Abstract
Purpose
In the mammalian retina, cannabinoid type 1 receptors (CB1Rs) are well-positioned to alter inhibitory synaptic function from amacrine cells and, thus, might influence visual signal processing in the inner retina. However, it is not known if CB1R modulates amacrine cells feedback inhibition at retinal bipolar cell (BC) terminals.
Methods
Using whole-cell voltage-clamp recordings, we examined the pharmacological effect of CB1R activation and inhibition on spontaneous inhibitory postsynaptic currents (sIPSCs) and glutamate-evoked IPSCs (gIPSCs) from identified OFF BCs in light-adapted rat retinal slices.
Results
Activation of CB1R with WIN55212-2 selectively increased the frequency of GABAergic, but not glycinergic sIPSC in types 2, 3a, and 3b OFF BCs, and had no effect on inhibitory activity in type 4 OFF BCs. The increase in GABAergic activity was eliminated in axotomized BCs and can be suppressed by blocking CB1R with AM251 or GABAA and GABAρ receptors with SR-95531 and TPMPA, respectively. In all OFF BC types tested, a brief application of glutamate to the outer plexiform layer elicited gIPSCs comprising GABAergic and glycinergic components that were unaffected by CB1R activation. However, blocking CB1R selectively increased GABAergic gIPSCs, supporting a role for endocannabinoid signaling in the regulation of glutamate-evoked GABAergic inhibitory feedback to OFF BCs.
Conclusions
CB1R activation shape types 2, 3a, and 3b OFF BC responses by selectively regulate GABAergic feedback inhibition at their axon terminals, thus cannabinoid signaling might play an important role in the fine-tuning of visual signal processing in the mammalian inner retina.
Keywords: feedback inhibition, cannabinoid, bipolar cells, inner retina, amacrine cells
Endocannabinoids (eCBs) are lipid-derived messengers that, by activating primarily cannabinoid type 1 receptors (CB1Rs), serve as regulators of excitatory and inhibitory synaptic function throughout the brain.1–3 In the retina, expression of CB1R has been found in the inner and outer synaptic and nuclear layers of several species, including rats and humans.4–12 For instance, in salamander and goldfish retinas,7,10,13,14 activation of CB1R reportedly inhibits different voltage-gated Ca2+ and K+ channels in photoreceptors and bipolar cells (BCs) and might modulate glutamatergic transmission in both the outer and inner retina. Activation of CB1R has also been shown to increase the intrinsic excitability of retinal ganglion cell in xenopus tadpoles,15 inhibit voltage-activated Ca2+ channels in cultured rat ganglion cells,16 modulate spontaneous transmitter release in cultured amacrine cells from embryonic chick retina,17 and, more recently, to reduce spontaneous excitatory and inhibitory inputs onto rat and mouse retinal ganglion cells.18–20 Although this evidence suggests that CB1Rs are well positioned to affect both excitatory and inhibitory transmission within the inner retina, little is known about the role of CB1R in modulating feedback inhibition from amacrine to retinal BCs.
BCs, responsible for transmitting, filtering, and separating aspects of visual information on its way from photoreceptors to ganglion cells,21 can be separated into two main groups based on their response to light: ON and OFF BCs.22,23 Inhibitory inputs from diverse GABAergic and glycinergic amacrine cells in the inner retina are known to shape both ON and OFF BC responses.24 In the OFF pathway, the classic view is that glycinergic inhibitory input to OFF BCs stems mainly from AII amacrine cells, a key component in signal transmission within the rod pathway.25–27 However, depending on the OFF BC type21,28–31 and the degree of light adaptation, inhibitory input from both glycinergic and GABAergic sources contributes to the functional requirements of the retina.32–34 Although little is known regarding the exact localization and function of CB1R within the OFF pathway, the dense labeling of CB1R in the inner plexiform layer (IPL),7,9,35 the apparent localization of CB1R at the cone-type 1 OFF BC synapse,35 in some recoverin-positive OFF BCs,19 and in subsets of GABAergic9 and putative glycinergic amacrine cells,19 suggest that CB1Rs can control OFF BC function by regulating their activity and/or inhibitory input in the inner retina, but this remains currently unproven. Here, we sought to address these issues by recording GABAergic and glycinergic postsynaptic currents (IPSCs) from identified OFF BCs in acute light-adapted rat retinal slices.
Materials and Methods
Sprague Dawley rats were raised in the animal facility of the Universidad de Valparaiso and held at 20 to 25°C under a 12-hour light/dark cycle with water and food ad libitum. Retinal slices (200 µm thickness) were prepared from 25 to 30-day-old rats irrespective of sex and weight, as previously described.29,36 Animal handling and use followed a protocol approved by the bioethics committee of the Universidad de Valparaiso, in accordance with the bioethics and biosafety regulation of the Chilean Research Council (CONICYT). Briefly, rats were anesthetized deeply by isoflurane inhalation and euthanized by decapitation. Eyes were quickly removed and the retinas were carefully separated from the sclera and maintained in extracellular solution containing (in mM): 119 NaCl, 23 NaHCO3, 1.25 NaH2PO4, 2.5 KCl, 2.5 CaCl2, 1.5 MgSO4, 20 glucose, and 2 Na+ pyruvate, aerated with 95% O2 and 5% CO2, reaching a pH of 7.4. Retinas were embedded in type VII agarose (Sigma) and cut with a vibratome (Leica VT1000S). To obtain viable axotomized BCs37 (Supplementary Fig. S1), the angle of the blade was adjusted to produce vertical or wedged slices. All experiments were performed at room temperature under conditions of low photopic background illumination (100 lux), in which OFF BCs display a significant amount of spontaneous background activity.33
Spontaneous inhibitory postsynaptic currents (sIPSCs) and glutamate-evoked IPSCs (gIPSCs) were recorded from OFF BCs voltage clamped at 0 mV using borosilicate patch electrodes (10-13 MΩ, 1.5 mm OD, 0.84 mm ID, WPI) filled with internal solution containing (in mM): 125 K+ gluconate, 10 KCl, 10 HEPES, 2 EGTA, 2 Na2ATP, 2 NaGTP, and 1% Lucifer yellow. The pH was adjusted to 7.4 with KOH. In some experiments (Supplementary Fig. S3), nifedipine (30 µM) was added to the extracellular solution to isolated voltage-gated K+ currents, whereas an internal solution containing (in mM): 90 Cs-methanesulfonate, 20 TEA-Cl, 10 HEPES, 10 EGTA, 10 Na2-phosphocreatine, 2 MgATP, and 0.2 NaGTP with pH adjusted to 7.4 with CsOH was used to record voltage-activated Ca2+ currents. BC subtypes were classified according to different parameters, including their axonal morphology and stratification within the OFF sublamina and by their electrophysiological response to glutamate in the outer plexiform layer (OPL), as previously described (Supplementary Fig. S1).29 The sIPSCs were also recorded from different subtypes of amacrine cells voltage-clamped at 0 mV with patch electrodes (7-8 MΩ) containing K+ gluconate internal solution. Although AII amacrine cells were distinguished by their smaller somata, a prominent primary dendrite protruding into the IPL, and a narrowly distributed dendritic arbor, other subtypes of amacrine cells were divided according to their dendritic stratification within different portions of the IPL as ON, OFF, or ON-OFF amacrine cells.
For gIPSC recordings, BCs were stimulated with L-glutamate (500 µM), applied to the OPL from a single-barrel glass pipette operated by a custom-made picospritzer operating at 2 to 3 psi. SR-95531 (SR, 10 µM) to block GABAA receptors, 1,2,5,6-tetrahydropyridin-4yl-methylphosphonic acid (TPMPA; 50 µM) to block GABAρ receptors, and strychnine (5 µM) to block glycine receptors were added to the bath solution as needed. To activate or inhibit CB1Rs, either WIN 55,212-2 (WIN, 1 µM) or AM251 (5 µM) were added to the bath solution. Except where indicated, the effects of the CB1R agonist and antagonist on sIPSC and gIPSC were recorded for at least 10 minutes after the establishment of a stable baseline. Drug application was performed using a pressurized superfusion system (Automate Scientific). Reagents were obtained from Tocris Bioscience, except for WIN and L-glutamate that were obtained from Sigma-Aldrich.
