Abstract
Simple Summary
Bone health is an important factor in broiler production. Among the key nutrients affecting bone health, phosphorus (P) plays a great role. Enterococcus faecium has been widely used as feed additive to promote growth performance of broilers. There were reports suggesting that E. faecium improved skeletal health of rats. However, the effect of E. faecium on the bones of broilers remains unclear. The present study is to investigate the effect of E. faecium on P absorption and utilization in broilers and the associated changes in the gut microbiota. Dietary inclusion with E. faecium did not improve broiler performance in this study but improved P absorption and bone mineralization. In E. faecium-treated broilers, the expression of intestinal type IIb sodium-dependent phosphate cotransporter (NaP-IIb) mRNA was upregulated and the concentration of serum alkaline phosphatase was increased. Dietary supplementation with E. faecium changed the gut microbiota populations of broilers and increased the relative abundance of SCFA (short-chain fatty acid)-producing bacteria. The changed populations of microbiota improved intestinal P absorption and bone forming metabolic activities. In conclusion, dietary inclusion with E. faecium facilitates increased utilisation of P in broilers.
Abstract
Modern broiler chickens have ongoing bone health problems. Phosphorus (P) plays an important role in bone development and increased understanding of P metabolism should improve the skeletal health of broilers. Enterococcus faecium has been widely used as a probiotic in broiler production and is shown to improve skeletal health of rats, but its effect on the bones of broilers remains unclear. This study investigated the effect of E. faecium on P absorption and utilization in broilers and the associated changes in the gut microbiota using 16S rDNA sequencing. Dietary supplementation with E. faecium improved P absorption through upregulation of the expression of intestinal NaP-IIb mRNA and increased the concentration of serum alkaline phosphatase. These actions increased P retention and bone mineralization in E. faecium-treated broilers. The positive effects of E. faecium on P metabolism were associated with changes in the populations of the intestinal microbiota. There was increased relative abundance of the following genera, Alistipes, Eubacterium, Rikenella and Ruminococcaceae and a decrease in the relative abundance of Faecalibacterium and Escherichia-Shigella. Dietary supplementation with E. faecium changed gut microbiota populations of broilers, increased the relative abundance of SCFA (short-chain fatty acid)-producing bacteria, improved intestinal P absorption and bone forming metabolic activities, and decreased P excretion. E. faecium facilitates increased utilisation of P in broilers.
Keywords: broiler, phosphorus, Enterococcus faecium, microbiota, 16S rDNA
1. Introduction
Skeletal disorders and associated welfare problems are an ongoing issue for fast growing broiler chickens and a major concern throughout the global poultry industry [1,2]. Calcium (Ca) and phosphorus (P) are the most important minerals in bone development and comprise the inorganic component of bone tissue, providing hardness and strength to the skeleton [3]. Diets deficient or imbalanced in these co-dependent minerals severely decrease growth performance and nutrient retention of broilers [4,5]. Many studies have investigated the absorption and metabolism of Ca and P, and there is a greater understanding of the regulatory mechanisms controlling Ca metabolism than P metabolism [5]. In addition, eutrophication due to high P excretion is becoming more and more serious, which intensifies concerns about P utilization and the sustainability of broiler production [6].
The addition of probiotics to poultry diets has increased significantly in recent years as the use of antibiotic growth promoters has declined [7]. This has prompted much research into the use of new probiotic feed additives. E. faecium, a lactic acid bacterium and normal inhabitant in the gut, is a probiotic that can promote growth performance, can reduce mortality, can improve intestinal morphology and can beneficially modulate the gut microbiota of broilers [8,9,10]. These characteristics of E. faecium, along with the ability to increase the efficiency of intermediary metabolism [11] and to improve meat quality [12], have made it an attractive poultry feed additive. Moreover, some probiotics have shown beneficial effects on the skeletal health of broilers [13,14] and rodents [15]. These observations are consistent with probiotics modifying the gut environment, including the gut microbiota, and/or enhancing mineral absorption. Although the mode of action(s) of probiotics are poorly understood [7], modulation of the gut microbiota is likely to be important.
There is little research on the effect of E. faecium on bone health. However, it has been suggested that E. faecium can prevent whole body bone mineral density loss in arthritic rats [16]. It is therefore likely that E. faecium will have some positive effect on the bone health of broilers. The objective of the present study was to investigate the effects of dietary E. faecium on performance traits, bone strength, P absorption and gut microbiota of broilers and to explore the regulatory mechanism of E. faecium on P absorption and utilisation in broilers.
2. Materials and Methods
2.1. Ethics Statement
Feeding trials were conducted according to the guidelines for animal experiments set out by the National Institute of Animal Health, and all animal procedures were approved by the Chinese Academy of Agricultural Sciences (statement no. AEC-CAAS-20191106).
2.2. Experimental Design
A total of 120 1-day-old male, Arbor Acres (AA) broilers, were purchased from the Huadu Broiler Breeding Co. (Beijing, China) and housed in the Nankou experimental farm of the Feed Research Institute, CAAS, Beijing, China. The day-old chicks (body weight, 47.2 ± 0.31 g) were randomly divided into two groups: control and treatment. Each group had 6 cages (replicates) with 10 birds per cage. The chickens were reared in two stages, starter (1–21 days) and grower (22–42 days), and fed a basal (control) corn-soybean meal diet (Table 1) in pellet form, to which 6.75 × 109 cfu/g of E. faecium was added before pelleting for the treatment group. Microcapsules of E. faecium CGMCC 2516 (viable count ≥15 × 1010 cfu/g; Challenge Group, Beijing, China) were used in this study. E. faecium CGMCC 2516 were cultured in de Man Rogosa and Sharpe (MRS) medium, adding suitable concentrations of anhydrous calcium chloride. The culture was dried under the condition of 45 °C and was solid microencapsulated using coating technology [17,18].
Table 1.
