Abstract
Purpose
To investigate the effects and mechanisms of the peroxisome proliferator-activated receptor alpha (PPAR-α) agonist fenofibrate on the formation of ocular surface squamous metaplasia induced by topical benzalkonium chloride (BAC) in a mouse model.
Methods
Ocular surface squamous metaplasia was induced in 16 days by topical BAC application in mice. During the period of induction, mice were divided into four groups: no additional treatment (BAC+UT), topical vehicle (BAC+Vehicle), topical fenofibrate (BAC+Feno), or topical fenofibrate plus intraperitoneal injection of MK886 (BAC+Feno+MK886). The parameters of tear film were evaluated on day 16, and eye specimens were collected. Histologic investigation; PAS assays; immunostaining for cytokeratin 10 (K10), Ki67, and F4/80; and PCR assays for TNF-α and IL-6 were performed. Cell Counting Kit 8 (CCK-8) assays were performed to evaluate the inhibitory effects of fenofibrate on RAW264.7 cells.
Results
Fenofibrate suppressed the formation of BAC-induced instable tear film. In the BAC+Feno group, the expression of K10 and Ki67 was lower than in the other three groups. The number of goblet cells was reduced in eyes of the BAC+UT and BAC+Vehicle groups but was maintained in eyes of the BAC+Feno group. The number of F4/80-positive cells and the levels of TNF-α and IL-6 mRNA were significantly reduced in the cornea of the BAC+Feno group. These effects of fenofibrate could be attenuated by MK886. The cell viability of RAW264.7 cells could be significantly inhibited by fenofibrate in a dose-dependent pattern.
Conclusions
Topical application of fenofibrate suppressed the formation of ocular surface squamous metaplasia, which might be mediated through the PPAR-α signaling pathway.
Keywords: squamous metaplasia, peroxisome proliferator-activated receptor-α, fenofibrate, macrophage, inflammation
As the most common ocular surface disease, dry eye has been extensively investigated in the past decades. Various findings suggest that, despite its multifactorial etiology, dry eye is an inflammation-based disease of the lacrimal gland functional unit, which includes the cornea, conjunctiva, main and accessory lacrimal glands, and meibomian glands.1 Tear hyperosmolarity stimulates a cascade of events in the epithelial cells of the ocular surface, involving several inflammatory signaling pathways and cytokines such as TNF-α and interleukins. These cytokines activate and recruit inflammatory cells to the ocular surface which then become an additional source of inflammatory mediators. Such mediators, acting together with tear hyperosmolarity, can lead to reduced expression of glycocalyx mucins, apoptotic death of surface epithelial cells, and loss of goblet cells. Damage is reinforced by inflammatory mediators from activated inflammatory cells. These result in tear film instability, which exacerbates and amplifies tear hyperosmolarity and completes the vicious cycle of events that aggravates ocular surface damage.2 Antiinflammatory therapy has long been considered to be one of the most important aspects in the treatment of dry eye.
Squamous metaplasia is defined as pathological transition of a nonkeratinized, stratified epithelium into a non-secretory, keratinized epithelium. It is a commonly occurring pathological process in mucous membranes such as respiratory epithelium3 and urothelium,4 as well as ocular surface epithelium.5,6 In the eye, squamous metaplasia is commonly seen in patients with long-term deficiency of tear film7 and is considered to be a hallmark of severe ocular surface disorders.8–10 In recent years, evidence has revealed a link between pathologic squamous metaplasia and chronic inflammation of the ocular surface mediated by certain types of lymphocyte/cytokines.11,12 However, treatments by commercially available antiinflammatory eye drops or operations such as amniotic membrane patching cannot obtain satisfactory results in the management of ocular surface squamous metaplasia. Further investigation is needed to find new agents with pharmacological activity for the treatment of squamous metaplasia.
Peroxisome proliferator-activated receptors (PPARs) are members of the nuclear hormone receptor superfamily of transcription factors that can be activated by lipophilic ligands. PPARs have three isoforms: α, β, and γ. PPAR-α is mainly expressed in tissues and organs in which fatty acid oxidation is active, such as liver, kidney, and skeletal muscle. PPAR-α regulates genes involved in the regulation of not only lipid metabolism but also inflammation.13 The role of PPARs in the ocular surface has been brought into focus in recent years. PPAR-γ may inhibit the expression of inducible nitric oxide synthase and MMP-9, as well as the production of TNF-α, IL-6, and IL-β.14 A few studies have also shown that PPAR-γ plays a vital role in regulating meibocyte differentiation and lipid synthesis in meibomian gland epithelial cells.15–17 However, the role of PPAR-α in the ocular surface is still unclear. Our earlier data indicate downregulation of PPAR-α expression in corneal epithelium of mice with dry eye induced by sleep deprivation.18 However, further investigation is needed to elucidate the critical role of PPAR-α in the pathogenesis of dry eye and ocular surface squamous metaplasia. We hypothesized that activation of PPAR-α by agonists, such as fibrates, could alleviate squamous metaplasia by a mechanism similar to that of PPAR-γ. In this study, a mouse model of ocular surface squamous metaplasia induced by topical benzalkonium chloride was applied and the effects of fenofibrate were evaluated to provide a better understanding of the role of PPAR-α and its agonist fenofibrate in epithelial squamous metaplasia.