All recordings were acquired using PClamp 10.4 (Molecular Devices) and signals were filtered at 3 kHz on an EPC7-plus patch clamp amplifier (HEKA Elektronik), digitized, and sampled at 10 kHz (Digidata 1550; Molecular Devices). The calculated liquid junction potential of 14 mV was corrected before the recordings. The sIPSC frequency and amplitude were analyzed using the event detection tool of Clampfit (Molecular Devices), with a detection threshold of twice the mean amplitude of the electrical noise. IPSCs with a duration of <5 ms were considered electrical artifacts and were eliminated from the analysis. Of 173 intact OFF BCs recorded, only 89 exhibited spontaneous activity with these characteristics and were considered for the analysis. Cumulative plots were constructed by pooling 150 consecutive events sampled per cell. The gIPSCs charge was calculated by integrating the area under the current trace in Origin 8 Pro software (Origin Lab). Data used for statistical analysis had a normal distribution according to the Shapiro-Wilk test. Results are shown as the mean ± SEM, and, unless otherwise indicated, statistical comparisons were made with a paired two-tailed Student's t-test (P < 0.05). Within the figures, asterisks indicate the following: *P < 0.05, **P < 0.01, and ***P < 0.001.
Results
OFF BCs Display Inhibitory Spontaneous Activity With Low and High Frequency Activity Patterns
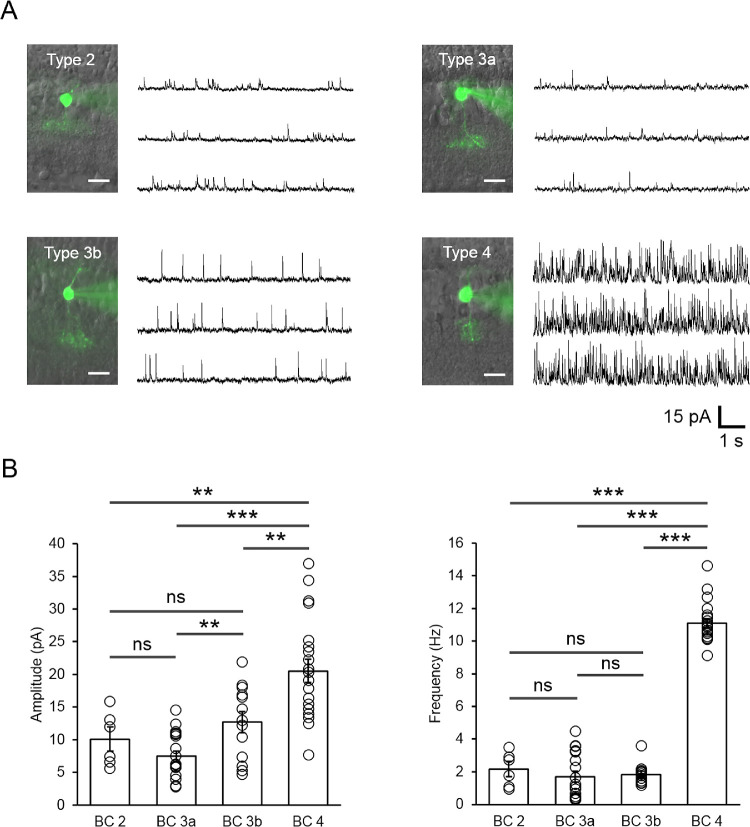
Voltage-clamp recordings from 89 intact and 11 axotomized BCs were obtained to examine the functional consequences of CB1R activation on the regulation of inhibitory feedback to morphologically and physiologically identify OFF BCs in light-adapted rat retinal slices. Fourteen cells, including axotomized cells, were classified as type 2, 35 as type 3a, 22 as type 3b, and 29 as type 4 OFF BCs. As previously reported in rat retinas,29 type 1 OFF BCs was difficult to encounter, and the two cells classified as type 1 were excluded from our analysis. Type 4 OFF BCs displayed a pattern of sIPSCs with significantly higher frequency compared to other OFF BC types recorded (Figs. 1A,B; type 4: 11.08 ± 0.28 Hz, n = 21 vs. type 2: 2.21 ± 0.41 Hz, n = 6, unpaired, P < 0.0001; type 3a: 1.70 ± 0.33 Hz, n = 19, unpaired, P < 0.0001; and type 3b: 1.84 ± 0.18 Hz, n = 13; unpaired, P < 0.0001). Likewise, the amplitude of sIPSCs was significantly higher in type 4 OFF BCs compared to other OFF BC types (Fig. 1B; type 4: 20.5 ± 1.78 pA; n = 21 vs. type 2: 10.08 ± 1.69 pA, n = 6, unpaired, P = 0.0044; type 3a: 7.46 ± 0.82 pA, n = 19, unpaired, P < 0.0001; and type 3b: 12.68 ± 1.62 pA, n = 13, unpaired, P = 0.0025). Although a significant difference in the amplitude of sIPSCs between types 3a (7.46 ± 0.83 pA, n = 19) and 3b (12.68 ± 1.62 pA, n = 13; unpaired, P = 0.0028; Fig. 1B) was observed, likely due to differences in GABA/glycine receptor function, for the purposes of this study, types 2, 3a, and 3b OFF BCs were grouped and will be referred to as cells with a low frequency of sIPSC (low frequency cell [LFC]; <5 Hz), whereas type 4 OFF BCs will be referred to as cells with high frequency cell (HFC) activity (>9 Hz).
Figure 1.
Rat OFF BCs display different backgrounds of spontaneous inhibitory activity under light-adapted conditions. (A) Fluorescence images (left) and sample traces (right) showing that type 2 and 3a/b OFF BCs display low background spontaneous inhibitory activity compared to type 4 OFF BCs. Scale bars: 10 µm. (B) Summary plot showing the difference in the amplitude and frequency of sIPSCs in the different OFF BC types recorded. Note that type 3a and 3b OFF BCs differ in their sIPSC amplitude, but not in frequency, whereas type 4 displays a comparatively higher amplitude and frequency compared to the other cell types. In all subsequent figures, types 2 and 3 OFF BCs are grouped as LFCs and type 4 OFF BC is referred to as HFCs. Summary data display mean ± SEM and open circles represent a single cell. **P < 0.01, ***P < 0.001; ns, not significant.
OFF BCs Receive Differential GABAergic and Glycinergic Inputs
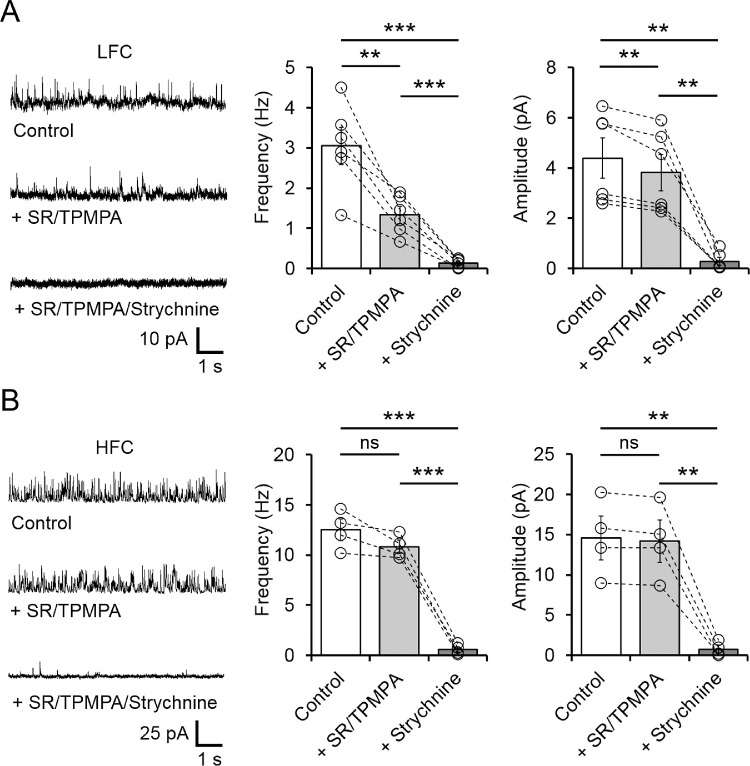
OFF BCs receive both GABAergic and glycinergic inhibition,30,31,38 whose contribution depends on the BC type and on the level of light adaptation.33 Under our experimental conditions, the amplitude and frequency of sIPSCs recorded in the LFC group were partially reduced by bath application of the GABAA and GABAρ receptor antagonists SR-95531 (10 µM) and TPMPA (50 µM), respectively (Amplitude: from 4.39 ± 0.73 to 3.82 ± 0.66 pA, n = 6, P = 0.009; Frequency: from 3.05 ± 0.47 to 1.33 ± 0.21 Hz, n = 6, P = 0.004; Fig. 2A). The remaining component was eliminated by addition of the competitive glycine receptor antagonist strychnine (5 µM; Fig. 2A), indicating that inhibition in the LFC group is mediated by both GABAergic and glycinergic inputs. Both GABAergic and glycinergic currents were abolished in axotomized LFC OFF BCs (Supplementary Fig. S1), reflecting synaptic feedback inputs from amacrine cells to axons and synaptic terminals in the inner retina. In contrast, spontaneous feedback IPSCs recorded in the HFC group were unaffected by bath application of GABA receptor antagonists (Frequency control: 12.51 ± 0.94 vs. SR/TPMPA: 10.82 ± 0.57 Hz, n = 4, P = 0.088; Amplitude Control: 14.59 ± 2.36 to 14.20 ± 2.27 pA, n = 4, P = 0.095), but were eliminated by strychnine (Frequency; P = 0.0010; Amplitude; P = 0.0061; Fig. 2B) or in axotomized HFC OFF BC (Supplementary Fig. S1), indicating that inhibitory activity in this population is mainly mediated by glycinergic amacrine cells in the inner retina.