Ingredient and nutrient composition of basal broiler diets.
| Ingredient | Starter (1–21 Days) (g/kg) | Grower (22–42 Days) (g/kg) |
|---|---|---|
| Corn | 593.1 | 604.2 |
| Soybean meal | 298.8 | 288.7 |
| Cotton seed meal | 50.0 | 30.0 |
| Soybean oil | 15.1 | 39.8 |
| L-Lysine | 1.5 | 0.9 |
| DL-Methionine | 1.4 | 1.6 |
| Limestone | 12.7 | 10.2 |
| CaHPO4 | 19.4 | 16.6 |
| NaCl | 3.0 | 3.0 |
| Choline chloride | 2.0 | 2.0 |
| Premix 1) | 1.3 | 1.3 |
| Zeolite powder | 1.7 | 1.7 |
| Total | 1000 | 1000 |
| Calculated nutrient level | ||
| Metabolic energy (MJ/kg) | 12.35 | 13.02 |
| Crude protein | 211.8 | 198.4 |
| Calcium | 10.1 | 8.5 |
| Available phosphorus | 4.5 | 4.0 |
| Total phosphorus | 6.9 | 6.3 |
| Lysine | 11.4 | 10.5 |
| Methionine | 4.9 | 4.8 |
| Methionine+Cysteine | 8.3 | 8.1 |
| Threonine | 7.7 | 2.2 |
1) The premix provided the following per kg diet: vitamin A 10,000 IU, vitamin D3 2000 IU, vitamin E 10 IU, vitamin K3 2.5 mg, vitamin B1 1 mg, vitamin B2 6 mg, vitamin B3 10 mg, vitamin B5 40 mg, vitamin B6 3 mg, vitamin B11 0.3 mg, vitamin B12 0.01 mg, biotin 0.12 mg, Cu (as copper sulphate) 8 mg, Fe (as ferrous sulphate) 80 mg, Mn (as manganese sulphate) 60 mg, Zn (as zinc sulphate) 40 mg, Se (as sodium selenite) 0.15 mg and I (as potassium iodide) 0.35 mg.
2.3. Bird Management
Birds were raised in accordance with the AA Broiler Management Guide [19]. Chicks were vaccinated for Marek’s disease at day-old and for Newcastle disease and infectious bronchitis at 7 days post-hatching. Room temperature was maintained at 33 °C for days 0–3 and was gradually reduced to 24 °C and maintained at 24 °C till the end of the study. The photoperiod was controlled to 16 h of light and 8 h of darkness. Relative humidity was set at 60–70% during the first week and then at 50–60% for the rest of the experiment.
2.4. Sample Collection and Parameter Determination
From days 18 to 21 and days 39 to 42 of the experiment, excreta from each replicate was collected, mixed and dried in an oven at 105 °C to a constant weight. The dried excreta were ashed in a muffle furnace at 550 °C for 4 h. The P content of the ash samples was determined using the vanadate-molybdate method [20].
On day 21 and day 42, body weight (BW) and feed intake were measured to calculate the average daily gain (ADG), average daily feed intake (ADFI) and the ratio of feed/gain (F/G). On those days, one broiler close to the cage average body weight was randomly selected from each replicate. The chosen birds were electrically stunned and manually slaughtered within 5 min [21]. Blood was collected from the jugular vein, and serum was obtained after centrifuging at 3000× g for 10 min at 4 °C and stored at −20 °C for further analysis. Serum alkaline phosphatase (ALP) and P were determined with a Hitachi 7600 automatic biochemical analyser using kits purchased from Nanjing Jiancheng Biological Engineering Institute.
After the blood sampling on day 42, the duodenum (about 10 cm distal to the pylorus), jejunum (about 10 cm preceding the Meckel’s diverticulum) and ileum (about 10 cm preceding the ileocecal junction) were separated [22] and flushed gently with saline solution. The mucosa samples were scraped with a coverslip and snap-frozen in liquid nitrogen for analysis of mRNA.
The right tibiae were cleaned and dried for determination of tibia weight and tibia breaking strength [23]. The bones were then ashed in a muffle furnace at 550 °C for 16 h [20]. After that, the tibia P content was measured using the vanadate-molybdate method.
2.5. RNA Extraction, Reverse Transcription and Real-Time Quantitative PCR
Total RNA was extracted using TRNzol-A + (TIANGEN, Beijing, China). The concentration of total RNA was estimated by a spectrophotometer (Ultrospec 2100 pro, GE Healthcare, Chicago, IL, USA), and the purity was determined by agarose gel electrophoresis. Five hundred nanagrams of total RNA were reverse transcribed into cDNA using the Fast Quant RT Kit (with gDNase) (TIANGEN). qPCR was conducted using the iCycler iQ5 system. The specific primers for NaP-IIb, type III sodium-dependent phosphate cotransporter-1,2 (PiT-1, 2) and β-actin are listed in Table 2. β-actin was used as internal reference gene. Relative gene expression was calculated using the 2-ΔΔCt method [24]. All the samples were analysed in triplicate, and the operational program for qPCR strictly followed the Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) [25].
Table 2.
Primer sequences of chicken NaP-IIb; PiT-1, 2; and β-actin.
| Gene | Primer Sequence (5’-3’) | Accession Number |
|---|---|---|
| NaP-IIb | F: CTGGATGCACTCCCTAGAGC R: TTATCTTTGGCACCCTCCTG |
NM_204474.1 |
| PiT-1 | F: GCTCGTGGCTTCGTTCTTG R: GACCATTTGACGCCTTTCT |
XM_015297502.1 |
| PiT-2 | F: GCAGCAGATACATCAACTC R: ATTTCCACTCCACCCTC |
NM_001305398·1 |
| β-actin | F: GAGAAATTGTGCGTGACATCA R: CCTGAACCTCTCATTGCCA |
NM_205518.1 |
2.6. Illumina Sequencing Analysis
The faecal samples were collected on day 42 and snap-frozen in liquid N2 prior to further processing. Gene sequencing (16S rDNA) was performed by OE Biotech Co., Ltd. (Shanghai, China). Total genomic DNA from frozen faecal samples was isolated using the GenElute™°Stool DNA Isolation Kit (Sigma-Aldrich, St. Louis, MO, USA); then, the V3–V4 hypervariable region of the 16S rDNA genes was amplified. The PCR products were collected and sequenced using the Illumina MiSeq platform (Illumina, San Diego, CA, USA). High-quality reads were clustered into operational taxonomic units (OTUs) based on sequences with ≥97% similarity and then analysed using the QIIME platform.
