heat-stressed and normal Holstein cows. Cell Stress Chaperones. 2014;19:973–981. doi: 10.1007/s12192-014-0521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Reinhardt T.A., Sacco R.E., Nonnecke B.J., Lippolis J.D. Bovine milk proteome: Quantitative changes in normal milk exosomes, milk fat globule membranes and whey proteomes resulting from Staphylococcus aureus mastitis. J. Proteomics. 2013;82:141–154. doi: 10.1016/j.jprot.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 126.Sun J., Aswath K., Schroeder S.G., Lippolis J.D., Reinhardt T.A., Sonstegard T.S. MicroRNA expression profiles of bovine milk exosomes in response to Staphylococcus aureus infection. BMC Genomics. 2015;16:806. doi: 10.1186/s12864-015-2044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sun Q., Chen X., Yu J., Zen K., Zhang C.Y., Li L. Immune modulatory function of abundant immune-related microRNAs in microvesicles from bovine colostrum. Protein Cell. 2013;4:197–210. doi: 10.1007/s13238-013-2119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang G.J., Liu Y., Qin A., Shah S.V., Deng Z.B., Xiang X., Cheng Z., Liu C., Wang J., Zhang L., et al. Thymus exosomes-like particles induce regulatory T cells. J. Immunol. 2008;181:5242–5248. doi: 10.4049/jimmunol.181.8.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Arntz O.J., Pieters B.C., Oliveira M.C., Broeren M.G., Bennink M.B., de Vries M., van Lent P.L., Koenders M.I., van den Berg W.B., van der Kraan P.M., et al. Oral administration of bovine milk derived extracellular vesicles attenuates arthritis in two mouse models. Mol. Nutr. Food Res. 2015;59:1701–1712. doi: 10.1002/mnfr.201500222. [DOI] [PubMed] [Google Scholar]
- 130.Nordgren T.M., Heires A.J., Zempleni J., Swanson B.J., Wichman C., Romberger D.J. Bovine milk-derived extracellular vesicles enhance inflammation and promote M1 polarization following agricultural dust exposure in mice. J. Nutr. Biochem. 2019;64:110–120. doi: 10.1016/j.jnutbio.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu X., Zhan Z., Xu L., Ma F., Li D., Guo Z., Li N., Cao X. MicroRNA-148/152 impair innate response and antigen presentation of TLR-triggered dendritic cells by targeting CaMKIIα. J. Immunol. 2010;185:7244–7251. doi: 10.4049/jimmunol.1001573. [DOI] [PubMed] [Google Scholar]
- 132.Shi C., Zhang M., Tong M., Yang L., Pang L., Chen L., Xu G., Chi X., Hong Q., Ni Y., et al. miR-148a is associated with obesity and modulates adipocyte differentiation of mesenchymal stem cells through Wnt signaling. Sci. Rep. 2015;5:9930. doi: 10.1038/srep09930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lujambio A., Calin G.A., Villanueva A., Ropero S., Sánchez-Céspedes M., Blanco D., Montuenga L.M., Rossi S., Nicoloso M.S., Faller W.J., et al. A microRNA DNA methylation signature for human cancer metastasis. Proc. Natl. Acad. Sci. USA. 2008;105:13556–13561. doi: 10.1073/pnas.0803055105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Long X.R., He Y., Huang C., Li J. MicroRNA-148a is silenced by hypermethylation and interacts with DNA methyltransferase 1 in hepatocellular carcinogenesis. Int. J. Oncol. 2014;44:1915–1922. doi: 10.3892/ijo.2014.2373. [DOI] [PubMed] [Google Scholar]
- 135.Melnik B.C., Schmitz G. DNA methyltransferase 1-targeting miRNA-148a of dairy milk: A potential bioactive modifier of the human epigenome. Funct. Food Health Dis. 2017;7:9. doi: 10.31989/ffhd.v7i9.379. [DOI] [Google Scholar]
- 136.Melnik B.C., Schmitz G. MicroRNAs: Milk’s epigenetic regulators. Best Pract. Res. Clin. Endocrinol. Metab. 2017;31:427–442. doi: 10.1016/j.beem.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 137.Zhang Y., Wang X., Zhong M., Zhang M., Suo Q., Lv K. MicroRNA let-7a ameliorates con A-induced hepatitis by inhibiting IL-6-dependent Th17 cell differentiation. J. Clin. Immunol. 2013;33:630–639. doi: 10.1007/s10875-012-9840-7. [DOI] [PubMed] [Google Scholar]
- 138.Chen Z., Zhang Y., Zhou J., Tian Y., Zhou Q., Mao Y., Yang Z., Loor J.J., Gou D. Tea tree oil prevents mastitis-associated inflammation in lipopolysaccharide-stimulated bovine mammary epithelial cells. Front. Vet. Sci. 2020 doi: 10.21203/rs.3.rs-18655/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lipkens Z., Piepers S., De Visscher A., De Vliegher S. Evaluation of test-day milk somatic cell count information to predict intramammary infection with major pathogens in dairy cattle at drying off. J. Dairy Sci. 2019;102:4309–4321. doi: 10.3168/jds.2018-15642. [DOI] [PubMed] [Google Scholar]
- 140.Bourdon C., Bardou P., Aujean E., Le Guillou S., Tosser-Klopp G., Le Provost F. RumimiR: A detailed microRNA database focused on ruminant species. Database. 2019;2019:baz099. doi: 10.1093/database/baz099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Huan T., Rong J., Liu C., Zhang X., Tanriverdi K., Joehanes R., Chen B.H., Murabito J.M., Yao C., Courchesne P., et al. Genome-wide identification of microRNA expression quantitative trait loci. Nat. Comm. 2015;6:6601. doi: 10.1038/ncomms7601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Goulart L.F., Bettella F., Sønderby I.E., Schork A.J., Thompson W.K., Mattingsdal M., Steen V.M., Zuber V., Wang Y., Dale A.M., et al. MicroRNAs enrichment in GWAS of complex human phenotypes. BMC Genomics. 2015;16:304. doi: 10.1186/s12864-015-1513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Do D.N., Li R., Dudemaine P.-L., Ibeagha-Awemu E.M. MicroRNA roles in signalling during lactation: An insight from differential expression, time course and pathway analyses of deep sequence data. Sci. Rep. 2017;7:44605. doi: 10.1038/srep44605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Do D.N., Dudemaine P.-L., Li R., Ibeagha-Awemu E.M. Co-expression network and pathway analyses reveal important modules of miRNAs regulating milk yield and component traits. Int. J. Mol. Sci. 2017;18:1560. doi: 10.3390/ijms18071560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ammah A.A., Do D.N., Bissonnette N., Gévry N., Ibeagha-Awemu E.M. Co-expression network analysis identifies miRNA–mRNA networks potentially regulating milk traits and blood metabolites. Int. J. Mol. Sci. 2018;19:2500. doi: 10.3390/ijms19092500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Billa P.A., Faulconnier Y., Ye T., Chervet M., Le Provost F., Pires J.A.A., Leroux C. Deep RNA-Seq reveals miRNome differences in mammary tissue of lactating Holstein and Montbéliarde cows. BMC Genomics. 2019;20:621. doi: 10.1186/s12864-019-5987-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Le Guillou S., Leduc A., Laubier J., Barbey S., Rossignol M.N., Lefebvre R., Marthey S., Laloë D., Le Provost F. Characterization of Holstein and Normande whole milk miRNomes highlights breed specificities. Sci. Rep. 2019;9:20345. doi: 10.1038/s41598-019-56690-7. [DOI] [PMC free article] [PubMed] [Google Scholar]