Figure 2.
Spontaneous activity in LFC comprises GABAergic and glycinergic inputs, whereas spontaneous activity in HFC is mainly mediated by glycinergic inputs. (A) sIPSC sample traces (left) and summary plot of frequency (middle) and amplitude (right) from the LFC group showing that sIPSC are partially reduced by GABA receptors antagonists (TPMPA, 50 µM/SR 10 µM) and the remaining component is eliminated by blocking glycine receptors with strychnine (5 µM). (B) The sIPSCs in HFC are mainly mediated by glycine receptors, as GABA receptors antagonists (TPMPA, 50 µM/SR 10 µM) had no significant effect on the frequency and amplitude of sIPSCs. Blocking glycine receptors with strychnine completely eliminated sIPSC in the HFC group. Summary data show mean ± SEM and open circles represent a single cell. **P < 0.01, ***P < 0.001; ns, not significant.
Activation of CB1R Selectively Modified GABAergic, But Not Glycinergic IPSCs in OFF BCs
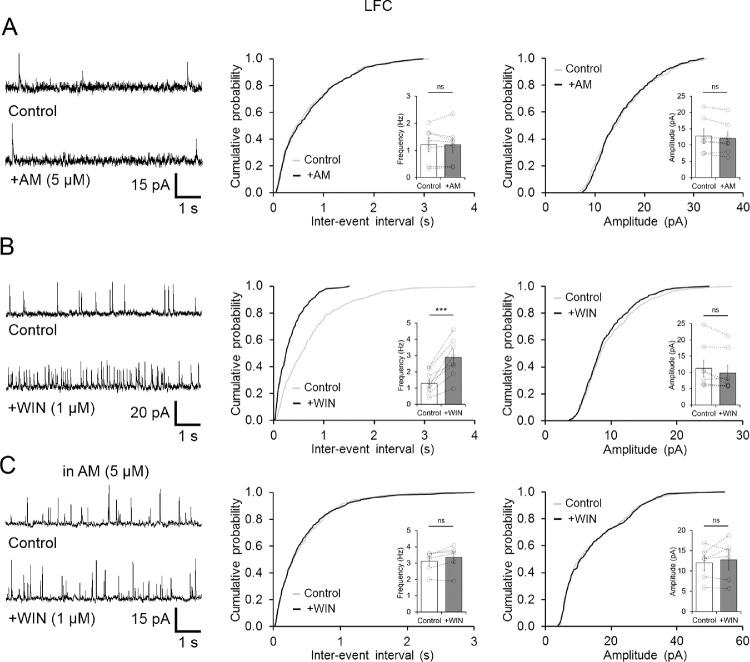
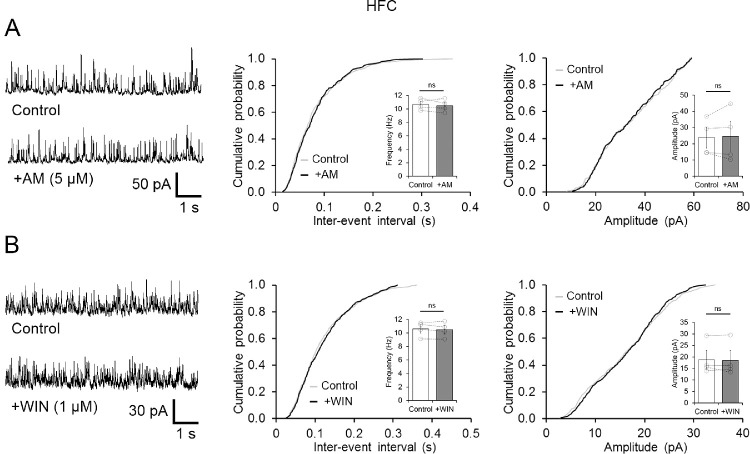
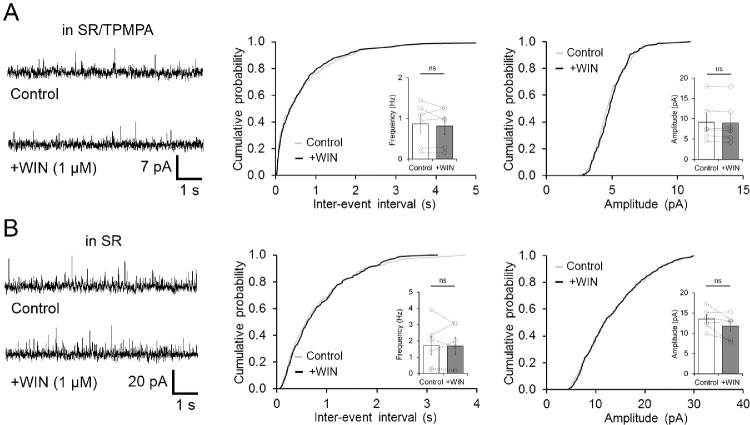
In rat retina, CB1Rs seem to be expressed throughout the entire inner retina and in a subset of amacrine cells,5,11 suggesting that CB1Rs participated in the control of GABAergic and glycinergic signaling to OFF BCs. In order to examine whether under light-adapted conditions retinal eCBs could be released to regulate feedback activity, a CB1R antagonist (AM251, 5 µM) was applied, whereas sIPSC were recorded in both OFF BC groups. Bath application of AM251 for 10 minutes, however, did not produce any significant change in the frequency of sIPSC in the LFC group (n = 7, P = 0.9413, Fig. 3A) nor in the HFC group (n = 4, P = 0.4748, Fig. 4A). Likewise, no changes in the amplitude of sIPSC in both OFF BC groups were observed (LFC: n = 7, P = 0.0622; Fig. 3A; HFC: n = 4, P = 0.7632; Fig. 4A). This result argues against a basal tone of eCBs in rat retina that regulates spontaneous inhibitory activity at OFF BCs. Conversely, in the LFC group, bath application of the specific CB1 receptor agonist WIN (1 µM) significantly increased sIPSC frequency in intact OFF BCs (1.28 ± 0.27 to 2.89 ± 0.47 Hz, n = 8, P < 0.0006) but not their amplitude (11.26 ± 2.53 to 9.8 ± 2.32 pA, n = 8, P = 0.0604; Fig. 3B). Importantly, this increase in the frequency of sIPSC was absent in axotomized LFC OFF BCs (Supplementary Fig. S1C), reflecting a direct effect of CB1R on inhibitory feedback to the axon and synaptic terminals of LFC BCs. Moreover, this effect was mediated by activation of CB1R, as in the continuous presence of the CB1R antagonist AM251 (5 µM), WIN no longer increased the frequency of sIPSCs (n = 6, P = 0.053; Fig. 3C). In contrast, in the HFC group, activation of CB1R did not exert any effect on sIPSC frequency (10.6 ± 0.61 to 10.49 ± 0.65 Hz, n = 4, P = 0.484) nor on their amplitude (18.8 ± 4.06 to 18.5 ± 4.31 pA; n = 4, P = 0.6840; Fig. 4B), indicating that CB1Rs selectively influence spontaneous inhibitory feedback to LFC but not HFC. Moreover, when GABAergic activity was blocked with SR-95531 and TPMPA, bath application of WIN had no effects on the frequency of LFC sIPSCs (Control: 0.87 ± 0.24 vs. WIN: 0.81 ± 0.21 Hz, n = 6, P = 0.4911; Fig. 5A), nor on HFC sIPSCs (10.01 ± 0.45 to 9.93 ± 0.53, n = 4, P = 0.5993; Supplementary Fig. S2). Similarly, blockage of GABAARs alone with SR-95531 also eliminated the effect of WIN in the frequency of LFC sIPSCs (Control: 1.72 ± 0.57 vs. WIN: 1.69 ± 0.53 Hz, n = 6, P = 0.9248; Fig. 5B), indicating that GABAergic, but not glycinergic feedback inhibition in OFF BCs is modulated by the activation of CB1Rs.