2.7. Statistical Analysis
The statistical analyses were performed using SPSS 17.0. The data were statistically analysed by T-Test (independent samples). For the indexes with significant main effect, Duncan’s method was used to compare the mean values among groups. A p-value less than 0.05 was considered significant.
3. Results
3.1. Growth Performance
The birds grew normally and remained in good health throughout the experiment. The effects of dietary E. faecium on growth performance of broilers is shown in Table 3. In the starter stage (1–21 days), dietary supplementation with E. faecium decreased (p < 0.05) the ADFI of broilers but did not affect (p > 0.05) BW, ADG and F/G. In the grower stage (22–42 days), E. faecium groups showed a tendency to numerically increase BW and ADG and to decrease ADFI and F/G of broilers.
Table 3.
Dietary E. faecium supplementation and broiler growth performance.
| Treatment | BW(g) | ADG(g/d) | ADFI(g/d) | F/G |
|---|---|---|---|---|
| Starter (1–21 days) | ||||
| Control | 771.11 ± 20.45 | 38.56 ± 1.02 | 51.04 ± 0.52 a | 1.374 ± 0.06 |
| E. faecium | 792.75 ± 18.28 | 39.64 ± 0.91 | 44.75 ± 1.49 b | 1.251 ± 0.02 |
| p Value | 0.460 | 0.453 | 0.019 | 0.102 |
| Grower (22–42 days) | ||||
| Control | 2207 ± 38.89 | 73.73 ± 2.79 | 152.47 ± 3.69 | 2.116 ± 0.03 |
| E. faecium | 2262 ± 25.48 | 76.36 ± 1.36 | 151.95 ± 5.07 | 2.071 ± 0.10 |
| p Value | 0.267 | 0.422 | 0.936 | 0.670 |
a,b Mean values with dissimilar superscript letters within the same list are significantly different (p < 0.05). BW, body weight; ADG, average daily gain; ADFI, average daily feed intake; F/G, feed/gain.
3.2. Ash and P of Excreta, Serum P and ALP Concentrations
Supplementation with E. faecium did not affect excreta the ash content during either growth stage or the P excreta content of starter chicks but did decrease (p < 0.05) P excretion in the grower stage (Table 4). No difference in serum P concentration was observed between the two groups of broilers. However, serum ALP increased significantly (p < 0.05) in both the starter and grower stages when compared to the control group.
Table 4.
Dietary E. faecium supplementation and excreta ash and P content, serum P concentration and alkaline phosphatase (ALP) of broilers.
| Treatment | Ash (g/kg Excreta) | P (g/kg Excreta) | P (mmol/L) | ALP (U/L) |
|---|---|---|---|---|
| Starter (1–21 days) | ||||
| Control | 14.86 ± 0.36 | 1.24 ± 0.02 | 1.50 ± 0.06 | 3291 ± 178 b |
| E. faecium | 15.11 ± 0.32 | 1.31 ± 0.05 | 1.64 ± 0.04 | 4099 ± 123 a |
| p Value | 0.611 | 0.151 | 0.073 | 0.010 |
| Grower (22–42 days) | ||||
| Control | 15.58 ± 0.17 | 1.38 ± 0.03 a | 1.47 ± 0.05 | 1843 ± 176 b |
| E. faecium | 15.68 ± 0.21 | 1.28 ± 0.01 b | 1.52 ± 0.06 | 2787 ± 166 a |
| p Value | 0.172 | 0.050 | 0.513 | 0.005 |
a,b Mean values with dissimilar superscript letters within the same list are significantly different (p < 0.05).
3.3. P and Ash of Bone, and Tibia Strength
In the starter stage, P and ash content of bone were not influenced by E. faecium supplementation (Table 5), but in the grower stage, the probiotic significantly (p < 0.05) increased both parameters, while tibia strength was not affected in the starter or grower stage.
Table 5.
Dietary E. faecium supplementation on P and ash content of bone, ash of bone and tibia strength of broilers.
| Treatment | P of Bone, % | Ash of Bone, % | Tibia Strength, g |
|---|---|---|---|
| Starter (1–21 days) | |||
| Control | 8.54 ± 0.55 | 53.51 ± 0.59 | 12868 ± 798 |
| E. faecium | 8.80 ± 0.16 | 54.25 ± 0.83 | 13408 ± 2440 |
| p Value | 0.847 | 0.491 | 0.840 |
| Grower (22–42 days) | |||
| Control | 8.34 ± 0.34 b | 52.16 ± 0.27 b | 23632 ± 1253 |
| E. faecium | 10.99 ± 0.40 a | 56.32 ± 1.84 a | 22896 ± 1712 |
| p Value | 0.002 | 0.035 | 0.736 |
a,b Mean values with unlike superscript letters within the same list are significantly different (p < 0.05).
3.4. NaP-IIb and PiT-1, 2 mRNA Expressions in the Duodenum, Jejunum and Ileum of Broilers
Dietary supplementation of E. faecium significantly increased (p < 0.05) NaP-IIb mRNA expression levels in the duodenum, jejunum and ileum (Table 6). However, PiT-1 and PiT-2 mRNA expressions were not affected in the duodenum and jejunum but increased in the ileum of the E. faecium-treated group.
Table 6.
Dietary E. faecium supplementation and NaP-IIb and PiT-1, 2 mRNA expression in small intestinal segments of broilers.
| Treatment | NaP- IIb | PiT-1 | PiT-2 |
|---|---|---|---|
| Duodenum | |||
| Control | 1.000 ± 0.20 b | 5.861 ± 4.87 | 3.969 ± 2.97 |
| E. faecium | 2.335 ± 0.24 a | 6.106 ± 6.58 | 4.547 ± 0.73 |
| p Value | 0.013 | 0.255 | 0.878 |
| Jejunum | |||
| Control | 3.930 ± 1.13 b | 3.703 ± 3.08 | 1.791 ± 1.34 |
| E. faecium | 11.291 ± 1.16 a | 10.176 ± 4.16 | 3.705 ± 1.67 |
| p Value | 0.007 | 0.279 | 0.406 |
| Ileum | |||
| Control | 1.117 ± 0.13 b | 0.893 ± 0.08 b | 1.111 ± 0.05 a |
| E. faecium | 4.265 ± 0.59 a | 8.87 ± 2.08 a | 3.523 ± 0.54 b |
| p Value | 0.029 | 0.019 | 0.046 |
a,b Mean values with dissimilar superscript letters within the same list are significantly different (p < 0.05).