Methods
Animals and Experimental Procedures
This study used 75 healthy male BALB/c mice (body weight 18–20 g; Shanghai Laboratory Animal Center, Shanghai, China), which had no clinically observable ocular surface disease. Mice were held in a standard environment throughout the study as follows: room temperature, 25°C ± 1°C; relative humidity, 65% ± 10%; and alternating 12-hour light/dark cycles (7 AM to 7 PM). All procedures were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and protocols in this study were approved by the Animal Ethics Committee of Xiamen University.
Fifteen mice were kept untreated as normal control. For 16 days, 60 mice received topical administrations of 5 µl 0.2% BAC (dissolved in PBS) twice daily (8 AM and 4 PM) to the right eyes for the induction of tear film instability.19 During the period of induction, these 60 mice were further divided into four groups (15 in each group): no additional treatment (BAC+UT), topical fenofibrate (200 µM; BAC+Feno), topical vehicle (saline containing the same volume of dimethyl sulfoxide as 200-µM fenofibrate; BAC+Vehicle), or topical fenofibrate (200-µM) plus intraperitoneal injection of MK886 (2mg/kg·d), an antagonist of PPAR-α (BAC+Feno+MK886). The topical treatment was performed at the right eye twice daily (12 AM and 8 PM) with 5 µl of solution for 16 days. The injection of MK886 was performed once daily at 10 AM (2 hours before the topical treatment).
On days 0 and 16, the mice were evaluated under slit-lamp microscope for tear film break-up time (BUT), inflammatory index of the cornea, and corneal epithelial staining scores; also, Schirmer’s test was conducted following the methods described below. Eye specimens were dissected on day 16 for histological analysis and real-time PCR as described below.
Evaluation of Inflammation in the Cornea
The severity of corneal inflammatory response was evaluated and scored by a single masked ophthalmologist under a slit lamp (BQ 900 IM9900; Haag-Streit, Köniz, Switzerland) as previously described.20 The inflammatory index was analyzed based on three parameters: ciliary hyperemia (absent, score 0; present but less than 1 mm, score 1; present between 1 and 2 mm, score 2; present at more than 2 mm, score 3); central corneal edema (absent, score 0; present with visible iris details, score 1; present without visible iris details, score 2; present without visible pupil, score 3); and peripheral corneal edema (absent, score 0; present with visible iris details, score 1; present without visible iris details, score 2; present with no visible iris, score 3). The final inflammatory index was obtained by summing the scores of the three parameters divided by a factor of 9.
Tear Film Break-Up Time and Corneal Fluorescein Sodium Staining
One microliter of 0.1% liquid fluorescein sodium was dropped into the conjunctival sac. After three blinks, BUTs were recorded in seconds using the slit lamp. Four quadrants of corneal surface staining were examined using cobalt blue light 90 seconds after the corneal surface was stained by the fluorescent paper. The four quadrants were scored as previously described21: absent, score 0; slight punctate staining with fewer than 30 spots, score 1; punctate staining with more than 30 spots but not diffuse, score 2; severe diffuse staining but no positive plaque, score 3; or positive fluorescein plaque, score 4. The scores of each quadrant were added to arrive at a final score (16 points total).
Phenol Red Thread Tear Test
The tear volume was measured by the Phenol Red Thread Tear Test using Zone-Quick Diagnostic Threads (Showa Yakuhin Kako Co., Ltd., Tokyo, Japan).22 Animals were kept immobile by intraperitoneal injection of pentobarbital (1 mg). One millimeter of the thread was placed on the palpebral conjunctiva for 15 seconds at a specified point approximately 1/3 of the distance from the lateral canthus of the lower eyelid. The red portion of the thread was measured and considered to be the final length.
Immunostaining
On day 16, five of the tissues (eyeball and orbit) were embedded in optimal cutting temperature (OCT) compound (Tissue-Tek; Sakura Finetek Japan, Tokyo, Japan) and stored at –80°C. Frozen sections (6 µm thick) were cut using a cryotome (CM 1850UV; Leica Microsystems AG, Wetzlar, Germany) and stored at –80°C. Sections were fixed in acetone and further permeated with 0.2% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA).
For immunofluorescent labeling of K10, sections were blocked and incubated at 4°C overnight with antibody of K10 (1:300; Abcam, Cambridge, UK). After further incubation in Alexa Fluor 488-conjugated secondary antibody (1:1000; Thermo Fisher Scientific, Waltham, MD, USA), sections were rinsed, counterstained with 4′,6-diamidino-2-phenylindole and photographed using a confocal laser scanning microscope (Fluoview 1000; Olympus, Tokyo, Japan).
For immunohistochemical staining of Ki67 and F4/80, the activity of endogenous peroxidases was quenched with 0.6% hydrogen peroxide for 30 minutes. After blocking with 2% BSA, the antibodies of Ki67 (1:400) and F4/80 (1:200) (Abcam) were applied and incubated at 4°C for 14 to 18 hours. Sections were further incubated with biotinylated anti-rabbit or anti-rat IgG (1:50) using Vectastain Elite ABC kits according to the manufacturer's protocol (Vector Laboratories, Burlingame, CA, USA). The reaction product was further developed with diaminobenzidine. Sections were photographed with a light microscope (Eclipse 50i; Nikon, Tokyo, Japan). Counterstaining with hematoxylin was not performed to avoid the interference of nuclear staining during examination.