Figure 3.
Activation of CB1R increases GABAergic sIPSCs in OFF BCs. (A) Sample traces and cumulative probability plots, including summary bar graphs (inset), showing that blockage of CB1R with AM251 (5 µM) had no effect on spontaneous activity in LFC OFF BCs. (B), Bath application of 1 µM WIN produced a leftward shift consistent with an increase in sIPSC frequency, but no changes in the amplitude of spontaneous activity in types 2 and 3 OFF BCs were observed. (C) The CB1R inverse agonist (AM251, 5 µM) eliminated the effect of WIN on sIPSC frequency in LFC OFF BCs. Summary data display mean ± SEM and open circles in summary bar graphs represent a single cell. ***P < 0.001; ns, not significant.
Figure 4.
Activation of CB1R does not alter glycinergic sIPSCs recorded from type 4 OFF BCs. (A) Sample traces and cumulative probability plots, including summary bar graphs (inset) showing that blockage of CB1R with AM251 (5 µM) had no effect on spontaneous activity in type 4 OFF BCs. (B) Bath application of 1 µM WIN also had no effect on the spontaneous activity in type 4 OFF BCs. Summary data display mean ± SEM and open circles in bar graphs represent a single cell. ns, not significant.
Figure 5.
Inhibition of GABA receptors eliminates the effect of CB1R activation on sIPSCs in LFC OFF BCs. (A) Sample traces and cumulative probability plots, including summary bar graphs (inset), showing that activation of CB1R with WIN (1 µM) had no effect on spontaneous activity when both GABAARs and GABACRs were blocked with SR-95531 and TPMPA, respectively. (B), Blockage of GABAARs alone also eliminated the WIN-mediated increase in the frequency of sIPSC in LFC BCs. Summary data display mean ± SEM and open circles represent a single cell. ns, not significant.
Inhibitory Inputs to ON-OFF Amacrine Cell Subtypes are Reduced by CB1R Activation
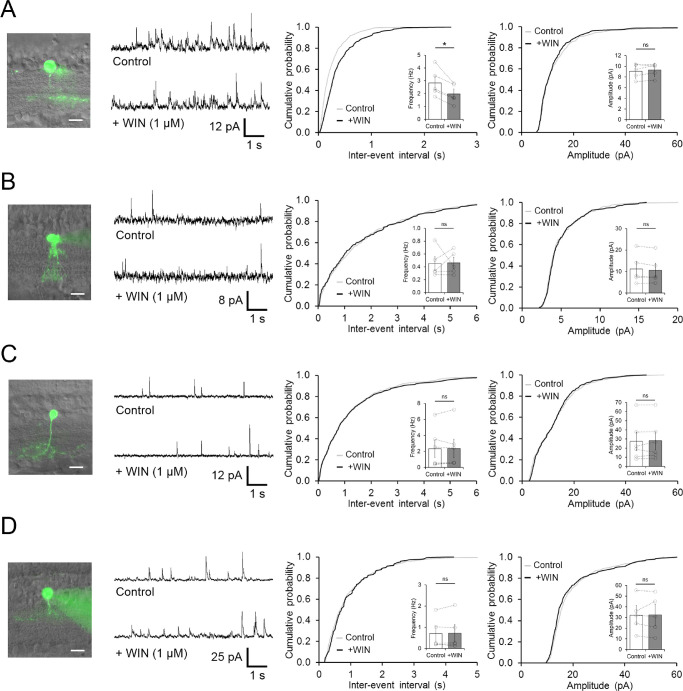
Typically, CB1R activation reduces inhibitory transmission throughout the brain1,2 and in the retinas.18,19 However, our observation that activation of CB1R increases rather than decreases spontaneous GABA release onto LFC OFF BCs suggests that other mechanism could be involved. To test whether CB1Rs regulates disinhibition between amacrine cells in the inner retina and, thus, modifies spontaneous feedback to OFF BCs, we recorded spontaneous inhibitory inputs from different amacrine cell subtypes, including the well-characterized glycinergic AII amacrine cells.39–41 Unlike inhibitory inputs to OFF BCs (Fig. 3), we found that activation of CB1R with WIN reduced rather than increased the frequency of sIPSC onto one ON-OFF amacrine cell subtype (Control: 2.84 ± 0.54 Hz vs. WIN: 1.98 ± 0.38 Hz, n = 5, P = 0.0129; Fig. 6A), whereas no significant effects were found in other amacrine cells subtypes, including AII amacrine cells (Figs. 6B−D). Although these results suggest that inhibitory interactions between amacrine cells in the inner retina is modulated by CB1R activation, it remains to be determined whether or not these ON-OFF amacrine cells are involved in the regulation of LFC BCs output.
Figure 6.
CB1R activation reduces spontaneous inhibitory activity in ON-OFF amacrine cell subtypes. (A) Activation of CB1R with WIN significantly reduces the frequency, but not the amplitude of spontaneous inhibitory activity recorded from morphologically identified ON-OFF subtypes of amacrine cells. (B), Inhibitory activity from morphological identified AII amacrine cells is unaffected by bath application of 1 µM WIN. (C,D) Bath application of WIN has no effect on the inhibitory activity in both morphological identified ON C and OFF D subtypes of amacrine cell recorded. All panels display representative fluorescence images of recorded amacrine cells (left), samples traces (middle), and cumulative probability plots (right), including summary bar graphs (inset). Images scale bars: 10 µm. Bars indicate mean ± SEM and open circles represent a single cell. *P < 0.05. ns, not significant.