3.5. Gut Microbiota Analysis
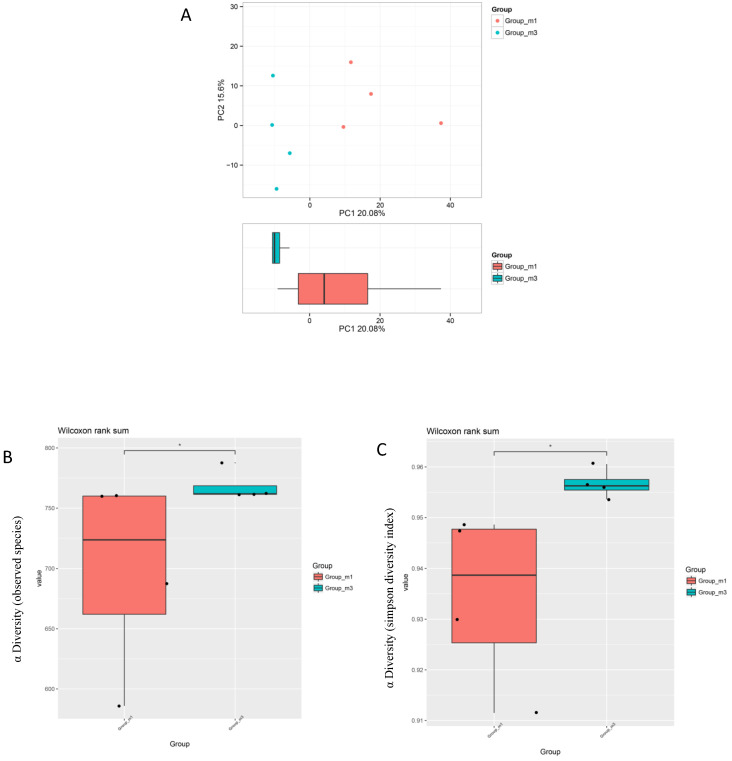
As revealed by principal component analysis (PCA), dietary supplementation with E. faecium changed the populations of the gut microbiota of broilers (Figure 1A). In addition, E. faecium led to a significant increase in the observed species and Simpson indices with respect to the control values (Figure 1B,C), suggesting that E. faecium exerted stronger positive effects on the α diversity of the gut microbiota of broilers.
Figure 1.
E. faecium-modulated gut microbiota of broilers: (A) principal component analysis (PCA) scores indicated the difference in gut microbiota populations and (B,C) observed species and Simpson diversity indexes of the gut microbiota. m1, control. m3, E. faecium. ns, not significant. * p < 0.05.
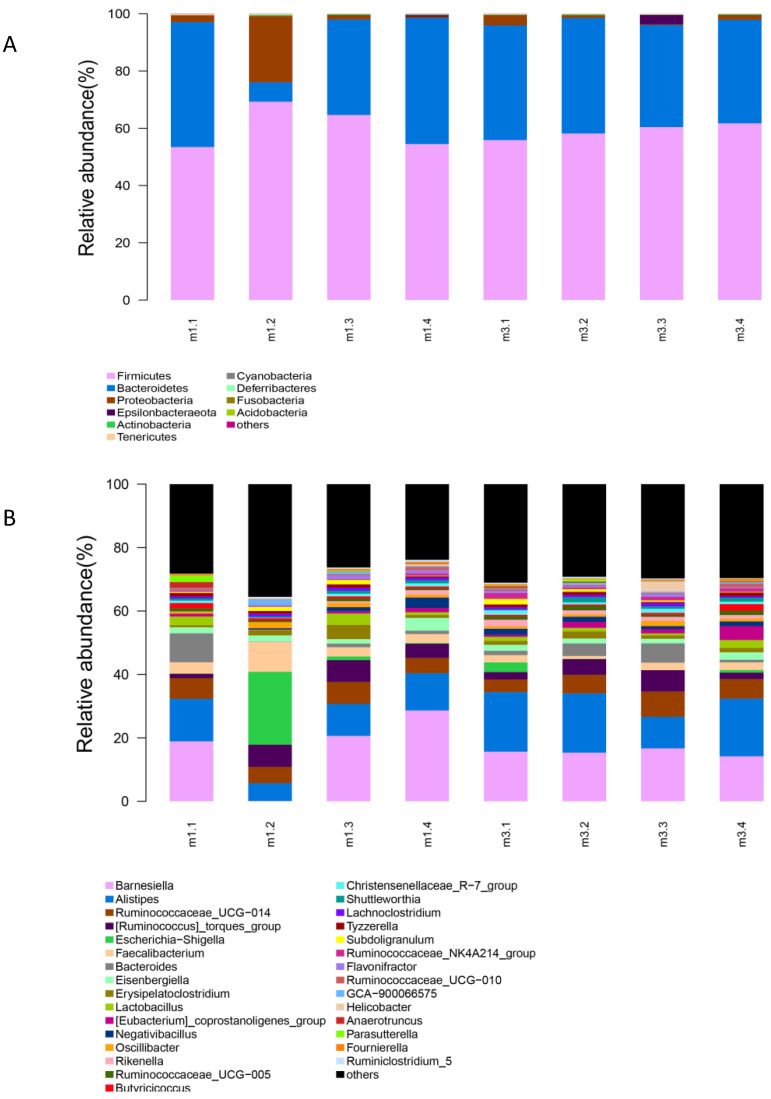
The gut microbiota composition at phylum and genus levels is shown in Figure 2. The predominant phyla were Firmicutes, Bacteroidetes, Proteobacteria, Epsilonbacteraeota, Actinobacteria and Tenericutes, representing 59.8%, 35.0%, 4.25%, 0.49%, 0.21% and 0.20% of the total sequences, respectively (Figure 2A). There were no significant differences in phylum level between E. faecium-treated and control birds. Compared to the control group, a higher abundance of Bacteroidetes and a lower abundance of Proteobacteria were observed in the E. faecium-treated group. The genus level analysis revealed that E. faecium mainly increased the relative abundance of Alistipes, Eubacterium, Rikenella and Ruminococcaceae, while the relative abundances of Faecalibacterium and Escherichia-Shigella were decreased (Figure 2B).