Cell Culture and Cell Proliferation Assay
RAW264.7 murine macrophages were purchased from the Cell Bank of China Science Academy (Shanghai, China) and maintained in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin. Cells were seeded in 96-well culture plates in the growth medium until 70% confluence with bacterial lipopolysaccharide (LPS) (1 µg/ml) pre-incubation for 8 hours or without LPS. The culture medium was replaced with DMEM supplemented with fenofibrate at concentrations of 0, 0.01, 0.1, 1, 10, 50, and 100 µM. The RAW264.7 cell proliferation was measured after 48 hours using a Cell Counting Kit 8 (CCK-8; Dojindo Molecular Technologies, Kumamoto, Japan). Briefly, the CCK-8 was added for 2 hours, after which the oculus dexter values were recorded.
Human corneal epithelial (HCE) cells were obtained from RIKEN Biosource Center (Tokyo, Japan) and cultured in supplemented hormonal epithelial medium, comprised of DMEM-F12 supplemented with 15% FBS, bovine insulin (5 µg/ml), recombinant human epidermal growth factor (10 ng/ml), and 1% penicillin and streptomycin. For CCK-8 assay, the cells were planted in 96-well culture plates until 70% confluency. The medium was then removed and changed with the medium supplemented with fenofibrate at concentrations of 0, 0.01, 0.1, 1, 10, 50, and 100 µM. The cell proliferation was measured after 48 hours by CCK-8 assay as discussed above.
RNA Isolation and Real-Time PCR
Total RNA was extracted by using TRIzol reagent (Thermo Fisher Scientific). Reverse transcription was performed with Oligo 18T primers and reverse transcription reagents according to the manufacturer's protocol (Takara Bio Inc., Shiga, Japan). Quantitative real-time PCR was performed with mRNA special primers including the following: for TNF-α, 5′-GAGTGACAAGCCTGTAGCCCATGTTGTAGCA-3′ (forward) and 5′-GAATGATAAAGTAGACCTGCCCAGACT-3′ (reverse); for IL-6, 5′-ATGAACTCCTTCTCCACAAGCGC-3′ (forward) and 5′-GAAGAGCCCTCAGGCTGGACTG-3′ (reverse). PCR reactions were performed on a Bio-Rad CFX96 Touch Real-Time PCR Detection System (Hercules, CA, USA) with SYBR Premix Ex Taq (Takara Bio) at 95°C for 10 minutes, followed by 45 cycles at 95°C for 10 seconds, 57°C for 30 seconds, and 75°C for 10 seconds, after which melt curve analysis was performed at once from 65°C to 95°C. All of the reactions were performed in triplicate, and the average Ct values greater than 38 were regarded as negative.
Light Microscopy and PAS Assay
Hematoxylin and eosin (HE) staining was performed in the cryosections. Five specimens of the whole orbit tissue in each group were embedded in paraffin, cross-sectioned, and stained with PAS reagents (PAS Staining System 395B-1 KT; Sigma-Aldrich) and hematoxylin. The number of goblet cells in the conjunctival fornix was counted in six representative slices of homologous positions from each orbit tissue. These sections were examined using the light microscope mentioned above.
Statistical Analysis