Depolarization of BCs Elicits GABAergic and Glycinergic IPSCs in OFF BCs
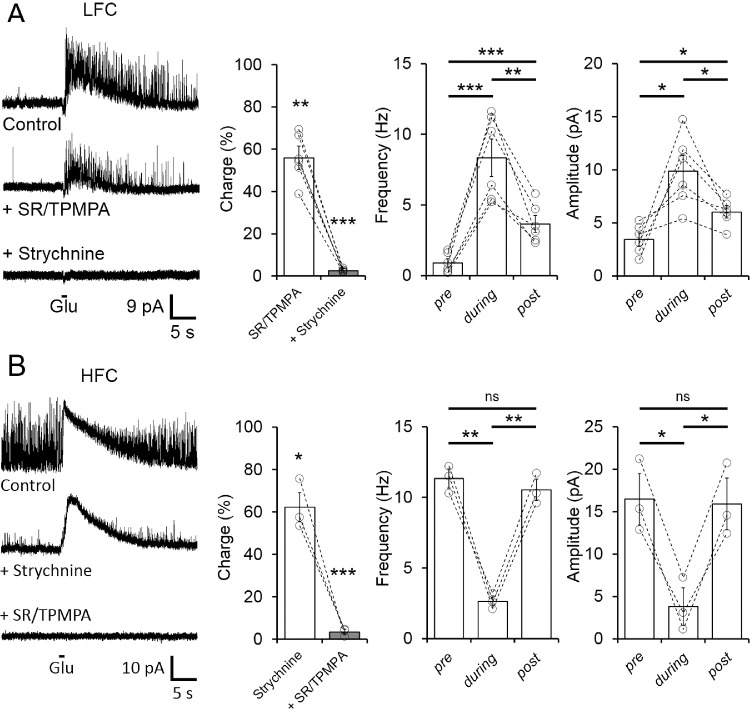
To mimic a local decrement of light intensity, a brief puff of glutamate was applied to the OPL close to the recorded cell (∼10 µm). The gIPSCs were observed in both LFC (Fig. 7A) and HFC (Fig. 7B). In LFC OFF BCs, gIPSCs were partially reduced by bath application of GABA receptor antagonists (SR/TPMPA to 55.91 ± 4.93% of control; n = 6; P = 0.002; Fig. 7A), and the remaining component was eliminated by strychnine to 2.56 ± 0.32% of control, n = 6, P < 0.0001; Fig. 7A). Similarly, gIPSC elicited in the HFC group were partially reduced by blocking glycine receptors (to 62.19 ± 6.83% of control, n = 3, P = 0.0311; Fig. 7B) and GABA antagonists eliminated the strychnine-insensitive component (to 3.41 ± 1.07% of control, n = 3, P = 0.0001; Fig. 7B), indicating that in both the LFC and HFC groups, OFF BC depolarization elicited gIPSCs comprising GABAergic and glycinergic inhibitory inputs. Interestingly, during the brief application of glutamate to the OPL, we also found that sIPSCs observed in LFC were significantly increased (Frequency from 0.88 ± 0.30 to 8.33 ± 1.33 Hz, n = 6, P < 0.0007; Amplitude from 3.45 ± 0.62 to 9.86 ± 1.50 pA, n = 6, P = 0.0154; Fig. 7A), an effect that was maintained for up to 20 seconds after the stimulus onset. In contrast, in the HFC group, the frequency and amplitude of sIPSC decreased significantly during the stimulus (Frequency from 11.33 ± 0.55 to 2.63 ± 0.32 Hz, n = 3, P = 0.0084; Amplitude from 16.47 ± 2.47 to 3.81 ± 1.80 pA, n = 3, P = 0.0126), and returned to the original values 20 seconds poststimulus (Fig. 7B).
Figure 7.
Brief application of glutamate to the OPL depolarizes OFF BCs and elicits IPSCs comprising GABAergic and glycinergic activity. (A) Sample traces (left panel) and summary plot (middle panel) showing that glutamate(Glu)-evoked inhibitory activity in the LFC group was partially reduced by GABA receptor antagonists (TPMPA, 50 µM/SR 10 µM) and the remaining component was eliminated by the glycine receptor antagonist strychnine (5 µM). Note that during glutamate-induced IPSCs, a significant increase in the frequency and amplitude of inhibitory activity was observed (right panels). (B) Glutamate-elicited IPSCs in HFC cells (left panel) were reduced by strychnine application and eliminated by GABA receptor antagonists (middle panel). Unlike LFC A, glycinergic spontaneous activity in the HFC decreases during the glutamate-evoked response (right panel). Summary data consists of mean ± SEM and open circles represent a single cell. The charge transfer is indicated as percentage of control; *P < 0.05, **P < 0.01, ***P < 0.001. ns, not significant.
Depolarization of BCs Induces Endocannabinoid Release That Regulates Evoked GABAergic Inhibitory Feedback to OFF BCs
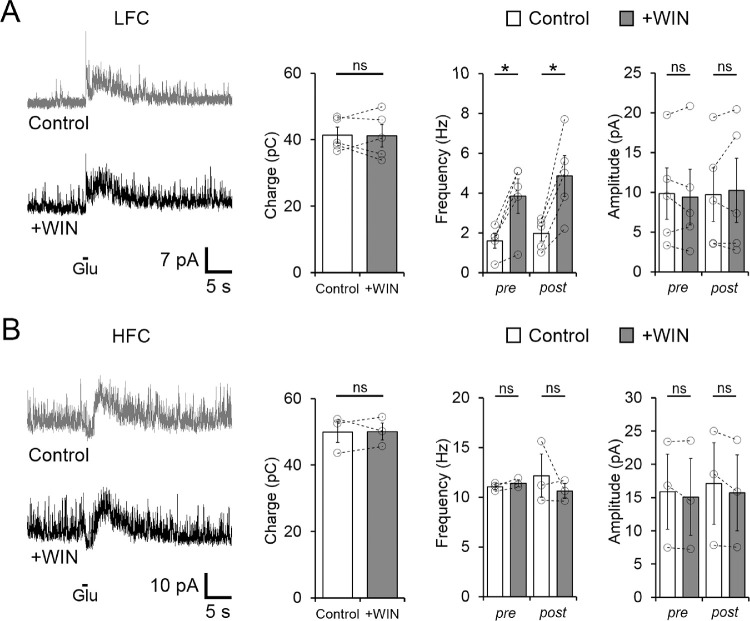
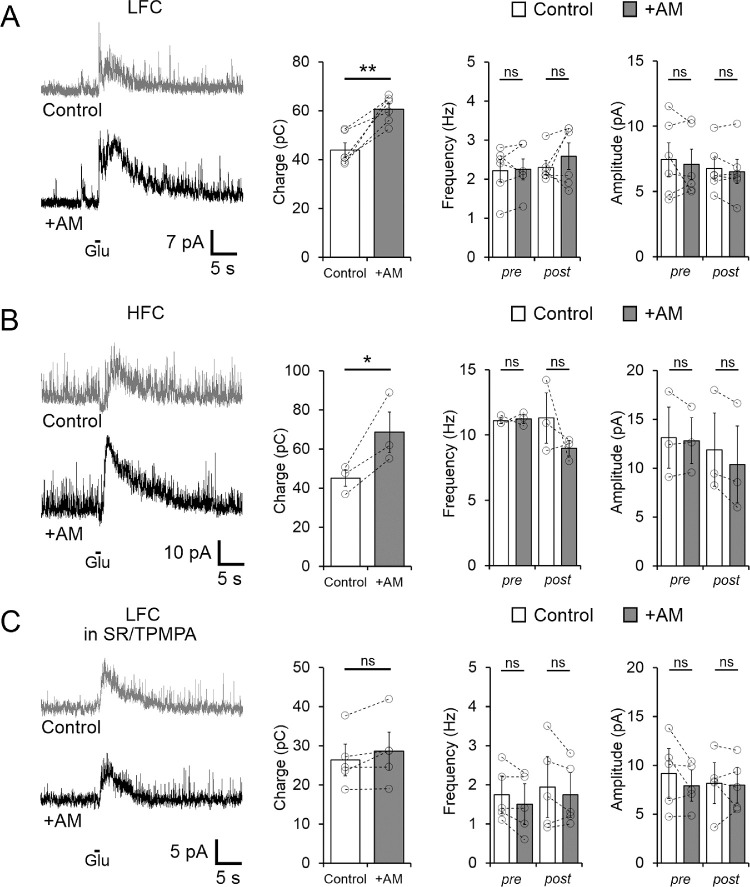
In order to determine whether gIPSCs could also be influenced by the activation of CB1Rs, we compared the charge transfer of the gIPSC before and after bath application of the CB1R agonist WIN (1 µM). Surprisingly, in the LFC group, WIN did not cause changes in the total charge of gIPSC (Control: 41.38 ± 2.38 vs. WIN: 41.15 ± 3.39 pC, n = 5, P = 0.9018; Fig. 8A). However, in the same cells, WIN significantly increased the sIPSC frequency (pre gIPSC from 1.60 ± 0.37 to 3.84 ± 0.87 Hz, P = 0.011, n = 5; Fig. 8A), indicating that different types of amacrine cells are responsible for spontaneous and evoked inhibitory inputs onto OFF BCs in the LFC group, and that CB1R signaling might differentially regulate them. Likewise, in the HFC group, the charge transfer of gIPSCs also remained unchanged after bath application of WIN (P = 0.955) and no differences in sIPSC frequency were observed (Fig. 8B), suggesting that the amacrine cells providing inhibitory inputs to the HFC group are not regulated by CB1R signaling. However, the effect of WIN on sIPSC but not on gIPSC in the LFC group opens the possibility that depolarization of BCs and/or amacrine cell activation by the application of glutamate to the OPL could engage eCB release, which, in turn, activates CB1Rs located in OFF BCs and/or amacrine cells to regulate gIPSCs. If this were the case, blocking CB1Rs should increase the charge of gIPSCs. To test this possibility, we evaluated the effect of bath application of AM251 on gIPSCs and found a significant increase in the total charge in both LFC and HFC (LFC control: 43.91 ± 2.93 vs. AM251: 60.57 ± 2.43 pC, n = 6, P = 0.0032; HFC control: 44.98 ± 4.19 vs. AM251: 68.55 ± 10.34 pC, n = 3, P = 0.0363; Figs. 9A,B). No further difference in the frequency of sIPSCs recorded in the presence of AM251, pre- and post-glutamate-evoked IPSCs, was observed in both the LCF (pre: P = 0.813; post: P = 0.358; Fig. 9A), and the HFC groups (pre: P = 0.8075; post: P = 0.364; Fig. 9B), which is consistent with an absence of an eCB tone in regulating inhibitory spontaneous activity under our experimental conditions (Fig. 3A).