Figure 2.
The gut microbiota composition is shown at the phylum level (A) and genus level (B). m1, control. m3, E. faecium.
We compared the gut microbiota of the two experimental groups using linear discriminant analysis effect size (LEfSe) to identify the specific microbiota linked to E. faecium treatment. Peptoclostridium, Ruminococcaceae, Papillibacter and Eubacterium were more abundant in the E. faecium groups (Figure 3A,B).
Figure 3.
The taxonomic cladogram (A) and the LDA (linear discriminant analysis) score (B) obtained from linear discriminant analysis effect size (LEfSe) analysis of the gut microbiota in different groups. m1, control. m3, E. faecium diet.
4. Discussion
The current study showed that dietary supplementation of E. faecium improved the growth performance of broilers but not significantly. These results were consistent with previous studies which suggested that inclusion of a probiotic had no effects or a slight improvement on growth performance of broilers [9,26]. However, in other studies using various probiotic strains, significant improvement in growth performance of broilers has been demonstrated [27,28,29,30]. The different outcomes in broiler growth performance are a response to many factors including the probiotic strain used and the experimental conditions. Chickens raised in less optimal conditions usually have inflammatory immune reactions in the intestinal mucosa and abnormal gut pH, which would exert bad influence on the growth performance of birds and give opportunities to probiotics to get the largest effect [31,32].
Bone metabolism largely mediates P and Ca metabolism, which are closely related but differ in relation to the endocrine control of absorption and renal reabsorption/excretion [33]. Dietary P is absorbed and accumulated in the small intestinal mucosa and then released into the blood gradually [34], where concentrations are maintained by homeostasis [35], as was evident in the current study. Previous studies in rats, humans and chickens have suggested that probiotics could promote intestinal absorption of P [36,37,38,39]. In this study, E. faecium increased the P content of bone. This result reflects increased P accretion in bone, where the bone-forming cells, osteoblasts, are responsible for the deposition of the bone matrix [40]. The concentration of ALP in serum, a marker of osteoblast activity, is usually elevated when bone formation rates are increased [41]. In the current study, ALP levels in serum of the E. faecium group were significantly higher than the control in both starter and grower stages, resulting in greater retention of P.
The increase in P deposition reflects both increased absorption and reduced excretion. The decreased concentration of P in excreta may reflect both enhanced intestinal absorption and renal reabsorption. Much of the intestinal absorption of P is accomplished by the sodium-dependent transporter NaP-IIb, which is primarily expressed in the duodenum of broilers and is the most important P transporter in the small intestine [42]. In our study, mRNA expression levels of NaP-IIb were increased in the small intestine of E. faecium-treated broilers, indicating that increased P absorption occurred in this study. The elevation of NaP-IIb expression levels in the E. faecium group may have resulted from increased available P in the intestine. The expression of NaP-IIb mRNA increases when the level of dietary P increases and is concentration dependent [22]. Some probiotics produce phytase [43], which would enhance phytate digestibility, releasing P for absorption. Perhaps E. faecium produces a phytase or facilitates increased phytase activity by the intestinal microbiota.
In the current study, the populations of ceacal microbiota was changed in the E. faecium group compared with the control group. E. faecium treatment resulted in a dramatic elevation in observed species and Simpson indices, indicating that the richness and diversity of the microbiota was significantly increased. Previous studies have shown that inclusion of E. faecium in broiler diets beneficially alters the gut microbiota [8,44]. Our results demonstrate that dietary E. faecium can modulate the intestinal flora of broilers, as indicated by 16S rDNA-based analysis. As a probiotic, E. faecium can exert antagonistic functions via competition for nutrients, metabolites and an occupying effect. In this study, the proliferation of potentially pathogenic bacteria, Faecalibacterium and Escherichia-Shigella, was inhibited. This is consistent with previous studies in which reduced relative abundance of Faecalibacterium and Escherichia-Shigella was also observed in E. faecium-feeding piglets [45,46]. Alistipes, initially isolated from human gut, is a strict anaerobe that produces succinic acid as the principal metabolic end-product of glucose fermentation [47,48]. Mice studies suggest that members of this genus affect host physiology, including sites distal to the gastrointestinal tract [49]. Rikenella has been isolated from faeces and caeca of a variety of animals including chickens. It is an obligate anaerobe and yields propionic and succinic acids together with moderate amounts of acetic acid from glucose fermentation [50]. The other two upregulated genera, Eubacterium and Ruminococcaceae, are generally associated with increased butyrate production [51], which is an important energy substrate for intestinal enterocytes. In general, the upregulated genera in the E. faecium group all are associated with increased SCFA production. SCFA production followed by a decrease in gut pH leads to increased mineral solubilization [52], which would help P absorption via the elevation of NaP-IIb expression levels because when the content of available P in the intestine is increased, the expression of NaP-IIb mRNA will increase accordingly [22]. Bone mineralization enhanced by changes in gut microbiota has also been proven in rats. Report showed that dietary supplementation with synbiotics exerted a synergistic effect on bone mineralization of rats, which was associated with higher counts of SCFA production genera and a reduced pH in the intestine [53]. Additionally, increased circulating concentrations of SCFA can interact with the skeleton to directly inhibit bone resorptive osteoclast differentiation and to activate bone forming osteoblasts, thus increasing bone mass and preventing bone loss [54,55].