Analysis of the significance of differences between groups was performed by using one-way ANOVA followed by a post hoc analysis Tukey test to compare the differences between the groups. P < 0.05 was considered statistically significant.
Results
Fenofibrate Ameliorated the Severity of Tear Film Instability
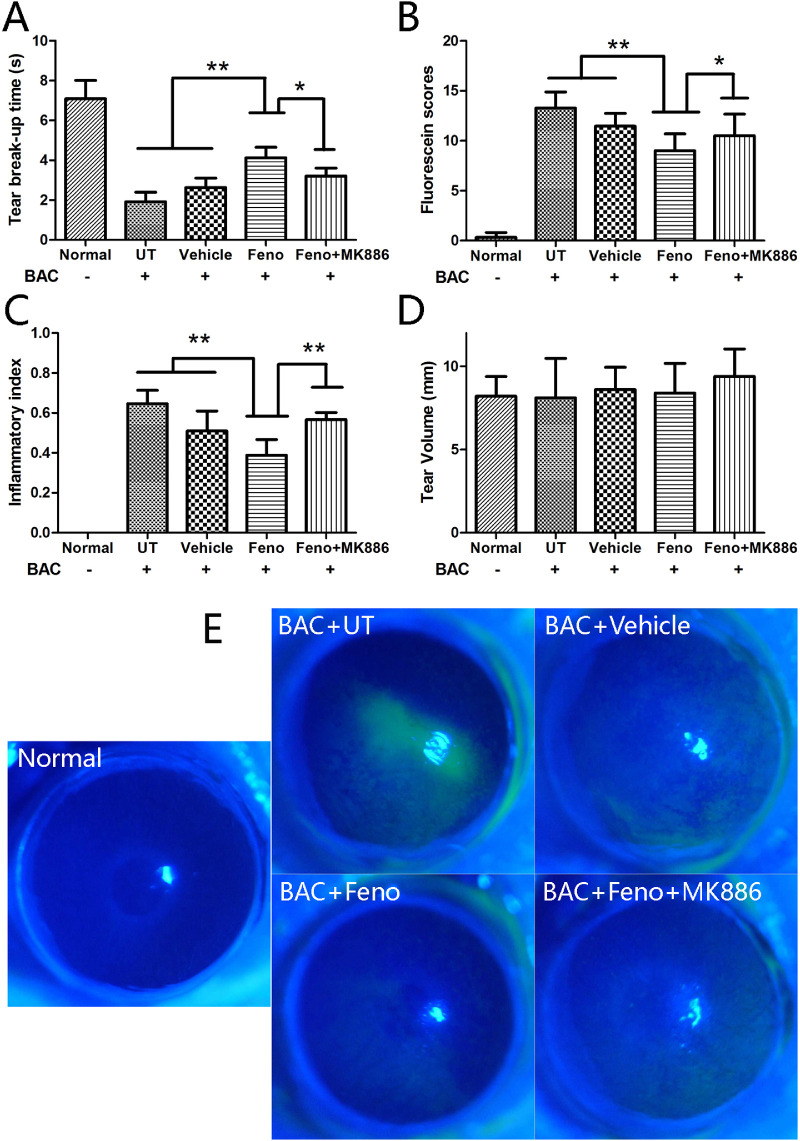
The effects of fenofibrate on the formation of tear film instability were evaluated by using the BAC-induced experimental murine dry eye settings that we established and reported19 but with modifications necessary for this study. With statistical significance, fenofibrate suppressed the formation of tear film instability after the BAC treatment for 16 days (P < 0.01), with the manifestations of longer BUT, less epithelial damage, and reduced inflammatory response in the cornea (Figs. 1A–1C). Interestingly, these effects of fenofibrate appeared to be attenuated by intraperitoneal injection of MK886 (P < 0.05); however, medicating for 16 days did not change the tear volumes among the groups (Fig. 1D). The average BUT and tear volume in the normal group were 7.1 seconds and 8.2 mm, respectively. Representative images of corneal fluorescein sodium staining are shown in Figure 1E.
Figure 1.
Fenofibrate significantly suppressed tear film instability after medication for 16 days (P < 0.01) and presented with longer BUT (A), lower fluorescein sodium staining scores (B), and reduced inflammatory index (C) in the cornea. The BUT values were longer and the fluorescein staining score and inflammatory index were lower in the BAC+Feno group when compared with the BAC+Feno+MK886 group (P < 0.05). (D) No significant difference of tear volume was observed among groups (n = 15; data presented as mean ± SD; **P < 0.01, *P < 0.05). (E) Representative images of corneal fluorescein sodium staining are provided in different groups on day 16.
Fenofibrate Inhibited Corneal Squamous Metaplasia
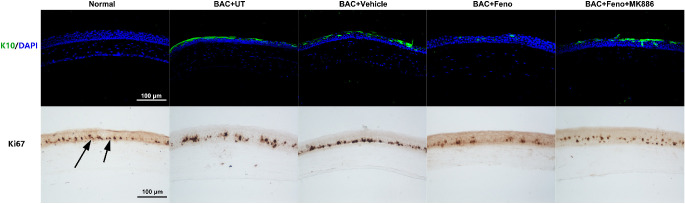
The expression of K10 keratin, an epidermal keratinocyte-specific intermediate filament, was negative in normal cornea. In the BAC+UT and BAC+Vehicle groups, K10-positive cells were apparently present in superficial cell layers of the corneal epithelium on day 16, whereas K10-positive cells were extremely sparse in eyes of the BAC+Feno group. MK886-treated eyes appeared to have more K10-positive cells than in the BAC+Feno group (Fig. 2).
Figure 2.
Representative images showing the expression and location of the K10- and Ki67-positive cells on day 16 after treatment. Levels of both K10- and Ki67-positive cells were lower in eyes of the BAC+Feno group than in eyes of the BAC+UT and BAC+Vehicle groups. MK886-treated eyes appeared to have more K10- and Ki67-positive cells than those in the BAC+Feno group.
Ki67 is known to be present during active phases of the cell cycle but absent from resting cells.23 It was demonstrated that the number of Ki67-positive cells was high in the basal and suprabasal epithelium of the eyes in the BAC+UT, BAC+Vehicle, and BAC+Feno+MK886 groups on day 16, whereas a lower number was observed in the BAC+Feno group and normal control group in the basal layer (Fig. 2).