Figure 8.
Activation of CB1Rs does not alter exogenous glutamate-evoked IPSCs, but increases the frequency of spontaneous GABAergic IPSCs. (A) Sample traces (left panel) and summary plot (middle panel) showing that bath application of 1 µM WIN did not exert any effect on the glutamate-evoked IPSC charge transfer, but in the same cell WIN significantly increased the frequency, but not the amplitude of GABAergic sIPSCs even after the exogenous application of glutamate (right panel). (B) Glutamate-elicited IPSCs in HFC cells (left panel) were unaffected by bath application of 1 µM WIN (middle panel). Summary data display mean ± SEM and open circles represent a single cell. *P < 0.05. ns, not significant.
Figure 9.
Inhibition of CB1R enhances exogenous glutamate-evoked IPSCs, but not spontaneous IPSCs. (A) Sample traces (left panel) and summary plot (right panel) showing glutamate-evoked IPSCs in LFC OFF BCs before and after bath application of the CB1R inverse agonist AM251. Although an increase in the charge transfer of evoked IPSCs was observed (middle panel) in the same cell sIPSC frequency and amplitude remained unchanged (right panel). (B) Charge transfer of glutamate-elicited IPSCs in type 4 OFF BCs (HFC; left panel) was also significantly increased by bath application of 5 µM AM251 (middle panel). No further effect of AM251 on sIPSC frequency was observed (right panel). (C) In the continuous presence of GABA receptor antagonists (SR/TPMPA), the charge transfer of glutamate-elicited IPSCs in types 2 and 3 OFF BCs (HFC; left panel) remained unchanged (middle panel), indicating that AM251 affected GABAergic but not glycinergic evoked transmission. Summary data display mean ± SEM and open circles represent a single cell. *P < 0.05, **P<0.01. ns, not significant.
Moreover, in the presence of GABA receptor antagonists (SR/TPMPA) to eliminate fast GABAergic inputs, blockage of CB1R with AM251 did not affect the charge transfer of isolated glycinergic gIPSC in the LFC group (Control: 26.34 ± 3.14 vs. in AM251: 28.58 ± 3.79, n = 5, P = 0.097; Fig. 9C) nor the frequency (P = 0.08) or amplitude of sIPSCs (P = 0.223), consistent with the idea that cannabinoid signaling selectively regulates GABAergic but not glycinergic evoked inhibitory transmission onto OFF BCs in the LFC group. Moreover, these results support the idea that during the depolarization of BCs by glutamate application to the OPL, eCBs are produced and released to modulate either glutamate release directly from OFF BC terminals or GABA release from amacrine cells. To further evaluate whether CB1R controls glutamate release and/or OFF BC activity directly, voltage-activated Ca2+ and K+ currents were recorded from LFC OFF BCs. CB1R activation, however, had no significant effects on Ca2+ (P = 0.0976) and K+ currents in BCs (P = 0.1117; Supplementary Fig. S3), whereas it significantly reduced voltage-activated Ca2+ and K+ currents in a subset of retinal ganglion cells (Ca2+: P = 0.0182; K+: P = 0.0025; Supplementary Fig. S3), suggesting that LFC OFF BCs do not express functional CB1Rs to alter glutamate release, and leaving open the possibility that CB1Rs located downstream of OFF BC might be responsible for the increase in the gIPSCs.
Discussion
The present study identifies CB1R as a regulator of visual signaling in the inner retina, exerting differential effects on amacrine cells that mediate spontaneous and evoked inhibitory feedback onto different types of OFF BCs. We report that activation of CB1Rs selectively increases spontaneous GABAergic, but not glycinergic feedback inhibition onto types 2, 3a, and 3b OFF BCs. Moreover, we provide evidence that OFF BC depolarization induced eCB-mediated effects on evoked feedback IPSCs, a phenomenon that was also cell- and synapse-specific, affecting GABAergic but not glycinergic inhibitory signaling. Although our study does not directly identify the amacrine cell subtypes regulated by CB1R in the OFF pathway, it suggests a specialization of cannabinoid signaling to selectively regulate GABAergic feedback inhibition onto a subset of BCs and inhibitory inputs to ON-OFF amacrine cells, supporting the notion that cannabinoid signaling plays an important role in the fine-tuning of visual processing in the inner retina.
Different Amacrine Cell Types Provide Inhibitory Input to OFF BCs
The relative contribution of GABA and glycine receptors to the regulation of OFF BC output depends on the degree of light adaptation and the OFF BC subtype.33 Under our experimental conditions, we found that types 2, 3a, and 3b OFF BCs in rat retinal slices receive both GABAergic and glycinergic inputs, whereas type 4 OFF BCs receive mainly glycinergic inputs (Fig. 2). Although the glycinergic input observed in type 4 OFF BCs could reflect AII amacrine cell signaling via ON pathway activation, we cannot rule out other sources of glycinergic input, because at least eight different types of glycinergic amacrine cells can be distinguished in rat retina.42 Potential candidates are glycinergic amacrine cell type 7, which has a sustained light response and its arborization stratifies in the ON-OFF sublayer of the IPL,43 and type 8, which receives input from ON cone BCs through gap junctions, and provide inhibitory input via glycine receptor to OFF cone BCs. Regarding types 2, 3a, and 3b OFF BCs, potential candidates for glycinergic inputs are types 2 and 6 amacrine cells, due to their transient OFF light response.43
For the GABAergic amacrine cells, the picture is more complex due to the lack of morphological and physiological characterization of most wide-field GABAergic amacrine cells that make synaptic contact with OFF BCs. The low frequency of sIPSC in types 2, 3a, and 3b OFF BCs (Fig. 1) could reflect a low rate of spontaneous GABA release arise from subtypes of GABAergic amacrine cells17 or the contribution of serial inhibition between GABAergic amacrine cells, which has been shown to operate under light-adapted conditions.33 In contrast, the contribution of GABAergic inputs to glutamate-evoked IPSCs (Fig. 7A), suggests that additional GABAergic amacrine cells activated by the OFF cone pathway are involved in the inhibitory inputs to these OFF BCs. Further studies are required to determine the specific subtype of glycinergic and GABAergic amacrine cells that make synaptic contact with different OFF BCs subtypes.