5. Conclusions
In conclusion, regardless of the mechanism(s), E. faecium facilitates increased utilisation of P in broilers. Dietary supplementation with E. faecium increased the relative abundance of SCFA-producing bacteria, improved intestinal absorption of P and bone forming metabolic activities, and decreased P excretion. Further research is required to more clearly define the metabolic actions of probiotics to permit their strategic use. The results of this study are, however, another illustration of the benefits of supplementing poultry diets with probiotics.
Author Contributions
W.L.B. and A.Z. (Aijuan Zheng) contributed to the conception and design of the work. H.C. and G.L. contributed to the design of the work. W.W. and A.Z. (Anrong Zhang) executed the experiments. Z.C., W.C., A.Z. (Aijuan Zheng) and X.D. contributed to the analysis and interpretation of the data. W.W. and A.Z. (Aijuan Zheng) drafted the manuscript. W.W., W.L.B. and A.Z. (Aijuan Zheng) contributed to the final approval of the version for publication. All authors have read and agreed to the published version of the manuscript.
Funding
Sponsored by (No. 2018YFD0500600) the National Key Research and Development Program of China.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Rath N.C., Huff G.R., Huff W.E., Balog J.M. Factors regulating bone maturity and strength in poultry. Poult. Sci. 2000;79:1024–1032. doi: 10.1093/ps/79.7.1024. [DOI] [PubMed] [Google Scholar]
- 2.Fleming, Robert H. Nutritional factors affecting poultry bone health. P Nutr. Soc. 2008;67:177–183. doi: 10.1017/S0029665108007015. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro R., Heaney R.P. Co-dependence of calcium and phosphorus for growth and bone development under conditions of varying deficiency. Bone. 2003;32:532–540. doi: 10.1016/S8756-3282(03)00060-7. [DOI] [PubMed] [Google Scholar]
- 4.Gautier A.E., Walk C.L., Dilger R.N. Influence of dietary calcium concentrations and the calcium-to-non-phytate phosphorus ratio on growth performance, bone characteristics, and digestibility in broilers. Poult. Sci. 2017;96:2795–2803. doi: 10.3382/ps/pex096. [DOI] [PubMed] [Google Scholar]
- 5.Li X., Zhang D., Bryden W.L. Calcium and phosphorus metabolism and nutrition of poultry: Are current diets formulated in excess? Anim. Prod. Sci. 2017;57:2304–2310. doi: 10.1071/AN17389. [DOI] [Google Scholar]
- 6.Li X., Zhang D., Yang T.Y., Bryden W.L. Phosphorus bioavailability: A key aspect for conserving this critical animal feed resource with reference to broiler nutrition. Agriculture. 2016;6:25. doi: 10.3390/agriculture6020025. [DOI] [Google Scholar]
- 7.Bajagal Y.S., Klieve A.V., Dart P.J., Bryden W.L. FAO Animal Production and Health Paper No. 179. FAO; Rome, Italy: 2016. [(accessed on 16 February 2020)]. Probiotics in animal nutrition: Production, impact and regulation. Available online: http://www.fao.org/3/a-i5933e.pdf. [Google Scholar]
- 8.Cao G.T., Zeng X.F., Chen A., Zhou L., Zhang L., Xiao Y.P., Yang C.M. Effects of a probiotic, Enterococcus faecium, on growth performance, intestinal morphology, immune response, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poult. Sci. 2013;92:2949–2955. doi: 10.3382/ps.2013-03366. [DOI] [PubMed] [Google Scholar]
- 9.Luo J.J., Zheng A.J., Meng K., Chang W.H., Bai Y.G., Li K., Cai H., Liu G., Yao B. Proteome changes in the intestinal mucosa of broiler (Gallus gallus) activated by probiotic Enterococcus faecium. J. Proteom. 2013;91:226–241. doi: 10.1016/j.jprot.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 10.Huang L.Q., Luo L.Q., Zhang Y.R., Wang Z., Xia Z.F. Effects of the dietary probiotic, Enterococcus faecium NCIMB11181, on the intestinal barrier and system immune status in Escherichia coli O78-challenged broiler chickens. Probiotics Antimicrob. Proteins. 2019;11:946–956. doi: 10.1007/s12602-018-9434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng A.J., Luo J.J., Meng K., Li J.K., Bryden W.L., Chang W.H., Zhang S., Wang L.X.N., Liu G., Yao B. Probiotic (Enterococcus faecium) induced responses of the hepatic proteome improves metabolic efficiency of broiler chickens (Gallus gallus) BMC Genom. 2016;17:89. doi: 10.1186/s12864-016-2371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng A.J., Luo J.J., Meng K., Li J.K., Zhang S., Li K., Liu G., Cai H., Bryden W.L., Yao B. Proteome changes underpin improved meat quality and yield of chickens (Gallus gallus) fed the probiotic Enterococcus faecium. BMC Genom. 2014;15:1167. doi: 10.1186/1471-2164-15-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan F.F., Murugesan G.R., Cheng H.W. Effects of probiotic supplementation on performance traits, bone mineralization, cecal microbial composition, cytokines and corticosterone in laying hens. Animal. 2018;13:33–41. doi: 10.1017/S175173111800109X. [DOI] [PubMed] [Google Scholar]
- 14.Yan F.F., Mohammed A., Murugesan R., Cheng H.W. Effects of a dietary synbiotic inclusion on bone health in broilers subjected to cyclic heat stress episodes. Poult. Sci. 2018;98:1083–1089. doi: 10.3382/ps/pey508. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez C.J., Guss J.D., Luna M., Goldring S.R. Links between the microbiome and bone. J. Bone Miner. Res. 2016;31:1638–1646. doi: 10.1002/jbmr.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mccabe L.R., Britton R.A., Parameswaran N. Prebiotic and probiotic regulation of bone health: Role of the intestine and its microbiome. Curr. Osteoporos. Rep. 2015;13:363–371. doi: 10.1007/s11914-015-0292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang T.T., Li A.K., Tao H.H., Yi J.M. Study on the production technology and properties of microencapsulated Enterococcus faecium. JNWAFU. 2009;37:51–62. [Google Scholar]
- 18.Wang T.T., Li A.K., Yi J.M., Tao H.H., Wang Y. Screening, identification and characterization of Enterococcus faecium for feed use. J. Chin. Cereal Oils Assoc. 2010;25:89–93, 97. (In Chinese) [Google Scholar]
- 19.Aviagen. [(accessed on 10 June 2019)]; Available online: http://cn.aviagen.com/assets/Tech_Center/AA_Broiler/AA-Broiler-Pocket-Guide-2020-EN.pdf.