Fenofibrate Retained Goblet Cell Density
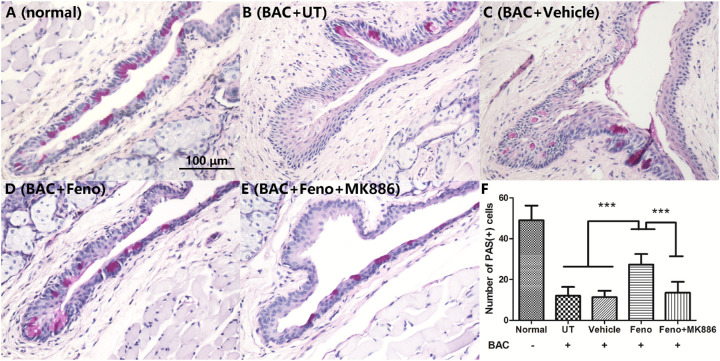
Most of the PAS-positive cells in normal mouse resided in the superficial epithelium of conjunctival fornix (Fig. 3A). The number of PAS-positive cells significantly decreased in the eyes of the BAC+UT and BAC+Vehicle groups, but the treatment of topical fenofibrate did increase the number of goblet cells significantly (P < 0.001). However, the effect of fenofibrate could be attenuated by MK886 (P < 0.001). Interestingly, the PAS-positive cells resided mainly in the superficial layer of fenofibrate-treated fornical conjunctiva. In contrast, the majority of PAS-positive cells could be observed in the basal and suprabasal epithelium of fornical conjunctiva in BAC-treated eyes without fenofibrate (Figs. 3B–3F).
Figure 3.
Representative images of the PAS assy. Most of the PAS-positive cells in normal mice resided in the superficial epithelium of the conjunctival fornix (A). The number of PAS-positive cells was significantly decreased in the BAC+UT (B), BAC+Vehicle (C), and BAC+Feno+MK886 (E) groups but was partially maintained in the BAC+Feno group (D). The average number of goblet cells in each group is shown (E). Scale bar: 100 µm. Data are presented as mean ± SD; n = 5; ***P < 0.001, **P < 0.01.
Fenofibrate Had an Antiinflammatory Effect
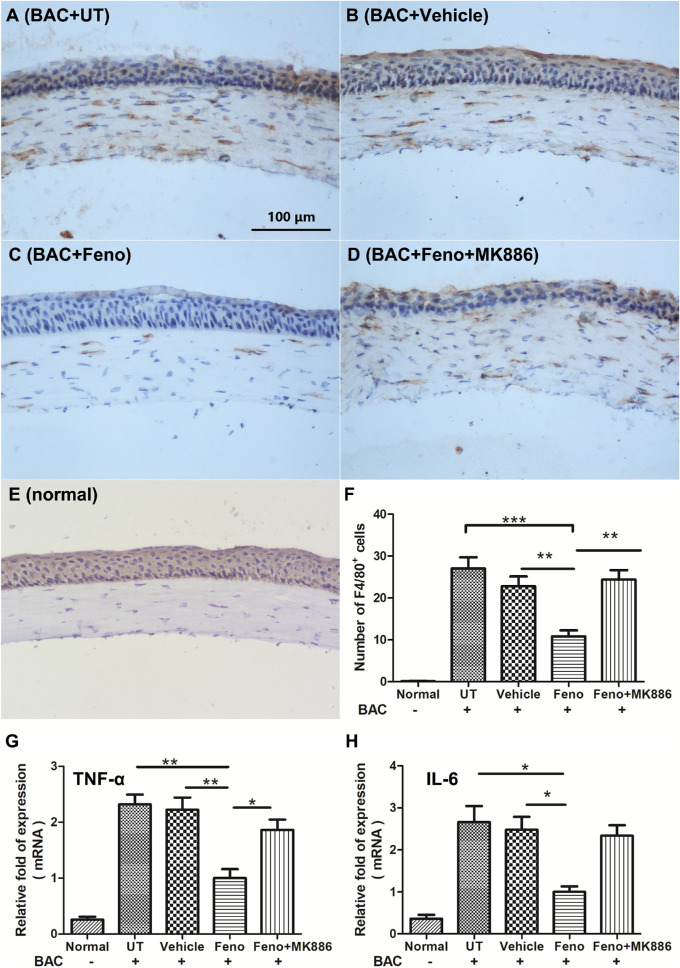
We detected F4/80, which is considered one of the key markers of classically activated macrophages. F4/80-positive cells were recorded in the corneal stroma in all of the BAC-treated groups on D16, but no F4/80-positive cells were found in the central stroma of normal cornea. Fenofibrate significantly decreased the number of F4/80-positive cells in the cornea (Fig. 4). Further, quantitative RT-PCR confirmed the downregulation of inflammatory factors TNF-α and IL-6 level in the mouse cornea of the BAC+Feno group on day 16.
Figure 4.
Representative images showing the infiltration of F4/80-positive cells in the cornea (A–E) The number of F4/80-positive cells in the fenofibrate-treated group was significantly lower than in the other three groups (F). Quantitative analysis of mRNA transcripts in the cornea on day 16 showed that the levels of both TNF-α (G) and IL-6 (H) were significantly reduced in the fenofibrate-treated group when compared with the untreated group and vehicle group. In addition, the reduction of TNF-α expression by fenofibrate treatment could be interrupted by MK886. Scale bar: 100 µm. Data are presented as mean ± SD; n = 5; ***P < 0.001, **P < 0.01, *P < 0.05.