Cannabinoid Signaling Selectively Modulates GABAergic Inhibition in OFF BCs
Typically, CB1R activation inhibits neurotransmitter release at synapses using two main mechanisms, inhibition of presynaptic Ca2+ influx through voltage-gated Ca2+ channels and inhibition of adenylyl cyclase, and downregulation of the cAMP/PKA pathway.1,2 Consistent with this idea and the extensive expression of CB1R in the IPL, including BC terminals and amacrine cells processes,5–10,12,35 it has been reported that CB1R activation reduces L-type VGCC currents in goldfish BCs,7 and also reduces GABAergic and glycinergic inputs to rat and mouse ganglion cells.18,19 However, our observation that activation of CB1R increases rather than decreases spontaneous GABA release onto types 2 and 3 OFF BCs (Fig. 3), without affecting the Ca2+ and K+ conductances in OFF BCs (Supplementary Fig. S3), suggest that additional mechanism could be involved. For instance, in cultured amacrine cells from chicken embryos,17 amacrine cells showing a low initial rate of spontaneous GABA release responded to CB1R agonists with an increase in release, caused by a Gi/o-mediated reduction in cAMP. Alternatively, CB1Rs activation in the amacrine cells connect to types 2 and 3 OFF BCs might lead to phospholipase C-dependent Ca2+ mobilization from internal stores,44 which, in turn, stimulates GABA release onto OFF BCs. Moreover, our observation that inhibitory inputs onto ON-OFF amacrine cells are reduced rather than increased upon CB1R activation (Fig. 6), opens the possibility that cannabinoid signaling regulates serial inhibition between amacrine cells in the inner retina, and thereby also feedback inhibition onto OFF BCs (Fig. 10A). However, further experiments are required to determine the exact mechanism(s) by which CB1R increase spontaneous feedback activity onto OFF BCs.
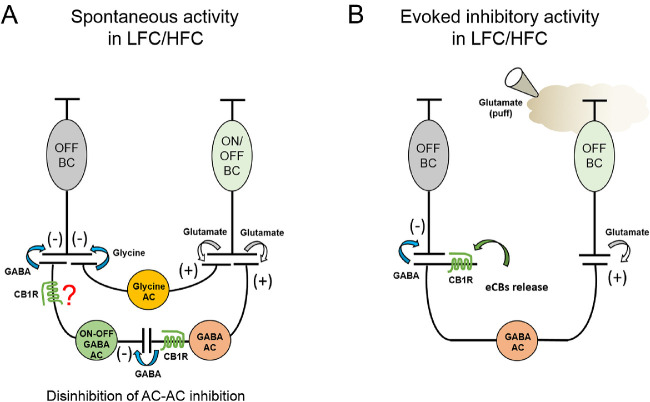
Figure 10.
Potential mechanisms underlying CB1R-mediated effects in the OFF BCs. (A) Under light-adapted conditions, CB1R activation selectively increases GABAergic sIPSCs by a direct regulation of GABA release from a subset of amacrine cells (question mark) or indirectly by regulating serial lateral inhibition between GABAergic amacrine cells that mediates inhibitory inputs to types 2 and 3 OFF BCs, rather than by regulating glutamate release from OFF BCs. By shutting down inhibitory inputs between GABAergic amacrine cells (disinhibition), CB1Rs could influence the intrinsic excitability and, thus, produce an increase in the GABA release probability onto OFF BCs. (B), Depolarization of OFF BCs by brief application of glutamate in the OPL boosts amacrine cell activity, thus engaging the release of eCBs, which likely activates CB1Rs localized in a subtype of GABAergic amacrine cells that regulate evoked feedback IPSCs. Alternatively, a strong activation of GABAergic amacrine cells might produce a CB1R-dependent self-inhibition that inhibits neuronal firing,45 thereby reducing GABA release onto OFF BCs. AC, amacrine cell; Gly, glycine; ON and OFF BC, ON and OFF bipolar cell; (+), glutamate; (-), GABA/glycine.
The observation that activation of CB1Rs did not exert any effect on glycinergic spontaneous and evoked IPSCs suggests a selective expression of CB1R in GABAergic, but not at glycinergic amacrine cells that signal onto types 2, 3a, and 4 OFF BCs. Recent evidence, however, suggests that CB1Rs are present in glycinergic AII amacrine cells and that their activation reduces spontaneous glycinergic signaling to ganglion cells.19 If this were the case, our pharmacological and electrophysiological data could reflect a spatial segregation of CB1R within a single glycinergic amacrine cell across multiple sublaminas within the IPL to differentially regulate inhibitory inputs to ganglion cells,19 but not OFF BC output.
Unlike sIPSCs, we found that inhibition rather than activation of CB1R enhanced glutamate-evoked IPSCs (Fig. 9). These observations suggest that during OFF BC depolarization elicited by glutamate stimulation, eCBs are produced in the inner retina to regulate evoked inhibitory inputs (Fig. 10B). In the mouse retina, type 1 OFF BC is a unique BC type defined by diacylglycerol lipase alpha (DLGα) expression,35 an enzyme implicated in the production of the eCB 2-Arachidonoylglycerol (2-AG). DGLα is also widely and diffusely distributed throughout the IPL likely in a subset of amacrine and ganglion cells.35 Similarly, fatty acid amide hydrolase (FAAH), an enzyme known to hydrolyze the eCB anandamide (AEA), has also been found in dendrites of ganglion cells that project to the OFF sublayer of the IPL9 and in some amacrine cells.35 Although the exact identity of the eCB involved in the regulation of evoked IPSCs and the localization of CB1R within the OFF pathway remains elusive, different scenarios could explain the increase in evoked transmission. In one scenario, activation of amacrine and/or ganglion cells by glutamate release from OFF BCs could induce 2-AG or AEA release that acts retrogradely on CB1Rs located in OFF BC synaptic terminals to decrease glutamate release onto the amacrine cells that mediate evoked inhibitory transmission. However, this possibility is unlikely as CB1R activation had no significant effect on voltage-activated Ca2+ and K+ channels in rat OFF BCs (Supplementary Fig. S3). Alternatively, activation of amacrine cells could produce a CB1R-dependent self-inhibition that decreases neuronal firing, thereby reducing GABA release onto OFF BCs. Such eCB-mediated nonretrograde self-inhibition has been reported in some neocortical GABAergic interneurons45 and a fraction of pyramidal neurons.46 Whether activation of CB1Rs can trigger similar forms of nonretrograde self-inhibition at GABAergic amacrine cells in mammalian retina remains to be determined.
Supplementary Material
Acknowledgments
The authors thank Jeff Diamond for a critical review of a first version of the manuscript.
Supported by the Chilean government through FONDECYT Regular #1151091 (AEC), #1171228 (OS and AHV), #1171006 (MF), FONDECYT Initiation #11191211 (AHV), and by the Millennium Institute Centro Interdisciplinario de Neurociencia de Valparaiso (CINV, P09-022F; OS and AEC), and Nucleus Biology of Neuropsychiatric Diseases (NuMIND, NC 130011; AEC and MF), two Millennium Scientific Initiative of the Ministry of Economy, Development and Tourism, Chile.