- 20.Liu S.B., Liao X.D., Lu L., Li S.F., Wang L., Zhang L.Y., Jiang Y., Luo X.G. Dietary non-phytate phosphorus requirement of broilers fed a conventional corn-soybean meal diet from 1 to 21 d of age. Poult. Sci. 2016;96:151–159. doi: 10.3382/ps/pew212. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L., Yue H.Y., Zhang H.J., Xu L., Wu H.J., Yan H.J., Gong Y.S., Qi G.H. Transport stress in broilers: I. Blood metabolism, glycolytic potential, and meat quality. Poult. Sci. 2009;88:2033–2041. doi: 10.3382/ps.2009-00128. [DOI] [PubMed] [Google Scholar]
- 22.Hu Y.X., Liao X.D., Wen Q., Lu L., Zhang L.Y., Luo X.G. Phosphorus absorption and gene expression levels of related transporters in the small intestine of broilers. Br. J. Nutr. 2018;119:1346–1354. doi: 10.1017/S0007114518000934. [DOI] [PubMed] [Google Scholar]
- 23.Frost T.J., Roland D.A. Research note: Current methods used in determination and evaluation of tibia strength: A correlation study involving birds fed various levels of cholecalciferol. Poult. Sci. 1991;70:1640–1643. doi: 10.3382/ps.0701640. [DOI] [PubMed] [Google Scholar]
- 24.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−△△ct method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J.F., Kubista M., Mueller R., Nolan T., Pfaffl M.W., Shipley G.L., et al. The MIQE guidelines: Minimum information for publication of quantitative Real-Time PCR experiments. Clin. Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 26.Shewita R., Elkatcha M., Soltan M., Sedeik M. Impact of using Enterococcus faecium as a probiotic in broiler diets. AJVS. 2016;51:102–113. doi: 10.5455/ajvs.241876. [DOI] [Google Scholar]
- 27.Shokryazdan P., Jahromi M.F., Liang J.B., Ramasamy K., Sieo C.C., Ho Y.W. Effects of a Lactobacillus salivarius mixture on performance, intestinal health and serum lipids of broiler chickens. PLoS ONE. 2017;12:e0175959. doi: 10.1371/journal.pone.0175959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L., Zhang L.L., Zhan X.A., Zeng X.F., Zhou L., Cao G.T., Chen A., Yang C. Effects of dietary supplementation of probiotic, Clostridium butyricum, on growth performance, immune response, intestinal barrier function, and digestive enzyme activity in broiler chickens challenged with Escherichia coli K88. J. Anim. Sci. Biotechno. 2016;7:3. doi: 10.1186/s40104-016-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gadde U., Oh S.T., Lee Y.S., Davis E., Zimmerman N., Rehberger T., Lillehoj H.S. The effects of direct-fed microbial supplementation, as an alternative to antibiotics, on growth performance, intestinal immune status, and epithelial barrier gene expression in broiler chickens. Probiotics Antimicrob. Proteins. 2017;9:397–405. doi: 10.1007/s12602-017-9275-9. [DOI] [PubMed] [Google Scholar]
- 30.Abdel-Hafeez H.M., Saleh E.S.E., Tawfeek S.S., Youssef I.M.I., Abdeldaim A.S.A. Effects of probiotic, prebiotic, and synbiotic with and without feed restriction on performance, hematological indices and carcass characteristics of broiler chickens. Asian-Australas. J. Anim. Sci. 2016;30:672–682. doi: 10.5713/ajas.16.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugiharto S., Yudiarti T., Isroli I., Widiastuti E., Kusumanti E. Dietary supplementation of probiotics in poultry exposed to heat stress—A review. Ann. Anim. Sci. 2016;17:591–604. doi: 10.1515/aoas-2016-0062. [DOI] [Google Scholar]
- 32.Alloui M.N., Szczurek W., Sylwester Ś. The usefulness of prebiotics and probiotics in modern poultry nutrition: A review. Ann. Anim. Sci. 2013;13:17–32. doi: 10.2478/v10220-012-0055-x. [DOI] [Google Scholar]
- 33.Shini S., Zhang D., Aland R.C., Li X., Dart P.J., Callaghan M.J., Speight R.E., Bryden W.L. Probiotic Bacillus amyloliquefaciens H57 ameliorates subclinical necrotic enteritis in broiler chicks by maintaining intestinal mucosal integrity and improving feed efficiency. Poult. Sci. 2020 doi: 10.1016/j.psj.2020.05.034. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wasserman R.H., Taylor A.N. Intestinal absorption of phosphate in the chick: Effect of vitamin D3 and other parameters. J. Nutr. 1973;103:586–599. doi: 10.1093/jn/103.4.586. [DOI] [PubMed] [Google Scholar]
- 35.Proszkowiec-Weglarz M., Angel R. Calcium and phosphorus metabolism in broilers: Effect of homeostatic; mechanism on calcium and phosphorus digestibility. J. Appl. Poult. Res. 2013;22:609–627. doi: 10.3382/japr.2012-00743. [DOI] [Google Scholar]
- 36.Pérez-Conesa D., López G., Ros G. Effect of probiotic prebiotic and synbiotic follow-up infant formulas on large intestine morphology and bone mineralisation in rats. J. Sci. Food Agr. 2007;87:1059–1068. doi: 10.1002/jsfa.2812. [DOI] [Google Scholar]
- 37.Rodrigues F.C., Castro A.S.B., Rodrigues V.C., Fernandes S.A., Fontes E.A.F., Oliveira T.T.D., Martino H.S.D., de Luces Fortes Ferreira C.L. Yacon flour and Bifidobacterium longum modulate bone health in rats. J. Med. Food. 2012;15:664–670. doi: 10.1089/jmf.2011.0296. [DOI] [PubMed] [Google Scholar]