Fenofibrate Inhibited RAW264.7 Cells In Vitro
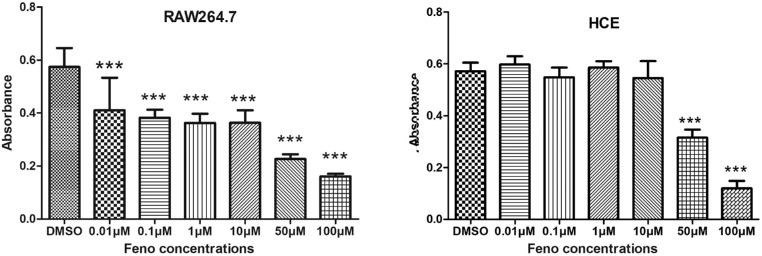
To understand whether the antiinflammatory effect of fenofibrate was induced through specific inhibition of F4/80-positive macrophages, we compared the effects of fenofibrate between RAW264.7 murine macrophages and HCE cells. At concentrations from 0 to 100 µM, fenofibrate inhibited the cell growth of RAW264.7 cells pretreated with LPS for 8 hours in a concentration-dependent manner, with a minimum effective concentration of 0.01 µM. However, fenofibrate had no inhibitory effects on the resting RAW264.7 cells. Fenofibrate did not significantly affect the growth of HCE cells at concentrations lower than 50 µM (Fig. 5).
Figure 5.
Specific inhibitory effects of fenofibrate on RAW264.7 cells. Fenofibrate inhibited the cell growth of RAW264.7 cells pretreated with LPS for 8 hours in a concentration-dependent manner with a minimum effective concentration of 0.01 µM. The HCE cells were not significantly affected by fenofibrate. Data are presented as mean ± SEM; n = 8; ***P < 0.001.
Discussion
Increasing evidence indicates that PPAR-α is important in the regulation of inflammatory processes; however, the role of PPAR-α in the ocular surface is still unclear and requires further elucidation. Previously, our data indicated downregulation of PPAR-α expression in the corneal epithelium of mice with dry eyes induced by sleep deprivation.18 In cultured corneal epithelium sheets, the PPAR-α agonist fenofibrate increased PPAR-α and restored microvilli morphology, whereas the PPAR-α antagonist MK886 attenuated these changes.18 In this study, we found novel evidence suggesting that fenofibrate could ameliorate the severity of ocular surface squamous metaplasia and suppress the formation of tear film instability in a murine model. The beneficial effects of fenofibrate might be mediated through the inhibition of F4/80-positive macrophages and subsequent downregulation of proinflammatory factors. These effects of fenofibrate could be attenuated by MK886.
Increased production and activation of proinflammatory cytokines that infiltrate the ocular surface tissues have been extensively reported in dry eye. Inflammation is considered one of the key mechanisms of dry eye, as both a cause and a consequence; therefore, antiinflammatory therapy has been considered to be a key point in management of dry eye. Moreover, inflammatory factors such as TNF-α and interleukins are critical in the formation of squamous metaplasia,24 presenting with K10 expression in the ocular surface epithelium. Our data indicate that fenofibrate has the ability to ameliorate the severity of dry eye and attenuate the severity of squamous metaplasia, probably via the inhibition of macrophages with downregulation of TNF-α and IL-6.
Two major macrophage phenotypes have been described—specifically, classically activated macrophages (CAMs) and alternatively activated macrophages (AAMs). CAMs, also referred to as M1 macrophages, are induced by stimulation with IFN-γ and microbial products, such as LPS. These macrophages produce several proinflammatory cytokines such as TNF-α and IL-6, recruiting more inflammatory cells and triggering downstream inflammatory cascades. M1 macrophages also have enhanced antigen-presenting ability. In contrast, AAMs, also referred to as M2 macrophages, are induced during Th2-type responses, such as allergic responses or those elicited by helminthic infection and during allergic responses. Activation of M2 macrophages is dependent upon stimulation with IL-4/IL-13, as well as some helminth antigens.25 In our study, fewer F4/80-positive cells were recorded in the fenofibrate-treated corneal stroma compared with those in other BAC-treated eyes. Furthermore, only the RAW264.7 cells pretreated with LPS in vitro could be inhibited by fenofibrate in a concentration-dependent manner, indicating that fenofibrate had an impact on M1 macrophages but not resting macrophages. These results were in principle consistent with the previous study by Ann et al.,26 who demonstrated that upregulation of β-defensin 1 was responsible for the inhibition of J774 macrophages by fenofibrate. Additional mechanisms of fenofibrate in the inhibition of M1 macrophages are still under investigation. Meanwhile, the effects of fenofibrate on M2 macrophages, as well as on other types of inflammatory cells, also require further study.
Macrophages play a vital role in initiating, maintaining, and resolving host inflammatory responses; however, macrophages also have deleterious effects on the host by inducing proinflammatory cytokines. These deleterious effects aggravate tissue damage and are responsible for many pathologic conditions associated with acute and chronic inflammation.27 Also, suppression of M1 macrophages or induction of M2 macrophages achieved by medication or surgery can contribute to the alleviation of several ocular surface diseases.28,29 However, the role of macrophages in the development of epithelial squamous metaplasia is still unclear except for some types of squamous carcinoma.30,31 You et al.32 revealed the increase of M1 macrophage markers in an experimental murine dry eye model induced by desiccating stress without the reduction of M2 macrophage markers, whereas Lee et al.33 found that M1 macrophages were predominant and accompanied by suppressed M2 phenotypes in another experimental dry eye model induced by subcutaneous scopolamine. However, the precise role of different phenotypes of macrophages was still unknown. Our data also revealed the specific inhibition of F4/80-positive macrophages by fenofibrate in vivo, raising the possibility that activated macrophages in the early stages might be a critical component in the pathogenesis of squamous metaplasia and even dry eye. Inhibition of M1 macrophages might be an alternative therapeutic target in the management of dry eye, but this hypothesis requires extensive research in the future.