Disclosure: A.H. Vielma, None; F. Tapia, None; A. Alcaino, None; M. Fuenzalida, None; O. Schmachtenberg, None; A.E. Chávez, None
References
- 1. Hashimotodani Y, Ohno-Shosaku T, Kano M. Endocannabinoids and synaptic function in the CNS. Neuroscientist. 2007; 13: 127–137. [DOI] [PubMed] [Google Scholar]
- 2. Castillo PE, Younts TJ, Chavez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. 2012; 76: 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Katona I, Freund TF. Multiple functions of endocannabinoid signaling in the brain. Annu Rev Neurosci. 2012; 35: 529–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yazulla S. Endocannabinoids in the retina: From marijuana to neuroprotection. Prog Retin Eye Res. 2008; 27: 501–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bouskila J, Burke MW, Zabouri N, Casanova C, Ptito M, Bouchard JF. Expression and localization of the cannabinoid receptor type 1 and the enzyme fatty acid amide hydrolase in the retina of vervet monkeys. Neuroscience. 2012; 202: 117–130. [DOI] [PubMed] [Google Scholar]
- 6. Bouskila J, Javadi P, Elkrief L, Casanova C, Bouchard JF, Ptito M. A comparative analysis of the endocannabinoid system in the retina of mice, tree shrews, and monkeys. Neural Plast. 2016; 2016: 3127658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Straiker A, Stella N, Piomelli D, Mackie K, Karten HJ, Maguire G. Cannabinoid CB1 receptors and ligands in vertebrate retina: Localization and function of an endogenous signaling system. Proc Natl Acad Sci USA. 1999; 96: 14565–14570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Straiker AJ, Maguire G, Mackie K, Lindsey J. Localization of cannabinoid CB1 receptors in the human anterior eye and retina. Invest Ophthalmol Vis Sci. 1999; 40: 2442–2448. [PubMed] [Google Scholar]
- 9. Yazulla S, Studholme KM, McIntosh HH, Deutsch DG. Immunocytochemical localization of cannabinoid CB1 receptor and fatty acid amide hydrolase in rat retina. J Comp Neurol. 1999; 415: 80–90. [DOI] [PubMed] [Google Scholar]
- 10. Yazulla S, Studholme KM, McIntosh HH, Fan SF. Cannabinoid receptors on goldfish retinal bipolar cells: electron-microscope immunocytochemistry and whole-cell recordings. Vis Neurosci. 2000; 17: 391–401. [DOI] [PubMed] [Google Scholar]
- 11. Zabouri N, Bouchard JF, Casanova C. Cannabinoid receptor type 1 expression during postnatal development of the rat retina. J Comp Neurol. 2011; 519: 1258–1280. [DOI] [PubMed] [Google Scholar]
- 12. Glaser ST, Deutsch DG, Studholme KM, Zimov S, Yazulla S. Endocannabinoids in the intact retina: 3 H-anandamide uptake, fatty acid amide hydrolase immunoreactivity and hydrolysis of anandamide. Vis Neurosci. 2005; 22: 693–705. [DOI] [PubMed] [Google Scholar]
- 13. Fan SF, Yazulla S. Biphasic modulation of voltage-dependent currents of retinal cones by cannabinoid CB1 receptor agonist WIN 55212-2. Vis Neurosci. 2003; 20: 177–188. [DOI] [PubMed] [Google Scholar]
- 14. Fan SF, Yazulla S. Reciprocal inhibition of voltage-gated potassium currents (I K(V)) by activation of cannabinoid CB1 and dopamine D1 receptors in ON bipolar cells of goldfish retina. Vis Neurosci. 2005; 22: 55–63. [DOI] [PubMed] [Google Scholar]
- 15. Miraucourt LS, Tsui J, Gobert D, et al.. Endocannabinoid signaling enhances visual responses through modulation of intracellular chloride levels in retinal ganglion cells. Elife. 2016; 5: e15932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lalonde MR, Jollimore CA, Stevens K, Barnes S, Kelly ME. Cannabinoid receptor-mediated inhibition of calcium signaling in rat retinal ganglion cells. Mol Vis. 2006; 12: 1160–1166. [PubMed] [Google Scholar]
- 17. Warrier A, Wilson M. Endocannabinoid signaling regulates spontaneous transmitter release from embryonic retinal amacrine cells. Vis Neurosci. 2007; 24: 25–35. [DOI] [PubMed] [Google Scholar]
- 18. Middleton TP, Protti DA. Cannabinoids modulate spontaneous synaptic activity in retinal ganglion cells. Vis Neurosci. 2011; 28: 393–402. [DOI] [PubMed] [Google Scholar]
- 19. Wang XH, Wu Y, Yang XF, et al.. Cannabinoid CB1 receptor signaling dichotomously modulates inhibitory and excitatory synaptic transmission in rat inner retina. Brain Struct Funct. 2016; 221: 301–316. [DOI] [PubMed] [Google Scholar]
- 20. Middleton TP, Huang JY, Protti DA. Cannabinoids modulate light signaling in ON-sustained retinal ganglion cells of the mouse. Front Neural Circuits. 2019; 13: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Euler T, Haverkamp S, Schubert T, Baden T. Retinal bipolar cells: elementary building blocks of vision. Nat Rev Neurosci. 2014; 15: 507–519. [DOI] [PubMed] [Google Scholar]
- 22. Nelson R, Kolb H. Synaptic patterns and response properties of bipolar and ganglion cells in the cat retina. Vision Res. 1983; 23: 1183–1195. [DOI] [PubMed] [Google Scholar]
- 23. Werblin FS, Dowling JE. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969; 32: 339–355. [DOI] [PubMed] [Google Scholar]
- 24. Diamond JS. Inhibitory interneurons in the retina: Types, circuitry, and function. Annu Rev Vis Sci. 2017; 3: 1–24. [DOI] [PubMed] [Google Scholar]
- 25. Daw NW, Jensen RJ, Brunken WJ. Rod pathways in mammalian retinae. Trends Neurosci. 1990; 13: 110–115. [DOI] [PubMed] [Google Scholar]
- 26. Demb JB, Singer JH. Functional circuitry of the retina. Annu Rev Vis Sci. 2015; 1: 263–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sassoe-Pognetto M, Wassle H, Grunert U. Glycinergic synapses in the rod pathway of the rat retina: cone bipolar cells express the alpha 1 subunit of the glycine receptor. J Neurosci. 1994; 14: 5131–5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wassle H. Types of bipolar cells in the mouse retina. J Comp Neurol. 2004; 469: 70–82. [DOI] [PubMed] [Google Scholar]
- 29. Vielma AH, Schmachtenberg O. Electrophysiological fingerprints of OFF bipolar cells in rat retina. Sci Rep. 2016; 6: 30259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ivanova E, Muller U, Wassle H. Characterization of the glycinergic input to bipolar cells of the mouse retina. Eur J Neurosci. 2006; 23: 350–364. [DOI] [PubMed] [Google Scholar]
- 31. Euler T, Wassle H. Different contributions of GABAA and GABAC receptors to rod and cone bipolar cells in a rat retinal slice preparation. J Neurophysiol. 1998; 79: 1384–1395. [DOI] [PubMed] [Google Scholar]
- 32. Eggers ED, Mazade RE, Klein JS. Inhibition to retinal rod bipolar cells is regulated by light levels. J Neurophysiol. 2013; 110: 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mazade RE, Eggers ED. Light adaptation alters the source of inhibition to the mouse retinal OFF pathway. J Neurophysiol. 2013; 110: 2113–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mazade RE, Eggers ED. Light adaptation alters inner retinal inhibition to shape OFF retinal pathway signaling. J Neurophysiol. 2016; 115: 2761–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hu SS, Arnold A, Hutchens JM, et al.. Architecture of cannabinoid signaling in mouse retina. J Comp Neurol. 2010; 518: 3848–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chavez AE, Grimes WN, Diamond JS. Mechanisms underlying lateral GABAergic feedback onto rod bipolar cells in rat retina. J Neurosci. 2010; 30: 2330–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Euler T, Masland RH. Light-evoked responses of bipolar cells in a mammalian retina. J Neurophysiol. 2000; 83: 1817–1829. [DOI] [PubMed] [Google Scholar]
- 38. Eggers ED, McCall MA, Lukasiewicz PD. Presynaptic inhibition differentially shapes transmission in distinct circuits in the mouse retina. J Physiol. 2007; 582: 569–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marc RE, Anderson JR, Jones BW, Sigulinsky CL, Lauritzen JS. The AII amacrine cell connectome: a dense network hub. Front Neural Circuits. 2014; 8: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Demb JB, Singer JH. Intrinsic properties and functional circuitry of the AII amacrine cell. Vis Neurosci. 2012; 29: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Graydon CW, Lieberman EE, Rho N, Briggman KL, Singer JH, Diamond JS. Synaptic transfer between rod and cone pathways mediated by AII amacrine cells in the mouse retina. Curr Biol. 2018; 28: 2739–2751 e2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Menger N, Pow DV, Wassle H. Glycinergic amacrine cells of the rat retina. J Comp Neurol. 1998; 401: 34–46. [DOI] [PubMed] [Google Scholar]
- 43. Pang JJ, Gao F, Wu SM. Physiological characterization and functional heterogeneity of narrow-field mammalian amacrine cells. J Physiol. 2012; 590: 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Navarrete M, Araque A. Endocannabinoids mediate neuron-astrocyte communication. Neuron. 2008; 57: 883–893. [DOI] [PubMed] [Google Scholar]
- 45. Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004; 431: 312–316. [DOI] [PubMed] [Google Scholar]
- 46. Marinelli S, Pacioni S, Cannich A, Marsicano G, Bacci A. Self-modulation of neocortical pyramidal neurons by endocannabinoids. Nat Neurosci. 2009; 12: 1488–1490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.