- 38.Narva M., Nevala R., Poussa T., Korpela R. The effect of Lactobacillus helveticus fermented milk on acute changes in calcium metabolism in postmenopausal women. Eur. J. Nutr. 2004;43:61–68. doi: 10.1007/s00394-004-0441-y. [DOI] [PubMed] [Google Scholar]
- 39.Mutu R., Kocabagli N., Alp M., Acar N., Eren M., Gezen S.S. The effect of dietary probiotic supplementation on tibial bone characteristics and strength in broilers. Poult. Sci. 2006;85:1621. doi: 10.1093/ps/85.9.1621. [DOI] [PubMed] [Google Scholar]
- 40.Gong Y., Slee R.B., Fukai N., Rawadi G., Roman-Roman S., Reginato A.M., Wang H., Cundy T., Glorieux F.H., Lev D., et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/S0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 41.Rawadi G., Vayssière B., Dunn F., Baron R., Romanroman S. BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J. Bone Miner. Res. 2003;18:1842–1853. doi: 10.1359/jbmr.2003.18.10.1842. [DOI] [PubMed] [Google Scholar]
- 42.Liu S.B., Hu Y.X., Liao X.D., Lu L., Li S.F., Zhang L.Y., Tan H.Z., Yang L., Suo H.Q., Luo X.G. Kinetics of phosphorus absorption in ligated small intestinal segments of broilers. J. Anim. Sci. 2016;94:3312. doi: 10.2527/jas.2016-0430. [DOI] [PubMed] [Google Scholar]
- 43.Andrabi S.T., Bhat B., Gupta M., Bajaj B.K. Phytase-producing potential and other functional attributes of lactic acid bacteria isolates for prospective probiotic applications. Probiotics Antimicrob. Proteins. 2016;8:121–129. doi: 10.1007/s12602-016-9220-3. [DOI] [PubMed] [Google Scholar]
- 44.Gheisar M.M., Hosseindoust A., Kim I.H. Effects of dietary Enterococcus faecium on growth performance, carcass characteristics, faecal microbiota, and blood profile in broilers. Vet. Med. Czech. 2016;61:28–34. doi: 10.17221/8680-VETMED. [DOI] [Google Scholar]
- 45.Xiang M., Li Y.Y., Guo Q.P., Ren Y.X., Liang S.Z., Huang Y. Effects of Enterococcus faecium on dominant phyla and genera of colonic microbial communities in suckling piglets. J. South. Agric. 2019;50:477–484. [Google Scholar]
- 46.Bednorz C., Guenther S., Oelgeschlager K., Kinnemann B., Pieper R., Hartmann S., Tedin K., Semmler T., Neuman K., Schierack P., et al. Feeding the probiotic Enterococcus faecium strain NCIMB 10415 to piglets specifically reduces the number of Escherichia coli pathotypes that adhere to the gut mucosa. Appl. Environ. Microb. 2013;79:7896–7904. doi: 10.1128/AEM.03138-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song Y.L., Kononen E., Rautio M., Liu C.X., Bryk A., Eerola E., Finegold S.M. Alistipes onderdonkii sp. nov. and Alistipes shahii sp. nov., of human origin. Int. J. Syst. Evol. Microbiol. 2006;56:1985–1990. doi: 10.1099/ijs.0.64318-0. [DOI] [PubMed] [Google Scholar]
- 48.Rautio M., Eerola E., Nen-Tunkelrott M.V.I., Molitoris D., Lawson P.A., Collins M.D., Lawson P., Collins M.D., Jousimies-Somer H. Reclassification of Bacteroides putredinis (Weinberg et al., 1937) in a new genus Alistipes gen. nov., as Alistipes putredinis comb. nov., and description of Alistipes finegoldii sp. nov., from human sources. Syst. Appl. Microbiol. 2003;26:182–188. doi: 10.1078/072320203322346029. [DOI] [PubMed] [Google Scholar]
- 49.Mcintosh C.M., Chen L.Q., Shaiber A., Eren A.M., Alegre M. Gut microbes contribute to variation in solid organ transplant outcomes in mice. Microbiome. 2018;6:96. doi: 10.1186/s40168-018-0474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Collins M.D., Shah H.N., Mitsuoka T. Reclassification of Bacteroides microfusus (Kaneuchi and Mitsuoka) in a new genus Rikenella, as Rikenella microfusus comb. nov. Syst. Appl. Microbiol. 1985;6:79–81. doi: 10.1016/S0723-2020(85)80015-1. [DOI] [Google Scholar]
- 51.Chen D.F., Jin D.C., Huang S.M., Wu J.Y., Xu M.Q., Liu T.Y., Dong W., Liu X., Wang S., Zhong W., et al. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Lett. 2020;469:456–467. doi: 10.1016/j.canlet.2019.11.019. [DOI] [PubMed] [Google Scholar]
- 52.Kolsoom P., Rosita J., Golgis K., Reza E. Effect of probiotics supplementation on bone mineral content and bone mass density. Sci. World J. 2014;2014:595962. doi: 10.1155/2014/595962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scholz-Ahrens K.E., Adolphi B., Rochat F., Barclay D., de Vrese M., Açil Y. Effects of probiotics, prebiotics, and synbiotics on mineral metabolism in ovariectomized rats—Impact of bacterial mass, intestinal absorptive area and reduction of bone turn-over. Nfs J. 2016;3:41–50. doi: 10.1016/j.nfs.2016.03.001. [DOI] [Google Scholar]
- 54.Lucas S., Omata Y., Hofmann J., Bottcher M., Ijazovic A., Sarter K., Albrecht O., Schulz O., Krishnacoumar B., Krönke G., et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 2018;9:55. doi: 10.1038/s41467-017-02490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iwami K., Moriyama T. Effects of short chain fatty acid, sodium butyrate, on osteoblastic cells and osteoclastic cells. Int. J. Biochem. 1993;25:1631–1635. doi: 10.1016/0020-711X(93)90522-G. [DOI] [PubMed] [Google Scholar]