The main functions of goblet cells are synthesizing, storing, and secreting some of the mucous components of the tear film.34 The cell number could be significantly reduced under chronic or severe ocular surface insults such as BAC application.19,35 Our data showed that the number of goblet cells could apparently be maintained by fenofibrate while being significantly reduced in the other three BAC-treated groups. Increased goblet cells, as well as decreased inflammatory cytokines, might be factors important to improving tear film stability. Unfortunately, no studies so far have explored the direct effects of fenofibrate on cultured goblet cells. For the corneal epithelium, low concentrations of fenofibrate had no effect on cell proliferation, but concentrations higher than 50 µM showed a toxic effect (Fig. 5).
In summary, topical application of fenofibrate demonstrated clinically observable suppression of the formation of BAC-induced tear film instability, in addition to decreasing the inflammatory response and alleviating the ocular surface squamous metaplasia. These effects could be attenuated by MK886, a PPAR-α antagonist. Fenofibrate could reduce the inflammatory response by the inhibition of macrophage proliferation and offers great potential for use as a preventive agent in patients with high risks of dry eye.
Acknowledgments
The authors thank Wei Li (Eye Institute of Xiamen University, China) for the valuable suggestions regarding the instructions in the experiment.
This research was supported in part by grants from the National Natural Science Foundation of China (81570816 and 81570815), Xiamen Science and Technology Program for Public Wellbeing (3502Z20174003), Xiamen Medical and Health Project (3502Z20189024), Huaxia Translational Medicine Fund for Young Scholars (2018-A-006), and Research Project of Health and Family Planning for Youth in Fujian Province (2017-2-117, 2018-2-79). Sponsors or funding organizations had no role in the design or conduct of this research.
Disclosure: H. He, None; M. Liang, None; L. Li, None; S. Luo, None; X. Fang, None; H. He, None; X. Xiao, None; H. Wu, None; Z. Lin, None
Reference
- 1. Li X, Xu B, Wang Y, Wei L. Anti-inflammatory effect of peroxisome proliferator-activated receptor-γ (PPAR-γ) on non-obese diabetic mice with Sjogren's syndrome. Int J Clin Exp Pathol. 2014; 7: 4886–4894. [PMC free article] [PubMed] [Google Scholar]
- 2. Bron AJ, de Paiva CS, Chauhan SK, et al.. TFOS DEWS II pathophysiology report. Ocul Surf. 2017; 15: 438–510. [DOI] [PubMed] [Google Scholar]
- 3. Leube RE, Rustad TJ.. Squamous cell metaplasia in the human lung: molecular characteristics of epithelial stratification. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991; 61: 227–253. [DOI] [PubMed] [Google Scholar]
- 4. Lagwinski N, Thomas A, Stephenson AJ, et al.. Squamous cell carcinoma of the bladder: a clinicopathologic analysis of 45 cases. Am J Surg Pathol. 2007; 31: 1777–1787. [DOI] [PubMed] [Google Scholar]
- 5. Tseng SC. Staging of conjunctival squamous metaplasia by impression cytology. Ophthalmology. 1985; 92: 728–733. [DOI] [PubMed] [Google Scholar]
- 6. Beitch I. The induction of keratinization in the corneal epithelium. A comparison of the “dry” and vitamin A-deficient eyes. Invest Ophthalmol. 1970; 9: 827–843. [PubMed] [Google Scholar]
- 7. Li W, Hayashida Y, Chen YT, et al.. Air exposure induced squamous metaplasia of human limbal epithelium. Invest Ophthalmol Vis Sci. 2008; 49: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li W, Chen YT, Hayashida Y, et al.. Down-regulation of Pax6 is associated with abnormal differentiation of corneal epithelial cells in severe ocular surface diseases. J Pathol. 2008; 214: 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Soong HK, Martin NF, Wagoner MD, et al.. Topical retinoid therapy for squamous metaplasia of various ocular surface disorders. A multicenter, placebo-controlled double-masked study. Ophthalmology. 1988; 95: 1442–1446. [DOI] [PubMed] [Google Scholar]
- 10. Murube J, Rivas L.. Impression cytology on conjunctiva and cornea in dry eye patients establishes a correlation between squamous metaplasia and dry eye clinical severity. Eur J Ophthalmol. 2003; 13: 115–127. [DOI] [PubMed] [Google Scholar]
- 11. Li S, Gallup M, Chen YT, McNamara NA. Molecular mechanism of proinflammatory cytokine-mediated squamous metaplasia in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2010; 51: 2466–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen YT, Li S, Nikulina K, Porco T, Gallup M, McNamara N. Immune profile of squamous metaplasia development in autoimmune regulator-deficient dry eye. Mol Vis. 2009; 15: 563–576. [PMC free article] [PubMed] [Google Scholar]
- 13. Pascual G, Glass CK. Nuclear receptors versus inflammation: mechanisms of transrepression. Trends Endocrinol Metab. 2006; 17: 321–327. [DOI] [PubMed] [Google Scholar]
- 14. Chen Y, Zhang X, Yang L, et al.. Decreased PPAR-γ expression in the conjunctiva and increased expression of TNF-α and IL-1β in the conjunctiva and tear fluid of dry eye mice. Mol Med Rep. 2014; 9: 2015–2023. [DOI] [PubMed] [Google Scholar]
- 15. Jester JV, Potma E, Brown DJ. PPARγ regulates mouse meibocyte differentiation and lipid synthesis. Ocul Surf. 2016; 14: 484–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim SW, Xie Y, Nguyen PQ, et al.. PPARγ regulates meibocyte differentiation and lipid synthesis of cultured human meibomian gland epithelial cells (hMGEC). Ocul Surf. 2018; 16: 463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim SW, Brown DJ, Jester JV. Transcriptome analysis after PPARgamma activation in human meibomian gland epithelial cells (hMGEC). Ocul Surf 2019; 17: 809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tang L, Wang X, Wu J, et al.. Sleep deprivation induces dry eye through inhibition of PPARα expression in corneal epithelium. Invest Ophthalmol Vis Sci. 2018; 59: 5494–5508. [DOI] [PubMed] [Google Scholar]
- 19. Lin Z, Liu X, Zhou T, et al.. A mouse dry eye model induced by topical administration of benzalkonium chloride. Mol Vis. 2011; 17: 257–264. [PMC free article] [PubMed] [Google Scholar]
- 20. Laria C, Alio JL, RuizMoreno JM. Combined non-steroidal therapy in experimental corneal injury. Ophthalmic Res. 1997; 29: 145–153. [DOI] [PubMed] [Google Scholar]
- 21. Pauly A, Brignole-Baudouin F, Labbe A, Liang H, Warnet JM, Baudouin C. New tools for the evaluation of toxic ocular surface changes in the rat. Invest Ophthalmol Vis Sci. 2007; 48: 5473–5483. [DOI] [PubMed] [Google Scholar]
- 22. Sakamoto R, Bennett ES, Henry VA, et al.. The phenol red thread tear test: a cross-cultural study. Invest Ophthalmol Vis Sci. 1993; 34: 3510–3514. [PubMed] [Google Scholar]
- 23. Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000; 182: 311–322. [DOI] [PubMed] [Google Scholar]
- 24. Li S, Nikulina K, DeVoss J, et al.. Small proline-rich protein 1B (SPRR1B) is a biomarker for squamous metaplasia in dry eye disease. Invest Ophthalmol Vis Sci. 2008; 49: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murray PJ, Wynn TA. Obstacles and opportunities for understanding macrophage polarization. J Leukoc Biol. 2011; 89: 557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ann SJ, Chung JH, Park BH, et al.. PPARα agonists inhibit inflammatory activation of macrophages through upregulation of β-defensin 1. Atherosclerosis. 2015; 240: 389–397. [DOI] [PubMed] [Google Scholar]
- 27. Duffield JS. The inflammatory macrophage: a story of Jekyll and Hyde. Clin Sci (Lond). 2003; 104: 27–38. [DOI] [PubMed] [Google Scholar]
- 28. Bauer D, Hennig M, Wasmuth S, et al.. Amniotic membrane induces peroxisome proliferator-activated receptor-γ positive alternatively activated macrophages. Invest Ophthalmol Vis Sci. 2012; 53: 799–810. [DOI] [PubMed] [Google Scholar]
- 29. He H, Li W, Chen SY, et al.. Suppression of activation and induction of apoptosis in RAW264.7 cells by amniotic membrane extract. Invest Ophthalmol Vis Sci. 2008; 49: 4468–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meng CD, Zhu DD, Jiang XD, et al.. Overexpression of interleukin-l7 in tumor-associated macrophages is correlated with the differentiation and angiogenesis of laryngeal squamous cell carcinoma. Chin Med J (Engl). 2012; 125: 1603–1607. [PubMed] [Google Scholar]
- 31. El-Rouby DH. Association of macrophages with angiogenesis in oral verrucous and squamous cell carcinomas. J Oral Pathol Med. 2010; 39: 559–564. [DOI] [PubMed] [Google Scholar]
- 32. You IC, Coursey TG, Bian F, Barbosa FL, de Paiva CS, Pflugfelder SC. Macrophage phenotype in the ocular surface of experimental murine dry eye disease. Arch Immunol Ther Exp (Warsz). 2015; 63: 299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee HS, Amouzegar A, Dana R. Kinetics of corneal antigen presenting cells in experimental dry eye disease. BMJ Open Ophthalmol. 2017; 1: e000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pflugfelder SC, De Paiva CS, Villarreal AL, Stern ME. Effects of sequential artificial tear and cyclosporine emulsion therapy on conjunctival goblet cell density and transforming growth factor-β2 production. Cornea. 2008; 27: 64–69. [DOI] [PubMed] [Google Scholar]
- 35. Xiao X, He H, Lin Z, et al.. Therapeutic effects of epidermal growth factor on benzalkonium chloride-induced dry eye in a mouse model. Invest Ophthalmol Vis Sci. 2012; 53: 191–197. [DOI] [PubMed] [Google Scholar]